Abstract
Macrophage infiltration into adipose tissue is a key pathological factor inducing adipose tissue dysfunction and contributing to obesity-induced inflammation and metabolic disorders. In this review, we aim to present the most recent research on macrophage heterogeneity in adipose tissue, with a focus on the molecular targets applied to macrophages as potential therapeutics for metabolic diseases. We begin by discussing the recruitment of macrophages and their roles in adipose tissue. While resident adipose tissue macrophages display an anti-inflammatory phenotype and promote the development of metabolically favorable beige adipose tissue, an increase in pro-inflammatory macrophages in adipose tissue has negative effects on adipose tissue function, including inhibition of adipogenesis, promotion of inflammation, insulin resistance, and fibrosis. Then, we presented the identities of the newly discovered adipose tissue macrophage subtypes (e.g. metabolically activated macrophages, CD9+ macrophages, lipid-associated macrophages, DARC+ macrophages, and MFehi macrophages), the majority of which are located in crown-like structures within adipose tissue during obesity. Finally, we discussed macrophage-targeting strategies to ameliorate obesity-related inflammation and metabolic abnormalities, with a focus on transcriptional factors such as PPARγ, KLF4, NFATc3, and HoxA5, which promote macrophage anti-inflammatory M2 polarization, as well as TLR4/NF-κB-mediated inflammatory pathways that activate pro-inflammatory M1 macrophages. In addition, a number of intracellular metabolic pathways closely associated with glucose metabolism, oxidative stress, nutrient sensing, and circadian clock regulation were examined. Understanding the complexities of macrophage plasticity and functionality may open up new avenues for the development of macrophage-based treatments for obesity and other metabolic diseases.
Keywords: macrophages, adipose tissue, plasticity, obesity, metabolic diseases
1. Introduction
Obesity has become a global pandemic, and its prevalence is increasing at an alarming rate (1). The rise in the prevalence of obesity significantly increases the risk of chronic metabolic diseases, such as cardiovascular disease, diabetes, hypertension, and cancer, and have a detrimental impact on both health and quality of life. Clarifying the pathogenesis of obesity is crucial for the prevention, treatment, and management of chronic metabolic diseases associated with obesity.
Obesity is characterized by an increase in the accumulation of macrophages in adipose tissue, which is accompanied by adipose tissue dysfunction, such as reduced adipogenesis and lipid storage capacity, adipocyte necrosis, inflammation, insulin resistance, and fibrosis (2). Adipose tissue stores excess energy in two ways: adipocyte hypertrophy and proliferation. Adipocyte proliferation is the healthy development of adipose tissue driven by preadipocyte proliferation and differentiation, whereas adipocyte hypertrophy is a pathological expansion of existing adipocytes with increased lipid storage and is closely related to adipocyte dysfunction (3). Hypertrophic adipocytes secrete a large number of chemokines, recruit immune cells, particularly macrophages, and cause chronic low-grade inflammation, insulin resistance, and the release of a large amount of free fatty acids into the circulation, eventually leading to obesity-related metabolic disorders (4).
A growing body of studies have indicated that innate immune cells play an important role in modulating adipose tissue activities during obesity (5). Among these cells, macrophages were the first and most important immune cells discovered infiltrating adipose tissue during obesity (6, 7). Macrophage infiltration has a significant impact on adipose tissue function and is a major cause of obesity-related metabolic diseases. Therefore, understanding the molecular mechanisms governing adipose tissue macrophages is critical for the prevention and treatment of obesity and other related metabolic diseases. Here, we review the current literature on adipose tissue macrophages with a particular emphasis on the heterogeneity and polarization of these cells during obesity in adipose tissue. We discuss the fundamental roles of macrophages in adipose tissue, highlighting macrophage-targeting strategies and assessing their therapeutic potential for treating obesity and related metabolic diseases.
2. Adipose tissue macrophages
2.1. Increased macrophage recruitment to adipose tissue in obesity
The primary sources of adipose tissue macrophages are tissue-resident macrophages and monocyte-derived recruited macrophages. Unlike most tissue-resident macrophages, which are derived from yolk sac primitive precursors and function to regulate tissue remodeling and maintain tissue homeostasis (8), a recent fate mapping study revealed that adipose tissue resident macrophages are derived from definitive embryonic hematopoietic precursors (9). These resident ATMs are phenotypically F4/80hiCD11b+CD169+ cells that can be further subdivided into three subtypes: MHCIIlow, MHCII+CD11c-, and MHCII+CD11c+. In response to HFD, the MHCII+CD11c+ ATMs were rapidly increased in adipose tissue and replenished by bone marrow-derived monocytes, implying that recruited monocytes are the major cells contributing to increased ATMs in obesity.
Infiltration of monocyte-derived macrophages into adipose tissue during obesity was firstly reported in mouse models obesity and humans in 2003 (6, 7). The infiltrated macrophages were derived from bone marrow (7) and were contributed by increased diapedesis of blood monocytes (10). In contrast, weight loss by surgery reduced macrophage infiltration in adipose tissue of patients with obesity (11). Chemokine and its receptor interaction play crucial roles in the recruitment of circulating monocytes into adipose tissue during obesity. For example, monocyte chemoattractant protein (MCP-1 or CCL2), a chemokine produced in both adipocytes and the stromal vascular (SV) portion of adipose tissue, is significantly elevated in both blood and adipose tissue in obesity (12–17). Mice lacking CCL2 (18) or its receptor, CC chemokine receptor 2 (CCR2) (19) or using CCR2 inhibitor (20, 21), have lower adipose tissue macrophage infiltration and improved metabolic function in db/db and HFD-induced obese mice. Conversely, mice overexpressing CCL2 in adipose tissue have enhanced macrophage infiltration into adipose tissue and an unfavorable metabolic profile (18, 22). Moreover, mice with CCR2 deficiency in bone marrow cells or macrophages had lower macrophage numbers in adipose tissue after high-fat diet (HFD) feeding, indicating that CCR2 plays a crucial role in macrophage recruitment into adipose tissue during obesity (23, 24).
In addition to CCL2/CCR2, other chemokines and their receptors may play a role in the increased macrophage accumulation in adipose tissue in obesity. For instance, CCL chemokines (such as CCL3, CCL4, CCL5, CCL7, CCL8, CCL11, CCL18) and its receptors (such as CCR1, CCR3 and CCR5) have been linked to increased adipose tissue in obese (25) and human individuals (26, 27). Indeed, a dual CCR2/5 antagonist significantly reduces M1 macrophage infiltration into adipose tissue in HFD-induced obese mice, as well as improving adipose tissue inflammation and insulin resistance (IR) (28). Furthermore, CXCL12 produced by adipocytes interacts with its receptor CXCR4 to mediate macrophage recruitment into adipose tissue during HFD-induced obesity (29). In addition, other chemokines such as haptoglobin and C3a have also been reported to mediate macrophage recruitment into adipose tissue during obesity (30, 31). These studies taken together have demonstrated the therapeutic potential of focusing on macrophage recruitment into adipose tissue.
2.2. Adipose tissue macrophages polarized to pro-inflammatory phenotype in obesity
Increased macrophage infiltration into adipose tissue forms a crown-like structure (CLS) around necrotic adipocytes (32, 33). The number of CLS is strongly correlated with the expression of inflammatory cytokines like TNF-α (32), indicating that infiltrating macrophages have a pro-inflammatory effect on adipose tissue in obesity. Lumeng et al. used PKH26 dye to label resident macrophages in adipose tissue and found that newly recruited adipose tissue macrophages (ATMs) in HFD-induced obese mice had a pro-inflammatory M1 phenotype (F4/80+CD11c+), whereas resident macrophages had an alternative activated M2 phenotype (F4/80+CD206+) (34–36).
Further examination of CD11c+ ATMs from epididymal WAT (eWAT) revealed a mixed M1/M2 profile that was divided into three subtypes: resident ATMs as MGL1+CD11c- expressing cells, CLS-associated MGL1-/CD11c+ ATMs, and MGL1med/CD11c+ ATMs (37). Similar to this work, resident ATMs in human adipose tissue have been shown to display M2 markers like CD206 and CD163, but they are also able to produce inflammatory cytokines (38, 39), indicating that these ATMs are mixed M1- and M2-polarized. Additionally, the number of ATMs in subcutaneous and omental adipose tissue of patients with obesity is higher than in lean subjects (40, 41). These findings collectively indicate that adipose tissue remodeling in obesity is connected to both an M1 and M2 progression.
Moreover, macrophage infiltration into adipose tissue during obesity is preferentially located in visceral adipose tissue in humans (42–44) and mice (33, 45), implying that visceral adipose tissue is the major adipose depot harboring the pro-inflammatory macrophages in obesity. The pro-inflammatory ATMs are one of the key cell types responsible to produce pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, which contribute to obesity-related adipose tissue inflammation. In addition to the recruitment of circulating monocytes into adipose tissue, a local proliferation of macrophages in CLS also contributes to the increased ATMs in adipose tissue during obesity (46–48). These proliferating macrophages express M2 macrophage markers including CD206 and CD301 and form resident ATMs in the interstitial space (49). Even though these proliferating macrophages are M2 phenotype, their presence maintained adipose tissue inflammation in obese mice even after weight loss (50).
The accumulation of macrophages in adipose tissue is not only a defining feature of obesity, but also a major cause of obesity-related metabolic diseases such as liver steatosis and IR (51–57). As a result, reducing the number of macrophages in adipose tissue slows the onset of obesity and improves insulin sensitivity and glucose metabolism (58, 59), indicating that macrophages in adipose tissue play crucial roles in the development of obesity and metabolic disorders.
3. Adipose tissue macrophage subtypes and functions in adipose tissue
3.1. Newly identified macrophage subtypes in adipose tissue
In addition to previously classified pro-inflammatory and alternatively activated macrophages using F4/80 and CD11c or CD206 markers, a new class of ATMs known as M3 ATMs (CD11c-CD206-MGL1-) that also localize to the CLS and uniquely express chemokine receptor Ccr7 has been reported (60). The presence of M3-like ATM suggests that different pathways may contribute to macrophage inflammation in the context of obesity. Additionally, another new type of ATM known as metabolically activated macrophages (MMe) were reported, which is produced when exposed to high levels of glucose, insulin, and palmitate. Rather than expressing classical M1 markers, MMe overexpress ATP binding cassette transporter (ABCA1), cluster of differentiation 36 (CD36), and perilipin 2 (PLIN2), which are regulated by peroxisome proliferator activated receptor gamma (PPARγ) (61). Moreover, MMe macrophages accumulated in CLS showed both beneficial and detrimental effects in response to high-fed diet feeding (62). For example, during the early stages of HFD-induced obesity, MMe macrophages increased adipose tissue inflammation by upregulating inflammatory markers such as TNF-α, IL-6, and IL-1β, as well as genes involved in lipid metabolism. In contrast, despite strong expression of pro-inflammatory and lipid metabolism genes in MMe macrophages, they are more active in the clearance of dead adipocytes via lysosomal exocytosis, hence inhibiting ectopic fat accumulation and IR in late-onset HFD-induced obesity. Mechanistically, TLR2, NOX2 and MyD88 have been proposed to modulate the positive and negative impact of MMe macrophages in HFD-induced obesity. Subsequent research suggested that MMe aggregation in breast adipose tissue may play a role in the development of triple-negative breast cancer (63).
Recent research using single-cell sequencing has revealed a much broader range of ATM phenotypes ( Figure 1 ; Table 1 ). For example, CD9+ATM, which also localizes in CLS in both mice and humans, was discovered to contain large amounts of intracellular lipids in lysosomal-like structures and to express genes associated with lysosome-dependent lipid metabolism, may have the same capacity as MMe to clear dead adipocytes via the lysosomal pathway. However, CD9+ATM is distinct from MMe because it contains traditional M1/M2 markers like CD206 and CD11b (64). Adoptive transfer of CD9+ ATM to lean mice leads to the up-regulation of genes related with obesity, suggesting that CD9+ ATM may promote the development obesity and metabolic diseases (64). Triggering receptor expressed on myeloid cells 2 (TREM2), a pathologically induced immune signaling in Alzheimer’s disease, metabolic diseases, and cancer, has been found to express in ATMs (69). A new subtype of macrophages termed as lipid-associated macrophages (LAM) was discovered in both mouse and human adipose tissue characterized by TREM2 expression (65). Despite the fact that mice with TREM2 deficiency had fewer LAM macrophages in CLS, they exhibited accelerated obesity with massive adipocyte hypertrophy, insulin resistance, and hyperlipidemia upon HFD feeding (65). In addition, single-cell sequencing studies have shown that CD9+TREM2+ ATMs have more specific surface markers CD45+CD11b+CD11c+CD9+TREM2+ for better identification (70). In addition, a new subset of ATMs expressing Duffy antigen receptors for chemokines (DARC+ ATMs) was also discovered to be recruited to CLS in eWAT under obesity conditions (66). DARC+ATMs were generated in response to IL-22 stimulation and exhibited high levels of IL-22 receptor and M2-like anti-inflammatory properties to reduce adipose tissue inflammation in obesity (66).
Figure 1.
ATMs plasticity in adipose tissue. In lean adipose tissue, anti-inflammatory M2-like macrophages are predominant and maintain homeostasis. In obese adipose tissue, an increase in pro-inflammatory M1 ATMs forms a crown-like structure (CLS) surrounding dead adipocytes. Recent research has uncovered new macrophage subtypes, particularly in CLS.
Table 1.
Newly identified adipose tissue subtypes.
| Macrophages phenotype | Category | Location | Marker | Function | Reference |
|---|---|---|---|---|---|
| MMe (metabolically activated macrophages) | Recruited macrophages | CLS | ABCA1, CD36, PLIN2 | Removing dead adipocytes through lysosomal exocytosis | (61) (62) (63) |
| CD9+ macrophages | Recruited macrophages | CLS | CD9, LPL, PLIN2, CD63, LAMP2, CD16, CD206 | Promotion of obesity | (64) |
| LAM (lipid-associated macrophages) | Recruited macrophages | CLS | TREM2, LIPA, LPL, CTSB, CTSL, FABP4, FABP5, LGALS1, LGALS3, CD9, CD36 | Preventing metabolic disorders when adipocyte homeostasis is lost | (65) |
| DARC+ macrophages | Recruited macrophages | CLS | DARC, Ly6C(low), M2-related marker(high) | Anti-inflammation and reducing immune cell infiltration. | (66) |
| MFehi macrophages | Resident macrophages | Intercellular space; CLS (a small number) |
CD163, TFRC, HO-1, FTL1, FTH1, CP, SLC40A1, F4/80, CD11c(high), CD206(low) | Coping with iron metabolic disorders | (67) (68) |
Other than CLS, several distinct ATM phenotypes in adipose tissue have been reported. For instance, in the intercellular space of adipose tissue, a distinct ATMs population known as “MFehi” with higher cellular iron content and an iron-recycling gene expression profile was found (67). These “MFehi” ATMs displayed M2-like alternatively activation markers such as CD163 and MGL1/2 and decreased M1 markers (67). As a result, MFehi ATMs can manage high iron loads by storing iron, regulating iron-handling genes, and protecting adipocytes from iron overload (68). More research is needed to characterized these newly discovered macrophage subtypes and to determine the potential mechanisms that link these cells to obesity and related metabolic disorders.
3.2. Role of ATMs in adipose tissue function
The interactions between recruited pro-inflammatory macrophages and adipocytes are often harmful to the functions of adipocytes, including adipogenesis and lipid metabolism, inflammation, and related metabolic dysfunctions ( Figure 2 ). In contrast, the resident macrophages in non-obese state are considered metabolically ‘favorable’ ATMs, which play important role in maintaining adipose tissue homeostasis via clearance of dead adipocytes. They are also critical for beige adipogenesis and thermogenesis, which lead to improved metabolic functions ( Figure 2 ). Here we focus on reviewing the recent literature on ATMs and major adipose tissue functions.
Figure 2.
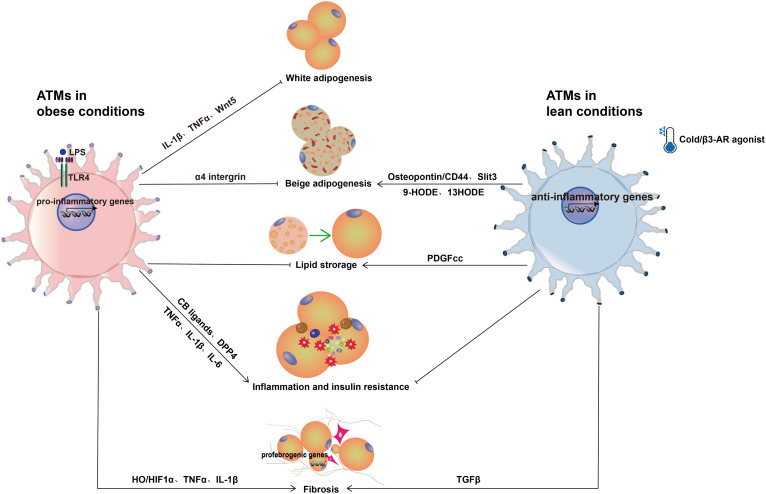
Role of ATMs in adipose tissue. In obese conditions, pro-inflammatory macrophages show detrimental effects on adipose tissue function such as inhibition of adipogenesis, promoting inflammation, insulin resistance, and fibrosis. The pro-inflammatory cytokines TNF-α and IL-1β and the protein factor Wnt5a inhibit preadipocyte differentiation when released by pro-inflammatory macrophages. In addition, TNF-α and IL-1β reduce the insulin sensitivity of adipocytes. Through LPS-TLR4 the LPS-induced CB ligands-CB1 signaling pathways, pro-inflammatory macrophages also aggravate adipose tissue inflammation. Moreover, macrophages secrete the enzyme DPP4, which causes both hyperglycemia and inflammation. In addition to inducing preadipocytes to produce abundant ECM, pro-inflammatory macrophages overproduce NO, which increases HIF-1α accumulation and promotes profibrogenic responses in preadipocytes, resulting in adipose tissue fibrosis. In lean conditions, ATMs are anti-inflammatory and play an important role in the formation and activation of beige adipocytes. In response to cold stimulation, ATMs polarize to an alternative activation state and promote the biogenesis of beige adipocytes via macrophage-secreted cytokine Slit3 and a sympathetic neuron-adipocyte signaling axis. Similar to cold stimulation, β3-AR agonists enhance the conversion of existing white adipocytes into beige adipocytes. Furthermore, β3-AR agonists induce alternative activation of macrophages to release osteopontin and the PPARγ ligands 9-HODE and 13-HODE, which stimulates beige adipocyte development.
3.2.1. Role of ATMs in adipogenesis and lipid metabolism
The differentiation of preadipocytes to adipocytes is essential for the growth of adipose tissue in obesity. The expansion of white adipose tissue can dramatically enhance metabolic function and health. However, when immune cells, particularly pro-inflammatory macrophages, infiltrate adipose tissue, its potential to expand is inhibited. In vitro culture of preadipocytes with macrophage-conditioned medium elicits a pro-inflammatory response in both murine and human preadipocytes and impairs their differentiation to adipocytes (71–75), suggesting that macrophage-secreted factors contribute to its inhibitory effect on adipogenesis. Among the pro-inflammatory cytokines produced by macrophages, TNF-α and IL-1β have shown a direct inhibition on preadipocyte differentiation, however, neither TNF-α nor IL-1β neutralization reverses the anti-adipogenic effect of macrophage-conditioned medium (72, 76, 77), suggesting that other soluble factors could play a role. Wnt5a has been demonstrated to be expressed in human ATMs and circulating monocytes, and inhibition of Wnt5a activity in J774A.1 macrophage-conditioned medium improved mesenchymal precursor cells differentiation into adipocytes (78), suggesting that Wnt5a is a possible factor secreted by macrophages to suppress adipogenesis. Mechanistically, pro-inflammatory macrophages suppressed PPARγ activity in adipocytes by S-nitrosylation at cysteine 168, resulting in proteasome-dependent degradation of PPAR and decreased adipogenesis (79).
Beige adipose tissue is an inducible thermogenic type of adipose tissue that resides within white subcutaneous adipose tissue in mice and humans (80). Beige adipocytes can be induced by cold exposure, β3-adrenergic receptor (β3-AR) agonist, and PPAR ligands (81) via beige adipogenesis and white adipocytes conversion. Several studies have found that macrophages are critical players in the formation and activation of beige adipocytes (82–84). For instance, it has been demonstrated that pro-inflammatory macrophages directly interact with beige adipocytes via α4 integrin and VCAM-1, triggering a persistent inflammatory cycle in adipose tissue and inhibiting beige adipogenesis in obesity (85). In contrast, cold stimulation results in the production of the type 2 cytokines IL-4 and IL-13 by eosinophils, which activate macrophages and promotes the biogenesis of beige adipocytes (86). Furthermore, a recent study discovered the cytokine Slit3 secreted from anti-inflammatory macrophages promotes WAT beiging in response to cold via the sympathetic neuron-adipocyte signaling axis (87). In line with this discovery, subcutaneous WAT browning was significantly induced by injecting anti-inflammatory macrophages in obese mice induced by the HFD (88). However, a recent study found that conditionally and partially depleting adipose tissue CD206+ macrophages increased proliferation and differentiation of beige progenitors in normal and cold stimulated conditions (89, 90), suggesting that CD206+ ATMs inhibit beige adipogenesis. This might be as a result of mixed populations of CD206+CD11c+ and CD206+CD11c - ATMs present in CD206+ macrophages. More research is needed to determine which subtype has the inhibitory effect on beige adipogenesis. Similar to cold stimulation, β3-AR agonist is a potent inducer of the conversion of existing white adipocytes into beige adipocytes (91). Recent data also point to a role for resident macrophages in promoting beige differentiation in response to β3-AR activation through the clearance of dead adipocytes, the secretion of the chemokine osteopontin to recruit PDGFRα+CD44+ beige progenitors into subcutaneous adipose depot, and the production of the PPARγ ligands 9-HODE and 13-HODE via ALOX15 activity (92, 93). Overall, resident ATMs support beige adipogenesis and offer a potential therapeutic strategy to enhance metabolic health in obesity. More research is necessary to test these findings in human settings.
The classical function of adipose tissue is to store surplus energy as triglyceride during food intake and release free fatty acids during fasting. Several early in vitro studies reported that LPS-stimulated macrophages activate the lipolysis of 3T3-L1 adipocytes (94), which is accompanied by an inhibition of lipoprotein lipase (95) and a decrease in fatty acids synthesis (96). Moreover, LPS/IFNγ-activated macrophages are related to increased mitochondrial activity in human adipocytes, indicating that macrophage activation state may influence adipocyte bioenergetics (97). A recent study discovered that adipose tissue resident macrophages, rather than recruited CCR2+ macrophages, have an evolutionarily conserved role in lipid storage in adipocytes (98). In response to HFD feeding, these resident macrophages produce higher levels of PDGFcc, which promotes white adipocyte hypertrophy and hence prevents ectopic fat deposition in the liver and other tissues. Blocking PDGFcc reduces lipid accumulation in white adipocytes while increasing thermogenesis in brown adipocytes, indicating a vital role of PDGFcc in regulating lipid metabolism. Further study is needed to evaluate whether pharmacological inhibition of PDGFcc has therapeutic promise for obesity treatment.
3.2.2. Role of ATMs in inflammation and related metabolic disorders
Increased ATM accumulation in obesity is one of the key contributors contributing to obesity-induced inflammation both locally and systemically. Newly recruited pro-inflammatory macrophages release a considerable amount of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1β and impede insulin signaling transduction in adipocytes (99–101). Consistently, the infiltration of pro-inflammatory macrophages precedes the IR in obese mice in vivo (6, 102), suggesting a causal role for inflammation in the development of IR in obesity. Insulin-resistant adipocytes release more free fatty acids and activate ATMs, resulting in a vicious loop that exacerbates inflammation via TLR4 (103). Moreover, TLR2 and TLR9 deficiency promotes HFD-induced adiposity, visceral adipose inflammatory responses, and IR in mice (104, 105), indicating that TLRs play a significant role in adipose tissue inflammation and IR in obesity. LPS derived from gut microbiota is another potential factor for inducing inflammatory responses in adipose tissue. On the one hand, LPS activates ATMs via TLR4 and amplifies inflammation by adipocyte-macrophage interactions (106). On the other hand, LPS causes robust productions of endogenous ligands for cannabinoid (CB) receptors in ATMs (107), which contributes to chronic inflammation in visceral adipose tissue, hyperglycemia, and IR (108). Furthermore, CB1 receptor blockage reduced LPS-induced pro-inflammatory responses in macrophages, alleviated adipose tissue inflammation and glucose intolerance (108, 109). In addition, other inflammatory mediators or proteins also contribute to adipose inflammation in obesity. DPP4, an enzyme that effectively increases blood glucose levels by degrading incretin peptides, was found to be more abundant in F4/80+ macrophages in CLS in adipose tissue than in adipocytes (110, 111). DPP4 inhibition dramatically reduced pro-inflammatory macrophage migration while producing an anti-inflammatory phenotype shift in adipose tissue macrophages, reducing obesity-induced inflammation and IR (112).
Additionally, pro-inflammatory macrophages play an important role in the development of adipose tissue fibrosis in obesity, which is another important pathogenic feature of obesity. Adipose tissue fibrosis is characterized by an increase in the expression and remodeling of extracellular matrix (ECM) proteins in WAT (113). The fibrotic deposition in adipose tissue has been observed as bundles of collagen fibers (Collagen I, III) in subcutaneous fat and thin fibrous lobule-like bands (Collagen VI) surrounding adipocytes in omental fat from subjects with obesity (114, 115). Collagens and fibronectin are expressed more abundantly in adipose tissue SV fractions than in adipocytes (114), indicating that SV fractions may be the primary cell types for fibrotic protein synthesis. Marcelin et al. have investigated the cellular origins of WAT fibrosis and discovered that pro-fibrotic cells originate from PDGFR+CD9high cells within adipose tissue SV fractions (116). Human preadipocytes cultured in vitro with LPS-activated macrophages had a pro-inflammatory phenotype and produced abundant ECM consisting of collagen 1, tenascin-C, and fibronectin (77, 117). Furthermore, macrophages dramatically increased the levels of ECM breakdown enzymes such as matrix metalloproteinases in both preadipocytes and adipocytes via the pro-inflammatory cytokines TNF-α and IL-1β (118, 119). In contrast to in vitro studies, anti-inflammatory macrophages have been linked to increased adipose tissue fibrosis in individuals with IR (115). Mechanistically, TGF-β has been shown to induce myofibroblast-like cells from adipose tissue progenitor cells (preadipocytes) treated with ATMs (120). Hypoxia is an additional essential component contributing to adipose fibrosis. The expansion of adipose tissue in obesity is associated with adipose tissue hypoxia, as has been demonstrated in adipose tissue of several obese mouse models (ob/ob, KKAy, diet-induced) (121–123) and human subjects with obesity (124). Mechanistically, adipose tissue hypoxia increases HIF-1α expression and stability, which triggers profibrogenic transcription in preadipocytes (125). Furthermore, pro-inflammatory macrophages overproduced NO, which elevated HIF-1α accumulation and promoted profibrogenic responses in preadipocytes, resulting in adipose tissue fibrosis (126). Collectively, these findings suggest to the possibility of targeting pro-inflammatory macrophage-mediated inflammatory pathways to diminish obesity-induced inflammation, IR and fibrosis.
4. Targeting macrophages to improve metabolic health
Given that ATMs play critical roles in both the onset and progression of obesity-related metabolic disorders, strategies that target the phenotypic flexibility of macrophages to fulfill tissue environment needs have demonstrated great therapeutic promise. The following is a summary of the prospective treatment targets for obesity and related metabolic diseases that can be delivered to macrophages ( Table 2 ; Figure 3 ).
Table 2.
Targeting macrophages for improving metabolic health.
| Molecular targets | Approach | Phenotype |
|---|---|---|
| IKKβ | Myeloid cell specific IKKβ deletion (127) | ↓ IR after HFD. |
| TLR4 | Hematopoietic cell specific TLR4 deletion (128) | ↓ IR, ↓adipose and liver inflammation |
| Fas | Myeloid/hematopoietic cell-specific Fas deletion (129) | ↓ skeletal muscle IR, no effect on inflammation in liver and AT. |
| MyD88 | Myeloid cell-specific MyD88 deletion (130) | ↓ atherosclerosis, IR, and systemic inflammation after HFD. |
| TRAF3 | Myeloid cell-specific TRAF3 deletion (131) | ↓inflammation and IR in HFD-obese mice; ↑ inflammation in liver and adipose in lean mice. |
| ERV1 | Myeloid cell-specific overexpression (132) | ↓adiposity and inflammation after HFD. |
| NOX2 | Myeloid cell-specific NOX2 deletion (133) | ↓adiposity and adipose inflammation |
| HIF1α | Myeloid cell-specific HIF1α deletion (134) | ↓systemic IR and inflammation after HFD |
| HO-1 | HO-1+/- mice (135) | ↓adiposity, adipose inflammation, and IR after HFD |
| NFATc3 | NFATc3-/- mice (136) | ↓hepatic steatosis and inflammation after HFD |
| HoxA5 | HoxA5 overexpressed mice (137) | ↓adiposity and inflammation after HFD |
| Insulin receptor (IR) | Mice with macrophage IR deletion (138) | ↓IR after HFD |
| PTPB1 | Macrophage PTPB1 deletion (139) | ↓IR, liver damage and chronic inflammation |
| IRS2 | Mice with macrophage IRS2 deletion (140) | ↓adiposity and glucose intolerance after HFD |
| mTORC1 | Myeloid cell-specific TSC1 deletion to constitutively activate mTROC1 (141) | ↓obesity, glucose intolerance, and AT inflammation after HFD |
| SIRT1 | Myeloid cell-specific SIRT1 deletion (142–145) | ↓glucose tolerance, ↑ liver steatosis and AT inflammation |
| TREM2 | TREM2 overexpressed mice (146) | ↑AT inflammation, adiposity, IR after HFD. |
| Catalase | Global catalase deficiency (147) | ↑oxidative stress, inflammation, and IR |
| PPARγ | Skeletal muscle and liver specific PPARγ depletion (148, 149) | ↑IR in muscle and liver. |
| KLF4 | Myeloid cell-specific KLF4 deletion (150) | ↑adiposity, glucose intolerance, and IR after HFD |
| GRIP1 | Myeloid cell-specific GRIP1 deletion (151) | ↑ adipose inflammation, hyperglycemia, and IR |
| ATG7 | Myeloid cell-specific ATG7 deletion (152) | ↑adipose inflammation and hyperglycemia. |
| PDK1/FoxO1 | Pdk1 deletion in macrophages; constitutive activation of nuclear Foxo1 (153) | ↑ adipose inflammation and IR |
| Estrogen receptor α (ERα) | Macrophage ERα deletion (154) | ↑adiposity, IR and atherosclerotic lesion area |
| SIRT6 | Myeloid cell specific SIRT6 deletion (155) | ↑ adipose and liver inflammation and IR |
| PER1/PER2 | Myeloid cell-specific deletion of core clock genes Period1 (PER1) and Period2 (PER2) (156) | ↑adipose inflammation and IR after HFD. |
IR, insulin resistance; HFD, high-fat diet; ↑ increase; ↓ reduce.
Figure 3.
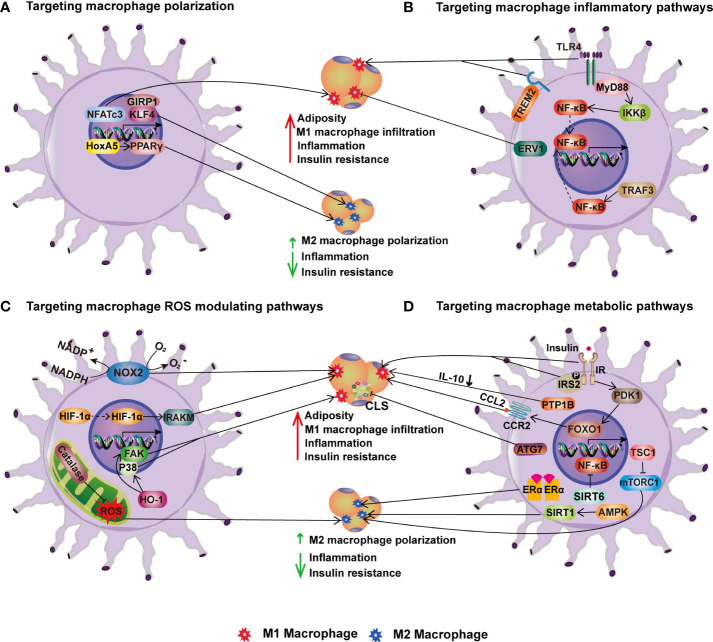
Targeting macrophages for improving metabolic health. (A) Targeting macrophage polarization. In HFD-induced obesity, transcription factors NFATc3, KLF4 and its coactivator GIRP1 enhance M1 macrophage polarization and infiltration into adipose tissue, inflammation, and insulin resistance. HoxA5 and PPAR, on the other hand, increase M2 macrophage polarization and thereby ameliorate obesity-induced inflammation and insulin resistance. (B) Targeting macrophage inflammatory pathways. TLR4-MyD88-IKK signaling and TRAF3 activation enhance adipose tissue M1 macrophage infiltration, inflammation, and IR in obesity via NF-B. On contrary, overexpression of ERV1 in macrophages reduces adiposity, hepatic and adipose inflammation, and hyperglycemia caused by HFD. (C) Targeting macrophage ROS modulating pathways. NOX2, HIF-1α, and HO-1 in macrophages increase obesity-induced adiposity, inflammation, and insulin resistance, whereas catalase inhibits inflammation via increasing M2 macrophage polarization. (D) Targeting macrophage metabolic pathways. The stimulation of macrophage insulin pathways such as IR-IRS2 and PDK1-FoxO1 signaling promotes HFD-induced obesity and insulin resistance. PTP1B, an insulin signaling negative regulator, induces IR by lowering IL-10. In contrast, mTORC1 activation improves M2 macrophage polarization and protects mice from HFD-induced obesity, inflammation, and insulin resistance. In addition, the ATG7-mediated autophagy pathway reduces CLS numbers and adipose tissue inflammation in obesity. Furthermore, other metabolic pathways regulated by ERα, SIRT1 and SIRT6 enhance M2 macrophage polarization, reducing inflammation and IR in obesity.
4.1. Targeting macrophage polarization
ATMs have been shown to negatively modulate insulin action via CD11c+ pro-inflammatory macrophages (157), indicating that pro-inflammatory macrophages are a target for the treatment of obesity-related insulin resistance. Fatty acids are one of the major factors controlling the activation of ATMs.For example, saturated free fatty acids/TLR signalling, TNF/TNF receptor signalling induce the classically activation of macrophages (158–163), while unsaturated fatty acids like oleic acid, linoleic acid, DHA, and n-3 PUFA induce alternatively activated phenotype. Furthermore, omega-3 PUFA can increase lipolysis and fatty acid re-esterification in alternatively activated macrophages (164). These findings indicate that consuming unsaturated fatty acids may polarize ATMs to alternatively activated phenotype, thereby regulating lipid metabolism or alleviating the symptoms of obesity-related diseases.
Rosiglitazone, a PPARγ activator, also encourages alternatively activated macrophage infiltration into adipose tissue in mice receiving HFD (165–168). PPARγ deficiency in macrophages promotes the predominance of pro-inflammatory macrophages and the decrease of alternatively activated macrophages in adipose tissue in obesity (148, 169), indicating that PPARγ is essential in controlling macrophage alternative activation. Moreover, an intact IL-4 and IL-13 signaling is required for maturation of alternatively activated ATMs and reducing diet-induced obesity and IR in mice (170, 171). However, myeloid cell-specific knockout of IL4R alpha decreased insulin sensitivity in lean mice while improving parameters of glucose homeostasis and partially protecting against adipose tissue inflammation in obese mice (172), indicating IL-4R signaling likely plays a significant role in maintaining the alternative activation of macrophage in lean conditions but not in obesity.
A number of transcription factors have been found to influence ATMs polarization. For instance, Krüppel-like factor 4 (KLF4) has been demonstrated to promote monocyte differentiation in vivo (173). Moreover, KLF4 is strongly induced in alternatively activated macrophages by STAT6 while being reduced in pro-inflammatory macrophages by NF-kB inhibition (150). Consistently, KLF4-deficient macrophages displayed increased pro-inflammatory cytokine expression, and myeloid-specific KLF4 deficiency predisposed mice to diet-induced obesity, glucose intolerance, and IR (150), indicating a crucial role for KLF4 in regulating macrophage polarization and maintenance of adipose tissue homeostasis. Similar metabolic problems were brought on by the knockdown of the protein known as glucocorticoid receptor-interacting protein 1 (GRIP1), which acts as a coactivator for KLF4 (151). Contrarily, nuclear factors of activated T cells (NFATc3) play a different role in controlling the transcription of various genes in immune cells. Nfatc3-/- mice showed adipose tissue macrophage polarization toward alternative activation, which significantly reduced hepatic steatosis and inflammation in HFD mice, indicating the potential role of NFATc3 in promoting adipose tissue inflammation (136). Homeobox A5 (HoxA5), a developmental transcription factor, has been demonstrated to support adipocyte differentiation by inhibiting the PKA/HSL pathway (174). HoxA5 has also been shown to reduce endoplasmic reticulum stress and inflammatory responses in adipocytes by blocking the eIF2/PERK signaling pathway (137). Additionally, Hoxa5 transcriptionally activated the PPARγ pathway to promote alternative activation of macrophage and WAT browning (137), which in turn alleviated obesity-induced chronic inflammation. These findings imply that Hoxa5 may represent a promising therapeutic target for the management of obesity.
Notably, some therapeutic options and drugs have been developed to treat obesity-related metabolic diseases by regulating macrophage polarization. For obese patients who have failed to respond to exercise and dietary changes, bariatric surgery is an option. Studies have shown that after bariatric surgery, ATMs is biased toward the alternative activation with an increase of CD163 expression (40). However, subsequent research expressed concern on this notion, claiming that modifications in CD163-positive cells do not precisely reflect metabolic improvements following weight loss (175). Further research into the mechanism of bariatric surgery is required. Metformin, the most popular anti-diabetic medication, is crucial for macrophage polarization. Metformin was shown to decrease pro-inflammatory markers like CD11c and MCP-1 in the adipose tissue of HFD mice (176). Additionally, in vitro metformin treatment to pro-inflammatory macrophages improved metabolic disorders in brown adipocytes (177). Dipeptidyl peptidase-4 (DDP4) inhibitors Linagliptin and Sitagliptin are both used primarily to control blood glucose levels in patients with type 2 diabetes. These two drugs have been shown to decrease obesity-induced inflammation and IR by inhibiting pro-inflammatory and promoting alternative activated macrophages because DDP4 is largely expressed in pro-inflammatory macrophages and its expression was significantly increased in obese mice (112, 178). Similar mechanisms are shared by a number of sodium-glucose cotransporter 2 inhibitors, including empagliflozin. Through the phenotypic switch of macrophages to alternative activation in the liver and WAT, empagliflozin can reduce body weight by inducing WAT browning and reducing inflammation associated with obesity (179, 180). In conclusion, targeting macrophage polarization is a feasible and worthwhile direction that may benefit the vast majority of patients suffering from metabolic diseases.
4.2. Targeting macrophage inflammatory pathways
Adipose tissue inflammation is a major contributor to obesity-related metabolic diseases such as IR and hepatic steatosis. In adipose tissue, ATMs play dominant role in producing pro-inflammatory cytokines, which cause inflammation in obesity. NF-kB is one of the main masters of inflammatory responses. IKK is a crucial enzyme that activates NF-kB in myeloid cells. Mice with myeloid cell-specific IKKβ deletion preserved insulin sensitivity when fed with HFD (127). Furthermore, mice with hematopoietic cell-specific deletion of TLR4 demonstrated an improvement in peripheral insulin sensitivity after HFD feeding, which is associated with to a notable decrease in macrophage infiltration and inflammatory cytokines in both adipose tissue and the liver (128). MyD88, a TLR4 downstream signaling protein, is crucial in triggering inflammatory response. MyD88 deficiency in myeloid cells reduced macrophage infiltration to adipose tissue and their polarization to pro-inflammatory phenotype (130). Along with this, there is a considerable reduction in atherosclerosis, insulin resistance, and systemic inflammation induced by HFD feeding. Another typical intracellular signaling protein for TLRs is TNF receptor-associated factor 3 (TRAF3), which is anti-inflammatory in lean but pro-inflammatory in obese conditions. This is supported by research showing that myeloid cell-specific TRAF3 deletion reduced the number of macrophages in eWAT, as well as IR and the expression of pro-inflammatory cytokines in the liver and adipose tissue of obese mice (131). In contrast, TRAF3 deletion increased the expression of pro-inflammatory cytokines in the liver and adipose tissue of lean mice. Moreover, activation of the Fas signaling pathway may also be a crucial element of the inflammatory response. In HFD-induced obese mice, ob/ob mice, and mice acutely treated LPS, myeloid/hematopoietic cell-specific Fas-depletion preserved skeletal muscle insulin sensitivity, which was contributed by the decreased TNF-α levels in circulation (129). However, there was no difference in immune cell infiltration or local cytokine expression in adipose, liver, or skeletal muscle, indicating that the protective role of myeloid Fas depletion is more closely linked to a reduction of systemic inflammation.
Contrary to the inflammatory triggers listed above, it has been shown that TLR4 signaling from the triggering receptor expressed on myeloid cells 2 (TREM2) negatively modulates the inflammatory response in macrophages (181). A recent study has found that TREM2 may be involved in the inflammatory response in adipose tissues. Following HFD feeding, mice with TREM2 overexpression showed elevated macrophage and T cell recruitment into adipose tissue as well as increased adiposity, IR, and hepatic steatosis (146). These findings suggest that TREM2 acts as a novel regulator of adipogenesis and that inhibiting TREM2 signaling may be a therapeutic target for obesity and IR. To fully understand the underlying mechanisms of TREM2 in regulating the inflammatory response in adipose tissues, additional research on macrophage-specific deletion of TRME2 is required. Moreover, endogenous lipids known as specialized pro-resolving mediators (SPMs), which include resolvins, protectins, and maresins, mediate the resolution of inflammation (182). Mice overexpressing the human resolvin E1 receptor (ERV1) in myeloid cells displayed reduced adiposity, hepatic and adipose inflammation, and hyperglycemia induced by HFD (132). Resolvin E1, a natural ERV1 agonist, administration replicated the pro-resolving effects obtained from ERV1 overexpression. This protective metabolic impact is in part explained by systemic activation of resolution programs leading to increased synthesis of specialized pro-resolving mediators. Taking together, targeting inflammatory pathways in macrophages offers a great potential for controlling adipose tissue inflammation and the ensuing metabolic disorders induced by obesity.
4.3. Targeting reactive oxygen species modulating pathways in macrophages
Oxidative stress and chronic inflammation are the important underlying factors for obesity-associated metabolic diseases. The imbalance between the oxidative and anti-oxidant systems of the cells and tissues results in the overproduction of oxygen free radicals and reactive oxygen species (ROS). Oxidative stress increases lipid peroxidation products, protein carbonylation which leads to cellular dysfunction. As the NADPH oxidase catalytic subunit, NOX2 has been demonstrated to be involved in obesity-induced IR, hyperlipidemia, and liver steatosis (183). Mice lacking myeloid-NOX2 showed reduced adiposity, adipose inflammation, and macrophage infiltration compared to controls when given a 16-week HFD diet (133). These results support the idea that NOX2 signaling in macrophages plays a role in the pathogenesis of obesity-induced metabolic disorders. Potentially, obesity may be reduced by targeted suppression of monocyte/macrophage NADPH oxidase in adipose tissue to maintain metabolic function.
Hypoxia is also a factor in the increased oxidative stress associated with obesity. The transcription factor hypoxia inducible factor-1 (HIF-1) regulates the expression of numerous hypoxic responsive genes by nuclear translocation and mediates adaptive responses to oxidative stress. HIF-1α has been demonstrated to contribute to oxidative stress and fibrosis in obese people (184). Additionally, macrophages in CLS and adipocytes are both hypoxic and inflammatory (185). In fact, mice with myeloid-specific HIF-1α deletion had enhanced adipose tissue vasculature development, which mitigated systemic IR and HFD-induced inflammation (134). Furthermore, a recent study identified interleukin-1 receptor-associated kinase M as the mechanism underlying HIF-1α-induced adipose tissue dysfunction in obesity (186), supporting the notion that HIF-1α in myeloid cells is crucial to obesity-related pathological growth of adipose tissue and systemic IR.
Additionally, heme oxygenase-1 (HO-1) is a stress-inducible enzyme that is crucial in several pathophysiological conditions, particularly inflammation and oxidative damage. Heme oxygenase (HO-1) expression was highly induced in the visceral adipose tissue, especially the SV fraction of HFD-fed mice. Myeloid HO-1 haploinsufficiency attenuated HFD-induced adiposity, adipose inflammation, and IR, due to impaired macrophage migration toward adipose tissue and reduced angiogenesis (135). Mechanistically, HO-1+/- macrophages displayed decreased chemoattractant-induced p38 phosphorylation and focal adhesion kinase expression (135). These findings point to a unique role of the myeloid cell HO-1 in adipose macrophage infiltration and IR development during obesity.
In contrast to the preceding factors, catalase, an important oxidative stress regulator, has been shown to control ATM polarization under both resting and metabolic stress conditions. Global catalase deficiency or use of the catalase inhibitor 3-aminotriazole causes oxidative stress, increased inflammation and IR in both lean and HFD-induced obese mice (147). Catalase inhibition increased pro-inflammatory macrophage accumulation but decreased alternatively activated macrophage accumulation in eWAT, indicating that endogenous catalase may be a critical regulator of obesity-related inflammation and IR.
4.4. Targeting macrophage metabolic pathways
Obesity-associated metabolic problems appear to be caused by a combination of metabolic endotoxemia and metabolic stress induced by chronic exposure to excessive amounts of nutrients. Because immune cell metabolism and function are inextricably connected, addressing the different metabolic pathways of macrophages could provide a unique opportunity to modify its phenotype and subsequent biological roles in obesity.
4.4.1. Insulin pathway as a target
Despite previous research, the main impact of macrophage insulin action on obesity and related metabolic disorders is still debated. Mice lacking macrophage insulin receptor were protected from the onset of obesity-related IR after HFD feeding (138). This protection was accompanied by lower macrophage counts in WAT and serum tumor TNF-α levels, which reflect a marked decrease in the local and systemic inflammation linked to obesity. These findings suggest that insulin action in myeloid cells plays an unexpectedly important role in regulating macrophage invasion into WAT and the development of obesity-associated IR. In line with this study, mice with macrophage insulin receptor substrate 2 (IRS2) deletion demonstrated protection from HFD-induced obesity and glucose intolerance due to increased energy expenditure via enhanced BAT activity and WAT beiging (140). Additionally, IRS2-deficient macrophages exhibited a transcriptional profile that was anti-inflammatory (140), indicating a crucial role for macrophage IRS2 signaling in ATM polarization and energy homeostasis. These findings may open therapeutic opportunities for the treatment of obesity. However, protein tyrosine phosphatase-1B (PTP1B), an intracellular protein that inhibits insulin and leptin signaling, has been shown to promote inflammation caused by obesity. Mice deficient in macrophage PTP1B displayed improved glucose and insulin tolerance, reduced liver damage and chronic inflammation after HFD feeding (139). The beneficial effect of PTP1B deletion in macrophages is due to increased IL-10 levels, which are inversely related to serum insulin and alanine transferase levels. These findings suggest that inhibiting myeloid PTP1B could be used to treat obesity-related inflammation and diabetes.
4.4.2. Nutrient sensing pathways as a target
Many studies have been conducted on the function of mTORC1 in obesity and associated inflammation. These studies have demonstrated the link between mTORC1 activation and obesity. Despite having no impact on the HFD-induced obesity, pharmacological mTORC1 inhibition by rapamycin worsened the inflammation and glucose intolerance, as shown by the rise in adipose tissue pro-inflammatory macrophages and elevated mRNA levels of pro-inflammatory cytokines such as TNF-α, IL-6, and MCP-1 (187). Additionally, macrophages derived from bone marrow exhibited pro-inflammatory phenotype as a result of in vitro mTORC1 inhibition (187). These results suggest that mTORC1 activity is a key regulator of macrophage plasticity and inflammation in adipose tissue. To further investigate the role of myeloid cell mTORC1 activation in obesity-induced inflammation, mice with myeloid cell specific TSC1 deletion and thus constitutive mTORC1 activation were generated. Mice lacking Tsc1 in macrophages exhibited protection from HFD-induced obesity, glucose intolerance, and adipose tissue inflammation (141). This protection was accompanied by mTORC1-dependent alternative activation of macrophages, indicating a protective role for mTORC1 activation in HFD-induced obesity and metabolic disorders. Unlike mTORC1, myeloid cell deficiency of mTORC2 obtained by Rictor deletion had no impact on HFD-induced obesity, adipose tissue inflammation, or systemic IR (188). However, mice lacking Rictor showed increased susceptibility to LPS-induced septic shock, indicating that mTORC2 is more important in diseases associated with severe inflammation than obesity-induced chronic low-grade inflammation.
Autophagy, a crucial cellular response pathway for sensing nutrient levels, is essential for cell survival and metabolism. When bred to ob/+ mice to induce metabolic stress, mice with myeloid cell-specific deletion of autophagy-related 7 (ATG7) displayed increased CLS numbers, activated NLRP3 inflammasome and IL-1β production in adipose tissue, as well as hyperglycemia (152). This was attributed to mitochondrial dysfunction in autophagy-deficient Macrophages, suggesting a critical role for macrophage autophagy in regulating adipose inflammation and insulin sensitivity in obesity.
As one of the key pathways regulating glucose and energy homeostasis, the 3-phosphoinositide-dependent protein kinase 1 (PDK1)/forkhead transcription factor (FoxO1) pathway has also been investigated in adipose tissue macrophages. PDK1 deletion in macrophages resulted in increased pro-inflammatory macrophages in adipose tissue and IR, which was reversed by inactivating nuclear FoxO1 (153). Furthermore, constitutively activating nuclear FoxO1 increased pro-inflammatory macrophages in adipose tissue via CCR2 and IR on HFD (153). Accordingly, PDK1 inhibits FoxO1 to regulate macrophage infiltration, and the PDK1/FoxO1 pathway in macrophages is essential for regulating macrophage polarization and insulin sensitivity in obesity.
Additionally, estrogen receptor alpha (ERα) plays a significant role in the control of glucose homeostasis (189). Even with a normal diet, mice with myeloid-specific ERα deletion displayed increased adiposity, IR, and atherosclerotic lesion area (154). Moreover, ERα deficiency reduced the response of isolated macrophages to IL-4-mediated alternative activation but promoted the inflammatory response to palmitate (154). This suggests that macrophage ER is important for suppressing inflammation and maintaining insulin sensitivity, making it a potential therapeutic target to combat obesity and IR.
4.4.3. Sirtuins as a target
Myeloid cell Sirtuin 1 (SIRT1) has been shown to play a protective role in studies of metabolic diseases caused by obesity. When given an HFD, mice with myeloid cell Sirt1 deletion exhibited pro-inflammatory macrophage polarization in adipose tissue and increased adipose tissue macrophage hypoxia and inflammatory response (142–144), which impaired glucose tolerance and exacerbated liver steatosis (143, 145). In line with this, dietary quercetin has been demonstrated to reduce macrophage infiltration, control macrophage polarization, and regulate inflammation through the AMPK1/SIRT1 pathway, resulting in a reduction in HFD-induced IR and an increase in glucose uptake in adipose tissue (190). Similar to SIRT1, myeloid cell-specific SIRT6 knockout mice displayed increased pro-inflammatory macrophage infiltration in adipose and liver, as well as decreased insulin sensitivity via the NF-κB/STAT3 signaling pathway (155). These findings indicate that SIRT1 or SIRT6 in macrophages may be potential targets for combating obesity-induced tissue inflammation and IR.
4.4.4. Circadian pathways as a target
Numerous studies have linked metabolic disorders like obesity to circadian clocks. Circadian clock dysregulation induces pro-inflammatory macrophages and potentiates adipose tissue inflammation in mice with Period1 (PER1) and Period2 (PER2) deletion in macrophages, according to a previous study (156). High MCP-1 levels in mice with myeloid cell-specific PER1/PER2 disruption attracted pro-inflammatory macrophage infiltration and increased inflammation and IR in HFD-induced adipose tissue (156). Mechanistically, PPARγ2 levels were decreased in PER1/2-disrupted macrophages and restoration of PPARγ2 levels reduced the infiltration of pro-inflammatory macrophages in adipose tissue, suggesting that PPARγ may link the molecular clock genes and obesity-related inflammation.
5. Concluding remarks and perspectives
Increased ATMs are the major contributor to adipose tissue inflammation in obesity. Efforts have been made to target macrophage recruitment to improve metabolic health and have shown a great promise in obese mouse models. For instance, blocking CCL2-CCR2 has been shown to reduce macrophage recruitment in adipose tissue and mitigated the obesity-induced inflammation and IR. Moreover, a dual CCR2/CCR5 antagonist reduced macrophage-mediated inflammation and prevented IR, providing a therapeutic potential for metabolic diseases linked to obesity. Another promising strategy is to promote the polarization of ATMs toward alternative activation. Several transcription factors, including PPARγ, KLF4, and HoxA5, have been shown to promote alternative activation of macrophages in adipose tissue and could be potential pharmacological targets. Additionally, strategies at targeting myeloid TLR4/NF-κB-mediated inflammatory pathways, ROS generating enzyme NOX2 and hypoxia adaptation factor HIF1α, and factors regulating glucose metabolism also appear to have a positive impact ( Table 2 ; Figure 3 ). Further research is needed to validate the findings of mouse studies in humans.
The recent single cell RNA-sequencing studies have identified a broad spectrum of ATM subtypes, suggesting a heterogeneity and functional plasticity of ATMs in obesity. It remains to be determined the differences in the development, phenotype, and function of these newly discovered macrophages within adipose tissue. Also, understanding the regulatory factors and intracellular pathways that underpin functional differences between subtypes would provide new molecular targets. Finally, the development of new technologies that can target specific macrophage subtypes would considerably boost the translational potential of the aforementioned findings for the treatment of obesity and metabolic diseases.
Author contributions
Conceptualization: DG, HG and SL; literature search: XL, YR, KC, WW, and DG; writing: XL, YR, and DG; review and editing: XL, YR, KC, WW, DG, HG, and SL. All authors contributed to the article and approved the submitted version.
Funding Statement
This work is supported the National Natural Science Foundation of China (NSFC) (Grant No. 81873665) to DG.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism (2019) 92:6–10. doi: 10.1016/j.metabol.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 2. Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci (2019) 20(9):2358. doi: 10.3390/ijms20092358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meister BM, Hong SG, Shin J, Rath M, Sayoc J, Park JY. Healthy versus unhealthy adipose tissue expansion: the role of exercise. J Obes Metab Syndr (2022) 31(1):37–50. doi: 10.7570/jomes21096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hammarstedt A, Gogg S, Hedjazifar S, Nerstedt A, Smith U. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol Rev (2018) 98(4):1911–41. doi: 10.1152/physrev.00034.2017 [DOI] [PubMed] [Google Scholar]
- 5. Michailidou Z, Gomez-Salazar M, Alexaki VI. Innate immune cells in the adipose tissue in health and metabolic disease. J Innate Immun (2022) 14(1):4–30. doi: 10.1159/000515117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest (2003) 112(12):1821–30. doi: 10.1172/JCI200319451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest (2003) 112(12):1796–808. doi: 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature (2015) 518(7540):547–51. doi: 10.1038/nature13989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Q, Ruedl C. Obesity retunes turnover kinetics of tissue-resident macrophages in fat. J Leukoc Biol (2020) 107(5):773–82. doi: 10.1002/JLB.1MA1219-275R [DOI] [PubMed] [Google Scholar]
- 10. Curat CA, Miranville A, Sengenès C, Diehl M, Tonus C, Busse R, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes (2004) 53(5):1285–92. doi: 10.2337/diabetes.53.5.1285 [DOI] [PubMed] [Google Scholar]
- 11. Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes (2005) 54(8):2277–86. doi: 10.2337/diabetes.54.8.2277 [DOI] [PubMed] [Google Scholar]
- 12. Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, et al. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem (2003) 278(47):46654–60. doi: 10.1074/jbc.M309895200 [DOI] [PubMed] [Google Scholar]
- 13. Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A (2003) 100(12):7265–70. doi: 10.1073/pnas.1133870100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond) (2005) 29(1):146–50. doi: 10.1038/sj.ijo.0802839 [DOI] [PubMed] [Google Scholar]
- 15. Dahlman I, Kaaman M, Olsson T, Tan GD, Bickerton AS, Wåhlén K, et al. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Clin Endocrinol Metab (2005) 90(10):5834–40. doi: 10.1210/jc.2005-0369 [DOI] [PubMed] [Google Scholar]
- 16. Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes (2005) 54(8):2305–13. doi: 10.2337/diabetes.54.8.2305 [DOI] [PubMed] [Google Scholar]
- 17. Chen A, Mumick S, Zhang C, Lamb J, Dai H, Weingarth D, et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res (2005) 13(8):1311–20. doi: 10.1038/oby.2005.159 [DOI] [PubMed] [Google Scholar]
- 18. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest (2006) 116(6):1494–505. doi: 10.1172/JCI26498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest (2006) 116(1):115–24. doi: 10.1172/JCI24335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tamura Y, Sugimoto M, Murayama T, Ueda Y, Kanamori H, Ono K, et al. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol (2008) 28(12):2195–201. doi: 10.1161/ATVBAHA.108.168633 [DOI] [PubMed] [Google Scholar]
- 21. Mulder P, van den Hoek AM, Kleemann R. The CCR2 inhibitor propagermanium attenuates diet-induced insulin resistance, adipose tissue inflammation and non-alcoholic steatohepatitis. PLoS One (2017) 12(1):e0169740. doi: 10.1371/journal.pone.0169740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem (2006) 281(36):26602–14. doi: 10.1074/jbc.M601284200 [DOI] [PubMed] [Google Scholar]
- 23. Ito A, Suganami T, Yamauchi A, Degawa-Yamauchi M, Tanaka M, Kouyama R, et al. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J Biol Chem (2008) 283(51):35715–23. doi: 10.1074/jbc.M804220200 [DOI] [PubMed] [Google Scholar]
- 24. Kim J, Chung K, Choi C, Beloor J, Ullah I, Kim N, et al. Silencing CCR2 in macrophages alleviates adipose tissue inflammation and the associated metabolic syndrome in dietary obese mice. Mol Ther Nucleic Acids (2016) 5(1):e280. doi: 10.1038/mtna.2015.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, et al. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-kappaB and c-jun NH2-terminal kinase pathways. Diabetes (2009) 58(1):104–15. doi: 10.2337/db07-1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, et al. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab (2008) 93(8):3215–21. doi: 10.1210/jc.2007-2630 [DOI] [PubMed] [Google Scholar]
- 27. Eriksson Hogling D, Petrus P, Gao H, Bäckdahl J, Dahlman I, Laurencikiene J, et al. Adipose and circulating CCL18 levels associate with metabolic risk factors in women. J Clin Endocrinol Metab (2016) 101(11):4021–9. doi: 10.1210/jc.2016-2390 [DOI] [PubMed] [Google Scholar]
- 28. Huh JH, Kim HM, Lee ES, Kwon MH, Lee BR, Ko HJ, et al. Dual CCR2/5 antagonist attenuates obesity-induced insulin resistance by regulating macrophage recruitment and M1/M2 status. Obes (Silver Spring) (2018) 26(2):378–86. doi: 10.1002/oby.22103 [DOI] [PubMed] [Google Scholar]
- 29. Kim D, Kim J, Yoon JH, Ghim J, Yea K, Song P, et al. CXCL12 secreted from adipose tissue recruits macrophages and induces insulin resistance in mice. Diabetologia (2014) 57(7):1456–65. doi: 10.1007/s00125-014-3237-5 [DOI] [PubMed] [Google Scholar]
- 30. Maffei M, Funicello M, Vottari T, Gamucci O, Costa M, Lisi S, et al. The obesity and inflammatory marker haptoglobin attracts monocytes via interaction with chemokine (C-c motif) receptor 2 (CCR2). BMC Biol (2009) 7:87. doi: 10.1186/1741-7007-7-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mamane Y, Chung Chan C, Lavallee G, Morin N, Xu LJ, Huang J, et al. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes (2009) 58(9):2006–17. doi: 10.2337/db09-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res (2005) 46(11):2347–55. doi: 10.1194/jlr.M500294-JLR200 [DOI] [PubMed] [Google Scholar]
- 33. Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res (2008) 49(7):1562–8. doi: 10.1194/jlr.M800019-JLR200 [DOI] [PubMed] [Google Scholar]
- 34. Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes (2007) 56(1):16–23. doi: 10.2337/db06-1076 [DOI] [PubMed] [Google Scholar]
- 35. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest (2007) 117(1):175–84. doi: 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes (2008) 57(12):3239–46. doi: 10.2337/db08-0872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet–induced obesity in mice. Diabetes (2010) 59(5):1171–81. doi: 10.2337/db09-1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Györi G, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) (2007) 31(9):1420–8. doi: 10.1038/sj.ijo.0803632 [DOI] [PubMed] [Google Scholar]
- 39. Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C, et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation (2008) 117(6):806–15. doi: 10.1161/CIRCULATIONAHA.107.724096 [DOI] [PubMed] [Google Scholar]
- 40. Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, et al. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab (2009) 94(11):4619–23. doi: 10.1210/jc.2009-0925 [DOI] [PubMed] [Google Scholar]
- 41. Fjeldborg K, Pedersen SB, Møller HJ, Christiansen T, Bennetzen M, Richelsen B. Human adipose tissue macrophages are enhanced but changed to an anti-inflammatory profile in obesity. J Immunol Res (2014) 2014:309548. doi: 10.1155/2014/309548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu R, Kim CS, Kwon BS, Kawada T. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obes (Silver Spring) (2006) 14(8):1353–62. doi: 10.1038/oby.2006.153 [DOI] [PubMed] [Google Scholar]
- 43. Harman-Boehm I, Blüher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab (2007) 92(6):2240–7. doi: 10.1210/jc.2006-1811 [DOI] [PubMed] [Google Scholar]
- 44. Michaud A, Drolet R, Noël S, Paris G, Tchernof A. Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism (2012) 61(5):689–98. doi: 10.1016/j.metabol.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 45. Okamoto Y, Higashiyama H, Rong JX, McVey MJ, Kinoshita M, Asano S, et al. Comparison of mitochondrial and macrophage content between subcutaneous and visceral fat in db/db mice. Exp Mol Pathol (2007) 83(1):73–83. doi: 10.1016/j.yexmp.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 46. Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab (2014) 19(1):162–71. doi: 10.1016/j.cmet.2013.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zheng C, Yang Q, Cao J, Xie N, Liu K, Shou P, et al. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis (2016) 7(3):e2167. doi: 10.1038/cddis.2016.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Muir LA, Kiridena S, Griffin C, DelProposto JB, Geletka L, Martinez-Santibañez G, et al. Frontline science: rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages. J Leukoc Biol (2018) 103(4):615–28. doi: 10.1002/JLB.3HI1017-422R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haase J, Weyer U, Immig K, Klöting N, Blüher M, Eilers J, et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia (2014) 57(3):562–71. doi: 10.1007/s00125-013-3139-y [DOI] [PubMed] [Google Scholar]
- 50. Zamarron BF, Mergian TA, Cho KW, Martinez-Santibanez G, Luan D, Singer K, et al. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes (2017) 66(2):392–406. doi: 10.2337/db16-0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ahlin S, Sjöholm K, Jacobson P, Andersson-Assarsson JC, Walley A, Tordjman J, et al. Macrophage gene expression in adipose tissue is associated with insulin sensitivity and serum lipid levels independent of obesity. Obes (Silver Spring) (2013) 21(12):E571–6. doi: 10.1002/oby.20443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes (2006) 55(6):1554–61. doi: 10.2337/db06-0133 [DOI] [PubMed] [Google Scholar]
- 53. Makkonen J, Westerbacka J, Kolak M, Sutinen J, Cornér A, Hamsten A, et al. Increased expression of the macrophage markers and of 11beta-HSD-1 in subcutaneous adipose tissue, but not in cultured monocyte-derived macrophages, is associated with liver fat in human obesity. Int J Obes (Lond) (2007) 31(10):1617–25. doi: 10.1038/sj.ijo.0803635 [DOI] [PubMed] [Google Scholar]
- 54. Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, Ferrante AW, Jr, et al. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy pima indians. Diabetes (2009) 58(2):385–93. doi: 10.2337/db08-0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes (2010) 59(7):1648–56. doi: 10.2337/db09-0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Klimcakova E, Roussel B, Kovacova Z, Kovacikova M, Siklova-Vitkova M, Combes M, et al. Macrophage gene expression is related to obesity and the metabolic syndrome in human subcutaneous fat as well as in visceral fat. Diabetologia (2011) 54(4):876–87. doi: 10.1007/s00125-010-2014-3 [DOI] [PubMed] [Google Scholar]
- 57. Le KA, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-kappaB stress pathway. Diabetes (2011) 60(11):2802–9. doi: 10.2337/db10-1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Koppaka S, Kehlenbrink S, Carey M, Li W, Sanchez E, Lee DE, et al. Reduced adipose tissue macrophage content is associated with improved insulin sensitivity in thiazolidinedione-treated diabetic humans. Diabetes (2013) 62(6):1843–54. doi: 10.2337/db12-0868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aouadi M, Tencerova M, Vangala P, Yawe JC, Nicoloro SM, Amano SU, et al. Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. Proc Natl Acad Sci U S A (2013) 110(20):8278–83. doi: 10.1073/pnas.1300492110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care (2011) 14(4):341–6. doi: 10.1097/MCO.0b013e328347970b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab (2014) 20(4):614–25. doi: 10.1016/j.cmet.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, Peris E, Cui C, Hoffman A, et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep (2017) 20(13):3149–61. doi: 10.1016/j.celrep.2017.08.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tiwari P, Blank A, Cui C, Schoenfelt KQ, Zhou G, Xu Y, et al. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J Exp Med (2019) 216(6):1345–58. doi: 10.1084/jem.20181616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci USA (2018) 115(22):E5096–105. doi: 10.1073/pnas.1802611115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell (2019) 178(3):686–98.e14. doi: 10.1016/j.cell.2019.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim EY, Noh HM, Choi B, Park JE, Kim JE, Jang Y, et al. Interleukin-22 induces the infiltration of visceral fat tissue by a discrete subset of Duffy antigen receptor for chemokine-positive M2-like macrophages in response to a high fat diet. Cells (2019) 8(12):1587. doi: 10.3390/cells8121587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Orr JS, Kennedy A, Anderson-Baucum EK, Webb CD, Fordahl SC, Erikson KM, et al. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes (2014) 63(2):421–32. doi: 10.2337/db13-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hubler MJ, Erikson KM, Kennedy AJ, Hasty AH. MFe(hi) adipose tissue macrophages compensate for tissue iron perturbations in mice. Am J Physiol Cell Physiol (2018) 315(3):C319–29. doi: 10.1152/ajpcell.00103.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Deczkowska A, Weiner A, Amit I. The physiology, pathology, and potential therapeutic applications of the TREM2 signaling pathway. Cell (2020) 181(6):1207–17. doi: 10.1016/j.cell.2020.05.003 [DOI] [PubMed] [Google Scholar]
- 70. Harasymowicz NS, Rashidi N, Savadipour A, Wu CL, Tang R, Bramley J, et al. Single-cell RNA sequencing reveals the induction of novel myeloid and myeloid-associated cell populations in visceral fat with long-term obesity. FASEB J (2021) 35(3):e21417. doi: 10.1096/fj.202001970R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Constant VA, Gagnon A, Landry A, Sorisky A. Macrophage-conditioned medium inhibits the differentiation of 3T3-L1 and human abdominal preadipocytes. Diabetologia (2006) 49(6):1402–11. doi: 10.1007/s00125-006-0253-0 [DOI] [PubMed] [Google Scholar]
- 72. Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology (2007) 148(2):868–77. doi: 10.1210/en.2006-0687 [DOI] [PubMed] [Google Scholar]
- 73. Liu LF, Craig CM, Tolentino LL, Choi O, Morton J, Rivas H, et al. Adipose tissue macrophages impair preadipocyte differentiation in humans. PLoS One (2017) 12(2):e0170728. doi: 10.1371/journal.pone.0170728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cao D, Ma F, Ouyang S, Liu Z, Li Y, Wu J. Effects of macrophages and CXCR2 on adipogenic differentiation of bone marrow mesenchymal stem cells. J Cell Physiol (2019) 234(6):9475–85. doi: 10.1002/jcp.27634 [DOI] [PubMed] [Google Scholar]
- 75. Moratal C, Raffort J, Arrighi N, Rekima S, Schaub S, Dechesne CA, et al. IL-1beta- and IL-4-polarized macrophages have opposite effects on adipogenesis of intramuscular fibro-adipogenic progenitors in humans. Sci Rep (2018) 8(1):17005. doi: 10.1038/s41598-018-35429-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of wnt, tumor necrosis factor-alpha, and inflammation. Diabetes (2009) 58(7):1550–7. doi: 10.2337/db08-1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gagnon A, Yarmo MN, Landry A, Sorisky A. Macrophages alter the differentiation-dependent decreases in fibronectin and collagen I/III protein levels in human preadipocytes. Lipids (2012) 47(9):873–80. doi: 10.1007/s11745-012-3696-8 [DOI] [PubMed] [Google Scholar]
- 78. Bilkovski R, Schulte DM, Oberhauser F, Mauer J, Hampel B, Gutschow C, et al. Adipose tissue macrophages inhibit adipogenesis of mesenchymal precursor cells via wnt-5a in humans. Int J Obes (Lond) (2011) 35(11):1450–4. doi: 10.1038/ijo.2011.6 [DOI] [PubMed] [Google Scholar]
- 79. Yin R, Fang L, Li Y, Xue P, Li Y, Guan Y, et al. Pro-inflammatory macrophages suppress PPARgamma activity in adipocytes via s-nitrosylation. Free Radic Biol Med (2015) 89:895–905. doi: 10.1016/j.freeradbiomed.2015.10.406 [DOI] [PubMed] [Google Scholar]
- 80. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell (2012) 150(2):366–76. doi: 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kaisanlahti A, Glumoff T. Browning of white fat: agents and implications for beige adipose tissue to type 2 diabetes. J Physiol Biochem (2019) 75(1):1–10. doi: 10.1007/s13105-018-0658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, et al. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab (2015) 22(2):279–90. doi: 10.1016/j.cmet.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 83. Sakamoto T, Nitta T, Maruno K, Yeh YS, Kuwata H, Tomita K, et al. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am J Physiol Endocrinol Metab (2016) 310(8):E676–87. doi: 10.1152/ajpendo.00028.2015 [DOI] [PubMed] [Google Scholar]
- 84. Machida K, Okamatsu-Ogura Y, Shin W, Matsuoka S, Tsubota A, Kimura K. Role of macrophages in depot-dependent browning of white adipose tissue. J Physiol Sci (2018) 68(5):601–8. doi: 10.1007/s12576-017-0567-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chung KJ, Chatzigeorgiou A, Economopoulou M, Garcia-Martin R, Alexaki VI, Mitroulis I, et al. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat Immunol (2017) 18(6):654–64. doi: 10.1038/ni.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell (2014) 157(6):1292–308. doi: 10.1016/j.cell.2014.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang YN, Tang Y, He Z, Ma H, Wang L, Liu Y, et al. Slit3 secreted from M2-like macrophages increases sympathetic activity and thermogenesis in adipose tissue. Nat Metab (2021) 3(11):1536–51. doi: 10.1038/s42255-021-00482-9 [DOI] [PubMed] [Google Scholar]
- 88. Liu PS, Lin YW, Burton FH, Wei LN. Injecting engineered anti-inflammatory macrophages therapeutically induces white adipose tissue browning and improves diet-induced insulin resistance. Adipocyte (2015) 4(2):123–8. doi: 10.4161/21623945.2014.981438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nawaz A, Aminuddin A, Kado T, Takikawa A, Yamamoto S, Tsuneyama K, et al. CD206(+) M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat Commun (2017) 8(1):286. doi: 10.1038/s41467-017-00231-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Igarashi Y, Nawaz A, Kado T, Bilal M, Kuwano T, Yamamoto S, et al. Partial depletion of CD206-positive M2-like macrophages induces proliferation of beige progenitors and enhances browning after cold stimulation. Sci Rep (2018) 8(1):14567. doi: 10.1038/s41598-018-32803-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jiang Y, Berry DC, Graff JM. Distinct cellular and molecular mechanisms for beta3 adrenergic receptor-induced beige adipocyte formation. Elife (2017) 6:e30329. doi: 10.7554/eLife.30329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab (2013) 18(3):355–67. doi: 10.1016/j.cmet.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee YH, Kim SN, Kwon HJ, Maddipati KR, Granneman JG. Adipogenic role of alternatively activated macrophages in beta-adrenergic remodeling of white adipose tissue. Am J Physiol Regul Integr Comp Physiol (2016) 310(1):R55–65. doi: 10.1152/ajpregu.00355.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pekala PH, Price SR, Horn CA, Hom BE, Moss J, Cerami A. Model for cachexia in chronic disease: secretory products of endotoxin-stimulated macrophages induce a catabolic state in 3T3-L1 adipocytes. Trans Assoc Am Physicians (1984) 97:251–9. [PubMed] [Google Scholar]
- 95. Mahoney JR, Jr, Beutler BA, Le Trang N, Vine W, Ikeda Y, Kawakami M, et al. Lipopolysaccharide-treated RAW 264.7 cells produce a mediator that inhibits lipoprotein lipase in 3T3-L1 cells. J Immunol (1985) 134(3):1673–5. [PubMed] [Google Scholar]
- 96. Kurokawa J, Arai S, Nakashima K, Nagano H, Nishijima A, Miyata K, et al. Macrophage-derived AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab (2010) 11(6):479–92. doi: 10.1016/j.cmet.2010.04.013 [DOI] [PubMed] [Google Scholar]
- 97. Keuper M, Sachs S, Walheim E, Berti L, Raedle B, Tews D, et al. Activated macrophages control human adipocyte mitochondrial bioenergetics via secreted factors. Mol Metab (2017) 6(10):1226–39. doi: 10.1016/j.molmet.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cox N, Crozet L, Holtman IR, Loyher PL, Lazarov T, White JB, et al. Diet-regulated production of PDGFcc by macrophages controls energy storage. Science (2021) 373(6550):eabe9383. doi: 10.1126/science.abe9383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Permana PA, Menge C, Reaven PD. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun (2006) 341(2):507–14. doi: 10.1016/j.bbrc.2006.01.012 [DOI] [PubMed] [Google Scholar]
- 100. Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab (2007) 292(1):E166–74. doi: 10.1152/ajpendo.00284.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gao D, Madi M, Ding C, Fok M, Steele T, Ford C, et al. Interleukin-1beta mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am J Physiol Endocrinol Metab (2014) 307(3):E289–304. doi: 10.1152/ajpendo.00430.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Maeda T, Noge I, Kagawa Y. Infiltration of M1 macrophages into adipose tissue of ddY-h mice preceding spontaneous appearances of insulin resistance. Biol Pharm Bull (2013) 36(5):825–32. doi: 10.1248/bpb.b12-01014 [DOI] [PubMed] [Google Scholar]
- 103. Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol (2005) 25(10):2062–8. doi: 10.1161/01.ATV.0000183883.72263.13 [DOI] [PubMed] [Google Scholar]
- 104. Davis JE, Braucher DR, Walker-Daniels J, Spurlock ME. Absence of Tlr2 protects against high-fat diet-induced inflammation and results in greater insulin-stimulated glucose transport in cultured adipocytes. J Nutr Biochem (2011) 22(2):136–41. doi: 10.1016/j.jnutbio.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 105. Hong CP, Yun CH, Lee GW, Park A, Kim YM, Jang MH. TLR9 regulates adipose tissue inflammation and obesity-related metabolic disorders. Obes (Silver Spring) (2015) 23(11):2199–206. doi: 10.1002/oby.21215 [DOI] [PubMed] [Google Scholar]
- 106. Nakarai H, Yamashita A, Nagayasu S, Iwashita M, Kumamoto S, Ohyama H, et al. Adipocyte-macrophage interaction may mediate LPS-induced low-grade inflammation: potential link with metabolic complications. Innate Immun (2012) 18(1):164–70. doi: 10.1177/1753425910393370 [DOI] [PubMed] [Google Scholar]
- 107. Liu J, Batkai S, Pacher P, Harvey-White J, Wagner JA, Cravatt BF, et al. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J Biol Chem (2003) 278(45):45034–9. doi: 10.1074/jbc.M306062200 [DOI] [PubMed] [Google Scholar]
- 108. Mehrpouya-Bahrami P, Chitrala KN, Ganewatta MS, Tang C, Murphy EA, Enos RT, et al. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci Rep (2017) 7(1):15645. doi: 10.1038/s41598-017-15154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Miranville A, Herling AW, Biemer-Daub G, Voss MD. Reversal of inflammation-induced impairment of glucose uptake in adipocytes by direct effect of CB1 antagonism on adipose tissue macrophages. Obes (Silver Spring) (2010) 18(12):2247–54. doi: 10.1038/oby.2010.81 [DOI] [PubMed] [Google Scholar]
- 110. Zhong J, Rao X, Deiuliis J, Braunstein Z, Narula V, Hazey J, et al. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes (2013) 62(1):149–57. doi: 10.2337/db12-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Varin EM, Mulvihill EE, Beaudry JL, Pujadas G, Fuchs S, Tanti JF, et al. Circulating levels of soluble dipeptidyl peptidase-4 are dissociated from inflammation and induced by enzymatic DPP4 inhibition. Cell Metab (2019) 29(2):320–34.e5. doi: 10.1016/j.cmet.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 112. Zhuge F, Ni Y, Nagashimada M, Nagata N, Xu L, Mukaida N, et al. DPP-4 inhibition by linagliptin attenuates obesity-related inflammation and insulin resistance by regulating M1/M2 macrophage polarization. Diabetes (2016) 65(10):2966–79. doi: 10.2337/db16-0317 [DOI] [PubMed] [Google Scholar]
- 113. Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol (2009) 29(6):1575–91. doi: 10.1128/MCB.01300-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes (2010) 59(11):2817–25. doi: 10.2337/db10-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, et al. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab (2010) 299(6):E1016–27. doi: 10.1152/ajpendo.00329.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Marcelin G, Ferreira A, Liu Y, Atlan M, Aron-Wisnewsky J, Pelloux V, et al. A PDGFRalpha-mediated switch toward CD9(high) adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metab (2017) 25(3):673–85. doi: 10.1016/j.cmet.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 117. Keophiphath M, Rouault C, Divoux A, Clément K, Lacasa D. CCL5 promotes macrophage recruitment and survival in human adipose tissue. Arterioscler Thromb Vasc Biol (2010) 30(1):39–45. doi: 10.1161/ATVBAHA.109.197442 [DOI] [PubMed] [Google Scholar]
- 118. O'Hara A, Lim FL, Mazzatti DJ, Trayhurn P. Microarray analysis identifies matrix metalloproteinases (MMPs) as key genes whose expression is up-regulated in human adipocytes by macrophage-conditioned medium. Pflugers Arch (2009) 458(6):1103–14. doi: 10.1007/s00424-009-0693-8 [DOI] [PubMed] [Google Scholar]
- 119. Gao D, Bing C. Macrophage-induced expression and release of matrix metalloproteinase 1 and 3 by human preadipocytes is mediated by IL-1beta via activation of MAPK signaling. J Cell Physiol (2011) 226(11):2869–80. doi: 10.1002/jcp.22630 [DOI] [PubMed] [Google Scholar]
- 120. Bourlier V, Sengenès C, Zakaroff-Girard A, Decaunes P, Wdziekonski B, Galitzky J, et al. TGFbeta family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages. PloS One (2012) 7(2):e31274. doi: 10.1371/journal.pone.0031274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab (2007) 293(4):E1118–28. doi: 10.1152/ajpendo.00435.2007 [DOI] [PubMed] [Google Scholar]
- 122. Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) (2008) 32(3):451–63. doi: 10.1038/sj.ijo.0803744 [DOI] [PubMed] [Google Scholar]
- 123. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes (2007) 56(4):901–11. doi: 10.2337/db06-0911 [DOI] [PubMed] [Google Scholar]
- 124. Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes (2009) 58(3):718–25. doi: 10.2337/db08-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab (2013) 18(4):470–7. doi: 10.1016/j.cmet.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jang JE, Ko MS, Yun JY, Kim MO, Kim JH, Park HS, et al. Nitric oxide produced by macrophages inhibits adipocyte differentiation and promotes profibrogenic responses in preadipocytes to induce adipose tissue fibrosis. Diabetes (2016) 65(9):2516–28. doi: 10.2337/db15-1624 [DOI] [PubMed] [Google Scholar]
- 127. Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med (2005) 11(2):191–8. doi: 10.1038/nm1185 [DOI] [PubMed] [Google Scholar]
- 128. Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab (2009) 10(5):419–29. doi: 10.1016/j.cmet.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wueest S, Mueller R, Blüher M, Item F, Chin AS, Wiedemann MS, et al. Fas (CD95) expression in myeloid cells promotes obesity-induced muscle insulin resistance. EMBO Mol Med (2014) 6(1):43–56. doi: 10.1002/emmm.201302962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yu M, Zhou H, Zhao J, Xiao N, Roychowdhury S, Schmitt D, et al. MyD88-dependent interplay between myeloid and endothelial cells in the initiation and progression of obesity-associated inflammatory diseases. J Exp Med (2014) 211(5):887–907. doi: 10.1084/jem.20131314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chen Z, Shen H, Sun C, Yin L, Tang F, Zheng P, et al. Myeloid cell TRAF3 promotes metabolic inflammation, insulin resistance, and hepatic steatosis in obesity. Am J Physiol Endocrinol Metab (2015) 308(6):E460–9. doi: 10.1152/ajpendo.00470.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sima C, Montero E, Nguyen D, Freire M, Norris P, Serhan CN, et al. ERV1 overexpression in myeloid cells protects against high fat diet induced obesity and glucose intolerance. Sci Rep (2017) 7(1):12848. doi: 10.1038/s41598-017-13185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pepping JK, Vandanmagsar B, Fernandez-Kim SO, Zhang J, Mynatt RL, Bruce-Keller AJ. Myeloid-specific deletion of NOX2 prevents the metabolic and neurologic consequences of high fat diet. PloS One (2017) 12(8):e0181500. doi: 10.1371/journal.pone.0181500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Takikawa A, Mahmood A, Nawaz A, Kado T, Okabe K, Yamamoto S, et al. HIF-1alpha in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes (2016) 65(12):3649–59. doi: 10.2337/db16-0012 [DOI] [PubMed] [Google Scholar]
- 135. Huang JY, Chiang MT, Yet SF, Chau LY. Myeloid heme oxygenase-1 haploinsufficiency reduces high fat diet-induced insulin resistance by affecting adipose macrophage infiltration in mice. PloS One (2012) 7(6):e38626. doi: 10.1371/journal.pone.0038626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hu L, He F, Huang M, Peng M, Zhou Z, Liu F, et al. NFATc3 deficiency reduces the classical activation of adipose tissue macrophages. J Mol Endocrinol (2018) 61(3):79–89. doi: 10.1530/JME-18-0070 [DOI] [PubMed] [Google Scholar]
- 137. Cao W, Zhang T, Feng R, Xia T, Huang H, Liu C, et al. Hoxa5 alleviates obesity-induced chronic inflammation by reducing ER stress and promoting M2 macrophage polarization in mouse adipose tissue. J Cell Mol Med (2019) 23(10):7029–42. doi: 10.1111/jcmm.14600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mauer J, Chaurasia B, Plum L, Quast T, Hampel B, Blüher M, et al. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PloS Genet (2010) 6(5):e1000938. doi: 10.1371/journal.pgen.1000938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Grant L, Shearer KD, Czopek A, Lees EK, Owen C, Agouni A, et al. Myeloid-cell protein tyrosine phosphatase-1B deficiency in mice protects against high-fat diet and lipopolysaccharide-induced inflammation, hyperinsulinemia, and endotoxemia through an IL-10 STAT3-dependent mechanism. Diabetes (2014) 63(2):456–70. doi: 10.2337/db13-0885 [DOI] [PubMed] [Google Scholar]
- 140. Rached MT, Millership SJ, Pedroni SMA, Choudhury AI, Costa ASH, Hardy DG, et al. Deletion of myeloid IRS2 enhances adipose tissue sympathetic nerve function and limits obesity. Mol Metab (2019) 20:38–50. doi: 10.1016/j.molmet.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Paschoal VA, Belchior T, Amano MT, Burgos-Silva M, Peixoto AS, Magdalon J, et al. Constitutive activation of the nutrient sensor mTORC1 in myeloid cells induced by Tsc1 deletion protects mice from diet-induced obesity. Mol Nutr Food Res (2018) 62(17):e1800283. doi: 10.1002/mnfr.201800283 [DOI] [PubMed] [Google Scholar]
- 142. Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol (2010) 30(19):4712–21. doi: 10.1128/MCB.00657-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ka SO, Song MY, Bae EJ, Park BH. Myeloid SIRT1 regulates macrophage infiltration and insulin sensitivity in mice fed a high-fat diet. J Endocrinol (2015) 224(2):109–18. doi: 10.1530/JOE-14-0527 [DOI] [PubMed] [Google Scholar]
- 144. Takikawa A, Usui I, Fujisaka S, Ikutani M, Senda S, Hattori S, et al. Deletion of SIRT1 in myeloid cells impairs glucose metabolism with enhancing inflammatory response to adipose tissue hypoxia. Diabetol Int (2016) 7(1):59–68. doi: 10.1007/s13340-015-0213-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Jeon BT, Kim KE, Heo RW, Shin HJ, Yi CO, Hah YS, et al. Myeloid-specific deletion of SIRT1 increases hepatic steatosis and hypothalamic inflammation in mice fed a high-fat diet. Metab Brain Dis (2014) 29(3):635–43. doi: 10.1007/s11011-014-9542-3 [DOI] [PubMed] [Google Scholar]
- 146. Park M, Yi JW, Kim EM, Yoon IJ, Lee EH, Lee HY, et al. Triggering receptor expressed on myeloid cells 2 (TREM2) promotes adipogenesis and diet-induced obesity. Diabetes (2015) 64(1):117–27. doi: 10.2337/db13-1869 [DOI] [PubMed] [Google Scholar]
- 147. Park YS, Uddin MJ, Piao L, Hwang I, Lee JH, Ha H. Novel role of endogenous catalase in macrophage polarization in adipose tissue. Mediators Inflammation (2016) 2016:8675905. doi: 10.1155/2016/8675905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature (2007) 447(7148):1116–20. doi: 10.1038/nature05894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest (2007) 117(6):1658–69. doi: 10.1172/JCI31561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, et al. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest (2011) 121(7):2736–49. doi: 10.1172/JCI45444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Coppo M, Chinenov Y, Sacta MA, Rogatsky I. The transcriptional coregulator GRIP1 controls macrophage polarization and metabolic homeostasis. Nat Commun (2016) 7:12254. doi: 10.1038/ncomms12254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lee HY, Kim J, Quan W, Lee JC, Kim MS, Kim SH, et al. Autophagy deficiency in myeloid cells increases susceptibility to obesity-induced diabetes and experimental colitis. Autophagy (2016) 12(8):1390–403. doi: 10.1080/15548627.2016.1184799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kawano Y, Nakae J, Watanabe N, Fujisaka S, Iskandar K, Sekioka R, et al. Loss of Pdk1-Foxo1 signaling in myeloid cells predisposes to adipose tissue inflammation and insulin resistance. Diabetes (2012) 61(8):1935–48. doi: 10.2337/db11-0770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, et al. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc Natl Acad Sci USA (2011) 108(39):16457–62. doi: 10.1073/pnas.1104533108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lee Y, Ka SO, Cha HN, Chae YN, Kim MK, Park SY, et al. Myeloid sirtuin 6 deficiency causes insulin resistance in high-fat diet-fed mice by eliciting macrophage polarization toward an M1 phenotype. Diabetes (2017) 66(10):2659–68. doi: 10.2337/db16-1446 [DOI] [PubMed] [Google Scholar]
- 156. Xu H, Li H, Woo SL, Kim SM, Shende VR, Neuendorff N, et al. Myeloid cell-specific disruption of Period1 and Period2 exacerbates diet-induced inflammation and insulin resistance. J Biol Chem (2014) 289(23):16374–88. doi: 10.1074/jbc.M113.539601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab (2008) 8(4):301–9. doi: 10.1016/j.cmet.2008.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Pamir N, McMillen TS, Kaiyala KJ, Schwartz MW, LeBoeuf RC. Receptors for tumor necrosis factor-alpha play a protective role against obesity and alter adipose tissue macrophage status. Endocrinology (2009) 150(9):4124–34. doi: 10.1210/en.2009-0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kohlstedt K, Trouvain C, Namgaladze D, Fleming I. Adipocyte-derived lipids increase angiotensin-converting enzyme (ACE) expression and modulate macrophage phenotype. Basic Res Cardiol (2011) 106(2):205–15. doi: 10.1007/s00395-010-0137-9 [DOI] [PubMed] [Google Scholar]
- 160. Camell C, Smith CW. Dietary oleic acid increases m2 macrophages in the mesenteric adipose tissue. PLoS One (2013) 8(9):e75147. doi: 10.1371/journal.pone.0075147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Pini M, Touch S, Poirier H, Dalmas E, Niot I, Rouault C, et al. Adipose tissue adaptive response to trans-10,cis-12-conjugated linoleic acid engages alternatively activated M2 macrophages. FASEB J (2016) 30(1):241–51. doi: 10.1096/fj.15-276675 [DOI] [PubMed] [Google Scholar]
- 162. Talamonti E, Pauter AM, Asadi A, Fischer AW, Chiurchiù V, Jacobsson A. Impairment of systemic DHA synthesis affects macrophage plasticity and polarization: implications for DHA supplementation during inflammation. Cell Mol Life Sci (2017) 74(15):2815–26. doi: 10.1007/s00018-017-2498-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bashir S, Sharma Y, Elahi A, Khan F. Amelioration of obesity-associated inflammation and insulin resistance in c57bl/6 mice via macrophage polarization by fish oil supplementation. J Nutr Biochem (2016) 33:82–90. doi: 10.1016/j.jnutbio.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 164. Rombaldova M, Janovska P, Kopecky J, Kuda O. Omega-3 fatty acids promote fatty acid utilization and production of pro-resolving lipid mediators in alternatively activated adipose tissue macrophages. Biochem Biophys Res Commun (2017) 490(3):1080–5. doi: 10.1016/j.bbrc.2017.06.170 [DOI] [PubMed] [Google Scholar]
- 165. Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Müller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem (2008) 283(33):22620–7. doi: 10.1074/jbc.M710314200 [DOI] [PubMed] [Google Scholar]
- 166. Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes (2009) 58(11):2574–82. doi: 10.2337/db08-1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Spencer M, Yang L, Adu A, Finlin BS, Zhu B, Shipp LR, et al. Pioglitazone treatment reduces adipose tissue inflammation through reduction of mast cell and macrophage number and by improving vascularity. PLoS One (2014) 9(7):e102190. doi: 10.1371/journal.pone.0102190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kumar D, Goand UK, Gupta S, Shankar K, Varshney S, Rajan S, et al. Saroglitazar reduces obesity and associated inflammatory consequences in murine adipose tissue. Eur J Pharmacol (2018) 822:32–42. doi: 10.1016/j.ejphar.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 169. Bassaganya-Riera J, Misyak S, Guri AJ, Hontecillas R. PPAR gamma is highly expressed in F4/80(hi) adipose tissue macrophages and dampens adipose-tissue inflammation. Cell Immunol (2009) 258(2):138–46. doi: 10.1016/j.cellimm.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol (2013) 31:317–43. doi: 10.1146/annurev-immunol-032712-095906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Lin SY, Yang CP, Wang YY, Hsiao CW, Chen WY, Liao SL, et al. Interleukin-4 improves metabolic abnormalities in leptin-deficient and high-fat diet mice. Int J Mol Sci (2020) 21(12):4451. doi: 10.3390/ijms21124451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ackermann J, Arndt L, Kirstein M, Hobusch C, Brinker G, Klöting N, et al. Myeloid cell-specific IL-4 receptor knockout partially protects from adipose tissue inflammation. J Immunol (2021) 207(12):3081–9. doi: 10.4049/jimmunol.2100699 [DOI] [PubMed] [Google Scholar]
- 173. Alder JK, Georgantas RW, 3rd, Hildreth RL, Kaplan IM, Morisot S, Yu X, et al. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo . J Immunol (2008) 180(8):5645–52. doi: 10.4049/jimmunol.180.8.5645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Cao W, Xu Y, Luo D, Saeed M, Sun C. Hoxa5 promotes adipose differentiation via increasing DNA methylation level and inhibiting PKA/HSL signal pathway in mice. Cell Physiol Biochem (2018) 45(3):1023–33. doi: 10.1159/000487343 [DOI] [PubMed] [Google Scholar]
- 175. Cinkajzlova A, Lacinová Z, Kloučková J, Kaválková P, Trachta P, Kosák M, et al. An alternatively activated macrophage marker CD163 in severely obese patients: the influence of very low-calorie diet and bariatric surgery. Physiol Res (2017) 66(4):641–52. doi: 10.33549/physiolres.933522 [DOI] [PubMed] [Google Scholar]
- 176. Jing Y, Wu F, Li D, Yang L, Li Q, Li R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol Cell Endocrinol (2018) 461:256–64. doi: 10.1016/j.mce.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 177. Pescador N, Francisco V, Vázquez P, Esquinas EM, González-Páramos C, Valdecantos MP, et al. Metformin reduces macrophage HIF1alpha-dependent proinflammatory signaling to restore brown adipocyte function in vitro. Redox Biol (2021) 48:102171. doi: 10.1016/j.redox.2021.102171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zheng W, Zhou J, Song S, Kong W, Xia W, Chen L, et al. Dipeptidyl-peptidase 4 inhibitor sitagliptin ameliorates hepatic insulin resistance by modulating inflammation and autophagy in ob/ob mice. Int J Endocrinol (2018) 2018:8309723. doi: 10.1155/2018/8309723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine (2017) 20:137–49. doi: 10.1016/j.ebiom.2017.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Xu L, Nagata N, Chen G, Nagashimada M, Zhuge F, Ni Y, et al. Empagliflozin reverses obesity and insulin resistance through fat browning and alternative macrophage activation in mice fed a high-fat diet. BMJ Open Diabetes Res Care (2019) 7(1):e000783. doi: 10.1136/bmjdrc-2019-000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, et al. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol (2006) 177(6):3520–4. doi: 10.4049/jimmunol.177.6.3520 [DOI] [PubMed] [Google Scholar]
- 182. Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab (2014) 19(1):21–36. doi: 10.1016/j.cmet.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest (2004) 114(12):1752–61. doi: 10.1172/JCI21625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol (2009) 29(16):4467–83. doi: 10.1128/MCB.00192-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Fujisaka S, Usui I, Ikutani M, Aminuddin A, Takikawa A, Tsuneyama K, et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1alpha-dependent and HIF-1alpha-independent manner in obese mice. Diabetologia (2013) 56(6):1403–12. doi: 10.1007/s00125-013-2885-1 [DOI] [PubMed] [Google Scholar]
- 186. Poblete JMS, Ballinger MN, Bao S, Alghothani M, Nevado JB, Jr, Eubank TD, et al. Macrophage HIF-1alpha mediates obesity-related adipose tissue dysfunction via interleukin-1 receptor-associated kinase m. Am J Physiol Endocrinol Metab (2020) 318(5):E689–700. doi: 10.1152/ajpendo.00174.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Paschoal VA, Amano MT, Belchior T, Magdalon J, Chimin P, Andrade ML, et al. mTORC1 inhibition with rapamycin exacerbates adipose tissue inflammation in obese mice and dissociates macrophage phenotype from function. Immunobiology (2017) 222(2):261–71. doi: 10.1016/j.imbio.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 188. Festuccia WT, Pouliot P, Bakan I, Sabatini DM, Laplante M. Myeloid-specific rictor deletion induces M1 macrophage polarization and potentiates in vivo pro-inflammatory response to lipopolysaccharide. PLoS One (2014) 9(4):e95432. doi: 10.1371/journal.pone.0095432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia (2006) 49(3):588–97. doi: 10.1007/s00125-005-0105-3 [DOI] [PubMed] [Google Scholar]
- 190. Dong J, Zhang X, Zhang L, Bian HX, Xu N, Bao B, et al. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKalpha1/SIRT1. J Lipid Res (2014) 55(3):363–74. doi: 10.1194/jlr.M038786 [DOI] [PMC free article] [PubMed] [Google Scholar]