Abstract
Few prospective studies have documented the seropositivity among those children infected with severe acute respiratory syndrome coronavirus 2. From 2 April 2021 to 24 June 2021, we prospectively enrolled children between the ages of 2 and 17 years at three North Carolina healthcare systems. Participants received at least four at-home serological tests detecting the presence of antibodies against, but not differentiating between, the nucleocapsid or spike antigen. A total of 1,058 participants were enrolled in the study, completing 2,709 tests between 1 May 2021 and 31 October 2021. Using multilevel regression with poststratification techniques and considering our assay sensitivity and sensitivity, we estimated that the seroprevalence of infection-induced antibodies among unvaccinated children and adolescents aged 2–17 years in North Carolina increased from 15.2% (95% credible interval, CrI 9.0–22.0) in May 2021 to 54.1% (95% CrI 46.7–61.1) by October 2021, indicating an average infection-to-reported-case ratio of 5. A rapid rise in seropositivity was most pronounced in those unvaccinated children aged 12–17 years, based on our estimates. This study underlines the utility of serial, serological testing to inform a broader understanding of the regional immune landscape and spread of infection.
Keywords: Adolescent, child, COVID-19, paediatrics, SARS-CoV-2, seroepidemiologic studies, serology
Introduction
The mortality rate of acute coronavirus disease 2019 (COVID-19) has typically followed a strong age gradient with children being at lower risk of severe clinical outcomes [1–5]. However, a growing body of evidence supports the finding that the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among children and adolescents is much higher than previously thought [6, 7]. While infections have been shown to be milder in children, children infected with SARS-CoV-2 are still susceptible to severe outcomes such as those associated with multisystem inflammatory syndrome (MIS-C) [8]; some portion of those infected go on to experience post-acute COVID-19 sequala with studies indicating 4% of seropositive children reporting at least one symptom for longer than 12 weeks [9]. Children also play a major role in the transmission of the virus [10, 11], and thus understanding the true burden of disease in this section of the population is crucial.
The United States National Commercial Laboratory Seroprevalence Survey has monitored infection-induced seroprevalence in all age groups since August 2020, with a recent focus on children aged ≤17 years [7, 12, 13]. The median estimated seroprevalence of infection among children from eight states increased from 8% in August 2020 to 37% in May 2021 [6]. Based on these data, Couture and colleagues, in a cross-sectional analysis of residual serum samples, estimated 5–9 times more SARS-CoV-2 infections in children than reported by case-based surveillance [6]. Although these surveys highlight the prevalence of SARS-CoV-2 infections among children, they are limited by their reliance on residual clinical specimens that may not be representative of the general population [6, 7, 13].
We describe trends in prevalence of infection-induced antibodies using serial at-home serological testing in a cohort of 2- to 17-year-old children enrolled in a prospective, syndromic surveillance study in North Carolina. We used seroprevalence estimates to calculate infection-to-reported case ratios for a better understanding of the local burden of SARS-CoV-2 infections.
Materials and methods
Study design and participants
The COVID-19 Community Research Partnership (CCRP) is a multi-site, prospective cohort study combining electronic symptom surveillance with serological surveillance [14, 15]. From 2 April 2021 to 24 June 2021, we enrolled children between the ages of 2 and 17 years at three North Carolina healthcare systems based in the cities of Charlotte, Winston-Salem, and Raleigh. Caregivers consented and participants aged ≥13 years assented electronically. Caregivers provided demographic information at enrolment. From enrolment through 31 December 2021, participants received daily electronic surveys soliciting symptoms of COVID-19-like illness and receipt of COVID-19 vaccines. Additionally, participants were mailed four or more serology tests to be completed once monthly using fingerprick blood. A smartphone application was used to upload results. Testing was completed by 31 October 2021.
Ethics approval
This study was approved by the institutional revenue board for Wake Forest University School of Medicine (IRB00064912).
Antibody detection
An Innovita Biological Technology SARS-CoV-2 lateral flow assay (Beijing, China) was used to detect SARS-CoV-2 immunoglobulin G (IgG) with a sensitivity of 84.5% and a specificity of 99%; the assay detected but did not differentiate between anti-spike and anti-nucleocapsid IgG. Infection-induced antibodies were defined as the presence of a positive IgG prior to any reported vaccine dose.
Statistical analysis
To estimate seroprevalence from infection over time, a Bayesian framework was employed accounting for assay sensitivity and specificity. Participants from North Carolina and South Carolina were pooled for the analysis. The proportion of respondents with infection-induced antibodies was estimated for each study month. To estimate the North Carolina seroprevalence from infection using the cohort data, multilevel regression with poststratification (MRP) was performed. A multilevel logistic regression was fitted with an intercept and random effects for age group in years [2–4, 5–11, 12–17], sex (male and female), and race (Black, White, and other), with adjustments for sensitivity and specificity of the assay [16]. Prevalence estimates were obtained by weighting the corresponding model estimates by the subgroup total using the National Center for Health Statistics Vintage 2020 Bridged-Race Postcensal Population Estimates [17]. Reported cases and vaccinations were retrieved from the North Carolina Health and Human Services dashboard [18]. Variant periods were defined as pre-Delta, Delta, and Omicron (pre-BA.4/BA.5), based on variant predominance in North Carolina following DeWitt et al. [19]. Infection-to-reported case ratios were calculated by dividing the estimated population seroprevalence for a 1-month timeframe by the cumulative reported cases for the prior month, in order to account for the delayed antibody response to a SARS-CoV-2 infection [20].
All analyses were conducted with R version 4.1.3 (2022-03-10) and Stan version 2.28.1 [21].
Results
A total of 1,058 participants from 35 and 6 counties in North Carolina and South Carolina, respectively, consented to both syndromic and serological surveillance. Participants were predominately White and non-Hispanic and returned a median of serology two tests (IQR 1–4) within a median of 32 days (IQR 27–38) (Table 1). During the study period, there was a substantial rise in community cases commensurate with the emergence of the Delta variant, with reported cases rising beginning in July 2021 and peaking in early September 2021 (Fig. 1). Seroconversion before vaccination occurred an average of 59.2 days after enrolment. Adjusting for sensitivity and specificity, the cohort infection-induced seroprevalence rose from 14.2% (95% credible interval, 9.2–19.8) in May 2021 to 38.7% (31.7–46.2) in October 2021 (Fig. 2a). MRP estimated infection-induced seroprevalence increased from 15.2% (9.0–22.0) in May to 54.1% (46.7–61.1) in October 2021 (Table 2), with the largest absolute increase occurring among the 12- to 17-year-olds. By October 2021, MRP estimated infection-induced antibody prevalence was 28.7% (15.8–43.7), 27.2% (19.5–36.1), and 92% (77.6–99.6) among unvaccinated children aged 2–4, 5–11, 12–17 years old, respectively (Fig. 2b). During this period, the estimated infection-to-case ratio increased from 3.1 (1.9–4.5) in May 2021 to a peak of 6.1 (5.1–6.9) in August 2021 before declining to 4.8 (4.1–5.4) in October 2021 (Fig. 2c and Table 2). In posterior estimates by unique cell, Black children less than 12 years of age tended to have point estimates of seroprevalence higher than any demographic in these age groups (Fig. 3a). Analysis of the regression coefficients suggests that those aged 12–17 years old were associated with a higher probability of infection-induced antibodies after June 2021 (Fig. 3b).
Table 1.
Overall characteristics and key demographics
| Characteristic | N = 1,058 (%) |
|---|---|
| Age (years), no. (%) | |
| 2–4 | 146 (13.8) |
| 5–11 | 495 (46.8) |
| 12–17 | 417 (39.4) |
| Sex, no. (%) | |
| Female | 537 (50.7) |
| Male | 516 (48.8) |
| Unknown | 5 (0.5) |
| Race, no. (%) | |
| Other | 114 (10.8) |
| Black or African American | 71 (6.7) |
| White | 873 (82.5) |
| Ethnicity, no. (%) | |
| Hispanic or Latino | 62 (5.9) |
| Not Hispanic/Latino | 956 (90.3) |
| Not specified or unknown | 40 (3.8) |
| Prior COVID-19 diagnosis (Y), no. (%) | 93 (8.9) |
| Seroconverted prior to vaccination (Y), no. (%) | 195 (18.4) |
Figure 1.
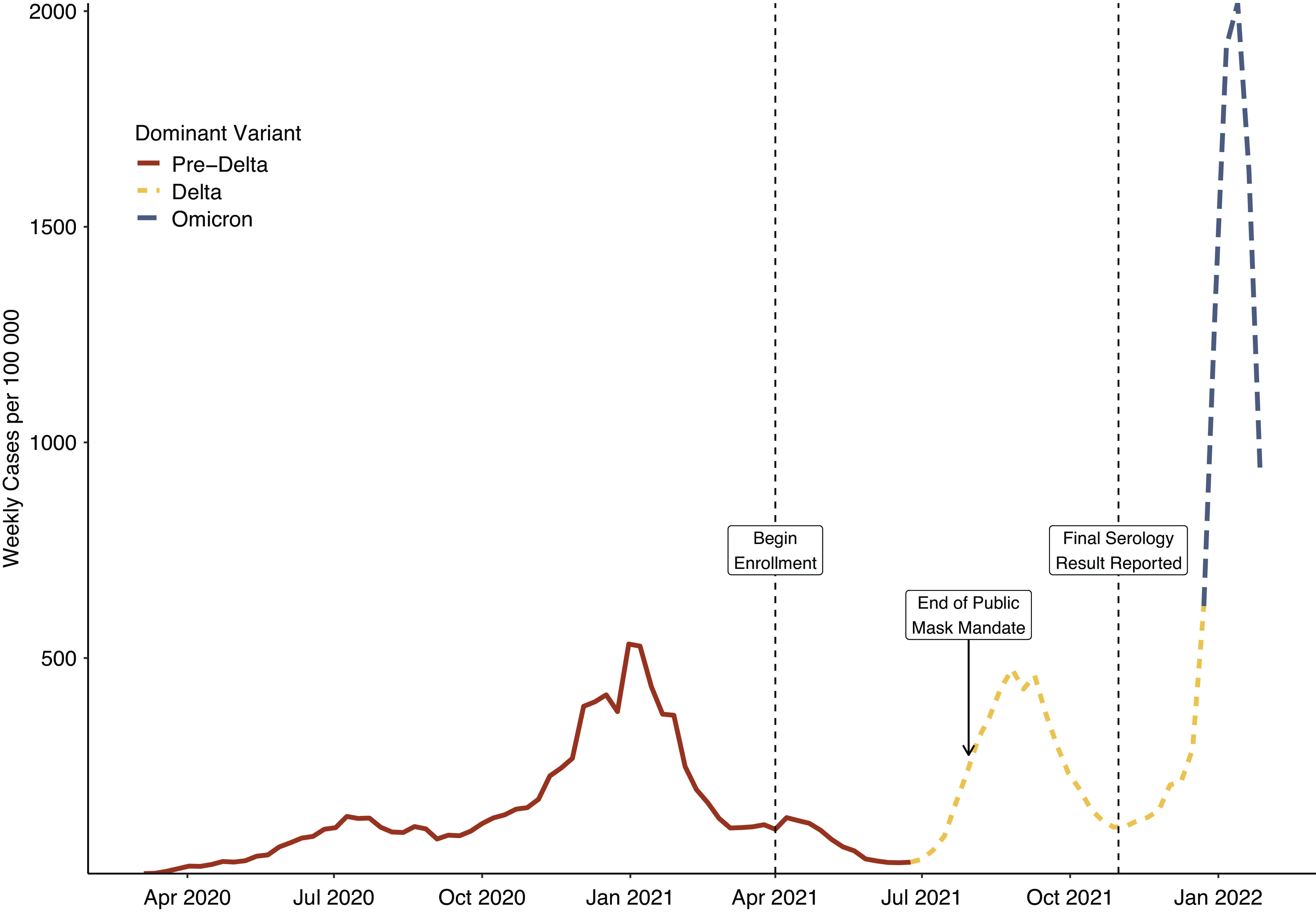
Reported COVID-19 weekly cases per 100,000 residents in the state of North Carolina from 1 March 2020 to 1 February 2022, with the dominant variant represented.
Figure 2.
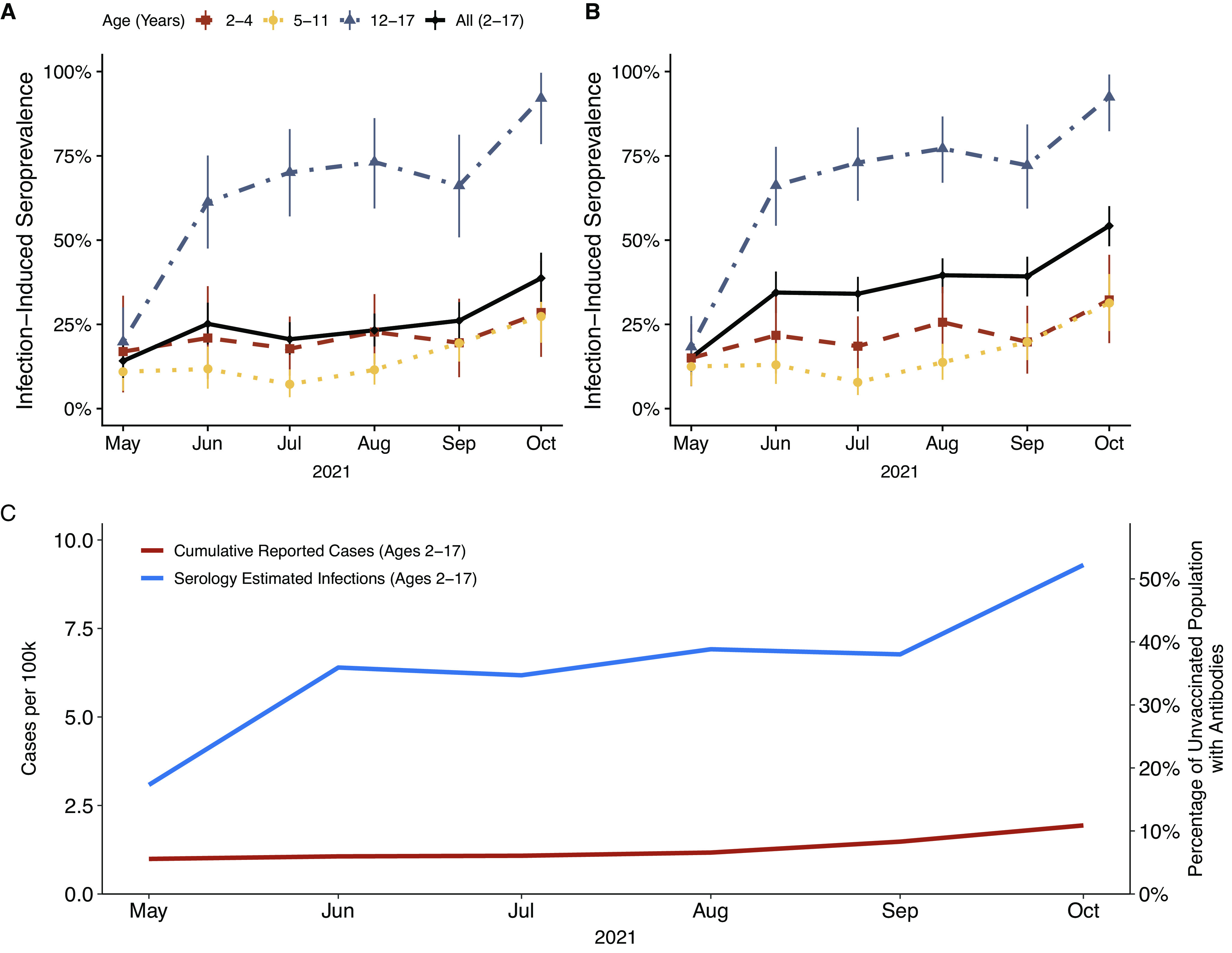
Estimated prevalence of infection-induced SARS-CoV-2 antibodies in study cohort overall and by age group (a). Estimated prevalence of infection-induced SARS-CoV-2 antibodies in North Carolina by age group estimated by multilevel regression with poststratification (b). All estimates are corrected for assay sensitivity and specificity and reflect 95% credible intervals. Comparison of reported SARS-CoV-2 cases with serology-estimated number of infections among North Carolina children aged 2–17 years who did not have any doses of vaccine (c).
Table 2.
Comparison of reported severe cute respiratory syndrome coronavirus 2 cases versus serology-based estimated infections and infection-to-case ratios among children in North Carolina
| Month (2021) | Estimated seroprevalence (95% CrI) | Cumulative reported cases prior montha | Average estimated infections | Infection/reported case ratio (95% CrI) |
|---|---|---|---|---|
| May | 15.2 (9.0–22.0) | 99,046 | 308,500 (183,400–446,600) | 3.1 (1.9–4.5) |
| June | 34.4 (27.2–41.9) | 106,251 | 639,700 (505,500–777,500) | 6.0 (4.8–7.3) |
| July | 34.2 (28.1–40.1) | 108,016 | 620,100 (509,100–727,600) | 5.7 (4.7–6.7) |
| August | 40.5 (34.5–46.4) | 116,979 | 708,300 (602,100–810,000) | 6.1 (5.1–6.9) |
| September | 39.2 (32.3–45.9) | 147,831 | 676,200 (556,400–791,800) | 4.6 (3.8–5.4) |
| October | 54.1 (46.7–61.1) | 193,676 | 926,600 (800,800–1,046,700) | 4.8 (4.1–5.4) |
Abbreviation: CrI, credible interval.
Cumulative coronavirus disease 2019 cases reported by the North Carolina Department of Health and Human Services based on the prior month (e.g., seroprevalence estimate for May are compared to cases in April to allow time for development of IgG antibodies).
Figure 3.
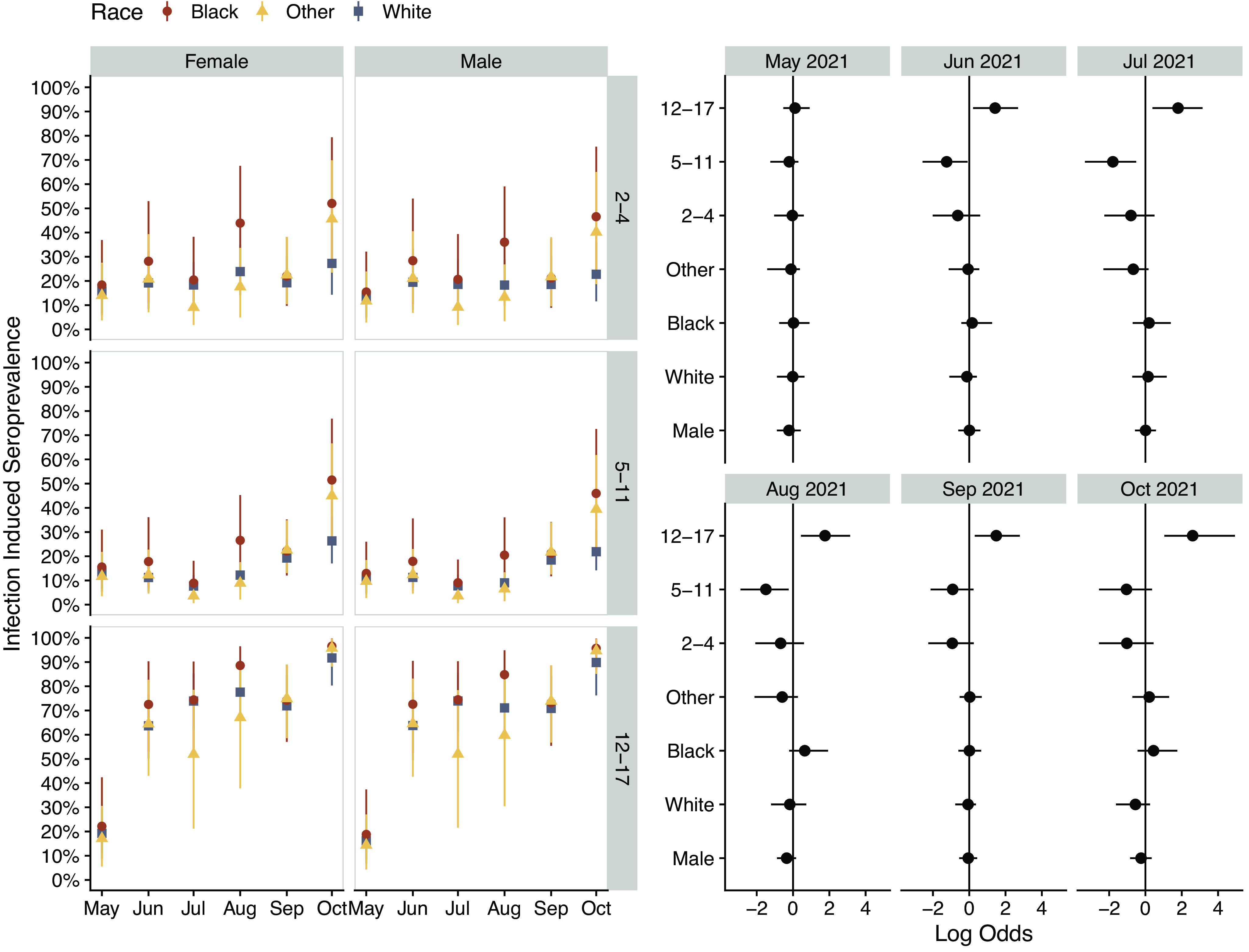
Modelled prevalence of infection-induced SARS-CoV-2 antibodies by age for North Carolina by demographic in 2021 (a). Simultaneous estimated model parameters (posterior log odds) for likelihood of seropositivity by month of data collection (b). Group level (random) effects are shown for race and age while the population (fixed) effect is shown for sex. All model estimates consider assay sensitivity and specificity and reflect 95% credible intervals.
Discussion
Our findings support the mounting evidence of a high seroprevalence of SARS-CoV-2 infection among children and adolescents [2, 3]. By October 2021, we estimate that more than 50% of children aged 2–17 years old in North Carolina had infection-induced antibodies. The greatest increase in seroprevalence occurred in the fall, coincident with the return to school and end of public masking mandates in North Carolina and South Carolina [22, 23]. Among adolescents aged 12–17 years who remained unvaccinated, the majority had infection-induced antibodies by June 2021, with an estimated 90% seropositive by October 2021. Despite increases in the availability of testing during 2021, the estimated infection-to-case ratios of 3.1–6.1 demonstrate that reported COVID-19 cases likely grossly underestimate the actual spread of infection.
The seroprevalence of infection-induced antibodies among children has exceeded that of adult age groups in the United States since November 2020 [1]. As of September 2021, the estimated seroprevalence was 30% (95% CI: 26–33%) among U.S. children aged 1–4 years, 38% (95% CI: 36–40%) among those aged 5–11 years, and 40% (95% CI: 38–41%) among adolescents aged 12–17 years [13]. In North Carolina, Couture and colleagues previously estimated ratios of SARS-CoV-2 infections to reported COVID-19 cases of 7.7 in August 2020, declining to 4.7 in May 2021 [6]. We report similar seroprevalence estimates for younger children as well as the continued underestimation of cases among children late in the second year of the pandemic. However, we estimated a much higher seroprevalence among adolescents by fall 2021. While national studies tend to report lower seroprevalence estimates than local studies [24], this variation may be also explained by important differences in study design. Like prior seroprevalence studies in children [25, 26], national surveys repeatedly assayed convenience samples of residual sera from vaccinated and unvaccinated children, with likely overrepresentation of children with increased access to or need for healthcare. In contrast, we conducted longitudinal surveillance in a more generalisable, community-based cohort, censoring for vaccination. Thus, our study more likely captured those who were asymptomatic or had mild infections and did not present for testing and thus would likely not have residual sera available for passive public health surveillance. Furthermore, many national cross-sectional surveys of residual sera employed serologic assays that detected only anti-nucleocapsid antibodies [7, 13]. It has been noted that not all children with SARS-CoV-2 infection develop these antibodies to nucleocapsid, and among those that do, the response may be short-lived compared with anti-spike antibodies [27, 28]. As such, our study likely captured more infections through the use of an assay detecting both anti-spike and anti-nucleocapsid antibodies. Lastly, the national surveys could not capture and thus could not stratify for important demographic variables known to impact seroprevalence estimates and infection-to-case ratios [3, 4].
We found that those unvaccinated adolescents aged 12–17 had the highest prevalence of infection-induced antibodies and further had several periods of large increases in seroprevalence. The estimated higher prevalence may be a product of differing contact patterns amongst this age group when compared with the younger age groups. Prior studies of contact patterns conducted in European countries have indicated that those aged 10–19 years have the highest number of reported contacts when compared to other age groups [29] – a pattern that was also seen in other high-income countries in a meta-analysis of contact pattern studies [30]. Understanding the role of contact patterns through the use of seroprevalence studies may help to shed light on the effectiveness of various non-pharmaceutical interventions or public health measures, especially when there is a high degree of asymptotic or pauci-symptomatic disease [31–33].
Likely the most profound change in contact patterns in children and adolescents during the study period was the widespread return to in-person education [34]. Due to varying masking requirements and mitigation measures employed by individual school districts, our study is unable to determine what proportion of the observed increase in seroprevalence was due to in-school transmission versus community transmission. Partnering with district and state educational leadership and with public health officials in North Carolina, the ABC Science Collaborative examined the impact of re-opening schools on the transmission of SARS-CoV-2 amongst children and adolescents [35]. In a study of in-school contact tracing between August 2020 and July 2021 in a private pre-kindergarten through 12th grade school, Thakkar and colleagues found that despite high rates of community incidence of SARS-CoV-2 infections, in-school transmission was limited when multilayered mitigation measures were in place, including masking, health screenings, and contact tracing [36]. Similarly, an analysis of United States–based, cross-sectional internet survey data from the COVID-19 Symptom Survey paired with reported case rates found that while in-person schooling increases the relative risk of COVID-19, these risks are attenuated or disappear depending on the number of school-based mitigation measures reported [37].
Our study highlights the opportunity to use Bayesian techniques with a relatively small number of prospectively enrolled participants conducting at-home testing, even among children and adolescents, to make small area inferences and better capture local disease dynamics for infections other than SARS-CoV-2. While traditional national sentinel-based frameworks leverage existing networks of healthcare providers, studies have shown that these frameworks may under-ascertain the disease dynamics in lower socio-economic populations who do not have access to these resources [38]. Minority recruitment and adjustment for underrepresentation are important for a more complete understanding of the impact a pathogen may have in a community and the associated interventions to improve health equity. For example, our study suggests higher rates of infection-induced antibodies amongst Black children younger than 12 years old, complementing similar studies with findings of higher prevalence of infection [26, 39] and MIS-C among children from minority racial groups [40]. Si and colleagues have shown how routine sampling of the IgG of hospital patients undergoing elective procedures can be used to complement existing state-based data in order to estimate levels of infection and vaccination derived immunity using similar Bayesian MRP frameworks [41]; however, a sampling of elective procedures likely fails to capture the immunology landscape amongst children and those from lower socio-economic backgrounds. Further still, without broader surveys, there is a risk that these blind spots to lower socio-economic population and children could be propagated forward in other modelling efforts [42], underlining the utility of studies such as ours.
Our study has a few limitations. The surveillance was limited to North Carolina and South Carolina, limiting generalisability of results. However, this geographic focus is important because public health measures may need to be deployed given local transmission dynamics, underlying comorbidities, and probability of adverse outcomes. For example, individuals in the Southeast United States are often disproportionately affected by many chronic diseases and infection [43, 44]. Our study recruitment strategy included email, advertisements, and social media posts, both in English and Spanish. Additionally, we established community-based partnerships with faith-based organisations to recruit minorities at community events. Despite these recruitment efforts, minorities were disproportionately underrepresented in our study, underscoring the importance of recruitment and retention strategies to improve representation. Prior studies have shown that minority groups, once enrolled, participate at rates similar to other racial and ethnic groups [14, 43]. A review of recruitment and retention strategies for low-income and minority populations indicates that language barriers, participant perceptions, and trust of the research were important considerations in improving representation in recruitment [45]. Different racial and ethnic groups appear to respond to different recruitment strategies, highlighting the likely need for a diversified approach to participant recruitment [46]. For future paediatric studies, recruitment strategies should utilise mixed approaches including the use of well-child visits or health fairs as an opportunity for study enrolment, especially in underrepresented communities [46].
Unlike prior national surveys [6, 7, 13], we were able to adjust for important variables such as race. Post-survey correction techniques and any small area estimates are ultimately limited by the representativeness of the survey respondents. Low response rates from a given demographic will result in higher levels of uncertainty in the inferences for those cells and will propagate to higher uncertainty in the overall estimates. Handling small cell sizes in a Bayesian framework will typically result in shrinkage towards the group mean rather than poststratification which can suffer from much higher variances [47], further underscoring the need for improved representation in studies. As this study ended prior to the emergence of the Omicron and subsequent variants, we are unable to make inferences on the role of specific variants on seropositivity. Finally, when estimating the infection-to-reported case ratios, we assumed that reported cases were amongst only those who were unvaccinated. This assumption could have the effect of reducing our effective susceptible population, biasing our infection-to-reported cases down.
Conclusion
Although children have milder illness compared to adults with COVID-19, they play a major role in transmission, even if asymptomatic [11, 15]. Accurate estimates of the cumulative incidence of infection among children are therefore vital to understanding the impact on the larger community. By prospectively following a relatively small but regionally generalisable cohort for infection-induced antibodies, we were able to estimate the local burden of SARS-CoV-2 infections among children. As the pandemic evolves, similar unbiased regional serological surveillances can complement national surveys to elucidate the dynamics of SARS-CoV-2 infections in children and inform public health measures for individual communities and their associated immune landscapes.
COVID-19 Community Research Partnership
Wake Forest School of Medicine: Thomas F. Wierzba, PhD, MPH, MS, John Walton Sanders, MD, MPH, David Herrington, MD, MHS, Mark A. Espeland, PhD, MA, John Williamson, PharmD, Morgana Mongraw-Chaffin, PhD, MPH, Alain Bertoni, MD, MPH, Martha A. Alexander-Miller, PhD, Paola Castri, MD, PhD, Allison Mathews, PhD, MA, Iqra Munawar, MS, Austin Lyles Seals, MS, Brian Ostasiewski, Christine Ann Pittman Ballard, MPH, Metin Gurcan, PhD, MS, Alexander Ivanov, MD, Giselle Melendez Zapata, MD, Marlena Westcott, PhD, Karen Blinson, Laura Blinson, Mark Mistysyn, Donna Davis, Lynda Doomy, Perrin Henderson, MS, Alicia Jessup, Kimberly Lane, Beverly Levine, PhD, Jessica McCanless, MS, Sharon McDaniel, Kathryn Melius, MS, Christine O’Neill, Angelina Pack, RN, Ritu Rathee, RN, Scott Rushing, Jennifer Sheets, Sandra Soots, RN, Michele Wall, Samantha Wheeler, John White, Lisa Wilkerson, Rebekah Wilson, Kenneth Wilson, Deb Burcombe, Georgia Saylor, Megan Lunn, Karina Ordonez, Ashley O’Steen, MS, Leigh Wagner.
Atrium Health: Michael S. Runyon, MD, MPH, Lewis H. McCurdy, MD, Yhenneko J. Taylor, PhD, Lydia Calamari, MD, Hazel Tapp, PhD, Michael Brennan, DDS, Lindsay Munn, PhD RN, Timothy Hetherington, MS, Lauren C. Lu, Connell Dunn, Melanie Hogg, MS, CCRA, Andrea Price, Marina Leonidas, Melinda Manning, Frank X. Gohs, MS, Anna Harris, MPH, Jennifer S. Priem, PhD, MA, Pilar Tochiki, Nicole Wellinsky, Crystal Silva, Tom Ludden, PhD, Jackeline Hernandez, MD, Kennisha Spencer, Laura McAlister.
Wake Med Health and Hospitals: William H. Lagarde, MD, LaMonica Daniel, BSCR.
George Washington University Data Coordinating Center: Sharon L. Edelstein, ScM, Michele Santacatterina, PhD, Greg Strylewicz, PhD, Brian Burke, MS, Mihili Gunaratne, MPH, Meghan Turney, MA, Shirley Qin Zhou, MS, Ashley H. Tjaden, MPH, Lida Fette, MS, Asare Buahin, Matthew Bott, Sophia Graziani, Ashvi Soni, MS, Guoqing Diao, PhD, Jone Renteria, MS.
George Washington University Mores Lab: Christopher Mores, PhD, Abigail Porzucek, MS.
Oracle Corporation: Rebecca Laborde, Pranav Acharya.
Vysnova Partners: Anne McKeague, PhD, Johnathan Ward, MS, Diana P. Naranjo, MA, Nana Darko, MPH, Kimberly Castellon, BS, Ryan Brink, MSCM, Haris Shehzad, MS, Derek Kuprianov, Douglas McGlasson, MBA, Devin Hayes, BS, Sierra Edwards, MS, Stephane Daphnis, MBA, Britnee Todd, BS.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Conceptualization: K.L.D., A.A., D.U., P.C.; Writing – review & editing: K.L.D., M.G., W.S.W., A.A., W.H.L., D.U., P.C., R.S.; Funding acquisition: M.G., W.S.W., A.A., W.H.L.; Project administration: M.G., W.R., W.S.W., A.B., W.H.L., D.U.; Data curation: W.R., A.B., D.U.; Resources: A.A.; Supervision: A.A., D.U.; Writing – original draft: A.A.; Formal analysis: D.U.; Methodology: D.U.
Financial support
This publication was supported by the CARES Act, of the U.S. Department of Health and Human Services (HHS; Contract # NC DHHS GTS #49927). The partnership is listed in clinicaltrials.gov (NCT04342884).
Competing interest
The authors have no relevant conflicts of interest to disclose.
References
- [1].Cruz AT, Zeichner SL (2020). COVID-19 in children: initial characterization of the pediatric disease. Pediatrics 145(6), e20200834 [cited 2022 July 5]. 10.1542/peds.2020-0834 [DOI] [PubMed] [Google Scholar]
- [2].Spychalski P, Błażyńska-Spychalska A, Kobiela J (2020). Estimating case fatality rates of COVID-19. Lancet Infectious Diseases 20(7), 774–775 [cited 2020 December 31]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7270730/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Meyerowitz-Katz G, Merone L (2020). A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. International Journal of Infectious Diseases 101, 138–148 [cited 2020 December 31]. Available from: http://www.sciencedirect.com/science/article/pii/S1201971220321809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thors V, Bjornsdottir KL, Love T, Haraldsson A (2022). SARS-CoV-2 infections in Icelandic children: close follow-up of all confirmed cases in a Nationwide study. Pediatric Infectious Disease Journal 41, 835–840 [cited 2022 July 14]. 10.1097/INF.0000000000003626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Viner RM et al. (2020). Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatrics 175, 143–156 [cited 2021 January 6]. Available from: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2771181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Couture A et al. (2022). Severe acute respiratory syndrome coronavirus 2 Seroprevalence and reported coronavirus disease 2019 cases in US children, August 2020–May 2021. Open Forum Infectious Diseases 9, ofac044 [cited 2022 June 24]. 10.1093/ofid/ofac044/6517552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clarke KEN (2022). Seroprevalence of infection-induced SARS-CoV-2 antibodies — United States, September 2021–February 2022. MMWR Morbidity and Mortality Weekly Report 71, 606–608 [cited 2022 July 14]. Available from: https://www.cdc.gov/mmwr/volumes/71/wr/mm7117e3.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Farooqi KM et al. (2021). Longitudinal outcomes for multisystem inflammatory syndrome in children. Pediatrics 148(2), e2021051155 [cited 2022 July 14]. 10.1542/peds.2021-051155 [DOI] [PubMed] [Google Scholar]
- [9].Radtke T, Ulyte A, Puhan MA, Kriemler S (2021) Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA 326, 869–871 [cited 2021 August 17]. 10.1001/jama.2021.11880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yonker LM et al. (2020). Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. Journal of Pediatrics 227, 45–52.e5 [cited 2022 December 9]. Available from: https://www.jpeds.com/article/S0022-3476(20)31023-4/fulltext#%20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Park YJ et al. (2020). Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerging Infectious Diseases 26(10), 2465–2468. Available from: http://wwwnc.cdc.gov/eid/article/26/10/20-1315_article.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Centers for Disease Control and Prevention (2022). Nationwide COVID-19 Infection-Induced Antibody Seroprevalence (Commercial laboratories) [Internet]. COVID Data Tracker [cited 2022 October 25]. Available from: https://covid.cdc.gov/covid-data-tracker/#national-lab.
- [13].Clarke KEN et al. (2022) Pediatric Infection-Induced SARS-CoV-2 Seroprevalence Estimation using Commercial Laboratory Specimens: How Representative Is It of the General U.S. Pediatric Population? [Internet]. Rochester, NY [cited 2022 June 30]. Available from: https://papers.ssrn.com/abstract=4092074.
- [14].Ahmed A et al. (2022) Feasibility of at-home virological and serological testing for SARS-CoV-2 in children. Open Forum Infectious Diseases 9(9), ofac459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sanders JW, The COVID-19 community research partnership (2022). The COVID-19 community research partnership; a multistate surveillance platform for characterizing the epidemiology of the SARS-CoV-2 pandemic. Biology Methods and Protocols 7, bpac033 [cited 2022 November 30]. 10.1093/biomethods/bpac033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gelman A, Carpenter B (2020). Bayesian analysis of tests with unknown specificity and sensitivity. Journal of the Royal Statistical Society: Series C (Applied Statistics) 69(5), 1269–1283 [cited 2020 November 20]. 10.1111/rssc.12435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].National Center for Health Statistics (2022). Vintage 2020 postcensal estimates of the resident population of the United States (April 1, 2010, July 1, 2010–July 1, 2020), by year, county, single-year of age (0, 1, 2, …, 85 years and over), bridged race, Hispanic origin, and sex. Prepared under a collaborative arrangement with the U.S. Census Bureau [Internet] [cited 2022 June 24]. Available from: https://www.cdc.gov/nchs/nvss/bridged_race.htm.
- [18].North Carolina Department of Health and Human Services. NCDHHS_COVID-19_Dashboard [Internet]. NCDHHS COVID-19 Response [cited 2022 October 25]. Available from: https://covid19.ncdhhs.gov/dashboard.
- [19].DeWitt ME et al. (2023). COVID-19 symptoms by variant period in the North Carolina COVID-19 community research partnership, North Carolina, USA. Emerging Infectious Diseases Journal 29(1), 207–211 [cited 2023 January 4]. Available from: https://wwwnc.cdc.gov/eid/article/29/1/22-1111_article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Iyer AS et al. (2020). Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Science Immunology 5(52), eabe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carpenter B et al. (2017). Stan: A probabilistic programming language. Journal of Statistical Software 76, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Anderson B (2021). Cooper moves to end North Carolina’s statewide mask mandate [Internet]. AP NEWS [cited 2023 March 14]. Available from: https://apnews.com/article/health-government-and-politics-education-coronavirus-pandemic-north-carolina-7b40a831d37699df58796bbcbab1a036.
- [23].Guzman J (2021). South Carolina governor orders end to local mask mandates [Internet]. The Hill [cited 2023 March 14]. Available from: https://thehill.com/changing-america/well-being/prevention-cures/553196-south-carolina-governor-orders-end-to-local-mask/.
- [24].Bobrovitz N et al. (2021). Global seroprevalence of SARS-CoV-2 antibodies: a systematic review and meta-analysis. PLoS One 16(6), e0252617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Levorson RE et al. (2021). A cross-sectional investigation of SARS-CoV-2 seroprevalence and associated risk factors in children and adolescents in the United States. PLoS One 16(11), e0259823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hobbs CV et al. (2021). Estimated SARS-CoV-2 Seroprevalence among persons aged <18 years - Mississippi, May–September 2020. MMWR Morbidity and Mortality Weekly Report 70(9), 312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Amjadi MF et al. (2021). Anti-membrane and anti-spike antibodies are long-lasting and together discriminate between past COVID-19 infection and vaccination [Internet]. Infectious Diseases (except HIV/AIDS) [cited 2021 November 11]. 10.1101/2021.11.02.21265750. [DOI]
- [28].Whitaker HJ et al. (2021). Nucleocapsid antibody positivity as a marker of past SARS-CoV-2 infection in population serosurveillance studies: impact of variant, vaccination, and choice of assay cut-off [Internet]. medRxiv [cited 2022 June 24]. Available from: 10.1101/2021.10.25.21264964v1. [DOI]
- [29].Mossong J et al. (2008). Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Medicine 5(3), e74 [cited 2020 June 22]. 10.1371/journal.pmed.0050074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mousa A et al. (2021). Social contact patterns and implications for infectious disease transmission—A systematic review and meta-analysis of contact surveys. eLife 10, e70294 [cited 2022 December 8]. 10.7554/eLife.70294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brooks-Pollock E, Read JM, McLean AR, Keeling MJ, Danon L (2022). Mapping social distancing measures to the reproduction number for COVID-19. Philosophical Transactions of the Royal Society B: Biological Sciences 376(1829), 20200276. [cited 2022 December 8]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8165600/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Davies NG et al. (2020). Age-dependent effects in the transmission and control of COVID-19 epidemics. Nature Medicine 26(8), 1205–1211. [DOI] [PubMed] [Google Scholar]
- [33].Goldstein E, Lipsitch M, Cevik M (2021). On the effect of age on the transmission of SARS-CoV-2 in households, schools, and the community. Journal of Infectious Diseases 223(3), 362–369 [cited 2022 July 5]. 10.1093/infdis/jiaa691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].General Assembly of North Carolina (2021). Session [Internet]. Senate Bill 220, Session Law 2021-4. Available from: https://www.ncleg.gov/Sessions/2021/Bills/Senate/PDF/S220v4.pdf.
- [35].Zimmerman KO, Jackman JG, Benjamin DK Jr (2022). The ABC science collaborative. From research to policy: Reopening K–12 schools in North Carolina during the COVID-19 pandemic. Pediatrics 149(Supplement_2), e2021054268E [cited 2023 March 8]. 10.1542/peds.2021-054268E [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thakkar PV, Zimmerman KO, Benjamin DK Jr, Kalu IC (2022). SARS-CoV-2 infections and incidence at a North Carolina pre-Kindergarten-12 school during in-person education: August 2020 to January 2021. Journal of School Health 92(5), 461–468 [cited 2023 March 8]. 10.1111/josh.13148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lessler J et al. (2021). Household COVID-19 risk and in-person schooling. Science 372(6546), 1092–1097 [cited 2023 March 9]. 10.1126/science.abh2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Scarpino SV, Scott JG, Eggo RM, Clements B, Dimitrov NB, Meyers LA (2020). Socioeconomic bias in influenza surveillance. PLoS Computational Biology 16(7), e1007941 [cited 2021 February 22]. 10.1371/journal.pcbi.1007941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zinszer K et al. (2021). Seroprevalence of SARS-CoV-2 antibodies among children in school and day care in Montreal, Canada. JAMA Network Open 4(11), e2135975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hobbs CV et al. (2022). Active surveillance with Seroprevalence-based infection rates indicates racial disparities with pediatric SARS-CoV-2 requiring hospitalization in Mississippi, March 2020–February 2021. Pediatric Infectious Disease Journal 41(9), 736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Si Y, Covello L, Wang S, Covello T, Gelman A (2022). Beyond vaccination rates: a synthetic random proxy metric of Total SARS-CoV-2 immunity seroprevalence in the community. Epidemiology 33(4), 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zelner J, Masters NB, Naraharisetti R, Mojola SA, Chowkwanyun M, Malosh R (2022). There are no equal opportunity infectors: epidemiological modelers must rethink our approach to inequality in infection risk. PLoS Computational Biology 18(2), e1009795 [cited 2022 March 11]. 10.1371/journal.pcbi.1009795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Oates GR, Jackson BE, Partridge EE, Singh KP, Fouad MN, Bae S (2017). Sociodemographic patterns of chronic disease: How the mid-south region compares to the rest of the country. American Journal of Preventive Medicine 52(1S1), S31–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Raghupathi W, Raghupathi V (2018). An empirical study of chronic diseases in the United States: A visual analytics approach to public health. International Journal of Environmental Research and Public Health 15(3), 431 [cited 2022 December 9]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5876976/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nicholson LM, Schwirian PM, Groner JA (2015). Recruitment and retention strategies in clinical studies with low-income and minority populations: progress from 2004–2014. Contemporary Clinical Trials 45, 34–40 [cited 2023 March 9]. Available from: https://www.sciencedirect.com/science/article/pii/S1551714415300458. [DOI] [PubMed] [Google Scholar]
- [46].Yancey AK, Ortega AN, Kumanyika SK (2006). Effective recruitment and retention of minority research participants. Annual Review of Public Health 27(1), 1–28 [cited 2023 March 9]. 10.1146/annurev.publhealth.27.021405.102113 [DOI] [PubMed] [Google Scholar]
- [47].Downes M, Gurrin LC, English DR, Pirkis J, Currier D, Spittal MJ, Carlin JB (2018). Multilevel regression and poststratification: a modeling approach to estimating population quantities from highly selected survey samples. American Journal of Epidemiology 187(8), 1780–1790 [cited 2020 May 2]. Available from: https://academic.oup.com/aje/article/187/8/1780/4964985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


