Abstract
Fucose is a monosaccharide commonly found in mammalian, insect, microbial and plant glycans. The removal of terminal α-l-fucosyl residues from oligosaccharides and glycoconjugates is catalysed by α-l-fucosidases. To date, glycoside hydrolases (GHs) with exo-fucosidase activity on α-l-fucosylated substrates (EC 3.2.1.51, EC 3.2.1.-) have been reported in the GH29, GH95, GH139, GH141 and GH151 families of the Carbohydrate Active Enzymes (CAZy) database. Microbes generally encode several fucosidases in their genomes, often from more than one GH family, reflecting the high diversity of naturally occuring fucosylated structures they encounter. Functionally characterised microbial α-l-fucosidases have been shown to act on a range of substrates with α-1,2, α-1,3, α-1,4 or α-1,6 fucosylated linkages depending on the GH family and microorganism. Fucosidases show a modular organisation with catalytic domains of GH29 and GH151 displaying a (β/α)8-barrel fold while GH95 and GH141 show a (α/α)6 barrel and parallel β-helix fold, respectively. A number of crystal structures have been solved in complex with ligands, providing structural basis for their substrate specificity. Fucosidases can also be used in transglycosylation reactions to synthesise oligosaccharides. This mini review provides an overview of the enzymatic and structural properties of microbial α-l-fucosidases and some insights into their biological function and biotechnological applications.
Keywords: carbohydrate-active enzymes, fucose, fucosidases, glycoside hydrolases, gut bacteria
Introduction
Fucose (Fuc) is a 6-deoxy sugar that can be present as d or l enantiomer in nature. d-fucose (6-deoxy-d-galactose) is frequently found in plant glycosides such as convolvulin from Convolvulaceae plants and in antimicrobials including curamycin produced by Streptomyces curacoi [1]. l-fucose (6-deoxy-l-galactose) is ubiquitously found in mammals, plants, insects and microbes as part of oligosaccharides, glycoproteins such as mucins, or lipid forming glycoconjugates via α linkage [1], whilst β-l-fucose is rare and only seldomly reported in bacteria [2]. These structures are involved in a myriad of physiological processes, including immune recognition [3], development and neural functions [4,5] plant immunity [6,7] or host-microbe interactions (for a review see [8]). For example, Fuc has been implicated in bacteria colonisation by modulating chemotaxis [9], swimming motility [10], pathogenesis [11] or by acting as nutrient source for commensal or pathogenic bacteria [12–14]. In nature, Fuc can be linked to other sugar residues via various linkages in the non-reducing end through the action of fucosyltransferases [15,16]. Core Fuc, Le-type Fuc and O-Fuc have different biological functions and are associated with different diseases [17]. Terminal Fuc can be α-1,2 linked to β-Galactose (Gal) from lactose (Lac) or N-acetyllactosamine (LacNAc) in human milk oligosaccharides (HMOs) [18] and blood group antigens [14]. Terminal Fuc can also be α-1,3-linked to β-Glucose (Glc) and β-N-acetylglucosamine (GlcNAc) from HMOs [18], to β-GlcNAc from Lewis antigens [14] and β-Gal from HMOs [19] and to β-GlcNAc in animal antennary N-glycans [20]. Terminal Fuc can also be found α-1,4 linked to β-GlcNAc from HMOs and Lewis antigens, and in plant antennary N-glycans [16]. Core Fuc is present in plants [16] and invertebrate N-glycans [21,22] where it is α-1,3-linked to the innermost GlcNAc. Core α-1,3/α-1,6-difucosylation is found in N-glycans from Schistosoma mansoni, Caenorhabditis elegans, insects and plants [16]. Human N-glycan core fucosylation is exclusively via α-1,6 linkage [23,24].
Reflecting the high diversity of naturally-occuring fucosylated structures, microbes produce a range of α-l-fucosidases (EC 3.2.1.51) of diverse substrate specificity cleaving the nonreducing terminal α-l-fucose from these glycoconjugates. According to the Carbohydrate Active Enzymes database (CAZy database, www.cazy.org), α-l-fucosidases are found into sequence-based families GH29, GH95, GH139, GH141, and GH151, a majority of which are from microbial sources, while GH1 [25] and GH30 [26] families contain β-d-fucosidases. This mini-review focuses on the structure and function of α-l-fucosidases from microorganisms.
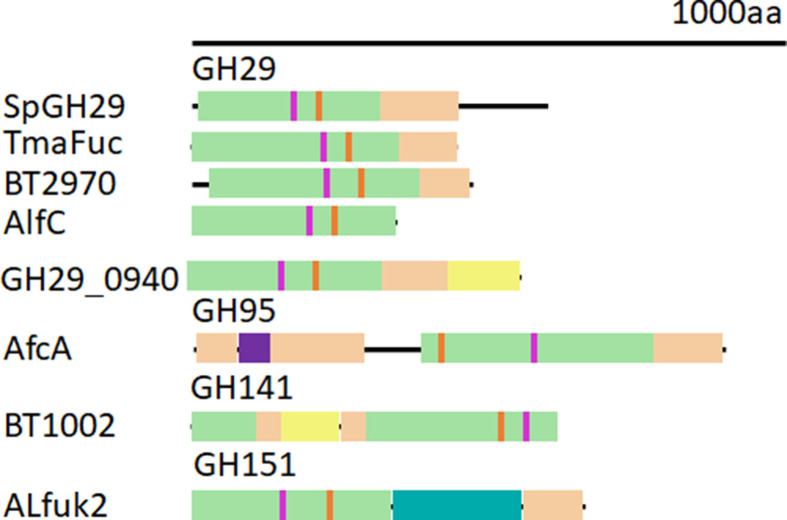
The most studied α-l-fucosidases belong to the GH29 (covering EC 3.2.1.51, EC 3.2.1.111, EC 3.2.1.63, EC 3.2.1.127) and GH95 (covering EC 3.2.1.51, EC 3.2.1.63) families employing retaining and inverting catalytic mechanisms, respectively. The GH141 (covering EC 3.2.1.51, EC 3.2.1.8) and GH151 (EC 3.2.1.51) fucosidases belong to relatively new founded families and their catalytic mechanisms remain to be demonstrated experimentally although the latter is probably a retaining enzyme based on reported transglycosylation activity and crystal structures (see below). Generally, fucosidases found in these four GH families are multimodular proteins including a catalytic domain and one or more terminal β-sandwich domains that may have carbohydrate binding properties (Figure 1). GH29 enzymes usually contain a N-terminal catalytic domain and one [27–34] or two [35–38] C-terminal β-sandwich domains apart for AlfC from Lactobacillus casei which lacks a C-terminal domain [23]. Some of these ancillary domains have been annotated as CBM32 [36] or CBM35 [32] or other types [37] although their role in carbohydrate binding remains to be experimentally validated. The modularity of GH95 enzymes is featured by a catalytic domain flanked by two β-sandwich domains [39–41] (Figure 1). There is only one example of functionally characterised GH141 fucosidase covering a N-terminal β-sandwich domain and a C-terminal catalytic domain [42]. More recently, the first crystal structure of a GH151 fucosidase was determined, showing a N-terminal catalytic domain, a central β-barrel domain and a C-terminal β-sandwich fold [43]. The catalytic domains of GH29 and GH151 fucosidases adopt a TIM- barrel fold (β/α)8, while GH95 and GH141 catalytic domains display a (α/α)6 barrel and parallel β-helix fold, respectively (www.cazy.org) (see Figure 2). GH139 (EC 3.2.1.-) fucosidases are poorly characterised and their catalytic mechanisms and 3D structures are still unknown.
Figure 1. Schematic modular representation of microbial α-l-fucosidases from different GH families.
Catalytic modules are shown in green and β-sandwich domains that may have carbohydrate binding properties in light brown. If a second β-sandwich domain is present, such as in GH29_0940, it is coloured yellow. AfcA has an additional helical barrel domain, colored purple. For clarity, the AfcA N-terminal domain of unknown function and the C-terminal bacterial Ig-like domain are not shown. These extend the total length of AfcA to 1959 amino acids. ALfuk2 also has a Rossman fold domain, colored teal.
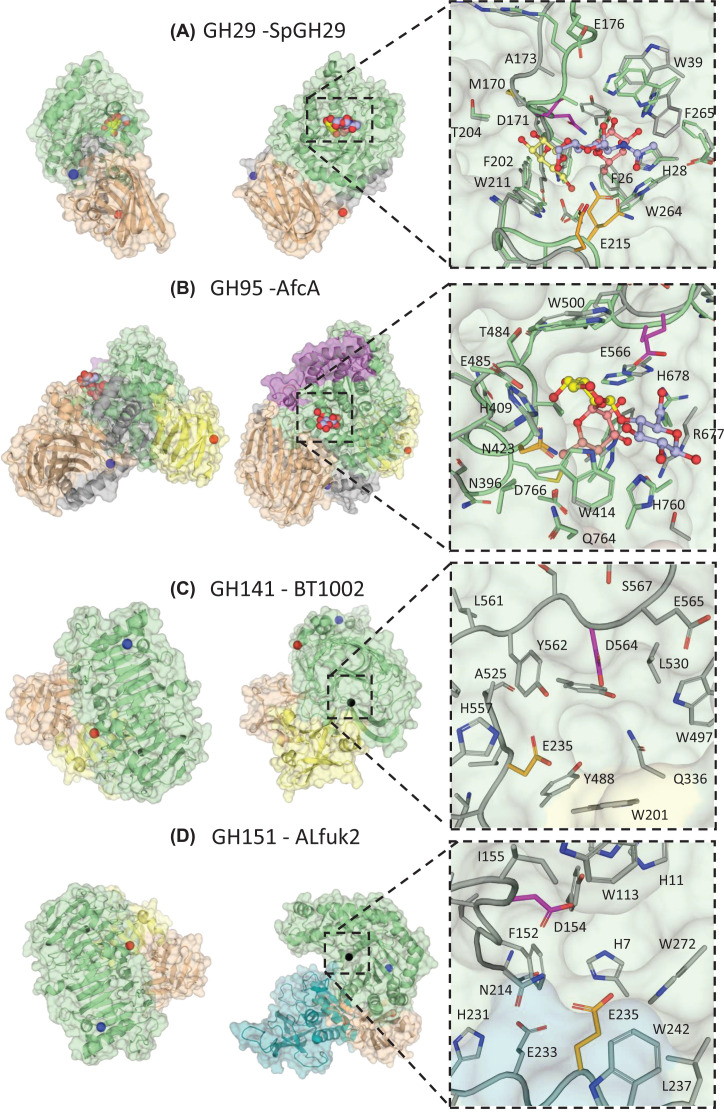
Figure 2. Crystal structures of microbial α-l-fucosidases from different GH families with close up of active sites.
Catalytic modules are shown in green and β-sandwich domains that may have carbohydrate binding properties in light brown and yellow. Catalytic nucleophile residues are coloured magenta and catalytic acid/base residues are coloured in orange. Where possible WT apo crystal structures (grey) have been aligned to their corresponding inactive mutant crystal structures (green) to highlight residue movements upon binding to a substrate like ligand. The N- and C-termini are indicated with blue and red spheres, respectively. Surface representation views are related by a 90° rotation around the y axis. If a substrate complex is not available, the location of the active site is indicated with a black sphere. (A) GH29 fucosidase (SpGH29, apo PDB = 6ORG; D171N; E215Q mutant in complex with LeX PDB = 6ORF). The catalytic domain comprises residues 11-317 and the C terminal β-sandwich module comprises residues 318-451. The bound ligand is shown with Fuc (light red), Gal (yellow) and GlcNAc (light blue). (B) GH95 fucosidase (AfcA, apo PDB = 2EAB; E566A mutant in complex with substrate PDB: 2EAD). The catalytic domain comprises residues 80-133 and 387-778, the N-terminal domain (in light brown) residues 9-79 and 134-293, and the C-terminal β-sandwich module (in yellow) residues 779-896. There is a helical barrel protruding from the N-terminal domain, residues 80-133. The substrate is shown with Gal (yellow), Fuc (light red) and Glc (light blue). C) GH141 fucosidase (BT1002, apo PDB = 5MQP). The catalytic domain comprises residues 1-108 and 296-619, the ancillary β-sandwich domain, residues 109-295 (in yellow for residues 151-251 and in wheat for residues 109-251 and 252-295, according to visual separation into sub domains). D) GH151 fucosidase (ALfuk2, apo PDB = 6TVK). The catalytic domain covers residues 1-336, the C-terminal domain (in wheat), residues 560-660 and the Rossman fold domain (in teal), residues 341-558.
According to the CAZy database (updated on 15 November 2022), there are a total of 9867 annotated GH29 sequences, 96% of which are of bacteria origin, including from the Terrabacteria group (42%), FCB (Fibrobacteres-Chlorobi-Bacteroidetes super phylum) group (24%) and Proteobacteria group (27%) in agreement with previous analyses [44]. Compared with GH29 fucosidases, about half of sequences (4890) are assigned to the GH95 family, 97% of which are from bacteria, with a similar distribution as for the GH29 family between the Terrabacteria (46%), FCB (29%), Proteobacteria (18%) groups. In contrast, GH139, GH141 and GH151 are smaller families comprising 254, 1043 and 203 members, respectively, mostly from bacterial origin (95% of GH139, 98% of GH141 and 99% of GH151). Altogether, these data indicate that about 96.5% of known fucosidase sequences are of bacterial origin [45]. There is also high variation and level of redundancy of putative fucosidase-encoding genes within a given bacterial genome with up to 21 GH29 encoding genes and up to 10 GH95 encoding genes found per genome, while the reported number of genes encoding GH139, GH141 and GH151 does not exceed two per genome (see Supplementary Table S1). In this mini review, we will describe the enzymatic and structural properties of α-l-fucosidases produced by microbes and provide an overview of their biological function and biotechnological applications.
Enzymatic and structural properties of fucosidases
GH29 fucosidases
Based on sequence analysis, GH29 fucosidases are predicted to be extracellular (secreted, membrane-attached or periplasm) or intracellular, depending on the metabolic pathways of microbes inhabiting various environments. However, this is rarely validated experimentally and the presence or absence of a signal peptide does not always accurately reflect their location [46]. Functionally characterised GH29 fucosidases from microbes are active within a broad pH range, from 3.3 to 9, with a majority of enzymes showing a preference for neutral conditions (Table 1). The optimum temperature for GH29 gut microbial fucosidases is around 37°C while marine-derived microbial fucosidases optimum temperatures are normally below 30°C (Table 1). The highest optimal temperature for microbial GH29 fucosidases reported so far is 95°C, which is for Ssα-fuc isolated from Sulfolobus solextreme P2 in hot springs (Table 1).
Table 1. Physiochemical and kinetic parameters of functionally characterised GH29 α-l-fucosidases.
| Taxonomy | Source | Organism | GenBank ID | SP | Subfamily | Opt pH | OptT/°C | Km (μM) | kcat (s−1) | kcat/ Km (s−1 μM−1) | Refs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Archaea | Hot springs | Sulfolobus solfataricus P2 | Ssα-fuc/AAK43160.1 AAK43159.1 | N | A | 3.3–6.3 | 95 | 28 ± 4 | 287 ± 11 | 10.25 | [111] |
| FCB Group | Gut | Bacteroides thetaiotaomicron VPI-5482 | BT1625/AAO76732.1 | Y | B | – | – | 3200 ± 700 | 0.37 ± 0.03 | (113 ± 3) × 10−6 | [53] |
| FCB Group | Gut | Bacteroides thetaiotaomicron VPI-5482 | BT2970/AAO78076.1 | Y | A | 6 | 37 | 2600 ± 500 | 0.53 ± 0.03 | (2 ± 0.5) × 10−4 | [47,53] |
| FCB Group | Gut | Bacteroides thetaiotaomicron VPI-5482 | BT4136/AAO79241.1 | Y | B | – | – | 4500 ± 400 | 0.45 ± 0.03 | (98 ± 12) × 10−6 | [53] |
| FCB Group | Warm spring | Emticicia oligotrophica | Eo0918/AFK04462.1 | N | B3 | 6–7 | 30–45 | 750 ± 110 | 3.73 ± 0.31 | 4.9 × 10−3 2 | [113] |
| FCB Group | Warm spring | Emticicia oligotrophica | Eo3066/AFK02389.1 | N | A3 | 6–7 | 30–45 | 8630 ± 1730 | 0.04 ± 0.01 | 4.6 × 10−6 2 | [113] |
| FCB Group | Warm spring | Emticicia oligotrophica | Eo3812/AFK05193.1 | N | A3 | 6–7 | 30–45 | 8410 ± 2060 | 3.61 ± 0.23 | 4.3 × 10−4 2 | [113] |
| FCB Group | Oral | Tannerella forsythia ATCC 43037 | TfFuc1/AEW21393.1 | N | A3 | 9 | – | 670 ± 200 | 17.27 ± 0.681 | 0.026 2 | [114] |
| FCB Group | Lymphoma patient | Elizabethkingia meningoseptica FMS-007 | cFase I/WP_047034007.1 | Y | A3 | 4.5 | 55 | 600 ± 50 | 0.14 ± 0.003 | (232 ± 6.7) × 10−6 | [55] |
| FCB Group | Marine | Wenyingzhuangia fucanilytica CZ1127T | Alf1_Wf/ANW96380.1 | Y | A | 7 | 30 | 3300 ± 420 | 5.44 | 1.65 × 10−3 | [32] |
| FCB Group | Marine | Wenyingzhuangia fucanilytica CZ1127T | FucWf1/ANW96121.1 | Y | A | 6.3 | 25 | 500 | 19.94 ± 4.131 | 0.040 2 | [46] |
| FCB Group | Marine | Wenyingzhuangia fucanilytica CZ1127T | FucWf2/ANW96113.1 | Y | A | 6.3 | 25 | 670 | 5.88 ± 0.821 | 8.8 × 10−3 2 | [46] |
| FCB Group | Marine | Wenyingzhuangia fucanilytica CZ1127T | FucWf3/ANW96108.1 | Y | A | 6.3 | 30 | 2210 | 0.20 ± 0.341 | 9.0 × 10−5 2 | [46] |
| FCB group | Marine | Flavobacterium algicola 12076 | OUC-Jdch16/MW767957.1 | Y | A3 | 6 | 25 | 1043 | 16.251 | 0.016 2 | [122] |
| FCB group | Plant | Spirosoma linguale DSM74 | SlFuc29/ADB37178.1 | Y | A3 | 5 | 50 | 180 ± 42 | 154.4 | 0.88 | [123] |
| Proteobacteria | Plant | Xanthomonas campestris pv.campestris str. ATCC 33913 | NixE/AAM42160.1 | Y | A | 5 | 37 | 700 ± 100 | 6.1 ± 2.0 | 8.7 × 10−3 2 | [117] |
| Proteobacteria | Marine | Paraglaciecola sp. | Fp231/MW623630.1 | Y | A | 5.6–6.0 | 25 | 140 ± 10 | 31 ± 0.5 | 0.221 | [57] |
| Proteobacteria | Marine | Vibrio sp. strain EJY3 | VejFCD/AEX22740.1 | N | A3 | – | – | 6700 ± 500 | 4.6 ± 1.4 | 6.9 × 10−4 2 | [37] |
| PVC group | Gut | Akkermansia muciniphila MucT (ATCC BAA-835) | Amuc_0010/ACD03857.1 | Y | A3 | 5.6 | – | 841.23 ± 46.72 | 378.33 | 0.45 | [75] |
| Terrabacteria group | Gut | Bifidobacterium longum subsp. infantis ATCC 15697 | Blon_2336/ACJ53394.1 | N | B | 6–7.5 | 37 | 709 ± 149 | 0.285 ± 0.024 | (407.73 ± 51.34) × 10−6 | [63] |
| Terrabacteria group | Gut | Bifidobacterium longum subsp. infantis ATCC 15697 | Blon_0248/ACJ51376.1 | N | A | 6–7.5 | 37 | 131 ± 10 | 0.110 ± 0.026 | (833.31 ± 134.64) × 10−6 | [63] |
| Terrabacteria group | Gut | Bifidobacterium longum subsp. infantis ATCC 15697 | Blon_0426/ACJ51546.1 | N | A | 6–7.5 | 37 | 180 ± 30 | 4.481 ± 0.329 | (24.95 ± 1.69) × 10−3 | [63] |
| Terrabacteria group | Streptosporangium roseum | SrFucNaFLD/ACZ87343.1 | Y | A | 5.6–7.5 | 37 | 10.59 ± 2.64 | 0.104 ± 0.0261 | 9.8 × 10−3 2 | [118] | |
| Terrabacteria group | Gut | Lactobacillus casei BL23 | AlfA/CAQ67115.1 | N | A | 7.5 | 39 | 270 | 0.8551 | 3.2 × 10−3 2 | [119] |
| Terrabacteria group | Gut | Lactobacillus casei BL23 | AlfB/CAQ67877.1 | N | A | 7 | 41 | 2900 | 4.71 | 1.6 × 10−3 2 | [119] |
| Terrabacteria group | Gut | Lactobacillus casei BL23 | AlfC/CAQ67984.1 | N | A | 7 | 41 | 5200 | 16.281 | 3.1 × 10−3 2 | [119] |
| Terrabacteria group | Hot spring | Paenibacillus sp.3179 | PsFuc/QEX52072.1 | N | A3 | 7.4 | 50 | 1110 ± 750 | (3 ± 1) × 10−3 1 | 2.7 × 10−3 2 | [124] |
| Terrabacteria group | Gut | Ruminococcus gnavus E1 | E1_10125/- | Y | B | 6 | – | 237.9 ± 39.69 | (18 ± 0.88)× 10−4 | 7.61 × 10−3 | [33] |
| Terrabacteria group | Gut | Ruminococcus gnavus ATCC 29149 | ATCC_03833/ WP_004844769.1 | N | A | 6 | – | 179.1 ± 28.77 | 83.6 ± 2.97 | 467 | [33] |
| Thermotogae | Thermotoga maritima | Thma/AAD35394.1 | N | A | 7 | – | 550 ± 30 | 12.6 ± 0.47 | 0.023 ± 0.007 | [74] | |
| Unclassified | Soil | Soil metagenome | Mfuc1/AIC77298.1 | N | A | 7 | – | 110 ± 10 | 1.33 ± 01 | 0.012 2 | [74] |
| Soil | Soil metagenome | Mfuc2/AIC77299.1 | N | A | 7 | – | 140 ± 10 | 1.92 ± 0.081 | 0.014 2 | [74] | |
| Soil | Soil metagenome | Mfuc4/AIC77301.1 | N | A | 7 | – | 71 ± 10 | 0.64 ± 0.021 | 9.0 × 10−3 2 | [74] | |
| Soil | Soil metagenome | Mfuc5/AIC77302.1 | N | A | 7 | – | 1900 ± 40 | 1.58 ± 0.031 | 8.3 × 10−4 2 | [74] | |
| Soil | Soil metagenome | Mfuc6/AIC77303.1 | N | A | 9 | – | 400 ± 50 | 0.47 ± 0.041 | 1.2 × 10−3 2 | [74] | |
| Soil | Soil metagenome | Mfuc7/AIC77304.1 | N | A | 6 | – | 280 ± 50 | 1.83 ± 0.131 | 6.5 × 10−3 2 | [74] |
Note: kinetic parameters were obtained using aryl-Fuc substrates.
Estimated from reported Vmax (μmol/L/min/mg) and molecular weight (g/mol, MW) using kcat (s−1) = Vmax × MW/1000/60.
Based on kcat /Km.
–, Data unavailable.
Predicted based on sequence analysis.
FCB, Fibrobacteres-Chlorobi-Bacteroidetes super phylum; Opt, optimal; PVC, Planctomycetes-Verrucomicrobia-Chlamydiae bacterial superphylum; SP, signal peptide.
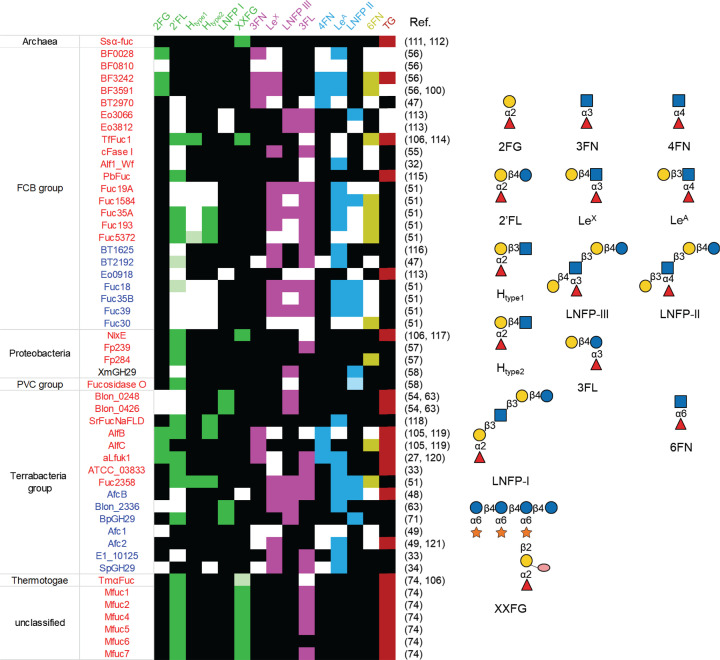
GH29 enzymes display broad substrate specificities covering α-1,2, α-1,3, α-1,4 and α-1,6 fucosylated linkages. Based on sequence homology and substrate specificity, GH29 enzymes are divided into two subfamilies, GH29A and GH29B [47]. In general, GH29A enzymes show higher activity towards synthetic aryl substrates such as 4-nitrophenyl α-l-fucopyranoside (pNP-Fuc) or 2-chloro-4-nitrophenyl-α-l-fucopyranoside (CNP-Fuc) compared with GH29B enzymes, while it is common for GH29B not to be active on these chromogenic substrates [46,48–51]. The Km values against aryl-Fuc for functionally characterised GH29 enzymes are in the μM to mM range, and the kcat values vary from 10−3 to 102 s−1. Their catalytic efficiency as estimated from kcat/Km varies from 10−6 to 102 s−1 μM−1 (Table 1). In addition, GH29B enzymes usually act on α-1,3/4 fucosylated linkages rather than α-1,2, whereas members of the GH29A subfamily show a more relaxed linkage specificity (Figure 3). To date, crystal structures are available from 16 microbial GH29 enzymes originating from 12 different microorganisms. Among them, BT2192 from B. thetaiomicron VPI-5482 [29] and BpGH29 from Bacteroides plebeius DSM 17135 [38] have α-galactosidase activities while ClAgl29A and ClAgl29B from Cecembia lonarensis LW9 were shown to be α-glucosidases [52]. GH29 enzymes are characterised by the lack of α-helix (α5) between β5 and β6 of TIM barrels [30,36]. The catalytic nucleophile and acid/base residues are located at the end of β4 and β6 strands, respectively. While the catalytic nucleophile in GH29 is a conserved Asp, the general acid/base residue is subfamily-dependent. In GH29B enzymes, the acid/base residue based is generally conserved based on sequence alignment with experimentally validated E249 of BT4136 and BT1625 from B. thetaiomicron VPI-5482. In SpGH29 from Streptococcus pneumoniae TIGR4, the assignment of E215 as acid/base was also confirmed by X-ray crystallography [34] (Figure 2A). Here, the D171 (nucleophile) and E215 (acid/base) of SpGH29 are located between the Fuc and GlcNAc residues, corresponding to the -1 and pseudo +1 subsite, respectively. The Gal within +2′ subsite makes hydrophobic interactions with W211 and hydrogen bonds to the nucleophile and D257, which, together with the -1 subsite, contributes to the α-1,3/4 fucosidase activity [34]. In contrast to GH29B fucosidases, the acid/base residues of GH29A enzymes show poor alignment across primary sequences, although they can be spatially overlapped with the acid/base residues from GH29B enzymes in their substrate-bound states but not free states [53]. However, the GH29A/B classification does not always accurately predicts linkage preferences [46,54,55] as enzymes from the same subfamily can show various substrate specificities (Table 1 and Figure 3).
Figure 3. Substrate specificity of microbial GH29 α-l-fucosidases.
The α1,2 substrates are colored in green, α1,3 in pink, α1,4 in sky blue, and α1,6 in olive. Light versions of the above colors indicate trace activity. Black boxes correspond to no enzymatic activity and empty boxes indicate lack of data. GH29A and GH29B α-l-fucosidases are coloured in red and blue, respectively; FCB, Fibrobacteres-Chlorobi-Bacteroidetes super phylum; PVC, Planctomycetes-Verrucomicrobia-Chlamydiae bacterial superphylum; TG, transglycosylation capability. Glycan structures presentation according to Symbol Nomenclature for Glycans (SNFG) [109,110].
Some functionally characterised bacterial GH29 fucosidases have only been reported to be active against artificial substrates, such as BF0810 from Bateroides fragilis NCTC 9343 [56], Fp240 and Fp251 from Paraglaciecola sp. [57]. Further investigation is required to determine their specificity towards natural substrates. GH29 fucosidases often present limited activity towards Lewis antigen glycan epitopes decorated with a sialic acid [48,58,59], which is ubiquitously found in antennary human N- and O-glycans. In contrast, the GH29 fucosidase E1_10125 from the gut symbiont Ruminococcus gnavus E1, was found to be active towards Lewis antigen glycan epitopes irrespective of the presence of terminal sialic acid [33]. Interestingly, E1_10125 showed stronger binding affinity and catalytic efficiency towards sialyl-Lewis X (sLeX) than Lewis X (LeX), as shown by isothermal titration calorimetry, saturation transfer difference NMR and kinetic assays [33]. X-ray crystallography, molecular dynamics simulation and docking showed that sLeX could be accommodated within the binding site of E1_10125 fucosidase. It is likely that other microbial fucosidases may also be able to accommodate a terminal sialic acid in their binding pocket although this remain to be demonstrated experimentally [50]. In addition, microbial GH29 fucosidases have been reported to carry out transglycosylation reactions due to their retaining mechanism of action, as recently reviewed elsewhere [60,61].
GH95 fucosidases
Compared to the GH29 family, GH95 fucosidases have been far less characterised. According to the CAZy database, currently the crystal structures of four GH95 fucosidases have been solved (PDB code: 4UFC, 2EAB, 2RDY, 7KMQ) [39–41]. The catalytic domain of GH95 adopts an (α/α)6-barrel fold, as illustrated with AfcA from Bifidobacterium bifidum JCM 1254 (Figure 2B). Here, the general acid residue E566 and base residues N421/N423 were experimentally validated by site-directed mutagenesis and structurally analysis [39]. These catalytic residues, together with other conserved residues such as E485 and D766, are part of a deep negatively-charged substrate-binding pocket (Figure 2B). The crystal structure of the complex between E566A inactive mutant and 2′-fucosyllactose (2′FL) revealed tighter interactions with Fuc and Gal moiety than with Glc, and site-directed mutagenesis further supported the importance of the hydrogen bond between Gal and E485 for catalysis [39] (Figure 2B).
The optimal pH for most characterised GH95 fucosidases has been shown to be between pH 6 to 7 [33,41,46,62–64] with some enzymes showing an optimum pH 5 [65–67] whereas BcFucA from Bacillus cereus 2-8 [68] and Afc3 from Clostridium perfringens ATCC 13124 [49] showed optimal pH of 4 and 8, respectively (Table 2). The optimal temperature of GH95 fucosidases varies from 25°C for FucWf5 from Wenyingzhuangia fucanilytica CZ1127T [46] to 60°C for both AfcA from B. bifidum JCM 1254 [65] and Afc3 [49] (Table 2).
Table 2. Physicochemical parameters and substrate specificity of functionally characterised GH95 α-l-fucosidases.
| Microbes | Protein name | GenBank ID | SP | Opt pH | Opt. T/°C | Fucosidase activity reported on | No reported activity on | Refs |
|---|---|---|---|---|---|---|---|---|
| Bacteroides fluxus YIT 12057 | BfGH95 | EGF57198.1 | Y | 6.0 | – | CNP-Fuc, 2'FL, 3FL, XXFG, XLFG | – | [62] |
| Bacteroides ovatus ATCC 8483 | BACOVA_ 03438 | ALJ48339.1 | Y | – | – | corn glucuronoarabinoxylan# | 2′FL and CNP-Fuc* | [40] |
| Bacteroides thetaiotaomicron VPI-5482 | BT1010 | AAO76117.1 | Y | – | – | chain A of RGII# | pNP linked β-d-Glc/ α-l- Rha/ α-l-Arap/β-d-Xyl/ α-d-Gal | [42] |
| Bacteroides uniformis ATCC 8492 | BuGH95 | EDO56039.1 | Y | 6.0 | – | CNP-Fuc, 2'FL, 3'FL, XXFG, XLFG | – | [62] |
| Bifidobacterium bifidum JCM 1254 | AfcA | AAQ72464.1 | Y | 5.0 | 60 | 2'FL, LNFP-I, Htype2 | 3FL, LNFP-II, LNFP-V, Atype 2 tri, Btype 2 tri, 6-fucosyl-N, N'-diacetylchitobiose | [65] |
| Bifidobacterium longum subsp. infantis ATCC 15697 | Blon_2335 | ACJ53393.1 | N | 6–7.5 | 37 | CNP-Fuc, 2′FL, 3′FL, 2FG | – | [63] |
| Cellvibrio japonicus Ueda107 | CjAfc95A | ACE83895.1 | Y | 6.5 | – | CNP-Fuc, XLFG, XXFG, lettuce xyloglucan | pNP linked β-d-Glc/β-d-Gal/β-d-Xyl/ α-l-Ara | [64] |
| Clostridium perfringens ATCC 13124 | Afc3 | ABG82552.1 | Y | 8 | 60 | 2FG, PGM | pNP-Fuc, LeA, LeX, 3FN, 4FN, 6FN | [49] |
| Dysgonomonas gadei ATCC BAA-286 | DgGH95 | EGJ99268.1 | Y | 6.0 | – | CNP-Fuc, 2′FL, 3′FL, 6FN, XXFG, XLFG | – | [62] |
| Ruminococcus gnavus ATCC 29149 | RUMGNA_00842 | QHB24557.1 | Y | 6 | – | pNP-Fuc, 2′FL, 3FL | LeA, LeX | [33] |
| Streptococcus pneumoniae TIGR4 | SpGH95 | AAK75733.1 | N | – | – | 2FL, 2FG, Htype1, Htype2, Htype3, LeB, LeY | 3FN, 4FN, 6FN, 3FL, Atype 2 tetra, Btype 2 tetra, LeA, LeX | [34] |
| Xanthomonas citri pv. citri str. 306 | XAC1774 (XacAfc95) | AAM36638.1 | Y | 6.0 | 55 | pNP-Fuc | pNP linked α-d-Gal/α-d-Glc/α-d-Man/ α-d-Xyl/α-l-Araf/α-l-Arap/α-l -Rha/β-d-Cellobioside/β -d-Fuc/β-d-Gal/β-d-Glc/β -d-Man/β-d-Xyl, arabinan, arabinogalactan, arabinoxylan, Avicel PH-101, β-1,4-glucobiose, CM-cellulose, CM-curdlan, curdlan, galactan, galactomannan, polygalacturonic acid, glucomannan, galactan, laminarin, lichenan, mannan, pachyman, CM-pachyman, pectin, pululan, reduced pululan, RG-I, RG, xanthan gum, xylan, amyloid xyloglucan, xyloglucan, β-glucan | [41] |
| Arabidopsis thaliana | AtFuc95A | CAB36703.1 | Y | 5 | – | 2′FL, XXFG, xyloglucan | pNP-Fuc, 3′FL, LNFP-II, LNFP-III, α-1,6 fucosylated chitopentaose | [66] |
| Aspergillus nidulans FGSC A4 | AN8149.2 | EAA59171.1 | Y | – | – | Cotton xyloglucan oligomers | pNP-Fuc | [69] |
| Wenyingzhuangia fucanilytica CZ1127 | FucWf5 | ANW96103.1 | N | 6.3 | 25 | pNP-Fuc, terminal α-1,3/4 fucose in fucoidan fragments | – | [46] |
–, data unavailable.
FCB, Fibrobacteres-Chlorobi-Bacteroidetes super phylum; Opt, optimal; PVC, Planctomycetes-Verrucomicrobia-Chlamydiae bacterial superphylum; SP, signal peptide.
l-Gal release.
*trace activity.
Individually, GH95 enzymes have been shown to have strict substrate specificities, acting preferentially on α-1,2 fucose linkages found in HMOs, mammalian O-glycans and fucosylated xyloglucan in dicots [34,49,64–67,69]. Some GH95 enzymes revealed a more relaxed activity on α-1,3/4/6 fucose linkages [62,63,70,71]. In addition, two GH95 enzymes were shown to have β-l-galactosidase activity [40,42] (Table 2).
GH139 and GH141 fucosidases
Currently, there are two functionally characterised GH141 enzymes in the CAZy database. BT1002 from B. thetaiotaomicron VPI-5482, the founding member of the GH141 family, is an endo-acting enzyme releasing 2-O-methyl-d-xylose-α-1,3-l-fucose disaccharide from the chain A of the complex pectin rhamnogalacturonan-II (RG-II) [42]. The catalytic domain of BT1002 folds into a right-handed parallel β helix (Figure 2C). The solvent-exposed surface representation of the catalytic centre of BT1002 reveals an extended catalytic pocket that may assist the accommodation of the disaccharide containing xylose and Fuc. Site directed mutagenesis revealed that putative nucleophile D523 and general acid/base D564 located in the binding pocket were critical for l-Rhap-α−1,3-d-Apif-α−1,4-d-MeXylp-l-Fucp hydrolysis [42]. The second member of the GH141 family is in fact a xylanase, Cthe_2195 from Acetivibrio thermocellus ATCC 27405 (previously known as Clostridium thermocellum) [72], which showed no activity on aryl-Fuc substrate.
The only characterised member of the GH139 family, BT0984 from B. thetaiotaomicron VPI-5482 is a α-2-O-methyl-l-fucosidase targeting 2-O-methyl-l-Fuc-α-1,2-d-Galp linkage from chain B of RG-II glycan [42]. The catalytic mechanism and crystal structure of GH139 enzymes remain to be determined.
GH151 fucosidases
Some initially classified GH29 enzymes including Blon_0346 from Bifidobacterium longum subsp. infantis ATCC 15697 [63], α-l-fucosidase isoenzyme iso2 from Paenibacillus thiaminolyticus [73], and Mfuc3 isolated from soil bacteria [74] were recently reclassified into the new GH151 family due to low sequence identity with all other known GH families. GH151 fucosidases have been shown to be active on aryl-Fuc and disaccharides where Fuc is linked to Gal via α-1,2 linkage or to GlcNAc via α-1,2/3/4/6 linkages, but no activity was detected on fucosyl trisaccharides or hexasaccharide Globo H with l-Fuc-α-1,2- d-Galp epitope [43]. Recently the first crystal structure of a GH151 fucosidase, ALfuk2, has been reported from Paenibacillus thiaminolyticus [43] (Figure 2D). The catalytic domain of Alfuk2 formed the (β/α)8 barrel with the nucleophile D154 and general acid/base E235, assigned based on site-directed mutagenesis, apo structural analysis, protein-ligand docking and a mixed quantum mechanical/molecular mechanical (QM/MM) calculation, located in terminal position of β4 and β6 strands, respectively [43]. Interestingly, GH151 revealed a unique oligomeric assembly across α-l-fucosidases families and the involvement of active site complementation from adjacent monomers with catalytic residues forming the active site cavity together with His503 from an adjacent monomer (Figure 1D). Mutation of His503 to Ala affected the substrate binding, enzymatic activity and optimal pH of 6.5, suggesting new catalytic features requiring further investigation [43].
Insights into the biological role of microbial fucosidases
Gut microbes such as Bifidobacteria species [63], B. thetaiotaomicron [47], R. gnavus [33] or Akkermansia muciniphila [75] have been shown to produce multiple fucosidases that cleave Fuc from host glycans, underscoring their importance for the fitness and adaptation of these bacteria to the gut environment (Supplementary Table S1). The capability of removing α-l-fucosyl residues from free oligosaccharides and glycoconjugates conferred fucosidase-possessing microbes a competitive advantage in mucin glycan foraging [14], and in turn help maintain intestinal homeostasis [76,77]. Fucosidases from commensal bacteria also play a role in cross-feeding with other members of the gut microbiota [78,79] or enteric pathogens such as Salmonella enterica serovar Typhimurium, Clostridium difficile, [80], Campylobacter jejuni [81,82] and other pathogens [83] facilitating their infection. Recently, α-l-fucosidases from the GH29 family were identified and characterised from the metagenome of faecal samples of breastfed infants. This analysis revealed a remarkably high number of GH29 α-l-fucosidases present in the infant intestinal environment with high sequences identity (above 98% identity) with α-l-fucosidases from B. thetaiotaomicron, Bacteroides caccae, Phocaeicola vulgatus, Phocaeicola dorei, R. gnavus, and Streptococcus parasanguinis (Supplementary Table S1). These enzymes showed different substrate specificities toward HMOs, blood group antigens, and glycoproteins [51]. GH95 fucosidases were also identified in the infant faecal microbiome from B. longum subsp. infantis, B. thetaiotaomicron, B. caccae, R. gnavus, P. vulgatus, and P. dorei (Supplementary Table S1). The variety of α-l-fucosidases may provide these species with an advantage in colonising the gut of infants and adults.
Novel tools have been developed to further investigate the biological roles of microbial fucosidases. For example, activity-based probes (ABP) have been used to identify their functional state, spatial and temporal distribution [84]. Cyclophellitol epoxides/aziridine, 2-deoxy-2-fluoro glycosides and quinone methide have been employed to design covalent inhibitors of glycosidases [85]. Fucopyranose-configured cyclophellitol aziridines have been applied for in vitro and in vivo labelling of bacterial and mammal GH29 fucosidases [86]. More recently, a 2-deoxyl-fluoro fucosyl fluoride derivative named YL209 has been developed to match the versatile linkage specificity of GH29 enzymes, potentially extending its application to the identification of gut microbial fucosidases [87]. Lately, an ortho-quinone methide based probe with an azide mini-tag has been developed to label both retaining and inverting bacterial fucosidases [88].
Biotechnological applications of microbial fucosidases
With the development of glycan analytical tools, glycan profiling has gained momentum in the last decade as a potential strategy to monitor the state of diseases [89]. Some of the main glycan biomarker targets are human serum N-glycans containing two types of fucosylation, antennary LeX or sLeX epitopes and Fuc-α-1,6-GlcNAc (6FN). The fucosylation pattern of human serum N-glycans are indicators of immunological responses to diseases including cancer [90], diabetes [91], and Helicobacter pylori infection [92]. Fucosidases with distinct substrate specificities have been employed as one of the exoglycosidases used to validate and monitor these glycan biomarkers in a number of human studies [72,93–98].
Another application of fucosidases is modulation of core fucosylation status in glycoproteins, such as antibodies, which is crucial for their functions such as antigen recognition [99]. So far, only human fucosidase FucA1 has been shown to release core fucose from intact glycoproteins albeit with low enzymatic activity [100]. No bacterial α-l-fucosidase has been described with the capability to remove the core Fuc from intact glycosylated IgG. However, recent work characterised four fucosidases showing high capacity to hydrolyse α-1,6-linked Fuc from the disaccharide 6FN [51]. These α-l-fucosidases might have applications in the development of therapeutic proteins with modified core fucosylation, although their capacity to act on core fucosylation in glycosylated antibodies needs further analysis. Recent glycosidase and glycoligase tools based on the site-specific GH29 core α-1,6-l-fucosidase AlfC from L. casei, have been developed to aid glycoengineering of antibodies for core fucosylation of the Fab and Fc fragments [23,101,102].
GH29 fucosidases also show potential for the enzymatic synthesis of valuable oligosaccharides (Figure 3) through transfucosylation including fucosylated HMOs [103] and antibody glycans [101], as recently reviewed [60,61]. For example, α-l-fucosidases AlfB and AlfC from L. casei were used to synthesise fucosyl-α-1,3-N-GlcNAc, 6FN, the glycoamino acid fucosyl-α-1,6-N-GlcNAc-Asn, and several 6′-fucosyl-glycans [104,105]. Fucosyl-N-GlcNAc disaccharides have also been recently produced using the tranglycosylation activity of α-l-fucosidases isolated from B. fragilis [56]. The HMOs, 2′FL, 3-fucosyllactose (3FL), and lacto-N-fucopentaose II (LNFP-II) have been synthesised in low amounts using the transfucosylation activity of α-l-fucosidases isolated from Thermotoga maritima, Clostridium perfringens, and a soil-derived metagenome library [74,106]. A GH95 fucosidase AfcA from B. bifidum JCM 1254 has also been engineered to perform the reverse reaction by site-directed mutagenesis with the N423H mutant acting as a fucosynthase [107,108], although this approach so far is limited to α-1,2-oligosaccharide synthesis.
Conclusions and perspectives
Fucosylated glycans influence a wide range of biological processes in health and diseases. Despite recent advances in the structure and function relationships of GH29 enzymes, our biochemical and structural understanding of the range of microbial α-l-fucosidases and of their natural substrates remains limited compared to the wealth of sequencing data available in metagenomic databases. Further enzymatic investigations of bacterial fucosidases should shed light on the type of fucosylated structures accessible to microbes and the specificity of α-l-fucosidases towards substrates with different modifications and linkages. A combination of metagenomics and glycomics approaches is warranted to advance our knowledge into the biological roles of microbial α-l-fucosidases. Harnessing the diversity of microbial α-l-fucosidases will provide powerful tools that can be exploited for glycan analysis, biomarker detection or new glycan-targeted therapies.
Summary
Microbial α-l-fucosidases from soil, marine or gut origin are of great biological and biotechnological importance.
Enzymatic investigations of GH29 α-l-fucosidases advanced our knowledge of the range of substrates and glycan utilisation strategies used by microbes to adapt to their environment while α- l-fucosidases from other GH families have been under-studied.
α-l-Fucosidases have been developed as glycoenzyme tools for glycan analysis, biomarkers for diagnosis or glycan-targeted therapies as well as oligosaccharide synthesis and glycoengineering on glycoproteins.
Further biochemical and structural characterisation of the variety of α-l-fucosidases produced by microbes is required to enhance our understanding of the mechanisms underpinning host–microbe interactions and harness the potential of these enzymes for biotechnological and biomedical applications.
Supplementary Material
Abbreviations
- 2FG
Fucα1-2Gal
- 2FL
2-Fucosyllactose (Galβ1-4(Fucα1-2)Glc)
- 2′FL
2′-Fucosyllactose ((Fucα1-2)Galβ1-4Glc)
- 3FL
3-Fucosyllactose (Galβ1-4(Fucα1-3)Glc)
- 3FN
Fucα1,3GlcNAc
- 3′FL
3′-Fucosyllactose ((Fucα1-3)Galβ1-4Glc))
- 4FN
Fucα1,4GlcNAc
- 6FN
Fucα1,6GlcNAc
- ABP
activity-based probes
- Araf
arabinofuranoside
- Arap
arabinopyranoside
- Atype2 tetra
blood group antigen A tetraose type 2 (GalNAcα1-3[Fucα1-2]Galβ1-4GlcNAc)
- Atype2 tri
blood group antigen A triose type 2 (GalNAcα1-3[Fucα1-2]Gal)
- Btype2 tetra
blood group antigen B tetraose type 2 (Galα1-3[Fucα1-2]Galβ1-4GlcNAc)
- Btype2 tri
blood group antigen B triose type 2 (Gal1-3[Fucα1-2]Gal)
- CAZy
carbohydrate active enzymes
- CNP-Fuc
2-chloro-4-nitrophenyl α-l-fucose
- Fuc
α-l-fucose (6-deoxyl-l-galactose)
- Gal
β-Galactose
- GH
glycoside hydrolase
- Glc
β-Glucose
- GlcNAc
β-N-acetylglucosamine
- HMO
human milk oligosaccharide
- Htype1
blood group antigen H triose type 1 (Fucα1-2)Galβ1-3GlcNAc
- Htype2
blood group antigen H triose type 2 (Fucα1-2)Galβ1-4GlcNAc
- Htype4
blood group antigen H tetraose type 4 (Fucα1-2)Galβ1-3GalNAcβ1-3Gal
- Lac
lactose
- LacNAc
N-acetyllactosamine
- LeA
lewis A antigen triose
- LeB
lewis B antigen tetraose (Fucα1-2Galβ1-3[Fucα1-4]GlcNAc)
- LeX
lewis X antigen triose
- LeY
lewis Y antigen tetraose (Fucα1-2Galβ1-4[Fucα1-3]GlcNAc)
- LNFP-I
lacto-N-fucopentaose I
- LNFP-II
lacto-N-fucopentaose II
- LNFP-III
lacto-N-fucopentaose III
- Man
mannopyranoside
- PGM
porcine gastric mucin
- pNP-Fuc
p-nitrophennol-fucosylpyranose
- RG-I
rhamnogalacturonan I
- RG-II
rhamnogalacturonan II
- Rha
rhamopyranose
- sLeX
sialyl lewis X antigen tetraose
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
N.J. gratefully acknowledges the support of the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Grant Gut Microbes and Health [grant number BB/R012490/1]. H.W. was supported by fund from Guangdong Basic and Applied Basic Research Foundation [grant number 2022A1515110917].
Open Access
Open access for this article was enabled by the participation of John Innes Centre in an all-inclusive Read & Publish agreement with Portland Press and the Biochemical Society under a transformative agreement with JISC.
References
- 1.Flowers H.M. (1981) Chemistry and biochemistry of D- and L-fucose. Adv. Carbohydr. Chem. Biochem. 39, 279–345 10.1016/S0065-2318(08)60208-5 [DOI] [PubMed] [Google Scholar]
- 2.Sutherland I.W. (1971) Enzymic hydrolysis of colanic acid. Eur. J. Biochem. 23, 582–587 10.1111/j.1432-1033.1971.tb01657.x [DOI] [PubMed] [Google Scholar]
- 3.Maverakis E., Kim K., Shimoda M., Gershwin M.E., Patel F., Wilken R.et al. (2015) Glycans in the immune system and the altered glycan theory of autoimmunity: a critical review. J. Autoimmun. 57, 1–13 10.1016/j.jaut.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker D.J. and Lowe J.B. (2003) Fucose: Biosynthesis and biological function in mammals. Glycobiology 13, 41R–53R 10.1093/glycob/cwg054 [DOI] [PubMed] [Google Scholar]
- 5.Schneider M., Al-Shareffi E. and Haltiwanger R.S. (2017) Biological functions of fucose in mammals. Glycobiology 27, 601–618 10.1093/glycob/cwx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strasser R. (2022) Recent developments in deciphering the biological role of plant complex N-glycans. Front. Plant Sci. 13, 1–8 10.3389/fpls.2022.897549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Paasch B.C., Chen J., Day B. and He S.Y. (2019) An important role of l-fucose biosynthesis and protein fucosylation genes in Arabidopsis immunity. New Phytol. 222, 981–994 10.1111/nph.15639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garber J.M., Hennet T. and Szymanski C.M. (2021) Significance of fucose in intestinal health and disease. Mol. Microbiol. 115, 1086–1093 10.1111/mmi.14681 [DOI] [PubMed] [Google Scholar]
- 9.Dwivedi R., Nothaft H., Garber J., Xin Kin L., Stahl M., Flint A.et al. (2016) L-fucose influences chemotaxis and biofilm formation in Campylobacter jejuni. Mol. Microbiol. 101, 575–589 10.1111/mmi.13409 [DOI] [PubMed] [Google Scholar]
- 10.Li J., Chen J., Wang L., Lin Y., Zhang X., Liu J.et al. (2022) Characterization of the response of Escherichia coli to l-fucose in bacterial swimming motility. J. Basic Microbiol. 62, 584–592 10.1002/jobm.202200054 [DOI] [PubMed] [Google Scholar]
- 11.Pacheco A.R., Munera D., Waldor M.K., Sperandio V. and Ritchie J.M. (2012) Fucose sensing regulates bacterial intestinal colonization. Nature 492, 113–117 10.1038/nature11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garber J.M., Nothaft H., Pluvinage B., Stahl M., Bian X., Porfirio S.et al. (2020) The gastrointestinal pathogen Campylobacter jejuni metabolizes sugars with potential help from commensal Bacteroides vulgatus. Commun. Biol. 3, 1–11 10.1038/s42003-019-0727-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crost E.H., Tailford L.E., Le Gall G., Fons M., Henrissat B. and Juge N. (2013) Utilisation of mucin glycans by the human gut symbiont Ruminococcus gnavus is strain-dependent. PloS ONE 8, e76341 10.1371/journal.pone.0076341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tailford L.E., Crost E.H., Kavanaugh D. and Juge N. (2015) Mucin glycan foraging in the human gut microbiome. Front. Genet 6, 1–18 10.3389/fgene.2015.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staudacher E., Altmann F., Wilson I.B.H. and März L. (1999) Fucose in N-glycans: From plant to man. Biochim. Biophys. Acta - Gen Subj. 1473, 216–236 10.1016/S0304-4165(99)00181-6 [DOI] [PubMed] [Google Scholar]
- 16.Ma B., Simala-Grant J.L. and Taylor D.E. (2006) Fucosylation in prokaryotes and eukaryotes. Glycobiology 16, 158R–184R 10.1093/glycob/cwl040 [DOI] [PubMed] [Google Scholar]
- 17.Kizuka Y. (2022) Metabolic utilization and remodeling of glycan biosynthesis using fucose analogs. Biochim. Biophys. Acta - Gen. Subj. 1866, 130243 10.1016/j.bbagen.2022.130243 [DOI] [PubMed] [Google Scholar]
- 18.Orczyk-Pawiłowicz M. and Lis-Kuberka J. (2020) The impact of dietary fucosylated oligosaccharides and glycoproteins of human milk on infant well-being. Nutrients 12, 1105, 10.3390/nu12041105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita K., Tachibana Y. and Kobata A. (1976) Oligosaccharides of human milk. Isolation and characterization of three new disialylfucosyl hexasaccharides. Arch. Biochem. Biophys. 174, 582–591 10.1016/0003-9861(76)90387-8 [DOI] [PubMed] [Google Scholar]
- 20.Nakano M., Kakehi K., Tsai M.H. and Lee Y.C. (2004) Detailed structural features of glycan chains derived from α1-acid glycoproteins of several different animals: The presence of hypersialylated, O-acetylated sialic acids but not disialy1 residues. Glycobiology 14, 431–441 10.1093/glycob/cwh034 [DOI] [PubMed] [Google Scholar]
- 21.Wuhrer M., Balog C.I.A., Koeleman C.A.M., Deelder A.M. and Hokke C.H. (2005) New features of site-specific horseradish peroxidase (HRP) glycosylation uncovered by nano-LC-MS with repeated ion-isolation/fragmentation cycles. Biochim. Biophys. Acta - Gen. Subj. 1723, 229–239 10.1016/j.bbagen.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 22.Seismann H., Blank S., Braren I., Greunke K., Cifuentes L., Grunwald T.et al. (2010) Dissecting cross-reactivity in hymenoptera venom allergy by circumvention of α-1,3-core fucosylation. Mol. Immunol. 47, 799–808 10.1016/j.molimm.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Klontz E.H., Li C., Kihn K., Fields J.K., Beckett D., Snyder G.A.et al. (2020) Structure and dynamics of an α-fucosidase reveal a mechanism for highly efficient IgG transfucosylation. Nat. Commun. 11, 1–14 10.1038/s41467-020-20044-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Höti N., Yang S., Hu Y., Shah P., Haffner M.C. and Zhang H. (2018) Overexpression of α [1,6] fucosyltransferase in the development of castration-resistant prostate cancer cells. Prostate Cancer Prostatic Dis. 21, 137–146 10.1038/s41391-017-0016-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dion M., Fourage L., Hallet J.N. and Colas B. (1999) Cloning and expression of a β-glycosidase gene from Thermus thermophilus. Sequence and biochemical characterization of the encoded enzyme. Glycoconj. J. 16, 27–37 10.1023/A:1006997602727 [DOI] [PubMed] [Google Scholar]
- 26.Yoshida S., Park D.S., Bae B., MacKie R., Cann I.K.O. and Nair S.K. (2011) Structural and functional analyses of a glycoside hydrolase family 5 enzyme with an unexpected β-fucosidase activity. Biochemistry 50, 3369–3375 10.1021/bi200222u [DOI] [PubMed] [Google Scholar]
- 27.Kovaľová T., Bene E., Lipovová P. and Dohnálek J. (2019) Active site complementation and hexameric arrangement in the GH family 29 ; a structure - function study of α-L-fucosidase isoenzyme 1 from Paenibacillus thiaminolyticus. Glycobiology 29, 59–73 [DOI] [PubMed] [Google Scholar]
- 28.van Bueren A.L., Arde A., Fayers-Kerr J., Luo B., Zhang Y., Sollogoub M.et al. (2010) Analysis of the reaction coordinate of alpha-L-fucosidases: a combined structural and quantum mechanical approach. J. Am. Chem. Soc. 132, 1804–1806 10.1021/ja908908q [DOI] [PubMed] [Google Scholar]
- 29.Guillotin L., Lafite P. and Daniellou R. (2014) Unraveling the substrate recognition mechanism and specificity of the unusual glycosyl hydrolase family 29 BT2192 from Bacteroides thetaiotaomicron. Biochemistry 53, 1447–1455 10.1021/bi400951q [DOI] [PubMed] [Google Scholar]
- 30.Sulzenbacher G., Bignon C., Nishimura T., Tarling C.A., Withers S.G., Henrissat B.et al. (2004) Crystal structure of Thermotoga maritima α-L-fucosidase: Insights into the catalytic mechanism and the molecular basis for fucosidosis. J. Biol. Chem. 279, 13119–13128 10.1074/jbc.M313783200 [DOI] [PubMed] [Google Scholar]
- 31.Sakurama H., Fushinobu S., Hidaka M., Yoshida E., Honda Y., Ashida H.et al. (2012) 1,3-1,4-α-L-Fucosynthase that specifically introduces Lewis a/x antigens into type-1/2 chains. J. Biol. Chem. 287, 16709–16719 10.1074/jbc.M111.333781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong S., Chang Y., Shen J., Xue C. and Chen F. (2017) Purification, expression and characterization of a novel α-L-fucosidase from a marine bacteria Wenyingzhuangia fucanilytica. Protein Expr. Purif. 129, 9–17 10.1016/j.pep.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 33.Wu H., Rebello O., Crost E.H., Owen C.D., Walpole S., Bennati-Granier C.et al. (2021) Fucosidases from the human gut symbiont Ruminococcus gnavus. Cell. Mol. Life Sci. 78, 675–693 10.1007/s00018-020-03514-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hobbs J.K., Pluvinage B., Robb M., Smith S.P. and Boraston A.B. (2019) Two complementary α-fucosidases from Streptococcus pneumoniae promote complete degradation of host-derived carbohydrate antigens. J. Biol. Chem. 294, 12670–12682 10.1074/jbc.RA119.009368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao H., Walton J.D., Brumm P. and Phillips G.N. (2014) Structure and substrate specificity of a eukaryotic fucosidase from fusarium graminearum. J. Biol. Chem. 289, 25624–25638 10.1074/jbc.M114.583286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Summers E.L., Moon C.D., Atua R. and Arcus V.L. (2016) The structure of a glycoside hydrolase 29 family member from a rumen bacterium reveals unique, dual carbohydrate-binding domains. Acta Crystallogr. Sect. Struct. Biol. Commun. 72, 750–761 10.1107/S2053230X16014072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong H., Kim D.H., Seo H., Kim K.J.K.H.K.J. and Kim K.J.K.H.K.J. (2021) Dual α-1,4- and β-1,4-Glycosidase Activities by the Novel Carbohydrate-Binding Module in α- l -Fucosidase from Vibrio sp. Strain EJY3. J. Agric. Food Chem. 69, 3380–3389 10.1021/acs.jafc.0c08199 [DOI] [PubMed] [Google Scholar]
- 38.Robb C.S., Hobbs J.K., Pluvinage B., Reintjes G., Klassen L., Monteith S.et al. (2022) Metabolism of a hybrid algal galactan by members of the human gut microbiome. Nat. Chem. Biol. 18, 501–510 10.1038/s41589-022-00983-y [DOI] [PubMed] [Google Scholar]
- 39.Nagae M., Tsuchiya A., Katayama T., Yamamoto K., Wakatsuki S. and Kato R. (2007) Structural basis of the catalytic reaction mechanism of novel 1,2-α-L-fucosidase from Bifidobacterium bifidum. J. Biol. Chem. 282, 18497–18509 10.1074/jbc.M702246200 [DOI] [PubMed] [Google Scholar]
- 40.Rogowski A., Briggs J.A., Mortimer J.C., Tryfona T., Terrapon N., Lowe E.C.et al. (2015) Glycan complexity dictates microbial resource allocation in the large intestine. Nat. Commun. 6, 7481 10.1038/ncomms8481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vieira P.S., Bonfim I.M., Araujo E.A., Melo R.R., Lima A.R., Fessel M.R.et al. (2021) Xyloglucan processing machinery in Xanthomonas pathogens and its role in the transcriptional activation of virulence factors. Nat. Commun. 12, 1–15 10.1038/s41467-021-24277-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ndeh D., Rogowski A., Cartmell A., Luis A.S., Baslé A., Gray J.et al. (2017) Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 544, 65–70 10.1038/nature21725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovaľová T., Kovaľ T., Stránský J., Kolenko P., Dušková J., Švecová L.et al. (2022) The first structure-function study of GH151 α-l-fucosidase uncovers new oligomerization pattern, active site complementation, and selective substrate specificity. FEBS J. 289, 4998–5020 10.1111/febs.16387 [DOI] [PubMed] [Google Scholar]
- 44.You J., Lin S. and Jiang T. (2019) Origins and evolution of the α-L-fucosidases: from bacteria to metazoans. Front. Microbiol. 10, 1–9 10.3389/fmicb.2019.01756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drula E., Garron M.L., Dogan S., Lombard V., Henrissat B. and Terrapon N. (2022) The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. 50, D571–D577 10.1093/nar/gkab1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silchenko A.S., Rubtsov N.K., Zueva A.O., Kusaykin M.I., Rasin A.B. and Ermakova S.P. (2022) Fucoidan-active α-L-fucosidases of the GH29 and GH95 families from a fucoidan degrading cluster of the marine bacterium Wenyingzhuangia fucanilytica. Arch. Biochem. Biophys. 728, 109373 10.1016/j.abb.2022.109373 [DOI] [PubMed] [Google Scholar]
- 47.Sakurama H., Tsutsumi E., Ashida H., Katayama T., Yamamoto K. and Kumagai H. (2012) Differences in the Substrate Specificities and Active-Site Structures of Two α- L -Fucosidases (Glycoside Hydrolase Family 29) from Bacteroides thetaiotaomicron. Biosci. Biotechnol. Biochem. 76, 1022–1024 10.1271/bbb.111004 [DOI] [PubMed] [Google Scholar]
- 48.Ashida H., Miyake A., Kiyohara M., Wada J., Yoshida E., Kumagai H.et al. (2009) Two distinct α-L-fucosidases from Bifidobacterium bifidum are essential for the utilization of fucosylated milk oligosaccharides and glycoconjugates. Glycobiology 19, 1010–1017 10.1093/glycob/cwp082 [DOI] [PubMed] [Google Scholar]
- 49.Fan S., Zhang H., Chen X., Lu L., Xu L. and Xiao M. (2016) Cloning, characterization, and production of three α- l -fucosidases from Clostridium perfringens ATCC 13124. J. Basic Microbiol. 56, 347–357 10.1002/jobm.201500582 [DOI] [PubMed] [Google Scholar]
- 50.Grootaert H., van Landuyt L., Hulpiau P. and Callewaert N. (2020) Functional exploration of the GH29 fucosidase family. Glycobiology 00, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moya-Gonzálvez E.M., Peña-Gil N., Rubio-del-Campo A., Coll-Marqués J.M., Gozalbo-Rovira R., Monedero V.et al. (2022) Infant gut microbial metagenome mining of α- l -fucosidases with activity on fucosylated human milk oligosaccharides and glycoconjugates. Microbiol. Spectr. 10, e0177522 10.1128/spectrum.01775-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shishiuchi R., Kang H., Tagami T., Ueda Y., Lang W., Kimura A.et al. (2022) Discovery of α- L -glucosidase raises the possibility of α- L -glucosides in nature. ACS Omega 7, 47411–47423 10.1021/acsomega.2c06991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaikh F.A., Lammerts Van Bueren A., Davies G.J. and Withers S.G. (2013) Identifying the catalytic acid/base in GH29 α-l-fucosidase subfamilies. Biochemistry 52, 5857–5864 10.1021/bi400183q [DOI] [PubMed] [Google Scholar]
- 54.Ashida H., Fujimoto T., Kurihara S., Nakamura M., Komeno M., Huang Y.et al. (2020) 1,6-α-L-Fucosidases from Bifidobacterium longum subsp. infantis ATCC 15697 Involved in the Degradation of Core-fucosylated N-Glycan. J. Appl. Glycosci. 67, 23–29 10.5458/jag.jag.JAG-2019_0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li T., Li M., Hou L., Guo Y., Wang L., Sun G.et al. (2018) Identification and characterization of a core fucosidase from the bacterium Elizabethkingia meningoseptica. J. Biol. Chem. 293, 1243–1258 10.1074/jbc.M117.804252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu P., Zhang H., Wang Y., Chen X., Jin L., Xu L.et al. (2020) Screening and characterization of an α-L-fucosidase from Bacteroides fragilis NCTC9343 for synthesis of fucosyl-N-acetylglucosamine disaccharides. Appl. Microbiol. Biotechnol. 104, 7827–7840 10.1007/s00253-020-10759-w [DOI] [PubMed] [Google Scholar]
- 57.Schultz-Johansen M., Stougaard P., Svensson B. and Teze D. (2022) Characterization of five marine family 29 glycoside hydrolases reveals an α-L-fucosidase targeting specifically Fuc(α1,4)GlcNAc. Glycobiology 32, 529–539 10.1093/glycob/cwab132 [DOI] [PubMed] [Google Scholar]
- 58.Wong-madden S.T. and Landry D. (1995) Purification and characterization of novel glycosidases from the bacterial genus Xanthomonas. Glycobiology 5, 19–28 10.1093/glycob/5.1.19 [DOI] [PubMed] [Google Scholar]
- 59.Klamer Z., Staal B., Prudden A.R., Liu L., Smith D.F., Boons G.et al. (2017) Mining high-complexity motifs in glycans: a new language to uncover the fine specificities of lectins and glycosidases. Anal. Chem 89, 12342–12350 10.1021/acs.analchem.7b04293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wan L., Zhu Y., Zhang W. and Mu W. (2020) α-l-Fucosidases and their applications for the production of fucosylated human milk oligosaccharides. Appl. Microbiol. Biotechnol. 104, 5619–5631 10.1007/s00253-020-10635-7 [DOI] [PubMed] [Google Scholar]
- 61.Zeuner B. and Meyer A.S. (2020) Enzymatic transfucosylation for synthesis of human milk oligosaccharides. Carbohydr. Res. 493, 108029 10.1016/j.carres.2020.108029 [DOI] [PubMed] [Google Scholar]
- 62.Déjean G., Tauzin A.S., Bennett S.W., Creagh A.L. and Brumer H. (2019) Adaptation of syntenic xyloglucan utilization loci of human gut Bacteroidetes to polysaccharide side chain diversity. Appl. Environ. Microbiol. 85, 1–17 10.1128/AEM.01491-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sela D.A., Garrido D., Lerno L., Wu S., Tan K., Eom H.J.et al. (2012) Bifidobacterium longum subsp. infantis ATCC 15697 α-fucosidases are active on fucosylated human milk oligosaccharides. Appl. Environ. Microbiol. 78, 795–803 10.1128/AEM.06762-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larsbrink J., Thompson A.J., Lundqvist M., Gardner J.G., Davies G.J. and Brumer H. (2014) A complex gene locus enables xyloglucan utilization in the model saprophyte Cellvibrio japonicus. Mol. Microbiol. 94, 418–433 10.1111/mmi.12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katayama T., Fujita K. and Yamamoto K. (2005) Novel bifidobacterial glycosidases acting on sugar chains of mucin glycoproteins. J. Biosci. Bioeng. 99, 457–465 10.1263/jbb.99.457 [DOI] [PubMed] [Google Scholar]
- 66.Léonard R., Pabst M., Bondili J.S., Chambat G., Veit C., Strasser R.et al. (2008) Identification of an Arabidopsis gene encoding a GH95 alpha1,2-fucosidase active on xyloglucan oligo- and polysaccharides. Phytochemistry 69, 1983–1988 10.1016/j.phytochem.2008.03.024 [DOI] [PubMed] [Google Scholar]
- 67.Ishimizu T., Hashimoto C., Takeda R., Fujii K. and Hase S. (2007) A novel α1,2-L-fucosidase acting on xyloglucan oligosaccharides is associated with endo-β-mannosidase. J. Biochem. 142, 721–729 10.1093/jb/mvm186 [DOI] [PubMed] [Google Scholar]
- 68.Li Q., Jiang C., Tan H., Zhao X., Li K. and Yin H. (2021) Characterization of recombinant E. coli expressing a novel fucosidase from Bacillus cereus 2-8 belonging to GH95 family. Protein Expr. Purif. 186, 105897 10.1016/j.pep.2021.105897 [DOI] [PubMed] [Google Scholar]
- 69.Bauer S., Vasu P., Persson S., Mort A.J. and Somerville C.R. (2006) Development and application of a suite of polysaccharide-degrading enzymes for analyzing plant cell walls. Proc. Natl. Acad. Sci. U.S.A. 103, 11417–11422 10.1073/pnas.0604632103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garrido D., Ruiz-Moyano S., Kirmiz N., Davis J.C., Totten S.M., Lemay D.G.et al. (2016) A novel gene cluster allows preferential utilization of fucosylated milk oligosaccharides in Bifidobacterium longum subsp. longum SC596. Sci. Rep. 6, 35045 10.1038/srep35045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shani G., Hoeflinger J.L., Heiss B.E., Masarweh C.F., Larke J.A., Jensen N.M.et al. (2022) Fucosylated Human Milk Oligosaccharide Foraging within the Species Bifidobacterium pseudocatenulatum Is Driven by Glycosyl Hydrolase Content and Specificity. Appl. Environ. Microbiol. 88, 1–18 10.1128/AEM.01707-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heinze S., Mechelke M., Kornberger P., Liebl W., Schwarz W.H. and Zverlov V.V. (2017) Identification of endoxylanase XynE from Clostridium thermocellum as the first xylanase of glycoside hydrolase family GH141. Sci. Rep. 7, 1–10 10.1038/s41598-017-11598-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benešová E., Lipovová P., Krejzová J., Kovalová T., Buchtová P., Spiwok V.et al. (2015) Alpha-L-Fucosidase Isoenzyme iso2 from Paenibacillus thiaminolyticus. BMC Biotechnol. 15, 1–7 10.1186/s12896-015-0160-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lezyk M., Jers C., Kjaerulff L., Gotfredsen C.H., Mikkelsen M.D. and Mikkelsen J.D. (2016) Novel α-L-fucosidases from a soil metagenome for production of fucosylated human milk oligosaccharides. PloS ONE 11, e0147438 10.1371/journal.pone.0147438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kostopoulos I., Elzinga J., Ottman N., Klievink J.T., Blijenberg B., Aalvink S.et al. (2020) Akkermansia muciniphila uses human milk oligosaccharides to thrive in the early life conditions in vitro. Sci. Rep. 10, 1–17 10.1038/s41598-020-71113-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pickard J.M., Maurice C.F., Kinnebrew M.A., Abt M.C., Schenten D., Golovkina T.V.et al. (2014) Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature 514, 638–641 10.1038/nature13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lei C., Sun R., Xu G., Tan Y., Feng W., McClain C.J.et al. (2022) Enteric VIP-producing neurons maintain gut microbiota homeostasis through regulating epithelium fucosylation. Cell Host Microbe 30, 1417–1434.e8 10.1016/j.chom.2022.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horigome A., Hashikura N., Yoshida K., Xiao J. and Odamaki T. (2022) 2′-Fucosyllactose increases the abundance of blautia in the presence of extracellular fucosidase-possessing bacteria. Front. Microbiol. 13, 913624 10.3389/fmicb.2022.913624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shuoker B., Pichler M.J., Jin C., Hiroka S., Wu H., Gascueña A.M.et al. (2022) Sialidases and Fucosidases of Akkermansia muciniphila are crucial for growth on mucin and nutrient sharing with mucus-associated gut bacteria. bioRxiv 10.1101/2022.09.10.507281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ng K.M., Ferreyra J.A., Higginbottom S.K., Lynch J.B., Kashyap P.C., Gopinath S.et al. (2013) Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502, 96–99 10.1038/nature12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stahl M., Friis L.M., Nothaft H., Liu X., Li J., Szymanski C.M.et al. (2011) L-fucose utilization provides Campylobacter jejuni with a competitive advantage. Proc. Natl. Acad. Sci. U.S.A. 108, 7194–7199 10.1073/pnas.1014125108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luijkx Y.M.C.A., Bleumink N.M.C., Jiang J., Overkleeft H.S., Wösten M.M.S.M., Strijbis K.et al. (2020) Bacteroides fragilis fucosidases facilitate growth and invasion of Campylobacter jejuni in the presence of mucins. Cell. Microbiol. 22, 1–12, 10.1111/cmi.13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sorbara M.T. and Pamer E.G. (2019) Interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal. Immunol. 12, 1–9, 10.1038/s41385-018-0053-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nandakumar M., Hsu Y.L., Lin J.C.Y., Lo C., Lo L.C. and Lin C.H. (2015) Detection of human α-L-fucosidases by a quinone methide-generating probe: enhanced activities in response to Helicobacter pylori infection. Chem. Bio. Chem 16, 1555–1559 10.1002/cbic.201500178 [DOI] [PubMed] [Google Scholar]
- 85.Rempel B.P. and Withers S.G. (2008) Covalent inhibitors of glycosidases and their applications in biochemistry and biology. Glycobiology 18, 570–586 10.1093/glycob/cwn041 [DOI] [PubMed] [Google Scholar]
- 86.Jiang J., Kallemeijn W.W., Wright D.W., Van Den Nieuwendijk A.M.C.H., Rohde V.C., Folch E.C.et al. (2015) In Vitro and in vivo comparative and competitive activity-based protein profiling of GH29 α-L-fucosidases. Chem. Sci. 6, 2782–2789 10.1039/C4SC03739A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luijkx Y.M.C.A., Jongkees S., Strijbis K. and Wennekes T. (2021) Development of a 1,2-difluorofucoside activity-based probe for profiling GH29 fucosidases. Org. Biomol. Chem. 19, 2968–2977 10.1039/D1OB00054C [DOI] [PubMed] [Google Scholar]
- 88.Luijkx Y.M.C.A., Henselijn A.J., Bosman G.P., Cramer D.A.T., Giesbers K.C.A.P., van Veld E.M.et al. (2022) Detection of bacterial α-l-fucosidases with an ortho-quinone methide-based probe and mapping of the probe-protein adducts. Molecules 27, 1615, 10.3390/molecules27051615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mitra I., Snyder C.M., Zhou X., Campos M.I., Alley W.R., Novotny M.V.et al. (2016) Structural characterization of serum N-glycans by methylamidation, fluorescent labeling, and analysis by microchip electrophoresis. Anal. Chem. 88, 8965–8971 10.1021/acs.analchem.6b00882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pinho S.S. and Reis C.A. (2015) Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 10.1038/nrc3982 [DOI] [PubMed] [Google Scholar]
- 91.Thanabalasingham G., Huffman J.E., Kattla J.J., Novokmet M., Rudan I., Gloyn A.L.et al. (2013) Mutations in HNF1A result in marked alterations of plasma glycan profile. Diabetes 62, 1329–1337 10.2337/db12-0880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu T.W., Ho C.W., Huang H.H., Chang S.M., Popat S.D., Wang Y.T.et al. (2009) Role for α-L-fucosidase in the control of Helicobacter pylori-infected gastric cancer cells. Proc. Natl. Acad. Sci. U.S.A. 106, 14581–14586 10.1073/pnas.0903286106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liquori G.E., Mastrodonato M., Mentino D., Scillitani G., Desantis S., Portincasa P.et al. (2012) In situ characterization of O-linked glycans of Muc2 in mouse colon. Acta Histochem. 114, 723–732 10.1016/j.acthis.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 94.Nwosu C., Yau H.K. and Becht S. (2015) Assignment of core versus antenna fucosylation types in protein N-glycosylation via procainamide labeling and tandem mass spectrometry. Anal. Chem. 87, 5905–5913 10.1021/ac5040743 [DOI] [PubMed] [Google Scholar]
- 95.Demus D., Jansen B.C., Gardner R.A., Urbanowicz P.A., Wu H., Štambuk T.et al. (2021) Interlaboratory evaluation of plasma N-glycan antennary fucosylation as a clinical biomarker for HNF1A-MODY using liquid chromatography methods. Glycoconj. J. 38, 375–386 10.1007/s10719-021-09992-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Demus D., Urbanowicz P.A., Gardner R.A., Wu H., Juszczak A., Štambuk T.et al. (2022) Development of an exoglycosidase plate-based assay for detecting α1-3,4 fucosylation biomarker in individuals with HNF1A-MODY. Glycobiology 32, 230–238 10.1093/glycob/cwab107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rebello O.D., Nicolardi S., Lageveen-Kammeijer G.S.M., Nouta J., Gardner R.A., Mesker W.E.et al. (2020) A matrix-assisted laser desorption/ionization—mass spectrometry assay for the relative quantitation of antennary fucosylated N-glycans in human plasma. Front. Chem. 8, 1–15 10.3389/fchem.2020.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rohrer J.S., Basumallick L. and Hurum D.C. (2016) Profiling N-linked oligosaccharides from IgG by high-performance anion-exchange chromatography with pulsed amperometric detection. Glycobiology 26, 582–591 10.1093/glycob/cww006 [DOI] [PubMed] [Google Scholar]
- 99.Li W., Yu R., Ma B., Yang Y., Jiao X., Liu Y.et al. (2015) Core fucosylation of IgG B cell receptor is required for antigen recognition and antibody production. J. Immunol. 194, 2596–2606 10.4049/jimmunol.1402678 [DOI] [PubMed] [Google Scholar]
- 100.Prabhu S.K., Li C., Zong G., Zhang R. and Wang L.X. (2021) Comparative studies on the substrate specificity and defucosylation activity of three α-L-fucosidases using synthetic fucosylated glycopeptides and glycoproteins as substrates. Bioorganic Med. Chem. 42, 116243 10.1016/j.bmc.2021.116243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li C., Zhu S., Ma C. and Wang L.X. (2017) Designer α1,6-fucosidase mutants enable direct core fucosylation of intact N-glycopeptides and N-glycoproteins. J. Am. Chem. Soc. 139, 15074–15087 10.1021/jacs.7b07906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giddens J.P., Lomino J.V., DiLillo D.J., Ravetch J.V. and Wang L.X. (2018) Site-selective chemoenzymatic glycoengineering of Fab and Fc glycans of a therapeutic antibody. Proc. Natl. Acad. Sci. U.S.A. 115, 12023–12027 10.1073/pnas.1812833115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saumonneau A., Champion E., Peltier-Pain P., Molnar-Gabor D., Hendrickx J., Tran V.et al. (2015) Design of an α-l-transfucosidase for the synthesis of fucosylated HMOs. Glycobiology 26, 261–269 10.1093/glycob/cwv099 [DOI] [PubMed] [Google Scholar]
- 104.Becerra J.E., Rodríguez-Díaz J., Gozalbo-Rovira R., Palomino-Schätzlein M., Zúñiga M., Monedero V.et al. (2020) Unique microbial catabolic pathway for the human core N-glycan constituent fucosyl-α-1,6-N-acetylglucosamine-asparagine. MBio 11, 1–18 10.1128/mBio.02804-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodríguez-Díaz J., Carbajo R.J., Pineda-Lucena A., Monedero V. and Yebra M.J. (2013) Synthesis of fucosyl-N-acetylglucosamine disaccharides by transfucosylation using α-L-Fucosidases from Lactobacillus casei. Appl. Environ. Microbiol. 79, 3847–3850 10.1128/AEM.00229-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zeuner B., Muschiol J., Holck J., Lezyk M., Gedde M.R., Jers C.et al. (2018) Substrate specificity and transfucosylation activity of GH29 α-L-fucosidases for enzymatic production of human milk oligosaccharides. N. Biotechnol. 41, 34–45 10.1016/j.nbt.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 107.Sugiyama Y., Gotoh A., Katoh T., Honda Y., Yoshida E., Kurihara S.et al. (2016) Introduction of H-antigens into oligosaccharides and sugar chains of glycoproteins using highly efficient 1,2-α-L-fucosynthase. Glycobiology 26, 1235–1247 10.1093/glycob/cww085 [DOI] [PubMed] [Google Scholar]
- 108.Sugiyama Y., Katoh T., Honda Y., Gotoh A., Ashida H., Kurihara S.et al. (2017) Application study of 1,2-α-l-fucosynthase: introduction of Fucα1-2Gal disaccharide structures on N-glycan, ganglioside, and xyloglucan oligosaccharide. Biosci. Biotechnol. Biochem. 81, 283–291 10.1080/09168451.2016.1254532 [DOI] [PubMed] [Google Scholar]
- 109.Varki A., Cummings R.D., Aebi M., Packer N.H., Seeberger P.H., Esko J.D.et al. (2015) Symbol nomenclature for graphical representations of glycans. Glycobiology 25, 1323–1324 10.1093/glycob/cwv091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neelamegham S., Aoki-Kinoshita K., Bolton E., Frank M., Lisacek F., Lütteke T.et al. (2019) Updates to the symbol nomenclature for glycans guidelines. Glycobiology 29, 620–624 10.1093/glycob/cwz045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cobucci-Ponzano B., Trincone A., Giordano A., Rossi M. and Moracci M. (2003) Identification of an archaeal α-L-fucosidase encoded by an interrupted gene: Production of a functional enzyme by mutations mimicking programmed -1 frameshifting. J. Biol. Chem. 278, 14622–14631 10.1074/jbc.M211834200 [DOI] [PubMed] [Google Scholar]
- 112.Curci N., Strazzulli A., Iacono R., De Lise F., Maurelli L., Di Fenza M.et al. (2021) Xyloglucan oligosaccharides hydrolysis by exo‐acting glycoside hydrolases from hyperthermophilic microorganism saccharolobus solfataricus. Int. J. Mol. Sci. 22, 3325 10.3390/ijms22073325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu S., Kulinich A., Cai Z.P., Ma H.Y., Du Y.M., Lv Y.M.et al. (2016) The fucosidase-pool of Emticicia oligotrophica: Biochemical characterization and transfucosylation potential. Glycobiology 26, 871–879 10.1093/glycob/cww030 [DOI] [PubMed] [Google Scholar]
- 114.Megson Z.A., Koerdt A., Schuster H., Ludwig R., Janesch B., Frey A.et al. (2015) Characterization of an α-L-fucosidase from the periodontal pathogen Tannerella forsythia. Virulence 6, 282–292 10.1080/21505594.2015.1010982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shi R., Ma J., Yan Q., Yang S., Fan Z. and Jiang Z. (2020) Biochemical characterization of a novel α-L-fucosidase from Pedobacter sp. and its application in synthesis of 3′-fucosyllactose and 2′-fucosyllactose. Appl. Microbiol. Biotechnol. 104, 5813–5826 10.1007/s00253-020-10630-y [DOI] [PubMed] [Google Scholar]
- 116.Briliūtė J., Urbanowicz P.A., Luis A.S., Baslé A., Paterson N., Rebello O.et al. (2019) Complex N-glycan breakdown by gut Bacteroides involves an extensive enzymatic apparatus encoded by multiple co-regulated genetic loci. Nat. Microbiol. 4, 1571–1581 10.1038/s41564-019-0466-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dupoiron S., Zischek C., Ligat L., Carbonne J., Boulanger A., Dugé De Bernonville T.et al. (2015) The N-Glycan cluster from Xanthomonas campestris pv. campestris: A toolbox for sequential plant N-Glycan processing. J. Biol. Chem. 290, 6022–6036 10.1074/jbc.M114.624593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bishnoi R., Mahajan S. and Ramya T.N.C. (2018) An F-type lectin domain directs the activity of Streptosporangium roseum alpha-L-fucosidase. Glycobiology 28, 860–875 10.1093/glycob/cwy079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rodríguez-Díaz J., Monedero V. and Yebra M.J. (2011) Utilization of natural fucosylated oligosaccharides by three novel α-L-fucosidases from a probiotic lactobacillus casei strain. Appl. Environ. Microbiol. 77, 703–705 10.1128/AEM.01906-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Benešová E., Lipovová P., Dvořáková H. and Králová B. (2013) α-L-fucosidase from paenibacillus thiaminolyticus: Its hydrolytic and transglycosylation abilities. Glycobiology 23, 1052–1065 10.1093/glycob/cwt041 [DOI] [PubMed] [Google Scholar]
- 121.Zeuner B., Vuillemin M., Holck J., Muschiol J. and Meyer A.S. (2018) Loop engineering of an α-1,3/4-L-fucosidase for improved synthesis of human milk oligosaccharides. Enzyme Microb. Technol. 115, 37–44 10.1016/j.enzmictec.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 122.Zhou W., Jiang H., Liang X., Qiu Y., Wang L. and Mao X. (2021) Discovery and characterization of a novel α-L-fucosidase from the marine-derived Flavobacterium algicola and its application in 2′-fucosyllactose production. Food Chem. 369, 130942 10.1016/j.foodchem.2021.130942 [DOI] [PubMed] [Google Scholar]
- 123.Pozzo T., Higdon S.M., Pattathil S., Hahn M.G. and Bennett A.B. (2018) Characterization of novel glycosyl hydrolases discovered by cell wall glycan directed monoclonal antibody screening and metagenome analysis of maize aerial root mucilage. PLoS ONE 13, 1–19 10.1371/journal.pone.0204525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thøgersen M.S., Christensen S.J., Jepsen M., Pedersen L.H. and Stougaard P. (2020) Transglycosylating β-d-galactosidase and α-l-fucosidase from Paenibacillus sp. 3179 from a hot spring in East Greenland. Microbiologyopen 9, 1–15 10.1002/mbo3.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.