Abstract
Maternally derived antibodies are believed to protect infants against infection, but there is little direct evidence for a protective role of passively acquired antibodies against malaria. A longitudinal study of malaria infection in 143 infants was conducted in a region of southern Ghana where Plasmodium falciparum is endemic. Infants born in the high-transmission season were less likely to become infected in the first 20 weeks of life than children born in the low-transmission season. Plasma, obtained at birth, was tested for immunoglobulin G (IgG) and IgG subclasses to P. falciparum schizonts and recombinant circumsporozoite antigen, MSP-119, MSP-2, AMA-1, and Pf155 (also called ring-infected erytrocyte surface antigen). Antibody levels at birth were not associated with resistance to malaria infection. On the contrary, antibodies at birth were positively associated with infection, indicating that high levels of maternally derived antibodies represent a marker for intensity of exposure to malaria infection in infants. However, all five children who experienced high-density infections (>100 parasites/μl of blood) were seronegative for MSP-119 at the time of infection.
In populations in which malaria is endemic, immunity is acquired in an age- and exposure-related manner such that the greatest burden of disease falls on young children (21). Nevertheless, infants appear to be relatively protected from clinical malaria for the first 3 to 6 months of life (7, 19). In an area of moderate, stable malaria transmission in southern Ghana, malaria infections occur throughout the first year of life but the vast majority of infections in infants are of low parasite density and are not accompanied by clinical symptoms (33). The risk of infection increases significantly from the age of about 18 weeks (33), while the risk of a clinical attack of malaria remains low throughout the first 6 months of life (22). Numerous mechanisms have been proposed to explain the low risk of malaria in neonates, although few detailed prospective studies have been performed. Our studies in Ghana (22, 33) indicate that lack of exposure to infective bites is an unlikely explanation, but physiological factors (presence of fetal hemoglobin, a temporary decline in erythropoiesis in the perinatal period, and lack of p-aminobenzoic acid in a breast milk diet) and immunological mechanisms are worthy of further investigation.
Mechanisms of protective immunity to malaria are poorly understood, as are the target antigens of protective immune responses. If neonatal resistance to malaria could be firmly attributed to maternal antibody, this would not only strengthen the evidence for effective humoral immunity to malaria but also allow us to determine which specificities, and isotypes, of antibody are involved in protective immunity. On the other hand, if protection in infants is not antibody dependent, then other immunological mechanisms might be implicated and should be investigated.
Previous studies have provided evidence for associations, at a population level, between decreasing levels of maternally derived malaria-specific immunoglobulin G (IgG) and increasing risk of clinical malaria (10, 28), but the numbers of subjects studied were too small for any meaningful statistical analysis to be performed. More recently, somewhat larger studies have provided conflicting results with regard to the protective effects of maternal antibody. In a study of 198 newborns in Tanzania, there was no association between cord blood antibodies to Plasmodium falciparum circumsporozoite antigen (CSP) or two different antigenic fragments of merozoite surface protein 1 (MSP-1) and age at which malaria parasitemia was first detected (18). Similarly, Achidi et al. found no correlation between cord blood antibodies to CSP or to the erythrocyte antigen Pf155 (also called ring-infected erythrocyte surface antigen) and age of onset of clinical malaria in 117 Nigerian infants (1) and a prospective study of 100 Liberian infants found no association between total antimalarial antibody levels at birth and risk of clinical malaria (16). In contrast, both the Liberian study (16) and a study of 60 Kenyan infants (8) have shown significant associations between the presence at birth of antibodies to the 19-kDa C-terminal fragment of MSP-1 (MSP-119) and resistance to clinical malaria over the first year of life. However, as maternal antibodies are unlikely to persist throughout the first year, interpretation of these studies is not straightforward.
To look for potential protective effects of maternally derived antibody, we have conducted a longitudinal, prospective study of a birth cohort of 143 children from southern Ghana. We looked for malaria parasitemia by microscopy and PCR of blood samples collected at least every 4 weeks from birth, and active case detection for clinical malaria was conducted every 2 weeks. The prevalence of asymptomatic and clinical malaria infection over the first 20 weeks of age was compared with levels of serum antibodies to erythrocytic and preerythrocytic stage antigens of P. falciparum at birth.
MATERIALS AND METHODS
Study area.
The study was conducted in Prampram, a coastal fishing village approximately 50 km east of Accra, Ghana. Malaria transmission is perennial but peaks in July and August, after the long rainy season. The predominant vector is Anopheles gambiae. The entomologic inoculation rate is approx 5 to 10 infectious bites per year; 92% of infections are due to P. falciparum, and 8% are due to Plasmodium malariae (2).
Study design.
Ethical permission for the study was obtained from the Ghanaian Ministry of Health. Informed consent was obtained from mothers and/or guardians of the children prior to commencement of the study. Mothers were recruited into the study in the last trimester of pregnancy. Maternal blood samples were collected by venipuncture, and children's samples were collected at birth, 2, 4, and 6 weeks after birth, and then every 4 weeks by heel prick. Thick and thin blood films were stained with Giemsa's stain. Parasite density was scored as the number of parasites per 300 white blood cells (WBC) and converted to parasites per microliter based on an average WBC count of 13,000/μl of whole blood in African infants (32). Slides were classified as negative only after a minimum of 1,000 WBC had been counted. Heparinized blood samples were separated into plasma and an erythrocyte pellet, both of which were stored at −20°C. Children visited the government health center at monthly intervals for full clinical examination and blood sampling. Children were visited at home by a field worker in intervening weeks; health questionnaires were completed, and axillary temperature was measured. The following symptoms of ill health were recorded as either present or absent at each visit: refusing to feed, vomiting, diarrhea, cough, and fever. Whenever a fever was detected (temperature of ≥37.5°C or a history of fever reported by the mother), a blood film was made and examined for parasites. Children who were parasitemic and unwell were treated with a full course of chloroquine (25 mg/kg of body weight over 3 days).
Parasite DNA detection.
P. falciparum DNA was extracted from erythrocyte pellets using the method of Foley et al. (14), and the 7H8/6 multicopy, subtelomeric sequence was amplified as described previously (33); the assay was able to detect as few as 1.5 parasites per μl of whole blood.
Hemoglobin typing.
The hemoglobin variants HbS and HbC were detected using Acid Haemoglobin Titan Gel agarose electrophoresis kits (Helena Laboratories, Tyne and Wear, United Kingdom).
Malaria antigens for serology.
P. falciparum schizont extract was prepared from cultured erythrocytic parasites, clone 3D7 (34), by purification of mature, schizont-infected cells on a 60% Percoll (Pharmacia, Uppsala, Sweden) gradient. Schizonts were washed, pelleted, and lysed by two freeze-thaw cycles, and the supernatant was reserved.
MSP-119 was prepared as a glutathione S-transferase (GST) fusion protein from transfected Escherichia coli as described previously (9) and represents the Wellcome/K1 allelic sequence of P. falciparum MSP-119 (29). Purified E. coli-derived GST was used as a control.
Recombinant apical membrane antigen 1 (AMA-1), MSP-2, and Pf155 were produced as N-terminal hexa-His-tagged proteins in E. coli. The AMA-1 construct represents the full ectodomain of the 3D7 form of AMA-1; after purification by nickel chelate chromatography, it was refolded in vitro to generate the native disulfide-bonded conformation as described previously (6) and further purified by ion-exchange chromatography and reverse-phase high-performance liquid chromatography. Two allelic sequences of MSP-2, FC27 and IC1, representing the two major families of MSP-2, were used. Each form of recombinant MSP-2 corresponded to the mature full-length protein. Pf155 is a highly conserved antigen; the expressed sequence corresponded to the C-terminal 70% of the antigen from the FC27 isolate. The two forms of MSP-2 and recombinant Pf155 were also purified by nickel chelate chromatography followed by reverse-phase high-performance liquid chromatography and/or ion-exchange chromatography.
The CSP antigen was kindly provided by Sanjai Kumar (U.S. Navy Medical Research Institute, Rockville, Md.) and represents the full-length P. falciparum CS gene expressed in E. coli as a hexa-His fusion protein.
ELISA.
Plasma samples collected from mothers before delivery and from children at delivery or at 2 weeks of age were analyzed by enzyme-linked immunosorbent assays (ELISA). Microtiter plates (Immulon 4; Dynatech, Chantilly, Va.) were coated with optimal dilutions of antigen (determined by titration) in carbonate coating buffer (15 mM Na2CO3, 35 mM NaHCO3) and incubated overnight at 4°C. Plates were washed three times in phosphate-buffered saline (PBS) with 0.05% Tween 20 (Sigma, Poole, United Kingdom) (PBST), blocked with a 1% solution of fat-free milk powder in PBST for 3 h at room temperature, and washed again. One hundred microliters of plasma, diluted 1:1,000 (1:200 for anti-CSP antibody detection) with blocking buffer, was added to duplicate wells, incubated overnight at 4°C, and washed three times with PBST. Bound IgG was detected with 100 μl of rabbit anti-human IgG-horseradish peroxidase (Dako, High Wycombe, United Kingdom) (1:5,000 in PBST, 3 h at room temperature) per well; IgG subclasses were detected using highly subclass-specific, horseradish peroxidase-conjugated, monoclonal antibodies to human IgG1, IgG2, IgG3, and IgG4 (The Binding Site, Birmingham, United Kingdom). Plates were developed with o-phenylenediamine (Sigma) and H2O2, and optical density (OD) was read at 492 nm.
For GST fusion proteins, the OD for the GST control protein was subtracted from the OD for the fusion protein to provide a corrected OD. Control plasma samples from 40 nonimmune European donors were used to establish the normal range for background reactivity with each antigen, and the cutoff for a positive reaction was defined as the mean plus 2 standard deviations of values obtained with the control plasma.
Statistical methods.
In order to look for associations between malaria infection and the presence or absence of malaria-specific maternal antibodies, it was necessary that the period of follow-up be restricted to the period during which maternal antibodies persisted in a child's circulation. The duration of maternal antibodies varied between children and between antigens; however, as 75% of children became antibody negative for antibodies to crude P. falciparum schizont antigen by 22 weeks of age, we selected 20 weeks as the most appropriate follow-up period. Malaria infection was defined as the detection of any level of parasitemia by microscopy, a positive reaction in a P. falciparum-specific PCR, or a twofold increase in the ELISA OD for serum antibodies to P. falciparum schizont antigen in consecutive blood samples. Samples suspected of congenital infections (detected at delivery or at 2 weeks of age if the child was not seen at delivery) were excluded from the analysis. Prevalence of infection was analyzed as the rate of infection per follow-up visit; Poisson regression was used to analyze prevalence by controlling for baseline characteristics of the mother and child.
Maternal antibody was defined as the mean of the ODs for antibodies detected at delivery and at week 2; where one of these values was missing, the maternal antibody measurement was based on the one available value. Correlations between mother and child antibody levels were assessed by Spearman's rank correlation coefficient. Antibody levels were analyzed both as continuous and categorical variables in the Poisson regression model and examined for trend with prevalence of infection. Linear regression was used to assess the association between maternal antibody and baseline characteristics; Cox's proportional-hazards model was used to assess the association between maternal antibody and duration of antibody or time to first infection.
Statistical significance was defined as a P of ≤0.05, and all tests were two sided. Data analysis was conducted using STATA (release 6; Statacorp, College Station, Tex.).
RESULTS
One hundred ninety-five mother-child pairs were recruited into the study between April 1994 and April 1997, of which 156 infants were monitored beyond delivery and 148 were monitored beyond 2 weeks and from 143 of whom a plasma sample was collected within 2 weeks of birth. These 143 children form the basis of all subsequent analysis. One hundred nine of these (76%) were monitored for the entire first 6 months of life, and the remaining 34 made between two and five visits (of a possible six).
Baseline data for the 143 infants is shown in Table 1. Thirty-four percent of the children were born to mothers living in the lower half of the town, near the seashore, where malaria infection rates were generally higher than in the rest of the town (unpublished). Eight (6%) children weighed less than 2.5 kg at birth, which is the standard definition for low-birth-weight babies. Overall, 43 of 142 (31%) children tested carried at least one copy of the S or C sickle-cell hemoglobin alleles. None of the parameters measured at baseline were associated with risk of infection (Table 1).
TABLE 1.
Baseline characteristics of 143 pairs of mothers and neonates and their association with risk of malaria infection in the first 20 weeks of life
| Subject | Parameter | Median (range) or no. of subjects (%) | Association with prevalence of infection (P) |
|---|---|---|---|
| Mother | Age (yr) | 26 (15–45) | χ22 = 1.2 (0.56) |
| No. of previous pregnancies | 3 (0–11) | χ23 = 3.8 (0.29) | |
| No. of children alive | 2 (0–8) | χ25 = 5.5 (0.36) | |
| Child | Birth weight (kg) | 3.0 (2.0–4.2) | χ24 = 5.3 (0.26) |
| Coastal area of residencea | 42 (34) | χ21 = 0.9 (0.34) | |
| Male sex | 77 (54) | χ21 = 2.2 (0.14) | |
| Sickle-cell trait: | |||
| AA | 95 (69) | χ22 = 0.28 (0.87) | |
| AC | 16 (12) | χ22 = 0.28 (0.87) | |
| CC | 1 (1) | χ22 = 0.28 (0.87) | |
| AS | 24 (17) | χ22 = 0.28 (0.87) | |
| SS | 2 (1) | χ22 = 0.28 (0.87) | |
| Not known | 5 (4) | χ22 = 0.28 (0.87) |
Coastal indicates the lower half of the town, close to the seashore.
Age-related changes in prevalence of malaria infection and clinical malaria.
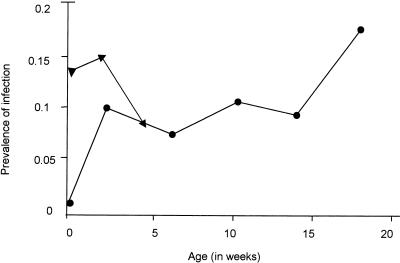
The prevalence of infections by age is shown in Fig. 1. The high initial prevalence of infection was assumed to be due to congenital infection. After these congenital infections were cleared, prevalence remained steady until about 18 weeks of age. This accords with previous data from this cohort, where the prevalence of infection was shown to increase significantly from 18 weeks of age onwards (33).
FIG. 1.
Rate of malaria infection by age. Rate of infections per 100 clinic visits, where infection is defined as a specimen that is blood film positive or PCR positive or as a twofold increase in OD for IgG to crude schizont antigen. ▴, includes congenital infections; ●, excludes congenital infections.
Infection was detected in between zero and four blood samples in the first 20 weeks of age in different infants. The prevalence of infection varied with season of birth, being higher in children born in the dry season (November to April) than in children born in the wet season (May to October) (Table 2). This difference was statistically significant (P = 0.03). This finding lends indirect support to the idea that children are relatively protected against malaria in the first few months of life, as children born in the dry season become vulnerable to infection a few months later when the risk of infection is highest while children born in the wet season become vulnerable to infection at the time when transmission is declining. The risk of congenital infection also varied significantly according to the time of year but was higher in the wet season than the dry season, with 33% of children born in May or June being congenitally infected compared with only 7% of children born between November and February (χ25 = 17.7, P = 0.003).
TABLE 2.
Prevalence of malaria infection varies by season of birth
| mo of birth | No. (%) of children | No. of clinic visits in first 20 wk of life | No. of infections in first 20 wk of life | Prevalencea | 95% CIb |
|---|---|---|---|---|---|
| January–February | 16 (12) | 91 | 13 | 143 | 82–246 |
| March–April | 15 (10) | 90 | 11 | 122 | 68–221 |
| May–June | 40 (28) | 238 | 19 | 80 | 51–125 |
| July–August | 22 (15) | 124 | 6 | 48 | 22–108 |
| September–October | 26 (18) | 146 | 11 | 75 | 42–136 |
| November–December | 24 (17) | 150 | 15 | 100 | 60–166 |
Prevalence is the infection rate per 1,000 clinic visits.
CI, confidence interval.
We have previously shown that, in children under 6 months of age, clinical malaria—defined as a febrile episode (temperature, ≥37.5°C) with parasitemia—can occur at parasite densities as low as 100 parasites/μl of blood (22). By this definition, there were two cases of clinical malaria (one child had a parasitemia level of 182 parasites/μl and a temperature of 37.5°C at 14 weeks of age, and another had 1,284 parasites/μl and a temperature of 38.1°C at 11 weeks of age). Fever was the only symptom reported for these children. There were five instances, with four different children, of asymptomatic parasitemia greater than 100 parasites/μl, with densities ranging from 102 to approximately 4,000 parasites/μl. Symptoms other than fever were reported with a frequency of between 0.4% (refusal to feed) and 7.5% (cough) of children observed, but none of these were associated with parasitemia.
Prevalence and levels of maternally acquired antibodies.
One hundred twelve of 127 (88%) mothers tested possessed antibodies to P. falciparum schizont extract, as determined by an OD greater than the cutoff value derived from negative control sera (Table 3). The prevalence of antibodies to defined malaria antigens ranged from 88% for AMA-1 to 46% for Pf155. Of the infants, 120 of 143 (84%) possessed antibodies to crude schizont antigen at birth, as determined by analysis of heel prick blood samples collected within 24 h of birth or by the age of 2 weeks. In neonates, the prevalence of antibodies to defined antigens ranged from 82% for AMA-1 to 28% for Pf155.
TABLE 3.
Prevalence and duration of IgG antibodies to malaria antigens in mother and child
| Antigen | No. of seropositive mothers/ total no. of mothers (%) | No. of seropositive neonates/total no. of neonates (%) | Median duration of maternal antibody (wk) (interquartile range) | Correlation (r) of ODs for mothers vs ODs for children (P) | Median OD for mothers | Median OD for neonates
|
||
|---|---|---|---|---|---|---|---|---|
| Weta | Dryb | P | ||||||
| P. falciparum schizont | 112/127 (88) | 120/143 (84) | 14 (6–22) | 0.71 (0.001) | 1.37 | 1.23 | 1.22 | 0.89 |
| Pf155 | 57/124 (46) | 40/142 (28) | 0 (0–0) | 0.77 (0.001) | 0.36 | 0.28 | 0.29 | 0.76 |
| AMA-1 | 108/123 (88) | 115/141 (82) | 10 (0–18) | 0.82 (0.001) | 1.67 | 1.62 | 1.35 | 0.06 |
| MSP-2–FC27 | 96/123 (78) | 84/141 (60) | 0 (0–6) | 0.68 (0.001) | 0.88 | 0.83 | 0.75 | 0.45 |
| MSP-2–IC1 | 104/123 (85) | 98/141 (70) | 4 (0–6) | 0.71 (0.001) | 1.46 | 1.00 | 1.18 | 0.13 |
| MSP-119 | 91/127 (72) | 96/143 (67) | 4 (0–14) | 0.84 (0.001) | 0.63 | 0.79 | 0.67 | 0.33 |
| CSP | 34/50 (68) | 23/64 (36) | 0 (0–0) | 0.18 (0.190) | 0.43 | 0.15 | 0.19 | 0.25 |
Median OD for children born in the wet season.
Median OD for children born in the dry season.
Antibody levels (expressed as OD values, or corrected OD values for MSP-119) in the children at birth are shown in Fig. 2. Although OD values for different antigens are not directly comparable, it is notable that median OD values for Pf155 and CSP are very low. This is especially true for CSP, as plasma samples were tested at a 1:200 dilution rather than at 1:1,000, which was used for all the other antigens.
FIG. 2.
Levels of IgG antibody to P. falciparum antigens in children at birth. Box, interquartile range; line through box, median; error bars, 95% confidence interval range; individual symbols, values lying outside the 95% range; Ag, antigen.
Correlations between OD values for mothers' and children's plasma samples (Table 3) were significant for all antigens with the exception of CSP, for which values for the children's plasma tended to be much lower than for the mothers', and far fewer infants than mothers were seropositive for CSP. An example of the correlation between mothers' and children's antibody levels is shown in Fig. 3a.
FIG. 3.
Maternally derived IgG antibodies to P. falciparum schizont antigen. (a) Comparison of OD values for IgG in mothers' sera during pregnancy (y axis) and children's sera at birth (x axis). (b) Comparison of rates of decay of maternally derived IgG in children with above- or below-median levels of antibody at birth. Proportions of children remaining antibody positive (y axis) over time (in weeks) (x axis) are shown. The difference between the two plots is statistically significant (Cox's proportional-hazards regression, P < 0.001).
Antibody responses to malaria antigens were assayed every 2 to 4 weeks. The duration of maternal antibody was defined as the last time point where OD values remained above the control cutoff, with samples from children in whom antibody levels rose as a result of an active immune response being excluded. Antibody duration varied according to the initial level of maternal antibody, persisting longer in those children who had high levels at birth (P < 0.001) (Fig. 3b). Maternal antibodies had disappeared from the majority of children by approximately 20 weeks of age.
Levels of maternally derived antibody were not significantly affected by a child's sickle-cell status or sex, the mother's age, or the season of birth (Table 3) but were significantly affected by the area of residence of the child. Children born in the lower part of the town (coastal area) had significantly higher levels of antibody to P. falciparum schizont antigen at birth than children born in the upper (inland) part of the town (mean ODs, 1.37 and 1.15, respectively; P = 0.03). Levels of antibodies to AMA-1 (OD, 1.66 versus 1.31; P = 0.02), MSP-2–FC27 (OD, 0.91 versus 0.70; P = 0.05), and MSP-2–3D7 (OD, 1.26 versus 1.00; P = 0.04) were also higher in children living in coastal than in inland areas.
Antibody subclass.
The subclass of maternally derived antibodies was determined by subclass-specific ELISA (Table 4) for all antigens except CSP, for which total IgG titers were so low that accurate determination of subclass was not possible with most sera. Plasma samples were categorized as positive or negative for a particular IgG subclass depending on whether OD values were higher than for European plasma. In some cases, samples with low levels of total IgG failed to give a positive response for any of the subclasses. Antibodies to the schizont extract tended to be of mixed subclasses, with IgG1, IgG3, and IgG4 being present in many sera and IgG2 being present in some samples; when present, IgG2 and IgG4 levels tended to be very low, with OD values only just above the cutoff defined by nonimmune sera. Antibodies to AMA-1, MSP-119, and Pf155 were also of various subclasses. As anticipated from previous studies (30, 31), IgG3 responses predominated for MSP-2–FC27 and MSP-2–IC1.
TABLE 4.
IgG subclass of maternally derived antibodies in neonatal plasma
| Antigen | No.a (%) of children who were seropositive for indicated subclass
|
|||
|---|---|---|---|---|
| IgG1 | IgG2 | IgG3 | IgG4 | |
| P. falciparum schizont | 69 (48) | 19 (13) | 74 (52) | 74 (52) |
| Pf155 | 8 (6) | 6 (4) | 13 (9) | 11 (8) |
| AMA-1 | 31 (22) | 6 (4) | 26 (18) | 20 (14) |
| MSP-2–FC27 | 2 (2) | 12 (8) | 46 (32) | 15 (10) |
| MSP-2–IC1 | 11 (8) | 3 (2) | 41 (29) | 15 (10) |
| MSP-119 | 21 (16) | 0 (0) | 21 (16) | 23 (16) |
The number of samples was 143 in all cases, except for MSP-119, where the number was 134.
Relationship between prevalence of infection and maternal antibody.
To determine whether maternally derived malaria-specific antibody confers protection against malaria infection in the first 5 months of life, we compared the levels of prevalence of infection in children with above- or below-median levels of IgG to each antigen at birth, adjusting for season of birth, area of residence, and other potential confounding factors (Table 5). There was no indication in these analyses of maternally derived antibodies having any significant protective effect against malaria infection. For IgG to crude schizont antigen, Pf155, CSP, and MSP-2–FC27, the relative risk was above 1.0, indicating that children with higher-than-average antibody levels were more likely to be infected in the first 5 months of life than children with lower-than-average antibody levels. For MSP-2–FC27, this increased risk was statistically significant. When the risk of infection was compared with the presence or absence of antibodies of the four different IgG subclasses for the six different antigens, there was, again, no indication of any significant protective effect of antibodies (Table 5). Of the 23 comparisons made, 4 comparisons were statistically significant (P ≤ 0.02); in each case, the relative risk was greater than 1.0, indicating that the presence of antibodies was associated with an increased risk of infection.
TABLE 5.
Relationship between rate of infection and presence of maternally derived antibodies to malaria antigensa
| Antigen | Total IgG
|
IgG1
|
IgG2
|
IgG3
|
IgG4
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| RRb | P | RRc | P | RRc | P | RRc | P | RRc | P | |
| P. falciparum schizont | 1.4 | 0.25 | 1.45 | 0.12 | 2.22 | 0.01 | 1.91 | 0.01 | 1.53 | 0.08 |
| Pf155 | 1.4 | 0.27 | 2.59 | 0.009 | 0.52 | 0.36 | 0.96 | 0.91 | 1.34 | 0.44 |
| AMA-1 | 1.0 | 0.90 | 1.05 | 0.88 | 0.38 | 0.34 | 0.84 | 0.61 | 1.02 | 0.97 |
| MSP-2–FC27 | 2.2 | 0.02 | 2.38 | 0.24 | 0.64 | 0.40 | 0.84 | 0.50 | 0.94 | 0.87 |
| MSP-2–IC1 | 0.86 | 0.62 | 0.74 | 0.54 | 0.46 | 0.44 | 0.82 | 0.46 | 1.15 | 0.70 |
| MSP-119 | 0.98 | 0.96 | 1.18 | 0.60 | — | — | 2.08 | 0.01 | 1.46 | 0.19 |
| CSP | 1.20 | 0.77 | NT | NT | NT | NT | ||||
Statistically significant values are highlighted in bold type. The number of samples was 143 in all cases, except with IgG subclasses to MSP-119, where the number was 134. —, no samples were positive for IgG2 subclass antibody; NT, samples not tested.
RR, rate ratio of infection in children with above-median antibody levels (OD values) compared with those with below-median antibody levels, adjusted for month of birth, mother's previous pregnancies, number of siblings alive, sickle-cell genotype, and area of residence.
RR, rate ratio of infection in children seropositive for an IgG subclass (defined as an OD of at least the mean plus standard deviations of values for nonimmune control sera) compared with that in children who were seronegative, adjusted for month of birth.
To be certain that we had not missed any protective effects of antibody, we reanalyzed the data using IgG levels as either a continuous variable or as five categories of increasing OD value (data not shown). Antibody levels were also compared with times to first infection, using survival analysis (data not shown). There was no indication in any of these analyses that maternally derived antibodies have any significant protective effect against malaria infection. When blood film-positive infections were considered a separate group, representing children with levels of parasitemia greater than 40 asexual stage parasites/μl of blood, a similar pattern was seen. The only statistically significant associations were for MSP-2–FC27 (children with higher-than-average levels of IgG to MSP-2–FC27 were 4.8 times more likely to be blood film positive than children with lower-than-average IgG [P = 0.001]) and for CSP (in a trend analysis, increasing levels of anti-CSP IgG were significantly associated with a markedly increased risk of infection [rate ratio of infection = 25.6, P < 0.001]). Finally, levels of maternal antibodies among children presenting with high levels of parasitemia (>100 parasites/μl) or among those who experienced a clinical malaria infection were not significantly different from levels shown by the cohort as a whole (data not shown). Four of the five children with high parasitemia had lower-than-average levels of antibody to MSP-119 at birth, and all five children (including the two who experienced clinical episodes of malaria) were seronegative for MSP-119 at the time their high-density or clinical infections were diagnosed; however, the number of children with high-density infections was too small for us to detect any significant difference in the mean OD for this group compared to that for the other 138 children (Student's t test result = 0.63, df = 141, P = 0.53).
DISCUSSION
It is clear from many epidemiological studies that neonates, and infants under the age of 3 to 6 months, are significantly protected from the severe consequences of malaria infection (7, 17, 22, 33), but the data on susceptibility to malaria infection per se are less clear. This longitudinal cohort study of malaria infection, as well as clinical malaria, reveals several interesting points. First, we have already shown that the risk of becoming infected with malaria increased significantly at about the age of 18 weeks, indicating that children under the age of 18 weeks had a lower risk of becoming infected than children above that age (33). This argues against the notion, proposed by Macdonald, that infants become colonized by malaria at a steady rate and that their apparent protection is simply due to inadequate exposure (19). Our ability to pick up this change in risk, which Macdonald could not see in his studies, may be linked to the use of a sensitive PCR assay for detecting very low-density parasitemias (11, 33). Second, children born during the wet (high-transmission) season had a significantly lower risk of becoming infected in the first 20 weeks of life than did children born in the dry (low-transmission) season; this can be explained only if children have some level of innate protection at birth that gradually wanes, leaving them vulnerable to infection some weeks or months later. Third, and perhaps surprisingly, the vast majority of malaria infections in children under 5 months of age are of very low density and completely asymptomatic.
Among the reasons frequently cited to explain the resistance to high parasitemia, clinical malaria, and severe disease in infants is the protective role of maternally derived antibodies. However, in a review of passively acquired resistance to malaria in infants, Brabin concluded that there was in fact “evidence for the lack of efficacy of passively acquired maternal antibody in reducing susceptibility (in infants)” but that “longitudinal studies under seasonal conditions would be required to interpret how infection risk alters in relation to passive immunity” (7). In this longitudinal study of the relationship between levels, antigen specificity, and IgG subclass of maternally derived antibodies to malaria and risk of malaria infection, we find no convincing evidence for any role for antimalarial antibodies in protection of neonates or infants from malaria infection. On the contrary, where significant associations were seen between antibody levels or antibody prevalence and risk of infection, infection risk was always higher in children with higher levels of antimalarial antibody. These few significant associations must, however, be regarded with caution due to the number of tests performed during this analysis. Nevertheless, these data confirm the results of a previous, interim, analysis of this cohort (33) and suggest that maternally derived antibody levels are in fact a marker for risk of infection rather than of protective immunity. These findings agree with those of a previous study in Tanzania, where levels of antibodies to CSP and to two fragments of MSP-1 were not found to be associated with protection against parasitemia (18).
The failure to detect a protective effect of maternal antibodies was not due to lack of statistical power in the study. The study had 80% power to detect a 40 to 50% reduction in malaria risk, i.e., rate ratios of 0.5 to 0.6, in children with maternal antibody levels above the median. In fact, the overwhelming trend was for rate ratios to be close to, or greater than, unity; the study was large enough to detect several instances of significantly increased rate ratios. The only examples of rate ratios below 0.6 were for the prevalence of IgG2 antibodies to recombinant antigens; however, the prevalence of IgG2 antibodies was very low and these differences were not statistically significant.
The lack of a protective effect of maternal antibodies was not due to either poor efficiency of transfer of antibodies across the placenta or selective transfer of certain IgG subclasses. Antibody levels of all subclasses and against all antigens, with the possible exception of anti-CSP antibodies, in the children's sera at birth were highly correlated with levels in mothers' sera, and median OD values were comparable.
Immunoepidemiological studies of older children and adults have suggested that serum antibody levels are a poor indicator of protective immunity, particularly when antibodies to crude blood-stage antigens are measured (20, 25). It seems likely that high levels of circulating antimalarial antibodies are indicative of boosting by recent parasitemia. This notion is supported in the present study by the finding that both the risk of infection and mean antimalarial antibody levels were higher in children living in the low-lying, humid area of the village, close to the seashore, than in children living in the higher, drier, and better-drained area of the village, where mosquito numbers tend to be lower.
Despite the fact that total antimalarial antibody levels do not correlate with resistance to infection, antibodies to some defined malaria antigens—including MSP-119, Pf155, and MSP-2—have been shown to be associated with resistance to clinical malaria in older children (3–5, 12, 26, 27, 30), yet we find no such association with resistance to infection in this study of neonates and infants. This difference may well be due to the fact that previous studies looked for resistance to symptomatic malaria rather than to malaria infection per se. Thus, it may be that maternally derived antibodies are able to protect infants from high parasitemia and clinical malaria but do not actually prevent infection. In this study, only five of the children experienced infections with densities greater than 100 parasites/μl in the first 20 weeks of life. These children had levels of total antimalarial antibodies, and antibodies to CSP, MSP-2, AMA-1, and Pf155, that were comparable with those in children who were either not infected or who developed only low-density infections. Similarly, previous studies in Nigeria (1) and in Liberia (16) have not shown any association between resistance to clinical malaria in infants and levels of antibodies to crude malaria antigens, CSP, or Pf155. However, four of the five children who developed high parasitemias in the first 20 weeks of life, including both of the children who experienced a clinical malaria infection, had below-average levels of antibodies to MSP-119 at birth (two of the five were seronegative for MSP-119 at birth) and all five children had become seronegative for MSP-119 by the time they experienced these high-density or clinical infections. Thus, although these numbers are small and not quite statistically significant, the study provides some, albeit weak, evidence that antibodies to MSP-119 may protect children against high levels of parasitemia or clinical disease. Two previous studies have shown associations between the presence of antibodies to MSP-119 at birth and protection against clinical malaria over the first year of life (8, 16), although the interpretation of these findings is complicated by the fact that maternal antibodies were unlikely to have persisted for the whole period of follow-up.
Despite the lack of any clear effect of antibodies in preventing malaria infection, it is obvious that many children were infected with malaria but were able to prevent rapid parasite growth and the development of clinical malaria. We cannot tell, from the data available, whether this restraint of parasite growth was due to control of parasite replication by antibody or to some other protective mechanism, although we have observed cases of seronegative children being persistently, subclinically infected with malaria (S. Franks et al. submitted for publication), indicating that parasite growth can be effectively controlled in the absence of antibody. There are numerous nonimmunological mechanisms that may contribute to control of malaria parasitemia in infants. Parasites have been shown to grow less well in erythrocytes containing fetal hemoglobin (24), and a breast milk diet may lack all the essential nutrients for parasite growth (15) or may contain inhibitory cytokines such as transforming growth factor β (23). Neonatal liver cells appear to be innately resistant to sporozoite invasion (L. Rénia, personal communication), and innate immune mechanisms such as opsonization by serum lectins (13) may lead to parasite clearance. Whatever the mechanism, it is clear that neonates are refractory to the development of high-density malaria parasitemias; all of these potential protective mechanisms are worthy of further study in order to identify new strategies for controlling malaria infections in older children and adults.
ACKNOWLEDGMENTS
We thank Sofia Hyder, Philippa Shield, Mallika Kaviratne, Salad Mohamud, Abdul-Rahman Hammond, Enid Owusu, and Ben Gyan for technical assistance and Allan Saul for providing 7H8/6 primers.
This study was funded by the Wellcome Trust (grant 040328); S.B. is funded by the United Kingdom Medical Research Council (grant G7508177).
REFERENCES
- 1.Achidi E A, Salimonu L S, Perlmann H, Perlmann P, Berzins K, Williams A I O. Lack of association between levels of transplacentally acquired Plasmodium falciparum-specific antibodies and age of onset of clinical malaria in infants in a malaria endemic area of Nigeria. Acta Trop. 1996;61:315–326. doi: 10.1016/0001-706x(96)00014-9. [DOI] [PubMed] [Google Scholar]
- 2.Afari E A, Appawu M A, Dunyo S, Baffoe-Wilmot A B A, Nkrumah F K. Malaria infection, morbidity and transmission in two ecological zones in Southern Ghana. Afr J Health Sci. 1995;2:312–316. [PubMed] [Google Scholar]
- 3.Al-Yaman F, Genton B, Anders R F, Falk M, Triglia T, Lewis D, Hii J, Beck H-P, Alpers M P. Relationship between humoral response to Plasmodium falciparum merozoite surface antigen-2 and malaria morbidity in a highly endemic area of Papua New Guinea. Am J Trop Med Hyg. 1994;51:593–602. doi: 10.4269/ajtmh.1994.51.593. [DOI] [PubMed] [Google Scholar]
- 4.Al-Yaman F, Genton B, Anders R, Taraika J, Ginny M, Mellor S, Alpers M P. Assessment of the role of the humoral response to Plasmodium falciparum MSP2 compared to RESA and SPf66 in protecting Papua New Guinean children from clinical malaria. Parasite Immunol. 1995;17:493–501. doi: 10.1111/j.1365-3024.1995.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 5.Al-Yaman F, Genton B, Kramer K J, Chang S P, Hui G S N, Baisor M, Alpers M P. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am J Trop Med Hyg. 1996;54:443–448. doi: 10.4269/ajtmh.1996.54.443. [DOI] [PubMed] [Google Scholar]
- 6.Anders R, Crewther P, Edwards S, Margetts M, Matthew M, Pllock B, Pye D. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine. 1998;16:240–247. doi: 10.1016/s0264-410x(97)88331-4. [DOI] [PubMed] [Google Scholar]
- 7.Brabin B. An analysis of malaria parasite rates in infants: 40 years after Macdonald. Trop Dis Bull. 1990;87:R1–R21. [PubMed] [Google Scholar]
- 8.Branch O H, Udhayakumar V, Hightower A W, Oloo A J, Hawley W, Nahlen B L, Bloland P B, Kaslow D C, Lal A A. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19 kilodalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitaemia and anaemia. Am J Trop Med Hyg. 1998;58:211–219. doi: 10.4269/ajtmh.1998.58.211. [DOI] [PubMed] [Google Scholar]
- 9.Burghaus P A, Holder A A. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol Biochem Parasitol. 1994;64:165–169. doi: 10.1016/0166-6851(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 10.Campbell C C, Martinez J M, Collins W E. Seroepidemiological studies of malaria in pregnant women and newborns from coastal El Salvador. Am J Trop Med Hyg. 1980;29:151–157. doi: 10.4269/ajtmh.1980.29.151. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Q, Lawrence G, Reed C, Stowers A, Ranford-Cartwright L, Creasey A, Carter R, Saul A. Measurement of Plasmodium falciparum growth rate in vivo: a test of malaria vaccines. Am J Trop Med Hyg. 1997;57:495–500. doi: 10.4269/ajtmh.1997.57.495. [DOI] [PubMed] [Google Scholar]
- 12.Egan A F, Morris J, Barnish G, Allen S, Greenwood B M, Kaslow D C, Holder A A, Riley E M. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19kDa C-terminal fragment of the merozoite surface antigen, PfMSP1. J Infect Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 13.Epstein J, Eichbaum Q, Sheriff S, Ezekowitz R A B. The collectins in innate immunity. Curr Opin Immunol. 1996;8:29–35. doi: 10.1016/s0952-7915(96)80101-4. [DOI] [PubMed] [Google Scholar]
- 14.Foley M, Ranford-Cartwright L C, Babiker H A. Rapid and simple method for isolating malaria DNA from fingerprick samples of blood. Mol Biochem Parasitol. 1992;53:241–244. doi: 10.1016/0166-6851(92)90026-g. [DOI] [PubMed] [Google Scholar]
- 15.Hawking F. Milk diet, p-aminobenzoic acid and malaria (P. berghei): preliminary communication. Br Med J. 1953;1:1201–1202. doi: 10.1136/bmj.1.4821.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogh B, Marbiah N T, Burghaus P A, Andersen P K. Relationship between maternally derived anti-Plasmodium falciparum antibodies and risk of infection and disease in infants living in an area of Liberia, West Africa, in which malaria is highly endemic. Infect Immun. 1995;63:4034–4038. doi: 10.1128/iai.63.10.4034-4038.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitua A, Smith T, Alonso P, Masanja H, Urassa H, Menendez C, Kimario J, Tanner M. Plasmodium falciparum malaria in the first year of life in an area of intense and perennial transmission. Trop Med Int Health. 1996;1:475–484. doi: 10.1046/j.1365-3156.1996.d01-89.x. [DOI] [PubMed] [Google Scholar]
- 18.Kitua A Y, Urassa H, Wechsler M, Smith T, Vounatsou P, Weiss N A, Alonso P L, Tanner M. Antibodies against Plasmodium falciparum vaccine candidates in infants in an area of intense and perennial transmission: relationships with clinical malaria and with entomological inoculation rates. Parasite Immunol. 1999;21:307–317. doi: 10.1046/j.1365-3024.1999.00230.x. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald G. The analysis of malaria parasite rates in infants. Trop Dis Bull. 1950;47:915–938. [PubMed] [Google Scholar]
- 20.Marsh K, Otoo L, Hayes R J, Carson D C, Greenwood B M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 21.McGregor I A. The development and maintenance of immunity to malaria in highly endemic areas. Clinics Trop Med Commun Dis. 1986;1:1–29. [Google Scholar]
- 22.McGuinness D, Koram K, Bennett S, Wagner G, Nkrumah F K, Riley E. Clinical case definitions for malaria: clinical malaria associated with very low parasite densities in African infants. Trans R Soc Trop Med Hyg. 1998;92:527–531. doi: 10.1016/s0035-9203(98)90902-6. [DOI] [PubMed] [Google Scholar]
- 23.Omer F M, Kurtzhals J A L, Riley E. Maintaining the immunological balance in parasitic infections: a role for TGFb? Parasitol Today. 1999;16:18–23. doi: 10.1016/s0169-4758(99)01562-8. [DOI] [PubMed] [Google Scholar]
- 24.Pasvol G, Weatherall D J, Wilson R J M. Effects of foetal haemoglobin on susceptibility of red cells to Plasmodium falciparum. Nature. 1977;270:171–173. doi: 10.1038/270171a0. [DOI] [PubMed] [Google Scholar]
- 25.Riley E M, Allen S J, Bennett S, Thomas P J, Andersson G, O'Donnell A, Lindsay S W, Good M F, Greenwood B M. Recognition of dominant T cell stimulating epitopes from the circumsporozoite protein of Plasmodium falciparum and relationship to malaria morbidity in Gambian children. Trans R Soc Trop Med Hyg. 1990;84:648–657. doi: 10.1016/0035-9203(90)90133-y. [DOI] [PubMed] [Google Scholar]
- 26.Riley E M, Allen S J, Troye-Blomberg M, Bennett S, Perlmann H, Andersson G, Smedman L, Perlmann P, Greenwood B M. Immune recognition of the malaria vaccine candidate antigen Pf155/RESA is associated with resistance to clinical disease: a prospective study in a malaria endemic region of West Africa. Trans R Soc Trop Med Hyg. 1991;85:436–443. doi: 10.1016/0035-9203(91)90207-f. [DOI] [PubMed] [Google Scholar]
- 27.Riley E M, Allen S J, Wheeler J, Blackman M J, Bennett S, Takacs B, Shönfeld H-J, Holder A A, Greenwood B M. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–338. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 28.Sehgal V M, Siddiqui W A, Alpers M P. A seroepidemiological study to evaluate the role of passive maternal immunity to malaria in infants. Trans R Soc Trop Med Hyg. 1989;83(Suppl.):105–106. doi: 10.1016/0035-9203(89)90616-0. [DOI] [PubMed] [Google Scholar]
- 29.Tanabe K, MacKay M, Goman M, Scaife J. Allelic dimorphism in a surface antigen gene of the malaria parasite isolated from Plasmodium falciparum merozoites. J Mol Biol. 1987;195:273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- 30.Taylor R R, Allen S J, Greenwood B M, Riley E. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg. 1998;58:406–413. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- 31.Taylor R R, Smith D B, Robinson V J, McBride J S, Riley E M. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect Immun. 1995;63:4382–4388. doi: 10.1128/iai.63.11.4382-4388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trape J-F, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, Legros F, Badji A, Ndiaye G, Ndiaye P, Brahimi K, Faye O, Druilhe P, Pereira da Silva L. The Dielmo Project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–137. doi: 10.4269/ajtmh.1994.51.123. [DOI] [PubMed] [Google Scholar]
- 33.Wagner G, McGuinness D, Koram K, Bennett S, Nkrumah F K, Riley E. High incidence of asymptomatic malaria infections in a birth cohort of children under 1 year of age in Ghana, detected by multicopy gene polymerase chain reaction. Am J Trop Med Hyg. 1998;59:115–123. doi: 10.4269/ajtmh.1998.59.115. [DOI] [PubMed] [Google Scholar]
- 34.Walliker D, Quakyi I A, Wellems T E, McCutchan T F, Szarfman A, London W T, Corcoran L M, Burkot T R, Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]