Abstract
Objective
This study aimed to determine the clinical features, risk factors, and effective antimicrobial therapy for Carbapenem-resistant Acinetobacter baumannii (CRAB) bloodstream infection (BSI).
Methods
This was a retrospective analysis of data from patients with CRAB bacteremia in a Chinese tertiary hospital between January 2012 and October 2021. Risk factors, predictors of 30-day mortality, and effective antimicrobial therapy for CRAB BSI were identified using logistic and cox regression analyses.
Results
Data from 276 patients with Acinetobacter baumannii (AB) BSI were included, of whom 157 (56.9%) had CRAB BSI. The risk factors that were significantly associated with CRAB BSI included previous intensive care unit (ICU) stay (P < 0.001), immunocompromised status (P < 0.001), cephalosporin use (P = 0.014), and fluoroquinolone use (P = 0.007). The 30-day mortality of the CRAB BSI group was 49.7% (78/157). ICU stay after BSI (P = 0.047), sequential organ failure assessment (SOFA) score ≥10 (P < 0.001), and multiple organ failure (MOF) (P = 0.037) were independent predictors of 30-day mortality. Among antibiotic strategies for the treatment of patients with CRAB BSI, we found that definitive regimens containing cefoperazone/sulbactam were superior to those without cefoperazone/sulbactam in reducing the 30-day mortality rate (25.4% vs 53.4%, P = 0.005). After propensity score matching, we observed a significant increase in the 30-day mortality (77.8%vs 33.3%, P = 0.036) in patients receiving tigecycline monotherapy compared to those receiving cefoperazone/sulbactam monotherapy. The mortality rate of patients receiving tigecycline with cefoperazone/sulbactam was also higher than that of patients receiving cefoperazone-sulbactam monotherapy; however, the difference was not significant (28.6%vs 19.0%, P = 0.375).
Conclusion
The severity of patient conditions was significantly associated with mortality in patients with CRAB BSI. Those Patients treated with cefoperazone/sulbactam had better clinical prognoses, and tigecycline should be used with caution.
Keywords: carbapenem-resistant, Acinetobacter baumannii, bacteremia, tigecycline, cefoperazone/sulbactam
Introduction
Acinetobacter baumannii (AB) is a common hospital-acquired pathogen worldwide, causing serious organismal infections, including pneumonia, bacteremia, meningitis, catheter-associated infections, and urinary tract infections.1–3 AB bloodstream infections (BSI) frequently occur in critically ill patients, significantly prolong hospitalization, and cause mortality rates comparable to other gram-negative bacteria, with crude mortality rates of 30% to 52%.4,5 Therefore, AB BSI has become a major global health crisis.
Carbapenem antibiotics are the standard treatment for severe AB infections.6 Unfortunately, in recent years, the global prevalence of carbapenem-resistant Acinetobacter baumannii (CRAB) has increased, including the incidence of CRAB BSI.7 Surveillance data collected globally over the past decade show that the resistance rate of AB to carbapenems is as high as 88% in Europe5 and 85% in Latin America.8 A national survey in China reported that the prevalence of CRAB increased from 31.0% in 2005 to 66.7% in 2014.9 The use of antibiotics in treating CRAB is highly limited. Presently, the recommended antibiotics for the treatment of CRAB BSI are β-lactam/sulbactam combinations, tigecycline, and polymyxin.10 However, these recommendations are not supported by large-scale clinical trials, and the current clinical evidence is insufficient. Moreover, the efficacy of some recommended treatment regimens for patients with CRAB BSI is not yet clear.11 Therefore, this study aimed to determine the risk factors and predictors of 30-day mortality and investigate effective antimicrobial therapy by analyzing data from patients with CRAB BSI. This study provides a more comprehensive understanding of clinical characteristics, patient mortality, and antimicrobial therapy to improve CRAB infection control and clinical care.
Materials and Methods
Study Design and Patient Population
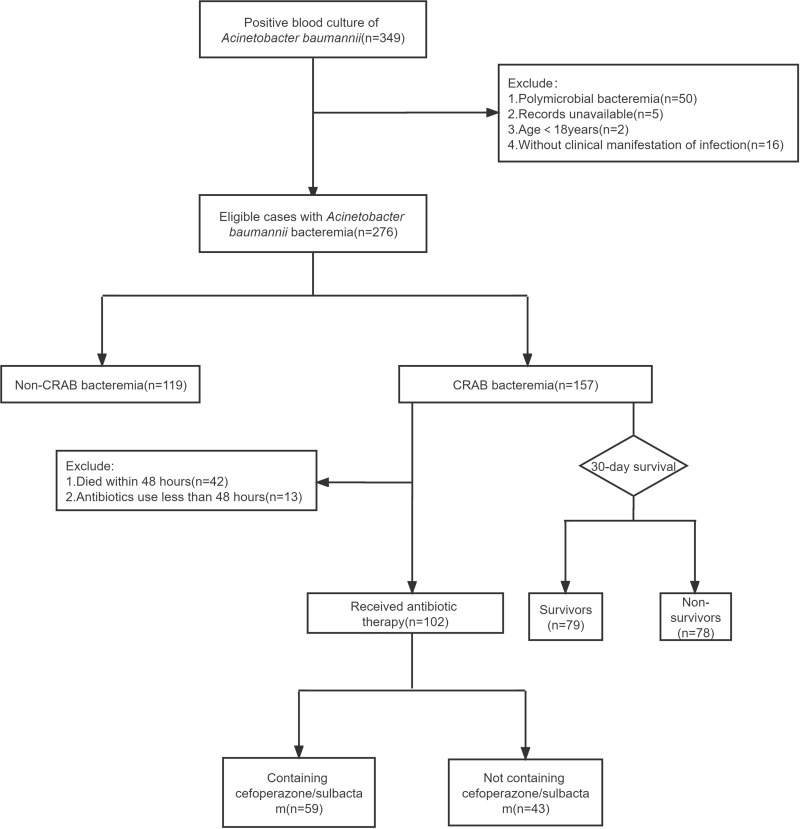
This retrospective case-control study was conducted at a 2400-bed tertiary general hospital in southern China. We collected data on patients with AB BSI from January 1, 2012, to October 31, 2021. Patients with polymicrobial bacteremia were excluded from this analysis. The analysis only included patient data from the first episode of AB BSI (Figure 1).
Figure 1.
Case identification flow chart.
Data Collection
The databases of the Laboratory Information System (LIS) and Hospital Information System (HIS) in our hospital were used to obtain clinical data on these patients. The collected data included the following: demographic characteristics, microbiological data, hospital exposure (surgical operation, hemodialysis, intensive care unit [ICU] stay, and invasive procedures before BSI), history of chronic illness, comorbid conditions (septic shock, gastrointestinal bleeding, and acute pancreatitis), source of BSI, the severity of illness, use of immunosuppressive agents and steroids before BSI, history of antibiotics use (antibiotic exposure and definitive antibiotic treatment), disease diagnosis, and clinical outcomes. Sequential [Sepsis-related] Organ Function Assessment (SOFA) and Pitt bacteremia scores were calculated at the onset of BSI to assess the severity of the condition. All-cause 30-day mortality after the onset of AB bacteremia was used as the primary clinical outcome.
Definitions
Patients with AB bacteremia were defined as patients with at least one positive blood culture while presenting with at least two of the following signs and symptoms: 1) body temperature greater than 38 °C or lower than 36 °C; 2) heart rate greater than 90 beats per minute; 3) respiratory rate greater than 20 breaths per minute; and 4) a rise in peripheral blood cell count exceeding 10×109 /L or a fall below 4×109 /L. The onset of BSI was defined as the day on which the first positive blood culture was collected. We determined the source of the infection according to the guidelines published by the Centers for Disease Control and Prevention,12 and if no other source could be identified, bacteremia was defined as a primary source.
Patients were considered to be immunosuppressed if they had the human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS), were in post-transplant, had received cancer chemotherapy, had received corticosteroid therapy with a dose of no less than 20 milligrams of prednisone per day or a cumulative dose of more than 600 milligrams of prednisone, had received biological immune modulators, or had received disease-modifying drugs (eg, cyclosporin, cyclophosphamide, hydroxychloroquine, methotrexate).
Prior antibiotic exposure was defined as the use of antibiotics for > 48 h within 30 days before AB BSI. Treatment that was started or continued on the day the findings of the antimicrobial susceptibility tests were obtained by clinicians was referred to as definitive therapy. CRAB infection was defined as AB infection due to an isolate that manifested non-susceptibility to at least one carbapenem (imipenem, meropenem, or doripenem). Failure of two or more organ systems (respiratory, renal, neurologic, hematologic, or hepatic) at the onset of BSI was defined as multiple organ failure (MOF). Septic shock was defined according to international definitions.13
Drug Dosage of the Treatment Protocol
All antibiotics involved in this study were administered by intravenous drip. Tigecycline at a dose of 100mg every 12h (q12h) for more than 48h. Cefoperazone/sulbactam (cefoperazone: sulbactam, 2:1) at a dose of 3g q6h or q8h for more than 48h. Carbapenem antibiotics which were administered for more than 48h include imipenem/cilastatin, meropenem and biapenem.
Imipenem/cistatin (imipenem: cistatin, 1:1) at a dose of 1g q6h or q8h; meropenem at a dose of 1g q8h and biapenem at a dose of 0.3g q8h.
Antimicrobial Susceptibilities
The VITEK 2 Compact system (bioMérieux, Marcy-l’Étoile, France) or MALDI-TOF MS (bioMérieux) were used to identify AB isolates, and the VITEK-2 Compact AST-GN16 (bioMérieux) or Kirby-Bauer test were used to determine in vitro antimicrobial susceptibilities. According to the Clinical and Laboratory Standards Institute (CLSI) standards, a minimum inhibitory concentration (MIC) ≥ 8 μg/mL for imipenem and meropenem was considered to indicate carbapenem resistance. Cefoperazone-sulbactam susceptibility was determined based on the breakpoints for ampicillin-sulbactam (MIC 16/8 μg/mL).14 The United States Food and Drug Administration breakpoints were used to determine tigecycline susceptibility.15 Susceptibility to other antibiotics was determined based on CLSI standards.
Statistical Analysis
Data normality for continuous variables was evaluated using Shapiro–Wilk tests. Normally distributed data are expressed as mean ± standard deviation (SD), and other data are expressed as the median and interquartile range (IQR), Categorical variables are expressed as accumulated frequencies and percentages. Groups were compared using the Mann–Whitney U-test for continuous variables and the chi-square test for categorical variables. Logistic regression analysis was used to determine the independent risk factors for CRAB BSI and predictors of 30-day mortality for CRAB BSI were identified using cox regression analysis. The relative efficacies of the different antimicrobial regimens were assessed by conducting propensity score matching (PSM) and cox regression analysis. The survival distribution function was estimated using the Kaplan-Meier product-limit method. All Statistical analyses were performed using SPSS (version25.0) software, and P < 0.05 was considered statistically significant.
Results
Microbiological Characteristics of AB
In the past decade, 276 patients with AB-BSIs were included in this study. According to the results of antimicrobial susceptibility tests, AB strains had the highest susceptibility to tigecycline, followed by cefoperazone/sulbactam, and levofloxacin, with a resistance rate of more than 50% to other antibiotics. We found, by comparing antimicrobial susceptibility profiles, that β-lactam/β-lactamase inhibitor combinations, cephalosporins, quinolones, aminoglycosides, tigecycline, and trimethoprim/sulfamethoxazole were significantly different between the CRAB and non-CRAB groups. In the CRAB group, except for tigecycline, cefoperazone/sulbactam, and levofloxacin (10%, 38.8%, and 65.6%, respectively), the incidence of other antibiotic resistance was more than 85% (Table 1).
Table 1.
Antibiotic Resistance in the CRAB Group and Non-CRAB Patient Group
| Antimirobial | TotalN=276 | CRABN=157 | Non-CRABN=119 | χ2 | P |
|---|---|---|---|---|---|
| Piperacillin/tazobactam | 55.3%(99/179) | 99.0%(98/99) | 1.3%(1/80) | 171.000 | <0.001 |
| Cefoperazone/sulbactam | 23.5%(19/81) | 38.8%(19/49) | 0(0/32) | 16.210 | <0.001 |
| Cefepime | 56.2%(154/274) | 97.4%(152/156) | 1.7%(2/118) | 250.179 | <0.001 |
| Ceftriaxone | 58.2%(131/225) | 100.0%(127/127) | 4.1%(4/98) | 209.227 | <0.001 |
| Ceftazidime | 60.3%(79/131) | 97.4%(76/78) | 5.7%(3/53) | 111.036 | <0.001 |
| Ciprofloxacin | 56.9%(128/225) | 98.4%(123/125) | 5.0%(5/100) | 197.608 | <0.001 |
| Levofloxacin | 38.4%(106/276) | 65.6%(103/157) | 2.5%(3/118) | 113.101 | <0.001 |
| Gentamicin | 53.8%(142/264) | 92.7%(140/151) | 1.8%(2/113) | 215.066 | <0.001 |
| Amikacin | 52.9%(27/51) | 89.7%(26/29) | 4.6%(1/22) | 36.373 | <0.001 |
| Tobramycin | 51.3%(119/232) | 89.4%(118/132) | 1.0%(1/100) | 177.943 | <0.001 |
| Tigecycline | 5.7%(10/177) | 10.0%(10/100) | 0(0/77) | 6.393 | 0.011 |
| Trimethoprim/sulfamethoxazole | 52.2%(144/276) | 87.8%(137/156) | 5.9%(7/119) | 181.695 | <0.001 |
Abbreviation: CRAB, carbapenem-resistant Acinetobacter baumannii.
Demographic Characteristics of Patients with AB BSI
We included data from 276 patients with AB BSI and analyzed their characteristics. The median age of patients was 62 years (IQR: 49.25–73.75), with men accounting for 69.6% of the total patients (192/276). The incidence of nosocomial infections was 93.5% (258/276), and approximately half of the patients (54.3%, 150/276) were diagnosed in the ICU. The most prevalent underlying diseases in these individuals were cerebrovascular and liver diseases (20.7%, 57/276), followed by malignant tumors (19.2%, 46/276). Comorbidities were prevalent, of which hypoproteinemia and septic shock were the most common (72.8%, 201/276; and 24.6%, 68/276, respectively). Respiratory tract infections (50.0%, 138/276) and abdominal infections (23.6%, 65/276) were the most common sources of CRAB bacteremia. The all-cause 30-day mortality rate in these individuals was 33.0% (91/276). Detailed patient data are shown in Table 2.
Table 2.
Characteristics of Patients with CRAB BSI and Non-CRAB BSI
| Variable | TotalN=276 | CRABN=157 | Non-CRABN=119 | P |
|---|---|---|---|---|
| Age(IQR) | 62(49.25–73.75) | 59(48–73) | 64(53–74) | 0.097 |
| Male | 192(69.6%) | 114(72.6%) | 78(65.5%) | 0.206 |
| Nosocomial infection | 258(93.5%) | 147(93.6%) | 111(93.3%) | 0.906 |
| ICU stay prior to BSI | 150(54.3%) | 123(78.3%) | 27(22.7%) | <0.001 |
| Hospital stay >30 days prior to BSI | 15(5.4%) | 13(8.3%) | 2(1.7%) | 0.029 |
| Surgical operation | 105(38.0%) | 59(37.6%) | 46(38.7%) | 0.855 |
| Hemodialysis | 50(18.1%) | 37(23.6%) | 13(10.9%) | 0.007 |
| Illness severity at time of BSI | ||||
| PITT(IQR) | 2(1–5) | 4(2–6) | 1(0–2) | <0.001 |
| SOFA(IQR) | 6.5(4–10) | 8(6–11) | 4(2–6) | <0.001 |
| Invasive procedure | 214(77.5%) | 146(93.0%) | 68(57.1%) | <0.001 |
| Urinary catheterization | 159(57.6%) | 128(81.5%) | 31(26.1%) | <0.001 |
| Gastrointestinal catheterization | 147(53.3%) | 115(73.2%) | 32(26.9%) | <0.001 |
| Central venous catheterization | 134(48.7%) | 100(64.1%) | 34(28.6%) | <0.001 |
| Drainage tube | 108(39.1%) | 70(44.6%) | 38(31.9%) | 0.033 |
| Tracheal intubation | 102(37.0%) | 84(53.5%) | 18(15.1%) | <0.001 |
| Tracheotomy | 36(13.0%) | 26(16.6%) | 10(8.4%) | 0.046 |
| Antimicrobial exposure within 30 days | ||||
| Piperacillin-tazobactam | 50(18.1%) | 35(22.3%) | 15(12.6%) | 0.038 |
| Cephalosporin antibiotics | 39(14.1%) | 28(17.8%) | 11(9.2%) | 0.042 |
| Fluoroquinolone antibiotics | 37(13.7%) | 33(21.0%) | 4(3.4%) | <0.001 |
| Cefoperazone-sulbactam | 36(13.0%) | 26(16.6%) | 10(8.4%) | 0.046 |
| Underlying disease | ||||
| Cerebrovascular diseases | 57(20.7%) | 32(20.4%) | 25(21.0%) | 0.899 |
| Liver disease | 57(20.7%) | 34(21.7%) | 23(19.3%) | 0.636 |
| Malignant tumor | 53(19.2%) | 26(16.6%) | 27(22.7%) | 0.201 |
| Diabetes | 48(17.4%) | 27(17.2%) | 21(17.6%) | 0.922 |
| Immunocompromised status | 46(16.7%) | 38(24.2%) | 8(6.7%) | <0.001 |
| Comorbid conditions | ||||
| Hypoproteinemia | 201(72.8%) | 126(80.3%) | 75(63.0%) | 0.001 |
| Septic shock | 68(24.6%) | 61(38.9%) | 7(5.9%) | <0.001 |
| Stroke | 48(17.4%) | 27(17.2%) | 21(17.6%) | 0.922 |
| Aute cerebral hemorrhage | 32(11.6%) | 23(14.6%) | 9(7.6%) | 0.069 |
| MOF | 32(11.6%) | 27(17.2%) | 5(4.2%) | 0.001 |
| Gastrointestinal bleeding | 28(10.1%) | 15(9.6%) | 13(10.9%) | 0.709 |
| Acute pancreatitis | 16(5.8%) | 11(7.0%) | 5(4.2%) | 0.323 |
| Source of BSI | ||||
| Respiratory tract infection | 138(50.0%) | 92(58.6%) | 46(38.7%) | 0.001 |
| Abdominal infection | 65(23.6%) | 32(20.4%) | 33(27.7%) | 0.154 |
| Primary BSI | 44(15.9%) | 18(11.5%) | 26(21.8%) | 0.020 |
| CVC-related infection | 20(7.2%) | 9(5.7%) | 11(9.2%) | 0.265 |
| Urinary infection | 9(3.3%) | 6(3.8%) | 3(2.5%) | 0.795 |
| Outcome | ||||
| 30-day mortality | 91(33.0%) | 78(49.7%) | 13(10.9%) | <0.001 |
Abbreviations: CRAB, carbapenem-resistant Acinetobacter baumannii; BSI, bloodstream infection; ICU, intensive care unit; SOFA, Sequential Organ Function Assessment score; PITT, Pitt bacteremia score; CVC, central venous catheter; MOF, multiple organ failure.
Risk Factors for the Emergence of CRAB BSI
Univariate analysis showed that previous ICU stays, hospital stay > 30 days before BSI, hemodialysis, higher PITT and SOFA scores, invasive procedure, antimicrobial exposure within 30 days, immunocompromised status, hypoproteinemia, septic shock, MOF, sources of respiratory tract infections, and primary BSI were associated with CRAB BSI. After adjusting for confounding factors, the results of multivariate logistic regression analysis revealed that a previous ICU stay [P < 0.001; odds ratio, OR(95% CI):11.990 (5.438–26.436)], immunocompromised status [P < 0.001; OR(95% CI):7.075(2.447–20.452)], exposure to cephalosporin antibiotics [P = 0.014; OR(95% CI):3.644(1.296–10.246)], and exposure to fluoroquinolone antibiotics [P = 0.007; OR(95% CI):5.813(1.632–20.703)] within 30 days before BSI were independent risk factors related to carbapenem resistance (Table 3).
Table 3.
Risk Factors for Patients with CRAB BSI
| Risk Factors | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR(95% CI) | P | OR(95% CI) | P | |
| Hospital exposure | ||||
| ICU stay prior to BSI | 12.327(6.951–21.861) | <0.001 | 11.990(5.438–26.436) | <0.001 |
| Hospital stay >30 days prior to BSI | 5.281(1.168–23.872) | 0.031 | 4.258(0.666–27.235) | 0.126 |
| Hemodialysis | 2.514(1.269–4.981) | 0.008 | 1.097(0.454–2.646) | 0.838 |
| Invasive procedure | 9.955(4.883–20.294) | <0.001 | 2.240(0.833–6.022) | 0.110 |
| Antimicrobial exposure within 30 days | ||||
| Piperacillin-tazobactam | 1.989(1.029–3.845) | 0.041 | 1.464(0.614–3.491) | 0.390 |
| Cephalosporin | 2.131(1.014–4.479) | 0.046 | 3.644(1.296–10.246) | 0.014 |
| Fluoroquinolone | 7.651(2.629–22.267) | <0.001 | 5.813(1.632–20.703) | 0.007 |
| Cefoperazone-sulbactam | 2.163(0.999–4.683) | 0.050 | 1.613(0.576–4.517) | 0.363 |
| Immunocompromised status | 4.431(1.981–9.911) | <0.001 | 7.075(2.447–20.452) | <0.001 |
| Hypoproteinemia | 2.385(1.388–4.097) | 0.002 | 1.191(0.571–2.486) | 0.642 |
| Respiratory tract infection | 2.246(1.380–3.655) | 0.001 | 0.915(0.440–1.900) | 0.811 |
| Primary BSI | 0.463(0.240–0.892) | 0.021 | 0.481(0.173–1.334) | 0.160 |
Abbreviations: CRAB, carbapenem-resistant Acinetobacter baumannii; BSI, bloodstream infection; ICU, intensive care unit.
Risk Factors Associated with 30-Day Mortality in Patients with CRAB BSI
For patients with CRAB BSI, the 30-day all-cause mortality rate was 49.7%. The cox regression analysis results showed that ICU stays after BSI [P = 0.047, OR(95% CI): 2.136(1.010–4.516)], SOFA ≥10 [P < 0.001, OR(95% CI): 3.343(2.012–5.556)], and MOF [P = 0.037, OR(95% CI): 1.804(1.035–3.144)] were significantly associated with the 30-day mortality. The data are presented in Table 4.
Table 4.
Analysis of the Risk Factors for 30-Day Mortality in Patients with CRAB BSI
| Variable | Survival(n=78) | Mortality(n=79) | Multivariable Analysis | |
|---|---|---|---|---|
| OR(95% CI) | P | |||
| ICU stay after BSI | 54(69.2%) | 71(89.9%) | 2.136(1.010–4.516) | 0.047 |
| SOFA≥10 | 13(16.7%) | 51(64.6%) | 3.343(2.012–5.556) | <0.001 |
| MOF | 3(3.9%) | 24(30.4%) | 1.804(1.035–3.144) | 0.037 |
| Immunocompromised status | 10(12.8%) | 28(35.4%) | – | 0.329 |
| Age>60 | 32(41.0%) | 44(55.7%) | – | 0.879 |
| Hemodialysis | 13(16.7%) | 24(30.4%) | – | 0.923 |
Abbreviations: CRAB, carbapenem-resistant Acinetobacter baumannii; BSI, bloodstream infection; ICU, intensive care unit; SOFA, Sequential Organ Function Assessment score; MOF, multiple organ failure.
The Effect of Definitive Antimicrobial Treatment on the Mortality of Patients with CRAB BSI
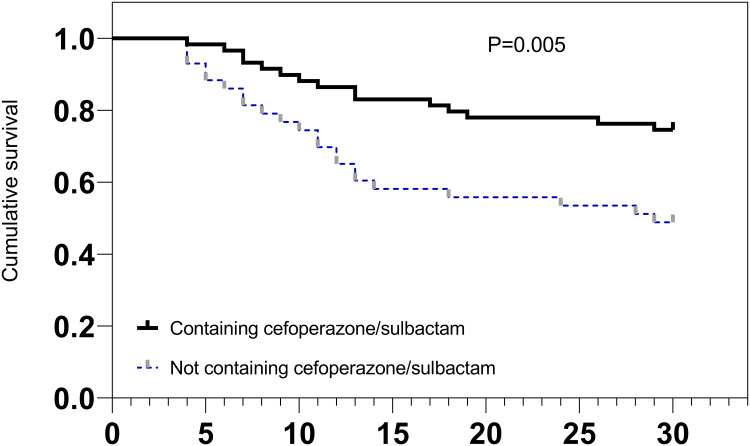
In this study, 59 patients with CRAB BSI received definitive therapy containing cefoperazone/sulbactam, including cefoperazone/sulbactam monotherapy and tigecycline combined with cefoperazone/sulbactam therapy, of whom 15 patients (25.4%) died within 30 days. Moreover, 43 patients with CRAB BSI received definitive therapy not containing cefoperazone/sulbactam, including tigecycline monotherapy, carbapenem monotherapy, and other regimens, of whom 22 patients (51.2%) died within 30 days (Table 5). Patients with CRAB BSI who received definitive therapy containing cefoperazone/sulbactam had significantly lower 30-day mortality rates than those who received definitive therapy without cefoperazone/sulbactam (P = 0.005), according to the Kaplan-Meier survival curve (Figure 2).
Table 5.
The Effect of Definitive Antimicrobial Regimens on the 30-Day Mortality of Patients with CRAB BSI
| Antimicrobial Regimen | No. of Deceased Patients/Total No. of Patients(%) | Cox Regression | |||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| OR(95% CI) | P | OR(95% CI) | P | ||
| Containing cefoperazone/sulbactam | 15/59(25.4) | – | – | – | – |
| Cefoperazone/sulbactam | 8/37(21.6) | Reference | – | Reference | – |
| Cefoperazone/sulbactam+Tigecycline | 7/22(31.8) | 1.524(0.552–4.206) | 0.416 | 1.687(0.582–4.889) | 0.335 |
| Not containing Cefoperazone/sulbactam | 23/43(53.4) | – | – | – | – |
| Tigecycline | 7/9(77.8) | 5.305(1.905–14.775) | 0.001 | 5.707(1.833–17.772) | 0.003 |
| Carbapenem | 10/21(47.6) | 2.660(1.048–6.753) | 0.040 | 3.087(1.162–8.198) | 0.024 |
| Other regimens | 6/13(46.2) | 2.365(0.819–6.830) | 0.112 | 3.439(1.032–11.466) | 0.044 |
Abbreviations: CRAB, carbapenem-resistant Acinetobacter baumannii; BSI, bloodstream infection;
Figure 2.
30-Day survival curve of patients with CRAB BSI treated with different definitive therapies.
In subsequent analysis, cefoperazone/sulbactam monotherapy served as a control group, and an additional multivariate cox regression analysis after controlling for confounders revealed that patients with CRAB BSI who had received tigecycline with cefoperazone/sulbactam therapy had a higher 30-day mortality rate [P = 0.221, OR (95% CI): 2.687(0.551–13.100)]; however, the result was not statistically significant. In addition, the definitive regimens not containing cefoperazone/sulbactam also showed a higher 30-day mortality rate compared to cefoperazone/sulbactam monotherapy, including tigecycline monotherapy [P = 0.033, OR(95% CI): 14.475(1.248–167.875)], carbapenem monotherapy [P = 0.010, OR(95% CI): 7.577(1.618–35.482)], and other regimens [P = 0.031, OR(95% CI):6.950(1.189–40.624)]. The highest 30-day mortality rate was observed with tigecycline monotherapy (77.8%, 7/9) (Table 5).
After the adjustment of PSM, the results are as before, patients with CRAB BSI who had received tigecycline with cefoperazone/sulbactam therapy had a higher 30-day mortality rate [P = 0.375, OR (95% CI): 1.784(0.497–6.409)] than those treated with cefoperazone/sulbactam monotherapy, however, the difference was not statistically significant. Meanwhile, the definitive regimens not containing cefoperazone/sulbactam also showed a higher 30-day mortality rate compared to cefoperazone/sulbactam monotherapy, including tigecycline monotherapy [P = 0.036, OR(95% CI): 4.607(1.105–19.210)], carbapenem monotherapy [P = 0.033, OR(95% CI): 9.646(1.203–77.328)], and other regimens [P = 0.046, OR(95% CI): 8.892(1.038–76.140)] (Table 6).
Table 6.
The Effect of Definitive Antimicrobial Regimens Adjusted by PSM on the 30-Day Mortality of Patients with CRAB BSI
| Antimicrobial Regimen | No. of Deceased Patients/Total No. of Patients(%) | Cox Regression | |||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| OR(95% CI) | P | OR(95% CI) | P | ||
| Cefoperazone/sulbactam | 4/21(19.0) | Reference | – | Reference | – |
| Cefoperazone/sulbactam+Tigecycline | 6/21(28.6) | 1.643(0.463–5.827) | 0.442 | 1.784(0.497–6.409) | 0.375 |
| Cefoperazone/sulbactam | 3/9(33.3) | Reference | – | Reference | – |
| Tigecycline | 7/9(77.8) | 3.352(0.856–13.128) | 0.082 | 4.607(1.105–19.210) | 0.036 |
| Cefoperazone/sulbactam | 1/18(5.6) | Reference | – | Reference | |
| Carbapenem | 8/18(44.4) | 9.758(1.218–78.173) | 0.032 | 9.646(1.203–77.328) | 0.033 |
| Cefoperazone/sulbactam | 1/13(7.7) | Reference | – | Reference | – |
| Other regimens | 6/13(46.2) | 7.088(0.851–59.012) | 0.070 | 8.892(1.038–76.140) | 0.046 |
Discussion
CRAB bacteremia has become a challenging clinical dilemma worldwide owing to its high antimicrobial resistance, limited therapeutic options, and high mortality rates.16 In this study, we summarized the clinical features of CRAB and non-CRAB bacteremia and identified the risk factors for CRAB bacteremia. Moreover, we evaluated the predictors of 30-day mortality and treatment effects of different antibiotic regimens in patients with CRAB BSI.
Carbapenem resistance is mainly caused by carbapenemase production by bacterial strains.17,18 Notably, these carbapenemase-producing strains are also highly prone to acquire other resistance mechanisms, including binding sites for quinolones and production of modification enzymes against aminoglycosides, eventually becoming highly resistant to other common antibiotics including aminoglycosides, quinolones, and broad-spectrum β-lactams.1,10,19 In the present study, in patients with CRAB BSI, except for tigecycline, cefoperazone/sulbactam, and levofloxacin, the incidence of other antibiotic resistance was more than 85%, which is consistent with previous studies.20
Our research revealed that some clinical characteristics were significantly different between patients with CRAB and non-CRAB bacteremia. Patients with CRAB BSI had more distinct hospital exposure before bacteremias, such as a hospital stay of more than 30 days, admission to the ICU, hemodialysis, and invasive procedures. This result may be attributed to AB biofilm formation, which allows the bacteria to survive for long periods in the hospital environment, particularly on the surfaces of medical devices.21 Moreover, bacteremia may develop after the disruption of the skin and mucosal barrier of patients through invasive procedures.22 Strains with high biofilm-forming capacity can develop significant resistance to several categories of antimicrobial agents, including carbapenems, possibly because biofilms evade antibiotics by shielding the bacteria, and isolates embedded within biofilms face stress due to high osmolarity, which causes changes in bacterial morphology, resulting in drug resistance.21 In addition, long hospital stays, ICU admissions, and hemodialysis indicate that the patient is in a severe condition and immunocompromised, increasing the chances of contracting CRAB. The condition of these patients was further aggravated after contracting CRAB BSI; they had higher PITT and SOFA scores and were more susceptible to shock and MOF at bacteremia onset and had higher mortality. Therefore, we suggest that hospitals should strengthen the monitoring of environmental hygiene and the prevention and control policies of nosocomial infections, improve the compliance of the medical staff and inpatient wards with regular hand-hygiene practices, and strictly adhere to the indications for invasive procedures to help control the spread of CRAB.
In addition, in our study, a multivariate analysis revealed that previous ICU stay, immunocompromised status, and cephalosporin and quinolone exposure were independent risk factors for the acquisition of CRAB BSI. Exposure to antibiotics is one of the most common risk factors for CRAB infection, and the use of carbapenems, cephalosporins, and fluoroquinolones before BSI can promote the occurrence of CRAB bacteremia.23,24 These drugs exert selective pressure to promote the growth of bacteria carrying mobile genetic elements, such as plasmids, transposons, and integrons, which harbor antimicrobial resistance determinants, including carbapenem resistance. Therefore, when these elements are transferred to AB, they can lead to carbapenem resistance.25
Patients in the ICU are typically in a critical condition with low immune function, and usually undergo invasive procedures and have already been exposed to multiple antibiotics, making them vulnerable to CRAB infection.26,27 However, invasive procedures were not an independent risk factor for the acquisition of CRAB in our study. This may be attributed to the fact that almost all patients had experienced invasive procedures during their ICU stay, and several patients had more than one invasive device installed at the same time. Therefore, these factors can interfere with each other and weaken their mutual influences.
In the present study, the 30-day all-cause mortality rate for patients with CRAB bacteremia was 49.7%. Moreover, the cox regression analysis revealed that ICU stay after BSI, MOF, and SOFA score ≥ 10 at the onset of CRAB bacteremia were risk factors for mortality. Because antibiotic options are limited for critically ill patients infected with CRAB in the ICU, the infection is difficult to control, making these patients more susceptible to MOF and consequently high SOFA scores, which leads to higher mortality.20,28
Most antibiotics are not effective against CRAB BSI infections, limiting the treatment options to only a few drugs, such as β-lactam/sulbactam combinations and tetracycline antibiotics recommended by the Infectious Diseases Society of America.10 Of these, cefoperazone-sulbactam and tigecycline are commonly used in China.29 A case-control study has revealed that the prognosis of patients with CRAB BSI was improved with cefoperazone-sulbactam treatment.30 In addition, a previous study has reported that patients with CRAB treated with cefoperazone-sulbactam had lower 30-day mortality rates than those treated with imipenem-cilastatin.31 Consistent with these studies, our results also suggest that cefoperazone-sulbactam regimens are more effective in patients with CRAB BSI compared to other regimens. Sulbactam can increase the susceptibility of CRAB to β-lactam antibiotics by inhibiting β-lactamases, especially OXA-type carbapenem hydrolases, and is subsequently often used in combination with cefoperazone or ampicillin.32 Sulbactam also has additional antibacterial effects as it exhibits direct bacteriostatic activity against AB by inhibiting penicillin-binding proteins 1 and 3.32 Furthermore, the resistance rate to sulbactam is very substantially low because spontaneous resistance in sulbactam-susceptible strains is due to the pbp3 mutation, which confers a fitness penalty to the organism.33 Collectively, these findings fully justify the rationality and effectiveness of cefoperazone-sulbactam in the treatment of individuals with CRAB BSI.
The effectiveness of tigecycline in treating CRAB bacteremia has been a controversial topic. A meta-analysis has revealed that tigecycline was generally safe and well-tolerated in the treatment of secondary bacteremia, with cure rates similar to those of comparative standard therapies.34 In contrast, several studies have shown less success.30,35,36 A retrospective study has reported that patients with CRAB BSI who received tigecycline therapy had significantly higher 28-day mortality rates than those treated with cefoperazone/sulbactam.30 Niu et al20 have also indicated that tigecycline use was an independent risk factor for 28-day mortality in patients with CRAB BSI. Moreover, the United States Food and Drug Administration (FDA) safety report warned of a higher mortality risk related to intravenous tigecycline compared to other antibiotics.37 Consistent with these studies and the FDA evaluation, in our study, tigecycline monotherapy resulted in higher mortality of patients with CRAB BSI. Notably, the FDA approval of tigecycline is limited to complex intra-abdominal, complex skin and skin structure infections, and community-acquired bacterial pneumonia (CABP) in adults,35 but not BSI. Moreover, tigecycline can rapidly achieve tissue distribution after administration, resulting in low serum concentrations. In critical infections with a high bacteremia risk, poor serum drug concentrations can only lead to an antibacterial rather than a bactericidal response, which may result in delayed clearance of bacteremia and higher mortality rates.38 However, in our further analysis, patients who received tigecycline combined with cefoperazone-sulbactam still had higher mortality than patients who had received cefoperazone/sulbactam monotherapy, which could not be explained by low serum levels and its bacteriostatic action. This suggests that the toxicity of tigecycline itself may have played a role in the high mortality rate. As shown in a previous study, septic shock, superinfections, and adverse events developed more often in patients who received tigecycline;39 therefore, combination therapy with tigecycline should also be considered carefully. Nevertheless, the difference in the mortality was not significant, which might be attributed to the insufficient sample size; therefore, this finding warrants further investigation. In addition, in our study, most patients who received tigecycline therapy were severely ill, and thereby had a worse disease progression. In conclusion tigecycline should be avoided when other effective antibiotic options are available.
Our study has some limitations. First, it was a retrospective study and, therefore, susceptible to selection and recall biases. Second, the dose and duration of exudation of the antimicrobial drugs, which can influence the treatment effect, were not included in our analysis. Third, the number of patients per treatment option was limited and the sample size was insufficient.
Conclusion
This study showed that CRAB BSI had more distinct hospital exposure and was associated with higher mortality rates than non-CRAB bacteremia; previous ICU stay, immunocompromised status, and quinolone and cephalosporin exposure were determined as risk factors associated with its emergence. Moreover, cefoperazone-sulbactam was significantly effective in patients with CRAB BSI, while critical illness and tigecycline use (either alone or in combination with cefoperazone-sulbactam) were associated with higher mortality. We advocate that tigecycline be used cautiously and recommend cefoperazone-sulbactam as the drug of choice for the treatment of CRAB BSI.
Funding Statement
This work was supported by the Science and Technology Research Project of Education Department of Jiangxi Province (No.GJJ180154), the Chinese Medical Science and Technology Research Projects of Jiangxi Provincial Administration of Traditional Chinese Medicine (No.2019A264).
Ethics Approval and Consent to Participate
Informed consent was acquired from each participant included in the study. This study was approved by the Second Affiliated Hospital of Nanchang University Medical Research Ethics Committee (No. Review-2021-116).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–582. doi: 10.1128/cmr.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Paulus T, Lugenheim M, et al. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J Infect. 2012;64(3):282–290. doi: 10.1016/j.jinf.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 3.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46(8):1254–1263. doi: 10.1086/529198 [DOI] [PubMed] [Google Scholar]
- 4.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Di. 2004;39(3):309–317. doi: 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 5.Nutman A, Glick R, Temkin E, et al. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin Microbiol Infect. 2014;20(12):O1028–O1034. doi: 10.1111/1469-0691.12716 [DOI] [PubMed] [Google Scholar]
- 6.Amin M, Navidifar T, Shooshtari FS, et al. Association between biofilm formation, structure, and the expression levels of genes related to biofilm formation and biofilm-specific resistance of Acinetobacter baumannii strains isolated from burn infection in Ahvaz, Iran. Infect Drug Resist. 2019;12:3867–3881. doi: 10.2147/idr.S228981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P, Niu W, Li H, et al. Rapid detection of Acinetobacter baumannii and molecular epidemiology of carbapenem-resistant A. baumannii in two comprehensive hospitals of Beijing, China. Front Microbiol. 2015;6:997. doi: 10.3389/fmicb.2015.00997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gales AC, Castanheira M, Jones RN, Sader HS. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010). Diagn Microbiol Infect Dis. 2012;73(4):354–360. doi: 10.1016/j.diagmicrobio.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 9.Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect. 2016;22(Suppl 1):S9–S14. doi: 10.1016/j.cmi.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 10.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Guidance on the treatment of AmpC β-lactamase-producing enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis. 2022;74(12):2089–2114. doi: 10.1093/cid/ciab1013 [DOI] [PubMed] [Google Scholar]
- 11.Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis. 2010;51(1):79–84. doi: 10.1086/653120 [DOI] [PubMed] [Google Scholar]
- 12.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 13.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones RN, Barry AL, Packer RR, Gregory WW, Thornsberry C. In vitro antimicrobial spectrum, occurrence of synergy, and recommendations for dilution susceptibility testing concentrations of the cefoperazone-sulbactam combination. J Clin Microbiol. 1987;25(9):1725–1729. doi: 10.1128/jcm.25.9.1725-1729.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casal M, Rodríguez F, Johnson B, et al. Influence of testing methodology on the tigecycline activity profile against presumably tigecycline-non-susceptible Acinetobacter spp. J Antimicrob Chemother. 2009;64(1):69–72. doi: 10.1093/jac/dkp169 [DOI] [PubMed] [Google Scholar]
- 16.Du X, Xu X, Yao J, et al. Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: a systematic review and meta-analysis. Am J Infect Control. 2019;47(9):1140–1145. doi: 10.1016/j.ajic.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 17.Vijayakumar S, Biswas I, Veeraraghavan B. Accurate identification of clinically important Acinetobacter spp.: an update. Future Sci OA. 2019;5(6):Fso395. doi: 10.2144/fsoa-2018-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turton JF, Ward ME, Woodford N, et al. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett. 2006;258(1):72–77. doi: 10.1111/j.1574-6968.2006.00195.x [DOI] [PubMed] [Google Scholar]
- 19.Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect. 2006;12(9):826–836. doi: 10.1111/j.1469-0691.2006.01456.x [DOI] [PubMed] [Google Scholar]
- 20.Niu T, Xiao T, Guo L, et al. Retrospective comparative analysis of risk factors and outcomes in patients with carbapenem-resistant Acinetobacter baumannii bloodstream infections: cefoperazone-sulbactam associated with resistance and tigecycline increased the mortality. Infect Drug Resist. 2018;11:2021–2030. doi: 10.2147/idr.S169432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy S, Chowdhury G, Mukhopadhyay AK, Dutta S, Basu S. Convergence of biofilm formation and antibiotic resistance in Acinetobacter baumannii infection. Front Med. 2022;9:793615. doi: 10.3389/fmed.2022.793615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo N, Xue W, Tang D, Ding J, Zhao B. Risk factors and outcomes of hospitalized patients with blood infections caused by multidrug-resistant Acinetobacter baumannii complex in a hospital of Northern China. Am J Infect Control. 2016;44(4):e37–e39. doi: 10.1016/j.ajic.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 23.Sheng WH, Liao CH, Lauderdale TL, et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis. 2010;14(9):e764–e769. doi: 10.1016/j.ijid.2010.02.2254 [DOI] [PubMed] [Google Scholar]
- 24.Huang ST, Chiang MC, Kuo SC, et al. Risk factors and clinical outcomes of patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect. 2012;45(5):356–362. doi: 10.1016/j.jmii.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 25.Chusri S, Silpapojakul K, McNeil E, Singkhamanan K, Chongsuvivatwong V. Impact of antibiotic exposure on occurrence of nosocomial carbapenem-resistant Acinetobacter baumannii infection: a case control study. J Infect Chemother. 2015;21(2):90–95. doi: 10.1016/j.jiac.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 26.García-Garmendia JL, Ortiz-Leyba C, Garnacho-Montero J, et al. Risk factors for Acinetobacter baumannii nosocomial bacteremia in critically ill patients: a cohort study. Clin Infect Dis. 2001;33(7):939–946. doi: 10.1086/322584 [DOI] [PubMed] [Google Scholar]
- 27.Ye JJ, Huang CT, Shie SS, et al. Multidrug resistant Acinetobacter baumannii: risk factors for appearance of imipenem resistant strains on patients formerly with susceptible strains. PLoS One. 2010;5(4):e9947. doi: 10.1371/journal.pone.0009947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appaneal HJ, Lopes VV, LaPlante KL, Caffrey AR. Treatment, clinical outcomes, and predictors of mortality among a national cohort of admitted patients with Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2022;66(3):e0197521. doi: 10.1128/aac.01975-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan X, He L, Hu B, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. 2016;22(Suppl 1):S15–S25. doi: 10.1016/j.cmi.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 30.Niu T, Luo Q, Li Y, Zhou Y, Yu W, Xiao Y. Comparison of tigecycline or cefoperazone/sulbactam therapy for bloodstream infection due to carbapenem-resistant Acinetobacter baumannii. Antimicrob Resist Infect Control. 2019;8:52. doi: 10.1186/s13756-019-0502-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi JY, Kim CO, Park YS, et al. Comparison of efficacy of cefoperazone/sulbactam and imipenem/cilastatin for treatment of Acinetobacter bacteremia. Yonsei Med J. 2006;47(1):63–69. doi: 10.3349/ymj.2006.47.1.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ku YH, Yu WL. Cefoperazone/sulbactam: new composites against multiresistant gram negative bacteria? Infect Genet Evol. 2021;88:104707. doi: 10.1016/j.meegid.2021.104707 [DOI] [PubMed] [Google Scholar]
- 33.Penwell WF, Shapiro AB, Giacobbe RA, et al. Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob Agents Chemother. 2015;59(3):1680–1689. doi: 10.1128/aac.04808-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardiner D, Dukart G, Cooper A, Babinchak T. Safety and efficacy of intravenous tigecycline in subjects with secondary bacteremia: pooled results from 8 Phase III clinical trials. Clin Infect Dis. 2010;50(2):229–238. doi: 10.1086/648720 [DOI] [PubMed] [Google Scholar]
- 35.Shin JA, Chang YS, Kim HJ, et al. Clinical outcomes of tigecycline in the treatment of multidrug-resistant Acinetobacter baumannii infection. Yonsei Med J. 2012;53(5):974–984. doi: 10.3349/ymj.2012.53.5.974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis. 2012;54(12):1699–1709. doi: 10.1093/cid/cis270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixit D, Madduri RP, Sharma R. The role of tigecycline in the treatment of infections in light of the new black box warning. Expert Rev Anti Infect Ther. 2014;12(4):397–400. doi: 10.1586/14787210.2014.894882 [DOI] [PubMed] [Google Scholar]
- 38.Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin Infect Dis. 2005;41(Suppl 5):S333–S340. doi: 10.1086/431674 [DOI] [PubMed] [Google Scholar]
- 39.Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother. 2011;66(9):1963–1971. doi: 10.1093/jac/dkr242 [DOI] [PubMed] [Google Scholar]