Abstract
OBJECTIVE
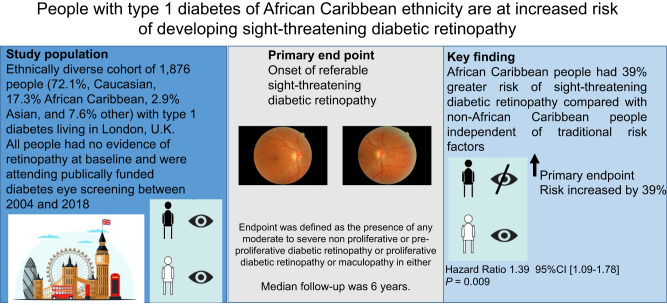
There is limited information on the effect of ethnicity on the development of referable sight-threatening diabetic retinopathy (STDR) in people with type 1 diabetes. This study describes the risk factors for STDR in a diverse cohort of people with type 1 diabetes attending a regional diabetes eye screening service.
RESEARCH DESIGN AND METHODS
Clinical and digital retinal imaging data from 1,876 people with type 1 diabetes (50% women, 72.1% Caucasian, 17.3% African Caribbean, 2.9% Asian, and 7.6% other) with no retinopathy at baseline, attending surveillance eye screening were reviewed. Referable STDR was defined as the presence of any moderate to severe nonproliferative or preproliferative diabetic retinopathy or proliferative diabetic retinopathy or maculopathy in either eye as per U.K. National Diabetic Eye Screening criteria. Median follow-up was 6 years.
RESULTS
The median (interquartile range) age of the cohort was 29 (21, 41) years. Of the cohort of 1,876 people, 359 (19%) developed STDR. People who developed STDR had higher baseline HbA1c, raised systolic blood pressure (SBP), longer diabetes duration, and were more often of African Caribbean origin (24% vs. 15.6%; P < 0.05 for all). In multivariable Cox regression analyses, African Caribbean ethnicity (hazard ratio [HR] 1.39, 95% CI 1.09–1.78, P = 0.009), baseline SBP (HR 1.01, 95% CI 1.00–1.01, P = 0.033), and baseline HbA1c (HR 1.01, 95% CI 1.00–1.01, P = 0.0001) emerged as independent risk factors for STDR.
CONCLUSIONS
We observed that people with type 1 diabetes of African Caribbean ethnicity are at significantly greater risk of STDR. Further research is required to understand the mechanisms that explain this novel observation.
Graphical Abstract
Introduction
Despite advances in clinical care over recent decades, there remains a significant clinical burden of diabetic retinopathy (DR) (1), which remains the leading cause of blindness in people aged between 25 and 75. Recent studies report a prevalence of DR of 48.4% in people with type 1 diabetes and 28.3% in people with type 2 diabetes (2,3). Similarly, a systematic review noted a prevalence of sight-threatening DR (STDR) of 6.17% and documented a threefold higher prevalence of DR in type 1 diabetes compared with type 2 diabetes (77.3% vs. 25.2%) (1).
The most recent meta-analysis predicted that the number of people worldwide with DR and STDR is projected to rise to 160.50 million and to 44.82 million in 2045 (1), respectively, and observed that people of African ancestry have the highest prevalence of STDR. The authors hypothesized ethnicity is a risk factor for STDR independent of geographical regions. The authors were, however, unable to ascertain whether people with type 1 diabetes of certain ethnic groups were specifically at a higher risk of STDR as the diagnosis of type of diabetes was not available.
A global pooled meta-analysis using individual participant data has reported the key risk factors for DR as longer diabetes duration, HbA1c, and raised blood pressure (4). After adjusting for these risk factors, people with type 1 diabetes were still 8.7 times more likely to have STDR compared with those with type 2 diabetes (3). Most studies that have reported the risk factors and predictors for progression of DR have been in Caucasian people with type 1 diabetes.
As there is a paucity of knowledge about the association between ethnicity and progression of DR to STDR in type 1 diabetes, we analyzed baseline and long-term follow-up data in an ethnically diverse cohort of people with type 1 diabetes undergoing routine surveillance for diabetes eye disease in South London.
Research Design and Methods
Clinical and demographic data were collected from 1,876 people with a diagnosis of type 1 diabetes, who had no baseline evidence of retinopathy in either eye, attending annual surveillance diabetes eye screening in South London between 2004 and 2018. The local general population of South East London is ethnically diverse, >30% of people being of African Caribbean heritage (4). There are currently no data on the prevalence of type 1 diabetes in people of African Caribbean heritage in London or nationally. More than 70% of our cohort was Caucasian, and national data (from a predominantly Caucasian general population) suggest a prevalence of type 1 diabetes of 0.6–0.7% in the U.K. based on recent estimates of ∼403,000 people with type 1 diabetes (5).
Diabetes-related clinical and biochemical data, anonymized at the source, for the cohort collected within a time span between 2004 and 2018 from electronic patient records at two large teaching hospitals in London were acquired. The following variables were available, including demographics (date of birth, date of death—if applicable, sex, ethnicity, which was self-reported, and date of diabetes diagnosis), anthropometrics (weight and height), systolic blood pressure (SBP) and diastolic blood pressure (DBP), and laboratory measurements (serum creatinine, urine albumin–to–creatinine ratio [ACR]), HbA1c, total cholesterol, and triglycerides. Serum creatinine measurements were used to calculate estimated glomerular filtration rate (eGFR) values according to the Chronic Kidney Disease Epidemiology Collaboration equation.
We collated data from the South East London Diabetes Eye Screening Program on status and results, with retinopathy and maculopathy grade assessments made from fundus photography of dilated pupils with a nonmydriatic digital camera as per national diabetes eye screening specifications (6).
The UK National Diabetes Eye Screening Committee (UK NSC) guidelines for the standardization of retinopathy grading were followed (6,7). The screening program is well established, with 100% of patients diagnosed with diabetes being offered annual screening. The program achieves a target of >85% of uptake annually; however, a 2% lower screening uptake in the most-deprived areas compared with the least-deprived areas has been observed, but people of African or Caribbean origin did not have lower screening attendance for eye screening in recent studies (6,8).
The UK NSC guidelines classify the condition as no retinopathy (R0), background retinopathy (R1), preproliferative retinopathy (R2), and proliferative retinopathy (R3), with or without referable maculopathy (M0 or M1, respectively), previous photocoagulation, and ungradable. Grades of R1M1, R2M0, R2M1, R3M0, and R3M1 are classified as referable STDR and require referral to secondary care hospital eye (ophthalmology) clinics or for more frequent assessment in a surveillance clinic. No retinopathy was defined as the UK NSC retinopathy grades of R0 and M0. The primary end point was defined as any of the UK NSC retinopathy grades R2, R3, or M1 in either eye, detected upon screening, as this is defined by the UK NSC as STDR requiring urgent referral.
The criteria used for the UK NSC grading and relationship to the Early Treatment Diabetic Retinopathy Study (ETDRS) scale have been described previously (7). In brief, R0 level identifies no detected DR (equivalent to ETDRS level 10), R1 level identifies a minimum of at least the presence of one microaneurysm and/or retinal hemorrhage and is equivalent to ETDRS levels 14–35. R2 level is the presence of multiple deep, round, or blot hemorrhages, and/or definite intraretinal microvascular abnormality, and/or venous beading, and/or reduplication, and is equivalent to levels 43–53 on the ETDRS scale. R3 level indicates the presence of proliferative DR (including fibrous proliferation), equivalent to a minimum of ETDRS level 61. M1 (maculopathy) represents the presence of any exudate in the central foveal area or a group of exudates within the macula region, or the presence of a microaneurysm or hemorrhage within the central foveal area in association with a reduced visual acuity taken to be a surrogate marker of diabetic maculopathy. M0 is present in the absence of any M1 features.
Specialist trained staff take digital color retinal photographs of two standard 45° fields (macula and disc centered) per eye after dilation of the pupils. Trained assessors in a central location grade the presence and severity of DR using a multilevel, internally and externally quality-assured grading process that meets national recommendations (6).
To create our baseline data set, we considered the date of the first diabetes eye screening review for each patient as the initial date of the study and extracted all other baseline values of the rest of the variables as the closest value to this date, within a 1-year span. All other variables that had not been measured within that span were considered missing. The follow-up duration was defined as the last available eye screening result. For people who developed the primary end point of STDR between the baseline eye screening and follow-up eye screening, the time period between these two screening events was used as the time taken for the primary end point to occur. Follow-up was to the last available date on eye screening data set or 5 March 2018, date of death, or date of incident STDR.
Exclusion criteria included documented history of DR at baseline, nondiabetic eye diseases, absence of annual follow-up eye screening, and pregnancy. Of the total cohort of 3,875 people with type 1 diabetes who had retinopathy assessments between 2004 and 2018, 2,219 people had no evidence of DR at baseline. Of these 2,219 people, 343 were excluded due to absence of follow-up. The prevalence of African Caribbean people in the 343 people excluded due to lack of follow-up data was 22%. A total 1,867 people with no baseline retinopathy and with annual follow-up eye photograph data were therefore included in the study. A total of two digital fundus photographs were taken per eye, and the total number of photographs per individual per year was four. Median follow-up was 6 years.
The diagnosis of type 1 diabetes was extracted from eye screening and primary care records. We measured socioeconomic status using the Index of Multiple Deprivation (IMD). Scores are ranked according to population deciles, with 1 indicating highest level of deprivation and 10 being the most affluent (9). This was a retrospective study conducted in line with local protocols using existing anonymized routine clinical data accessed directly by the clinical team and approved by hospital data governance committees.
Statistical Methods
Our primary end point was onset of STDR from baseline retinopathy grade R0 and maculopathy grade M0 to R2 and above and/or the onset of M1. Prognostic value of covariates was tested by univariable Cox regression and log-rank tests, and those with P values <0.2 were considered for the multivariable Cox regression models, unless they were deemed clinically relevant, in which case they were included in the multivariate analysis irrespective of statistical significance in the univariate model. To identify variables and combinations of variables with the best prognostic value, a step-down procedure was followed for model selection. For individuals who developed the primary end point of STDR between the baseline eye screening and follow-up eye screening, the time between these two screening events was used as the time taken for the primary end point to occur.
Only variables with <30% missing within the baseline data set were included in the analysis. All missing baseline data for continuous variables were imputed by predictive mean matching using the “mice” package in RStudio 1.3.959 (Posit Software). Analyses were performed in data sets, with and without imputation of missing values. For statistical comparisons of variables between retinopathy stages, one-way ANOVA or Wilcoxon rank sum tests were used for continuous variables and χ2 tests for categorical variables. For computation, presentation, assessment of performance, validation, and calibration of the models, we used R statistical functions. Data are presented as median and interquartile range (IQR) for variables without a normal distribution and mean ± SD for data with normal distribution with the appropriate parametric and nonparametric testing, respectively. Proportional hazard assumptions were checked by graphs of scaled Schoenfeld residuals, and Schoenfeld individual tests for model validation bias-corrected indexes and calibration was also performed. To examine the effect of all-cause mortality on the primary end point of STDR, competing risk analyses using Fine-Gray regression models were performed. All statistical analysis was done within RStudio 1.3.1073 software under R 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
The baseline characteristics of our study population of 1,876 (50% women) are summarized in Table 1. Of the cohort, 72.1% were Caucasians, 17.3% were African Caribbean, 2.9% were Asian, and 7.6% were other (including mixed ethnicities). Median (IQR) age and duration of diabetes were 29 (21, 41) years and 6 (2, 12) years, respectively. Median age at the time of diagnosis was 19 (IQR 11, 30) years. We did not have full information on smoking status or use of medications, such as blood pressure medications and lipid-lowering drugs, on the database. The median IMD decile of the cohort was 3 (IQR 2, 5). The median duration of diabetes at time of screening from time of diagnosis was 6 years (IQR 2, 12).
Table 1.
Baseline clinical and biochemical characteristics of 1,876 people with type 1 diabetes with no evidence of retinopathy
| Characteristic | N = 1,876 |
|---|---|
| Age, years | 29 (21, 41) |
| Duration of diabetes, years | 6 (2, 12) |
| Age at diabetes diagnosis, years | 19 (11, 30) |
| Sex | |
| Female | 940 (50) |
| Male | 936 (50) |
| Ethnicity | |
| Asian | 55 (2.9) |
| African Caribbean | 326 (17.3) |
| Caucasian | 1,352 (72.1) |
| Other | 143 (7.6) |
| BMI, kg/m2 | 24.33 ± 4.83 |
| HbA1c, mmol/mol | 70 (57, 95) |
| ACR, mg/mmol | 16 (6, 44) |
| Cholesterol, mmol/L | 4.58 ± 0.96 |
| SBP, mmHg | 120.05 ± 15.37 |
| DBP, mmHg | 72.17 ± 9.63 |
| eGFR, mL/min/1.73 m2 | 94 (76, 116) |
Data are presented as median (IQR), mean ± SD, or n (%).
Of the 1,867 people, 359 (19%) progressed to STDR at the end point, while 1,442 did not over the duration of follow-up. During the follow-up, 75 people died before reaching STDR and were therefore not included in our primary analyses. People who died were older, had longer duration of diabetes, higher SBP and DBP levels, higher urine ACR, and lower eGFR than those who did and did not progress to STDR (see Supplementary Table 1).
Table 2 summarizes the comparison of baseline characteristics between people who progressed to STDR and those who did not. Duration of diabetes, total cholesterol, SBP, and HbA1c were higher and eGFR was lower in those with progression to STDR. We also observed that people of African Caribbean origin were more likely to progress to STDR (Table 2).
Table 2.
Comparison of baseline clinical and biochemical characteristics in people with type 1 diabetes with and without development of STDR
| Characteristic | No progression | Progression | P value |
|---|---|---|---|
| n = 1,442 | n = 359 | ||
| Age, years | 29 (21, 39) | 28 (20, 40) | 0.6 |
| Sex | 0.7 | ||
| Female | 724 (50) | 184 (51) | |
| Male | 718 (50) | 175 (49) | |
| Ethnicity | |||
| Non–African Caribbean | 1,218 (84) | 273 (76) | |
| African Caribbean | 224 (16) | 86 (24) | <0.001 |
| Duration of diabetes, years | 5 (2, 11) | 8 (4, 14) | <0.001 |
| HbA1c, mmol/mol | 69 (56, 94) | 77 (61, 98) | <0.001 |
| BMI, kg/m2 | 23.7 (21.1, 26.4) | 24.0 (21.7, 27.0) | 0.2 |
| SBP, mmHg | 119.40 ± 15.31 | 121.16 ± 14.30 | 0.05 |
| DBP, mmHg | 71.91 ± 9.68 | 72.58 ± 9.40 | 0.2 |
| ACR, mg/mmol | 18 (6, 44) | 13 (5, 43) | 0.026 |
| Cholesterol, mmol/L | 4.54 ± 0.95 | 4.74 ± 0.99 | <0.001 |
| eGFR, mL/min/1.73m2 | 96 (77, 118) | 92 (75, 112) | 0.016 |
Data are presented as median (interquartile range), mean ± SD, or n (%). Data from 75 people who died during follow-up before the end of study are not included in this table.
We did not observe any significant impact of deprivation as determined by the IMD score on the development of STDR (median IMD score was 3 [IQR 2, 5]) in people with and without the end point. Similarly we also did not observe any differences in IMD scores between African Caribbean and non–African Caribbean people (IMD was 3 [IQR 2, 5] in both groups). In our study, African Caribbean people had similar median duration of follow-up attendances to eye screening and no differences in missing data compared with non–African Caribbean people.
Of the 359 people who reached end point of STDR during follow-up, 66 developed R2, 293 developed maculopathy grade M1 (with and without R2 or R3), and 27 (7.5%) developed R2 or R3 and M1. Only maculopathy without R2 or R3 developed in 266 people and only R2 and R3 without maculopathy developed in 19 and 16 people, respectively. Supplementary Table 2A reports the number for each component of the retinopathy end point in African Caribbean and non–African Caribbean people. We observed that people of African Caribbean origin compared with non–African Caribbean people had a nearly twofold higher risk of onset of ≥R2 (9.7% vs. 4.2%) and similarly for onset of M1, this was 21% vs. 15%, respectively (Supplementary Table 2B).
Table 3 reports the baseline characteristics in African Caribbean people versus people of non–African Caribbean origin. People of the African Caribbean group were younger, had shorter duration of diabetes, lower weight, more prevalent albuminuria, and higher baseline HbA1c compared with non–African Caribbean people. SBP and DBP, BMI, eGFR, and total cholesterol at baseline were comparable in both groups.
Table 3.
Baseline characteristics of 1,876 people with type 1 diabetes stratified according to African Caribbean and non–African Caribbean ethnicity
| Characteristic | Non–African Caribbean | African Caribbean | P value |
|---|---|---|---|
| n = 1,550 | n = 326 | ||
| Age, years | 30 (22, 41) | 27 (16, 38) | <0.001 |
| Duration of diabetes, years | 6 (2, 13) | 4 (2, 8) | <0.001 |
| Sex | 0.1 | ||
| Female | 760 (49) | 177 (54) | |
| Male | 784 (51) | 149 (46) | |
| HbA1c, mmol/mol | 68 (56, 90) | 85 (63, 114) | <0.001 |
| BMI, kg/m2 | 24.0 (21.4, 26.7) | 23.4 (21.0, 26.6) | 0.13 |
| Weight, kg | 70 (60, 82) | 66 (55, 78) | <0.001 |
| SBP, mmHg | 120.21 ± 15.15 | 119.25 ± 16.34 | 0.2 |
| DBP, mmHg | 72.18 ± 9.50 | 72.11 ± 10.25 | 0.9 |
| ACR mg/mmol | 13 (5, 44) | 39 (8, 44) | <0.001 |
| Cholesterol, mmol/L | 4.58 ± 0.96 | 4.57 ± 0.97 | 0.8 |
| eGFR, mL/min/1.73m2 | 95 (76, 115) | 92 (71, 123) | 0.9 |
Data are presented as median (interquartile range), mean ± SD, or n (%). Of the cohort of 1,876, 75 people died during follow-up, and their data are included in this table.
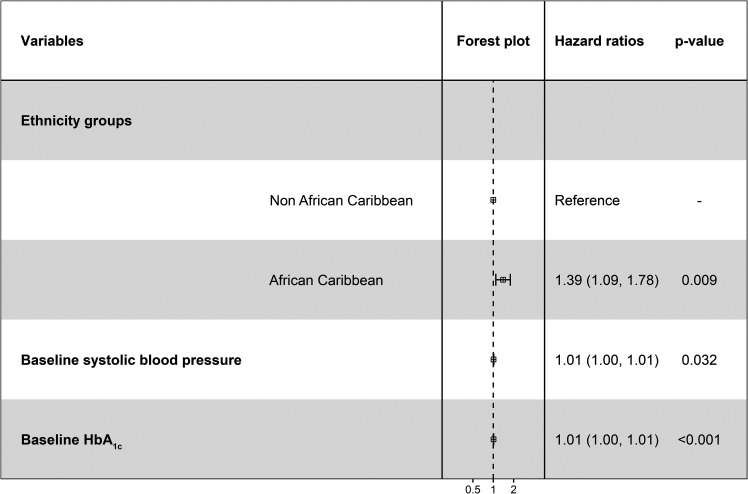
In multivariable Cox regression models, African Caribbean ethnicity (hazard ratio [HR] 1.39, 95% CI 1.09–1.78, P = 0.009), baseline SBP (HR 1.01, 95% CI 1.00–1.01, P = 0.033), and baseline HbA1c (HR 1.01, 95% CI 1.00–1.01, P = 0.0001) emerged as significant independent risk factors for progression to STDR (Fig. 1).
Figure 1.
Multivariable Cox regression analyses of variables associated with the development of STDR in an ethnically diverse cohort of people with type 1 diabetes.
Our results indicate a 1% increase in risk of STDR for each 1 mmol/mol rise in baseline HBA1c or 1 mmHg rise in SBP. Similarly, the increased hazard of 1.39 (95% CI 1.09–1.78, P = 0.009) suggests that people of African Caribbean origin with type 1 diabetes have a 39% greater risk of developing STDR than non–African Caribbean people and that this significant effect is independent of conventional risk factors for retinopathy such as baseline HBA1c or SBP or DBP.
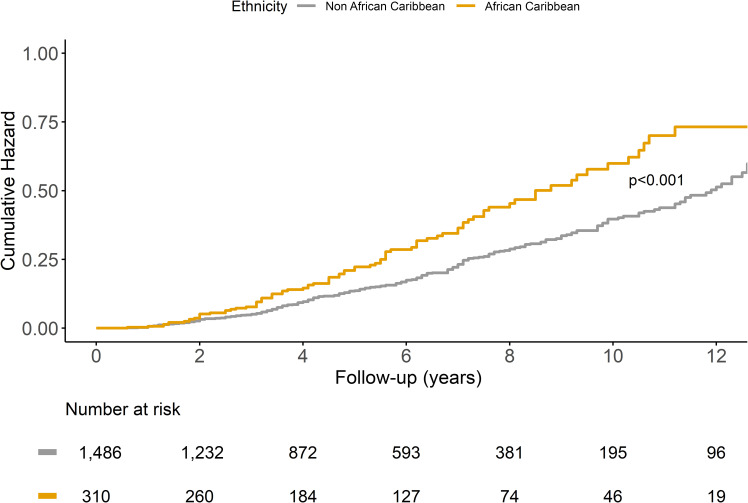
The observed notable association between the ethnicity and progression to STDR is further demonstrated in the Kaplan-Meier survival curve for STDR comparing African Caribbean versus other ethnicities (Fig. 2).
Figure 2.
Cumulative hazard for the development of STDR stratified by ethnicity.
In further analyses only in people of African Caribbean origin, those who developed STDR had higher HbA1c (median [IQR] 92 [70, 122] mmol/mol vs. 80 [61, 111] mmol/mol) and total cholesterol levels (mean ± SD, 4.46 ± 0.91 mmol/L vs. 4.84 ± 1.04 mmol/L) compared with people who did not, with no other statistically significant differences observed (Supplementary Table 3).
Of the 1,876 cohort, 75 people died over the duration of follow-up before reaching STDR or end of follow-up over the duration of follow-up and were therefore not included in our primary analyses. In competing risk analyses where death was a competing event with STDR, we observed a similar significant independent effect of African Caribbean ethnicity on higher risk of developing STDR (Supplementary Table 4).
Conclusions
We observed in a contemporary longitudinal data study in an ethnically diverse cohort of 1,876 people with type 1 diabetes, with no DR at baseline, the novel finding that African Caribbean ethnicity is a predictor of STDR independent of traditional risk factors for DR progression and socioeconomic status (10–12).
The majority of studies investigating the role of ethnicity in DR have been in people with type 2 diabetes. The Multi-Ethnic Study of Atherosclerosis in 778 people with type 2 diabetes aged 45–85 reported that the prevalence of STDR was higher in African Americans and Hispanic people, but these differences were not statistically significant when adjusted for risk factors such as duration of diabetes, HbA1c, and SBP and DBP (13). Similar observations of higher prevalence of DR among African Americans but no differences in STDR people with type 2 diabetes has been reported in the U.S. (14,15).
Cross-sectional studies in people with type 2 diabetes attending screening programs in the U.K. demonstrated that the prevalence of STDR was higher in African Caribbean people compared with Caucasians (9,16,17). A study of >50,000 people, including 3,323 people with type 1 diabetes, documented a greater prevalence of retinopathy in African Caribbean people; however, the observations in type 1 diabetes were not statistically significant. A cross-sectional study from the U.S., where data were combined from people with type 1 diabetes from two ethnicities (White American and Black American), reported that the prevalences of DR of any level or STDR were similar in both ethnic groups (16).
There are limited longitudinal studies on the progression rates of DR to STDR in people with type 1 diabetes from ethnically diverse populations. A 6-year follow-up study in 508 African American people (including children) with type 1 diabetes, of whom ∼60% had evidence of DR at baseline, observed that 21.6% of the cohort progressed to STDR. The increased risk of STDR was associated with lower socioeconomic status, older age, kidney disease, higher HbA1c, and worse hypertension control (15). In that study, compared with ours, baseline HbA1c was much higher, at 13.5% (124 mmol/mol), and 80% of people with baseline HbA1c of ≥16.2% (153 mmol/mol) showed progression of DR during follow-up. In contrast to our study, people below the age of 18 were included, and the progression of DR was the highest among people aged 10–19 years. Indeed, there is evidence that a rapid progression of DR is associated with a shorter duration of diabetes in African American people (17–19). The lower rates of STDR observed in historical studies of type 1 diabetes may be in part related to the competing risk of premature mortality, which can be greater in African Americans with type 1 diabetes, with one study demonstrating 18% of the cohort had died at 3 years of follow-up (20).
The mechanisms that explain our results of increased risk of STDR in African Caribbean people with type 1 diabetes need further investigation. Our study was not designed to identify the putative reasons or mechanisms; however, we can speculate there may be multiple explanations for our results. Low socioeconomic status has been linked to increased morbidity and mortality in diabetes; however, an association with progression of DR is uncertain, with conflicting data reported (17,21,22). In our study, we did not observe any significant impact of deprivation as measured by the IMD on onset of STDR. We cannot exclude that those with more deprivation may cluster in certain ethnic groups (23,24) and therefore may also be less likely to attend diabetes eye screening (6). Indeed, a 2% lower screening uptake in the most-deprived compared with the least-deprived areas has been observed (6). If this is the case, the findings we observed may be an underestimation of risk in certain ethnic groups. Conversely, no differences between African Caribbean and Caucasian people with diabetes attending eye screening in London has been reported (6,8). Further studies, including sensitivity analyses in ethnically diverse cohorts, are clearly needed to better understand the factors influencing eye screening attendance in all health care systems.
Genetic predisposition to progression of DR has been described (19,20) but has not been replicated in some studies (21). Whether our observation of enhanced risk of STDR in African Caribbean people is related to specific genetic markers requires further investigation.
A negative “legacy” impact of poor glycemic control may explain our results. Indeed, studies in younger people with type 1 diabetes have demonstrated higher HbA1c in African Caribbean people compared with their Caucasian counterparts, which persists even after adjustment for socioeconomic status and diabetes duration (25–27). Disparities and unequal access to technology to aid diabetes self-management and optimize glucose control, higher rates of hypoglycemia and ketoacidosis, and socioeconomic factors have been proposed as possible reasons for the observed ethnic differences in glycemic control (25–30). All these studies in numerically smaller cohorts with shorter duration of follow-up compared with our study were, however, unable to assess the impact of ethnicity on retinopathy progression.
There are several limitations of our study. The results from an urban, ethnically diverse environment are unlikely to be representative of national cohorts. Our median length of follow-up was 6 years; while this is longer than in some recent cohort studies, we acknowledge the need for longer follow-up studies. Although we did not observe a significant impact of deprivation as measured by the IMD on onset of STDR, other measures of socioeconomic status and deprivation are needed to further assess the impact of social and economic factors. We observed a numerically greater incidence of both retinopathy and maculopathy in African Caribbean individuals; however, our study was not designed to evaluate the relative contribution and impact of ethnicity on the components of the composite STDR end point
A major limitation of our study is the lack of data and information on medications and smoking status. These data were not captured in our study, and we cannot therefore exclude that the observed results may be related to differences in use of medications that have demonstrated benefits for retinal and other vascular complications of diabetes (31,32).
In our study, we did not have a formal laboratory diagnosis of type 1 diabetes as we relied on medical records. We cannot exclude that people with ketosis-prone diabetes may have been labeled with type 1 diabetes. It is recognized that clinical features, such as higher BMI, heavier body weight, or older age, are more prevalent in ketosis-prone diabetes (33). However, no significant differences in these parameters were observed between African Caribbean and non–African Caribbean people in our cohort.
The median age at diagnosis of diabetes in our cohort was 19 years, and, therefore, our results cannot be extrapolated to people with younger age of onset of type 1 diabetes.
We acknowledge as a further limitation only including baseline HbA1c in our analyses models and not time-weighted HbA1c. Further studies with time-weighted HbA1c data to see whether this correlates with STDR are needed. Ethnic differences in HbA1c are well documented, and we cannot exclude an effect of ethnicity on HbA1c measurements (34).
The strengths of our study include its contemporaneous nature and having a cohort with ethnic diversity representative of urban-dwelling people with type 1 diabetes. The use of standardized digital fundal photographic eye screening by a single provider with real-world clinic-based measures and assessments, access to linked data, including socioeconomic status, and laboratory data from a single unit with standardized processes are further strengths of our work.
In a recent study from our group, we observed enhanced greater risk of diabetic kidney disease progression in people with type 1 diabetes of African Caribbean ethnicity compared with non-African-Caribbean ethnicity (35), independent of traditional risk factors for kidney disease. The results we report in this study for STDR are consistent with our observations for kidney disease and suggest potential common pathways for faster progression of microvascular disease in people with type 1 diabetes of African Caribbean ethnicity.
A large number of people with type 1 diabetes are at risk for progression to STDR, with significant heterogeneity in the rates and risks of progression. Studies such as ours in a distinctive urban cohort of ethnically diverse people with type 1 diabetes can help identify new risk factors that aid risk stratification and better identify people at high risk of STDR. The information learned from such studies can facilitate changes in clinical care and pathways to enable earlier identification and treatment of modifiable risk factors to delay progression of DR (1,18,32).
In summary, we observed in a multi-ethnic cohort of people with type 1 diabetes managed in a publically funded health care system the novel finding of African Caribbean ethnicity as an independent predictor of STDR. This enhanced risk was independent of traditional risk factors for DR. Further research is required to better understand pathophysiology that may explain the association between African-Caribbean ethnicity and higher risk of progression to STDR in people with type 1 diabetes.
Article Information
Funding. This work was funded by a research grant from Guy’s and St Thomas’ Charity. S.A. was funded/supported by the National Institute for Health Research Biomedical Research Centre based at Guy’s and St Thomas’ National Health Service Foundation Trust and King’s College London.
The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.M. and S.A. contributed and led on data analysis and interpretation. P.W., N.F., L.W., A.N., P.V., S.M., J.C., and D.H. collected and interpreted the data and contributed to the manuscript. A.M., S.T., S.A., and J.K. designed the research study, interpreted the data, and drafted the article. All authors have reviewed the article and approved the final draft. A.M. and J.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.22185859.
References
- 1. Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology 2021;128:1580–1591 [DOI] [PubMed] [Google Scholar]
- 2. Mathur R, Bhaskaran K, Edwards E, et al. Population trends in the 10-year incidence and prevalence of diabetic retinopathy in the UK: a cohort study in the Clinical Practice Research Datalink 2004-2014. BMJ Open 2017;7:e014444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yau JWY, Rogers SL, Kawasaki R, et al.; Meta-Analysis for Eye Disease (META-EYE) Study Group . Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Winkley K, Thomas SM, Sivaprasad S, et al. The clinical characteristics at diagnosis of type 2 diabetes in a multi-ethnic population: the South London Diabetes cohort (SOUL-D). Diabetologia 2013;56:1272–1281 [DOI] [PubMed] [Google Scholar]
- 5. Gregory GA, Robinson TIG, Linklater SE, et al.; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group . Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol 2022;10:741–760 [DOI] [PubMed] [Google Scholar]
- 6. Gulliford MC, Dodhia H, Chamley M, et al. Socio-economic and ethnic inequalities in diabetes retinal screening. Diabet Med 2010;27:282–288 [DOI] [PubMed] [Google Scholar]
- 7. Stratton IM, Aldington SJ, Taylor DJ, Adler AI, Scanlon PH. A simple risk stratification for time to development of sight-threatening diabetic retinopathy. Diabetes Care 2013;36:580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olvera-Barrios A, Seltene M, Heeren TFC, et al. Effect of ethnicity and other sociodemographic factors on attendance at diabetic eye screening: a 12-month retrospective cohort study. BMJ Open 2021;11:e046264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McLennan D, Noble S, Noble M, Plunkett E, Wright G, Gutacke N. The English Indices of Deprivation 2019. Technical Report. London, Ministry of Housing, Communities and Local Government, 2019. [Google Scholar]
- 10. Helve J, Sund R, Arffman M, et al. Incidence of end-stage renal disease in patients with type 1 diabetes. Diabetes Care 2018;41:434–439 [DOI] [PubMed] [Google Scholar]
- 11. de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int 2020;98:839–848 [DOI] [PubMed] [Google Scholar]
- 12. Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010;21:2020–2027 [DOI] [PubMed] [Google Scholar]
- 13. Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 2006;141:446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris MI, Klein R, Cowie CC, Rowland M, Byrd-Holt DD. Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? A U.S. population study. Diabetes Care 1998;21:1230–1235 [DOI] [PubMed] [Google Scholar]
- 15. Rabb MF, Gagliano DA, Sweeney HE. Diabetic retinopathy in blacks. Diabetes Care 1990;13:1202–1206 [DOI] [PubMed] [Google Scholar]
- 16. Roy MS, Klein R, O’Colmain BJ, Klein BEK, Moss SE, Kempen JH. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch Ophthalmol 2004;122:546–551 [DOI] [PubMed] [Google Scholar]
- 17. Roy MS, Affouf M. Six-year progression of retinopathy and associated risk factors in African American patients with type 1 diabetes mellitus: the New Jersey 725. Arch Ophthalmol 2006;124:1297–1306 [DOI] [PubMed] [Google Scholar]
- 18. Krolewski AS, Warram JH, Rand LI, Christlieb AR, Busick EJ, Kahn CR. Risk of proliferative diabetic retinopathy in juvenile-onset type I diabetes: a 40-yr follow-up study. Diabetes Care 1986;9:443–452 [DOI] [PubMed] [Google Scholar]
- 19. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1989;107:237–243 [DOI] [PubMed] [Google Scholar]
- 20. Roy M, Rendas-Baum R, Skurnick J. Mortality in African-Americans with type 1 diabetes: the New Jersey 725. Diabet Med 2006;23:698–706 [DOI] [PubMed] [Google Scholar]
- 21. Klein R, Klein BE, Jensen SC, Moss SE. The relation of socioeconomic factors to the incidence of proliferative diabetic retinopathy and loss of vision. Ophthalmology 1994;101:68–76 [DOI] [PubMed] [Google Scholar]
- 22. Wagenknecht LE, Roseman JM, Alexander WJ. Epidemiology of IDDM in black and white children in Jefferson County, Alabama, 1979–1985. Diabetes 1989;38:629–633 [DOI] [PubMed] [Google Scholar]
- 23. Millett C, Dodhia H. Diabetes retinopathy screening: audit of equity in participation and selected outcomes in South East London. J Med Screen 2006;13:152–155 [DOI] [PubMed] [Google Scholar]
- 24. Benzeval M, Judge K, Smaje C. Beyond class, race, and ethnicity: deprivation and health in Britain. Health Serv Res 1995;30:163–177 [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobsen JJ, Black MH, Li BH, Reynolds K, Lawrence JM. Race/ethnicity and measures of glycaemia in the year after diagnosis among youth with type 1 and type 2 diabetes mellitus. J Diabetes Complications 2014;28:279–285 [DOI] [PubMed] [Google Scholar]
- 26. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Network Open 2018;1:e181851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Redondo MJ, Libman I, Cheng P, et al.; Pediatric Diabetes Consortium . Racial/ethnic minority youth with recent-onset type 1 diabetes have poor prognostic factors. Diabetes Care 2018;41:1017–1024 [DOI] [PubMed] [Google Scholar]
- 28. Delamater AM, Albrecht DR, Postellon DC, Gutai JP. Racial differences in metabolic control of children and adolescents with type I diabetes mellitus. Diabetes Care 1991;14:20–25 [DOI] [PubMed] [Google Scholar]
- 29. Muthuppalaniappan VM, Yaqoob MM. Ethnic/race diversity and diabetic kidney disease. J Clin Med 2015;4:1561–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agarwal S, Schechter C, Gonzalez J, Long JA. Racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther 2021;23:306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang B, Wang F, Zhang Y, et al. Effects of RAS inhibitors on diabetic retinopathy: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2015;3:263–274 [DOI] [PubMed] [Google Scholar]
- 32. Wong TY, Cheung CM, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Primers 2016;2:16012. [DOI] [PubMed] [Google Scholar]
- 33. Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med 2006;144:350–357 [DOI] [PubMed] [Google Scholar]
- 34. Selvin E. Are there clinical implications of racial differences in HbA1c? A difference, to be a difference, must make a difference. Diabetes Care 2016;39:1462–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mangelis A, Fountoulakis N, Corcillo A, et al. African Caribbean ethnicity is an independent predictor of significant decline in kidney function in people with type 1 diabetes. Diabetes Care 2022;45:2095–2102 [DOI] [PubMed] [Google Scholar]