Abstract
There is growing evidence that long-term central nervous system (CNS) inflammation exacerbates secondary deterioration of brain structures and functions and is one of the major determinants of disease outcome and progression. In acute CNS injury, brain microglia are among the first cells to respond and play a critical role in neural repair and regeneration. However, microglial activation can also impede CNS repair and amplify tissue damage, and phenotypic transformation may be responsible for this dual role. Mesenchymal stem cell (MSC)-derived exosomes (Exos) are promising therapeutic agents for the treatment of acute CNS injuries due to their immunomodulatory and regenerative properties. MSC-Exos are nanoscale membrane vesicles that are actively released by cells and are used clinically as circulating biomarkers for disease diagnosis and prognosis. MSC-Exos can be neuroprotective in several acute CNS models, including for stroke and traumatic brain injury, showing great clinical potential. This review summarized the classification of acute CNS injury disorders and discussed the prominent role of microglial activation in acute CNS inflammation and the specific role of MSC-Exos in regulating pro-inflammatory microglia in neuroinflammatory repair following acute CNS injury. Finally, this review explored the potential mechanisms and factors associated with MSC-Exos in modulating the phenotypic balance of microglia, focusing on the interplay between CNS inflammation, the brain, and injury aspects, with an emphasis on potential strategies and therapeutic interventions for improving functional recovery from early CNS inflammation caused by acute CNS injury.
Key Words: acute CNS injury, central nervous system inflammation, exosome, immune regulation, mesenchymal stem cell, mesenchymal stem cell-derived exosomes (MSC-Exos), microglia activation, microglia phenotypic transformation, molecular mechanism, neuroinflammation
Introduction
Neurological disorders remain the largest contributors to disability globally (Winkler, 2020). Highly persistent and recurrent acute central nervous system (CNS) injuries culminate in irreversible brain dysfunction, and complex and costly clinical treatments lead to a range of medical and social problems and a severe economic burden. It is estimated that more than 101 million people globally are affected by incident stroke each year, of which 6.55 million die and 143 million are left with permanent disability (Roth et al., 2020). No therapies are currently available to achieve complete or near-complete CNS regeneration and recovery, so there is an urgent need to develop strategies to treat the brain after injury. According to the pathology of stroke and the World Health Organization criteria (World Health Organization, 1971), acute CNS injuries can be divided into traumatic brain injury (TBI) and nontraumatic brain injury. Stroke, the most common nontraumatic brain injury, is the main cause of cerebral vessel obstruction (ischemic stroke, IS) or burst/hemorrhage (hemorrhagic stroke), with a sudden onset of hemiparesis as the main manifestation, which can lead to death or permanent neurological deficits. Hemorrhagic strokes are divided into intracerebral hemorrhage (ICH) and subarachnoid hemorrhage, depending on the site of the bleeding (Table 1).
Table 1.
Classification of acute CNS injury diseases, based on etiology and main lesion sites
| Acute CNS injuries | Cause of illness | Incidence rate | Case fatality rate |
|---|---|---|---|
| Stroke | Focal brain dysfunction that occurs suddenly due to blockage or rupture of a blood vessel (World Health Organization, 1971) | 42% (Feigin et al., 2019; Roth et al., 2020) | 43.3% (Roth et al., 2020) |
| IS | Death of the brain parenchyma due to lack of blood supply (World Health Organization, 1971) | 62.4% (Roth et al., 2020) | 17.7% (Roth et al., 2020) |
| ICH | Hemorrhage originating in the brain parenchyma (World Health Organization, 1971) | 27.9% (Roth et al., 2020) | 15.5% (Roth et al., 2020) |
| SAH | Hemorrhage originating from the subarachnoid space (World Health Organization, 1971) | 9.7% (Roth et al., 2020) | 2% (Roth et al., 2020) |
| TBI | Traumatic structural damage and/or brain dysfunction caused by external forces (Maas et al., 2017) | 37% (Majdan et al., 2016) | 30–40% (Kumar and Singh, 2021) |
CNS: Central nervous system; ICH: intracerebral hemorrhage; IS: ischemic stroke; SAH: subarachnoid hemorrhage; TBI: traumatic brain injury.
Within minutes after localized ischemic necrosis of brain tissue, hypoxia and energy deprivation trigger a series of ischemic chain reactions, such as oxidative stress and inflammation, leading to neuronal excitotoxicity and cell death. The release of signals from pathogen-associated or danger-associated molecular patterns, such as alarmin, by damaged cells causes the elevation of acute inflammatory mediators and proinflammatory cytokines (e.g., tumor necrosis factor [TNF]-α, interleukin [IL]-1β, IL-6), reactive oxygen species (ROS), and intercellular adhesion molecule (ICAM)-1, exacerbating brain edema, oxidative stress, and apoptosis, leading to disruption of the blood-brain barrier (BBB) and increasing the neuroinflammatory response in the brain (Barichello et al., 2015). Furthermore, in a sustained pathological state, the autoimmune response to inflammatory products, red blood cell metabolites, and neuronal antigens stimulates the activation of multiple effector cells in the ischemic area, such as microglia (Wolf et al., 2017), the immune regulatory cells of the central nervous system (CNS), which phagocytose and remove abnormal or excess proteins to maintain the balance of protein in the brain and prevent the onset of neurodegeneration. On the other hand, the microglia-driven inflammatory response further produces multiple proinflammatory mediators that together regulate encephalitis, thereby exacerbating postischemic brain injury (Loane and Byrnes, 2010; Figure 1)
Figure 1.
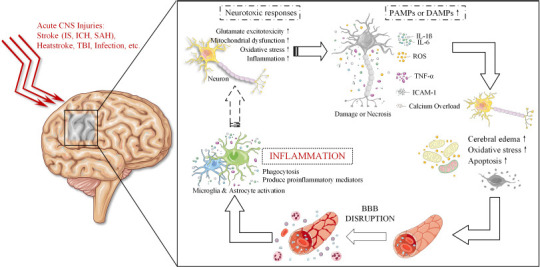
The vicious circle of neuroinflammation after acute central nervous system injury.
After acute CNS injury, neurons are deprived of oxygen and energy, which triggers a series of chain reactions, such as glutamate excitotoxicity, mitochondrial dysfunction, oxidative stress, and inflammation, leading to neuronal damage and necrosis. This leads to calcium overload and the release of acute inflammatory mediators such as TNF-α, IL-1β, IL-6, ROS, and ICAM-1, exacerbating cerebral edema, oxidative stress and apoptosis, leading to disruption of the BBB and increasing the inflammatory response in the brain. BBB: Blood-brain barrier; CNS: central nervous system; DAMPs: danger-associated molecular patterns; ICAM-1: intercellular adhesion molecule-1; ICH: intracerebral hemorrhage; IL: interleukin; IS: ischemic stroke; PAMPs: pathogen-associated molecular patterns; ROS: reactive oxygen species; SAH: subarachnoid hemorrhage; TBI: traumatic brain injury; TNF-α: tumor necrosis factor-α.
Research has shown that structural or functional disorders of the brain are at the root of neurological disorders (Pollak et al., 2018). Effective treatments for acute CNS-induced brain injury are lacking. Recently, an increasing number of studies have confirmed that the CNS inflammatory response is present throughout the pathology of CNS and peripheral injury and is an important factor in the early exacerbation of brain injury. Long-term and poorly controlled CNS inflammation can lead to secondary brain injury, which can cause progressive neurodegeneration (e.g., chronic encephalopathy) and even the development of recurrent encephalopathy lasting for several years. Therefore, the neuroinflammatory response to acute CNS injury has become a recent focus of research, and maintaining the inflammatory/anti-inflammatory balance of the body through appropriate anti-inflammatory interventions is an important strategy for the treatment of acute CNS injury.
In the past decade, many experimental studies have confirmed the beneficial role of mesenchymal stem cell-derived exosomes (MSC-Exos) in the treatment of acute CNS injuries such as IS and hemorrhagic stroke. In this review, we summarized the current therapeutic role of MSC-Exos in acute CNS injury, described the molecular and cellular mechanisms of MSC-Exos in regulating microglia, and demonstrated that MSC-Exos are a potential new therapeutic agent to treat CNS inflammation. We discussed novel roles for microglia subsets in different states and functions of the CNS. Finally, we speculated that the MSC-Exos-targeted microglia-mediated pro-inflammatory immune response may be a potential therapeutic opportunity and described some of the hurdles that must be overcome for its development.
Literature Search Strategy
An extensive review of the literature from 1970 to the present was conducted in September 2022 using multiple databases (GeenMedical, PubMed, Google Scholar, and ClinicalTrials.gov). The keywords used in the search were: “mesenchymal stem cells,” “microglia,” “exosome,” “neuroinflammation,” “neuroprotection,” “central nervous system,” “acute brain injury,” “ischemic stroke,” “traumatic brain injury,” “intracerebral hemorrhage,” “subarachnoid hemorrhage,” “heat stroke,” “miRNA,” and “human induced pluripotent stem cells.” A list of studies referencing the included studies was screened by the authors to identify other potentially useful studies. Eligible studies had to explore the relationship between CNS inflammation and MSC-Exos as well as the molecular and cellular mechanisms of the effects of MSC-Exos on reactive microglia phenotypes, and the studies’ findings were analyzed in this review.
Microglia & Acute Central Nervous System Inflammation
The acute response to CNS inflammation is a complex process involving multiple immune cells (Rickman et al., 2022). Glial cells, including microglia, astrocytes, oligodendrocytes, and neuron-glial antigen 2 (NG2) cells, are the most abundant and widely distributed cells in the CNS (Yang and Zhou, 2019). Among them, microglia are the most active key immunoreactive cells in the CNS inflammatory response (Yang and Zhou, 2019).
After CNS injury, the early inflammatory response is initially mediated by the activation of microglia, the brain’s neuroimmune cells (Wolf et al., 2017). Several studies (Sousa et al., 2018; Goshi et al., 2020; Zhang et al., 2021) have confirmed that the onset and progression of neuroinflammatory responses are closely linked to microglia. Although the “defense and repair” mechanisms of microglia in brain homeostasis are widely accepted, there is a lack of comprehensive understanding of microglia-driven inflammatory immune responses. In recent years, some scholars have argued that the microglia polarization phenotypes M1 (pro-inflammation) and M2 (anti-inflammation) have not been confirmed (Ransohoff, 2016; Friedman et al., 2018). Simple extreme activation states and a few of their markers cannot reflect the heterogeneity and complexity of microglia. Therefore, it has been proposed that restrictive polarizing hypothesis that hinders the progress of study should be discarded (Ransohoff, 2016; Rodríguez-Gómez et al., 2020). In this context, we replace the terms “resting” and “M1/M2 polarization” with a detailed description of microglia characteristics and annotations according to microglia detection methods. In this review, the microglia “pro-inflammatory response” was considered to be an overactivated immune response that might lead to neurotoxicity (García-Revilla et al., 2019; Rodríguez-Gómez et al., 2020).
Correlation between microglia and acute CNS inflammation
Microglia are intrinsic immune cells in the CNS and play an important role in the immune defense of the nervous system in normal development as well as in pathological situations (Wolf et al., 2017). Under nonpathological conditions, there are low-level but still active microglia in the CNS, often referred to as “steady-state/balanced microglia,” whose bodies appear to be stationary but are constantly monitoring the surrounding environment. The synapses of steady-state microglia are continuously elongated and contracted and use microfilm structures for immune surveillance of surrounding cells and to maintain homeostasis of the immune microenvironment in the brain (Colonna and Butovsky, 2017). When the CNS is in a pathological state, including infection, inflammation, trauma, ischemia, and neurodegeneration, multiple cells die, including neurons. These dying cells produce inflammatory mediators such as ROS and release cytokines, and microglia rapidly convert into reactive, but unstable, phenotypes, serving as a first line of defense (Nimmerjahn et al., 2005) and forming a barrier between normal and damaged tissue (Davalos et al., 2005). In addition to programmed cell death being activated in vulnerable neurons, activation and proliferation of microglia reactive phenotypes may also increase the appropriate coupling of phagocytosis and apoptosis via ‘find me’ signaling. This helps to further remove cellular debris, promote resolution of inflammation, and provide a relatively suitable microenvironment for neuronal repair (Wolf et al., 2017).
However, activation of an environmentally driven microglia-related pro-inflammatory response under excessively noxious stimuli is typically considered a negative event. In response to trauma and various immune stimuli, microglia release the pro-inflammatory factors IL-1β, TNF-α, and IL-15, which directly exacerbate neural injury and damage to neurons (Gao and Hong, 2008). This unstable internal environmental stimulus activates the acute inflammatory response of microglia, promoting the infiltration of inflammatory cells such as neutrophils, different subtypes of T cells, and monocytes/macrophages into ischemic areas for in situ proliferation and differentiation, amplifying the local inflammatory response and further exacerbating the permeability of the BBB, exerting a combined effect to exacerbate CNS injury (Kim et al., 2020). Furthermore, activated microglia trigger a wide range of signaling pathways through effectors such as cell surface receptors, signal transduction pathways, and transcription factors. For example, intracellular proteins (protein kinase B [AKT], mammalian target of rapamycin [mTOR], and p38 mitogen-activated protein kinase) are phosphorylated and activated, leading to nuclear factor kappa-B (NF-κB) phosphorylation and ubiquitination, which mediates the transcription and translation of inflammatory cytokines and chemokines (Fann et al., 2018). At the same time, the NF-κB pathway can activate calcium (Ca2+)-dependent enzymes (including calpain, cystatin, and enzymes that produce nitric oxide, ROS, and arachidonic acid metabolites) via oxidative stress, leading to a neurotoxic cascade of Ca2+ overload and the induction of nitrative stress, further exacerbating neuronal damage and CNS inflammation (Liu et al., 2019b).
Several studies have pointed out that, during the development of CNS injury such as Alzheimer’s disease (vonderEmbse et al., 2019), multiple sclerosis (Gillen et al., 2018), TBI (Liu et al., 2019c), and stroke (Liu et al., 2019a), microglia show different neurotoxicity and neuroprotective characteristics, which are related to the different activation phenotypes shown in the disease process (Wolf et al., 2017). The emergence of the concept of reactive phenotypic diversity explains the heterogeneity between microglia subsets. For example, some microglia-specific promoters, including CD11b, CD11c, LysM, CX3CR1, and Iba1, as well as some protein-encoding genes, including Crybb1, P2ry13, Tmem119, TSPO, and Itgb5, are associated with microglia’s ability to monitor neuronal activity, cellular metabolism, synthesis of cytokines (e.g., pro-inflammatory cytokines), chemokines, and growth factors, expression of antigen-presenting molecules, or demyelination (Wolf et al., 2017; Gerrits et al., 2020). Therefore, a targeted investigation of the main mechanisms triggering microglia phenotypic transformation during CNS inflammation may provide new ideas for the clinical treatment of acute CNS injury by modulating the microglial activation phenotype.
Molecular mechanisms of microglia activation
Microglia are the main cells that perform immune functions in the CNS, and their functions and properties are similar to those of peripheral macrophages (Chen et al., 2022). Characterizations using the M1/M2 phenotypes are considered to be a simplified conceptual framework based on the fact that microglia differentiate into two different extreme activation states in pathological situations, depending on the microenvironment (Hu et al., 2015b). However, microglial activation responds differently to specific conditions (Friedman et al., 2018), and microglia populations with different phenotypes display different molecular characteristics and physiological functions (García-Revilla et al., 2019). Friedman et al. (2018) compared microglia/myeloid cell expression profiles in mouse models of CNS disease, including neurodegenerative disease, aging, inflammation, and ischemic injury. One of the main conclusions of this study was that it identified genes related to inflammation that were highly induced by lipopolysaccharide-induced models of inflammation, such as Adam8, Ikbke, Irg1, Cd44, Ccl5, and Tspo. These key genes play an important role in microglia function under acute cerebral CNS inflammation. Hickman et al. (2013) analyzed microglia in five-month-old adult mice using single-cell RNA sequencing, which identified a group of highly enriched transcript clusters showing the homeostatic microglia “Sensome,” including P2ry12, Cx3cr1, Tlr2, HexB, Camp, and Ngp. Interestingly, RNAscope double fluorescence in situ hybridization data from brain sections from adult mice showed that HexB mRNA was expressed only in brain microglia. In addition, changes in the neuroprotective priming state were associated with an overall increase in gene expression in microglia that are involved in neuroprotection. This could trigger neuroprotective pathways such as the oxidative phosphorylation pathways associated with cellular metabolism or microglia programmed to inhibit inflammation by closing certain cellular metabolic pathways, such as the AKT–mTOR–HIF-1α pathway (Baik et al., 2019). This suggested that the molecular subtypes of different microglia were related to their neuroprotective and neurotoxic characteristics.
A recent study has pointed out that the switching of steady-state microglia to reactive microglia depends on the selective activation of pattern recognition receptors (García-Revilla et al., 2019). Among them, Toll-like receptor-4 (TLR-4) is an associated key pattern recognition receptor for maintaining brain homeostasis. Driven by TLR signaling, microglial cells first respond to CNS injury and infection by promoting the destruction of invading pathogens, a process that releases large amounts of proinflammatory factors (Hu et al., 2012) and neurotoxic transmitters to induce neurotoxic responses and initiate neuronal death and acute inflammation, contributing to increased BBB permeability. Furthermore, excessive activation of pro-oxidative microglia causes extensive inflammatory damage to neurons in the ischemic penumbra (Cunha et al., 2016). Subsequently, the anti-inflammatory and repair phases of neuroprotective microglia are rapidly initiated to repair trauma and restore tissue homeostasis, inhibit pro-inflammatory immune responses, and promote the expression of the repair-related genes Ym1/2, arginase1 (Arg1), CD206, and found in inflammatory zone-1 (Mecha et al., 2015). Considering the anti-inflammatory and neuroprotective properties of the microglia phenotype, Capiralla et al. (2012) provided evidence that regulating the microglia phenotype may be a novel strategy to reduce CNS inflammation and improve motor recovery after brain injury.
Recent studies have shown that signal transduction is involved in the modulation of acute CNS inflammation by activated microglia. Microglia activation and aggregation trigger the production of inflammatory mediators and lead to neuronal death through the TLR4/NF-κB p65 signaling pathway (Liu et al., 2020; Abd El-Rahman and Fayed, 2022). In addition, interferon regulatory factor (IRF)/signal transducer and activator of transcription (STAT)/suppressors of cytokine signaling, NF-κB activation, nuclear receptors (peroxisome proliferator-activated receptor [PPAR]-γ, PPAR-δ, and RXR), and redox signaling (Nrf2, NOX2, and HIF-1) likewise control microglial phenotypic transformation (Sica and Mantovani, 2012). Downregulation of IRF-5 signaling using short interfering RNA resulted in increased IRF-4 expression, and microglia showed significantly higher expression of the cell membrane marker CD206 and lower levels of CD68, with inhibition of proinflammatory responses and improved stroke prognosis in IRF-5 conditional knockout mice (Al Mamun et al., 2020). Recently, several other receptors have been found to play important regulatory roles in the microglia phenotypic transformation, including scavenger receptor A and nucleotide-binding oligomerization domain-like receptors. For example, one study found that enhanced activation of IL-33/ST2 (a member of the IL-1 receptor family) transduction after 3 days of middle cerebral artery occlusion (MCAO) could induce the anti-inflammatory properties of glutamate/aspartate transporter (GLAST)-CD11b+CD45Intermediate microglia (Yang et al., 2017b). In addition, blocking complex signaling pathways, such as the mTOR1 pathway could promote anti-inflammatory phenotypic transformation of microglia, thereby inhibiting the inflammatory response of primary microglia after MCAO (Wang et al., 2018). In addition, AMP-activated protein kinase (AMPK) induces nuclear factor erythroid 2-related factor 2 (Nrf2) by reducing the production of ROS and promoting the transformation of microglial anti-inflammatory function (Sag et al., 2008).
In addition to the common signal transduction factors, c-AMP response element binding protein, STAT-1/6, PPAR-γ and Krüppel-like factors all play a key role in microglial phenotype regulation (Sag et al., 2008; Sica and Mantovani, 2012; Ji et al., 2018). On the other hand, several studies have shown that some endogenous molecules and synthetic drugs can induce anti-inflammatory phenotypic changes in microglia, such as steroid hormones (Farahani et al., 2022), cannabinoids (Juknat et al., 2019), PPAR agonists (Ji et al., 2018), ion channel modulators (Luo et al., 2021), and ILs. The inhibition of the NF-κB pathway by fenugreek (phenolic compound of asparagus) significantly upregulates the expression of CD206+ microglia and inhibits the phosphorylation of c-Jun N-terminal kinase, thereby attenuating the production of inflammatory mediators and cytokines (Xiang et al., 2018).
Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Central Nervous System Inflammation Caused by Injury
Immunomodulatory effects and mechanisms of MSCs
It has been shown that BBB-permeable anti-inflammatory drugs do not significantly improve CNS injury outcomes (Mallah et al., 2020). Therefore, cellular therapy is considered one of the most promising approaches for the treatment of CNS diseases and neuroinflammatory disorders. MSCs are multipotent stem cells with immune system-regulating functions and are widely distributed in connective tissue. MSCs have multipotent properties such as the ability to secrete multiple factors, as well as differentiation potential, and they exert immunomodulatory effects. In the presence of inflammatory TNF-α and IL-1, MSCs are activated and exert immunosuppressive effects (Toupet et al., 2015). Previously, numerous studies on animal models of stroke, TBI, multiple sclerosis, nerve damage, and neurodegenerative diseases (Liu et al., 2019d) showed that infusion of MSCs inhibited apoptosis, preventing neuronal cell damage and counteracting brain injury. While early studies showed that MSCs promote tissue repair through direct differentiation, a large number of studies have now demonstrated that the main mechanism by which MSCs participate in brain remodeling and functional recovery is through their paracrine effect rather than cell replacement (Phinney et al., 2015).
The concept of MSC-derived exosomes and their regulation in acute CNS inflammation
Exos are small vesicles with a molecular diameter of about 40–160 nm (average about 100 nm) formed and secreted extracellularly by various cell types (Kalluri and LeBleu, 2020). Under physiological and pathological conditions, exos can carry proteins, lipids, and genetic material, mediate information exchange, and activate signaling pathways between parent and target cells. Additionally, exos are involved in the immune response, antigen presentation, inflammatory response, cell migration and differentiation, tumor invasion, apoptosis, and angiogenesis.
In CNS physiology, the release of exos is considered to be an important regulator of interactions between neurons, astrocytes, microglia, and oligodendrocytes. Exos act as an important carrier of CNS intercellular information, and they are involved in functional recovery and disease diagnosis. In addition, exos, as an important way for MSCs to exert paracrine effects and participate in tissue regeneration, are considered a novel and effective therapeutic route for the diagnosis and treatment of CNS injury (Keshtkar et al., 2018). In this section, we review studies showing MSC-Exos effects on microglial activation phenotypes.
The main characteristics of MSC-Exos
MSCs are multifunctional progenitor cells with self-renewal that originate from the early developmental mesoderm (Majumdar et al., 1998). In recent years, considerable attention has been given to the role of MSC-Exos in the brain. Unlike cell therapy, systemic administration of MSC-Exos delivers active ingredients of cell-based therapies to the CNS, avoiding immune rejection and reducing the risk of infection (Venkat et al., 2020). The small size of MSC-Exos reduces the risk of cerebrovascular embolism and provides a better therapeutic range. Haraszti et al. (2016) demonstrated that exos contain extracellular matrix proteins, heparin-binding proteins, receptors, immunoreactive proteins, and cell adhesion proteins. Exos also have the ability to penetrate the BBB in specific states of the organism, and Xu et al. demonstrated that inhibition of miR-132-containing exos in a zebrafish model increased BBB permeability and brain microvasculature (Xu et al., 2017). Similarly, Venkat et al. (2020) demonstrated that overexpression of miR-9-containing exos in an MCAO model increased BBB permeability and brain microvasculature. Furthermore, therapeutic molecules within MSC-Exos are protected by a natural lipid bilayer, which ensures stability, biocompatibility, low immunogenicity and the ability to overcome the body’s biological barriers (e.g., BBB) to reach the CNS (Rufino-Ramos et al., 2017).
Role of MSC-Exos in acute CNS diseases
In CNS lesions, MSC-Exos help maintain cellular homeostasis, clean up protein aggregates and other pathogenic factors in the nervous system, and play an important role in neuroprotection, regulation of synaptic activity, and microglial phenotypic transformation.
a. Anti-apoptosis
In oxygen and glucose deprivation-injured BV2 cells, exos derived from mouse bone marrow MSCs (BMSCs-Exo) could reduce microglial apoptosis by reducing the level of cell division protein kinase 6. In mice, tail vein injection of 200 μL of MSC-Exos effectively weakened the cerebral infarct volume in MCAO mice, reduced the ratio of cleaved-caspase 3/total Cleaved caspase 3 and cleaved-PARP/total PARP in brain tissue, and improved the brain injury induced by cerebral ischemia-reperfusion (I/R) injury (Cheng et al., 2021).
b. Immunomodulation
BMSC-Exos could reduce the inflammation induced by regulating the phenotype of Iba1+/CD32+ microglia in cerebral MCAO rats (in the presence of activated caspase-1 and positive propidium iodide staining) (Liu et al., 2021). During the pathological process of brain injury, cysteinyl leukotrienes (CysLTs) are produced in large quantities, and their receptor (mainly CysLT2R) is also up-regulated in reactive microglia and selectively activated by NMLTC4 (Wang et al., 2020). BMSC-Exos inhibit NMLTC4-induced phenotypic transformation of reactive CD86+ microglia to reduce brain injury by reversing the expression of CysLT2R-extracellular signal-related kinases (ERK) 1/2 (Zhao et al., 2020a).
Additionally, MSC-Exos could promote microglial phenotypic transformation from the inflammatory subtype to the neuroprotective subtype. For example, exos from adipose-derived MSCs (ADMSCs) could ameliorate TBI-induced angioedema and neuronal cell injury by regulating the number of Iba-1 and Arg-1 double-labeled microglia in lesions and increasing the secretion of the anti-inflammatory cytokine transforming growth factor (TGF)β and TNF-stimulated gene (TSG)-6 (Xu et al., 2020).
c. Neuroprotection and functional recovery
Twelve hours after TBI, BMSC-derived exos were shown to reduce the level of the pro-inflammatory cytokine IL-1β in a dose-dependent manner and alleviate CNS inflammation in mice; one month after TBI, they restored the rescue mode and spatial mode learning ability (Kim et al., 2016). Experiments using a catwalk system and the Morris water maze have shown long-term neuroprotective effects of spatial learning and memory functional recovery (Liu et al., 2021). The same methods have been used to demonstrate that tail vein injection of BMSC-Exos in TBI rats promoted the number of DCX (neonatal immature neuron marker)/BrdU+ (proliferation marker) and BrdU+/NeuN (mature neuron marker) neurons. This further improved spatial learning (Morris water maze) after TBI and modified neurological severity scores and reduced the number of activated GFAP+ astrocytes and CD68+ activated microglia and promoted neurological recovery (Zhang et al., 2015).
In an MCAO rat model induced with a modified nylon suture method, CD63+CD9+CD81+ BMSC-Exos could reduce neurological deficit scores, reduce the levels of TNF-α, IL-1β, and IL-6, increase amounts of B-cell CLL/lymphoma 2-associated X protein (Bax), and increase the number of Nissl bodies, which improved neurological and pathological changes and slowed neuronal apoptosis (Li et al., 2022). At 24 hours after transient MCAO induction, Xin et al. (2021) administered BMSC-Exos by tail vein injection in rats and found that it increased myelin formation and axonal remodeling in the ischemic boundary zone (IBZ) and promoted the recovery of symmetry defects caused by MCAO-induced unilateral brain injury. Similarly, BMSC-Exos downregulated levels of phosphatase and tensin homolog and activated the PI3K/Akt/mTOR inflammatory cascade signaling pathway, increased the density of axonal myelin bundles in the white matter of the IBZ, and significantly improved neurological function as well as neural plasticity and functional recovery after stroke (Xin et al., 2017). These results all support the neuroprotective effects of therapy with MSC-Exos, including functional recovery, reduction of cortical damage, reduction of apoptosis, reduction of the neuroinflammatory response, and modulation of cytokine release and microglia activation (Ni et al., 2019).
RNA molecules carried by MSCs-Exos and microglial phenotypic transformation
RNA molecules (mRNA, miRNA, lncRNAs, and snRNAs) are key to cell communication
Exos are now widely established as mediators of intercellular information exchange. Exos contain extracellular RNAs, including messenger RNAs (mRNAs) and many noncoding RNAs, including microRNAs (miRNAs), long noncoding RNAs (lncRNAs), circular RNAs (circRNAs), and small nuclear RNAs (snRNAs) (Yang et al., 2020). Exos protect RNAs from degradation by RNA enzymes, enable the delivery of RNA from parental to recipient cells, and regulate protein production in recipient cells or act as a template for protein production, playing a regulatory role in the maintenance of health and in disease progression (Gurunathan et al., 2019). With the gradual establishment of the competitive endogenous RNA hypothesis (Wang et al., 2019), the important roles of pseudogenes, lncRNAs, and circRNAs as competitive endogenous RNAs in the development of various diseases have been gradually elucidated (Wei et al., 2021). In addition to RNAs, exos also contain and deliver important membrane and cytoplasmic protein components, some of which are involved in RNA stabilization and translocation, translation, and transcription. Not only do the proteins and lipids they carry have direct biological activity and the mRNAs can be translated into proteins, but the miRNAs transferred are equally biologically active and can target and regulate mRNAs in recipient cells (Moghadasi et al., 2021; Figure 2).
Figure 2.
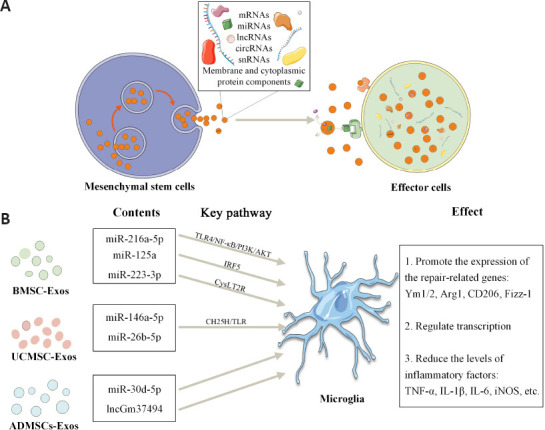
RNA molecules carried by mesenchymal stem cell-derived exosomes participate in the regulation of microglia.
(A) MSC-Exos contain and deliver important RNAs (including mRNAs, miRNAs, lncRNAs, circRNAs, snRNAs) and membrane and cytoplasmic protein components to effector cells in more than three ways: a) Exosomal membrane proteins can bind to target cell membrane proteins; b) Exosomal membrane proteins can be sheared in the extracellular matrix, and the fragments can act as ligands to bind to receptors; c) Exosome membranes can fuse directly with target cell membranes, releasing the contents. (B) Exosomes from different sources of MSCs play a role in regulating the microglial phenotype. Arg1: Arginase1; CH25H: cholesterol 25-hydroxylase; circRNA: circular RNA; Exos: exosomes; IRF: interferon regulatory factor; lncRNA: long noncoding RNA; miRNA: microRNA; mRNA: messenger RNA; MSCs: mesenchymal stem cells; NF-κB: nuclear factor kappa-B; snRNA: small nuclear RNA; TLR: Toll-like receptor.
The RNAs carried by MSC-Exos play an important role mainly by regulating CNS inflammation caused by microglia
Studies have shown that MSC-Exos mediate cellular communication by delivering informative substances such as proteins, lipids, and nucleic acids (Wei et al., 2021; Zhang et al., 2022). MSC-Exos contain high levels of miRNAs, and exosomal miRNAs are associated with several types of immune regulation, remodeling of the brain, and inhibition of neuroinflammatory responses (Lee et al., 2014; Harrell et al., 2021). The exos secreted by engineered MSCs can regulate the level of inflammation by transporting specific functional cargo, playing a beneficial role in regulating the phenotypes in microglia. It has been confirmed that miR-216a-5p (Liu et al., 2020), miR-125a (Chang et al., 2021), miR-146a-5p (Zhang et al., 2021), and miR-26b-5p (Li et al., 2020) in MSC-Exos could reduce the levels of inflammatory factors and pro-inflammatory microglia after acute CNS injury and have anti-inflammatory and nerve injury recovery effects. BMSCs are the most popular stem cells in the experimental treatment of CNS injury. At present, preclinical studies in animals have shown the advantages of BMSC-Exos in promoting functional recovery after spinal cord injury (SCI). Hypoxia-treated BMSC-Exos carrying miR-216a-5p mediated the expression of Arg1, CD206, and M1/M2 phenotypes in BV2 microglia by inhibiting the TLR4/NF-κB/PI3K/AKT inflammatory signaling cascade, promoting the recovery of function and behavior in contusion mice after SCI (Liu et al., 2020). Hypoxic preconditioning enhanced the release of BMSC-Exos and their uptake by microglia compared to normoxic conditions. BMSC-Exos carrying miR-125a reduced the levels of inflammatory factors IL-5, IL-13, CCL12, and TNF-α by inhibiting the expression of IRF-5, which promotes the upregulation of Arg1, Ym1, and fiz by microglia, and thus reduces SCI (Chang et al., 2021). Similarly, human umbilical cord (hUC)-MSC-Exos reduced microglia-mediated inflammation in the brains of MCAO mice by delivering miR-146a-5p to microglia and subsequently regulating microglial gene expression (Zhang et al., 2021). hUC-MSC-Exos miR-26b-5p prevented microglia from being overactivated and reduced the number of microglia expressing inducible nitric oxide synthase (iNOS) by targeting cholesterol 25-hydroxylase to inhibit the TLR pathway, thereby attenuating CNS inflammation after brain I/R injury (Li et al., 2020).
Furthermore, MSC-Exos can be designed to overexpress specific miRNAs that can be delivered in vivo for specific therapeutic targeting. MSC-Exos inhibit autophagy and reduce the production of pro-inflammatory factors by reversing the pro-inflammatory phenotype of microglia in the ischemic core, thereby reducing brain injury after MCAO. MSC-Exos can remain in the ischemic area for a long time, improving behavioral and functional recovery after injury. Exposure of microglia to OGD and CysLTs increased CD16/32+ microglia, whereas overexpression of miR-223-3p in BMSC-Exos increased CD206+ microglia, reversed the secretion of NMLTC4-induced pro-inflammatory factors, and downregulated the transcription and expression of CysLT2R, thereby reducing inflammation-mediated cerebral ischemia (Zhao et al., 2020b). Transfection of miR-30d-5p overexpression mimics in ADMSCs reversed OGD-induced and autophagy-mediated microglial secretion of the inflammatory factors TNF-α, IL-6, and iNOS, showing promising therapeutic effects on IS-induced neuroinflammatory injury (Jiang et al., 2018). ADSCs-Exos under hypoxic conditions are more effective than normal ADSCs-Exos in promoting neuronal functional recovery and alleviating CNS inflammatory responses after SCI in vivo. In addition, Shao et al. (2020) demonstrated that the ADSCs-Exos modified by lncGm37494 could more effectively inhibit the expression of lipopolysaccharide-induced inflammatory factors and reduce the ratio of iNOS+ microglia. The regulatory effects of lncExos therapy on microglial phenotypes needs to be further elucidated (Table 2).
Table 2.
Summary of studies in animal or clinical trials of MSCs-Exos modulating microglia phenotypic transformation in acute CNS injury
| Stem cell types | Animal/human studies | Disease models | Routes of administration | Treatment days | Efficacy | Genes and/or pathways of interest | References |
|---|---|---|---|---|---|---|---|
| AD-MSCs (hypoxic pre-treatment; human) | Rat | TBI | Tail vein injection | Three, 7, and 14 days after TBI | • Promote transformation of Iba-1 and Arg1 double-labeled microglia cell • Reduce neuroinflammation and immune response • Reduce cell apoptosis |
IL-6, TNF-α, TGF-β, TSG-6, and NF-κB P65 | Xu et al., 2020 |
| BM-MSCs (hypoxic pPre-treatment; mice) | Mice | SCI | Tail vein injection | Twenty-eight days after SCI | • Promote phenotypic transformation of Arg1+, CD206+ and YM1/2+ microglia • Promoting the functional and behavioral recovery of mice after SCI • Inhibition of neuroinflammation • BM-MSCs-Exos-carried miR-216a-5p promoted the transformation of microglia phenotype by inhibiting the activity of TLR4 |
TLR4, NF-κB, PI3K, AKT, and miR-216a-5p | Liu et al., 2020b |
| BM-MSCs (rat) | Rat | MCAO | Tail vein injection | Twenty-eight days after SCI | • Promote phenotypic transformation of CD16/32+ microglia • Reduction of cerebral ischaemia/reperfusion injury • Inhibition of neuroinflammation. • BM-MSCs-Exos-carried miR-223-3p inhibited microglia CD206+ phenotypic polarization by inhibiting CysLT |
CysLT, CysLT2R, and miR-125a | Zhao et al., 2020b |
| MSCs (rat) | Rat | MCAO | Tail vein injection | Seven days after MCAO | • Promote phenotypic transformation of CD206+ microglia • Reduction of cerebral ischaemia/reperfusion injury • Inhibition of neuroinflammation • MSC-Exos inhibits CysLT2R-ERK1/2-mediated CD86+ phenotype transformation in microglia |
CysLT, CysLT2R, and ERK | Zhao et al., 2020a |
| BM-MSCs (rat) | Rat | MCAO | Tail vein injection | Five weeks after stroke | • Promote phenotypic transformation of Iba1 /CD206+ microglia • BM-MSC-Exos improves neurological function after stroke • Relieved neuroinflammation and scorching |
NLRP3, Cl.caspase-1, IL-1β, and IL-18 | Liu et al., 2021 |
| hUC-MSCs (human) | Mice | I/R | Tail vein injection | Unspecified | • Promote phenotypic transformation of Arg1+ microglia • hUC-MSCs-Exos attenuate I/R-induced phenotypic activation and inflammatory response in iNOS+ microglia • hUCMSCs-exosomal miR-26b-5p reverses brain injury-induced CH25H expression and suppresses neuroinflammatory responses |
IL-6, CCL-2, TNF-α, iNOS, CH25H, miR-26b-5p, TLR-2, TLR-4, and TLR-6 | Li et al., 2020 |
| AD-MSCs (rat) | Human, Rat | IS; OGD | Tail vein injection | Three days after IS | • Reduce neuroinflammation and immune response • Low expression of miR-30d-5p in patients with IS • Therapeutic effect of AD-MSCs-Exos on OGD-induced inflammatory responses in primary microglia |
IL-4, IL-10, IL-6, TNF-α, miR-30d-5p, and iNOS | Jiang et al., 2018 |
| BM-MSCs (rat) | Rat | SCI | Intrathecal injection | Unspecified | • Promote phenotypic transformation of CD206+ microglia • MiR-125a carried by BM-MSCs-Exos reduced the SCI-induced neuroinflammatory response by inhibiting the expression of IRF5. |
Ym1, Arg1, Fizz, CD206, miR-125a, and IRF5 | Chang et al., 2021 |
| AD-MSCs (hypoxic pre-treatment; mice) | Mice | SCI | Tail vein injection | Twenty-eight days after SCI | • Promote phenotypic transformation of Iba1 /CD206+ microglia • Hypoxia-pretreated AD-MSCs-Exos effectively repaired SCI by delivery of lncGm37494. |
LncGm37494 and miR-130b-3p | Shao et al., 2020 |
| hUC-MSCs (human) | Mice | MCAO | Tail vein injection | Three days after cerebral ischemia | • Promote phenotypic transformation of CD206+ microglia • HU-MSC-Exos treatment significantly reverses OGD-activated microglia-mediated inflammatory responses |
miR-146a-5p, IL-6, NF-κB, IRAK1, and TRAF6 | Zhang et al., 2021 |
AD: Adipose-derived; BM: bone marrow; CH25H: Cholesterol 25-hydroxylase; CysLT2R: cysteinyl leukotriene receptor 2; ERK: extracellular regulated protein kinases; Exos: exosomes; I/R: ischemia/reperfusion; IL: interleukin; IRF: interferon regulatory factor; IS: ischemic stroke; MCAO: middle cerebral artery occlusion; MSCs: mesenchymal stem cells; NF-κB: nuclear factor kappa-B; OGD: oxygen and glucose deprivation; SCI: spinal cord injury; TBI: traumatic brain injury; TGF: transforming growth factor; TLR: Toll-like receptor; TNF-α: tumor necrosis factor-α; TSG: tumor necrosis factor α stimulating gene; UC: umbilical cord.
Specific molecular mechanisms by which MSC-Exos regulate microglial phenotypic transformation
MSCs are able to change their cellular properties and functions according to their environment and respond to changes in the immune microenvironment to maintain its stability (Liu et al., 2016). After CNS injury, the activation of immune/inflammatory cells releases inflammatory mediators, such as TNF-α and IL-1β, which rapidly change the microenvironment (Ma et al., 2014). MSCs are activated and migrate toward the lesion site, and their paracrine exos exert immunosuppressive effects and control the inflammatory process (Ma et al., 2014). At the same time, proinflammatory factors released from microglia enhance the ability of MSCs to produce inflammatory cytokines, which, in turn, alter the activation state of microglia (Rahmat et al., 2013). This suggests that a feedback loop may exist between endogenous MSCs and the regulation of their immune function by microglia.
It is thought that MSC-Exos regulate microglial activation mainly through three pathways: prostaglandin E2 (PGE2), TSG-6 (Prockop, 2013), and the glucocorticoid (GR) progesterone receptors (PR) (Schumacher et al., 2014). MSCs can be activated by proinflammatory signals and release PGE2, which drives a shift from the proinflammatory microglial phenotype to the anti-inflammatory phenotype (Prockop, 2013). Activated MSCs secrete TSG-6, which interacts with CD44 on microglia to reduce TLR2/NF-κB signaling, thereby reducing the secretion of proinflammatory mediators. Evidence has shown that TLRs play vital roles in cerebral ischemic injury. For example, TLR-2 is highly expressed in the brain, and TLR-2 deficiency helps to reduce hypoxia-ischemia injury in the brains of mice (Stridh et al., 2011). In addition, TLR-4 agonists may enhance the clearance of damaged tissues and abnormal protein aggregates associated with several different CNS diseases by upregulating the phagocytic activity of microglia (Leitner et al., 2019). A negative feedback loop between PGE2 and TSG-6 allows MSCs to act as modulators of the early stages of inflammation. The third pathway is linked by the microglial surface receptors GR and PR, which determine the phenotypic transformation of microglia (Abumaree et al., 2013). GR belongs to a superfamily of steroid receptors and functions as a ligand-dependent transcription factor (Vandevyver et al., 2014). Some evidence has shown that the over-activation of microglia could be significantly inhibited by the activation of microglial GR, which could protect dopaminergic neurons in the substantia nigra and regulate inflammation by regulating the transcriptional activity of NF-κB and AP-1 in microglia (Maatouk et al., 2018; Figure 3).
Figure 3.
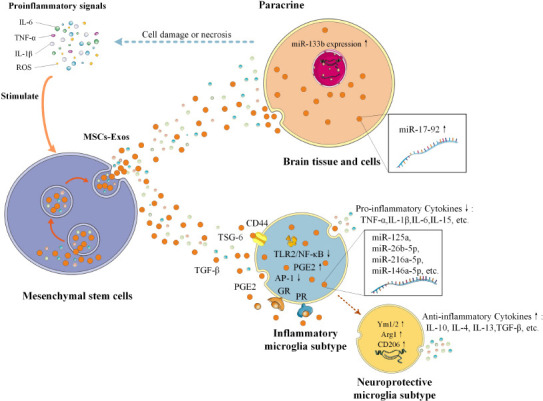
Mesenchymal stem cells regulate inflammation through multiple pathways.
MSCs can be activated by proinflammatory signals and regulate inflammation in two ways by secreting MSC-Exos: A) Acting directly on brain cells through paracrine signaling; B) Activated MSCs produce TSG-6, PGE2, TGFβ, GR, PR, and MSC-Exos with high expression of miR-125a, miR-26b-5p, miR-216a-5p, and miR-146a-5p to influence microglia subtype; Arg1: Arginase1; Exos: exosomes; GR: glucocorticoid receptor; IL: interleukin; miR: microRNA; MSCs: mesenchymal stem cells; NF-κB: nuclear factor kappa-B; PGE2: prostaglandin E2; PR: progesterone receptor; ROS: reactive oxygen species; TGF: transforming growth factor; TLR: Toll-like receptor; TNF-α: tumor necrosis factor-α; TSG: tumor necrosis factor α stimulating gene.
Therapeutic Effects and Prospects of Pretreated or Genetically Modified Mesenchymal Stem Cells
Regulation of MSCs in CNS inflammation
Optimizing the properties of MSC-Exos without modifying the original cell phenotype may increase their efficacy. For example, the in vivo hypoxic microenvironment under physiological conditions (≤ 2–8% O2) is different from the oxygen concentration in in vitro media and often does not mimic real in vivo hypoxic microenvironments. Recent studies have found that hypoxic pretreatment of MSCs can significantly enhance their biological function and activity, thus improving the transplant efficacy of MSCs in treating various diseases (Hu et al., 2016; Liu et al., 2020). A recent study reported that miR-216a-5p in hypoxic MSC-Exos played a key role in regulating microglial phenotypic transformation through inhibition of TLR-4/NF-κB and activation of the PI3K/AKT signaling pathway (Liu et al., 2020). Exos secreted by ADMSCs pretreated with hypoxia (1% O2) significantly inhibited inflammatory cytokines and promoted phenotypic changes of Arg1+ microglia, more effectively promoting SCI functional recovery than untreated exos (21% O2) (Shao et al., 2020). In MSC-Exos grown in ischemic medium (0% FBS, 1% O2) similar to that used for peripheral arterial disease, foreign bodies were found to contain many proangiogenic factors that may be beneficial to ischemic tissue (Anderson et al., 2016).
The regulatory effects of genetically modified MSCs on CNS inflammation
The therapeutic effects of MSCs are limited, and, in some cases, the anti-inflammatory functions of primitive MSCs are not sufficient to rescue neuroinflammatory damage. Due to the restrictive nature of the BBB, brain endothelial cells must undergo complex transcytosis to deliver macromolecules to the brain; otherwise, ligands and/or receptors are directed to the wrong plasma membranes and degraded, and specificity can be increased and controlled by modification of surface ligands. In protein and peptidomimetic mediated routes of administration, drugs are designed to target specific receptors on cells, such as surface coatings with biocompatible polymers, surface net charge modification, and the addition of brain-specific ligands and cell-penetrating peptides to obtain the ability to pass barriers or concentrate in target organs (Azarmi et al., 2020). For example, rabies virus glycoprotein-modified exos could effectively deliver miR-124 to infarct sites, and intravenous administration of rabies virus glycoprotein-exos loaded with miR-124 promoted cortical neural progenitor cells to gain neuronal recognition and attenuate ischemic brain injury through cortical neurogenesis (Yang et al., 2017a). In an MCAO mouse model, administration of RGD-C1c2 (a recombinant fusion protein of RGD-4c peptide [ACDCRGDCFC] and C1C2 [RGD-C1C2]) modified EVReN (RGD-EVReN) and then targeted high-level integrin αvβ3 in the diseased area of the ischemic brain effectively inhibiting post-stroke inflammation (Tian et al., 2021). The cyclo (Arg-Gly-ASP-D-Tyr-Lys) peptide (C[RGDYK])-conjugated MSC-Exos (RGD-Exos) have been demonstrated to improve ischemic stroke by intravenously delivering loaded miR-210 to the ischemic mouse brain (Zhang et al., 2019a). In addition, to improve the targeting properties of exos, curcumin has been loaded onto RGD-Exos, which effectively penetrates the BBB, inhibiting inflammatory responses and apoptosis in a mouse model of MCAO (Tian et al., 2018). In a rat model of TBI, MSCs genetically modified to overexpress the anti-inflammatory cytokine IL-10 showed enhanced immunomodulatory function and reduced cell death and CNS inflammatory markers along with superior functional recovery. By modifying these cells to optimize their therapeutic properties in the inflamed brain, it is possible to improve the use of these cells as a treatment for CNS brain injury (Cho et al., 2009). Cell-specific tropism, low immunogenicity, adeno-associated viruses, encapsulated protein nanocages, and exosome-mimetic nanovesicles are also seen as promising exos delivery systems for the treatment of CNS injury (Rufino-Ramos et al., 2017).
Multiple studies over the past decade have shown that MSC transplantation treatment and paracrine effects could ameliorate a variety of acute CNS injuries in a variety of rodent models. From animal studies to clinical trials, a growing body of data has suggested that MSCs can mediate microglia phenotypic transformation to ameliorate acute neuroinflammatory responses to CNS injury and exert neuroprotective and reparative effects.
Conclusions
Patients who survive CNS injury are often chronically disabled due to extensive neurological deficits and impaired tissue function, and there is an urgent need for new therapeutic approaches to improve the prognosis of patients with acute CNS injury. MSCs have been shown to alter the immune microenvironment through the release of various paracrine cytokines. Due to their nanometer size, biosafety, ability to cross the BBB, potential for targeted delivery, and lack of immune response, exos are promising carriers for the treatment of acute CNS injuries and drug delivery to the brain. A recent/ongoing phase-II clinical trial (Identifier number NCT03384433) aimed to investigate the therapeutic effects of allogenic MSC-Exos in patients with acute IS. Intranasal administration of MSC-Exos has been evaluated as a noninvasive and effective route to reach the brain (Long et al., 2017). MSC-Exos are expected to fill this therapeutic gap based on their potent short-term neuroprotective effects as well as their long-term neuroregenerative and immunomodulatory effects. Given the role of neuroprotective microglia in promoting specific brain injury and neuroinflammatory repair, pharmacotherapeutic strategies that alter the phenotypic and functional responses of microglia after brain injury offer considerable promise as new therapeutic options for acute CNS injury.
In addition, some drugs, such as metformin and azithromycin, induce activated microglia toward neuroprotective phenotypes in both in vitro and in vivo long-term stroke models (Petrelli et al., 2016; Kodali et al., 2021). Rosiglitazone and pioglitazone decreased the activity of MAPKs and NF-κB after stroke, reduced the size of cerebral infarcts and edema, and exerted neuroprotective effects in different animal models of stroke (Yeh et al., 2021). Induced pluripotent stem cell (iPSC)-derived MSCs (iMSCs) are a promising cell source for autologous cell therapy in regenerative medicine (Hu et al., 2015a). iPSCs are somatic cells reprogrammed to be pluripotent (Lee et al., 2017). One significant advantage over MSCs is that they can produce an unlimited number of early stage, patient-specific iMSCs and are of stable quality (Hu et al., 2015a). In an ICH rat model, the transplantation of iPSCs derived from patients with ICH was found to significantly improve cranial nerve function (Qin et al., 2013; Gao et al., 2018). iMSC-Exos have been shown to support angiogenesis and ameliorate ischemic injury following intramuscular injection into the ischemic limb of a mouse model (Hu et al., 2015a). Intracranial injection of 1.2 × 106 engineered hyaluronic acid (HA) hydrogel-assisted iPSC-MSC cells effectively inhibited the expression of inflammatory proteins in brain tissue of ICH rats and reduced infarct volumes (Chen et al., 2019). Similar to iPSCs, embryonic stem cells have regenerative properties and, in particular, a strong ability to secrete exos (Bi et al., 2022). Webb et al. (2018) studied the therapeutic potential of MSC-Exos of H9 pluripotent stem cells in an MCAO mouse model, finding poor effects in terms of tissue and functional recovery from acute cerebral infarction. Although studies have demonstrated the clinical potential of pluripotent stem cells in the treatment of CNS disorders, the potential mechanisms of ESC/iPSC-MSC-Exos therapy for improving the prognosis of CNS-injured animals remains uncertain. In addition, there is still a need to overcome the inefficient reprogramming of pluripotent cells and induce their stable differentiation before widespread application.
Exos are a key intercellular communication device. MSC-Exos avoid the high immunogenicity and teratogenicity of stem cell transplantation, and have an especially protective effect in craniocerebral diseases. However, the molecular mechanisms of the associated proteins and miRNAs they carry in regulating the phenotypes of reactive microglia remains to be elucidated. Until recently, response-related gene expression and motility in microglia have been gradually revealed. For example, two-photon microscopy can visualize physiological process in microglia in vivo. Analysis of mononuclear gene expression profiles may be a key driver for revealing microglia activation under neuroinflammatory conditions. Cell characterization (antibody/fluorescent protein targeting) techniques are used to explain the relationships between microglia heterogeneity and the communication of surrounding cells. Positron emission tomography can help us understand microglial activation in brain diseases with a neuroinflammatory component. Although the use of multiple modern technologies has provided breakthroughs in our understanding of microglial phenotypes, there is still a lack of comprehensive understanding on the diversity of microglial responses after brain inflammation. In addition, many aspects still need to be explored, such as finding predictive animal models that better reflect the pathological processes of human brain injury, improving the timing of therapeutic interventions, and cell type-specific miRNA systems in experimental conditions (exposure to hypoxia or normoxia). Whether MSC-Exos can be applied clinically still needs further exploration regarding the effectiveness and sensitivity of standardized dose administration, pharmacokinetics, and biological distribution. Furthermore, in order to prolong the research cycle of MSC-Exos therapy, it is necessary to investigate how to improve yield and purity so that they can be compared for maximum therapeutic effects. Another problem may be related to the uptake capacity of microglia. The uptake of large numbers of MSC-Exos by target cells may enhance their neuroprotective effects while maintaining the stability, integrity, and biological potency of these factors. The CNS inflammatory environment can be mimicked in vitro, such as by using pre-treatment of MSCs with inflammatory factors or genetic modification for specific mechanisms of microglial pro-inflammatory response signaling and its phenotypes. This can also be done by using exos secreted by transfected modified MSCs or by loading specific miRNAs into MSC-Exos that can be delivered in vivo to specifically target brain inflammation.
Acknowledgments:
Figures were modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 3.0 Generic License (https://creativecommons.org/licenses/by/3.0/). All the contents of the figures were created with Microsoft ® Visio ® 2019MSO (Version 2209) by the first author.
Footnotes
Conflicts of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Abd El-Rahman SS, Fayed HM. Improved cognition impairment by activating cannabinoid receptor type 2: Modulating CREB/BDNF expression and impeding TLR-4/NFκBp65/M1 microglia signaling pathway in D-galactose-injected ovariectomized rats. PLoS One. (2022);17:e0265961. doi: 10.1371/journal.pone.0265961. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Abumaree M, Al Jumah M, Kalionis B, Jawdat D, Al Khaldi A, Abomaray F, Fatani A, Chamley L, Knawy B. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev Rep. (2013);9:620–641. doi: 10.1007/s12015-013-9455-2. [DOI] [PubMed] [Google Scholar]
- 3.Al Mamun A, Chauhan A, Qi S, Ngwa C, Xu Y, Sharmeen R, Hazen AL, Li J, Aronowski JA, McCullough LD. Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc Natl Acad Sci U S A. (2020);117:1742–1752. doi: 10.1073/pnas.1914742117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, Montgomery EN, Mellema MS, Bardini RL, Contreras Z. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells. (2016);34:601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azarmi M, Maleki H, Nikkam N, Malekinejad H. Transcellular brain drug delivery:a review on recent advancements. Int J Pharm. (2020);586:119582. doi: 10.1016/j.ijpharm.2020.119582. [DOI] [PubMed] [Google Scholar]
- 6.Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI, Mook-Jung I. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer's disease. Cell Metab. (2019);30:493–507. doi: 10.1016/j.cmet.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Barichello T, Generoso JS, Goularte JA, Collodel A, Pitcher MR, Simões LR, Quevedo J, Dal-Pizzol F. Does infection-induced immune activation contribute to dementia? Aging Dis. (2015);6:342–348. doi: 10.14336/AD.2015.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi Y, Qiao X, Liu Q, Song S, Zhu K, Qiu X, Zhang X, Jia C, Wang H, Yang Z, Zhang Y, Ji G. Systemic proteomics and miRNA profile analysis of exosomes derived from human pluripotent stem cells. Stem Cell Res Ther. (2022);13:449. doi: 10.1186/s13287-022-03142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capiralla H, Vingtdeux V, Zhao H, Sankowski R, Al-Abed Y, Davies P, Marambaud P. Resveratrol mitigates lipopolysaccharide-and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J Neurochem. (2012);120:461–472. doi: 10.1111/j.1471-4159.2011.07594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang Q, Hao Y, Wang Y, Zhou Y, Zhuo H, Zhao G. Bone marrow mesenchymal stem cell-derived exosomal microRNA-125a promotes M2 macrophage polarization in spinal cord injury by downregulating IRF5. Brain Res Bull. (2021);170:199–210. doi: 10.1016/j.brainresbull.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Zheng ZV, Lu G, Chan WY, Zhang Y, Wong GKC. Microglia activation, classification and microglia-mediated neuroinflammatory modulators in subarachnoid hemorrhage. Neural Regen Res. (2022);17:1404–1411. doi: 10.4103/1673-5374.330589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen KH, Lin KC, Wallace CG, Li YC, Shao PL, Chiang JY, Sung PH, Yip HK. Human induced pluripotent stem cell-derived mesenchymal stem cell therapy effectively reduced brain infarct volume and preserved neurological function in rat after acute intracranial hemorrhage. Am J Transl Res. (2019);11:6232. [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng C, Chen X, Wang Y, Cheng W, Zuo X, Tang W, Huang W. MSCs-derived exosomes attenuate ischemia-reperfusion brain injury and inhibit microglia apoptosis might via exosomal miR-26a-5p mediated suppression of CDK6. Mol Med. (2021);27:67. doi: 10.1186/s10020-021-00324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho SW, Sun HJ, Yang J-Y, Jung JY, An JH, Cho HY, Choi HJ, Kim SW, Kim SY, Kim D. Transplantation of mesenchymal stem cells overexpressing RANK-Fc or CXCR4 prevents bone loss in ovariectomized mice. Mol Ther. (2009);17:1979–1987. doi: 10.1038/mt.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. (2017);35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunha C, Gomes C, Vaz AR, Brites D. Exploring new inflammatory biomarkers and pathways during LPS-induced M1 polarization. Mediators Inflamm. (2016);2016:6986175. doi: 10.1155/2016/6986175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan W-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. (2005);8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 18.Fann DY, Lim YA, Cheng YL, Lok KZ, Chunduri P, Baik SH, Drummond GR, Dheen ST, Sobey CG, Jo DG, Chen CL, Arumugam TV. Evidence that NF-κB and MAPK signaling promotes NLRP inflammasome activation in neurons following ischemic stroke. Mol Neurobiol. (2018);55:1082–1096. doi: 10.1007/s12035-017-0394-9. [DOI] [PubMed] [Google Scholar]
- 19.Farahani F, Khaksari M, Amiresmaili S, Iranpour M, Shahrokhi N, AsadiKaram G, Soltani Z. Possible involvement of female sex steroid hormones in intracellular signal transduction mediated by cytokines following traumatic brain injury. Brain Res Bull. (2022);178:108–119. doi: 10.1016/j.brainresbull.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, Culpepper WJ, Dorsey ER, Elbaz A, Ellenbogen RG. Global, regional, and national burden of neurological disorders 1990–2016:a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019);18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman BA, Srinivasan K, Ayalon G, Meilandt WJ, Lin H, Huntley MA, Cao Y, Lee S-H, Haddick PC, Ngu H. Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of Alzheimer's disease not evident in mouse models. Cell Rep. (2018);22:832–847. doi: 10.1016/j.celrep.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 22.Gao HM, Hong JS. Why neurodegenerative diseases are progressive:uncontrolled inflammation drives disease progression. Trends Immunol. (2008);29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L, Xu W, Li T, Chen J, Shao A, Yan F, Chen G. Stem cell therapy:a promising therapeutic method for intracerebral hemorrhage. Cell Transplant. (2018);27:1809–1824. doi: 10.1177/0963689718773363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Revilla J, Alonso-Bellido IM, Burguillos MA, Herrera AJ, Espinosa-Oliva AM, Ruiz R, Cruz-Hernández L, García-Domínguez I, Roca-Ceballos MA, Santiago M. Reformulating pro-oxidant microglia in neurodegeneration. J Clin Med. (2019);8:1719. doi: 10.3390/jcm8101719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerrits E, Heng Y, Boddeke EW, Eggen BJ. Transcriptional profiling of microglia;current state of the art and future perspectives. Glia. (2020);68:740–755. doi: 10.1002/glia.23767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillen KM, Mubarak M, Nguyen TD, Pitt D. Significance and in vivo detection of iron-laden microglia in white matter multiple sclerosis lesions. Front Immunol. (2018);9:255. doi: 10.3389/fimmu.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goshi N, Morgan RK, Lein PJ, Seker E. A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation. J Neuroinflammation. (2020);17:155. doi: 10.1186/s12974-020-01819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. (2019);8:307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. (2016);5:32570. doi: 10.3402/jev.v5.32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrell CR, Volarevic A, Djonov V, Volarevic V. Mesenchymal stem cell-derived exosomes as new remedy for the treatment of neurocognitive disorders. Int J Mol Sci. (2021);22:1433. doi: 10.3390/ijms22031433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. (2013);16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. (2015a);6:10. doi: 10.1186/scrt546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. (2012);43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 34.Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. (2015b);11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu X, Xu Y, Zhong Z, Wu Y, Zhao J, Wang Y, Cheng H, Kong M, Zhang F, Chen Q. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates:paracrine activity without remuscularization. Circ Res. (2016);118:970–983. doi: 10.1161/CIRCRESAHA.115.307516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji J, Xue TF, Guo XD, Yang J, Guo RB, Wang J, Huang JY, Zhao XJ, Sun XL. Antagonizing peroxisome proliferator-activated receptor γfacilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway. Aging Cell. (2018);17:e12774. doi: 10.1111/acel.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X. Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem. (2018);47:864–878. doi: 10.1159/000490078. [DOI] [PubMed] [Google Scholar]
- 38.Juknat A, Gao F, Coppola G, Vogel Z, Kozela E. miRNA expression profiles and molecular networks in resting and LPS-activated BV-2 microglia-Effect of cannabinoids. PLoS One. (2019);14:e0212039. doi: 10.1371/journal.pone.0212039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. (2020);367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles:novel frontiers in regenerative medicine. Stem Cell Res Ther. (2018);9:63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YR, Kim YM, Lee J, Park J, Lee JE, Hyun YM. Neutrophils return to bloodstream through the brain blood vessel after crosstalk with microglia during LPS-induced neuroinflammation. Front Cell Dev Biol. (2020);8:613733. doi: 10.3389/fcell.2020.613733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kodali M, Attaluri S, Madhu LN, Shuai B, Upadhya R, Gonzalez JJ, Rao X, Shetty AK. Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus. Aging Cell. (2021);20:e13277. doi: 10.1111/acel.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar A, Singh D. Heat stroke-related deaths in India: An analysis of natural causes of deaths, associated with the regional heatwave. J Therm Biol. (2021);95:102792. doi: 10.1016/j.jtherbio.2020.102792. [DOI] [PubMed] [Google Scholar]
- 44.Lee HK, Finniss S, Cazacu S, Xiang C, Brodie C. Mesenchymal stem cells deliver exogenous miRNAs to neural cells and induce their differentiation and glutamate transporter expression. Stem Cells Dev. (2014);23:2851–2861. doi: 10.1089/scd.2014.0146. [DOI] [PubMed] [Google Scholar]
- 45.Lee WH, Chen WY, Shao NY, Xiao D, Qin X, Baker N, Bae HR, Wei TT, Wang Y, Shukla P. Comparison of non-coding RNAs in exosomes and functional efficacy of human embryonic stem cell-versus induced pluripotent stem cell-derived cardiomyocytes. Stem Cells. (2017);35:2138–2149. doi: 10.1002/stem.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leitner GR, Wenzel TJ, Marshall N, Gates EJ, Klegeris A. Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders. Expert Opin Ther Targets. (2019);23:865–882. doi: 10.1080/14728222.2019.1676416. [DOI] [PubMed] [Google Scholar]
- 47.Li G, Xiao L, Qin H, Zhuang Q, Zhang W, Liu L, Di C, Zhang Y. Exosomes-carried microRNA-26b-5p regulates microglia M1 polarization after cerebral ischemia/reperfusion. Cell Cycle. (2020);19:1022–1035. doi: 10.1080/15384101.2020.1743912. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Li X, Bi T, Yang S. Exosomal microRNA-150-5p from bone marrow mesenchymal stromal cells mitigates cerebral ischemia/reperfusion injury via targeting toll-like receptor 5. Bioengineered. (2022);13:3030–3043. doi: 10.1080/21655979.2021.2012402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Kuwabara A, Kamio Y, Hu S, Park J, Hashimoto T, Lee JW. Human mesenchymal stem cell-derived microvesicles prevent the rupture of intracranial aneurysm in part by suppression of mast cell activation via a PGE2-dependent mechanism. Stem Cells. (2016);34:2943–2955. doi: 10.1002/stem.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Nolte K, Brook G, Liebenstund L, Weinandy A, Höllig A, Veldeman M, Willuweit A, Langen KJ, Rossaint R, Coburn M. Post-stroke treatment with argon attenuated brain injury, reduced brain inflammation and enhanced M2 microglia/macrophage polarization:a randomized controlled animal study. Crit Care. (2019a);23:198. doi: 10.1186/s13054-019-2493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Q, Zhang Y, Liu S, Liu Y, Yang X, Liu G, Shimizu T, Ikenaka K, Fan K, Ma J. Cathepsin C promotes microglia M1 polarization and aggravates neuroinflammation via activation of Ca2+-dependent PKC/p38MAPK/NF-κB pathway. J Neuroinflammation. (2019b);16:10. doi: 10.1186/s12974-019-1398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu R, Liao XY, Tang JC, Pan MX, Chen SF, Lu PX, Lu LJ, Zhang ZF, Zou YY, Bu LH, Qin XP, Wan Q. BpV(pic) confers neuroprotection by inhibiting M1 microglial polarization and MCP-1 expression in rat traumatic brain injury. Mol Immunol. (2019c);112:30–39. doi: 10.1016/j.molimm.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Liu W, Li R, Yin J, Guo S, Chen Y, Fan H, Li G, Li Z, Li X, Zhang X, He X, Duan C. Mesenchymal stem cells alleviate the early brain injury of subarachnoid hemorrhage partly by suppression of Notch1-dependent neuroinflammation:involvement of Botch. J Neuroinflammation. (2019d);16:8. doi: 10.1186/s12974-019-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, Jiang D, Gong F, Li L, Chen J, Zhao S, Kong F, Gu C, Fan J, Cai W. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. (2020);17:47. doi: 10.1186/s12974-020-1726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Zhang M, Liu H, Zhu R, He H, Zhou Y, Zhang Y, Li C, Liang D, Zeng Q. Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp Neurol. (2021);341:113700. doi: 10.1016/j.expneurol.2021.113700. [DOI] [PubMed] [Google Scholar]
- 56.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. (2010);7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, Prockop DJ, Shetty AK. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A. (2017)(114):E3536–3545. doi: 10.1073/pnas.1703920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo L, Song S, Ezenwukwa CC, Jalali S, Sun B, Sun D. Ion channels and transporters in microglial function in physiology and brain diseases. Neurochem Int. (2021);142:104925. doi: 10.1016/j.neuint.2020.104925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. (2014);21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maas AI, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM. Traumatic brain injury:integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. (2017);16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 61.Maatouk L, Compagnion AC, Sauvage MC, Bemelmans AP, Leclere-Turbant S, Cirotteau V, Tohme M, Beke A, Trichet M, Bazin V, Trawick BN, Ransohoff RM, Tronche F, Manoury B, Vyas S. TLR9 activation via microglial glucocorticoid receptors contributes to degeneration of midbrain dopamine neurons. Nat Commun. (2018);9:2450. doi: 10.1038/s41467-018-04569-y. Erratum in: Nat Commun. 2018;9:3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Majdan M, Plancikova D, Brazinova A, Rusnak M, Nieboer D, Feigin V, Maas A. Epidemiology of traumatic brain injuries in Europe:a cross-sectional analysis. Lancet Public Health. (2016);1:e76–83. doi: 10.1016/S2468-2667(16)30017-2. [DOI] [PubMed] [Google Scholar]
- 63.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. (1998);176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 64.Mallah K, Couch C, Borucki DM, Toutonji A, Alshareef M, Tomlinson S. Anti-inflammatory and neuroprotective agents in clinical trials for CNS disease and injury:where do we go from here? Front Immunol. (2020);11:2021. doi: 10.3389/fimmu.2020.02021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mecha M, Feliú A, Carrillo-Salinas FJ, Rueda-Zubiaurre A, Ortega-Gutiérrez S, de Sola RG, Guaza C. Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun. (2015);49:233–245. doi: 10.1016/j.bbi.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Moghadasi S, Elveny M, Rahman HS, Suksatan W, Jalil AT, Abdelbasset WK, Yumashev AV, Shariatzadeh S, Motavalli R, Behzad F, Marofi F, Hassanzadeh A, Pathak Y, Jarahian M. A paradigm shift in cell-free approach:the emerging role of MSCs-derived exosomes in regenerative medicine. J Transl Med. (2021);19:302. doi: 10.1186/s12967-021-02980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ni H, Yang S, Siaw-Debrah F, Hu J, Wu K, He Z, Yang J, Pan S, Lin X, Ye H. Exosomes derived from bone mesenchymal stem cells ameliorate early inflammatory responses following traumatic brain injury. Front Neurosci. (2019);13:14. doi: 10.3389/fnins.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. (2005);308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 69.Petrelli F, Muzzi M, Chiarugi A, Bagetta G, Amantea D. Poly (ADP-ribose) polymerase is not involved in the neuroprotection exerted by azithromycin against ischemic stroke in mice. Eur J Pharmacol. (2016);791:518–522. doi: 10.1016/j.ejphar.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 70.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DW, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. (2015);6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pollak TA, Drndarski S, Stone JM, David AS, McGuire P, Abbott NJ. The blood-brain barrier in psychosis. Lancet Psychiatry. (2018):79–92. doi: 10.1016/S2215-0366(17)30293-6. [DOI] [PubMed] [Google Scholar]
- 72.Prockop DJ. Concise review:two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. (2013);31:2042–2046. doi: 10.1002/stem.1400. [DOI] [PubMed] [Google Scholar]
- 73.Qin J, Gong G, Sun S, Qi J, Zhang H, Wang Y, Wang N, Wang QM, Ji Y, Gao Y. Functional recovery after transplantation of induced pluripotent stem cells in a rat hemorrhagic stroke model. Neurosci Lett. (2013);554:70–75. doi: 10.1016/j.neulet.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 74.Rahmat Z, Jose S, Ramasamy R, Vidyadaran S. Reciprocal interactions of mouse bone marrow-derived mesenchymal stem cells and BV2 microglia after lipopolysaccharide stimulation. Stem Cell Res Ther. (2013);4:12. doi: 10.1186/scrt160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ransohoff RM. A polarizing question:do M1 and M2 microglia exist? Nat Neurosci. (2016);19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 76.Rickman AD, Hilyard A, Heckmann BL. Dying by fire:noncanonical functions of autophagy proteins in neuroinflammation and neurodegeneration. Neural Regen Res. (2022);17:246–250. doi: 10.4103/1673-5374.317958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodríguez-Gómez JA, Kavanagh E, Engskog-Vlachos P, Engskog MK, Herrera AJ, Espinosa-Oliva AM, Joseph B, Hajji N, Venero JL, Burguillos MA. Microglia:agents of the CNS pro-inflammatory response. Cells. (2020);91717 doi: 10.3390/cells9071717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP. Global burden of cardiovascular diseases and risk factors, 1990–2019:update from the GBD 2019 study. J Am Coll Cardiol. (2020);76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rufino-Ramos D, Albuquerque PR, Carmona V, Perfeito R, Nobre RJ, Pereira de Almeida L. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J Control Release. (2017);262:247–258. doi: 10.1016/j.jconrel.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 80.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. (2008);181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schumacher M, Mattern C, Ghoumari A, Oudinet J, Liere P, Labombarda F, Sitruk-Ware R, De Nicola AF, Guennoun R. Revisiting the roles of progesterone and allopregnanolone in the nervous system:resurgence of the progesterone receptors. Prog Neurobiol. (2014);113:6–39. doi: 10.1016/j.pneurobio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Shao M, Jin M, Xu S, Zheng C, Zhu W, Ma X, Lv F. Exosomes from long noncoding RNA-Gm37494-ADSCs repair spinal cord injury via shifting microglial M1/M2 polarization. Inflammation. (2020);43:1536–1547. doi: 10.1007/s10753-020-01230-z. [DOI] [PubMed] [Google Scholar]
- 83.Sica A, Mantovani A. Macrophage plasticity and polarization:in vivo veritas. J Clin Invest. (2012);122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Skog J, Würdinger T, Van Rijn S, Meijer DH, Gainche L, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. (2008);10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sousa C, Golebiewska A, Poovathingal SK, Kaoma T, Pires-Afonso Y, Martina S, Coowar D, Azuaje F, Skupin A, Balling R, Biber K, Niclou SP, Michelucci A. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. (2018);19:e46171. doi: 10.15252/embr.201846171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stridh L, Smith PL, Naylor AS, Wang X, Mallard C. Regulation of toll-like receptor 1 and-2 in neonatal mice brains after hypoxia-ischemia. J Neuroinflammation. (2011);8:1–10. doi: 10.1186/1742-2094-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. (2018);150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 88.Tian T, Cao L, He C, Ye Q, Liang R, You W, Zhang H, Wu J, Ye J, Tannous BA. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics. (2021);11:6507. doi: 10.7150/thno.56367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Toupet K, Maumus M, Luz-Crawford P, Lombardo E, Lopez-Belmonte J, van Lent P, Garin MI, van den Berg W, Dalemans W, Jorgensen C. Survival and biodistribution of xenogenic adipose mesenchymal stem cells is not affected by the degree of inflammation in arthritis. PLoS One. (2015);10:e0114962. doi: 10.1371/journal.pone.0114962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vandevyver S, Dejager L, Libert C. Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocr Rev. (2014);35:671–693. doi: 10.1210/er.2014-1010. [DOI] [PubMed] [Google Scholar]
- 91.Venkat P, Zacharek A, Landschoot-Ward J, Wang F, Culmone L, Chen Z, Chopp M, Chen J. Exosomes derived from bone marrow mesenchymal stem cells harvested from type two diabetes rats promotes neurorestorative effects after stroke in type two diabetes rats. Exp Neurol. (2020);334:113456. doi: 10.1016/j.expneurol.2020.113456. [DOI] [PubMed] [Google Scholar]
- 92.vonderEmbse AN, Hu Q, DeWitt JC. Dysfunctional microglia:neuron interactions with significant female bias in a developmental gene x environment rodent model of Alzheimer's disease. Int Immunopharmacol. (2019);71:241–250. doi: 10.1016/j.intimp.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 93.Wang M, Mao C, Ouyang L, Liu Y, Lai W, Liu N, Shi Y, Chen L, Xiao D, Yu F. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. (2019);26:2329–2343. doi: 10.1038/s41418-019-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Huang Y, Xu Y, Ruan W, Wang H, Zhang Y, Saavedra JM, Zhang L, Huang Z, Pang T. A dual AMPK/Nrf2 activator reduces brain inflammation after stroke by enhancing microglia M2 polarization. Antioxid Redox Signal. (2018);28:141–163. doi: 10.1089/ars.2017.7003. [DOI] [PubMed] [Google Scholar]
- 95.Wang Y, Yang Y, Zhang S, Li C, Zhang L. Modulation of neuroinflammation by cysteinyl leukotriene 1 and 2 receptors: Implications for cerebral ischemia and neurodegenerative diseases. Neurobiol Aging. (2020);87:1–10. doi: 10.1016/j.neurobiolaging.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 96.Webb RL, Kaiser EE, Scoville SL, Thompson TA, Fatima S, Pandya C, Sriram K, Swetenburg RL, Vaibhav K, Arbab AS. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res. (2018);9:530–539. doi: 10.1007/s12975-017-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei H, Chen Q, Lin L, Sha C, Li T, Liu Y, Yin X, Xu Y, Chen L, Gao W. Regulation of exosome production and cargo sorting. Int J Biol Sci. (2021);17:163. doi: 10.7150/ijbs.53671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Winkler AS. The growing burden of neurological disorders in low-income and middle-income countries:priorities for policy making. Lancet Neurol. (2020);19:200–202. doi: 10.1016/S1474-4422(19)30476-4. [DOI] [PubMed] [Google Scholar]
- 99.Wolf SA, Boddeke H, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. (2017);79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- 100.World Health Organization (1971) Cerebrovascular diseases : prevention, treatment, and rehabilitation, report of a WHO meeting [held in Monaco from 25 to 29 May 1970]. World Health Organization. https://apps.who.int/iris/handle/10665/40898. [PubMed]
- 101.Xiang B, Xiao C, Shen T, Li X. Anti-inflammatory effects of anisalcohol on lipopolysaccharide-stimulated BV2 microglia via selective modulation of microglia polarization and down-regulation of NF-κB p65 and JNK activation. Mol Immunol. (2018);95:39–46. doi: 10.1016/j.molimm.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 102.Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. MicroRNA-17–92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. (2017);48:747–753. doi: 10.1161/STROKEAHA.116.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xin H, Liu Z, Buller B, Li Y, Golembieski W, Gan X, Wang F, Lu M, Ali MM, Zhang ZG. MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J Cereb Blood Flow Metab. (2021);41:1131–1144. doi: 10.1177/0271678X20950489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW, Yan Y, Han H, Du JL. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. (2017);27:882–897. doi: 10.1038/cr.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu C, Diao YF, Wang J, Liang J, Xu HH, Zhao ML, Zheng B, Luan Z, Wang JJ, Yang XP. Intravenously infusing the secretome of adipose-derived mesenchymal stem cells ameliorates neuroinflammation and neurological functioning after traumatic brain injury. Stem Cells Dev. (2020);29:222–234. doi: 10.1089/scd.2019.0173. [DOI] [PubMed] [Google Scholar]
- 106.Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q. Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics. (2020);10:3684. doi: 10.7150/thno.41580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. (2017a);7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang QQ, Zhou JW. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia. (2019);67:1017–1035. doi: 10.1002/glia.23571. [DOI] [PubMed] [Google Scholar]
- 109.Yang Y, Liu H, Zhang H, Ye Q, Wang J, Yang B, Mao L, Zhu W, Leak RK, Xiao B. ST2/IL-33-dependent microglial response limits acute ischemic brain injury. J Neurosci. (2017b);37:4692–4704. doi: 10.1523/JNEUROSCI.3233-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yeh JH, Wang KC, Kaizaki A, Lee JW, Wei HC, Tucci MA, Ojeda NB, Fan LW, Tien LT. Pioglitazone ameliorates lipopolysaccharide-induced behavioral impairment, brain inflammation, white matter injury and mitochondrial dysfunction in neonatal rats. Int J Mol Sci. (2021);22:6306. doi: 10.3390/ijms22126306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang H, Wu J, Wu J, Fan Q, Zhou J, Wu J, Liu S, Zang J, Ye J, Xiao M, Tian T, Gao J. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnology. (2019a);17:29. doi: 10.1186/s12951-019-0461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang K, Tu M, Gao W, Cai X, Song F, Chen Z, Zhang Q, Wang J, Jin C, Shi J. Hollow prussian blue nanozymes drive neuroprotection against ischemic stroke via attenuating oxidative stress, counteracting inflammation. and suppressing cell apoptosis. Nano Lett. (2019b);19:2812–2823. doi: 10.1021/acs.nanolett.8b04729. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. (2015);122:856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Z, Zou X, Zhang R, Xie Y, Feng Z, Li F, Han J, Sun H, Ouyang Q, Hua S, Lv B, Hua T, Liu Z, Cai Y, Zou Y, Tang Y, Jiang X. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging (Albany NY) (2021);13:3060–3079. doi: 10.18632/aging.202466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Z, Mi T, Jin L, Li M, Zhanghuang C, Wang J, Tan X, Lu H, Shen L, Long C, Wei G, He D. Comprehensive proteomic analysis of exosome mimetic vesicles and exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. (2022);13:312. doi: 10.1186/s13287-022-03008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao Y, Gan Y, Xu G, Yin G, Liu D. MSCs-derived exosomes attenuate acute brain injury and inhibit microglial inflammation by reversing CysLT2R-ERK1/2 mediated microglia M1 polarization. Neurochem Res. (2020a);45:1180–1190. doi: 10.1007/s11064-020-02998-0. [DOI] [PubMed] [Google Scholar]
- 117.Zhao Y, Gan Y, Xu G, Hua K, Liu D. Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci. (2020b);260:118403. doi: 10.1016/j.lfs.2020.118403. [DOI] [PubMed] [Google Scholar]


