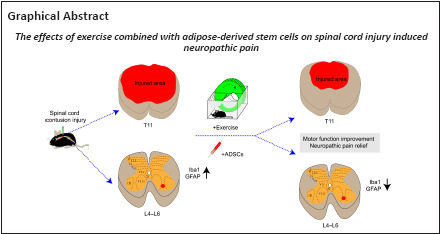
Key Words: adipose-derived stem cells, allodynia, exercise, glial fibrillary acidic protein, hyperalgesia, hypoalgesia, ionized calcium-binding adapter molecule 1, motor function, spinal cord injury
Abstract
Experimental studies have shown that exercise and human adipose-derived stem cells (ADSCs) play positive roles in spinal cord injury (SCI). However, whether ADSCs and/or exercise have a positive effect on SCI-induced neuropathic pain is still unclear. Thus, there is a need to explore the effects of exercise combined with administration of ADSCs on neuropathic pain after SCI. In this study, a thoracic 11 (T11) SCI contusion model was established in adult C57BL/6 mice. Exercise was initiated from 7 days post-injury and continued to 28 days post-injury, and approximately 1 × 105 ADSCs were transplanted into the T11 spinal cord lesion site immediately after SCI. Motor function and neuropathic pain-related behaviors were assessed weekly using the Basso Mouse Scale, von Frey filament test, Hargreaves method, and cold plate test. Histological studies (Eriochrome cyanine staining and immunohistochemistry) were performed at the end of the experiment (28 days post-injury). Exercise combined with administration of ADSCs partially improved early motor function (7, 14, and 21 days post-injury), mechanical allodynia, mechanical hypoalgesia, thermal hyperalgesia, and thermal hypoalgesia. Administration of ADSCs reduced white and gray matter loss at the lesion site. In addition, fewer microglia and astrocytes (as identified by expression of ionized calcium-binding adapter molecule 1 and glial fibrillary acidic protein, respectively) were present in the lumbar dorsal horn in the SCI + ADSCs and SCI + exercise + ADSCs groups compared with the sham group. Our findings suggest that exercise combined with administration of ADSCs is beneficial for the early recovery of motor function and could partially ameliorate SCI-induced neuropathic pain.
Introduction
As one of the most common and complex clinical problems associated with spinal cord injury (SCI), neuropathic pain (NP) occurs in approximately 75–80% of SCI patients and has a substantial effect on their quality of life (Finnerup et al., 2010). Various approaches, including acupuncture, analgesic drugs, transcutaneous electrical nerve stimulation, physiotherapy, and psychotherapy, are currently used to treat NP (Tong et al., 2021; Saleh et al., 2022).
Exercise is a physiotherapy-based approach that can be used to treat NP. Because of its low cost and non-invasive nature, exercise has recently received significant attention from researchers as a treatment for NP. Exercise can protect the spine (at or below the injury level) by inducing the release of various neurotrophic factors, modulating afferent input or different inflammatory mediators, and regulating neuronal plasticity (Richardson et al., 2019; Dugan et al., 2020, 2021; Li et al., 2020a). Cell therapy is another non-drug treatment that is used for patients with various neurological conditions, including SCI and NP (Eaton, 2004; Zhang et al., 2022). Human adipose-derived stem cells (ADSCs) have also received substantial attention from researchers owing to their availability, safety, and rich stem cell content. For example, ADSCs are a good treatment option for pain relief because they improve nerve healing, restore structural nerve damage, and decrease anti-inflammatory cytokine production (Fodor and Paulseth, 2016; Forouzanfar et al., 2018; Dehdashtian et al., 2020). Considering their modulation of inflammatory mediators, the combination of exercise and ADSCs may be an effective non-drug approach for managing and relieving SCI-induced NP.
Recently, several animal studies have evaluated the co-administration of various therapies for SCI (Sarveazad et al., 2017; Fadeev et al., 2021). Younsi et al. (2020) reported that treadmill training promotes the survival of transplanted neural precursor cells and modulates their differentiation. Co-therapy with stem cell and treadmill training leads to better functional recovery than individual treatment with either approach (Younsi et al. 2020). Similarly, Yousof et al. (2021) reported that combined treatment with ADSCs and pregabalin is much more effective for NP than monotherapy. Sarveazad et al. (2019) showed that co-administration of ADSCs and low-level laser treatment ameliorates SCI-induced NP, but the molecular mechanisms are still unclear.
To the best of our knowledge, no study has explored the effects of exercise and ADSCs on SCI induced NP recovery. Therefore, the aim of this study was to determine the effect of exercise combined with ADSC administration on SCI-induced NP in a contusion-induced mouse model of SCI. Changes in spinal cord segments below the injury level are important for SCI-induced NP. Treadmill training ameliorates thoracic SCI-induced allodynia via increased expression of brain-derived neurotrophic factor (BDNF) in the lumbar spinal cord (Hutchinson et al., 2004). Given that both exercise and ADSCs can modulate the expression of inflammatory mediators, and neuroinflammatory responses are implicated in SCI-induced NP in spinal segments below the injury level, we also assessed the effect of combined treatment with exercise and ADSCs on microglia and astrocyte activation in the lumbar spinal cord after thoracic SCI.
Methods
Animals
Our study included 95 adult female C57BL/6 mice weighing 19–26 g (8–10 weeks old). Based on anatomy of the urinary system, the female animals are easier to catheterize. The risk of urinary system infections can be reduced (Cheng et al., 2021), thus only female mice were chosen in this study. The mice were obtained from the Animal Research Center of Sun Yat-sen University (license No. SYXK (Yue) 2022-0002), and the animal experiments were approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (approval No. [2021]864) on December 29, 2021. Mice were kept 4–5 per cage with free access to food and water under a 12/12-hour light/dark cycle. The mice were randomly divided into five groups: sham (n = 15), SCI (n = 20, SCI + α-MEM injection), SCI + exercise (n = 20, SCI + exercise), SCI + ADSCs (n = 20, SCI + injection of 105 ADSCs), and SCI + exercise + ADSCs (n = 20, SCI + exercise + injection of 105 ADSCs)-based on their baseline withdrawal rates in the von Frey test prior to SCI induction and treatment. The study design and timeline are shown in Figures 1 and 2.
Figure 1.
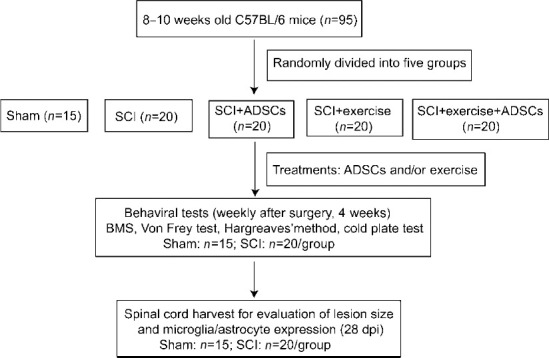
Study flow chart.
ADSCs: Adipose-derived stem cells; BMS: Basso Mouse Scale; dpi: days post-injury; SCI: spinal cord injury.
Figure 2.
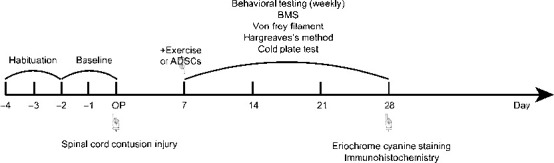
Experimental timeline.
ADSCs: Adipose-derived stem cells; BMS: Basso Mouse Scale.
Isolation of human adipose-derived stem cells
All participants provided written informed consent. Abdominal adipose samples were collected from healthy young adults (aged < 32 years) who underwent liposuction at the First Affiliated Hospital of Sun Yat-sen University. ADSCs were isolated by collagenase digestion and cultured as previously reported (Bunnell et al., 2008). Briefly, the ADSCs were cultured in alpha minimum essential medium (α-MEM, Shanghai Weike Biotechnology Co., Ltd., Shanghai, China) supplemented with 10% FBS and in a humidified 5% CO2 atmosphere at 37°C. When the cultures reached ~80% confluence, the cells were detached by treatment with 0.05% trypsin/ethylenediaminetetraacetic acid (EDTA, Shanghai Weike Biotechnology Co., Ltd.) and passaged, and the culture media were changed every 3 days.
Characterization of human adipose-derived stem cells by flow cytometry
ADSCs were identified by their surface antigens using flow cytometry (eBioscience, Inc., San Diego, CA, USA) and monoclonal antibodies to CD34 (Thermo Fisher Scientific, Waltham, MA, USA, Cat# 11-0349-41, RRID: AB_1518733), CD44 (Thermo Fisher Scientific, Cat# 11-0441-81, RRID: AB_465044), CD90 (Thermo Fisher Scientific, Cat# 11-0909-41, RRID: AB_10668827), CD105 (Thermo Fisher Scientific, Cat# 12-1057-41, RRID: AB_1311125), and HLA-DR (Thermo Fisher Scientific, Cat# 17-9956-41, RRID: AB_10671395). A mouse IgG monoclonal antibody was used as a negative control.
Spinal cord injury and treatment with human adipose-derived stem cells
Mice were anesthetized with ketamine (31 mg/kg, Ratiopharm, Ulm, Germany) mixed with xylazine (1.5 mg/kg, Ratiopharm) via intraperitoneal (IP) injection. Then the skin of the back was incised gently to expose the T8–T10 vertebrae. To adequately expose the T11 spinal cord segment (located at the T9 vertebra), laminectomy was performed. In the SCI groups, mice were subjected to moderate spinal cord contusion injury at the T11 spinal cord segment by applying a force of 50 kdyn (1 dyn = 10–5 N) (IH-0400 Impactor; Precision Systems & Instrumentation, Lexington, KY, USA) as described previously (Cheng et al., 2021), using a standard mouse tip impactor size (1.3 mm in diameter). For the ADSC treatment groups, 105 cells in a total volume of 3 μL were injected into the thoracic spinal cord dorsal horn using a 10 μL insulin pen. The injection was administered at two sites (the upper and lower boundaries of the injury site). After SCI, the incisions were closed. Then, Ringer’s solution (100 μL) was subcutaneously injected in the mice to prevent dehydration after SCI. Ampicillin (100 μL, 30 mg/kg; Ratiopharm) was subcutaneously injected to prevent infection. Furthermore, artificial bladder voiding was conducted twice per day until mice recovered reflexive bladder emptying.
Exercise
Running wheels (Kelly et al., 2014) were placed in each cage (Huaibei Jiubai Electronic Technology Co., Ltd. Huaibei, Anhui, China) containing mice in the exercise groups at 7 dpi. Running behavior in each 24-hour period was recorded via a counter connected to the wheel. The distance covered during each running session was about 10 cm. The mean total running distance over 3 weeks was about 421 m and 505 m in the SCI + exercise and SCI + ADSCs + exercise groups, respectively. To avoid inducing a stress response, the mice were habituated to the running wheel for at least 2 days before we the exercise protocol started.
Behavioral testing
Awake, unrestrained mice were subjected to behavioral tests by a single experienced investigator who was blinded to the group assignments. Mice were habituated to the testing environment for 1.5 hours every day for 2 days before testing, and at least 30 minutes immediately before each test.
Von Frey test
To assess mechanical sensitivity, the von Frey test was performed as previously described (Cheng et al., 2021). Mechanical hypersensitivity (allodynia) was assessed using light force filament (0.07 and 0.16 g, Touch Test Sensory Evaluators; North Coast Medical, Gilroy, CA, USA) stimulation, and mechanical hyposensitivity (hyperalgesia) was assessed using strong force filament (1 and 1.4 g, Touch Test Sensory Evaluators; North Coast Medical) stimulation. Withdrawal or movement of the tested hind paw was considered positive response. The percent response rate to stimulation was calculated after a total of 10 stimulations to the hind paws (five times per hind paw). The average of two preoperative values for each animal and each filament type was defined as baseline mechanical sensitivity. von Frey testing was performed 7 dpi and at weekly intervals thereafter for 4 weeks after SCI.
Thermal sensitivity
The Hargreaves method was used to evaluate thermal sensitivity as described previously (Cheng et al., 2021). Briefly, an infrared heat beam (Plantar Test; Ugo Basile, Milan, Italy) was used to stimulate the plantar surface of the mouse’s hind paw five times on each side. The latency of withdrawal from the heat beam in seconds was recorded. The average of two preoperative values per animal was defined as baseline thermal sensitivity. The Hargreaves test was performed 7 dpi and at weekly intervals thereafter for 4 weeks after SCI.
Cold sensitivity
A 2°C metal plate (Cold Hot Plate Test; Bioseb, Vitrolles, France) was used to evaluate cold sensitivity (Cheng et al., 2021). We recorded withdrawal latency from the cold plate in seconds. Shaking and lifting the hind paws and/or sudden changes in the pattern of movement were considered positive responses. The average of two preoperative values for every mouse was defined as baseline cold sensitivity. After surgery, cold sensitivity was evaluated 10 dpi (in the middle of the experimental period) and 28 dpi (at the end of the experiment).
Basso Mouse Scale assessment
We used the Basso Mouse Scale (BMS) to examine the motor function recovery (Basso et al., 2006). A BMS score of 0 indicates that the animal is completely paralyzed, whereas a BMS score of 9 indicates normal motor function. A BMS score of 3 reflects the mouse’s ability to support its own weight or occasionally take a step, indicating the animal can undergo behavioral testing. The BMS score was evaluated at baseline, 7 dpi, and at weekly intervals thereafter for 4 weeks after SCI.
Eriochrome cyanine staining
To explore the extent of thoracic spinal cord damage after SCI, Eriochrome cyanine (EC) staining was conducted to determine the degree of tissue sparing at the epicenter of the lesion via myelin staining. First, mice were euthanized and perfused transcardially with 0.9% saline. Next, the spinal tissue was fixed by pericardial perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer transcardially perfusion. The T11 spinal cord segments were collected and sliced into 25 μm-thick coronal sections using a cryostat (CM1900, Leica, Weztlar, Germany). Eriochrome cyanine staining (Servicebio, Wuhan, Hubei, China) of the sections was performed as described previously (Cheng et al., 2021). An XC30 camera mounted on an Olympus BX530 microscope (Olympus, Hamburg, Germany) was used to take images of the stained sections. The lesion size was calculated by dividing the remaining white matter area by the total cross-sectional area. Two independent investigators blinded to the group assignments analyzed the data using ImageJ software (version 1.8.0, National Institutes of Health, Bethesda, MD, USA; Schneider et al., 2012).
Immunohistochemistry
L4–L6 spinal cord segments were obtained at the end of the experiment, and 25 μm-thick frozen coronal spinal cord sections were used for immunohistochemical procedures (Cheng et al., 2021). Sections were labeled with rabbit anti-ionized calcium-binding adapter molecule 1 (Iba1; 1:500; Abcam, Cambridge, MA, USA, Cat# ab178846, RRID: AB_2636859) or rabbit anti-glial fibrillary acidic protein (GFAP; 1:1000; Abcam, Cat# ab7260; RRID: AB_305808) to evaluate the microglial and astroglial responses, respectively. To identify lamina I and II, some sections were double labeled with biotinylated isolectin B4 (IB4) (1:250; Sigma, St. Louis, MO, USA, Cat# MAB2058, RRID: AB_11214113; integrin B4 from Bandeiraea simplicifolia) and rabbit anti-protein kinase Cγ (PKCγ) (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA, Cat# sc-211, RRID: AB_632234). Donkey anti-rabbit secondary antibodies were conjugated to donkey anti-rabbit Alexa Fluor 488 (1:300; Abcam, Cat# ab150073; RRID: AB_2636877) for calcium-binding adapter molecule 1 (Iba1) and GFAP staining. Alexa Fluor 488-conjugated streptavidin (1:300; Jackson ImmunoResearch Laboratories, Suffolk, UK, Cat# 016-540-084; RRID: AB_2337249) and goat anti-rabbit Alexa Fluor® 594 (1:300; Abcam, Cat# ab150080, RRID: AB_2650602) were used to detect IB4 and PKCγ, respectively. All primary antibodies were incubated overnight at 4°C, and the secondary antibodies were incubated at room temperature for 2.5 hours.The right and left dorsal horns of three fluorescently labeled sections were visualized at 20× magnification under the same conditions for each mouse using a confocal laser scanning microscope (LSM 700, ZEISS, Oberkochen, Germany). Iba1 and GFAP expression levels in the lumbar dorsal horn lamina I–II were quantified using ImageJ software. The percentage of Iba1 and GFAP expression in lamina I–II was calculated by dividing Iba1 and GFAP expression in lamina I–II by their expression throughout the dorsal horn.
Statistical analysis
No statistical methods were used to predetermine sample sizes; however, our sample sizes were similar to those reported in a previous publication (Cheng et al., 2021). The results are presented as mean ± standard error of the mean (SEM). One-way analysis of variance with Fisher’s least significant difference post hoc test was used to analyze the lesion size and expression levels of Iba1 and GFAP. Two-way analysis of variance with Fisher’s least significant difference post hoc test was used to analyze the behavioral test data. GraphPad Prism 7 software (GraphPad Software, San Diego, CA, USA, www.graphpad.com) was utilized to conduct the statistical analyses. The statistical significance threshold was set to P < 0.05.
Results
Identification of ADSCs and ADSCs-Exos
ADSCs were isolated and characterized at passage 3 (P3) for cell transplantation after SCI. When they reached 80–90% confluence, ADSCs exhibited strong proliferative capacity and spindle-shaped morphology in a relatively homogeneous population (Figure 3A). Flow cytometry was performed to evaluate surface antigen expression, and the ADSCs stained positively for CD34, CD44, CD90, and CD105, but negatively for HLA-DR (Figure 3B–F).
Figure 3.
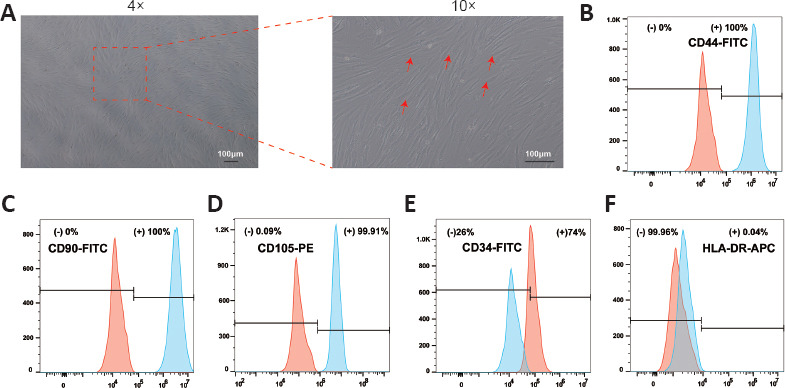
Identification of ADSCs.
(A) ADSCs exhibited characteristic spindle-like morphology, as indicated by red arrows. The mesenchymal markers CD34 (B), CD44 (C), CD90 (D), CD105 (E) and the negative control marker HLA-DR (F) were used to analyze the cells by flow cytometry. The indicated markers are presented as solid red peaks, and the isotype controls are presented as solid blue peaks. The experiment was independently repeated three times. ADSCs: Adipose-derived stem cells.
Exercise and ADSCs promote motor recovery in SCI mice
BMS scoring was used to evaluate motor recovery after SCI. Recovery was evident in all injured animals, with no obvious differences among the groups at 28 dpi (Figure 4). However, the animals in the SCI + ADSCs (7 dpi: P = 0.0075, 14 dpi: P = 0.0174, 21 dpi: P = 0.9857) and SCI + exercise + ADSCs (7 dpi: P < 0.001, 14 dpi: P = 0.0011, 21 dpi: P = 0.003) had higher BMS scores than animals in the SCI group at 7, 14, and 21 dpi.
Figure 4.
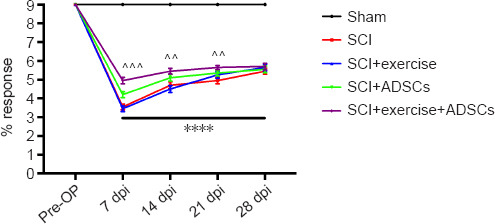
Recovery of motor function.
The Basso Mouse Scale (BMS) scores were higher in the SCI + ADSCs and SCI + exercise + ADSCs groups than in the SCI and SCI + exercise groups. The results are presented as the mean ± SEM. ****P < 0.0001, SCI group vs. Sham group; ^^P < 0.01, ^^^P < 0.001, SCI + exercise + ADSCs group vs. SCI group (two-way analysis of variance with Fisher’s least significant difference post hoc test). ADSCs: Adipose-derived stem cells; dpi: day post-injury; SCI: spinal cord injury.
Exercise and ADSCs ameliorate mechanical allodynia and hypoalgesia in SCI mice
After 7 dpi, SCI mice began to develop mechanical hyperalgesia and hypoalgesia (Figure 5). Light filament forces (0.07 and 0.16 g) were used to evaluate mechanical hypersensitivity (Figure 5A and B). Significantly lower response rates were observed in the SCI + ADSCs (0.07 g: P < 0.001; 0.16 g: P = 0.0164) and SCI + exercise + ADSCs (0.07 g: P < 0.001, 0.16 g: P = 0.0012) groups than in the SCI + exercise group at 7 dpi. In addition, analysis at different time points showed significantly lower response rates to the 0.07-g filament in the SCI + exercise + ADSCs group at 7–28 dpi compared with the other groups (P < 0.001). Compared with the single-treatment groups (SCI + exercise or SCI + ADSCs), the combination treatment group (SCI + exercise + ADSCs) exhibited greater relief from SCI-induced mechanical allodynia. When strong filament forces (1 and 1.4 g) were used to evaluate mechanical hyposensitivity (Figure 5C and D), no difference was observed between the SCI group and the SCI + exercise group, indicating that exercise does not have a positive effect on SCI-induced mechanical hypoalgesia. However, injured animals that were injected with ADSCs (SCI + ADSCs and SCI + exercise + ADSCs) exhibited dramatically higher response rates than animals in the SCI and SCI + exercise groups (P < 0.0001).
Figure 5.
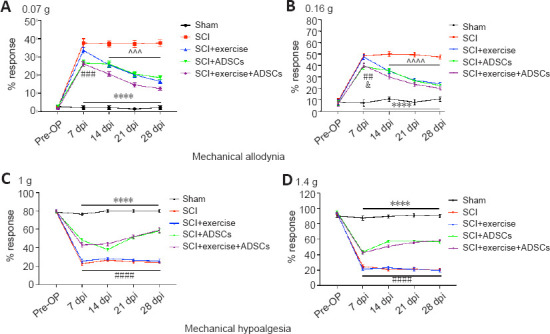
Exercise and ADSCs ameliorate mechanical sensitivity after SCI.
(A, B) von Frey filament testing with 0.07 g (A) and 0.16 g forces (B) was performed to detect mechanical allodynia. Animals in the SCI, SCI + exercise, SCI + ADSCs, and SCI + exercise + ADSCs groups developed mechanical allodynia after 7 days post-injury (dpi). However, animals in the SCI + ADSCs and SCI + exercise + ADSCs groups showed a lower withdrawal response rate compared with those in the SCI and SCI + exercise groups (P < 0.05, P < 0.01, or P < 0.001). Both exercise and ADSCs administration significantly ameliorated SCI-induced mechanical allodynia from 14 to 28 dpi (P < 0.0001, P < 0.001, or P < 0.0001). (C, D) Mechanical hypoalgesia was evaluated via using von Frey filaments with 1 (C) and 1.4 g forces (D). At 7 dpi, animals in the SCI, SCI + exercise, SCI + ADSCs, and SCI + exercise + ADSCs groups showed lower withdrawal response rates compared with the sham group, indicating mechanical hypoalgesia (P < 0.0001). Exercise had no influence on mechanical hypoalgesia, while ADSCs administration significantly improved SCI-induced mechanical hypoalgesia (P < 0.0001). The results are presented as the mean ± SEM. ****P < 0.0001, SCI group vs. Sham group; &P < 0.05, SCI + ADSCs group vs. SCI + exercise group; ##P < 0.01, ###P < 0.001, ####P< 0.0001, SCI + exercise + ADSCs group vs. SCI + exercise group; ^^^P < 0.001 and ^^^^P < 0.0001, SCI + exercise + ADSCs group vs. SCI group (two-way analysis of variance with Fisher’s least significant difference post hoc test). ADSCs: Adipose-derived stem cells; dpi: day post-injury; SCI: spinal cord injury.
ADSCs ameliorate thermal hyperalgesia and cold hypoalgesia in SCI mice
Animals were exposed to an infrared heat beam (Hargreaves method) and a cold plate (2°C) to examine thermal hypersensitivity and cold hyposensitivity after SCI (Figure 6). SCI animals had decreased withdrawal latency from 7 to 28 dpi compared with sham animals (P < 0.0001), indicating that injured animals developed thermal hyperalgesia (Figure 6A). Exercise did not influence SCI-induced thermal hypersensitivity, but ADSCs significantly improved the withdrawal latency compared with exercise alone (P < 0.0001). Moreover, SCI mice had an increased response latency in the cold plate test compared with sham animals at 10 dpi (P < 0.0001), indicating that SCI mice developed cold hypoalgesia (Figure 6B). Cold hypoalgesia was observed in SCI mice and did not change significantly throughout the course of the experiment. Similarly, SCI-induced cold hyposensitivity was not influenced by exercise, but ADSCs significantly reduced the withdrawal latency compared with exercise alone (P < 0.0001).
Figure 6.
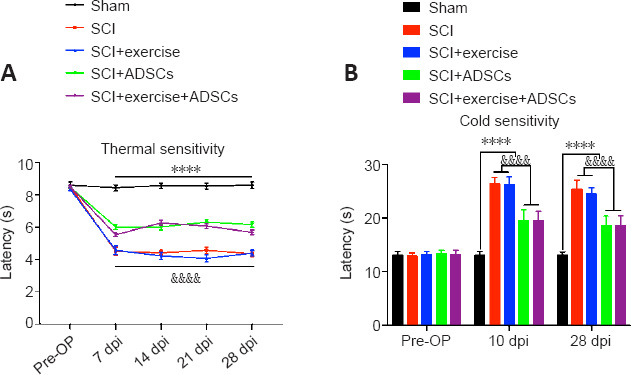
ADSCs improve thermal and cold sensitivity after SCI.
(A) Thermal and cold sensitivitywere assessed using Hargreaves’ method. SCI mice had a shorter withdrawal latency than the sham mice at all time points after injury (P < 0.0001). Exercise did not affect (P < 0.0001). (B) Injured mice exhibited a longer response latency to the cold plate (2°C) than did the sham mice (P < 0.0001) at 10 and 28 days post-injury (dpi). In addition, ADSC administration helped relieve cold sensitivity (P < 0.0001). The results are presented as the mean ± SEM. ****P < 0.0001, SCI group vs. Sham group; &&&&P < 0.0001, SCI + ADSCs group vs. SCI + exercise group (two-way analysis of variance with Fisher’s least significant difference post hoc test). ADSCs: Adipose-derived stem cells; dpi: day post-injury; SCI: spinal cord injury.
ADSCs have a neuroprotective effect after SCI
Previous studies have reported that exercise does not influence lesion size after SCI (Battistuzzo et al., 2012; Cheng et al., 2021). To determine whether ADSCs influence lesion size, myelin staining of T11 spinal cord sections was performed. There was no statistical significance in lesion size between animals in the SCI and SCI + exercise groups (Figure 7). A large part of the gray matter and a small part of the white matter were lost at the lesion epicenter in SCI mice. ADSCs dramatically reduced gray and white matter loss at the lesion site (P < 0.001), revealing that ADSCs have a neuroprotective effect after SCI.
Figure 7.
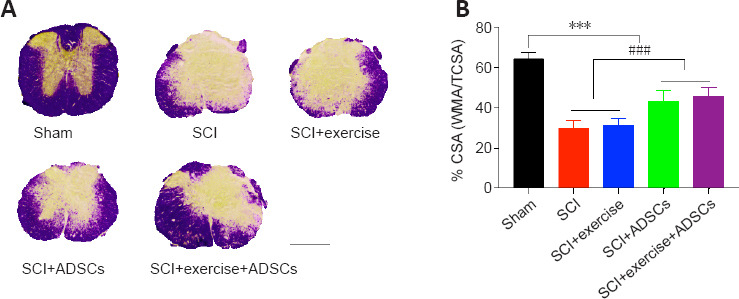
ADSCs have a neuroprotective effect on the spinal cord.
(A) Representative images of thoracic spinal cord lesion sizes in the different groups using Eriochrome cyanine staining (blue staining indicates white matter). The sham animal spinal cords were intact, whereas animals that had undergone SCI showed a decrease in white matter. Exercise did not appear to have an effect on tissue sparing, while ADSCs administration had a protective effect. Scale bar: 500 μm. (B) Lesion size was calculated as the percentage of white matter area (WMA) to the total cross-sectional area (TCSA). Compared with sham animals, SCI animals showed a significant loss of white matter at the lesion site (***P < 0.001). Exercise did not influence the lesion size, but ADSCs administration significantly reduced the lesion size (###P < 0.001). The results are presented as the mean ± SEM. One-way analysis of variance with Fisher’s least significant difference post hoc test was applied. ADSCs: Adipose-derived stem cells; SCI: spinal cord injury.
The injury-induced increase in Iba1 and GFAP expression levels is attenuated by ADSCs
Previous studies have demonstrated that inflammatory changes in the spinal cord dorsal horn below the lesion site are associated with the development of NP behaviors (Li et al., 2020b; Iqubal et al., 2021). Therefore, we quantified Iba1 and GFAP intensity in the lumbar spinal dorsal horn after SCI at the T11 level (Figures 8 and 9).
Figure 9.
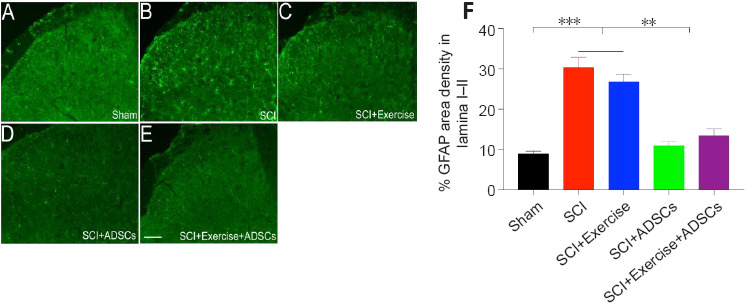
Administration of ADSCs decreases GFAP expression in lamina I and II of the lumbar spinal cord dorsal horn after SCI.
(A–E) Representative images of GFAP (green) expression indicating astrocytes in the different groups. SCI resulted in GFAP overexpression in the lumbar spinal dorsal horn. ADSC administration decreased SCI-induced GFAP overexpression in the lumbar spinal dorsal horn (especially lamina I–II). (F) Quantification of GFAP intensity in the lumbar spinal lamina I–II. GFAP expression was significantly up-regulated in the injured groups (SCI and SCI + exercise) compared with the sham group. There was no statistically significant difference in GFAP expression between the SCI group and the SCI + exercise group. ADSCs administration significantly reduced Iba1 expression in the dorsal horn, with no significant difference between the SCI + ADSCs and SCI + exercise + ADSCs groups. **P < 0.01, ***P < 0.001. The results are presented as the mean ± SEM. One-way analysis of variance with Fisher’s least significant difference post hoc test was applied. ADSCs: Adipose-derived stem cells; GFAP: glial fibrillary acidic protein; SCI: spinal cord injury.
IB4 and PKCγ were used to label lamina I and II, respectively (Figure 8A). Iba1 expression was dramatically up-regulated in the spinal dorsal horn at levels L4 to L6 after SCI and was mainly detected in lamina I–II (Figure 8B–F). Exercise slightly reduced Iba1 expression, albeit without statistical significance. Animals in the SCI + ADSCs and SCI + exercise + ADSCs groups showed a significant decrease in Iba1 expression in the L4–L6 dorsal horn comparing with those in the SCI and SCI + exercise groups (P < 0.01; Figure 8G).
Figure 8.
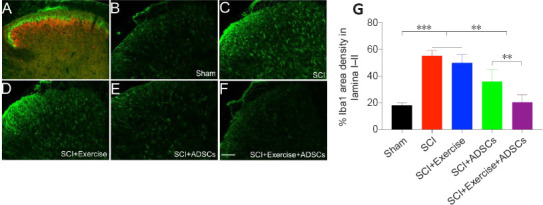
Administration of ADSCs decreases Iba1 expression in lamina I and II of the lumbar spinal cord dorsal horn after SCI.
(A) The different lamina in the dorsal horn of the lumbar spinal cord were distinguished via isolectin B4 (lamina I, green) and protein kinase Cγ (lamina II, red) staining. (B–F) Representative images of Iba1 expression in different groups. SCI resulted in an up-regulation of Iba1 expression in the lumbar spinal dorsal horn, especially in the superficial lamina (lamina I–II). ADSCs administration decreased SCI-induced Iba1 overexpression in the lumbar spinal dorsal lamina I–II. (G) Quantification of Iba1 intensity in the lumbar spinal dorsal horn. Iba1 expression was dramatically higher in the injured groups (SCI and SCI + exercise) than in the sham group. Exercise slightly decreased Iba1 expression, albeit without statistical significance. ADSCs administration significantly reduced Iba1 expression in dorsal horn. The combination of exercise and ADSCs administration was more effective in reducing Iba1 than ADSCs administration alone. **P < 0.01, ***P < 0.001. The results are presented as the mean ± SEM. One-way analysis of variance with Fisher’s least significant difference post hoc test was applied. ADSCs: Adipose-derived stem cells; Iba1: ionized calcium-binding adapter molecule 1; SCI: spinal cord injury.
Similarly, GFAP expression was significantly up-regulated in the L4–L6 superficial dorsal horn (lamina I–II) in animals in the SCI group compared with animals in the sham group (P < 0.001; Figure 9). Animals that received ADSCs (SCI + ADSCs and SCI + exercise + ADSCs groups) showed a significantly lower GFAP expression level in the L4–L6 dorsal horn compared with SCI animals (P < 0.01). GFAP expression was slightly decreased in the dorsal horn in the SCI + exercise group, albeit without statistical significance (Figure 9F).
Discussion
The combination of exercise and ADSC administration clearly enhanced motor function recovery at an early stage of SCI recovery and alleviated SCI-induced hypersensitivity and hyposensitivity compared with exercise alone or ADSCs alone. Injection with ADSCs had a neuroprotective effect and decreased lesion size after SCI compared with exercise alone. In addition, the combination of exercise and ADSC administration significantly decreased Iba1 and GFAP expression.
Rodent models of contusion SCI have been widely used to explore NP development and treatment (Burke et al., 2017; McFarlane et al., 2020). Similar to previous studies (McFarlane et al., 2020; Kishima et al., 2021), we established a moderate T11 contusion injury mouse model and evaluated mechanical, thermal, and cold sensitivity. We found that mice with moderate SCI developed obvious mechanical hypersensitivity, hyposensitivity, heat hypersensitivity, and cold hyposensitivity. These data indicate that the injured mice displayed not only an increase in sensitivity, but also a loss of sensory function. The reasons for these findings remain unclear. Injury causing loss of descending modulatory inhibition in the spinal cord can lead to hyperexcitability of nociceptive neurons, which could explain in part why SCI results in central NP. In addition, SCI can lead to abnormal neural plasticity or to excitability changes in supraspinal regions, which may integrate nociceptive and homeostatic functions, thus inducing NP in response to innocuous stimuli. Owing to the limited reports and understanding regarding the mechanisms associated with NP after SCI, it is understandable that treatments intended to ameliorate NP have been unsuccessful (Hutchinson et al., 2004). Interestingly, both exercise and administration of ADSCs partially resolved mechanical allodynia in our study. In addition, ADSC administration partially resolved thermal hyperalgesia and loss of sensory function (mechanical and cold hypoalgesia). Moreover, administration of ADSCs improved motor function at early time points following SCI, although this change was not statistically significant. Our results indicate that the combination of exercise and ADSCs has better therapeutic effects on SCI-induced NP than ADSCs alone. Consistent with our results, exercise improves mechanical hyperalgesia but has no effect on thermal hyperalgesia in rat contusion models (Hutchinson et al., 2004; Detloff et al., 2014). Non-peptidergic C fibers are associated with mechanical sensitivity, while peptidergic C fibers correspond to heat sensitivity. Detloff et al. (2014) demonstrated that the mechanical hyperalgesia-related increase in non-peptidergic but not peptidergic afferent distribution and density within the dorsal horn can be regulated by exercise. Whether the combination of exercise and ADSC administration can ameliorate NP via modulating non-peptidergic and/or peptidergic C fiber distribution and reducing abnormal plasticity is still unclear. Combined laser treatment and ADSC administration clearly enhances motor recovery and ameliorates SCI-induced mechanical allodynia and hyperalgesia via increasing GDNF, BDNF and GABA receptor expression (Sarveazad et al., 2019). Laser treatment may ameliorate allodynia and hyperalgesia by decreasing BDNF levels, while hADSCs may significantly increase GDNF expression levels. Alavi et al. (2021) also reported that ADSCs have anti-fibrotic, anti-inflammatory, anti-apoptotic, immunomodulatory, and angiogenic properties because they secrete various cytokines, and therefore improve locomotion and sensory recovery in animal models of SCI. Apart from ADSCs, the combination of exercise and transplantation of neural progenitor cells (NPCs) also helps improve NP after SCI. Dugan et al. (2020) suggested several monotherapies and combined therapies that decrease pro-inflammatory markers and spinal pathology, and therefore ameliorate SCI induced allodynia, hyperalgesia, and ongoing pain. Tashiro et al. (2018) demonstrated that treadmill running combined with NPC transplantation decreases mechanical and thermal hypersensitivity in mice with chronic, severe thoracic cord injury by downregulating CGRP-positive fibers and increasing GABAergic activity in the posterior horn. Kim et al. (2018) reported that the combination of treadmill training and bone marrow stromal cell transplantation provides neuroprotective effects after SCI via activation of the BDNF-ERK1/2 pathway. Taken together, these studies suggest that co-administration of different treatments is a promising direction for the treatment of patients with neurological diseases.
To the best of our knowledge, this is the first study to evaluate the effect of combined treatment with exercise and ADSC administration on NP after SCI in animals. Future studies will explore the mechanisms underlying exercise- and ADSC-induced amelioration of NP after SCI. It is unclear why exercise selectively ameliorated mechanical, but not thermal, sensitivity, while ADSCs influenced both mechanical and thermal sensitivity. In our study, the lesion sizes were smaller, and the loss of myelin was less prominent, in the SCI + ADSC group compared with the SCI + exercise group. The greater tissue sparing at the lesion site may reduce the loss of sensory function, which could explain why ADSC administration or the combination of exercise and ADSC administration improved motor function at an early stage of recovery (7 dpi). In a non-NP model, ADSCs combined with aspirin promoted the functional recovery of optic nerve pathways by ameliorating astrocyte activation and attenuating demyelination (Galeshi et al., 2019). In addition, ADSCs enhance nerve regeneration and promote angiogenesis in a rat model of peripheral nerve injury (Reichenberger et al., 2016).
It has been reported that microglia reactivity is important in the long-term maintenance of NP (Tawfik et al., 2007; Liang et al., 2021). Microglia activity is regarded as an inevitable factor for inducing hyperalgesia after peripheral nerve injury (PNI) (Raghavendra et al., 2003). Iba1 is a cytosolic calcium-binding protein that is mainly expressed in microglia. In different NP models, injury-induced Iba1 up-regulation was related to the development of mechanical hyperalgesia (Romero-Sandoval et al., 2008). In accordance with the results reported by Romero-Sandoval et al., we found that, following SCI, Iba1 expression was up-regulated in the entire dorsal horn of the lumbar spinal cord, especially in superficial lamina I–II. It remains unclear why Iba1 intensity was increased in the dorsal horn below the level of injury. Several studies have shown Iba1 to be significantly up-regulated in the lumbar spinal cord in response to various molecular signaling events induced by PNI, including activation of NF-κB and JAK-STAT3 (Arman et al., 2020; Nishihara et al., 2020; Zhou et al., 2021). Recently, Kishima et al. (2021) reported that phosphorylated p38 (p-p38)- and Iba1-positive areas were statistically significantly up-regulated in the dorsal horn of the lumbar spinal cord after T10 spinal cord contusion injury in rats, and that ROCK signaling might be involved in this process. PNI-induced astrocytic activation occurs secondary to microglial activation. Previous studies have suggested that interleukin-1β or matrix metalloprotease-2 can increase GFAP expression in the spinal cord and induce a pro-inflammatory astrocyte phenotype that is associated with allodynia in different pain models (Guo et al., 2007; Kawasaki et al., 2008). In our study we found that GFAP intensity was increased in the dorsal horn of lumbar spinal cord after thoracic SCI.
Interestingly, we also found that the combination of exercise and ADSC administration clearly ameliorated NP by reducing Iba1 and GFAP expression in the lumbar spinal cord dorsal horn. Although the underlying molecular mechanisms were not clarified in the current study, this is the first study to report changes in microglia and astrocyte reactivity in the spinal cord below the level of SCI and investigate the effects of combined treatment with exercise and ADSC administration on these changes. ADSCs suppress Iba1 and GFAP expression throughout the entire cortex after stroke (Zhou et al., 2015). Kazuya et al. (2021) found that ripasudil can ameliorate NP by reducing p-p38- and Iba1-immunoreactive cell intensity in the spinal cord dorsal horn of SCI rats. Estrogen can also alleviate SCI-induced NP via inhibition of microglia and astrocyte activation in the lumbar dorsal horn lamina I–II (Lee et al., 2018). Brown et al. (2021) also demonstrated that a persistent pro-inflammatory macrophage/microglia response in the superficial dorsal horn was involved in SCI-induced NP. Therefore, microglia and astrocyte reactivity in the lumbar segments of the spinal cord following thoracic SCI may contribute to persistent NP. The combination of exercise and ADSC administration helps inhibit this process. However, the underlying molecular mechanism requires further investigation. Some data suggest that exercise-mediated changes in BDNF and GDNF expression in the spinal cord account for the amelioration of mechanical allodynia (Hutchinson et al., 2004; Detloff et al., 2014). In contrast, engaging in exercise shortly after SCI results in BDNF-mediated mechanical hypersensitivity (Ulmann et al., 2008). BDNF, which is derived from activated microglia, may play an important role in central sensitization by reducing inhibitory synaptic transmission, enhancing excitatory input, and downregulating KCC2 (Lu et al., 2008). Furthermore, the combination of ADSC administration and laser treatment can also enhance motor function recovery, hyperalgesia, and allodynia by increasing GDNF mRNA expression in the spinal cord and increasing the number of axons around the lesion site after SCI (Sarveazad et al., 2019). ADSCs also help improve osteoarthritis-induced knee pain (Chen et al., 2021; Okamoto-Okubo et al., 2021). In summary, the combination of exercise and ADSC administration helps relieve SCI-induced NP, with complex underlying mechanisms. Future studies should explore the potential role of suppression of Iba1 and GFAP expression in the dorsal horn of the lumbar spinal cord in this process.
This study had some limitations that should be noted. First, the molecular mechanisms underlying the effects of ADSCs and exercise on SCI-induced NP were not investigated in detail in the current study. Future studies should use transgenic mice, drugs, and other measures to explore the molecular mechanisms underlying the observations made here. Moreover, lumbar dorsal root ganglions (DRGs) play an important role in the conduction of sensation. We did not investigate microglia and astrocyte activation in the L4–L6 DRGs in our study. Exploring whether the combination of exercise and ADSC administration influences IBA1 and GFAP expression in the L4–L6 DRGs may elucidate how they inhibit IBA1 and GFAP expression in the lumbar dorsal horn. Finally, Iba1 and GFAP expression levels were only detected at the end of the experiment (28 dpi) in this study. Iba1 and GFAP expression levels should be assessed at various time points (7, 14, and 21 dpi) throughout the experimental period to better define the relationship between Iba1 and GFAP expression, NP, and the effects of combined treatment with exercise and ADSC.
In conclusion, the combination of exercise and ADSC administration promoted early recovery of motor function and partially ameliorated SCI-induced NP in a rat model. Therefore, this combined treatment protocol may be a promising therapy for ameliorating NP in patients with SCI.
Acknowledgments:
We thank Prof. Zhongshu Tang of Zhongshan Ophthalmic Center, Sun Yat-sen University for providing imaging software.
Footnotes
Funding: This study was supported in part by the National Natural Science Foundation of China, Nos. 81971151 (to YW), 82201360 (to XC), 82102583 (to LW), and 82202739 (to GPM); China Postdoctoral Science Foundation, Nos. YJ20210208 and 2022M713592; Guangdong Basic and Applied Basic Research Foundation, China, No. 2021A1515110188 (to XC); the Natural Science Foundation of Guangdong Province, China, No. 2020A1515010306 (to LW); the Science and Technology Program of Guangzhou, China, No. 202102020040 (to LW).
Conflicts of interest: The authors declare that they have no conflict of interest.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Crow E, Li CH, Song LP; T-Editor: Jia Y
References
- 1.Arman A, Deng F, Goldys EM, Liu G, Hutchinson MR. In vivo intrathecal IL-1βquantification in rats: Monitoring the molecular signals of neuropathic pain. Brain Behav Immun. (2020);88:442–450. doi: 10.1016/j.bbi.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. (2006);23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 3.Battistuzzo CR, Callister RJ, Callister R, Galea MP. A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J Neurotrauma. (2012);29:1600–1613. doi: 10.1089/neu.2011.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown EV, Falnikar A, Heinsinger N, Cheng L, Andrews CE, DeMarco M, Lepore AC. Cervical spinal cord injury-induced neuropathic pain in male mice is associated with a persistent pro-inflammatory macrophage/microglial response in the superficial dorsal horn. Exp Neurol. (2021);343:113757. doi: 10.1016/j.expneurol.2021.113757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells:isolation, expansion, and differentiation. Methods. (2008);45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke D, Fullen BM, Stokes D, Lennon O. Neuropathic pain prevalence following spinal cord injury: A systematic review and meta-analysis. Eur J Pain. (2017);21:29–44. doi: 10.1002/ejp.905. [DOI] [PubMed] [Google Scholar]
- 7.Chen CF, Hu CC, Wu CT, Wu HH, Chang CS, Hung YP, Tsai CC, Chang Y. Treatment of knee osteoarthritis with intra-articular injection of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE®:a phase I/II, randomized, active-control, single-blind, multiple-center, clinical trial. Stem Cell Res Ther. (2021);12:562. doi: 10.1186/s13287-021-02631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng X, Xiao F, Xie R, Hu H, Wan Y. Alternate thermal stimulation ameliorates thermal sensitivity and modulates calbindin-D 28K expression in lamina I and II and dorsal root ganglia in a mouse spinal cord contusion injury model. FASEB J. (2021);35:e21173. doi: 10.1096/fj.202001775R. [DOI] [PubMed] [Google Scholar]
- 9.Dehdashtian A, Bratley JV, Svientek SR, Kung TA, Awan TM, Cederna PS, Kemp SW. Autologous fat grafting for nerve regeneration and neuropathic pain:current state from bench-to-bedside. Regen Med. (2020);15:2209–2228. doi: 10.2217/rme-2020-0103. [DOI] [PubMed] [Google Scholar]
- 10.Detloff MR, Smith EJ, Quiros Molina D, Ganzer PD, Houlé JD. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp Neurol. (2014);255:38–48. doi: 10.1016/j.expneurol.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dugan EA, Jergova S, Sagen J. Mutually beneficial effects of intensive exercise and GABAergic neural progenitor cell transplants in reducing neuropathic pain and spinal pathology in rats with spinal cord injury. Exp Neurol. (2020);327:113208. doi: 10.1016/j.expneurol.2020.113208. [DOI] [PubMed] [Google Scholar]
- 12.Dugan EA, Schachner B, Jergova S, Sagen J. Intensive locomotor training provides sustained alleviation of chronic spinal cord injury-associated neuropathic pain:a two-year pre-clinical study. J Neurotrauma. (2021);38:789–802. doi: 10.1089/neu.2020.7378. [DOI] [PubMed] [Google Scholar]
- 13.Eaton M. Cell therapy for neuropathic pain in spinal cord injuries. Expert Opin Biol Ther. (2004);4:1861–1869. doi: 10.1517/14712598.4.12.1861. [DOI] [PubMed] [Google Scholar]
- 14.Fadeev FO, Bashirov FV, Markosyan VA, Izmailov AA, Povysheva TV, Sokolov ME, Kuznetsov MS, Eremeev AA, Salafutdinov II, Rizvanov AA, Lee HJ, Islamov RR. Combination of epidural electrical stimulation with ex vivo triple gene therapy for spinal cord injury:a proof of principle study. Neural Regen Res. (2021);16:550–560. doi: 10.4103/1673-5374.293150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. (2010);150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Fodor PB, Paulseth SG. Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet Surg J. (2016);36:229–236. doi: 10.1093/asj/sjv135. [DOI] [PubMed] [Google Scholar]
- 17.Forouzanfar F, Amin B, Ghorbani A, Ghazavi H, Ghasemi F, Sadri K, Mehri S, Sadeghnia HR, Hosseinzadeh H. New approach for the treatment of neuropathic pain: Fibroblast growth factor 1 gene-transfected adipose-derived mesenchymal stem cells. Eur J Pain. (2018);22:295–310. doi: 10.1002/ejp.1119. [DOI] [PubMed] [Google Scholar]
- 18.Galeshi A, Ghasemi-Kasman M, Feizi F, Davoodian N, Zare L, Abedian Z. Co-administration of aspirin and adipose-derived stem cell conditioned medium improves the functional recovery of the optic pathway in a lysolecithin-induced demyelination model. Neuropsychiatr Dis Treat. (2019);15:2681–2694. doi: 10.2147/NDT.S218594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. (2007);27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson KJ, Gómez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. (2004);127((Pt 6)):1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- 21.Iqubal A, Ahmed M, Iqubal MK, Pottoo FH, Haque SE. Polyphenols as potential therapeutics for pain and inflammation in spinal cord injury. Curr Mol Pharmacol. (2021);14:714–730. doi: 10.2174/1874467213666201223111743. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. (2008);14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly SA, Rezende EL, Chappell MA, Gomes FR, Kolb EM, Malisch JL, Rhodes JS, Mitchell GS, Garland T. Exercise training effects on hypoxic and hypercapnic ventilatory responses in mice selected for increased voluntary wheel running. Exp Physiol. (2014);99:403–413. doi: 10.1113/expphysiol.2013.076018. [DOI] [PubMed] [Google Scholar]
- 24.Kim YM, Jin JJ, Lee SJ, Seo TB, Ji ES. Treadmill exercise with bone marrow stromal cells transplantation facilitates neuroprotective effect through BDNF-ERK1/2 pathway in spinal cord injury rats. J Exerc Rehabil. (2018);14:335–340. doi: 10.12965/jer.1836264.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishima K, Tachibana T, Yamanaka H, Kobayashi K, Okubo M, Maruo K, Noguchi K. Role of Rho-associated coiled-coil containing protein kinase in the spinal cord injury induced neuropathic pain. Spine J. (2021);21:343–351. doi: 10.1016/j.spinee.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Choi HY, Ju BG, Yune TY. Estrogen alleviates neuropathic pain induced after spinal cord injury by inhibiting microglia and astrocyte activation. Biochim Biophys Acta Mol Basis Dis. (2018);1864:2472–2480. doi: 10.1016/j.bbadis.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Wang Q, Ding J, Wang S, Dong C, Wu Q. Exercise training modulates glutamic acid decarboxylase-65/67 expression through TrkB signaling to ameliorate neuropathic pain in rats with spinal cord injury. Mol Pain. (2020a);16:1744806920924511. doi: 10.1177/1744806920924511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Ritzel RM, Khan N, Cao T, He J, Lei Z, Matyas JJ, Sabirzhanov B, Liu S, Li H, Stoica BA, Loane DJ, Faden AI, Wu J. Delayed microglial depletion after spinal cord injury reduces chronic inflammation and neurodegeneration in the brain and improves neurological recovery in male mice. Theranostics. (2020b);10:11376–11403. doi: 10.7150/thno.49199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang YH, Chen GW, Li XS, Jia S, Meng CY. Guanosine-5'-triphosphate cyclohydrolase 1 regulated long noncoding RNAs are potential targets for microglial activation in neuropathic pain. Neural Regen Res. (2021);16:596–600. doi: 10.4103/1673-5374.290914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Zheng J, Xiong L, Zimmermann M, Yang J. Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J Physiol. (2008);586:5701–5715. doi: 10.1113/jphysiol.2008.152348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFarlane K, Otto TE, Bailey WM, Veldhorst AK, Donahue RR, Taylor BK, Gensel JC. Effect of sex on motor function, lesion size, and neuropathic pain after contusion spinal cord injury in mice. J Neurotrauma. (2020);37:1983–1990. doi: 10.1089/neu.2019.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishihara T, Tanaka J, Sekiya K, Nishikawa Y, Abe N, Hamada T, Kitamura S, Ikemune K, Ochi S, Choudhury ME, Yano H, Yorozuya T. Chronic constriction injury of the sciatic nerve in rats causes different activation modes of microglia between the anterior and posterior horns of the spinal cord. Neurochem Int. (2020);134:104672. doi: 10.1016/j.neuint.2020.104672. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto-Okubo CE, Cassu RN, Joaquim JGF, Reis Mesquita LD, Rahal SC, Oliveira HSS, Takahira R, Arruda I, Maia L, Cruz Landim FD, Luna SPL. Chronic pain and gait analysis in dogs with degenerative hip joint disease treated with repeated intra-articular injections of platelet-rich plasma or allogeneic adipose-derived stem cells. J Vet Med Sci. (2021);83:881–888. doi: 10.1292/jvms.20-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafiei Alavi SN, Madani Neishaboori A, Hossein H, Sarveazad A, Yousefifard M. Efficacy of adipose tissue-derived stem cells in locomotion recovery after spinal cord injury:a systematic review and meta-analysis on animal studies. Syst Rev. (2021);10:213. doi: 10.1186/s13643-021-01771-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. (2003);306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 36.Reichenberger MA, Mueller W, Hartmann J, Diehm Y, Lass U, Koellensperger E, Leimer U, Germann G, Fischer S. ADSCs in a fibrin matrix enhance nerve regeneration after epineural suturing in a rat model. Microsurgery. (2016);36:491–500. doi: 10.1002/micr.30018. [DOI] [PubMed] [Google Scholar]
- 37.Richardson EJ, McKinley EC, Rahman AKMF, Klebine P, Redden DT, Richards JS. Effects of virtual walking on spinal cord injury-related neuropathic pain: A randomized, controlled trial. Rehabil Psychol. (2019);64:13–24. doi: 10.1037/rep0000246. [DOI] [PubMed] [Google Scholar]
- 38.Romero-Sandoval A, Chai N, Nutile-McMenemy N, Deleo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. (2008);1219:116–126. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saleh C, Ilia TS, Jaszczuk P, Hund-Georgiadis M, Walter A. Is transcranial magnetic stimulation as treatment for neuropathic pain in patients with spinal cord injury efficient?A systematic review. Neurol Sci. (2022);43:3007–3018. doi: 10.1007/s10072-022-05978-0. [DOI] [PubMed] [Google Scholar]
- 40.Sarveazad A, Babahajian A, Bakhtiari M, Soleimani M, Behnam B, Yari A, Akbari A, Yousefifard M, Janzadeh A, Amini N, Agah S, Fallah A, Joghataei MT. The combined application of human adipose derived stem cells and Chondroitinase ABC in treatment of a spinal cord injury model. Neuropeptides. (2017);61:39–47. doi: 10.1016/j.npep.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Sarveazad A, Janzadeh A, Taheripak G, Dameni S, Yousefifard M, Nasirinezhad F. Co-administration of human adipose-derived stem cells and low-level laser to alleviate neuropathic pain after experimental spinal cord injury. Stem Cell Res Ther. (2019);10:183. doi: 10.1186/s13287-019-1269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ:25 years of image analysis. Nat Methods. (2012);9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tashiro S, Nishimura S, Shinozaki M, Takano M, Konomi T, Tsuji O, Nagoshi N, Toyama Y, Liu M, Okano H, Nakamura M. The amelioration of pain-related behavior in mice with chronic spinal cord injury treated with neural stem/progenitor cell transplantation combined with treadmill training. J Neurotrauma. (2018);35:2561–2571. doi: 10.1089/neu.2017.5537. [DOI] [PubMed] [Google Scholar]
- 44.Tawfik VL, Nutile-McMenemy N, Lacroix-Fralish ML, Deleo JA. Efficacy of propentofylline, a glial modulating agent on existing mechanical allodynia following peripheral nerve injury. Brain Behav Immun. (2007);21:238–246. doi: 10.1016/j.bbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Tong C, Zhengyao Z, Mei L, Dongpo S, Qian H, Fengqun M. Pregabalin and gabapentin in patients with spinal cord injury-related neuropathic pain:a network meta-analysis. Pain Ther. (2021);10:1497–1509. doi: 10.1007/s40122-021-00302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. (2008);28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Younsi A, Zheng G, Scherer M, Riemann L, Zhang H, Tail M, Hatami M, Skutella T, Unterberg A, Zweckberger K. Treadmill training improves survival and differentiation of transplanted neural precursor cells after cervical spinal cord injury. Stem Cell Res. (2020);45:101812. doi: 10.1016/j.scr.2020.101812. [DOI] [PubMed] [Google Scholar]
- 48.Yousof SM, ElSayed DA, El-Baz AA, Sallam HS, Abbas F. Combined treatment of adipose derived-mesenchymal stem cells and pregabalin is superior to monotherapy for the treatment of neuropathic pain in rats. Stem Cells Int. (2021)(2021):8847110. doi: 10.1155/2021/8847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang K, Lu WC, Zhang M, Zhang Q, Xian PP, Liu FF, Chen ZY, Kim CS, Wu SX, Tao HR, Wang YZ. Reducing host aldose reductase activity promotes neuronal differentiation of transplanted neural stem cells at spinal cord injury sites and facilitates locomotion recovery. Neural Regen Res. (2022);17:1814–1820. doi: 10.4103/1673-5374.330624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou F, Gao S, Wang L, Sun C, Chen L, Yuan P, Zhao H, Yi Y, Qin Y, Dong Z, Cao L, Ren H, Zhu L, Li Q, Lu B, Liang A, Xu GT, Zhu H, Gao Z, Ma J, Xu J, Chen X. Human adipose-derived stem cells partially rescue the stroke syndromes by promoting spatial learning and memory in mouse middle cerebral artery occlusion model. Stem Cell Res Ther. (2015);6:92. doi: 10.1186/s13287-015-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Leung-Pitt Y, Deng H, Ren Y, You Z, Kem WR, Shen S, Zhang W, Mao J, Martyn JAJ. Nonopioid GTS-21 mitigates burn injury pain in rats by decreasing spinal cord inflammatory responses. Anesth Analg. (2021);132:240–252. doi: 10.1213/ANE.0000000000005274. [DOI] [PMC free article] [PubMed] [Google Scholar]


