Abstract
Lactoferrin (LF) is a multifunctional immunoregulatory protein that has been associated with host defense at mucosal surfaces through its antibacterial properties. The antibacterial and anti-inflammatory properties of LF were further explored with an animal model of experimental urinary tract infection. Bovine LF (bLF), human LF (hLF), and synthetic peptide sequences based on the antibacterial region of hLF (amino acid residues 16 to 40 [HLD1] and 18 to 40 [HLD2]) were given orally to female mice 30 min after the instillation of 108 Escherichia coli bacteria into the urinary bladder. The control groups received phosphate-buffered saline or water. C3H/Tif mice were treated with hLF or bLF, and C3H/HeN mice were treated with bLF only. The numbers of bacteria in the kidneys and bladder of C3H/Tif and C3H/HeN mice were significantly reduced 24 h later by the LF treatments compared to the findings for the control group. The hLF-treated group showed the strongest reduction compared with the vehicle-treated-group (P values were 0.009 and 0.0001 for the kidneys and bladder, respectively). The urinary leukocyte response was diminished in the hLF-treated group. The hLF treatment also significantly reduced the urinary interleukin-6 (IL-6) levels at 2 h and the systemic IL-6 levels at 24 h after infection (P values were 0.04 and < 0.002, respectively). In the bLF-treated animals, no such strong anti-inflammatory effects were obtained. In another series of experiments, C3H/Tif mice perorally treated with HLD1 or HLD2 also showed reduced numbers of bacteria in the kidneys compared with the vehicle-treated mice, although the results were significantly different only for HLD2 (P < 0.01). Analysis of urine from hLF-fed C3H/Tif mice showed that hLF was excreted into the urinary tract at 2 h after feeding. Testing of the in vitro bactericidal activity of LF (1 mg/ml) or the peptides (0.1 mg/ml) in mouse urine against the E. coli bacteria revealed moderate killing only by HLD2. In conclusion, these results demonstrate for the first time that oral administration of hLF or peptides thereof is effective in reducing infection and inflammation at a remote site, the urinary tract, possibly through transfer of hLF or its peptides to the site of infection via renal secretion. The antibacterial mechanism is suggested to involve bactericidal capacities of LF, fragments thereof, or its peptides.
Urinary tract infections (UTI) are among the most common infections in humans, occurring predominantly in females of all ages. Escherichia coli is the most common pathogen, causing 80% of uncomplicated UTI (38). The therapy of UTI consists of antibiotics (21). Since antibiotics have the disadvantage of adverse effects, less safety when used during pregnancy (20) and in children, and a high prevalence of antibiotic resistance among uropathogens (18, 19), new antimicrobial drugs need to be developed. An experimental model of ascending UTI, developed in mice by inoculation of E. coli into the urinary bladder, has been previously described (12). It has been shown with this model that live or formalin-killed E. coli can induce mucosal inflammation consisting of recruitment of polymorphonuclear leukocytes (PMN) and the appearance of interleukin-6 (IL-6) in the urine (8). The mucosal inflammation has been shown to be initiated by E. coli outer membrane components, especially type P fimbriae and lipopolysaccharide (LPS) (26).
There is evidence that breast-feeding protects infants against UTI during the first 6 months of life (31). Moreover, some studies have suggested that the protection conferred by mother's milk persists after the termination of breast-feeding (28). The reduction of UTI by breast-feeding has been attributed to the content in milk of secretory immunoglobulin A antibodies and oligosaccharides, which inhibit the adherence of enterobacteria to the uroepithelial cells (6, 34). However, milk contains several other, potentially protective factors (e.g., lactoferrin [LF], lysozyme, and complement factors) which could play a role in UTI, especially since intact human LF (hLF) and fragments thereof have been found in the urine of infants fed human milk (11, 16). In addition, two forms of LF (one intact and one “nicked”) of maternal origin have been isolated from the urine of preterm infants fed human milk (17).
The major milk whey protein, LF, is a multifunctional immunoregulatory protein that has been associated with anti-inflammatory activities and host defense at mucosal surfaces through its antibacterial and iron-binding properties (for reviews, see references 5 and 24). Lactoferricin (LFcin), a pepsin-cleaved fragment of hLF or bovine LF (bLF) located in the N-terminal lobe of the molecule and in a region distinct from the iron-binding sites, has been shown to contain the bactericidal domain of the protein (4). Human LFcin (hLFcin) consists of 47 amino acids corresponding to residues 1 to 47 of hLF, while bovine LFcin (bLFcin) contains 25 amino acids corresponding to residues 17 to 41 of bLF (4). In vitro studies have shown that bLF is more potent than hLF with regard to antibacterial activity. Moreover, bLFcin has been shown to be more effective than hLFcin, although they both express much stronger antibacterial activity than the parent molecules. The antibacterial region has been reported to correspond to residues 18 to 40 of hLF and 17 to 41 of bLF (4). It has been shown that a loop region consisting of amino acid residues 28 to 34 of hLF is essential for high-affinity binding to LPS (9). Furthermore, bLFcin has been proposed to be the region in bLF responsible for the suppression of the IL-6 response in a monocytic cell line (THP-1) when stimulated by LPS (29).
The aim of this study was to investigate the anti-infectious capacity of orally administered hLF, bLF, and synthetic peptide sequences based on the antibacterial region of hLF, corresponding to amino acid sequences 16 to 40 (HLD1) and 18 to 40 (HLD2), in experimentally induced UTI in mice. The proteins and peptides were orally administered to mice 30 min after the inoculation of E. coli into the urinary tract. Our results showed that orally given LF, particularly hLF, and the synthetic peptides increased resistance to UTI by reducing the number of bacteria in the urinary tract compared with the results for the control group.
MATERIALS AND METHODS
Bacteria.
E. coli strain O6:K5:H− (hereafter referred to as O6K5) was isolated from a child with acute pyelonephritis (37). The strain was donated to us by that research group and has been characterized elsewhere (33). The bacteria were cultured on lactose-bromthymol blue agar plates. For infection, bacteria were cultured in Luria broth supplemented with 0.1% CaCl2 at 37°C overnight (see Fig. 1 to 5) and for two more days (see Table 1). The bacteria were harvested by centrifugation and diluted in phosphate-buffered saline (PBS) to an optical density at 597 nm of 0.96 (measured with Vitatron DCP apparatus; Vital Scientific, Dieren, The Netherlands) (approximately 109 bacteria per ml). Adhesins specific for the globoseries of glycolipid receptors were identified by mannose-resistant agglutination of human erythrocytes (23). Adhesins specific for mannose-containing receptors were identified by mannose-reversed agglutination of guinea pig erythrocytes (10). The O6K5 strain expressed adhesins specific for the globoseries of glycolipids with the receptor site Galα1-4Galβ, which conferred the ability to attach to mouse uroepithelial and kidney cells, and mannose-specific adhesins with a lower degree of attachment to epithelial cells (13). The concentration of the bacteria was checked by determining viable counts.
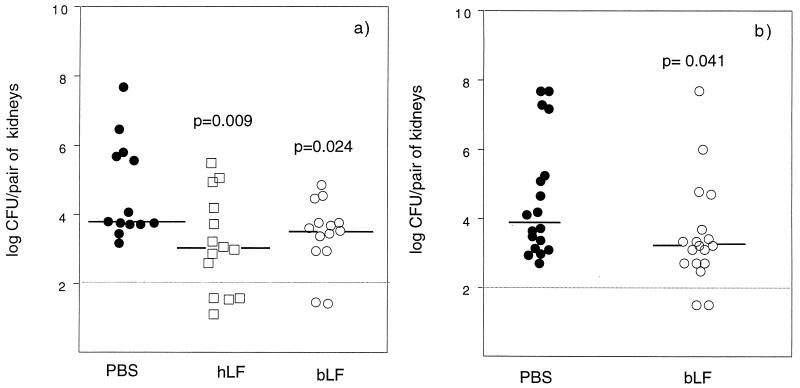
FIG. 1.
Recovery of bacteria from kidneys of C3H/Tif (a) and C3H/HeN (b) mice infected with E. coli O6K5. The mice were given 500 μg of hLF, bLF, or PBS perorally 30 min after the instillation of E. coli bacteria into the urinary tract. The number of bacteria in the kidneys was determined 24 h after inoculation and calculated as log10 CFU per pair of kidneys. Each symbol represents one mouse, and the bars denote the median values. Samples represented by symbols below the line were culture negative.
FIG. 5.
Serum IL-6 response 24 h after experimentally induced UTI in C3H/Tif (a) and C3H/HeN (b) mice orally treated with hLF and bLF as described in the legend to Fig 1. Serum samples were analyzed by a bioassay. Each symbol represents one mouse, and the bars denote the median values.
TABLE 1.
Effects of orally given hLF and hLF-derived peptides (HLD1 and HLD2) on the recovery of bacteria from the kidneys and bladder and the urinary PMN levels 24 h after the injection of E. coli O6K5 into the urinary tract of C3H/Tif mice
| Treatment | No. of animals | Median (rangea) log CFU in:
|
Median (rangea) PMN/ml of urine (104) | |
|---|---|---|---|---|
| Pair of kidneys | Bladder | |||
| hLF | 24 | 3.16 (2–5.2)b | 3.58 (2–6.29) | 58 (0–445) |
| HLD1 | 23 | 3.36 (2–5.2) | 3.30 (2–4.89) | 73 (5–497) |
| HLD2 | 25 | 3.56 (2–4.6)b | 3.23 (2–4.63) | 71 (6–332) |
| None (control)c | 23 | 4.32 (2.3–5.3) | 3.38 (2–6.27) | 39 (3–770) |
The minimum and maximum values are indicated.
The P value was <0.01, as determined by the Mann-Whitney U test.
The control consisted of mice perorally treated with water.
Mice.
C3H/Tif and C3H/HeN female mice at least 8 weeks old (Charles River, Margate Trent, United Kingdom, and Bomholtgård Breeding and Research Center Ltd., Ry, Denmark) were used. The animals were housed five to a cage and given pellets and tap water ad libitum.
LFs and peptides.
hLF and bLF were purchased from Sigma-Aldrich. The peptides with the sequences EATKCFQWQRNMRKVRGPPVSCIKR and TK(C)FQWQRNMRKVRGPPVS(C)IKR (see below), corresponding to amino acids 16 to 40 (HLD1) and 18 to 40 (HLD2) of hLF, were synthesized by a 9-fluorenyl-methoxy carbony continuous-flow strategy on a Biosearch Pioneer automated peptide synthesizer.
HLD1 and HLD2 were capped at the N (acetyl)- and C (NH2)-terminal ends in order to neutralize the otherwise charged ends. HLD1 was made cyclic by a disulfide bridge between the cysteine residues. In HLD2, the cysteine residues were changed to acetamidomethylcysteine [(C)] in order to avoid spontaneous disulfide bridging in this peptide. The purity of the peptides was > 95%, as measured by high-pressure liquid chromatography and electrospray mass spectrometry.
Experimental UTI.
Mice were infected under ether or methophane anesthesia by instillation of 108 E. coli O6K5 bacteria (100 μl) into the urinary bladder through a urethral catheter (polyethylene catheter, 0.61 mm; Intramedic; Becton Dickinson and Company, Sparks, Md.) (attached to a 20-mm needle on a tuberculin syringe). Immediately after inoculation, the catheter was withdrawn. Prior to inoculation (12), the urinary bladders were emptied by gentle compression of the abdomen. The urine from each mouse was cultured to assess the sterility and inspected microscopically to confirm the absence of a preexisting inflammatory response. Animals with bacteriuria or leukocyturia (more than 20 PMN/ml of urine) were excluded. At 30 min after the instillation, the mice received 500 μg of hLF, bLF, or peptides perorally. For the control groups, the protein or peptide solutions were changed to pure vehicle (PBS or water). Viable counts, leukocyte counts, and IL-6 levels were determined for urine samples collected at various times (0, 2, 6, and 24 h) after infection. Animals were killed 24 h after infection by cervical dislocation. Serum samples were collected by retro-orbital bleeding and stored at −20°C until assayed for cytokines and LF. The kidneys and bladders were removed aseptically and homogenized in 5 ml of PBS in disposable plastic bags. Serial 10-fold dilutions of 50 μl of tissue homogenate were cultured on lactose-bromthymol blue agar plates. The detection limit of the culturing was 100 bacteria per bladder or pair of kidneys.
E. coli adherence to mouse uroepithelial cells.
Uroepithelial cells from mice were collected by centrifugation (400 × g for 10 min at room temperature) of urine pooled from 30 mice (C3H/Tif). The sediment was washed once and suspended in PBS to a concentration of 105 cells/ml. A mixture of 200 μl of 109 CFU of E. coli O6K5 bacteria per ml suspended in PBS, 200 μl of uroepithelial cell suspension, and 100 μl of bLF, hLF, HLD1, or HLD2 (10 mg/ml) or PBS was incubated on a rotator for 30 min at 37°C. After incubation, 3 ml of cold PBS was added, and the cells were centrifuged, resuspended in PBS, and fixed with 1 drop of neutral buffered formalin (Histofix; Histolab, Göteborg, Sweden). In one experiment (C3H/HeN mice), a mixture of 250 μl of 109 CFU of E. coli O6K5 bacteria per ml suspended in PBS and 250 μl of various concentrations of bLF in PBS (10, 5, 2.5, and 1.25 mg/ml) was incubated on a rotator for 30 min at 37°C. The sediment was washed once and suspended in 300 μl of PBS; then, 250 μl of uroepithelial cell (C3H/HeN) suspension was added. The mixture was incubated for 1 h at 37°C with end-over-end rotation and thereafter was washed by repeated cycles of centrifugation and resuspension in PBS. The number of bacteria attached to at least 20 cells was determined. Adhesion was scored with an interference contrast microscope (Nicon Optiphot, at a ×500 magnification). The adherence value was defined as the mean number of adherent bacteria per cell.
Hemagglutination.
The hemagglutination assay was performed with 96-well microtiter plates (U shaped; Greiner Laborteknik, Frickenhausen, Germany) as previously described (35). Briefly, 25 μl of twofold serially diluted solutions of bLF, hLF, HLD1, and HLD2 (initial concentration, 10 mg/ml) in PBS was mixed with 25 μl of E. coli O6K5 bacteria suspended in PBS at a concentration of 1 × 109 or 2 × 109/ml. The plates were shaken at room temperature for 30 min. Then, 25 μl of human or guinea pig erythrocytes (washed three times and resuspended at a concentration of 2% [vol/vol] in PBS) was added. The plates were shaken and incubated for 2 h at room temperature. Hemagglutination was read by eye and by bright-field microscopy at a ×40 magnification.
Bacterial agglutination.
Twenty-five microliters of bLF, hLF, HLD1, or HLD2 at a concentration of 10 mg/ml was diluted twofold in a microtiter plate. An equal volume of PBS or α-methyl-d-mannoside (25 mg/ml in PBS) and 50 μl of an E. coli O6K5 bacterial suspension (109) were added. The plate was shaken and incubated at 37°C for 30 min. The plate was then kept at 4°C overnight. Agglutination was read by eye and by bright-field microscopy at a ×40 magnification. The reciprocal of the highest dilution giving bacterial agglutination was defined as the agglutination titer (35).
Antimicrobial activities of hLF, bLF, and peptides.
E. coli O6K5 was cultured three times overnight at 37°C in Luria broth supplemented with 0.1% CaCl2. One milliliter of the culture was transferred to a new tube containing Luria broth and incubated at 37°C for two more hours. The bacteria were washed by centrifugation, and the concentration was adjusted to 107 bacteria/ml in brain heart infusion (BHI; Difco, Sparks, Md.) diluted 1/100. hLF, bLF, or peptides were serially diluted in twofold steps in BHI (diluted with water, 1/100) or in mouse normal urine (undiluted or diluted in twofold steps in diluted BHI). The solutions were added (100 μl) in duplicate to a microtiter plate (Nunclon; Nunc, Roskilde, Denmark). The bacteria were added to the plate in a 10-μl volume of diluted BHI in order to give a final concentration of 105 bacteria/ml. The plate was incubated at 37°C in a humid chamber for 2 h. Thereafter, 5 μl was taken from each well and added as a drop to a blood agar plate, and the plate was incubated overnight at 37°C. The concentration of hLF, bLF, or peptides giving 99% reduction of the inoculum was defined as the minimum microbicidal concentration required to kill 99% of the inoculum (MMC99). In another experiment, 10 mM sodium phosphate buffer (pH 7.4) was used as the diluent. In this experiment, viable counts were determined at each concentration of hLF, bLF, or peptides after 2 h of incubation with bacteria.
IL-6 determination.
IL-6 in urine and serum samples was measured by a bioassay with the B9 hybridoma cell line, which is dependent on IL-6 for proliferation (1, 7). At 72 h after the addition of the samples, [3H]thymidine incorporation was determined after 4 h of incubation in the presence of the isotope. The samples were compared to an IL-6 standard. One unit per milliliter is the concentration required for half-maximal proliferation of B9 cells (approximately equivalent to 1 pg).
hLF determination by ELISA.
Urine and serum hLF levels were detected by a double-sandwich enzyme-linked immunosorbent assay (ELISA). Maxisorp plates (Nunc) were coated overnight at 4°C with the immunoglobulin G fraction of a rabbit antiserum to hLF (Dako, Glostrup, Denmark) at 18 μg/ml in 0.1 M bicarbonate buffer (pH 9.6). After the plates were washed and blocked for 1 h at room temperature with 5% fish gelatin in PBS, the samples undiluted or diluted 1:2 in dilution buffer (1% fish gelatin in PBS–0.05% Tween 20) and the hLF standard were incubated for 2 h at room temperature. The plates were washed three times with PBS–0.05% Tween 20 and incubated for 1 h at room temperature with rabbit anti-hLF antibodies conjugated to alkaline phosphatase (ICN Biomedicals, Inc., Chemical Credential, Costa Mesa, Calif.) and diluted 1:1,000 in dilution buffer. After the plates were washed three times with PBS-Tween 20, the substrate (1 mg of p-nitrophenyl phosphate per ml in 10 mmol of diethanolamine per liter–0.5 mmol of MgCl2 [pH 9.5]) was added, and color development was read with a microplate reader (Multiskan Bichromatic; Labsystem, Stockholm, Sweden) at 405 nm. The sensitivity of the method was approximately 1.5 ng/ml.
Statistics.
Differences between groups were analyzed for significance by the Mann-Whitney U test. The percentages of animals with detectable hLF concentrations in serum were analyzed by Fisher's exact test. Differences were considered significant for P values of <0.05.
RESULTS
Effects of peroral treatment with hLF and bLF in experimental UTI.
hLF and bLF were given perorally 30 min after the instillation of E. coli bacteria into the urinary tract. Two mouse strains (C3H/Tif and C3H/HeN) were used for the bLF treatment in order to compare the effects in similar but not identical strains. hLF was given only to C3H/Tif mice.
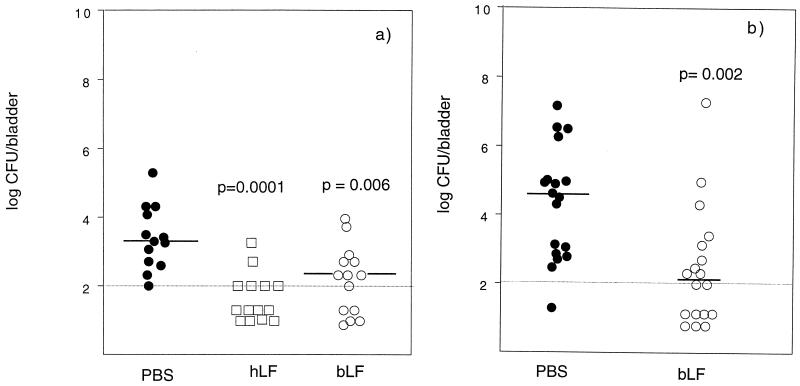
The numbers of bacteria present in the bladder and kidneys of the hLF- and bLF-treated mice were significantly reduced compared to those in the control groups at 24 h after inoculation with E. coli (Fig. 1 and 2). Twenty-nine percent of the hLF-treated mice had no detectable bacteria in the kidneys (Fig. 1a). The corresponding values for bLF treatment were 14 and 11% in C3H/Tif and C3H/HeN mice, respectively (Fig. 1). None of the controls was free from detectable bacteria at 24 h. The percentage of animals with no detectable bacteria in the urinary bladder with hLF treatment was 57% (Fig. 2a). The percentages of bLF-treated C3H/Tif and C3H/HeN mice free from detectable bacteria in the bladder were 35 and 39%, respectively, compared to 0 and 6% for the respective control groups (Fig. 2).
FIG. 2.
Recovery of bacteria from bladders of C3H/Tif (a) and C3H/HeN (b) mice infected with E. coli O6K5 as described in the legend to Fig. 1. The number of bacteria in the bladder was determined 24 h after inoculation and calculated as log10 CFU per bladder.
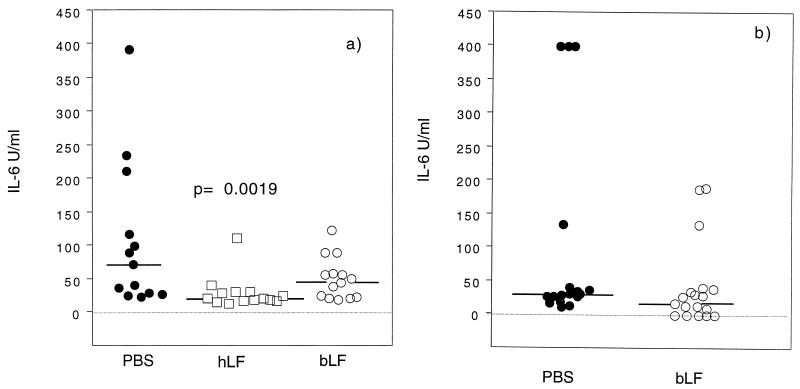
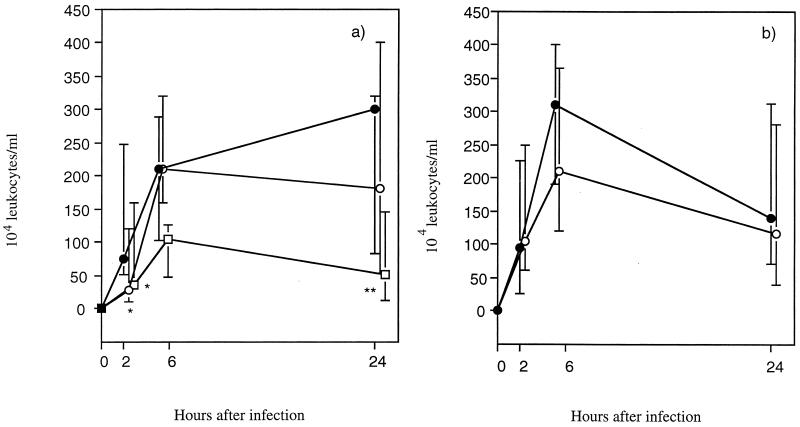
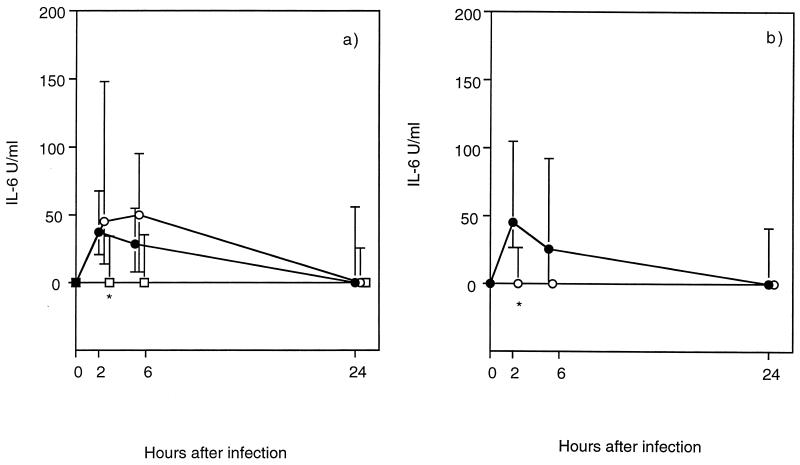
The levels of PMN and IL-6 in the urine were used as parameters of the inflammatory response. The number of PMN in the urine was reduced in the hLF-treated C3H/Tif mice compared with the PBS-treated mice (significantly reduced at 2 and 24 h) (Fig. 3). The effects of bLF treatment on the PMN levels were much weaker, showing a significant reduction only at 2 h after infection in the C3H/Tif mice (Fig. 3). The urinary IL-6 response was significantly reduced in the hLF-treated C3H/Tif mice at 2 h after the inoculation of bacteria (Fig. 4a). bLF treatment also induced a reduced IL-6 response, but only in the C3H/HeN mice at 2 h (Fig. 4b).
FIG. 3.
Kinetics of urinary leukocyte influx in E. coli O6K5-infected C3H/Tif (a) and C3H/HeN (b) mice treated perorally with hLF (□), bLF (○), or PBS (●) as described in the legend to Fig. 1. The number of leukocytes in the urine was counted with a Bürker chamber. The median values (symbols) and interquartile ranges (vertical bars) are indicated. P values were <0.05 (∗) and <0.01 (∗∗), as determined by the Mann-Whitney U test.
FIG. 4.
Kinetics of the IL-6 response in the urine of E. coli O6K5-infected C3H/Tif (a) and C3H/HeN (b) mice treated perorally with hLF (□), bLF (○), or PBS (●) as described in the legend to Fig. 1. Urine was analyzed by an IL-6 bioassay. The median values (symbols) and interquartile ranges (vertical bars) are indicated. The P value was <0.05 (∗), as determined by the Mann-Whitney U test.
The serum IL-6 levels were significantly reduced at 24 h in the hLF-treated C3H/Tif mice. No such change was established for the bLF-treated mice (Fig. 5).
Effects of peroral treatment with peptides in experimental UTI.
Since our results showed that hLF had a stronger effect than bLF with regard to both anti-infectious and anti-inflammatory activities, synthetic peptides derived from the antibacterial region of hLF were compared with hLF in the experimental UTI model.
Orally administrated HLD2 was comparable to hLF in reducing the number of bacteria in the kidneys of C3H/Tif mice infected with E. coli bacteria, compared to the results obtained for the control group (Table 1). HLD1 showed the same pattern, although it was not quite significant. The percentages of animals free from detectable bacteria in the kidneys after hLF, HLD1, HLD2, and water (control) treatments were 25, 17, 16, and 0%, respectively. The number of bacteria in the bladder was not significantly affected by the hLF or peptide treatment (Table 1). Neither was the number of PMN affected by hLF or peptides in this second series of experiments. However, an overall lower PMN response was observed in this series of experiments than in the first series of experiments (Fig. 3).
hLF levels in serum and urine from mice given hLF perorally.
The findings of antibacterial effects in the urinary tract suggested that hLF or peptides exerted their activity locally. Subsequently, serum and urine from mice given hLF perorally were analyzed for the presence of hLF.
Serum collected 24 h after infection from hLF-treated C3H/Tif in the second series of experimental UTI was analyzed. Eleven out of 24 hLF-treated mice (45%) had detectable serum hLF levels (P < 0.001; Fisher's exact test), while none in the control group did (0 of 24). The hLF levels varied between 1.7 and 88 ng/ml.
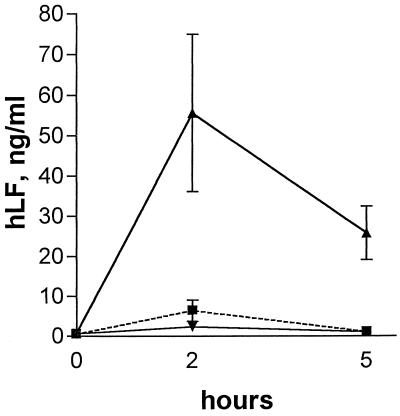
Urine from uninfected C3H/TiF mice given hLF (0.5 or 4 mg) or water was analyzed for hLF at 0, 2, and 5 h after the feeding (Fig. 6). The urinary hLF levels were highest at 2 h and were approximately 10-fold higher in the group treated with 4 mg than in the group treated with 0.5 mg.
FIG. 6.
Levels of hLF in urine from C3H/Tif mice treated perorally with 0.5 mg of hLF (▪), 4 mg of hLF (▴), or water (▾). The symbols represent the mean and standard error of urinary hLF levels in four to six animals per time point.
In vitro adherence of E. coli O6K5 to uroepithelial cells in the presence of hLF, bLF, or peptides.
In the following experiments, the capacity of hLF, bLF, or peptides to inhibit the adherence of E. coli O6K5 to mouse uroepithelial cells was assessed.
The results showed that, in contrast to hLF or peptides, only bLF interfered with the adherence of E. coli O6K5 to the uroepithelial cells of C3H/Tif mice. The concentration needed for bLF to significantly reduce adherence was determined to be at least 1.3 mg/ml in the in vitro assay with C3H/HeN mice (data not shown).
Agglutination of E. coli O6K5 in the presence of hLF, bLF, or peptides.
Agglutination of E. coli bacteria in the presence of hLF, bLF, or peptides could have some bearing on the antibacterial effect locally in the urinary tract during infection. However, the E. coli bacteria were agglutinated only in the presence of bLF. The agglutination was reversed by the addition of α-methyl-d-mannoside (data not shown). Inhibition of the hemagglutination of guinea pig but not human erythrocytes in the presence of E. coli O6K5 confirmed that the mannose-containing sugar residues of bLF interfered with the mannose-binding type 1 fimbriae of E. coli.
Antimicrobial activities of hLF, bLF, and peptides.
The bactericidal activities of hLF, bLF, and hLF-derived peptides in mouse urine (undiluted or diluted) and broth diluent were analyzed (Table 2). Determination of the concentration needed for 99% killing revealed that none of the substances was effective in undiluted urine at the concentrations used. With urine diluted 1/64 and 1/128, bactericidal activity was obtained with 100 and 25 μg of HLD2 per ml, respectively. HLD1 was active only at the highest concentration tested in the 1/128-diluted urine. A much stronger bactericidal effect was observed for the peptides in broth diluent, where 5 and 10 μg of HLD2 and HLD1 per ml, respectively, reduced the number of bacteria by more than 99%. A similar reduction was obtained with 100 to 200 times more bLF. hLF did not show the same reduction at the highest concentration tested.
TABLE 2.
Bactericidal activities against E. coli O6K5 of LFs and peptides after 2 h of incubation
| Agent | MMC99 (μg/ml) in:
|
||
|---|---|---|---|
| Urine diluted
|
BHI diluted 1/100 | ||
| 1/64 | 1/128 | ||
| hLFac | >1,000 | >1,000 | >2,000 |
| bLFac | >1,000 | >1,000 | 1,000 |
| HLD1bd | >100 | 100 | 10 |
| HLD2bd | 100 | 25 | 5 |
Concentrations tested in 1/100-diluted BHI: 2,000, 1,000, 500, 250, and 100 μg/ml.
Concentrations tested in 1/100-diluted BHI: 100, 25, 20, 10, 5, and 2.5 μg/ml.
Concentrations tested in mouse urine: 1,000 and 100 μg/ml.
Concentrations tested in mouse urine: 100 and 25 μg/ml.
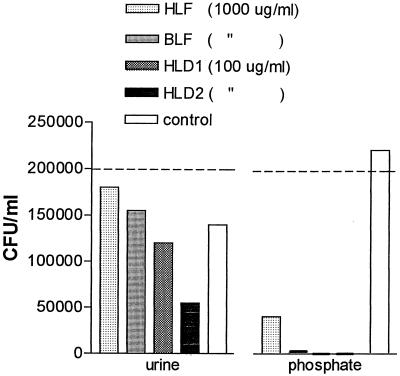
In a subsequent experiment, it was shown that a 60% reduction in the bacterial number was induced in the presence of HLD2 (100 μg/ml) compared with no peptide added (control) in urine at 2 h of incubation (Fig. 7). Urine by itself had a bacteriostatic or bactericidal effect on E. coli O6K5. In accordance with the bactericidal analysis using diluted BHI, both HLD1 and HLD2 killed 100% of the bacteria in phosphate buffer. hLF and bLF reduced the concentrations of bacteria to 18 and 2%, respectively, compared to the control.
FIG. 7.
Concentrations of E. coli O6K5 in undiluted urine (pH 6.1; conductance, 18.1 mS) and in 10 mM phosphate buffer (pH 7.4; conductance, 1.2 mS) in the presence of hLF, bLF, HLD1, or HLD2. The inoculum was 2 × 105 CFU/ml. The numbers of bacteria in the controls after 2 h of incubation were 1.4 × 105/ml in urine and 2.2 × 105/ml in phosphate buffer. The concentration of bacteria is expressed as CFU per milliliter at 2 h of incubation. No growth was obtained with HLD1 and HLD2 in phosphate buffer. All viable counts were determined in duplicate.
DISCUSSION
The present study showed that orally administrated hLF, bLF, or hLF-derived synthetic peptides could reduce and partly prevent experimentally induced UTI in mice when given 30 min after intravesical inoculation of E. coli into the urinary bladder. The inflammatory response induced by the bacteria (PMN and IL-6) was also reduced in the hLF-treated mice. The overall results indicated that hLF was somewhat more effective as an anti-infectious and anti-inflammatory factor than bLF. The effects of the hLF-derived peptides, HLD1 and HLD2, indicated that the amino acid sequence of the antibacterial region comprising residues 18 to 40 of the hLF molecule may be the active site.
The mechanism by which hLF acts as an anti-infective agent may be multifactorial. Since antibacterial (4, 36) and anti-inflammatory (29) activities have been described for hLF and hLFcin in vitro, it is possible that hLF exerts both of these properties in our infection model. Studies in vitro have shown that LF downregulates LPS-induced IL-6 production in a monocytic cell line (29). Thus, the anti-inflammatory capacity of hLF in our model could be a consequence of either its antibacterial activity or downregulation of IL-6 or both. Withdrawal of iron from E. coli as an anti-infectious activity of hLF does not seem to be a major mechanism, since the active peptide, HLD2, has no such binding capacity.
A prerequisite for the local anti-infective activity of LF or peptides is the transfer of the molecules from the gut into the urinary tract. Little is known about the metabolic pathway of ingested LF in mice. An intestinal LF receptor has been described and characterized for the mouse brush-border membrane (14, 15). This receptor, a glycoprotein (130 kDa), binds both hLF and bLF (14, 15). LF receptors (110 kDa) have been reported to be present in the brush border membranes of infant rhesus monkey and infant human intestines (27). However, these receptors bind only hLF and monkey LF.
By using a double-sandwich ELISA, we detected hLF in the serum and urine (Fig. 6) of hLF-fed mice as an immunoreactive protein. In this way, hLF or its fragments could act locally in the urinary tract. However, it remains to be determined whether this protein is absorbed mainly as an intact molecule or as more or less degraded peptide fragments. Recently, by use of surface-enhanced laser desorption-ionization affinity mass spectrometry, intact bLF and bLF fragments containing the bLFcin region were detected in the feces of adult mice fed milk enriched with bLF (22). Whether our orally administrated hLF-derived peptides (HLD1 and HLD2) are resistant to proteolysis in vivo during passage through the gastrointestinal tract is not known.
Since the adherence of pathogenic microorganisms to mucosal surfaces constitutes the first step in the infectious process (3), we investigated if hLF, bLF, or hLF-derived peptides interfered with this process. We found that hLF or hLF-derived peptides did not inhibit the in vitro adherence of E. coli to C3H/Tif mouse uroepithelial cells, even at a very high concentration. Mannosylated bLF, however, blocked adherence by up to 50% at the highest concentration tested (5 mg/ml). Thus, interference with adherence did not seem to be a major mechanism for the anti-infective effects of hLF or the peptides.
The attachment of bacteria to host tissue is mediated by adhesins recognizing receptor structures on the epithelial cell membrane. E. coli O6K5, used in this study, expressed both P fimbriae and type 1 fimbriae, as shown by the hemagglutination profile. It has been reported that bLF oligomannosidic glycans function as receptors for E. coli type 1 fimbriae (35). This report was supported by our findings of reduced adherence of E. coli to mouse uroepithelial cells in the presence of bLF. The fact that bLF inhibited the agglutination of guinea pig erythrocytes which contained the type 1 fimbrial receptor in the presence of E. coli O6K5 confirmed that bLF could interact with type 1 fimbriae.
The transfer of hLF into the urinary tract and possibly also of hLF fragments, such as LFcin, and the peptides suggested that their antibacterial effects could have been mediated on site. Our in vitro studies confirmed the antibacterial of hLF, bLF, and hLF-derived peptides against E. coli O6K5. As expected, HLD1 and HLD2 were much stronger bactericidal agents than the parent molecule. Although the bactericidal effect in mouse urine was moderate for HLD2, with 72 and 60% reductions in comparison with the results for the inoculum and the control (no peptide added), respectively, HLD2 was the most active agent (Fig. 7). In synergy with other antibacterial mechanisms and phagocytosis, moderate bactericidal activity may still be decisive for the outcome of infections.
A bacteriostatic effect of mouse urine against pyelonephritic and fecal isolates has been previously reported (12). We even observed bactericidal activity of mouse urine for E. coli O6K5 cultured overnight. However, within 2 h of incubation, 99% killing by HLD2 was seen at a 1/64 dilution of urine at the highest concentration tested (100 μg/ml). The low antimicrobial activity of HLD1 and HLD2 in undiluted mouse urine was probably an effect of the salt concentration, since the bactericidal activity of these peptides was inhibited by increasing the NaCl concentration from 0.035 M to the saline concentration (0.14 M) present in the diluent used for bactericidal testing (unpublished results). The antimicrobial activity of other natural cationic peptides, such as mouse β defensin 1, has also been reported to be salt sensitive (2). The milieu of the renal tubules, especially of the ascending limb of the loop of Henle or the distal tubule, with a low salt concentration, might be the site where the peptides are particularly active against bacteria.
Recently, it was reported that bLF given intravenously (10 mg) to mice 24 h before intravenous infection with E. coli reduced the lethality caused by the bacteria. A dramatic reduction in the number of bacteria was observed 5 h after infection in the kidneys and lungs (39). Since the intracellular killing of bacteria by macrophages could reduce the number of bacteria in infected organs, we do not exclude the possibility that hLF, bLF, or the peptides could enhance the phagocytic activity of macrophages in vivo. Studies in vitro have shown that macrophages may aquire LF through surface receptors and thereby enhance the killing of intracellular Legionella pneumophila, Mycobacterium microti, and Trypanosoma cruzi (25). With regard to the effects of LF on human neutrophils, it was shown by in vitro studies that bLF and its fragment, bLFcin, enhanced the phagocytic activity of these cells; this effect seemed to be due to direct binding of bLF to neutrophils and an opsonin-like activity (30). However, no influence on the phagocytic activity of PMN has been described for hLF, although it can bind to these cells (30, 32).
The HLD2 peptide significantly reduced the number of bacteria in the kidneys of the C3H/Tif mice. The number of mice with no detectable bacteria in the kidneys was the same for the HLD1-treated animals as for the HLD2-treated animals. Counting the two groups together, the frequency of mice with no detectable bacteria in the kidneys was significantly lower than the frequency in vehicle-treated animals. The cyclic HLD1 peptide is two amino acid residues longer at the N-terminal end than HLD2, which is a linear peptide. Whether these structural differences would have any effect on gastrointestinal uptake, degradation, and distribution in vivo needs to be studied further. Capping of the peptides was performed in order to neutralize the otherwise charged ends. Thus, the peptide sequences would agree more with the parental surface helical region.
In conclusion, this study has shown that perorally given hLF and peptides thereof could mediate anti-infective and anti-inflammatory activities at mucosal sites remote from the gut. These effects are suggested to be mediated by hLF and peptide fragments of hLF and by our synthetic peptides locally in the urinary tract. The bactericidal activity of hLF, hLF fragments, or synthetic peptides may partly contribute to the antibacterial effect.
ACKNOWLEDGMENTS
The skillful technical assistance of Helena Kahu, Ingela Delgado, and Lotta Sjögren is very much appreciated.
This study was financially supported by a grant from the Swedish Medical Research Council (no. 215) and by A+ Science Invest AB, Göteborg, Sweden.
REFERENCES
- 1.Arden L A, de Groot E R, Schaap O L, Landsdorp P M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 2.Bals R, Goldman M J, Wilson J M. Mouse β-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect Immun. 1998;66:1225–1232. doi: 10.1128/iai.66.3.1225-1232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beachey E H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J Infect Dis. 1981;143:325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy W M, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- 5.Brock J. Lactoferrin: a multifunctional immunoregulatory protein? Immunol Today. 1995;16:417–419. doi: 10.1016/0167-5699(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 6.Coppa G V, Gabrielli O, Giorgi P, Catassi C, Montanary M P, Varaldo P E, Nichols B L. Preliminary study of breastfeeding and bacterial adhesion to uroepithelial cells. Lancet. 1990;335:569–571. doi: 10.1016/0140-6736(90)90350-e. [DOI] [PubMed] [Google Scholar]
- 7.de Man P, Jodal U, Lincoln K, Svanborg-Edén C. Bacterial attachment and inflammation in the urinary tract. J Infect Dis. 1988;158:29–35. doi: 10.1093/infdis/158.1.29. [DOI] [PubMed] [Google Scholar]
- 8.de Man P, van Kooten C, Aarden L, Engberg I, Linder H, Svanborg-Edén C. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect Immun. 1989;57:3383–3388. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elass-Rochard E, Roseanu A, Legrand D, Trif M, Salmon V, Motas C, Montreuil J, Spik G. Lactoferrin-lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli O55B5 lipopolysaccharide. Biochem J. 1995;312:839–845. doi: 10.1042/bj3120839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firon N, Ofek I, Sharon N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumonie and Salmonella typhimurium. Carbohydr Res. 1983;120:235–249. doi: 10.1016/0008-6215(83)88019-7. [DOI] [PubMed] [Google Scholar]
- 11.Goldman A S, Garza C, Schanler R J, Goldblum R M. Molecular forms of lactoferrin in stool and urine from infants fed human milk. Pediatr Res. 1990;27:252–255. doi: 10.1203/00006450-199003000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg-Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagberg L, Hull R, Hull S, Falkow S, Freter R, Svanborg-Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983;40:265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu W L, Mazurier J, Montreuil J, Spik G. Isolation and partial characterization of a lactotransferrin receptor from mouse intestinal brush border. Biochemistry. 1990;29:535–541. doi: 10.1021/bi00454a030. [DOI] [PubMed] [Google Scholar]
- 15.Hu W L, Mazurier J, Montreuil J, Spik G. Lactotransferrin receptor of mouse intestinal brush border. Biochem J. 1988;248:435–441. doi: 10.1042/bj2490435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutchens T W, Henry J F, Yip T T. Purification and characterization of intact lactoferrin found in the urine of human milk-fed preterm infants. Clin Chem. 1989;35:1928–1933. [PubMed] [Google Scholar]
- 17.Hutchens T W, Henry J F, Yip T T. Structurally intact (78-kDa) forms of maternal lactoferrin purified from urine of preterm infants fed human milk: identification of a trypsin-like proteolytic cleavage event in vivo that does not result in fragment dissociation. Proc Natl Acad Sci USA. 1991;88:2994–2998. doi: 10.1073/pnas.88.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson J R, Stamm W E. Urinary tract infection in women: diagnosis and treatment. Ann Intern Med. 1989;111:906–917. doi: 10.7326/0003-4819-111-11-906. [DOI] [PubMed] [Google Scholar]
- 19.Jones R N. Impact of changing pathogens and antimicrobial susceptibility patterns in the treatment of serious infections in hospitalized patients. Am J Med. 1996;100:3S–12S. doi: 10.1016/s0002-9343(96)00102-7. [DOI] [PubMed] [Google Scholar]
- 20.Krieger J N. Complications and treatment of urinary tract infections during pregnancy. Urol Clin North Am. 1986;13:685–693. [PubMed] [Google Scholar]
- 21.Kunin C M. Use of antimicrobial agents in treating urinary tract infection. Adv Nephrol. 1985;14:39–65. [PubMed] [Google Scholar]
- 22.Kuwata H, Yip T T, Yamauchi K, Teraguchi S, Hayasawa H, Tomita M, Hutchens T W. The survival of ingested lactoferrin in the gastrointestinal tract of adult mice. Biochem J. 1998;334:321–323. doi: 10.1042/bj3340321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leffler H, Svanborg-Edén C. Chemical identification of a glycosphingolipid receptor for Escherichia coli attaching to human uroepithelial cells and agglutination of human erythrocytes. FEMS Microbiol Lett. 1980;8:127–134. [Google Scholar]
- 24.Levay P F, Viljoen M. Lactoferrin: a general review. Hematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- 25.Lima M F, Kierszenbaum F. Lactoferrin effects on phagocytic cell function. I. Increased uptake and killing of an intracellular parasite by murine macrophages and human monocytes. J Immunol. 1985;134:4176–4183. [PubMed] [Google Scholar]
- 26.Linder H, Engberg I, Mattsby-Baltzer I, Klaus J, Svanborg-Edén C. Induction of inflammation by Escherichia coli on the mucosal level: requirement for adherence and endotoxin. Infect Immun. 1988;56:1309–1313. doi: 10.1128/iai.56.5.1309-1313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonnerdal B. Lactoferrin receptors in intestinal brush border membranes. Adv Exp Med Biol. 1994;357:171–175. doi: 10.1007/978-1-4615-2548-6_16. [DOI] [PubMed] [Google Scholar]
- 28.Mårild S, Jodal U, Hanson L Å. Breastfeeding and urinary tract infection. Lancet. 1990;336:942. doi: 10.1016/0140-6736(90)92313-7. [DOI] [PubMed] [Google Scholar]
- 29.Mattsby-Baltzer I, Roseanu A, Motas C, Elverfors J, Engberg I, Hanson L Å. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr Res. 1996;40:257–262. doi: 10.1203/00006450-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Miyauchi H, Hashimoto S, Nakajima M, Shinoda I, Fukuwatari Y, Hayasawa H. Bovine lactoferrin stimulates the phagocytic activity of human neutrophils: identification of its active domain. Cell Immunol. 1998;187:34–37. doi: 10.1006/cimm.1997.1246. [DOI] [PubMed] [Google Scholar]
- 31.Pisacane A, Graziano L, Mazzarella G, Scarpellino B, Zona G. Breast-feeding and urinary tract infection. J Pediatr. 1992;120:87–89. doi: 10.1016/s0022-3476(05)80607-9. [DOI] [PubMed] [Google Scholar]
- 32.Pruzanski W, Saito S. The influence of natural and synthetic cationic substances on phagocytic activity of human polymorphonuclear cells. Exp Cell Res. 1978;117:1–13. doi: 10.1016/0014-4827(78)90421-4. [DOI] [PubMed] [Google Scholar]
- 33.Roberts J A, Marklund B I, Ilver D, Haslam D, Kaack M B, Baskin G, Louis M, Möllby R, Winberg J, Normark S. The Gal(α1-4) Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc Natl Acad Sci USA. 1994;91:11889–11893. doi: 10.1073/pnas.91.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svanborg-Edén C, Carlsson B, Hanson L Å. Anti-pili antibodies in breast milk. Lancet. 1979;ii:1235. doi: 10.1016/s0140-6736(79)92348-1. [DOI] [PubMed] [Google Scholar]
- 35.Teraguchi S, Kouichirou S, Fukuwatari Y, Shimamura S. Glycans of bovine lactoferrin function as receptors for the type 1 fimbrial lectin of Escherichia coli. Infect Immun. 1996;64:1075–1077. doi: 10.1128/iai.64.3.1075-1077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomita M, Takase M, Bellamy W, Shimamura S. A review: the active peptide of lactoferrin. Acta Paediatr Jpn. 1994;36:585–591. doi: 10.1111/j.1442-200x.1994.tb03250.x. [DOI] [PubMed] [Google Scholar]
- 37.Tullus K, Hörlin K, Svenson S B, Källenius G. Epidemic outbreaks of acute pyelonephritis caused by nosocomial spread of P fimbriated Escherichia coli in children. J Infect Dis. 1984;150:728–736. doi: 10.1093/infdis/150.5.728. [DOI] [PubMed] [Google Scholar]
- 38.Waren J W. Clinical presentations and epidemiology of urinary tract infections. In: Mobley H L T, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C.: American Society for Microbiology; 1996. pp. 3–27. [Google Scholar]
- 39.Zagulski T, Lipinski P, Zagulska A, Jarzabek Z. Antibacterial system generated by lactoferrin in mice in vivo is primarily a killing system. Int J Exp Pathol. 1998;79:117–123. doi: 10.1046/j.1365-2613.1998.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]