Ischemic stroke: Stroke is the second and third cause of death and disability, respectively, with an annual rate of 24.9 million cases worldwide (Chidambaram et al., 2022). Stroke is defined as the lack of blood supply to a specific area of the brain, accounting for 85% of all cases (Chidambaram et al., 2022). Changes in the gut microbiota have also been reported as risk factor for stroke (Pluta et al., 2021; Tan et al., 2021; Chidambaram et al., 2022). Post-stroke neurodegeneration is multifactorial characterized by neuronal death, amyloid plaques, neurofibrillary tangles, a neuroinflammatory response, a lack of acetylcholine, and a cognitive deficit with full-blown dementia (approximately 50% survivors) (Yuan et al., 2020; Pluta 2022).
Numerous microorganisms such as Chlamydophila pneumoniae, Borrelia burgdorferi, other spirochetes and the herpes simplex virus are associated with the pathogenesis of stroke (Pluta et al., 2021). Patients after a stroke show an imbalance of the intestinal microbiota, which is manifested by a reduced diversity of microbes in the stool, a lower number of beneficial (e.g. Clostridium symbiosum, Bifidobacterium longum, Faecalibacterium prausnitzii, Lactobacillus fermentum, Akkermansia muciniphila) and a greater number of pathogenic bacteria (e.g. Escherichia, Enterobacteriaceae, Parabacteroide, Oscillospira, Lactobacillus ruminis).
Two-way communication between the gut microbiome and the brain, known as the gut-brain axis, is involved in cognition and brain aging (Cryan et al., 2020). The contribution of gut microbiota products to stroke is unique as they influence both risk and outcome. Clinical trials show that patients with the best known risk factors for stroke have a significantly altered gut microbiota composition (Panther et al., 2022). Moreover, high-risk patients have a reduced number of butyrate-producing bacteria. Other human studies evaluating post-stroke outcome have also found reduced levels of short chain fatty acids in stroke patients compared with healthy controls (Tan et al., 2021). One of the differences between the microbiota of young and old mice is the reduction in the number of bacteria that produce short-chain fatty acids (Coman et al., 2020). When older mice receive a fecal transplant from young mice with focal brain ischemia, they show better functional regeneration than those who receive a fecal transplant from older mice (Lee et al., 2020a). These studies indicate that not only the presence, absence, or complete microbial load regulates the gut-brain axis, but rather the relative abundance and interaction between bacterial clusters. Clinical trials showed an increase in microbiota in two thirds cases (e.g. Streptococcus, Lactobacillus ruminis, Escherichia) and in one third cases a decrease in microbiota (e.g. Eubacterium, Roseburia) in stroke compared to healthy participants (Chidambaram et al., 2022). In an experimental stroke, the severity of a stroke depends on the composition of the gut microbiome, age, and diet. A growing body of evidence supports the role of the gut microbiota in stroke prognosis and recovery. Conversely, a stroke can cause intestinal dysbiosis. These data suggest that not only is the brain gut axis an important risk and outcome factor for stroke, but the specific metabolites may be important in this relationship. From this perspective, we present the role of the intestinal microbiota and its metabolites in experimental and clinical stroke.
Gut microbiome neurotransmitters and cognitive processes: Recent research shows that metabolites produced by the gut microbiota include neurotransmitters such as glutamate, gamma-aminobutyric acid, acetylcholine, and dopamine (Box 1) (Chen et al., 2021). Since neurotransmitters such as glutamate, gamma-aminobutyric acid, dopamine, and acetylcholine do not cross the blood-brain barrier, they are synthesized in the brain from its own pools of neurotransmitter precursors. In contrast, most of the precursors, such as acetate, tyrosine, and choline (Box 1), come from the diet, which enter the bloodstream and are transported across the blood-brain barrier and are taken up by appropriate cells in the brain that produce neurotransmitters. The dietary origin of these precursors enables the gut microbiome to influence host behavior by regulating the production of neurotransmitters in the brain.
Box 1.
Selected neurotransmitters and their precursors, associated with the gut microbiota
| Gut microbiota | Precursor | Neurotransmitter | Type of action | Impact on brain function |
|---|---|---|---|---|
| Lactobacillus plantarum, Bacteroides vulgatus, Campylobacter jejuni | Acetate | Glutamate | Excitatory | Activates the neurons death mechanism caused by the excessive level of glutamate with atrophy of the hippocampus and the development of dementia |
| Lactobacillus plantarum, Bacillus acetylcholine, Bacillus subtilis, Escherichia coli, Staphylococcus aureus | Choline | Acetylcholine | Excitatory | Acting on cognitive function, particularly closely related to learning and memory in the brain |
| Staphylococcus | Tyrosine | Dopamine | Excitatory | Deficiency causes anxiety, inner tension, decreased motivation, reluctance to act, fatigue, apathy and even depression |
| Bifidobacterium, Bacteroides fragilis, Parabacteroides, Eubacterium | Acetate | Gamma-aminobutyric acid | Inhibitory | Can lead to anxiety and depression |
Glutamate, the most abundant excitatory neurotransmitter in the brain, is responsible for sending signals between neurons. Since glutamate does not cross the blood-brain barrier, its synthesis in the brain depends on the cooperation of neurons and astrocytes, which use intermediate metabolites of the tricarboxylic acid cycle as precursors. However, gastrointestinal cells other than neurons can also produce glutamate (Box 1) and use it to transmit signals to the brain via the vagus nerve. Enteroendocrine cells form synapses with the vagus nerve, they are called neuropodal cells, which within milliseconds produce glutamate transporter 1 transcript in vesicles and release glutamate to transmit sensory inputs from the gut to the brain (Lee et al., 2020b).
Subsequently, it was discovered that gamma-aminobutyric acid is produced by Bacteroides fragilis, Parabacteroides, Eubacterium and Bifidobacterium (Box 1). However, gamma-aminobutyric acid also does not cross the blood-brain barrier. Therefore, gamma-aminobutyric acid produced by the intestinal microbes can act locally by the enteric nervous system or the vagus nerve. However, like the glutamate precursor, the gamma-aminobutyric acid precursor, acetate, can cross the blood-brain barrier and be involved in the metabolic cycle of gamma-aminobutyric acid in the brain.
It should be emphasized that acetylcholine dysregulation is closely related to stroke (Yuan et al., 2020). Acetylcholine has been shown to be produced by Bacillus acetylcholine, Lactobacillus plantarum, Bacillus subtilis, Escherichia coli and Staphylococcus aureus (Box 1). Acetylcholine does not cross the blood-brain barrier, but its precursor, choline, easily crosses the barrier to the brain, where it is used to synthesize this neurotransmitter.
Dopamine is made from tyrosine, which is rich in the diet and is transported to the brain across the blood-brain barrier. Dopamine is produced by Staphylococcus (Box 1) in the human gut, which can take up the L-3,4-dihydroxy-phenylalanine precursor and convert it to dopamine.
It is not fully understood how these neurotransmitters and their precursors are transported to the brain and how they affect its function. Some of the neurotransmitter precursors synthesized in the gut are small enough to cross the blood-brain barrier and can be transported from the blood to the brain. The data show an association between the metabolism of gut microbes and the synthesis of neurotransmitters in the brain. This shows that changes in the gut microbiota can affect the synthesis of neurotransmitters and their precursors, and this affects their respective concentrations in the brain, which may interfere with the cognitive functions of the brain host (Pluta et al., 2021; Chidambaram et al., 2022). Recent studies have shown that extracellular vesicles secreted by Paenalcaligenes hominis are transported through the blood to the hippocampus, with the consequence of cognitive impairment in mice (Lee et al., 2020b). Studies in germ-free mice showed skeletal muscle alterations accompanied by lower serum levels of choline, a precursor to acetylcholine that plays a pivotal role in muscle-to-nerve signaling and in stroke (Lee et al., 2020b; Yuan et al., 2020). It should be emphasized that transplanting fecal microbes from specific pathogen-free mice into germ-free mice or treating germ-free mice with short-chain fatty acids reverses skeletal muscle damage (Lee et al., 2020b). These data suggest that the gut microbiome can influence host behavior by regulating the levels of neurotransmitters and the expression of their receptors, which are involved in synaptic transmission and brain plasticity. Moreover, studies have shown that the administration of antibiotics changes the composition of the fecal microbiota of piglets and significantly reduces the level of aromatic amino acids (i.e., tyrosine) in the feces, blood and hypothalamus along with the levels of neurotransmitters, including dopamine, in the hypothalamus (Lee et al., 2020b). Interestingly, the prevention of coprophagy in small mammals changed the composition of the gut microbiota with cognitive decline, which is probably associated with changes in the level of neurotransmitters in the brain (Bo et al., 2020).
Conclusion: The data clearly indicate that intestinal dysbiosis may be directly or indirectly related to ischemic stroke and vice versa. It should be noted that not only does the brain gut axis affect the risk and outcome of stroke, but neurotransmitters and diet are important in this relationship (Figure 1). Enteroendocrine cells called neuropodal cells, by binding to the neurons of the vagus node, release glutamate, which triggers the transmission of stimuli through the vagus nerve (which has 80% afferent and 20% efferent fibers) to the brain (Chidambaram et al., 2022). The precursors of neurotransmitters such as acetate, tyrosine and choline come from the diet and are transported across the blood-brain barrier to the appropriate structures of the brain, where they are used for the production of, inter alia, glutamate (Figure 1). Stroke triggers excess glutamate in the ischemic focus, and additional glutamate stimulation from the gut microbiome or glutamate from a processed dietary precursor enhances the neurotoxic effects of glutamate, which ultimately leads to a vicious cycle (Figure 1). The presented data suggest that the neurotransmitters of the intestinal microbiota transmit their activity through the vagus nerve to the brain, while their precursors reach the brain through the blood-brain barrier. Among other things, this opens up an unexpected route to the development of a drug delivery method to the brain via the gut microbiota-vagal pathway.
Figure 1.
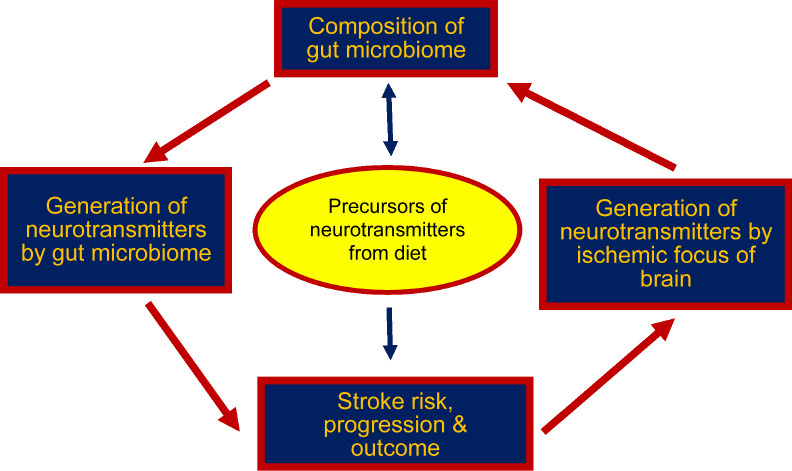
Vicious circle interaction between the gut microbiota and ischemic stroke.
The authors acknowledge the financial support from the Mossakowski Medical Research Institute, Polish Academy of Sciences, Warsaw, Poland (T3-RP) (to RP).
Footnotes
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Bo TB, Zhang XY, Kohl KD, Wen J, Tian SJ, Wang DH. Coprophagy prevention alters microbiome, metabolism, neurochemistry, and cognitive behavior in a small mammal. ISME J. (2020);14:2625–2645. doi: 10.1038/s41396-020-0711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Xu J, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. (2021);13:2099. doi: 10.3390/nu13062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chidambaram SB, Rathipriya AG, Mahalakshmi AM, Sharma S, Hediyal TA, Ray B, Sunanda T, Rungratanawanich W, Kashyap RS, Qoronfleh MW, Essa MM, Song BJ, Monaghan TM. The influence of gut dysbiosis in the pathogenesis and management of ischemic stroke. Cells. (2022);11:1239. doi: 10.3390/cells11071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coman V, Vodnar DC. Gut microbiota and old age:modulating factors and interventions for healthy longevity. Exp Gerontol. (2020);141:111095. doi: 10.1016/j.exger.2020.111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cryan JF, O'Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. (2020);19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, D'Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, Hassan A, Graf J, Petrosino J, Putluri N, Zhu L, Durgan DJ, Bryan RM, Jr, McCullough LD, Venna VR. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res. (2020a);127:453–465. doi: 10.1161/CIRCRESAHA.119.316448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KE, Kim JK, Han SK, Lee DY, Lee HJ, Yim SV, Kim DH. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome. (2020b);8:107. doi: 10.1186/s40168-020-00881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panther EJ, Dodd W, Clark A, Lucke-Wold B. Gastrointestinal microbiome and neurologic injury. Biomedicines. (2022);10:500. doi: 10.3390/biomedicines10020500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pluta R, Januszewski S, Czuczwar SJ. The role of gut microbiota in an ischemic stroke. Int J Mol Sci. (2021);22:915. doi: 10.3390/ijms22020915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pluta R. Brain ischemia as a bridge to Alzheimer's disease. Neural Regen Res. (2022);17:791–792. doi: 10.4103/1673-5374.322453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan C, Wu Q, Wang H, Gao X, Xu R, Cui Z, Zhu J, Zeng X, Zhou H, He Y, Yin J. Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN J Parenter Enter Nutr. (2021);45:518–529. doi: 10.1002/jpen.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan Y, Shan X, Men W, Zhai H, Qiao X, Geng L, Li C. The effect of crocin on memory, hippocampal, acetylcholine level, and apoptosis in a rat model of cerebral ischemia. Biomed Pharmacother. (2020);130:110543. doi: 10.1016/j.biopha.2020.110543. [DOI] [PubMed] [Google Scholar]


