Abstract
Vascular endothelial growth factor (VEGF) was discovered by its angiogenic activity. However, during evolution, it appeared earlier as a neurotrophic factor required for the development of the nervous system in invertebrates lacking a circulatory system. We aimed at reviewing recent evidence indicating that VEGF has neuroprotective effects in neurons exposed to a variety of insults. Of particular interest is the link established between VEGF and motoneurons, especially after the design of the VEGFδ/δ mutant mice. These mice are characterized by low levels of VEGF and develop muscle weakness and motoneuron degeneration resembling amyotrophic lateral sclerosis. The administration of VEGF through several routes to animal models of amyotrophic lateral sclerosis delays motor impairment and motoneuron degeneration and increases life expectancy. There are new recent advances in the role of VEGF in the physiology of motoneurons. Our experimental aims use the extraocular (abducens) motoneurons lesioned by axotomy as a model for studying VEGF actions. Axotomized abducens motoneurons exhibit severe alterations in their discharge activity and a loss of synaptic boutons. The exogenous administration of VEGF to axotomized abducens motoneurons, either from the transected nerve or intraventricularly, fully restores the synaptic and discharge properties of abducens motoneurons, despite being axotomized. In addition, when an anti-VEGF neutralizing antibody is delivered from the muscle to intact, uninjured abducens motoneurons, these cells display alterations in their discharge pattern and a loss of synaptic boutons that resemble the state of axotomy. All these data indicate that VEGF is an essential neurotrophic factor for motoneurons.
Key Words: abducens nucleus, amyotrophic lateral sclerosis, cell death, extracellular single-unit recordings, eye movements, neurodegeneration, oculomotor, trophic factors
Introduction
In last recent years, numerous investigations have widely revealed the important role played by neurotrophic factors, both in the development of the nervous system and the maintenance of the structural and functional phenotype of mature neurons, and particularly as mediators of neuronal plasticity induced by lesion or learning (Castrén 2013; Lewin and Carter, 2014; Xiao and Le, 2016; Lien et al., 2020). Neurotrophic factors are proteins secreted by target cells, which travel retrogradely towards their innervating neurons, where they regulate a myriad of biochemical, morphological, and physiological properties. The source of neurotrophic factors can also be anterograde (from afferents), paracrine (from glial or nearby neuronal cells), and autocrine (from the neuron itself). Among the variety of functions described for these molecules, the following can be highlighted: i) the regulation of ionic channels and thereby neuronal membrane excitability (Mitre et al., 2017); ii) synaptogenesis and the stabilization of synapses (Vicario-Abejón et al., 2002); iii) structural and functional changes operating during synaptic plasticity (Kowiański et al., 2018); and iv) stimulation of axonal regeneration (Bendella et al., 2018).
Among the neurotrophic factors of greatest interest currently in Neuroscience is the vascular endothelial growth factor (VEGF). VEGF was initially characterized by its proliferative activity on endothelial cells, acting on the vascular system by promoting vasculogenesis, angiogenesis, and increased vascular permeability (Yancopoulus et al., 2000; Apte et al., 2019). In addition to its well-characterized effects on blood vessels, there is extensive evidence indicating that VEGF is also a neuroprotective factor. For instance, VEGF protects hippocampal neurons from cell death after an induced seizure (Nicoletti et al., 2008), improves the behavioral deficits of status epilepticus (Ureña-Guerrero et al., 2020), and the brain damage caused by ischemia (Yang et al., 2018). Interestingly, it also promotes the regeneration of sectioned nerves (Beecher et al., 2018; Ding et al., 2018; Theis and Theiss, 2018; Xu et al., 2022). It also acts as a cellular rescuing factor in animal models of neurodegenerative diseases, such as Alzheimer’s disease (Guo et al., 2019; Ureña-Guerrero et al., 2020), and Parkinson’s disease (Yasuhara et al., 2005).
It is important to outline that the more recently discovered neurotrophic activity of VEGF appears to be evolutionarily more ancient than its angiogenic activity. Thus, invertebrate animals lacking blood vessels, such as Caenorhabditis elegans, express VEGF at the neuronal level, acting as a necessary molecule for the development of the nervous system. The same occurs in the fruit fly Drosophila melanogaster, which has a rudimentary vasculature but requires VEGF for proper neuronal development (Zacchigna et al., 2008).
This review aimed at revising the evidence that implies VEGF as a critical neurotrophic and neuroprotective factor, with a special focus on motoneurons and in particular on extraocular motoneurons.
Retrieval Strategy
The following inclusion criteria were used for literature screening: studies that discussed the co-occurrence of VEGF and neuroprotection or neurotrophic factors or receptors or motoneurons for literature search. English language and full-text articles published between 1992 and 2022 were included in this narrative review. Some oculomotor field articles older than 1992 were also quoted because they describe the seminal electrophysiological findings of abducens motoneurons. The authors searched the PubMed database to identify relevant publications by search terms. The authors screened the reference list of included studies to identify other potentially useful studies. Firstly, the authors screened the titles and abstracts, then, the full texts.
Vascular Endothelial Growth Factor Family and Receptors
The members of the VEGF family are dimeric glycoproteins of a molecular weight of around 40 kDa that have a high degree of homology with each other. In mammals, the VEGF family is composed of five factors: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and the placental growth factor (PlGF). The VEGF family has been implicated in numerous functions involving angiogenesis, lymphangiogenesis, development of the nervous system and its vascularization, neurogenesis, synaptic plasticity, neuronal electrophysiological properties, neurotransmission, neuronal survival, and neuroregeneration (Ruiz de Almodovar et al., 2009; Lladó et al., 2013; Chi et al., 2019; Kou et al., 2019; Latzer et al., 2019). They have also been considered factors with a promising therapeutic role in neurological diseases (Lange et al., 2016). VEGF-A was the first member discovered, for its actions on the vascular system, and is, therefore, the founding molecule of this family. The term VEGF refers to VEGF-A and they are used synonymously in the literature. VEGF-A stands out as the most studied VEGF family member, due to both its high angiogenic activity and its role as a powerful neuroprotective factor (Silva-Hucha et al., 2021). VEGF-B has weak angiogenic activity, but also exerts neuroprotection and can restore physiological alterations induced by injury (Poesen et al., 2008; Dhondt et al., 2011; Calvo et al., 2018). VEGF-C and VEGF-D promote lymphangiogenesis, and PlGF acts under pathological conditions stimulating angiogenesis (Laakkonen et al., 2019; Uccelli et al., 2019).
The signaling of VEGFs is mediated through binding to two classes of surface membrane receptors: receptors with tyrosine kinase activity and receptors lacking such activity. The first group comprises three structurally related receptors characterized by an intracellular domain endowed by tyrosine kinase activity, which are VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1), and VEGFR-3 (Flt-4). VEGF-A binds to VEGFR-1 and VEGFR-2, VEGF-B and PlGF bind to VEGFR-1, and VEGF-C and VEGF-D interact with VEGFR-3 and VEGFR-2 (Table 1; Stevens and Oltean 2019; Malekan and Ebrahimzadeh, 2022). The best characterized VEGFR is VEGFR-2 which is the major receptor mediating the angiogenic and neuroprotective activity of VEGF. The role of VEGFR-1 is not so clear, and it has been suggested that could act as a negative regulator of VEGFR-2 acting as a “decoy receptor” that traps VEGF preventing excessive activation of VEGFR-2 (Koch and Claesson-Welsh, 2012; Stevens and Oltean, 2019). VEGFR-3 is mainly involved in lymphangiogenesis (Takahashi and Shibuya, 2005; Malekan and Ebrahimzadeh, 2022).
Table 1.
VEGF family ligands and receptors
| Factor | VEGF-A | VEGF-B | PlGF | VEGF-C | VEGF-D |
|---|---|---|---|---|---|
| Receptor | VEGFR-1 | VEGFR-1 | VEGFR-1 | VEGFR-2 | VEGFR-2 |
| VEGFR-2 | NRP-1 | NRP-1 | VEGFR-3 | VEGFR-3 | |
| NRP-1 | NRP-2 | NRP-1 | NRP-1 | ||
| NRP-2 | NRP-2 | NRP-2 |
NRP: Neuropilins; PlGF: placental growth factor; VEGF: vascular endothelial growth factor.
In addition, VEGFs can also bind to receptors lacking tyrosine kinase activity, which are named neuropilins (NRP): NRP-1 and NRP-2. They act as co-receptors for VEGFR-1, VEGFR-2, and VEGFR-3, enhancing the binding of VEGFs to VEGFRs and increasing the efficacy of VEGFR-mediated intracellular signaling pathways. The specific ligands for NRPs are as follows: VEGF (i.e., VEGF-A) binds to NRP-1 and NRP-2, VEGF-B binds to NRP-1, PlGF binds to NRP-1 and NRP-2, and VEGF-C and VEGF-D interact with NRP-1 and NRP-2 (Table 1; Lange et al., 2016; Mercurio, 2019; Stevens and Oltean, 2019).
The VEGF-A gene produces mRNA variants by alternative splicing, leading to at least three major VEGF isoforms. The human VEGF isoforms contain 121, 165, or 189 amino acids. In rats and mice, these three proteins are one residue shorter (i.e., 120, 164, or 188) (Micheli et al., 2021; Fico et al., 2022). The present review will focus mainly on the VEGF165(164) isoform, unless otherwise specified, which is the prevalent isoform in the study of VEGF actions on neurons.
Low VEGF Levels and Motoneuronal Degeneration: Lessons from Transgenic Animals
A finding of great interest regarding VEGF is the relationship established by Oosthuyse et al. in 2001 between low levels of VEGF and amyotrophic lateral sclerosis (ALS). ALS is a neurodegenerative disease that causes progressive degeneration of the brainstem and spinal motoneurons, as well as cortical neurons. Consequently, ALS leads to severe and progressive muscle weakness with atrophy and paralysis, being generally fatal within 5 years after symptoms onset. The majority of ALS cases are sporadic, and only 10% have a family story, with a fifth of these caused by mutations in the gene encoding the superoxide dismutase (SOD1) (Morrice et al., 2018; Liu et al., 2022; Ruffo et al., 2022; Verma et al., 2022). The etiology of ALS is still unclear and several processes have been implicated in its pathogenesis such as oxidative stress, excitotoxicity, protein aggregation, glial cell toxicity, cytoskeleton alterations, and lack of trophic factors (Robberecht and Philips, 2013; Valko and Cieslac, 2019; Raffaele et al., 2021; Dhasmana et al., 2022; Liu and Henty-Ridilla, 2022). Rodents expressing a mutant SOD1 protein develop pathological features that resemble ALS. SOD1 transgenic mice and rats constitute thus a classical model for ALS investigations (Tovar-y-Romo et al., 2009; Todd and Petrucelli, 2022). Some studies have demonstrated a decrease in VEGF and VEGFR-2 levels in the spinal cord of SOD1 ALS mice (Lu et al., 2007; Lund et al., 2009).
The link between VEGF and ALS was established after the design of the transgenic VEGFδ/δ mutant mice, which is characterized by the deletion of the hypoxia response element in the promoter region of the VEGF gene (Oosthuyse et al., 2001). Hypoxia is a potent stimulator of VEGF transcription, and thereby VEGFδ/δ mutant mice lose the ability to increase VEGF levels in hypoxic situations. Surprisingly, this alteration caused them to develop early-onset motoneuron degeneration in adulthood. They exhibit progressive severe signs of muscle atrophy with little mobility. Histological examination of the spinal cord reveals progressive motoneuron degeneration and loss, and peripheral nerves show prominent signs of Wallerian degeneration. Although basal VEGF levels are normal in peripheral organs and cells outside the nervous system, spinal VEGF levels are reduced by 75%, indicating that VEGF deficiency selectively impaired motoneurons (Oosthuyse et al., 2001; Silva-Hucha et al., 2021).
The symptoms and neuropathological changes in VEGFδ/δ mutant mice resemble those found in ALS patients (Dhasmana et al., 2022; Felman et al., 2022) and are also similar to the well-established SOD1 animal model of ALS (Peggion et al., 2020; Alhindi 2022). The relevance of that work (Oosthuyse et al., 2001) is that it establishes for the first time a link between low levels of VEGF and motoneuron degeneration. Although other neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor, ciliary neurotrophic factor, or leukemia inhibitory factor, can ameliorate motoneuron degeneration in ALS mouse models, however, the loss of these molecules does not produce ALS-like neuropathology. Taken together, these findings reinforce the powerful role of VEGF in maintaining motoneuron integrity and point to a potential value of VEGF for the treatment of motoneuron diseases (Lambrechts and Carmeliet, 2006; Tovar-y-Romo et al., 2014). Interestingly, mice resulting from the crossing between VEGFδ/δ and SOD1 mutant mice exhibit a more severe motoneuron degeneration and an earlier onset of muscle weakness than the individual transgenic mice (Lambrechts et al., 2003). In the same line, crossbreeding of SOD1 mice with mice overexpressing VEGF results in double-mutant mice SOD1/VEGF+/+ exhibiting delayed motoneuron degeneration and motor deficiencies, and with a longer life expectancy than single SOD1 transgenics (Wang et al, 2007).
Vascular Endothelial Growth Factor Administration to Injured Motoneurons
VEGF delivery has been shown to improve the biochemical, morphological, and physiological alterations in severed motoneurons, both in vitro and in vivo. In vitro, in motoneuron cell cultures, exogenously administered VEGF has been reported to increase basal motoneuron survival in a dose-dependent manner. For instance, when NSC-34 cells (a motoneuron-like cell line) are exposed in vitro to cerebrospinal fluid from ALS patients, the addition of VEGF to the culture medium protects them from cell death and leads to better ultrastructural preservation (Kulsheshtha et al., 2011; Vijayalakshmi et al., 2015). Similarly, VEFG protects NSC-34 motoneuron-like cell death from the effects of mutant SOD1 protein, after transfecting these cells with adenovirus containing the mutant protein.
In vivo, several experiments have also demonstrated that VEGF administration protects injured motoneurons in a variety of animal models of the lesion. Several ways of administration have been used to deliver VEGF: viral vectors, neural stem cells, or direct exogenous infusion of the factor itself. In a pioneering study, injection of a VEGF-expressing lentiviral vector into various skeletal muscles in the SOD1 mouse model of ALS demonstrated that the construct is retrogradely transported and transduced in motoneurons, resulting in delayed onset and slowed progression of the disease, with a marked increase in life expectancy of these mutant mice (Azzouz et al., 2004). In a different administration protocol, intrathecal transplantation of immortalized human neural stem cells, genetically modified to overexpress VEGF, significantly delays the onset and prolongs the survival of SOD transgenic mice. Moreover, the implanted cells migrate to the anterior horn of the spinal cord and differentiate into motoneurons. The neuroprotective mechanism involves the downregulation of proapoptotic proteins and the upregulation of antiapoptotic proteins in the spinal cord tissue (Hwang et al., 2009). In another study, adeno-associated virus 9 vectors were used to deliver VEGF through intrathecal injection in SOD1 mice (Wang et al., 2016). The authors show that this treatment results in motor function improvement and a longer life span for SOD1 mice. Furthermore, VEGF contributes to motoneuron protection by the activation of the PI3-K/Akt survival pathway, leading to a decrease in the expression of apoptotic proteins and an increase in antiapoptotic proteins (Wang et al., 2016).
The direct administration of VEGF to vulnerable motoneurons, in both ALS mice and motoneurons exposed to excitotoxicity, has also revealed neuroprotection due to this factor. VEGF infused intracerebroventricularly by an osmotic pump significantly delays the onset of limb paralysis, improves motor performance, and prolongs motoneuron survival and life expectancy in SOD1 rats. These beneficial effects are mediated via VEGFR-2 signaling (Storkebaum et al., 2005). In ALS transgenic mice, intraperitoneal administration of VEGF also shows neuroprotective effects, such as improved motor behavior, delayed disease onset, and progression, decreased astrogliosis in the spinal cord ventral horn, restoration of neuromuscular junctions, and a longer survival time (Zheng et al., 2004, 2007). In an interesting study, Tovar-y-Romo et al. (2007) developed an in vivo (rat) model of progressive spinal motoneuron death due to the overactivation of AMPA receptors. VEGF administered together with AMPA through osmotic minipumps completely prevents motor deficits, and motoneuron death is reduced by more than 75%. Subsequently, the same group demonstrated that VEGF mediates this neuroprotective function in spinal motoneurons through VEGFR-2 signaling, activating the PI3-K pathway and inhibiting p38MAPK (Tovar-y-Romo and Tapia, 2010).
Two mechanisms of action for the neuroprotective role of VEGF have been proposed, which are non-mutually exclusive. The vascular hypothesis suggests that VEGF protects severed motoneurons due to its activity in the vascular system, inducing angiogenesis and increased blood vessel permeability, which would supply more oxygen and nutrients to vulnerable motoneurons improving their conditions. The second hypothesis is that VEGF is a neurotrophic factor and acts directly on motoneurons exerting neuroprotective activity. The neuroprotective mechanism involves the downregulation of proapoptotic proteins and the upregulation of antiapoptotic proteins (Hwang et al., 2009; Wang et al., 2016). In addition, neurotrophic action could activate intracellular pathways that ultimately lead to the maintenance of the morphofunctional characteristics of motoneurons (Tovar-y-Romo et al., 2007; Tovar-y-Romo and Tapia, 2010; Calvo et al., 2018, 2020). An insufficient supply of VEGF could be deleterious to motoneurons. Although no signs of angiogenesis were found in VEGFδ/δ mice (Oosthuyse et al., 2001), the authors report reduced spinal cord perfusion and indicate that both mechanisms could operate. However, there is more evidence pointing to VEGF as a neurotrophic factor that acts by itself promoting the neuroprotective effects that follow its administration, without the mediation of its vascular activity. The finding that VEGF addition to the culture medium in vitro protects motoneuron-like cells from deleterious conditions favors the neurotrophic hypothesis, as there is no vascular system in vitro (see above). Moreover, the administration of VEGF to animal models of motoneuron disease or after the lesion has demonstrated a lack of angiogenesis and no change in blood vessel permeability (Azzouz et al., 2004; Storkebaum et al., 2005; Tovar-y-Romo et al., 2007; Acosta et al., 2018).
In relation to the second hypothesis, it should be indicated that there is more evidence pointing to VEGF as a neurotrophic factor that acts by itself promoting the neuroprotective effects that follow its administration, without the mediation of vascular activity. These include studies carried out in different neuronal types and following a variety of insults. For instance, VEGF protects against seizure-induced cell death in the hippocampus (Nicoletti et al., 2009) and mitigates epileptic behavioral disorders (Ureña-Guerrero et al., 2020). VEGF also promotes the morphofunctional recovery of injured peripheral nerves as seen, for instance, after olfactory nerve bulbectomy (Beecher et al., 2018). VEGF exerts neuroprotective and neurostorative effects in animal models of neurodegenerative diseases (Ureña-Guerrero et al., 2020). In stroke, VEGF administration reduces infarct size and reduces hypoxic neuronal cell death (Cárdenas-Rivera et al., 2019). Thus, there is ample evidence indicating a direct neuroprotective and neurotrophic action of VEGF, not only in motoneurons, but also in other neuronal types exposed to different types of damage.
Extraocular Motoneurons and Vascular Endothelial Growth Factor
Extraocular motoneurons are responsible for generating eye movements. They lay in three brainstem nuclei: the mesencephalic oculomotor and trochlear nuclei, and the pontine abducens nucleus. Our group has carried out several studies in these motoneurons concerning VEGF that have contributed to a better understanding of the neurotrophic influence of this molecule in motoneurons. Extraocular motoneurons in rats and cats are endowed with the VEGF receptors VEGFR-1 and VEGFR-2, both at the level of the somatic membrane and in the axon terminals at the neuromuscular junction (Silva-Hucha et al., 2017, 2020; Calvo et al., 2018), and are therefore responsive to this factor. Moreover, extraocular muscles contain VEGF (Calvo et al., 2018; Silva-Hucha et al., 2020), so that VEGF might act as a target-derived neurotrophic factor for extraocular motoneurons. It has been demonstrated, in the rat sciatic nerve, that VEGF can be transported along the axons (Storkebaum et al., 2005). In this line, it should be noted that the possible sources of VEGF supply for extraocular motoneurons have been extensively studied in the work by Silva-Hucha et al. (2020). These authors emphasize the role played by the retrograde pathway arising in the target muscle, as well as the autocrine route since axotomy induces an increase in VEGF and VEGFR-2 in extraocular motoneurons. Glial cells appear to contribute negligibly as a paracrine source (Silva-Hucha et al., 2020). Motoneurons afferents as a likely source of VEGF have not been explored yet.
It should be noted that, although extraocular motoneurons have traditionally been considered to be more resistant to ALS than spinal and other cranial motoneurons, they also show signs of degeneration and patients also suffer from oculomotor deficits, although in later stages of the disease (Takahashi et al., 1993; Sharma et al., 2011; Kimura et al., 2014; Tjust et al., 2017). The influence of VEGF on extraocular motoneurons has been evaluated after the section of their axons, that is, by axotomy, an injury model that leads to numerous biochemical, structural, and physiological alterations (Rink et al., 2019; Alvarez et al., 2020).
Recovery of the Cholinergic Phenotype in Axotomized Extraocular Motoneurons after Vascular Endothelial Growth Factor Delivery
A general response of motoneurons to axotomy is the downregulation in the expression of the biosynthetic enzyme of their neurotransmitter (acetylcholine), i.e., choline acetyltransferase (Navarro et al., 2007). Extraocular motoneurons also exhibit a decrease in choline acetyltransferase after their axotomy, which is maximal by 7 days post-lesion in the rat (Morcuende et al., 2005, 2013). By immunocytochemical measurements, it has been demonstrated that 7 days post-axotomy, approximately only 50% of oculomotor, trochlear, and abducens motoneurons in the lesioned side are choline acetyltransferase-immunoreactive, as compared to the control side. The peripheral administration of VEGF to axotomized extraocular motoneurons prevents, in the three extraocular motor nuclei, the loss of the cholinergic phenotype, so that the number and the intensity of immunolabeling are similar on the lesioned and control side (Acosta et al., 2018). Moreover, in that study it was demonstrated that the density and permeability of blood vessels are not increased after VEGF treatment, pointing to a direct neurotrophic effect of VEGF on the motoneurons. Choline acetyltransferase mRNA levels, enzyme activity, and immunostaining are also decreased in spinal motoneurons of patients with ALS (Nagata et al., 1982; Virgo et al., 1992; Oda et al., 1995). Taken together, the results of these studies suggest the possibility that VEGF delivered to diseased motoneurons, in addition to its neurorescue effect, also preserves their neurotransmissive function (Acosta et al., 2018).
Although numerous works have demonstrated the neuroprotective role of VEGF in injured or diseased motoneurons, they have not investigated the functional state in which these motoneurons remain after being rescued from cell death, which is an important question, as motoneurons should remain with normal synaptic and discharge properties for their normal operation mode. This issue has been an important objective in studies of VEGF in extraocular motoneurons. In particular, physiological recordings have been carried out in the cat abducens nucleus after the axotomy of their motoneurons followed by the administration of VEGF, which was delivered either peripherally from the proximal stump of the transected axons (Figure 1A) or centrally following an intraventricular injection (Figure 1B). Another interesting approach in this model has been the administration of an anti-VEGF neutralizing antibody to control abducens motoneurons. In all these experiments, the discharge activity of abducens motoneurons has been recorded using the chronic alert animal preparation. This preparation offers the advantage of allowing the correlation of neuronal activity with motor behavior (eye movements) under physiological conditions, and also after experimental manipulations such as axotomy and administration of substances.
Figure 1.
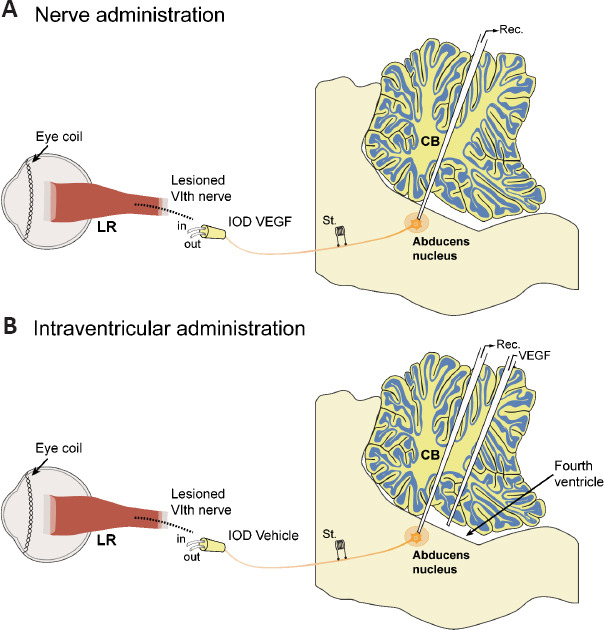
Schematic diagram illustrating the two methods of VEGF administration to axotomized abducens motoneurons.
(A) One approach consisted of the administration of VEGF through the transected sixth nerve inserted into an intraorbital device (IOD). (B) In a different experimental procedure, VEGF was administered in the fourth ventricle with a micropipette through the intact cerebellum (CB). Abducens motoneurons were electrophysiological recorded (Rec.) following their antidromic identification carried out after electrical stimulation (St.) to the nerve. Eye coils were used for the recording of eye movements. LR: Lateral rectus muscle, the extraocular muscle innervated by abducens motoneurons; VEGF: vascular endothelial growth factor.
Vascular Endothelial Growth Factor Administration to Axotomized Abducens Motoneurons from the Transected Nerve
Abducens motoneurons are characterized by a tonic-phasic discharge pattern that correlates with eye position and eye velocity. They discharge at higher rates for temporally-directed eye movements of the ipsilateral side (i.e., abduction, the on direction). During eye fixations, abducens motoneurons fire tonically with a frequency that increases for eye positions more lateral in the orbit (Figure 2A). They also exhibit a dynamic component, so that during rapid eye movements or saccades in the on direction, they discharge a high-frequency burst of spikes, whereas during off-directed saccades their firing decreases abruptly o ceases completely (Figure 2A) (Delgado-García et al., 1986; Davis-López de Carrizosa et al., 2011; Calvo et al., 2018, 2020, 2022).
Figure 2.
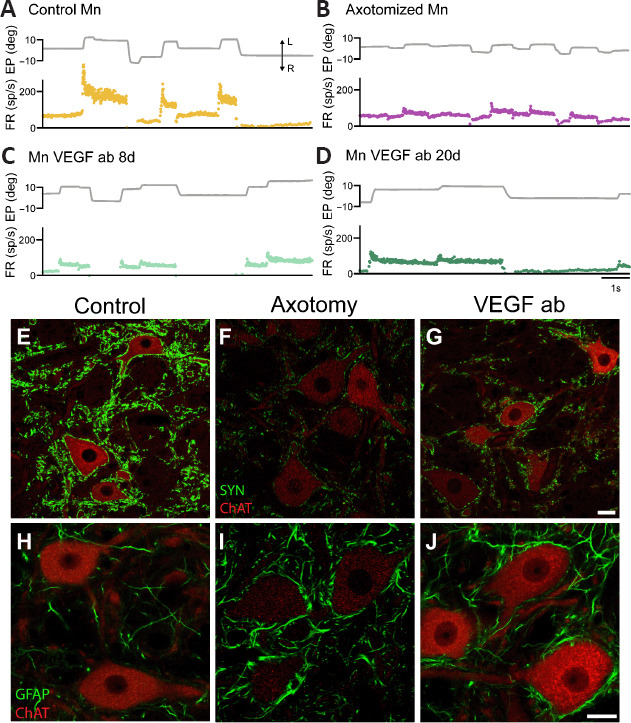
Changes in firing pattern and synaptic inputs in abducens motoneurons treated with anti-VEGF antibody.
(A) Firing rate (FR, in spikes/s) of a control motoneuron (Mn) during spontaneous eye movements (EP, eye position, in degrees). (B) Axotomized abducens motoneurons discharge at remarkably lower frequencies during both fixations and rapid eye movements or saccades. (C, D) Discharge activity of two (intact) motoneurons treated with the neutralizing antibody (ab) against VEGF during 8 or 20 days (C and D, respectively). Note that the blockade of VEGF with the antibody renders motoneurons into an axotomy-like state. The double arrow with L and R in panel A indicates leftward and rightward eye movement, respectively, in A–D. (E–G) Confocal microscopy images of double immunofluorescence in the abducens nucleus combining choline acetyltransferase (ChAT, in red), a marker for motoneurons, with synaptophysin (SYN, in green) to label synaptic boutons. Control motoneurons (E) appear surrounded by numerous synaptophysin-positive boutons. In contrast, after axotomy (F), motoneurons show a massive loss of synaptic boutons as observed by the weak synaptophysin labeling. Uninjured motoneurons treated with the anti-VEGF antibody (VEGF ab) exhibit also scarce synaptophysin-positive boutons (G), lesser than in control (E) and similar to the axotomy situation (F). (H–J) Confocal microscopy images of double immunofluorescence in the abducens nucleus combining (ChAT, in red) with GFAP (in green) as a marker of astrocytes. GFAP immunostaining was very weak in the control situation (H). In contrast, it increased markedly after axotomy (I). In the anti-VEGF antibody situation, although motoneurons are not injured, there was a glial reaction similar to the axotomy condition (J). Scale bars: 20 μm in G for E–G; 20 μm in J for H–J). Reprinted with permission from Calvo et al. (2022). VEGF: Vascular endothelial growth factor.
After the section of the VIth nerve, axotomized abducens motoneurons show noticeable alterations in their discharge pattern. They fire at an overall lower frequency, and bursts of action potentials during on-directed saccades are remarkably reduced (Figure 2B). Axotomized abducens motoneurons show less eye position and velocity sensitivities than controls. They also exhibit a loss of synaptic boutons –a process known as synaptic stripping (Figure 2E and F)- and a marked glial reaction around their cell bodies and in the neuropil (Figure 2H and I) (Delgado-García et al., 1988; Davis-López de Carrizosa et al., 2009, 2010; Calvo et al., 2018, 2020, 2022; Alvarez et al., 2020).
VEGF has been delivered chronically to axotomized motoneurons through the proximal stump of the sectioned VIth nerve (Figure 1A) and following two administration protocols: the immediate administration protocol, in which VEGF begins to be administered just after axotomy, and the delayed administration protocol, in which the onset of VEGF supply is carried out after 20 days of axotomy. In both protocols, it has been found that the exogenous administration of VEGF prevents (immediate administration protocol) and recovers (delayed administration protocol) all the alterations induced by axotomy. Thus, axotomized abducens motoneurons treated with VEGF show a normal tonic-phasic discharge pattern, and eye position and velocity sensitivities values are similar to controls. Moreover, they show a normal synaptic coverage and glial reaction is not present in the abducens nucleus (Calvo et al., 2018). Interestingly, the addition along with VEGF of specific inhibitors of VEGFRs has revealed that the two tyrosine kinase receptors of VEGF, VEGFR-1, and VEGFR-2, play different roles in the maintenance of specific afferents to abducens motoneurons (Calvo et al., 2018).
Previous works have studied the effects of BDNF, NT-3, or NGF administration on axotomized abducens motoneurons. In no case do these neurotrophic factors fully recover abducens motoneurons from the alterations induced by axotomy? BDNF restores the tonic signal but not the phasic component of the discharge. On the other hand, NT-3 recovers only the phasic firing, but not the tonic component. NGF produces an increase in firing irregularity during fixations and yields neuronal eye position and velocity sensitivities higher than controls (Davis-López de Carrizosa et al., 2009, 2010). Therefore, among all neurotrophic factors tested in this model, only VEGF fully reestablishes the discharge activity and synaptic composition of axotomized abducens motoneurons, indicating the crucial role of this factor in maintaining the normal physiology of motoneurons.
Intraventricular Administration of Vascular Endothelial Growth Factor to Axotomized Abducens Motoneurons
VEGF has also been administered into the fourth ventricle, which lies just above the medulla oblongata and the pons, where the abducens nucleus is located at a dorsal position (Figure 1B). The peculiarity of this study is the finding that a single dose of VEGF administered in the same surgical session as axotomy is sufficient for the long-term recovery of the morpho-physiological characteristics of axotomized abducens motoneurons (Calvo et al., 2020). VEGF-treated axotomized abducens motoneurons discharge with a normal firing pattern in relation to eye movements. Synaptic density is also preserved in axotomized neurons and glial reaction is absent in the abducens nucleus. A likely mechanism to explain the long-lasting effects of a single intraventricular dose of VEGF could be related to its heparin-binding capacity. VEGF has a heparin-binding domain, which can interact with the heparin-sulfate-rich extracellular matrix and thus remains for a long period in the tissue, prolonging its biological activity (Robinson and Stringer, 2001; Tokunaga et al., 2005; Krilleke et al., 2009).
Calvo et al. (2020) also showed that the intraventricular administration of VEGF does not induce an angiogenic response in the abducens nucleus or other brainstem structures, such as the medial vestibular nucleus, as occurs after the peripheral administration to axotomized extraocular motoneurons in the rat (Acosta et al., 2018). Moreover, it has been demonstrated that the angiogenic activity of VEGF is only promoted when VEGF is administered at high doses (60 μg of VEGF/7 days in rats). However, in all the experimental studies referenced in this review, the dose of VEGF that has been applied exogenously is much lower, again indicating that the neuroprotective activity of VEGF is more the consequence of a direct neurotrophic action on neurons than an indirect vascular effect due to increased nutrient supply. In addition, high doses of VEGF are not neuroprotective since angiogenesis along with increased blood vessel permeability can lead to tissue edema (Manoonkitiwongsa et al., 2004).
The intracerebroventricular delivery of VEGF has previously been shown to represent a viable methodological approach for VEGF to exert its neuroprotective activity in SOD1 rats (Storkebaum et al., 2005). However, the particularity of the work by Calvo et al. (2020) was the demonstration that a single dose of VEGF applied to the fourth ventricle is enough for VEGF to induce long-term neurotrophic and synaptotrophic effects on injured abducens motoneurons, at least for 50 days (maximum time interval studied). This finding could raise expectations for the therapeutic administration of VEGF (due to the potential of VEGF for the treatment of motoneuron disorders) since it would avoid the side effects of repetitive or high doses of VEGF.
Anti-Vascular Endothelial Growth Factor Antibody Administration to Abducens Motoneurons
Electrophysiological recordings of intact abducens motoneurons in the alert chronic cat, during 21 days of intramuscular administration of a neutralizing antibody against VEGF, have demonstrated that abducens motoneurons discharge with significantly lower eye position and eye velocity sensitivities during spontaneous eye movements than in control, i.e., before the onset of antibody treatment (Figure 2A, control; Figure 2C and D, 8 and 20 days of anti-VEGF antibody treatment, respectively). Their overall firing rate is reduced during eye fixations and rapid eye movements (saccades) in the on-direction, showing statistical similarity with the axotomy state (Figure 2B; Calvo et al., 2022). However, during the vestibulo-ocular reflex, neuronal discharge shows similar eye-related parameters to control and is not affected by the anti-VEGF antibody. This finding is not surprising given previous results indicating that the selective blockage of VEGFR-2 does not abolish the recovery of firing properties of axotomized abducens motoneurons treated with VEGF during vestibular eye movements, in contrast to what happens during spontaneous eye movements (Calvo et al., 2018). Moreover, it should be considered that the vestibulo-ocular reflex is highly conserved throughout evolution, and is present in all vertebrates acting through the classic three-neuron reflex arch as a rather fixed circuitry (Horn and Straka, 2021). Therefore, this fact suggests that vestibular synaptic inputs are a source of afferents well-preserved on vertebrate extraocular motoneurons and might be likely less influenced by trophic mechanisms.
In addition, the treatment with the anti-VEGF antibody to uninjured abducens motoneurons (chronically delivered from the muscle) leads to a loss of synaptic boutons surrounding their somatic membrane (i.e., synaptic stripping), as occurs during axotomy (Figure 2E, control; Figure 2F, axotomy; Figure 2G, anti-VEGF antibody) (Delgado-García et al., 1988; Calvo et al., 2018, 2020). However, the reduction in synaptic coverage in the anti-VEGF antibody situation is not as large as during axotomy, likely due to the maintenance of the vestibular inputs (Calvo et al., 2022). In addition, there are also signs of a glial reaction in the abducens nucleus, observed by an increase in GFAP-immunoreactive profiles, in spite that motoneurons are not injured (Figure 2H, control; Figure 2I, axotomy; Figure 2J, anti-VEGF antibody treatment). All these findings indicate that retrograde VEGF blockade causes uninjured abducens motoneurons to enter an axotomy-like state, highlighting the critical role of VEGF in preserving the morphophysiological properties of these motoneurons.
Conclusion
The essential role of VEGF for motoneuron survival was evidenced for the first time after the design of the VEGFδ/δ mutant mice, characterized by low levels of VEGF, mainly in the nervous tissue. These mutant mice develop a progressive muscle weakness and spinal motoneuron degeneration resembling ALS. Until now, the lack of other neurotrophic factors induced by genetic engineering in rodents has not produced deleterious effects on motoneurons, highlighting the relevance of VEGF for this neuronal type. Experiments administrating VEGF to animal models of ALS, using different delivery procedures, have demonstrated that VEGF significantly reduces and delays motor impairment and motoneuron degeneration and increases life expectancy. Electrophysiological recordings carried out in the behaving animal have proven that the discharge activity and synaptic inputs of extraocular (abducens) motoneurons, altered by axotomy, fully recover after VEGF administration, delivered from either the transected nerve or intraventricularly. Moreover, the administration of an anti-VEGF neutralizing antibody produces in intact, uninjured abducens motoneurons changes in their firing pattern and synaptic inputs that resemble the axotomy state. Altogether, it is proposed that VEGF plays an essential role in the survival and maintenance of the physiological properties of motoneurons.
Footnotes
Funding: This work was supported by the I+D+i project P20_00529 Consejería de Transformación Económica Industria y Conocimiento, Junta de Andalucía-FEDER. Research materials were also supported by project PGC2018-094654-B-100 and PID2021-124300NB-I00 funded by MCIN/AEI/FEDER “A way of making Europe”. P.M.C. was a scholar of Ministerio de Educación y Ciencia (BES-2016-077912) in Spain and is now a “Margarita Salas” postdoctoral fellow. RGH is a postdoctoral fellow from PAIDI-2019, “Talento Doctores” Junta de Andalucía in Spain, and is now a “Ramón y Cajal” fellow in Spain.
Conflicts of interest: The authors declare no conflicts of interest.
C-Editors: Zhao M, Liu WJ, Li CH; T-Editor: Jia Y
References
- 1.Acosta L, Morcuende S, Silva-Hucha S, Pastor AM, de la Cruz RR. Vascular endothelial growth factor (VEGF) prevents the downregulation of the cholinergic phenotype in axotomized motoneurons of the adult rat. Front Mol Neurosci. (2018);11:241. doi: 10.3389/fnmol.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhindi A, Boehm I, Chaytow H. Small junction, big problems: Neuromuscular junction pathology in mouse models of amyotrophic lateral sclerosis (ALS) J Anat. (2022);241:1089–1107. doi: 10.1111/joa.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez FJ, Rotterman TM, Akhter ET, Lane AR, English AW, Cope TC. Synaptic plasticity on motoneurons after axotomy:a necessary change in paradigm. Front Mol Neurosci. (2020);13:68. doi: 10.3389/fnmol.2020.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease:beyond discovery and development. Cell. (2019);176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. (2004);429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 6.Beecher K, Hafner LM, Ekberg J, St John JA, Chehrehasa F. Combined VEGF/PDGF improves olfactory regeneration after unilateral bulbectomy in mice. Neural Regen Res. (2018);13:1820–1826. doi: 10.4103/1673-5374.238713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendella H, Rink S, Grosheva M, Sarikcioglu L, Gordon T, Angelov DN. Putative roles of soluble trophic factors in facial nerve regeneration, target, reinnervation, and recovery of vibrissal whisking. Exp Neurol. (2018);300:100–110. doi: 10.1016/j.expneurol.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Calvo PM, de la Cruz RR, Pastor AM. Synaptic loss and firing alterations in axotomized motoneurons are restored by vascular endothelial growth factor (VEGF) and VEGF-B. Exp Neurol. (2018);304:67–81. doi: 10.1016/j.expneurol.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Calvo PM, de la Cruz RR, Pastor AM. A single intraventricular injection of VEGF leads to long-term neurotrophic effects in axotomized motoneurons. eNeuro. (2020);29:7. doi: 10.1523/ENEURO.0467-19.2020. ENEURO.0467-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvo PM, Hernández RG, de la Cruz RR, Pastor AM. VEGF is an essential retrograde trophic factor for motoneurons. Proc Natl Acad Sci U S A. (2022);119:e2202912119. doi: 10.1073/pnas.2202912119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cárdenas-Rivera A, Campero-Romero AN, Heras-Romero Y, Penagos-Puig A, Rincón-Heredia R, Tovar-Y-Romo LB. Early post-stroke activation of vascular endothelial growth factor receptor 2 hinders the receptor 1-dependent neuroprotection afforded by the endogenous ligand. Front Cell Neurosci. (2019);13:270. doi: 10.3389/fncel.2019.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castrén E. Trophic factors: Neurotrophic factors. Neuroscience in the 21st century. In: Pfaff DW, editor. From basic to clinical. New York: Springer; (2013). pp. 1555–1589. [Google Scholar]
- 13.Chi ZL, Adini A, Birsner AE, Bazinet L, Akula JD, D'Amato RJ. Neuropharmacology PR1P ameliorates neurodegeneration through activation of VEGF signaling pathway and remodeling of the extracellular environment. Neuropharmacology. (2019);148:96–106. doi: 10.1016/j.neuropharm.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 14.Davis-López de Carrizosa MA, Morado-Díaz CJ, Tena JJ, Benítez-Temiño B, Pecero ML, Morcuende SR and others. Complementary actions of BDNF and neurotrophin-3 on the firing patterns and synaptic composition of motoneurons. J Neurosci. (2009);29:575–587. doi: 10.1523/JNEUROSCI.5312-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis-López de Carrizosa MA, Morado-Díaz CJ, Morcuende S, de la Cruz RR, Pastor AM. Nerve growth factor regulates the firing patterns and synaptic composition of motoneurons. J Neurosci. (2010);30:8308–8319. doi: 10.1523/JNEUROSCI.0719-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis-López de Carrizosa MA, Morado-Díaz CJ, Miller JM, de la Cruz RR, Pastor AM. Dual encoding of muscle tension and eye position by abducens motoneurons. J Neurosci. (2011);3:2271–2279. doi: 10.1523/JNEUROSCI.5416-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado-García JM, del Pozo F, Baker R. Behavior of neurons in the abducens nucleus of the alert cat--I. Motoneurons. Neuroscience. (1986);17:929–952. doi: 10.1016/0306-4522(86)90072-2. [DOI] [PubMed] [Google Scholar]
- 18.Delgado-García JM, del Pozo F, Spencer RF, Baker R. Behavior of neurons in the abducens nucleus of the alert cat--III. Axotomized motoneurons. Neuroscience. (1988);24:143–160. doi: 10.1016/0306-4522(88)90319-3. [DOI] [PubMed] [Google Scholar]
- 19.Dhasmana S, Dhasmana A, Narula AS, Jaggi M, Yallapu MM, Chauhan SC. The panoramic view of amyotrophic lateral sclerosis: A fatal intricate neurological disorder. Life Sci. (2022);288:120156. doi: 10.1016/j.lfs.2021.120156. [DOI] [PubMed] [Google Scholar]
- 20.Ding Z, Cao J, Shen Y, Zou Y, Yang X, Zhou W, Guo Q, Huang C. Resveratrol promotes nerve regeneration via activation of p300 acetyltransferase-mediated VEGF signaling in a rat model of sciatic nerve crush injury. Front Neurosci. (2018);12:341. doi: 10.3389/fnins.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ, Sobue G. Amyotrophic lateral sclerosis. Lancet. (2022);400:1363–1380. doi: 10.1016/S0140-6736(22)01272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fico E, Rosso P, Triaca V, Segatto M, Lambiase A, Tirassa P. NGF prevents loss of TrkA/VEGFR2 cells, and VEGF isoform dysregulation in the retina of adult diabetic rats. Cells. (2022);11:3246. doi: 10.3390/cells11203246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H, Xia D, Liao S, Niu B, Tang J, Hu H, Qian H, Cao B. Vascular endothelial growth factor improves the cognitive decline of Alzheimer's disease via concurrently inducing the expression of ADAM10 and reducing the expression of β-site APP cleaving enzyme 1 in Tg2576 mice. Neurosci Res. (2019);142:49–57. doi: 10.1016/j.neures.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Horn AKE, Straka H. Functional organization of extraocular motoneurons and eye muscles. Annu Rev Vis Sci. (2021);7:793–825. doi: 10.1146/annurev-vision-100119-125043. [DOI] [PubMed] [Google Scholar]
- 25.Hwang DH, Lee HJ, Park IH, Seok JI, Kim BG, Joo IS, Kim SU. Intrathecal transplantation of human neural stem cells overexpressing VEGF provide behavioral improvement, disease onset delay and survival extension in transgenic ALS mice. Gene Ther. (2009);16:1234–1244. doi: 10.1038/gt.2009.80. [DOI] [PubMed] [Google Scholar]
- 26.Kimura T, Jiang H, Konno T, Seto M, Iwanaga K, Tsujihata M, Satoh A, Onodera O, Kakita A, Takahashi H. Bunina bodies in motor and non-motor neurons revisited:a pathological study of an ALS patient after long-term survival on a respirator. Neuropathology. (2014);34:392–397. doi: 10.1111/neup.12105. [DOI] [PubMed] [Google Scholar]
- 27.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. (2012);2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kou ZW, Mo JL, Wu KW, Qiu MH, Huang YL, Tao F, Lei Y, Lv LL, Sun FY. Vascular endothelial growth factor increases the function of calcium-impermeable AMPA receptor GluA2 subunit in astrocytes via activation of protein kinase C signaling pathway. Glia. (2019);67:1344–1358. doi: 10.1002/glia.23609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF:a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. (2018);38:579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krilleke D, Ng YS, Shima DT. The heparin-binding domain confers diverse functions of VEGF-A in development and disease:a structure-function study. Biochem Soc Trans. (2009);37:1201–1206. doi: 10.1042/BST0371201. [DOI] [PubMed] [Google Scholar]
- 31.Kulshreshtha D, Vijayalakshmi K, Alladi PA, Sathyaprabha TN, Nalini A, Raju TR. Vascular endothelial growth factor attenuates neurodegenerative changes in the NSC-34 motor neuron cell line induced by cerebrospinal fluid of sporadic amyotrophic lateral sclerosis patients. Neurodegener Dis. (2011);8:322–830. doi: 10.1159/000323718. [DOI] [PubMed] [Google Scholar]
- 32.Laakkonen JP, Lähteenvuo J, Jauhiainen S, Heikura T, Ylä-Herttuala S. Beyond endothelial cells: Vascular endothelial growth factors in heart, vascular anomalies and placenta. Vascul Pharmacol. (2019);112:91–101. doi: 10.1016/j.vph.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SL, Wyns S, Thijs V, Andersson J, van Marion I, Al-Chalabi A, Bornes S, Musson R, Hansen V, Beckman L, Adolfsson R, Pall HS, Prats H, Vermeire S, Rutgeerts P, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. (2003);34:383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- 34.Lambrechts D, Carmeliet P. VEGF at the neurovascular interface:therapeutic implications for motor neuron disease. Biochim Biophys Acta. (2006);1762:1109–1121. doi: 10.1016/j.bbadis.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Lange C, Storkebaum E, de Almodóvar CR, Dewerchin M, Carmeliet P. Vascular endothelial growth factor:a neurovascular target in neurological diseases. Nat Rev Neurol. (2016);12:439–454. doi: 10.1038/nrneurol.2016.88. [DOI] [PubMed] [Google Scholar]
- 36.Latzer P, Shchyglo O, Hartl T, Matschke V, Schlegel U, Manahan-Vaughan D, Theiss C. Blocking VEGF by bevacizumab compromises electrophysiological and morphological properties of hippocampal neurons. Front Cell Neurosci. (2019);13:113. doi: 10.3389/fncel.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewin GR, Carter BD. Heidelberg: Springer; (2014). Neurotrophic factors. [Google Scholar]
- 38.Lien BV, Brown NJ, Ransom SC, Lehrich BM, Shahrestani S, Tafreshi AR, Ransom RC, Sahyouni R. Enhancing peripheral nerve regeneration with neurotrophic factors and bioengineered scaffolds: A basic science and clinical perspective. J Peripher Nerv Syst. (2020);25:320–334. doi: 10.1111/jns.12414. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Henty-Ridilla JL. Multiple roles for the cytoskeleton in ALS. Exp Neurol. (2022);355:114143. doi: 10.1016/j.expneurol.2022.114143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lladó J, Tolosa L, Olmos G. Cellular and molecular mechanisms involved in the neuroprotective effects of VEGF on motoneurons. Front Cell Neurosci. (2013);7:181. doi: 10.3389/fncel.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu L, Zheng L, Viera L, Suswam E, Li Y, Li X, Estévez AG, King PH. Mutant Cu/Zn-superoxide dismutase associated with amyotrophic lateral sclerosis destabilizes vascular endothelial growth factor mRNA and downregulates its expression. J Neurosci. (2007);27:7929–7938. doi: 10.1523/JNEUROSCI.1877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lunn JS, Sakowski SA, Kim B, Rosenberg AA, Feldman EL. Vascular endothelial growth factor prevents G93A-SOD1-induced motor neuron degeneration. Dev Neurobiol. (2009);69:871–884. doi: 10.1002/dneu.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malekan M, Ebrahimzadeh MA. Vascular endothelial growth factor receptors [VEGFR] as target in breast cancer treatment:current status in preclinical and clinical studies and future directions. Curr Top Med Chem. (2022);22:891–920. doi: 10.2174/1568026622666220308161710. [DOI] [PubMed] [Google Scholar]
- 44.Manoonkitiwongsa PS, Schultz RL, McCreery DB, Whitter EF, Lyden PD. Neuroprotection of ischemic brain by vascular endothelial growth factor is critically dependent on proper dosage and may be compromised by angiogenesis. J Cereb Blood Flow Metab. (2004);24:693–702. doi: 10.1097/01.WCB.0000126236.54306.21. [DOI] [PubMed] [Google Scholar]
- 45.Mercurio AM. VEGF/neuropilin signaling in cancer stem cells. Int J Mol Sci. (2019);20:490. doi: 10.3390/ijms20030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Micheli L, Parisio C, Lucarini E, Vona A, Toti A, Pacini A, Mello T, Boccella S, Ricciardi F, Maione S, Graziani G, Lacal PM, Failli P, Ghelardini C, Di Cesare Mannelli L. VEGF-A/VEGFR-1 signalling and chemotherapy-induced neuropathic pain:therapeutic potential of a novel anti-VEGFR-1 monoclonal antibody. J Exp Clin Cancer Res. (2021);40:320. doi: 10.1186/s13046-021-02127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitre M, Mariga A, Chao MV. Neurotrophin signalling:novel insights into mechanisms and pathophysiology. Clin Sci (Lond) (2017);131:13–23. doi: 10.1042/CS20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morcuende S, Benítez-Temiño B, Pecero ML, Pastor AM, de la Cruz RR. Abducens internuclear neurons depend on their target motoneurons for survival during early postnatal development. Exp Neurol. (2005);195:244–256. doi: 10.1016/j.expneurol.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Morcuende S, Muñoz-Hernández R, Benítez-Temiño B, Pastor AM, de la Cruz RR. Neuroprotective effects of NGF, BDNF, NT-3 and GDNF on axotomized extraocular motoneurons in neonatal rats. Neuroscience. (2013);250:31–48. doi: 10.1016/j.neuroscience.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 50.Morrice JR, Gregory-Evans CY, Shaw CA. Animal models of amyotrophic lateral sclerosis: A comparison of model validity. Neural Regen Res. (2018);13:2050–2054. doi: 10.4103/1673-5374.241445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagata Y, Okuya M, Watanabe R, Honda M. Regional distribution of cholinergic neurons in human spinal cord transections in the patients with and without motor neuron disease. Brain Res. (1982);244:223–229. doi: 10.1016/0006-8993(82)90081-6. [DOI] [PubMed] [Google Scholar]
- 52.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. (2007);82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Nicoletti JN, Shah SK, McCloskey DP, Goodman JH, Elkady A, Atassi H, Hylton D, Rudge JS, Scharfman HE, Croll SD. Vascular endothelial growth factor is up-regulated after status epilepticus and protects against seizure-induced neuronal loss in hippocampus. Neuroscience. (2008);151:232–241. doi: 10.1061/j.neuroscience.2007.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oda Y, Imai S, Nakanishi I, Ichikawa T, Deguchi T. Immunohistochemical study on choline acetyltransferase in the spinal cord of patients with amyotrophic lateral sclerosis. Pathol Int. (1995);45:933–939. doi: 10.1111/j.1440-1827.1995.tb03418.x. [DOI] [PubMed] [Google Scholar]
- 55.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. (2001);28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 56.Peggion C, Scalcon V, Massimino ML, Nies K, Lopreiato R, Rigobello MP, Bertoli A. SOD1 in ALS:taking stock in pathogenic mechanisms and the role of glial and muscle cells. Antioxidants (Basel) (2020);11:614. doi: 10.3390/antiox11040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poesen K, Lambrechts D, Van Damme P, Dhondt J, Bender F, Frank N, Bogaert E, Claes B, Heylen L, Verheyen A, Raes K, Tjwa M, Eriksson U, Shibuya M, Nuydens R, Van Den Bosch L, Meert T, D'Hooge R, Sendtner M, Robberecht W, et al. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration. J Neurosci. (2008);28:10451–10459. doi: 10.1523/JNEUROSCI.1092-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raffaele S, Boccazzi M, Fumagalli M. Oligodendrocyte dysfunction in amyotrophic lateral sclerosis:mechanisms and therapeutic perspectives. Cells. (2021);10:565. doi: 10.3390/cells10030565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rink S, Bendella H, Akkin SM, Manthou M, Grosheva M, Angelov DN. Experimental studies on facial nerve regeneration. Anat Rec (Hoboken) (2019);302:1287–1303. doi: 10.1002/ar.24123. [DOI] [PubMed] [Google Scholar]
- 60.Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. (2013);14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- 61.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. (2001);114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 62.Ruffo P, Perrone B, Conforti FL. SOD-1 variants in amyotrophic lateral sclerosis: Systematic re-evaluation according to ACMG-AMP Guidelines. Genes (Basel) (2022);13:537. doi: 10.3390/genes13030537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. (2009);89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- 64.Sharma R, Hicks S, Berna CM, Kennard C, Talbot K, Turner MR. Oculomotor dysfunction in amyotrophic lateral sclerosis:a comprehensive review. Arch Neurol. (2011);68:857–861. doi: 10.1001/archneurol.2011.130. [DOI] [PubMed] [Google Scholar]
- 65.Silva-Hucha S, Hernández RG, Benítez-Temiño B, Pastor AM, de la Cruz RR, Morcuende S. Extraocular motoneurons of the adult rat show higher levels of vascular endothelial growth factor and its receptor Flk-1 than other cranial motoneurons. PLoS One. (2017);12:e0178616. doi: 10.1371/journal.pone.0178616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silva-Hucha S, Carrero-Rojas G, Fernández de Sevilla ME, Benítez-Temiño B, Davis-López de Carrizosa MA, Pastor AM, Morcuende S. Sources and lesion-induced changes of VEGF expression in brainstem motoneurons. Brain Struct Funct. (2020);225:1033–1053. doi: 10.1007/s00429-020-02057-y. [DOI] [PubMed] [Google Scholar]
- 67.Silva-Hucha S, Pastor AM, Morcuende S. Neuroprotective effect of vascular endothelial growth factor on motoneurons of the oculomotor system. Int J Mol Sci. (2021);22:814. doi: 10.3390/ijms22020814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stevens M, Oltean S. Modulation of Receptor Tyrosine Kinase Activity through Alternative Splicing of Ligands and Receptors in the VEGF-A/VEGFR Axis. Cells. (2019);8:288. doi: 10.3390/cells8040288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van Damme P, Rutten B, Man WY, De Mol M, Wyns S, Manka D, Vermeulen K, Van Den Bosch L, Mertens N, Schmitz C, Robberecht W, Conway EM, Collen D, Moons L, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. (2005);8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) (2005);109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 71.Theis V, Theiss C. VEGF - a stimulus for neuronal development and regeneration in the CNS and PNS. Curr Protein Pept Sci. (2018);19:589–597. doi: 10.2174/1389203719666180104113937. [DOI] [PubMed] [Google Scholar]
- 72.Tjust AE, Danielsson A, Andersen PM, Brännström T, Pedrosa Domellöf F. Impact of amyotrophic lateral sclerosis on slow tonic myofiber composition in human extraocular muscles. Invest Ophthalmol Vis Sci. (2017);58:3708–3715. doi: 10.1167/iovs.17-22098. [DOI] [PubMed] [Google Scholar]
- 73.Todd TW, Petrucelli L. Modelling amyotrophic lateral sclerosis in rodents. Nat Rev Neurosci. (2022);23:231–251. doi: 10.1038/s41583-022-00564-x. [DOI] [PubMed] [Google Scholar]
- 74.Tokunaga Y, Yamazaki Y, Morita T. Identification and localization of heparin-binding region of snake venom VEGF and its blocking of VEGF-A165. Pathophysiol Haemost Thromb. (2005);34((4-5)):194–196. doi: 10.1159/000092422. [DOI] [PubMed] [Google Scholar]
- 75.Tovar-y-Romo LB, Zepeda A, Tapia R. Vascular endothelial growth factor prevents paralysis and motoneuron death in a rat model of excitotoxic spinal cord neurodegeneration. J Neuropathol Exp Neurol. (2007);66:913–922. doi: 10.1097/nen.0b013e3181567c16. [DOI] [PubMed] [Google Scholar]
- 76.Tovar-y-Romo LB, Santa-Cruz LD, Tapia R. Experimental models for the study of neurodegeneration in amyotrophic lateral sclerosis. Mol Neurodegener. (2009);4:31. doi: 10.1186/1750-1326-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tovar-y-Romo LB, Tapia R. VEGF protects spinal motor neurons against chronic excitotoxic degeneration in vivo by activation of PI3-K pathway and inhibition of p38MAPK. J Neurochem. (2010);115:1090–1101. doi: 10.1111/j.1471-4159.2010.06766.x. [DOI] [PubMed] [Google Scholar]
- 78.Tovar-y-Romo LB, Ramírez-Jarquín UN, Lazo-Gómez R, Tapia R. Trophic factors as modulators of motor neuron physiology and survival:implications for ALS therapy. Front Cell Neurosci. (2014);8:61. doi: 10.3389/fncel.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uccelli A, Wolff T, Valente P, Di Maggio N, Pellegrino M, Gürke L, Banfi A, Gianni-Barrera R. Vascular endothelial growth factor biology for regenerative angiogenesis. Swiss Med Wkly. (2019);149:w20011. doi: 10.4414/smw.2019.20011. [DOI] [PubMed] [Google Scholar]
- 80.Ureña-Guerrero ME, Castañeda-Cabral JL, Rivera-Cervantes MC, Macias-Velez RJ, Jarero-Basulto JJ, Gudiño-Cabrera G, Beas-Zárate C. Neuroprotective and neurorestorative effects of Epo and VEGF:perspectives for new therapeutic approaches diseases. Curr Pharm Des. (2020);26:1263–1276. doi: 10.2174/1381612826666200114104342. [DOI] [PubMed] [Google Scholar]
- 81.Valko K, Ciesla L. Amyotrophic lateral sclerosis. Prog Med Chem. (2019);58:63–117. doi: 10.1016/bs.pmch.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 82.Verma S, Khurana S, Vats A, Sahu B, Ganguly NK, Chakraborti P, Gourie-Devi M, Taneja V. Neuromuscular Junction dysfunction in amyotrophic lateral sclerosis. Mol Neurobiol. (2022);59:1502–1527. doi: 10.1007/s12035-021-02658-6. [DOI] [PubMed] [Google Scholar]
- 83.Vicario-Abejón C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nat Rev Neurosci. (2002);3:965–74. doi: 10.1038/nrn988. [DOI] [PubMed] [Google Scholar]
- 84.Vijayalakshmi K, Ostwal P, Sumitha R, Shruthi S, Varghese AM, Mishra P, Manohari SG, Sagar BC, Sathyaprabha TN, Nalini A, Raju TR, Alladi PA. Role of VEGF and VEGFR2 receptor in reversal of ALS-CSF induced degeneration of NSC-34 motor neuron cell line. Mol Neurobiol. (2015);51:995, 1007. doi: 10.1007/s12035-014-8757-y. [DOI] [PubMed] [Google Scholar]
- 85.Virgo L, de Belleroche J, Rossi M, Steiner TJ. Characterisation of the distribution of choline acetyltransferase messenger RNA in human spinal cord and its depletion in motor neurone disease. J Neurol Sci. (1992);112:126–132. doi: 10.1016/0022-510x(92)90141-7. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA, Jin K. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci. (2007);27:304–307. doi: 10.1523/JNEUROSCI.4433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Duan W, Wang W, Di Wen, Liu Y, Liu Y, Li Z, Hu H, Lin H, Cui C, Li D, Dong H, Li C. scAAV9-VEGF prolongs the survival of transgenic ALS mice by promoting activation of M2 microglia and the PI3K/Akt pathway. Brain Res. (2016);1648((Pt A)):1–10. doi: 10.1016/j.brainres.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 88.Xiao N, Le QT. Neurotrophic factors and their potential applications in tissue regeneration. Arch Immunol Ther Exp (Warsz) (2016);64:89–99. doi: 10.1007/s00005-015-0376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu W, Zhang Z, Lu H, Wu Y, Liu J, Liu S, Yang W. Biocompatible polyurethane conduit grafted with vascular endothelial growth factor-loaded hydrogel repairs the peripheral nerve defect in rats. Macromol Biosci. (2022);22:e2100397. doi: 10.1002/mabi.202100397. [DOI] [PubMed] [Google Scholar]
- 90.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. (2000);407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 91.Yang J, Yang B, Xiu B, Qi J, Liu H. Effect of combination therapy with neuroprotective and vasoprotective agents on cerebral ischemia. Can J Neurol Sci. (2018);45:325–331. doi: 10.1017/cjn.2018.8. [DOI] [PubMed] [Google Scholar]
- 92.Yasuhara T, Shingo T, Muraoka K, Kameda M, Agari T, Wen Ji Y, Hayase H, Hamada H, Borlongan CV, Date I. Neurorescue effects of VEGF on a rat model of Parkinson's disease. Brain Res. (2005);1053:10–18. doi: 10.1016/j.brainres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 93.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. (2008);9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 94.Zheng C, Nennesmo I, Fadeel B, Henter JI. Vascular endothelial growth factor prolongs survival in a transgenic mouse model of ALS. Ann Neurol. (2004);56:564–567. doi: 10.1002/ana.20223. [DOI] [PubMed] [Google Scholar]
- 95.Zheng C, Sköld MK, Li J, Nennesmo I, Fadeel B, Henter JI. VEGF reduces astrogliosis and preserves neuromuscular junctions in ALS transgenic mice. Biochem Biophys Res Commun. (2007);363:989–993. doi: 10.1016/j.bbrc.2007.09.088. [DOI] [PubMed] [Google Scholar]


