Key Words: growth-associated protein 43 (GAP-43), immunohistochemistry, nerve guide, nerve tissue regeneration, peripheral nerve repair, spinal cord, tissue engineering
Abstract
Growth-associated protein 43 plays a key role in neurite outgrowth through cytoskeleton remodeling. We have previously demonstrated that structural damage of peripheral nerves induces growth-associated protein 43 upregulation to promote growth cone formation. Conversely, the limited regenerative capacity of the central nervous system due to an inhibitory environment prevents major changes in neurite outgrowth and should be presumably associated with low levels of growth-associated protein 43 expression. However, central alterations due to peripheral nerve damage have never been assessed using the growth-associated protein 43 marker. In this study, we used the tubulization technique to repair 1 cm-long nerve gaps in the rat nerve injury/repair model and detected growth-associated protein 43 expression in the peripheral and central nervous systems. First, histological analysis of the regeneration process confirmed an active regeneration process of the nerve gaps through the conduit from 10 days onwards. The growth-associated protein 43 expression profile varied across regions and follow-up times, from a localized expression to an abundant and consistent expression throughout the regeneration tissue, confirming the presence of an active nerve regeneration process. Second, spinal cord changes were also histologically assessed, and no apparent changes in the structural and cellular organization were observed using routine staining methods. Surprisingly, remarkable differences and local changes appeared in growth-associated protein 43 expression at the spinal cord level, in particular at 20 days post-repair and beyond. Growth-associated protein 43 protein was first localized in the gracile fasciculus and was homogeneously distributed in the left posterior cord. These findings differed from the growth-associated protein 43 pattern observed in the healthy control, which did not express growth-associated protein 43 at these levels. Our results revealed a differential expression in growth-associated protein 43 protein not only in the regenerating nerve tissue but also in the spinal cord after peripheral nerve transection. These findings open the possibility of using this marker to monitor changes in the central nervous system after peripheral nerve injury.
Introduction
Growth-associated protein 43 (GAP-43) is induced during periods of axonal extension and it is highly enriched on the inner surface of the growth cone membrane. GAP-43 or neuromodulin plays an important role in the activation of transduction signals related to the actin cytoskeletal remodeling of newly-formed neurites or axons (Benowitz and Routtenberg, 1997; Korshunova and Mosevitsky, 2010). In this regard, palmitoylation and basic residues are required for the early binding of GAP-43 to cellular membranes. Following this process, the phosphorylation of GAP-43 at Ser-41 by protein kinase C occurs, promoting cytoskeletal remodeling. In neurons, GAP-43 is sorted onto vesicles for fast axonal transport to the growth cone or presynaptic plasma membranes (Denny, 2006). During development, GAP-43 is considerably upregulated, playing key roles in neurite outgrowth and guidance (Benowitz and Routtenberg, 1997; Oestreicher et al., 1997; Schrama et al., 1997; Korshunova and Mosevitsky, 2010). In adulthood, high levels of GAP-43 persist in specific regions of the brain, such as the hippocampus, and associative cortices which are characterized by continuous neuronal plasticity (Benowitz and Routtenberg, 1997; Burello et al., 2012). In the central nervous system (CNS), GAP-43, together with synaptophysin, is considered a marker of neuronal plasticity (Benowitz and Routtenberg, 1997; Grijalva et al., 2021). Zhang et al. (2022) used GAP-43 as a potential biomarker associated with synaptic degeneration in patients with Alzheimer’s disease (AD) (Zhang et al., 2022). Following neural injury, GAP-43 is activated by cytokines and neurotrophic factors to promote neuroprotection and regeneration (Chung et al., 2020). Nevertheless, in healthy mixed peripheral nerves, GAP-43 was observed in 1% of the nerve fibers (Carriel et al., 2014). This localized and poorly distributed GAP-43 expression was attributed to structural changes that take place at the peripheral nerve endings and peripheral nerve plasticity (Carriel et al., 2014; Chen et al., 2014).
Injuries associated with structural damage of the CNS and peripheral nervous system (PNS) can activate several cellular and molecular processes, including the upregulation of GAP-43 to promote growth cone formation (Chen et al., 2010; Burello et al., 2012; Carriel et al., 2014). However, as far as the CNS is concerned, neuroregeneration is limited, mostly due to a persistent inhibitory environment that surrounds the injured degenerating fibers, which hinders any attempt at effective regeneration. Peripheral nerves have an acceptable capability to progressively regenerate their cells and extracellular matrix (ECM) after structural damage followed by surgical repair (Carriel et al., 2014).
Currently, different surgical techniques are used to repair damaged nerves. For repairing nerve injuries without significant defects, in situ suturing or direct repair is the gold standard technique. An alternative strategy to bridge short sensory nerve defects is the tubulization technique (Daly et al., 2012), and the nerve autograft is the gold standard method to bridge critical nerve defects (over 3 cm length). However, as successful peripheral nerve repair is still a challenge, more basic research is needed to elucidate the cellular and molecular processes involved during nervous system repair and regeneration. In this context, nerve guides arise as a useful model to evaluate these complex processes (Belkas et al., 2004; Daly et al., 2012).
In the last years, tissue engineering approaches with different peripheral nerve regeneration profiles were used to investigate the correlation between GAP-43 and neurofilament (NFL) expression (Carriel et al., 2014; Chato-Astrain et al., 2018). Interestingly, these studies demonstrated differential expression of GAP-43 and NFL. First, GAP-43 was observed to be expressed by immature and newly-formed axonal sprouts, but not by more mature axons, which expressed NFL (Carriel et al., 2014). Second, its expression was significantly higher in those approaches with higher regeneration profile, being a good indicator of positive peripheral nerve regeneration through bio-artificial conduits (Chato-Astrain et al., 2018). Although these studies demonstrated that GAP-43 can play an important role in peripheral nerve regeneration through tissue-engineered approaches, it is important to evaluate the expression and distribution of GAP-43 in the spinal cord after peripheral nerve repair and regeneration. This can contribute to elucidating the role of this molecule on CNS and peripheral nerve regeneration. For this reason, this study aimed to investigate the expression of GAP-43 in the spinal cord after peripheral nerve repair between 10–30 days of conduit-based nerve repair.
Methods
Laboratory animals and experimental groups
In this study, twelve 12-week-old male Wistar rats weighing 250–300 g were used. These animals were provided by JANVIER LABS (Le Genest-Saint-Isle, France, authorization number D53-103-02) and maintained in the Experimental Unit of the Instituto de Investigación Biosanitaria, ibs.GRANADA, Granada, Spain. Male rats were chosen in this study based on our previous study (Chato-Astrain et al., 2018). Animals were kept in individual cages in a temperature-controlled environment (21 ± 1°C) under a 12-hour light/dark cycle and with ad libitum access to tap water and standard rat chow.
To evaluate the axonal regeneration, we used the tubulization technique to repair a 1 cm long nerve gap in the rat sciatic nerve injury/repair model using NeuraGen® collagen conduits (Integra® Life Sciences Corp., Plainsboro, NJ, USA) following previous protocols (Carriel et al., 2011, 2013, 2014). First, animals were deeply anesthetized with an intraperitoneal injection of acepromazine (Calmo-Neosan®, Boehringer Ingelheim Animal Health España, S.A.U., Barcelona, Spain, 0.001 mg/g body weight), ketamine (Imalgene 1000®, Merial, Lyon, France, 0.15 mg/g body weight) and atropine (Pfizer, New York, NY, USA, 0.05 g/g body weight). 1 cm long left sciatic nerve was surgically removed from each animal. All the nerve defects were then bridged using collagen conduits 1.5 cm in length and 0.2 cm in internal diameter. In all cases, 0.2 cm of each nerve stump was inserted into the conduit leaving a gap of 1 cm (Figure 1). After the surgery, animals were kept in individual cages and received a postoperative analgesic treatment with metamizole in drinking water for 48 hours. Animals were euthanatized by applying an anesthetic overdose at 10, 20 or 30 days post-repair (n = 3 in each). In all cases, the right sciatic nerve was used as an unoperated control. In case of spinal cord, three non-operated rats (n = 3) were maintained in the same experimental conditions and euthanized at the last time point (30 days).
Figure 1.
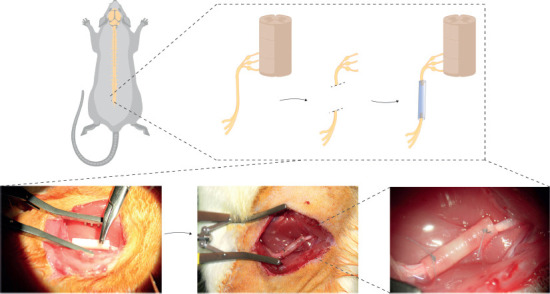
Schematic surgical procedure of peripheral nerve transection and repair using the tubulization technique.
These images show a schematic representation and macroscopic aspect of the tubulization procedure with NeuraGen® collagen conduit to repair 1 cm long nerve gaps in the sciatic nerve of Wistar rats.
All experimental animal procedures were conducted according to the Spanish and European regulations for animal experimentation (EU directive No. 63/2010, RD 53/2013). Moreover, these studies and research projects were approved by the Ethics and Animal Experimentation Committee of the University of Granada (No. 03/07/15/311 and 29/03/2022/052, Grant N° FIS P20-0318 respectively).
Histological and immunohistochemical analyses
For histological analyses, all animals were euthanized with an intraperitoneal overdose of the same anesthetic mixture described above followed by intracardiac perfusion of a fixative solution. First, we perfused 500 mL of physiological saline solution to remove the blood from the tissues and then 500 mL of 10% buffered formalin for the complete fixation of the animal. The fixed normal sciatic nerve and grafted conduits were immediately harvested and post-fixed in 10% buffered formalin for 24 hours. In the case of the spinal cord, the perfused vertebral column was dissected and placed in 10% buffered formalin for 24 hours and then the lumbar-sacral region (T13–L5) was carefully dissected and post-fixed for 24 hours (fixation for 48 hours). Fixed samples were dehydrated, diaphanized in xylene, and embedded in paraffin as follows: grafted conduits were cut for transversal evaluation of the central area of regeneration. The lumbosacral spinal cords were cut every 0.5 cm and embedded transversally. For histological analyses, embedded samples were serially cut at 7 µm and stained with hematoxylin and eosin (Thermo Shandon Limited, Runcorn, UK) (data not shown). To simultaneously evaluate histological pattern, collagen fibers and myelin, all sections were stained and analyzed with myelin-collagen histochemical method (MCOLL) as previously described (Carriel et al., 2013).
GAP-43 and NFL expression in grafted conduits, spinal cords, and controls were evaluated by indirect immunohistochemistry as previously described (Carriel et al., 2014). Briefly, rehydrated sections were pre-treated with ethylenediaminetetraacetic acid (EDTA) pH = 8 (GAP-43) or citrate pH = 6 (NFL) antigen unmasking. Endogenous peroxidase activity was blocked using 3% H2O2 in 0.1 M phosphate buffer solution (PBS) for 10 minutes, while the non-specific binding of the primary antibody was blocked using 1x Casein (Vector Laboratories, Burlingame, CA, USA, Cat# SP-5020) for 15 minutes. Then, sections were incubated with the primary antibody rabbit anti-GAP-43 (Merck-Sigma-Aldrich, Burlington, MA, USA, Cat# Sab4300525, RRID: AB_10622060) diluted at 1:50 in PBS at room temperature for 1 hour at room temperature. This antibody recognizes the peptide sequence Q–A–S–F–R of the protein GAP-43. For NFL detection, sections were incubated with mouse anti-neurofilament (MilliporeSigma, Burlington, MA, USA, Cat# N2912, RRID: AB_477262) diluted at 1:500 in PBS. Subsequently, sections were rinsed in PBS, incubated with commercially prediluted secondary anti-rabbit IgG antibody and anti-mouse IgG antibody conjugated with peroxidase molecules (Vector Laboratories, Burlingame, CA, USA, Cat# MP-7401-50 and MP-7500, respectively) for 30 minutes at room temperature, and rinsed in PBS. Antigen-antibody reaction was visualized with diaminobenzidine (Vector Laboratories, Burlingame, CA, USA, Cat# SK-4100) and contrasted with Mayer’s hematoxylin. As a technical negative control, we omitted the primary antibody, and all procedures were carried out simutaneously using the same conditions.
Quantitative histological and immunohistochemical analyses
Quantitative analyses were performed on histochemically or immunohistochemically stained sections. MCOLL myelin staining GAP-43 and NFL immunoreactions were determined in both peripheral nerves and spinal cords (n = 3 each). In the case of corticospinal tract, the right and left posterior, cuneiform fasciculus and gracile fasciculus were quantitatively analyzed. For these analyses, ImageJ software (version 1.53k, National Institute of Health, Bethesda, MD, USA) was used to select the adequate color with threshold function. Once positive reactions were isolated, the percentage of the area occupied by each histochemical or immunohistochemical reaction (or area fraction) was automatically measured as previously described (Carriel et al., 2014; Chato-Astrain et al., 2020; El Soury et al., 2021; García-García et al., 2021). The percentage of positive stained area was calculated in region of interest of the analyzed field (40× in peripheral nerves and 8× in spinal cords) in four independent images from three rats at each time point.
Statistical analysis
Histological analyses were blindly conducted for each treatment, whereas in vivo analyses were not performed blind to the conditions of the experiments. No statistical methods were used to predetermine sample sizes; however, our sample sizes were similar to those reported in previous publications (Carriel et al., 2014; Chato-Astrain et al., 2018). For each analysis, results obtained under each experimental condition were compared with those in the control group. As none of the distributions fulfilled the criteria of normality, the Shapiro-Wilk test and the non-parametric Mann-Whitney U tests were used for all comparisons. The comparisons were done with Real Statistics Resource Pack software (Release 7.2) (Dr. Charles Zaiontz, Purdue University, West Lafayette, IN, USA, www.real-statistics.com). P < 0.05 was considered statistically significant, and all tests were performed double-tailed.
Results
Histological regeneration pattern within repaired nerves
The time-course histological analyses showed an active regeneration process of the nerve gaps repaired with the collagen conduit from 10 days onwards (Figure 2). The regeneration process was observed in the conduit at all time points studied, and small newly-formed fascicles were detected. Furthermore, no signs of inflammatory or adverse reaction were observed in any animal evaluated.
Figure 2.
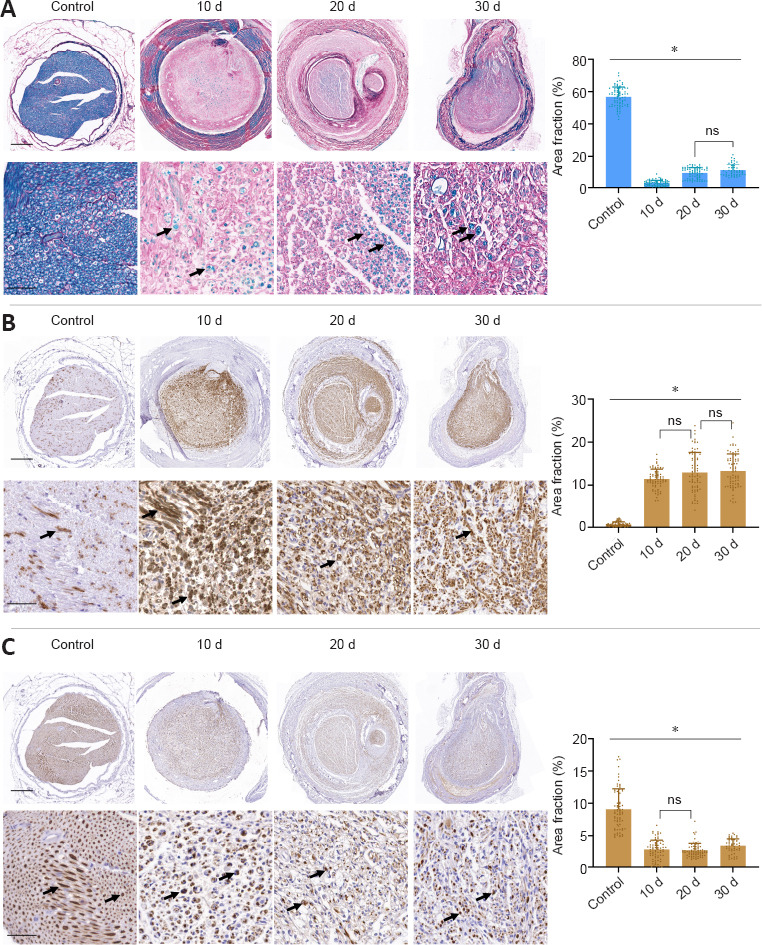
Time-course microscopic and quantitative results of peripheral nerve regeneration through the conduit.
Figures show MCOLL (A), GAP-43 (B) and NFL (C) cross-section staining at low and medium magnifications from the central portion of repaired nerves by the collagen conduit. In addition, a representative transversal histological section of a healthy nerve (control) was included. Positive reaction areas are indicated with arrows. Quantitative results were included as positive area fraction for each histological technique (data are expressed as the mean ± SD). Stainings were performed at least three times and in three samples (animals) at each time point as described in the Methods section. Statistically differences (P < 0.05) were found in all comparison “*” unless indicated above by “ns”. Scale bars: 200 μm and 50 μm in images with higher magnification. GAP-43: Growth-associated protein-43; MCOLL: myelin-collagen histochemical method; NFL: neurofilament.
Then, the MCOLL analysis revealed remarkable differences in the histological pattern during peripheral nerve regeneration (Figure 2A). At the first time point studied (10 days post-repair), small nerve fascicles were surrounded by a loose connective tissue which reflects an immature extracellular matrix composition. The newly-formed nerve fibers presented a low number of myelinated nerve fibres between the collagen networks. At 20 days post-repair, MCOLL results revealed clear signs of remyelination and collagen remodelling. At this point, the regeneration process was more organized. Nerve fibers were more abundant and with well-defined signs of myelination inside a well-structured ECM. Finally, at 30 days post-repair, a high density of newly-formed nerve fibers was observed. Nevertheless, the regeneration process was far to be completed. As expected, at these time points, regeneration was not comparable to that in the control group. These descriptive results were confirmed by quantitative area fraction analysis. This test confirmed that the newly-formed nerve tissue underwent a progressive maturation and remyelination process over time. However, the percentage of the area occupied by myelinated fibers (positive histochemical reaction) was significantly lower in operated animals than that in the control group as expected (P < 0.05). Histochemical reaction was significantly increased at 20–30 days post-repair compared with that at 10 days post-repair (P < 0.05), but there was no significant difference in histochemical reaction between 20 and 30 days post-repair (P = 0.078).
GAP-43 expression profile also showed remarkable differences among the time points studied. GAP-43 protein showed a significantly different positivity within the newly-formed nerve tissue from 10 to 30 days from that in the control group (Figure 2B). The staining pattern was fibrillar, highly specific and consistent with the regenerated tissue formed inside the conduits, confirming an active regeneration process of the peripheral nerve, as previously confirmed by histochemical analyses. The area fraction quantitative analyses showed a significant increase of GAP-43 in all operated animals compared with the control group (P < 0.05). In addition, quantitative data showed a progressive increase over time, but differences were only significant between 30 and 10 days post-repair (P = 0.005).
Axonal regeneration process was also assessed by identification of NFL expression. Unsurprisingly, we observed a decrease of NFL immunostaining in operated animals compared with the control group (Figure 2C). Indeed, less than 5% of the area showed positivity for NFL. NFL immunostaining increased over time, and there was significant difference in NFL expression between 30 days post-repair and 20 and 10 days post-repair (P = 0.01, P = 0.03 respectively). These findings were in line with the MCOLL and GAP-43 descriptive and quantitative results since immature nerve fibres were more abundant than mature and myelinated ones in the early stages of peripheral nerve regeneration.
Histological regeneration pattern at the spinal cord
Spinal cord changes during nerve regeneration were evaluated by hematoxylin-eosin staining and MCOLL histochemical method (Figure 3). In this study, the transversal section at low magnification revealed the complete cytoarchitecture of the spinal cord, and the white and grey substances were remarkably different as shown by MCOLL method. Hematoxylin-eosin staining results revealed no apparent changes at the cellular (neuronal and glial) level or fiber organization. However, the MCOLL technique at higher magnification suggested slight changes in the structural organization of the posterior cord, as compared with the control group (Figure 3).
Figure 3.
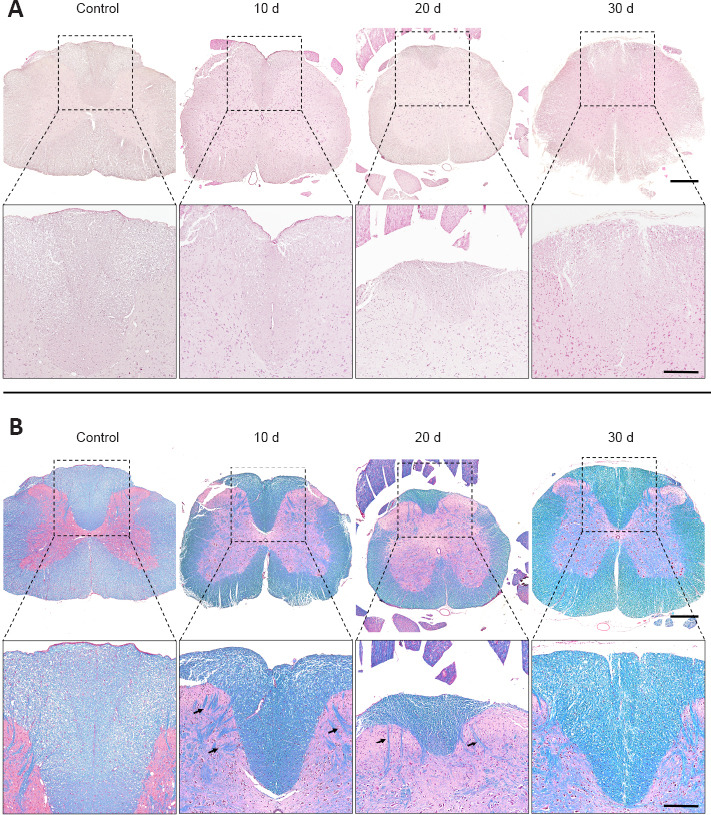
Time-course microscopic results of the lumbar section of the spinal cord after peripheral nerve damage and repair.
Hematoxylin-eosin (A) and myelin-collagen histochemical method (MCOLL) (B) cross-section staining images at low and medium magnifications from the central portion of the spinal cord. Arrows indicate MCOLL positive reaction in the grey matter. In addition, a representative transversal histological section of a healthy spinal cord (control) was included. Scale bars: 500 μm and 250 μm in images with higher magnification. Stainings were performed at least three times and in three samples (animals) at each time point as described in Methods section.
Interestingly, important differences were observed in GAP-43 expression at the spinal cord level during sciatic nerve regeneration process (Figure 4A and C). Histology revealed a considerable increase in GAP-43 immunoreaction in the grey and white matters, as compared with the control group, from 10 days onward. Surprisingly, these changes were especially evident in the white matter of the posterior columns. At 10 days post-repair, GAP-43 was found to be located in certain regions of the left cuneiform fasciculus, differing from the pattern found in the control group, where it was just observed in the descending pathway of the corticospinal tract of the posterior funiculus. Furthermore, GAP-43 staining at 20 days post-repair was consistent in most spinal cord areas, with a similar expression pattern in the cuneiform fasciculus and in the corticospinal tract (Figure 4A and C). At 30 days post-repair, GAP-43 expression exhibited an overall homogenous distribution, with a remarkable increase in the left posterior cord, covering the cuneiform fasciculus and the gracile fasciculus. These results were corroborated by the quantitative area fraction analyses conducted in the white posterior columns which revealed remarkable statistical differences in operated animals as compared with the control group (P < 0.05). Interestingly, the increase in GAP-43 area fraction positive reaction was statistically significant at 10 and 30 days only in the left posterior gracile fasciculus and cuneiform fasciculus (P < 0.05; Figure 4C).
Figure 4.
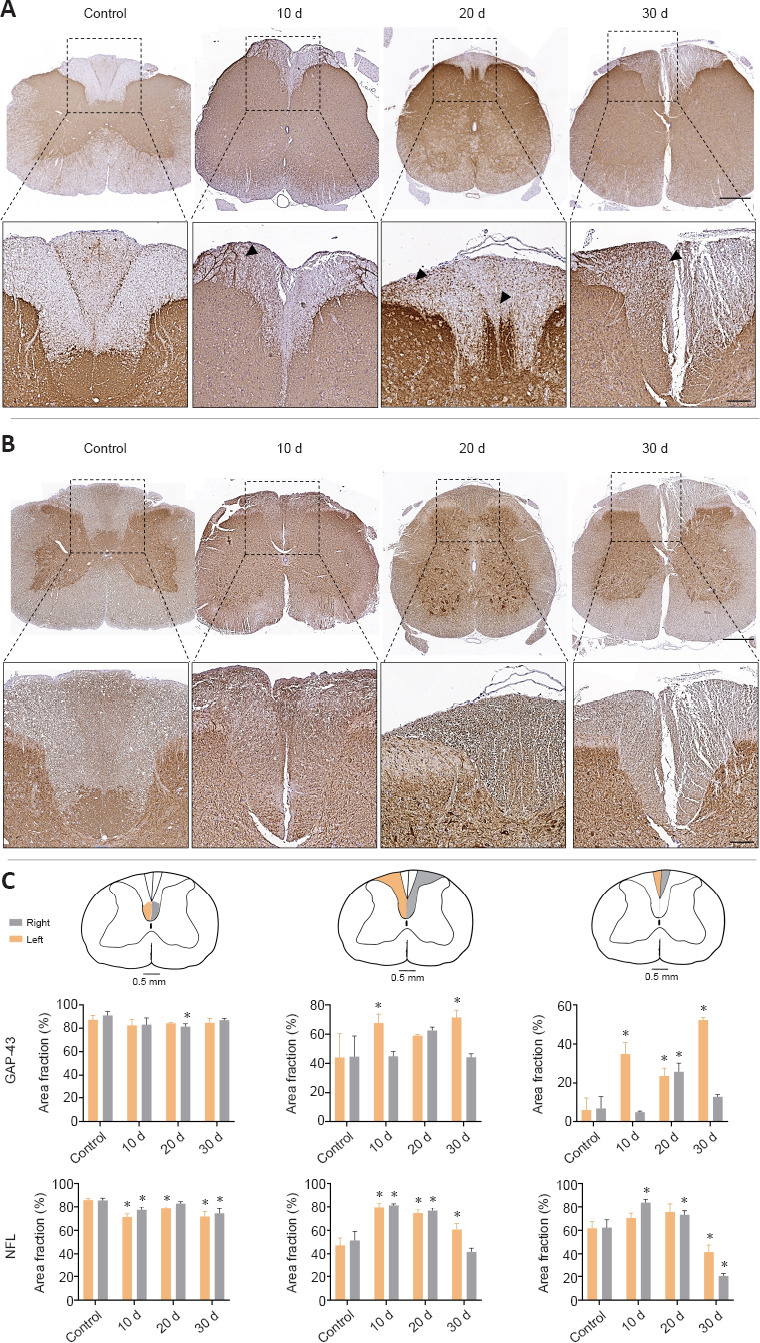
Immunohistochemical and quantitative evaluation of GAP-43 and NFL expression in the spinal cord after peripheral nerve damage and repair.
GAP-43 (A) and NFL (B) cross-section staining at low and medium magnifications from the central portion of the spinal cord after nerve injury. Arrows indicate GAP-43 positive cells. Quantitative analysis of the right (grey) and left (orange) posterior corticospinal tract, cuneiform fasciculus and gracile fasciculus were included in (C) (data are expressed as the mean ± SD). Scale bars: 500 μm and 150 μm in images with higher magnification. *P < 0.05, vs. control group. Stainings were performed at least three times and in three samples (animals) at each time point as described in the Methods section. GAP-43: Growth-associated protein-43; MCOLL: myelin-collagen histochemical method; NFL: neurofilament.
NFL expression in the spinal cord exhibited slight change at this level. Unsurprisingly, this protein was found in the white and grey matters, staining the neuronal somas and neurites. Histology revealed spatial distribution changes of NFL in different areas of the spinal cord following peripheral nerve repair. These changes were more evident in the corticospinal tract. In the cuneiform fasciculus and gracile fasciculus, NFL expression was similar to GAP-43 expression, showing a more homogeneous NFL distribution pattern (Figure 4B). Quantitative analyses supported these findings revealing a significant increase (P < 0.05) in NFL area fraction values over time, as compared with the control group, in the ascending pathways as described above. Moreover, there was no remarkable difference in NFL expression between the left and right sides of the spinal cord in animals subjected to sciatic nerve repair (Figure 4A and C).
Interestingly, here we found a correlation between the expression of NFL and GAP-43 in the spinal cord with the expression pattern observed within the implanted conduits.
Discussion
We reported the time-course histological expression pattern of GAP-43 and NFL proteins in repaired sciatic nerves and the spinal cord in rats. We histologically and quantitatively assessed sciatic nerve regenetaion at 10, 20, and 30 days post-repair.
In regenerative medicine, tissue-engineered substitutes should promote fast and active tissue regeneration (García-García et al., 2021). In this study, we used a commercially available collagen conduit (NeuraGen®) able to accomplish the specific anatomical needs of a tension-free nerve repair. Hence, this substitute should be able to create a closed microenvironment where tissue regeneration can occur by guiding proximal nerve sprouts to achieve distal organ reconnection (Carriel et al., 2013; Chato-Astrain et al., 2018).
First, analysis of the nerve regeneration process within the conduit revealed a clear sign of nerve tissue regeneration. It was classically described that the tubulization technique supports a progressive nerve tissue regeneration characterized by five phases (fluid, matrix, cell, axon, and myelinization) (Belkas et al., 2004). Histological evaluation of the repaired nerve confirmed that at 10–30 days post-repair, the tubulisation technique may support an active and progressive nerve tissue regeneration process. Histology confirmed that an ECM started to form, supporting cell migration, axonal growth, and early stages of the myelination process. These findings are in line with previous findings (Belkas et al., 2004). Indeed, the newly-formed nerve tissue was characterized by a relatively simple organization that initially lacks mature structures, such as mature myelin sheaths or a well-organized ECM and nerve fascicles. However, GAP-43 and NFL were allowed to accurately confirm axonal regrowth and their progressive maturation between 10 and 30 days post-repair. As shown in previous studies (Carriel et al., 2014; Chato-Astrain et al., 2018, 2020), GAP-43 expression is especially detected in immature growing axons supporting the progressive increase observed from 10 days onwards. Moreover, as expected, GAP-43 was considerably more abundant than NFL in the early stage of nerve regeneration process. These new findings corroborate that GAP-43 is needed for the early stage of axonal regeneration. When axonal sprouts acquire certain maturation, the establishment of the NFL cytoskeletal occurs. These new results are in line with findings from previous longer-term studies conducted after 12 weeks of peripheral nerve repair, indicating that GAP-43 is needed for the initial axonal regrowth and its expression is followed by NFL expression (Carriel et al., 2014).
Second, the histochemical evaluation of the spinal cord using hematoxylin and eosin staining and the MCOLL method revealed that a peripheral nerve injury and immediate repair do not cause relevant structural changes detected by light microscopy. The absence of structural changes at the spinal cord level is consistent with findings from previous neurophysiological studies since Wallerian degeneration hardly causes changes in the proximal nerve stump. Nevertheless, and very interestingly, analysis of GAP-43 expression in the spinal cord showed clear changes at the CNS level which were found to be directly correlated with the site of injury (left side), as well as with the regeneration profile at the PNS level.
It might be clear that, after a peripheral nerve injury, morphological, molecular and gene expression changes occur in the spinal cord. Peripheral nerve injury triggers in the central neurons a phenotype switch from a functional to a regenerative state required to start the axonal re-growth process (Geuna et al., 2009). In this study, we were able to detect changes in the GAP-43 expression pattern in the spinal cord, supporting the results observed at the peripheral level. These results are in agreement with previous studies, in which GAP-43 was upregulated in the spinal cord following peripheral nerve injuries (Chen et al., 2010; Liu and Wang, 2020). Surprisingly, in this study, we found that the GAP-43 expression pattern differed from the control group during the early stage of peripheral nerve regeneration. Changes were found in most spinal cord areas, but they started to be evident from 10 days onwards within the posterior white columns, concretely in the left (operated side) corticospinal tract and in the gracile fasciculus and cuneiform fasciculus. It is important to bear in mind that the rat spinal cord typically shows some cytoarchitectural differences compared with the human spinal cord. Within the rat dorsal column, it is possible to find not only ascending sensitive pathways (cuneiform fasciculus and gracile fasciculus) like in humans, but also descending motor ones, the posterior corticospinal tracts (Watson et al., 2009; Paxinos, 2014). On one hand, the ascending sensitive pathways include the gracile fasciculus which collects the fibers from the lower limb (Goll’s fascicle) (Waschke et al., 2018) and the cuneiform fasciculus which collects fibers from the upper limb (Burdach’s fascicle). Then, the fibers ascend ipsilaterally to the spinal bulb, where they establish synaptic junctions with the second neuron in the gracile and cuneiform nuclei. On the other hand, the descending motor pathway that changes nerve regeneration is the corticospinal tract, the principal motor pathway for voluntary movements which starts at the cortex and terminates on the lower motor neurons and interneurons of the spine controlling limb movements (Welniarz et al., 2017). Our findings demonstrated that GAP-43 immunostaining, rather than NFL immunostaining, can be used to identify early spinal cord response following peripheral nerve repair and regeneration. Overall results suggest that changes in the spinal cord start from 10 days onwards with differences in regeneration dynamics response. Specifically, the early response of the white columns suggests a faster or preferential regeneration-related plasticity of sensory pathways followed by motor and contralateral areas. Concerning the NFL expression profile within the spinal cord, the slight changes observed were in line with GAP-43 expression, and in this case, they could be related to volumetric or spatial adaptations of neurons following peripheral nerve damage and regeneration, instead of a real increase in the number of axons in these areas. These new results support the idea of a differential plasticity response and specificity between sensory and motor pathways in the rat spinal cord. However, to deeply study this phenomenon, future molecular experiments are still needed as routine light microscopy analyses are unable and not sensible enough to identify these complex changes (Liu and Wang, 2020).
This study was designed to evaluate the role of GAP-43 in the central nervous system, if any, during the peripheral nerve regeneration process. Our results showed that the expression pattern of GAP-43 protein was altered at the central level when nerve regeneration processes occurred peripherally. However, there were some limitations to methodological development in this study. First, this study was based on histological analysis that evaluates protein expression in tissue sections, so molecular-based and gene expression experiments would help to detect changes in expression and epigenetic modulations that are essential for the transition to a neural pro-regenerative phenotype. In addition, although animal studies provide invaluable information, it is important to bear in mind that the translation of these results to phylogenetically more complex organisms such as humans must be done with care (Robinson et al., 2019) as some anatomy and physiology processes are species-specific like the spatial distribution of the posterior cords in the spinal level as demonstrated above.
In conclusion, our results demonstrate that GAP-43 is crucial at the site of injury during the early stage of nerve regeneration where axonal sprouting process occurs and is even more abundant than NFL. In addition, this study also confirms that GAP-43 is reacquired by neurons at the spinal cord level where this protein probably contributes to modulating cytoskeletal changes needed to promote axonal repair and CNS remodelling. Furthermore, the differential expression of GAP-43 at the spinal cord suggests novel differences in the regeneration response of the sensory and motor pathways. Finally, it is still unknown the specific role of GAP-43 in the CNS dynamics and plasticity in long-term peripheral nerve regeneration studies. Therefore, spatiotemporal morphofunctional and molecular studies are still needed to elucidate the CNS preferential response to peripheral nerve injury and regeneration.
Footnotes
Funding: The study was financed by the Spanish “Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica, Ministerio de Economía y Competitividad (Instituto de Salud Carlos III)”, grant Nos: FIS PI17-0393, FIS PI20-0318, co-financed by the “Fondo Europeo de Desarrollo Regional ERDF-FEDER European Union”; grant No. P18-RT-5059 by “Plan Andaluz de Investigación, Desarrollo e Innovación (PAIDI 2020), Consejería de Transformación Económica, Industria, Conocimiento y Universidades, Junta de Andalucía, España”; and grant No. A-CTS-498-UGR18 by “Programa Operativo FEDER Andalucía 2014-2020, Universidad de Granada, Junta de Andalucía, España”, co-funded by ERDF-FEDER, the European Union (all to VC).
Conflicts of interest: None declared.
C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Benowitz LI, Routtenberg A. GAP-43:an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. (1997);20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 2.Carriel V, Garzon I, Alaminos M, Campos A. Evaluation of myelin sheath and collagen reorganization pattern in a model of peripheral nerve regeneration using an integrated histochemical approach. Histochem Cell Biol. (2011);136:709–717. doi: 10.1007/s00418-011-0874-3. [DOI] [PubMed] [Google Scholar]
- 3.Carriel V, Garzón I, Campos A, Cornelissen M, Alaminos M. Differential expression of GAP-43 and neurofilament during peripheral nerve regeneration through bio-artificial conduits. J Tissue Eng Regen Med. (2014);11:553–563. doi: 10.1002/term.1949. [DOI] [PubMed] [Google Scholar]
- 4.Chato-Astrain J, Campos F, Roda O, Miralles E, Durand-Herrera D, Sáez-Moreno JA, García-García S, Alaminos M, Campos A, Carriel V. In vivo evaluation of nanostructured fibrin-agarose hydrogels with mesenchymal stem cells for peripheral nerve repair. Front Cell Neurosci. (2018);12:501. doi: 10.3389/fncel.2018.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chato-Astrain J, Philips C, Campos F, Durand-Herrera D, García-García OD, Roosens A, Alaminos M, Campos A, Carriel V. Detergent-based decellularized peripheral nerve allografts:an in vivo preclinical study in the rat sciatic nerve injury model. J Tissue Eng Regen Med. (2020);14:789–806. doi: 10.1002/term.3043. [DOI] [PubMed] [Google Scholar]
- 6.Chen LJ, Ren YH, Liu L, Zhang XQ, Zhao Y, Wu WT, Li F. Upregulated expression of GAP-43 mRNA and protein in anterior horn motoneurons of the spinal cord after brachial plexus injury. Arch Med Res. (2010);41:513–518. doi: 10.1016/j.arcmed.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Chung D, Shum A, Caraveo G. GAP-43 and BASP1 in axon regeneration:implications for the treatment of neurodegenerative diseases. Front Cell Dev Biol. (2020);8:567537. doi: 10.3389/fcell.2020.567537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly W, Yao L, Zeugolis D, Windebank A, Pandit A. A biomaterials approach to peripheral nerve regeneration:bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. (2012);9:202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denny JB. Molecular mechanisms, biological actions, and neuropharmacology of the growth-associated protein GAP-43. Curr Neuropharmacol. (2006);4:293–304. doi: 10.2174/157015906778520782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Soury M, García-García ÓD, Moretti M, Perroteau I, Raimondo S, Lovati AB, Carriel V. Comparison of decellularization protocols to generate peripheral nerve grafts:a study on rat sciatic nerves. Int J Mol Sci. (2021);22:2389. doi: 10.3390/ijms22052389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-García ÓD, El Soury M, González-Quevedo D, Sánchez-Porras D, Chato-Astrain J, Campos F, Carriel V. Histological, biomechanical, and biological properties of genipin-crosslinked decellularized peripheral nerves. Int J Mol Sci. (2021);22:674. doi: 10.3390/ijms22020674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grijalva LE, Miranda MI, Paredes RG. Differential changes in GAP-43 or synaptophysin during appetitive and aversive taste memory formation. Behav Brain Res. (2021);397:112937. doi: 10.1016/j.bbr.2020.112937. [DOI] [PubMed] [Google Scholar]
- 13.Korshunova I, Mosevitsky M. Role of the growth-associated protein GAP-43 in NCAM-mediated neurite outgrowth. Adv Exp Med Biol. (2010);663:169–182. doi: 10.1007/978-1-4419-1170-4_11. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Wang H. Peripheral nerve injury induced changes in the spinal cord and strategies to counteract/enhance the changes to promote nerve regeneration. Neural Regen Res. (2020);15:189–198. doi: 10.4103/1673-5374.265540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paxinos G. San Diego: Academic Press; (2014). The rat nervous system. [Google Scholar]
- 16.Robinson NB, Krieger K, Khan FM, Huffman W, Chang M, Naik A, Yongle R, Hameed I, Krieger K, Girardi LN, Gaudino M. The current state of animal models in research:a review. Int J Surg. (2019);72:9–13. doi: 10.1016/j.ijsu.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Waschke J, Koch M, Kürten S, Schulze-Tanzil G, Spittau B. Texto de anatomía: Elsevier Health Sciences; (2018). Sobotta. [Google Scholar]
- 18.Watson C, Paxinos G, Kayalioglu G, Heise C. Chapter 15 - Atlas of the rat spinal cord. In: Watson C, Paxinos G, Kayalioglu G, editors. The spinal cord. San Diego: Academic Press; (2009). pp. 238–306. [Google Scholar]
- 19.Welniarz Q, Dusart I, Roze E. The corticospinal tract:evolution, development, and human disorders. Dev Neurobiol. (2017);77:810–829. doi: 10.1002/dneu.22455. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Lyu D, Jia J Alzheimer's Disease Neuroimaging Initiative. The trajectory of cerebrospinal fluid growth-associated protein 43 in the Alzheimer's disease continuum: a longitudinal study. J Alzheimers Dis. (2022);85:1441–1452. doi: 10.3233/JAD-215456. [DOI] [PubMed] [Google Scholar]


