Abstract
Background and Hypothesis
Cognitive remediation (CR) benefits cognition and functioning in psychosis but we do not know the optimal level of therapist contact, so we evaluated the potential benefits of different CR modes.
Study Design
A multi-arm, multi-center, single-blinded, adaptive trial of therapist-supported CR. Participants from 11 NHS early intervention psychosis services were independently randomized to Independent, Group, One-to-One, or Treatment-as-usual (TAU). The primary outcome was functional recovery (Goal Attainment Scale [GAS]) at 15-weeks post randomization. Independent and TAU arms were closed after an interim analysis, and three informative contrasts tested (Group vs One-to-One, Independent vs TAU, Group + One-to-One vs TAU). Health economic analyses considered the cost per Quality Adjusted Life Year (QALY). All analyses used intention-to-treat principles.
Study Results
We analyzed 377 participants (65 Independent, 134 Group, 112 One-to-One, 66 TAU). GAS did not differ for Group vs One-to-One: Cohen’s d: 0.07, −0.25 to 0.40 95% CI, P = .655; Independent vs TAU: Cohen’s d: 0.07, −0.41 to 0.55 95% CI, P = .777. GAS and the cognitive score improved for Group + One-to-One vs TAU favoring CR (GAS: Cohen’s d: 0.57, 0.19–0.96 95% CI, P = .003; Cognitive score: Cohens d: 0.28, 0.07–0.48 95% CI, P = .008). The QALY costs were £4306 for Group vs TAU and £3170 for One-to-One vs TAU. Adverse events did not differ between treatment methods and no serious adverse events were related to treatment.
Conclusions
Both active therapist methods provided cost-effective treatment benefiting functional recovery in early psychosis and should be adopted within services. Some individuals benefited more than others so needs further investigation.
Trial registration
ISRCTN14678860 https://doi.org/10.1186/ISRCTN14678860Now closed.
Keywords: therapist support, early intervention, goal achievement, functioning, cognitive training
Introduction
Cognitive function is the strongest predictor of social and occupational functioning 4 years later1–3 and limits opportunities offered by evidence-based rehabilitation.4 Cognitive remediation (CR) was developed using the simple model that boosting cognition benefits functioning. Although studies show only partial mediation, meta-analyses have shown durable benefits of CR5–9 and some national guidelines now recommend it.10–13 The CR White Paper14 highlighted four effective elements: cognitive exercise, developing problem-solving strategies, an active therapist, and facilitating transfer to real-world functioning. A recent meta-analysis demonstrated that CRs with all these elements improved cognitive and functioning benefits.9 One programme has all elements, Cognitive Interactive Remediation of Cognition and Thinking Skills or “CIRCuiTS”, and uniquely facilitates the link between cognitive functioning and everyday life by incorporating metacognitive training into the software and therapy interactions.15–17
Service users have positive views about CR therapists,18,19 and they facilitate therapeutic benefit,8,9 but the White Paper does not define an “active therapist”. Therapist access provided either in a group or one-to-one is usual, but a CR embedding the three other key elements (cognitive exercise, strategy training, and transfer to real-life functioning) might reduce the importance of a therapist.
This study was designed to identify how much therapist time would provide an efficient and cost-effective CR service within UK NHS Early Intervention Services (EIS) to inform implementation. We tested 3 widely used therapy modalities with different therapist involvement.16,20–23 EIS was chosen as an early benefit that may alleviate future problems and improve life opportunities. Although we know CR is effective in EIS,24–27 the chosen methods have different costs, and the balance between costs and outcomes is important for large-scale roll-out. EIS has comprehensive case management that includes regular contact with a care coordinator, medication management, psychiatric consultation, crisis management, physical health assessment, and psychological therapies so it offers a stringent test of the extra CR benefit. CR trials assess functional outcomes using self-report, clinician observation, or tests of functional capacity.28 We consulted clinical staff and service users about the outcomes that would persuade them that CR was worth investing in and they said it was whether CR helped patients to attain their personal goals. We therefore, chose a valid and psychometrically sound functional outcome scale that is sensitive to change in clinical trials and has greater face validity than global measures (Goal Attainment Scale [GAS]29,30). This choice has the benefit of capturing the heterogeneous personal goals and aspirations of EIS patients with some wishing to return to education, others aiming to start employment or wanting more social activities.
Methods
Study Design
A 4-arm multi-center, single-blinded, adaptive, randomized controlled trial comparing 3 CR implementation methods compared to treatment-as-usual (TAU) in people presenting with non-affective psychosis in UK NHS EIS. Outcomes were measured at weeks 0, 15, and 39. Treatment was provided independently (at home with phone contact and drop-in clinics), in groups or one-to-one within a 12-week time window. Camden and Kings Cross NHS Research Ethics Committee (ref. number 15/LO/1960) provided a favorable review. An Independent Data Monitoring Committee (IDMC) oversaw study progression, adverse events, and statistical analyses.
Study Sample
The inclusion criteria were: EIS care for at least 3 months, clinical stability judged by the clinical team, 16–45 years, a research diagnosis of non-affective psychosis assessed by the MINI.31 Exclusion criteria were communication difficulties in completing assessments, an organic condition affecting cognition, a learning disability or a definitive bipolar disorder diagnosis. Six sites (North London; South London; Cambridge; Warwick; Sussex; and Birmingham—see Supplementary) ensured a wide-ranging community backdrop of urbanicity and ethnicity.
Randomization and Masking
Consented participants were initially randomized in blocks of 15, stratified by the site in proportions 4:4:3:4 (Group: Independent: One-to-One: TAU) using a concealed sequence on an independent web-based King’s Clinical Trials Unit system following baseline assessment. We changed following slow recruitment to allow 11–15 participant blocks and later individual randomization with equal allocation, first to 4 and then 2 arms (Group, One-to-One) following an interim analysis. The outcome assessors, trial manager, and investigators were blind to the trial arm, including the senior trial statistician until primary analysis completion.
Intervention
The therapist-supported CR computerized CR CIRCuiTS programme was used. It was co-developed with service users and therapists15,32 and is based on cognitive practice, strategy use and metacognition engagement, a pedagogical factor that allows skill transfer to other situations. Cognitive tasks and exercises (modeling community skills such as traveling or texting) are graduated with movement to higher levels depending on performance. Therapists encourage participants to regulate and monitor their cognitive performance through improved metacognitive awareness using strategies and cognitive skills learned through the programme16 (see Supplementary p5s–6s). Therapy plans are based on the participant’s goals to facilitate therapeutic engagement. Treatment arms differed in therapist contact hours (see Supplementary p6s for detail). The arms were:
One-to-One (a single participant) receives 10.5 weeks of twice weekly therapy, up to 42 h in total, with sessions lasting 60 to 180 min, split into 3 parts: (1) 20–60 min of CR with a therapist; (2) 20–60 min of in vivo transfer work (ie, putting CR strategies into real life); (3) 20–60 min of independent CR, with (2) and (3) depending on the stage of independence
Group (max 4 participants) receives 14 weeks of 3 times weekly CR with a single shared therapist. Sessions last up to 90 min and begin and end with group activities related to goal setting and metacognition.
Independent participants receive one therapist session for orientation and up to 41 independent sessions. Therapists offer telephone contact or drop-in sessions on an as-needed basis not exceeding 1 h contact time per fortnight.
The therapy window was constrained to 12 weeks and missing sessions were not replaced. Therapists were trained graduate-level psychologists (25–30 h training for up to 12 weeks). They delivered all 3 treatment arms and were supervised weekly by an experienced clinical psychologist. Trial participants also received TAU (comprehensive case management).
Outcomes
The primary outcome was self-reported personal recovery goals at 15-weeks post randomization, measured in a structured way with the GAS weighted T-score;33–35 following a recent review.36 GAS is sensitive to change, has been the primary outcome for both pharmacological and psychological interventions in psychiatric disorders, and measures cognitive rehabilitation outcomes37 including CR.38 A baseline participant interview to establish up to 3 goals weighted on importance and difficulty following the scoring manual. Secondary outcomes were: Social and Occupational Functioning (SOFAS39); Total hours in structured activity (Time Use Survey;40); Negative symptoms (CAINS total score;41,42); a composite cognitive score (CANTAB tests:43 attention switching, paired visual information processing, reaction time, one touch stocking (testing spatial planning and working memory), spatial working memory, paired associate learning), as well as the Rey auditory verbal learning task, Wisconsin card sorting test, and the digit span task from the Wechsler Adult Intelligence Scale (see Supplementary p3s-4s); Self-esteem total score (Rosenberg Self Esteem Scale44); We also collected context information including socio-demographic information and symptoms (Positive and Negative Symptom Scale, PANSS45). Cost-effectiveness was estimated with both Quality Adjusted Life Years (QALYs46) derived from the EuroQOL-5D-5L and the use of services from the Client Service Receipt Inventory47 (see Supplementary for further detail).
Adverse Events
Adverse and serious events (see Supplementary for definitions) were reported to the trial clinician who assessed their importance and association with the trial and sent a report to the chair of the IDMC for final categorization.
Service User Involvement
Patient involvement is associated with study success48 and so we consulted people with experience of using mental health services at every trial stage, including the study question, primary outcome, design, protocol wording, information sheet, and consent form, in addition to consulting clinicians and carers. We ran focus groups to develop study leaflets to address the sensitive issue of explaining cognitive difficulties. We continued to involve service users as advisors (Patient Advisory Board) who were also critical reviewers, with some being authors of this publication.
Statistical Analysis
We analyzed the characteristics of people finally entering the trial and compared them descriptively with those from large observational studies of EIS to understand whether the sample was representative of those attending EIS.
Statistical Power
Following the interim analysis, power was recalculated using an expected 438 participants (see Supplementary p7s for initial calculation). Conventional 2-tailed significance, with 80% endpoint and follow-up data, a plausible correlation structure (0.5 correlation between follow-up measures, 0.2 between baseline and follow-up), but making no allowance for clustering, produced 79% power for an effect size of 0.3 for the Group vs One-to-One arm comparison. Since the focus of therapy was narrowly based on CR activity, both therapist and group interaction variance were expected to be negligible. Site, as a randomization stratifier, was included within the analyses.
An interim intention-to-treat (ITT) analysis, overseen by the IDMC, considered closing trial arms based on: (1) Treatment engagement: > 50% of individuals receiving less than 5 therapy hours, (2) Cost-effectiveness: > £500 for a one-point increase in cognition or one hour of structured activity (a reasonable cost adopted from a previous trial49), (3) Participant satisfaction: <25% of participants satisfied with therapy. Designed for 175 participants, it was carried out early with 100, to maximize power for informative contrasts.
Primary Analysis.
ITT analyses included all randomized participants, irrespective of the amount of therapy received, using Stata version 15. IDMC recommended three informative contrasts for future clinical service design prioritized to minimize false positive results. The sequence was: (1) determine any difference in the therapist-supported arms (Group vs One-to-One) and if no difference then arms are combined, (2) test whether independent CR improved the primary outcome (Independent vs TAU), and finally (3) a comparison of the combined therapist arms with TAU to consider the overall treatment effect. These pre-specified analyses (see Supplementary statistical analysis plan) were applied to the primary and all secondary outcomes with no adjustment for multiple contrasts. The contrasts specified show whether one of the therapist-supported modes was likely to be more beneficial and whether the least expensive treatment (contrast 2) could also be considered.
A linear mixed model estimated the mean weighted GAS T-score difference between arms at 15 weeks. Independent variables were treatment arm, time (post therapy or follow-up), time by treatment arm interaction, recruitment period (pre- or post-interim analysis), baseline GAS T-score, recruitment site, and a random patient-specific intercept. A dummy indicator for baseline missingness50 was included as a covariate. Standardized effect sizes were reported using a standard deviation of 10 in the GAS scoring guide.
Sensitivity analyses assessed, by simulation, the effect of closing arms following the interim analysis, under 2 scenarios (1) no treatment difference and (2) naïve effect estimates for the primary outcome. Additional analyses examined: (1) assumed lack of clustering by site/group, (2) missing at random assumption, and (3) effect of noncompliance to visit windows (see Supplementary).
An additional analysis estimated the effect of receiving treatment (CR hours) on the primary outcome. As those who received more CR were likely to be different from those who did little, we used random treatment assignment as an instrumental variable51 and assumed a common effect per hour of CR across the 3 active treatment arms. Estimated using the sem command in Stata, the model included site and baseline GAS as covariates in both stages of the instrumental variable regression to estimate the effect of an hour of CR.
Secondary Analysis.
These mirrored the primary analyses, but standardized effect sizes (Cohen’s d) were calculated by dividing mean differences by the pooled baseline sample standard deviation. The number of hours of structured activity was first log-transformed.
Health Economic Analyses
The health economic analyses followed standard procedures. EuroQOL-5D-5L ratings were converted to an EQ-5D-3L tariff using the established crosswalk method.52 QALYs were calculated using the area under the curve methods and were compared between groups while controlling for baseline utility.53 Unit costs were based on the Personal Social Services Research Unit 2020 costs and NHS Improvement 2018–2019 reference costs.46,54 As cost data are skewed, the analysis used non-parametric bootstrapping (1000 replications) to generate 95% confidence intervals around the mean differences in costs and outcomes between the groups at each time point. For the main analysis, NHS/PSS costs and QALYs were adjusted for baseline costs/baseline EQ5D-3L scores, trial arm, site, and period. The secondary analyses were the same except included a dummy indicator for baseline missingness.50 The 3 cost-effectiveness analyses were Group CR vs TAU, One-to-One CR vs TAU; and Group CR vs One-to-One with QALYs as the primary and GAS scores as the secondary outcome and included all randomized participants. Decisions about cost-effectiveness are based on cost-effectiveness acceptability curves (CEAC) with the key issue being how likely a treatment is to be cost-effective not standard significant differences between arms.
Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
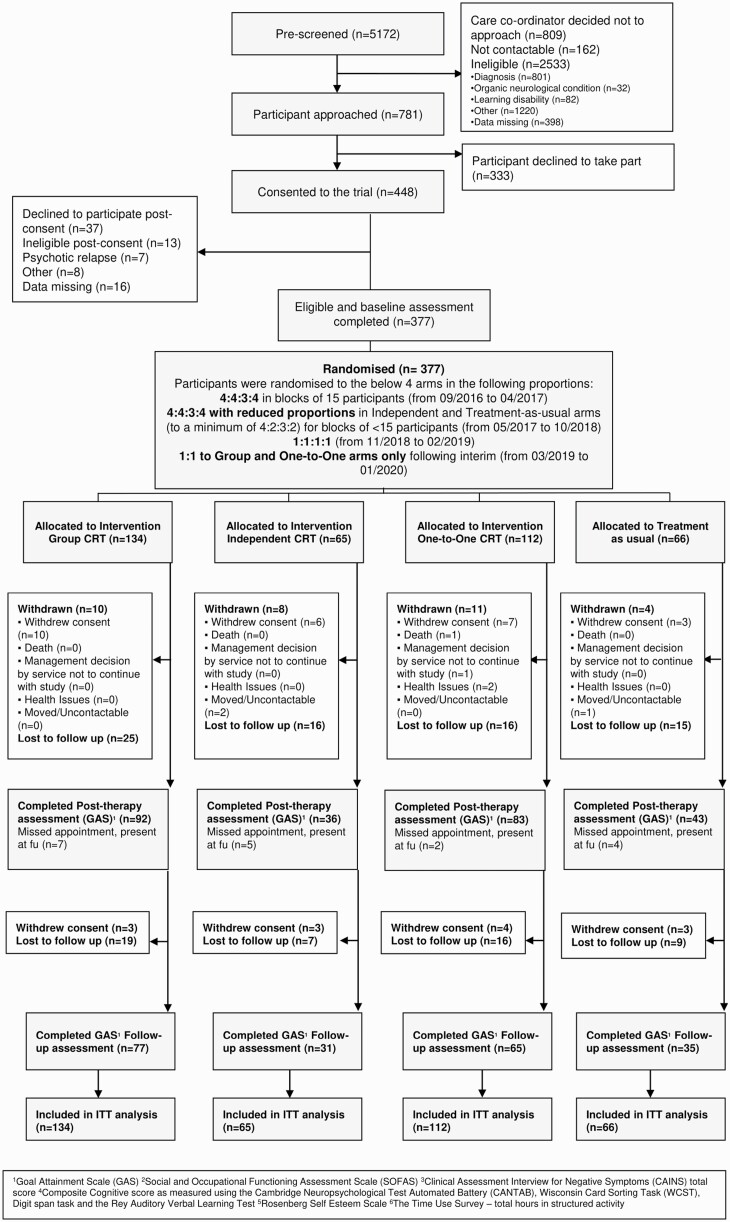
The Consort Flow diagram (figure 1) shows 448 eligible participants; 71 were excluded before randomization, leaving 377 participants (65 Independent, 134 Group, 112 One-to-One, 66 TAU). All those with at least one post therapy or follow-up GAS score were included in the primary analysis (Independent-41, Group-99, One-to-One-85, TAU-47).
Fig. 1.
Consort flow chart.
The first randomization was on September 14, 2016 and last on January 9, 2020 with the last assessment on October 26, 2020. The Independent arm failed the engagement criterion (50% received less than 5 h) and was the poorest for cost-effectiveness in the interim analysis. The IDMC recommended dropping it and TAU was also dropped because CR had been added to UK NICE guidance.
The sample was predominantly male with a mean age of 26 years. Most were single and largely unemployed. Symptom scores (PANSS and CAINS) were in line with those presenting to EIS.55,56 Their pre morbid IQ, current IQ, and duration of untreated psychosis were almost identical to previous large UK EIS studies.57,58 Our study sample showed a decrease from pre morbid (98.09) to current IQ (88.18) which is almost identical to changes noted in UK EI services57 (pre morbid IQ 95.78, current IQ 88.17,59 mean current IQ 84.16, pre morbid IQ 95.82), but also from an international sample of people with schizophrenia60 (pre morbid IQ 95.82, current IQ 84.16).
Baseline socio-demographic and clinical data (see table 1) were balanced across treatment arms as expected from randomization. Nine hundred and sixty-four GAS personal goals were identified at baseline, falling into seven categories (Daily Life Skills N285, Employment N107, Education N97, Health and Wellbeing N133, Relationships N133, Recreation/Hobbies N206 and Other eg, spiritual N3: see Supplementary table 1s for examples). The median dose of antipsychotic medication (converted to chlorpromazine equivalents following Leucht et al61) was 200 mg post therapy, with 31% of participants reducing and 18% increasing their total dose during the trial (Supplementary table 2s).
Table 1.
Baseline Characteristics
| Group (n = 134) |
Independent (n = 65) |
One-to-One (n = 112) | TAU (n = 66) |
All Participants (n = 377) | |
|---|---|---|---|---|---|
| Age at consent Mean (SD) | 25.19 (5.91) | 25.92 (5.56) | 26.39 (6.72) | 25.14 (5.55) | 25.67 (6.05) |
| Sex Female N (%) | 44 (32.8%) | 18 (27.7%) | 29 (25.9%) | 11 (16.7%) | 102 (27.1%) |
| Ethnicity White N (%) | 59 (44.0%) | 32 (49.2%) | 57 (50.9%) | 37 (56.1%) | 185 (49.1%) |
| Employment status Full-time education or employed N (%) |
47 (35.0%) | 18 (27.7%) | 35 (31.2%) | 23 (34.8%) | 123 (32.6%) |
| Living situation Own property (private, rented) or parental home N (%) |
119 (88.9%) | 53 (81.6%) | 92 (82.2%) | 57 (86.3%) | 321 (85.2%) |
| Relationship status Single N (%) | 120 (89.6%) | 54 (83.1%) | 98 (87.5%) | 59 (89.4%) | 331 (87.8%) |
| WTAR Standard score Mean (SD) | 99.19 (17.05) | 97.19 (17.55) | 99.00 (16.80) | 95.22 (18.22) | 98.09 (17.27) |
| WASI II Estimate IQ (FSIQ) score Mean (SD) | 88.81 (15.24) | 88.51 (18.95) | 87.79 (16.98) | 87.23 (17.72) | 88.18 (16.82) |
| PANSS Total score Mean (SD) | 55.24 (14.19) | 59.66 (19.18) | 57.35 (16.45) | 55.64 (14.14) | 56.70 (15.84) |
| CAINS Total score Mean (SD) | 17.42 (9.31) | 18.65 (9.68) | 18.62 (9.56) | 17.25 (8.44) | 17.95 (9.29) |
| Antipsychotic dosage (converted to chlorpromazine equivalents) Median (Upper–Lower quartiles) | 200 (100–301) | 240 (120–350) | 300·00 (150–450) | 180 (59–300) | 200 (100–370) |
Which treatment arms provide the most benefit?
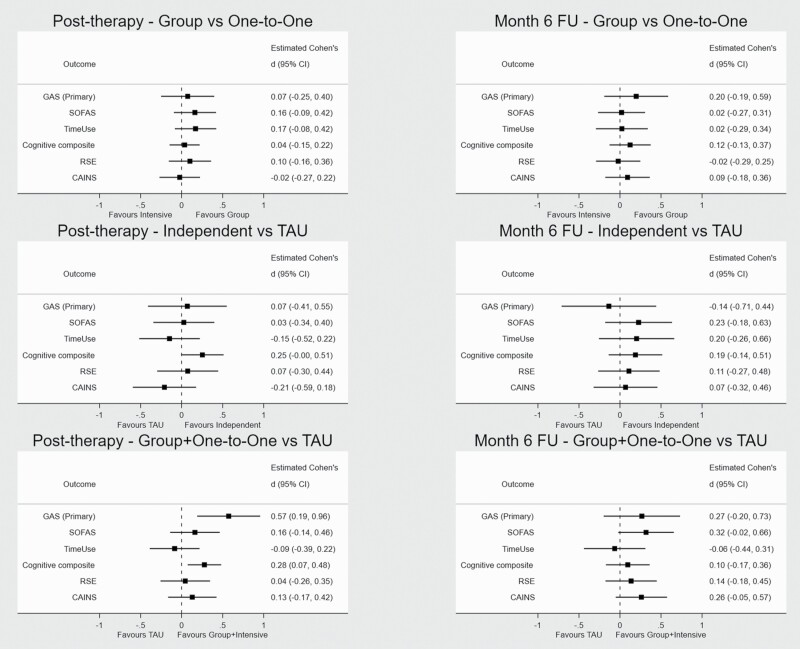
Supplementary tables 7s–16s provide summary information for all primary and secondary outcomes. Table 2 shows the results for the primary and secondary outcomes using the pre-specified contrasts at post therapy and at 6-month (post therapy) follow-up. Higher scores indicate a better outcome, except for the CAINS. Forest plots show standardized effects for primary and secondary outcomes for each contrast (figure 2). There was no difference between Group and One-to-One arm, or between TAU and the Independent arm for the GAS-T score. The pooled Group + One-to-One arms showed more benefit than TAU at post therapy (mean pooled GAS T-scores 5.7 points higher, 1.9–9.6 95% CI, Cohen’s d 0.57, 0.19–0.96 95% CI, P = .003).
Table 2.
Primary and Secondary Outcome Results
| Outcome | Contrast | Post therapy | 6-month (post therapy) Follow-up | ||||
|---|---|---|---|---|---|---|---|
| Estimated Mean Difference | P-value | 95% CI | Estimated Mean Difference | P-value | 95% CI | ||
| GAS T-score1 | Group vs One-to-One | 0.737 | .655 | −2.500, 3.975 | 1.975 | .319 | −1.913, 5.863 |
| Independent vs TAU | 0.695 | .777 | −4.104, 5.493 | −1.353 | .645 | −7.112, 4.407 | |
| Group + One-to-One vs TAU | 5.734 | .003 | 1.898, 9·571 | 2.665 | .262 | −1.988, 7.319 | |
| Global cognition composite score2 | Group vs One-to-One | 0.192 | .699 | −0.785, 1.170 | 0.659 | .333 | −0.675, 1.992 |
| Independent vs TAU | 1.348 | .054 | −0.026, 2.722 | 1.005 | .257 | −0.733, 2.744 | |
| Group + One-to-One vs TAU | 1.479 | .008 | 0.395, 2.564 | 0.507 | .484 | −0.912, 1.926 | |
| SOFAS score | Group vs One-to-One | 2.395 | .217 | −1.405, 6.194 | 0.287 | .894 | −3.952, 4.526 |
| Independent vs TAU | 0.400 | .887 | −5.100, 5.901 | 3.363 | .274 | −2.657, 9.384 | |
| Group + One-to-One vs TAU | 2.397 | .293 | −2.073, 6.867 | 4.701 | .066 | −0.314, 9.716 | |
| Time Use (hours per week) | Group vs One-to-One | 0.103 | .188 | −0.051, 0·257 | 0.014 | .887 | −0.179, 0.207 |
| Independent vs TAU | −0.090 | .433 | −0.315, 0.135 | 0.123 | .387 | −0.156, 0.403 | |
| Group + One-to-One vs TAU | −0.052 | .581 | −0.237, 0.133 | −0.039 | .735 | −0.267, 0.189 | |
| CAINS score | Group vs One-to-One | 0.218 | .852 | −2.072, 2.508 | −0.855 | .505 | −3.368, 1.658 |
| Independent vs TAU | 1.945 | .287 | −1.633, 5.523 | −0.630 | .732 | −4.240, 2.981 | |
| Group + One-to-One vs TAU | −1.205 | .389 | −3.946, 1.536 | −2.422 | .102 | −5.327, 0.483 | |
| RSE score | Group vs One-to-One | 0.571 | .440 | −0.879, 2.021 | −0.122 | .875 | −1.643, 1.398 |
| Independent vs TAU | 0.415 | .698 | −1.680, 2.510 | 0.614 | .568 | −1.496, 2.725 | |
| Group + One-to-One vs TAU | 0.249 | .774 | −1.452, 1.949 | 0.766 | .393 | −0.992, 2.524 | |
Note: Results in bold indicate treatment estimates that were statistically significant using a P < .05 threshold.
GAS, Goal Attainment Scale; SOFAS, Social and Occupational Functioning Assessment Scale; CAINS, Clinical Assessment Interview for Negative Symptoms; CANTAB, Cambridge Neuropsychological Test Automated Battery; RSE, Rosenberg Self Esteem Scale.
1GAS T-score calculated using formula with wi = the weight assigned to the ith goal; a product of participants perceived goal importance (rated 1–3) and difficulty (1–3) xi = the numerical rating achieved for the ith goal (between –2 and + 2) and ρ = 0.3 as recommended by the GAS guide. The score was calculated optimally for 3 goals but also for 1 or 2.
2The global cognition composite score includes CANTAB tests (Attention switching, Rapid visual information processing continuous performance, Simple and 5 choice reaction time, “One touch Stockings of Cambridge” Test of Planning, Spatial Working Memory) and the Rey Auditory Verbal Learning Test (RAVLT), Wisconsin Card Sorting Task (WCST) and Wechsler Adult Intelligence Scale (WAIS) Digit span. Some components were reverse scored and/or transformed to be approximately normally distributed. Z-scores were calculated, and these were then trimmed, to 3 or −3 before summing to give a composite score.
Fig. 2.
Forest plots for primary and secondary results at each of the contrasts for the 3-month post therapy and 6-month follow-up time points.
A preliminary homogeneity test indicated no difference in GAS benefit per hour of CR by delivery method. The instrumental variable analysis (ignoring method of delivery) estimated that the endpoint GAS T-score increased by 2.81 (0.90–4.71 95% CI, P = .004) post therapy or a 0.28 effect size (Cohens d, 0.09–0.47 95% CI) for each 10 h of CR. This reduces to 1.73 (−0.29 to 3.76 95% CI, Cohens d 0.17, −0.03 to 0.38 95% CI, P = .093) at follow-up.
The cognition composite score for the Group + One-to-One vs TAU comparison at post therapy also showed a benefit, with a small to medium-sized effect (mean increase of 1.48 points, 0.40–2.56 95% CI, Cohen’s d 0.28, 0.07–0.48 95% CI, P = .008).
Safety Assessment
Ninety-five adverse events (AE) and 59 serious adverse events (SAEs) were reported. Two AEs were related to the intervention (hearing voices from the CR computer; sending abusive texts to the therapist about compensation) but no SAE was trial related. SAEs were: 2 deaths, 55 mental state deteriorations requiring urgent assessment, and 2 medical hospital admissions (Supplementary tables 5s and 6s). AEs and SAEs were relatively balanced across trial arms with an average of 0.16 SAEs per participant (range 0.10–0.19).
Adherence:
Therapy dropout was considered as less than 6 sessions, and although many received 1 therapy session (92.6%), a substantial number failed the 6-session threshold (45% Independent, 37.6% Group, 21.6% One-to-One). When dropouts were removed there was little difference between arms in the proportion receiving a 20-session minimum dose defined in the White Paper (42.9% Independent, 48.2% Group, and 47.1% One-to-One, see Supplementary table 4s).
Sensitivity analyses demonstrated little to no bias introduced by dropping arms or other potential sources of bias (see Supplementary tables 19s–22s and figure 1s).
Resource use, EQ5D-3L and GAS scores for the health economic analyses are in Supplementary tables 23s and 24s. Table 3 shows the NHS costs for each arm at each timepoint and the complete case analysis including the outcomes (QALYs; GAS score), costs (NHS PSS perspective), and cost-effectiveness of Group vs One-to-One, Group vs TAU and One-to-One vs TAU).
Table 3.
Total Costs (NHS Perspective) and Complete Case Analysis: NHS PSS Costs, Outcome, and Cost-Effectiveness Results
| Total costs (NHS perspective) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables: costs (£) |
Group | Independent | One-to-One | TAU | ||||
| N | Mean (sd) | N | Mean (sd) | N | Mean (sd) | N | Mean (sd) | |
| Intervention | 133 | 266 (989) | 63 | 24 (11) | 111 | 740 (375) | 66 | 0 (0) |
| Baseline: NHS PSS costs1 | 134 | 5542 (12 274) | 64 | 5095 (10 139) | 110 | 4943 (10 749) | 66 | 3365 (6648) |
| Post Therapy: NHS PSS costs1 | 104 | 839 (1984) | 48 | 1433 (3199) | 92 | 1070 (2866) | 54 | 1875 (5818) |
| Post Therapy: NHS PSS costs1 + Intervention Costs | 104 | 1125 (1975) | 48 | 1456 (3201) | 92 | 1854 (2838) | 54 | 1875 (5818) |
| Follow-up: NHS PSS costs1 | 89 | 2201 (6689) | 45 | 2391 (4980) | 82 | 1686 (4305) | 51 | 2928 (7770) |
| Complete case analysis: NHS PSS costs, QALY outcome, and cost-effectiveness results | ||||||||
| Group vs One-to-One | (n = 64) | (n = 58) | Incremental cost/QALY gained (95% CI)2 | |||||
| NHS PSS costs1 | 2960 (6626) | 2564 (2487) | 150 (−1132 to 1905) | |||||
| QALYs3 | 0.5998 (0.1059) | 0.5601 (0.1661) | 0.0057 (−0.023 to 0.0392) | |||||
| NHS/PSS perspective: costs (£) per QALY gain (ICER)4 | 26 383 | |||||||
| Group vs TAU | (n = 64) | (n = 30) | Incremental cost/QALY gained (95% CI)2 | |||||
| NHS PSS costs1 | 2960 (6626) | 2724 (4974) | 257 (−1694 to 2615) | |||||
| QALYs3 | 0.5998 (0.1059) | 0.5046 (0.1954) | 0.0597 (0.0122–0.1005) | |||||
| NHS/PSS perspective: costs (£) per QALY gain (ICER)4 | 4306 | |||||||
| One-to-One vs TAU | (n = 58) | (n = 30) | Incremental cost/QALY gained (95% CI)2 | |||||
| NHS PSS costs1 | 2564 (2487) | 2724 (4974) | 260 (−1654 to 2239) | |||||
| QALYs3 | 0.5601 (0.1661) | 0.5046 (0.1954) | 0.0821 (0.0421–0.1219) | |||||
| NHS/PSS perspective: costs (£) per QALY gain (ICER)4 | 3170 | |||||||
| Complete case analysis: NHS PSS costs, GAS Score outcome, and cost-effectiveness results | ||||||||
| Group vs One-to-One | (n = 70) | (n = 62) | Incremental cost/GAS score unit gained2 | |||||
| NHS PSS costs1 | 3012 (6478) | 2578 (2582) | 195 (−1026 to 1751) | |||||
| GAS Score | 54.54 (11.91) | 53.09 (11.01) | 1.59 (−2.26 to 5.17) | |||||
| NHS/PSS perspective: costs (£) per additional unit on the GAS (ICER)4 | 123 | |||||||
| Group vs TAU | (n = 70) | (n = 32) | Incremental cost/GAS score unit gained2 | |||||
| NHS PSS costs1 | 3012 (6478) | 2916 (5088) | 328 (−1770 to 2870) | |||||
| GAS Score | 54.54 (11.91) | 51.12 (16.28) | 4·29 (−1.93 to 10.36) | |||||
| NHS/PSS perspective: costs (£) per additional unit on the GAS (ICER)4 | 76 | |||||||
| One-to-One vs TAU | (n = 62) | (n = 32) | Incremental cost/GAS score unit gained2 | |||||
| NHS PSS costs1 | 2578 (2582) | 2916 (5088) | 157 (−1556 to 2155) | |||||
| GAS Score | 53.09 (11.01) | 51.12 (16.28) | 2.33 (−3.91 to 9.18) | |||||
| NHS/PSS perspective: costs (£) per additional unit on the GAS (ICER)4 | 67 | |||||||
The costs per QALY for Group or One-to-One vs TAU were £4306 and £3170, respectively. There was uncertainty around the results (ie, the probability of cost-effectiveness at £20 000 per QALY (National Institute for Health and Care Excellence (NICE)62), shown in CEAC (ICER; see Supplementary figure 3s). The ICER for Group vs One-to-One was £26 383 per QALY gained. Despite less cost for the group intervention, this large difference is mainly accounted for by inpatient and other costs in the group arm.
Discussion
Cognitive remediation provided either in a Group or One-to-One was more beneficial at post treatment than TAU, but the benefits were reduced at follow-up. There were few differences between these CR methods in terms of costs or cost-effectiveness, and the treatment costs (therapist time) in comparison to the overall health service costs. Both types of provision are therefore recommended. The interventions may entail extra initial investment compared to usual care. However, the health economic results show that overall costs do not differ much from usual care due to cost offsets elsewhere in the system. This is crucial to consider when investigating potentially expensive interventions. QALYs were significantly higher compared to usual care and the probability of either being more cost-effective than usual care was high.
Anecdotal evidence while we were recruiting suggested that the group option was less popular, and there were more people who dropped out of this condition suggesting that more encouragement might be needed to engage in this intervention method. After removing dropouts there were few adherence differences or meeting the minimal 20-session dose between group and one-to-one. This suggests that, once people were engaged, group treatment was equally acceptable (Supplementary table 4s). The choice should therefore be made by the patient themselves if both are offered.
Fewer therapy hours may have contributed to the poor results in the independent arm as they received fewer sessions and hours of therapy than the other CR arms (Supplementary table 4s). As suggested in the White Paper and in meta-analyses8,9,14 encouragement by therapists may help adherence and the transfer of gains to functional outcomes. More formal therapist input was associated with treatment adherence, and this clearly affects therapeutic benefit. Therapy hours were constrained by our 12-week intervention window as sessions missed were not reinstated which might explain the lower number of therapy hours compared to other studies.
While the cost and QALY differences were not statistically significant between the treatment arms, the approach used in the economic analyses focuses on the probability that one intervention is more cost-effective than another. Here we found that both Group and One-to-One had a high probability of being more cost-effective than TAU, and the corresponding ICERs were below the lower threshold used by UK NICE (£20 000) for the adoption of a new NHS therapy. The ICER for Group CR vs One-to-one was substantially higher although below the upper threshold of £30 000.
The CR benefits wane, and although present, are no longer statistically significant at 6-month follow-up. We embedded the transfer of skills to real-world activities within the CIRCuiTS software with exercises for using a bus, cooking, or shopping as well as homework activities to aid transfer. However, COVID-19 might have had an impact on outcomes for a minority as opportunities to fulfill some GAS-defined recovery goals were unavailable when social distancing and lockdown were implemented in the United Kingdom. We had 25 post therapy (17 Group, 11 One-to-One) and 40 (18 Group and 22 One-to-One) follow-up assessments that occurred after the beginning of the pandemic (March 16, 2020). These results are therefore likely to be the minimum rather than the maximum of what might have been achieved. In addition we only included a handover note to the local EIS team on CR outcomes, and perhaps if we had included joint sessions with other healthcare professionals (like the Thinking Skills for Work programme63) or provided intermittent sessions over the follow-up (as suggested by our Patient Advisory Board), then the benefits might have been maintained.
Strengths and Limitations
This is the largest study of CR in people with a diagnosis of first-episode non-affective psychosis. Individuals were excluded at the prescreen or screening stages that might have affected confidence in the results, although the sample characteristics were remarkably similar to those of previous observational studies. As this study considered the therapist contact needed for wider implementation we chose a personalized measure of outcome—the GAS—favored by clinical staff and service users to record an individual’s functional recovery targets. This outcome requires rater consistency, although not more so than symptom measures such as PANSS. To ensure consistency we trained and supervised all these ratings that were made blind to group allocation. Missing outcome data could be a potential bias, but our broad sensitivity analyses suggest that our results are robust. While early slow recruitment required changes to randomization, all recruits were randomly allocated and balanced in characteristics suggesting no bias was introduced. We carried out the study in Early Intervention Services which have access to a wide range of recovery services and so the results may not be applicable to those in longer-stay services although this was a stringent test of any additional therapy added to case management.
Conclusions
The results suggest that providing therapist-led Group and One-to-One CR can improve the prospects for personal functional recovery in early psychosis, and both types of provision are cost-effective. Therapists seem to increase adherence which then increases the CR benefits. Future studies should investigate whether patient characteristics can inform the choice of group or one-to-one therapy.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Acknowledgments
TW and AP were part funded by the National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London (IS-BRC-1215-20018) and their NIHR Senior Investigator Awards (NF-SI-0514-10023 and NF-SI-0617-10120). EJ was part funded by the NIHR University College London Hospitals Biomedical Research Centre. NIHR Applied Research Collaborations (ARC) supported MB (West Midlands), JP (East of England) DF (Kent, Surrey, and Sussex). We would like to thank the Cantab team for their generosity in allowing us to use this online software.
Contributor Information
Til Wykes, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; South London and Maudsley NHS Foundation Trust, London, UK.
Dominic Stringer, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Janette Boadu, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Rose Tinch-Taylor, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Emese Csipke, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Matteo Cella, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; South London and Maudsley NHS Foundation Trust, London, UK.
Andrew Pickles, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Paul McCrone, School of Health Sciences, University of Greenwich, London, UK.
Clare Reeder, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Max Birchwood, Warwick Medical School, University of Warwick, Coventry, UK.
David Fowler, School of Psychology, University of Sussex, Brighton, UK.
Kathryn Greenwood, School of Psychology, University of Sussex, Brighton, UK.
Sonia Johnson, Faculty of Brain Sciences, University College London, London, UK.
Jesus Perez, Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, UK.
Rosa Ritunnano, Warwick Medical School, University of Warwick, Coventry, UK.
Andrew Thompson, Warwick Medical School, University of Warwick, Coventry, UK.
Rachel Upthegrove, School of Psychology, University of Birmingham, Birmingham, UK.
Jon Wilson, Norfolk and Suffolk NHS Foundation Trust, Norwich, UK.
Alex Kenny, Patient Advisory Board, King’s College London, London, UK.
Iris Isok, Patient Advisory Board, King’s College London, London, UK.
Eileen M Joyce, UCL Queen Square Institute of Neurology, University College London, London, UK.
Funding
This study was funded by an NIHR Applied Programme grant (RP-PG-0612-20002)
Conflict of Interest Disclosures
Prof Wykes and Dr Reeder developed the cognitive remediation software, CIRCuiTS, used in this study. No other authors have any conflict of interest to report.
Funding/Support: This study is funded by the National Institute for Health Research (NIHR) Programme grant for Applied Research (NIHR-PGfAR RP-PG-0612-20002).
Author contributions: Wykes, Joyce, Pickles, and McCrone had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Wykes, Joyce, Pickles, and McCrone.
Drafting the manuscript: Wykes, Joyce, Pickles, McCrone, Boadu, Stringer, Tinch-Taylor, Cella, and Csipke.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical and health economic analysis: Pickles, Stringer, Tinch-Taylor, McCrone, and Boadu.
Obtained funding: Wykes, Joyce, Pickles, McCrone, Reeder, Birchwood, Fowler, Johnson, and Perez.
Administrative, technical, or material support: KCL CTU.
Supervision: Wykes and Joyce.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See supplement.
Additional Contributions: We would like to thank all the research assistants and therapists who helped us to recruit and treat participants in this trial. We would also like to acknowledge Tiyi Morris who carried out the interim health economic analysis and Andrew Watson, Tjasa Velikonja and Leena Subramanian who provided coordination at various times. The UK Clinical Research Collaboration-registered King’s Clinical Trials Unit at King’s Health Partners, which is part funded by the NIHR Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King’s College London and the NIHR Evaluation, Trials and Studies Coordinating Centre supported our randomization. Most importantly, vital support was provided by our Patient Advisory Board and the independent Data Monitoring Committee who both had input into each stage of the study.
References
- 1. Leeson VC, Harrison I, Ron MA, Barnes TR, Joyce EM.. The effect of cannabis use and cognitive reserve on age at onset and psychosis outcomes in first-episode schizophrenia. Schizophr Bull. 2012;38(4):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wykes T, Dunn G.. Cognitive deficit and the prediction of rehabilitation success in a chronic psychiatric group. Psychol Med. 1992;22(2):389–398. [DOI] [PubMed] [Google Scholar]
- 3. Wykes T, Katz R, Sturt E, Hemsley D.. Abnormalities of response processing in a chronic psychiatric group: a possible predictor of failure in rehabilitation programmes? Br J Psychiatry. 1992;160(2):244–252. [DOI] [PubMed] [Google Scholar]
- 4. Wykes T. Predicting symptomatic and behavioural outcomes of community care. Br J Psychiatry. 1994;165(4):486–492. [DOI] [PubMed] [Google Scholar]
- 5. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P.. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. [DOI] [PubMed] [Google Scholar]
- 6. Grynszpan O, Perbal S, Pelissolo A, et al. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychol Med. 2011;41(1):163–173. [DOI] [PubMed] [Google Scholar]
- 7. Cella M, Price T, Corboy H, Onwumere J, Shergill S, Preti A.. Cognitive remediation for inpatients with psychosis: a systematic review and meta-analysis. Psychol Med. 2020;50(7):1062–1076. [DOI] [PubMed] [Google Scholar]
- 8. Kambeitz-Ilankovic L, Betz LT, Dominke C, et al. Multi-outcome meta-analysis (MOMA) of cognitive remediation in schizophrenia: revisiting the relevance of human coaching and elucidating interplay between multiple outcomes. Neurosci Biobehav Rev. 2019;107:828–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vita A, Barlati S, Ceraso A, et al. Effectiveness, core elements, and moderators of response of cognitive remediation for schizophrenia: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2021;78(8):848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institute for Health and Care Excellence. Rehabilitation for Adults with Complex Psychosis NICE Guideline; 2020. Published online August 19, 2020. https://wwwniceorguk/guidance/ng181/resources/rehabilitationfor-adults-with-complex-psychosis-pdf-66142016643013. Accessed 1 November, 2022.
- 11. Scottish Intercollegiate Guidelines Network. Management of Schizophrenia: A National Clinical Guideline; 2013. http://wwwsignacuk/sign-131-management-of-schizophreniahtml. Accessed 1 November, 2022.
- 12. American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia; 2020. https://wwwpsychiatryorg/psychiatrists/practice/clinical-practice-guidelines. Accessed 1 November, 2022.
- 13. Ärzteblatt D. Schizophrenia—Clinical Practice Guideline ; 2020. https://wwwaerzteblattde/int/archive/article/214278. Accessed 1 November, 2022.
- 14. Bowie CR, Bell MD, Fiszdon JM, et al. Cognitive remediation for schizophrenia: an expert working group white paper on core techniques. Schizophr Res. 2020;215:49–53. [DOI] [PubMed] [Google Scholar]
- 15. Reeder C, Pile V, Crawford P, et al. The feasibility and acceptability to service users of CIRCuiTS, a computerized cognitive remediation therapy programme for schizophrenia. Behav Cogn Psychother. 2016;44(3):288–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reeder C, Huddy V, Cella M, et al. A new generation computerised metacognitive cognitive remediation programme for schizophrenia (CIRCuiTS): a randomised controlled trial. Psychol Med. 2017;47(15):2720–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drake R, Day C, Picucci R, et al. A naturalistic, randomized, controlled trial combining cognitive remediation with cognitive-behavioural therapy after first-episode non-affective psychosis. Psychol Med; 2014;44(9):1889–1899. [DOI] [PubMed] [Google Scholar]
- 18. Rose D, Wykes T, Farrier D, Doran A-M, Sporle T, Bogner D.. What do clients think of cognitive remediation therapy?: a consumer-led investigation of satisfaction and side effects. Am J Psychiatr Rehabil. 2008;11(2):181–204. [Google Scholar]
- 19. Contreras NA, Lee S, Tan EJ, Castle DJ, Rossell SL.. How is cognitive remediation training perceived by people with schizophrenia? A qualitative study examining personal experiences. J Ment Health. 2016;25(3):260–266. [DOI] [PubMed] [Google Scholar]
- 20. Twamley EW, Thomas KR, Burton CZ, et al. Compensatory cognitive training for people with severe mental illnesses in supported employment: a randomized controlled trial. Schizophr Res. 2019;203:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loewy R, Fisher M, Schlosser D, et al. Improved cognition and positive symptoms with targeted auditory processing training in recent-onset schizophrenia. Paper presented at: Early Intervention in Psychiatry, 2016. [Google Scholar]
- 22. Donohoe G, Dillon R, Hargreaves A, et al. Effectiveness of a low support, remotely accessible, cognitive remediation training programme for chronic psychosis: cognitive, functional and cortical outcomes from a single blind randomised controlled trial. Psychol Med. 2018;48(5):751–764. [DOI] [PubMed] [Google Scholar]
- 23. Cella M, Reeder C, Wykes T.. Group cognitive remediation for schizophrenia: exploring the role of therapist support and metacognition. Psychol Psychother. 2016;89(1):1–14. [DOI] [PubMed] [Google Scholar]
- 24. Nuechterlein KH, Ventura J, Subotnik KL, et al. A randomized controlled trial of cognitive remediation and long-acting injectable risperidone after a first episode of schizophrenia: improving cognition and work/school functioning. Psychol Med. 2022;52(8):1517–1526. [DOI] [PubMed] [Google Scholar]
- 25. Vidarsdottir OG, Roberts DL, Twamley EW, Gudmundsdottir B, Sigurdsson E, Magnusdottir BB.. Integrative cognitive remediation for early psychosis: results from a randomized controlled trial. Psychiatry Res. 2019;273:690–698. [DOI] [PubMed] [Google Scholar]
- 26. Bellani M, Ricciardi C, Rossetti MG, Zovetti N, Perlini C, Brambilla P.. Cognitive remediation in schizophrenia: the earlier the better? Epidemiol Psychiatr Sci. 2020;29:E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loewy R, Fisher M, Ma S, et al. Durable cognitive gains and symptom improvement are observed in individuals with recent-onset schizophrenia 6 months after a randomized trial of auditory training completed remotely. Schizophr Bull. 2022;48(1):262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lejeune JA, Northrop A, Kurtz MM.. A meta-analysis of cognitive remediation for schizophrenia: efficacy and the role of participant and treatment factors. Schizophr Bull. 2021;47(4):997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Swildens W, van Busschbach JT, Michon H, et al. Effectively working on rehabilitation goals: 24-month outcome of a randomized controlled trial of the Boston psychiatric rehabilitation approach. Can J Psychiatry. 2011;56(12):751–760. [DOI] [PubMed] [Google Scholar]
- 30. Sanches SA, Swildens WE, Schaefer B, et al. Effectiveness of the Boston University approach to psychiatric rehabilitation in improving social participation in people with severe mental illnesses: a randomized controlled trial. Front Psychiatry. 2020;11:571640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. [PubMed] [Google Scholar]
- 32. Medalia A, Revheim N.. Computer assisted learning in psychiatric rehabilitation. Psychiatr Rehabil Ski. 1999;3(1):77–98. [Google Scholar]
- 33. Kiresuk TJ, Smith A, Cardillo JE.. Goal Attainment Scaling: Applications, Theory, and Measurement. Hillsdale, NJ, England: Lawrence Erlbaum, Inc.; 1994. [Google Scholar]
- 34. Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil. 2009;23(4):362–370. [DOI] [PubMed] [Google Scholar]
- 35. Kiresuk TJ, Sherman RE.. Goal attainment scaling: a general method for evaluating comprehensive community mental health programs. Community Ment Health J. 1968;4(6):443–453. [DOI] [PubMed] [Google Scholar]
- 36. Logan B, Jegatheesan D, Viecelli A, Pascoe E, Hubbard R.. Goal attainment scaling as an outcome measure for randomised controlled trials: a scoping review. BMJ Open. 2022;12(7):e063061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rockwood K, Joyce B, Stolee P.. Use of goal attainment scaling in measuring clinically important change in cognitive rehabilitation patients. J Clin Epidemiol. 1997;50(5):581–588. [DOI] [PubMed] [Google Scholar]
- 38. Strawbridge R, Tsapekos D, Hodsoll J, et al. Cognitive remediation therapy for patients with bipolar disorder: a randomised proof-of-concept trial. Bipolar Disord. 2021;23(2):196–208. [DOI] [PubMed] [Google Scholar]
- 39. Morosini PL, Magliano L, Brambilla La, Ugolini S, Pioli R.. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social funtioning. Acta Psychiatr Scand. 2000;101(4):323–329. [PubMed] [Google Scholar]
- 40. Lader D, Short S, Gershuny J.. The Time Use Survey, 2005. London: Office for National Statistics; 2006. [Google Scholar]
- 41. Forbes C, Blanchard JJ, Bennett M, Horan WP, Kring A, Gur R.. Initial development and preliminary validation of a new negative symptom measure: the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res. 2010;124(1-3):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blanchard JJ, Gur RE, Horan WP, Kring AM.. Manual for the Clinical Assessment Interview for Negative Symptoms (CAINS). Berkeley, USA: CANSAS Collaborative Group; 2012. [Google Scholar]
- 43. Barnett JH, Robbins TW, Leeson VC, Sahakian BJ, Joyce EM, Blackwell AD.. Assessing cognitive function in clinical trials of schizophrenia. Neurosci Biobehav Rev. 2010;34(8):1161–1177. [DOI] [PubMed] [Google Scholar]
- 44. Rosenberg M. Society and the Adolescent Self-Image. Revised edn with a New Introduction. Middletown: Wesleyan University Press; 1989. [Google Scholar]
- 45. Kay, S. R., , Fiszbein, A., , Opler. L. A.. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophrenia Bulletin 2012;13(13):261-276. [DOI] [PubMed] [Google Scholar]
- 46. NHS Improvement. NHS Improvement. National Cost Collection for the NHS; 2018. https://webarchivenationalarchivesgovuk/20200501111101/https://improvementnhsuk/resources/national-cost-collection/#ncc1819. Accessed 29 October, 2019.
- 47. Beecham J, Knapp M.. Costing psychiatric intervention. In: Thornicroft G, ed. Measuring Mental Health Needs. London: Gaskell; 2001. [Google Scholar]
- 48. Ennis L, Wykes T.. Impact of patient involvement in mental health research: longitudinal study. Br J Psychiatry. 2013;203(5):381–386. [DOI] [PubMed] [Google Scholar]
- 49. Patel A, Knapp M, Romeo R, et al. Cognitive remediation therapy in schizophrenia: cost-effectiveness analysis. Schizophr Res. 2010;120(1-3):217–224. [DOI] [PubMed] [Google Scholar]
- 50. White IR, Thompson SG.. Adjusting for partially missing baseline measurements in randomized trials. Stat Med. 2005;24(7):993–1007. [DOI] [PubMed] [Google Scholar]
- 51. Dunn G, Emsley R, Liu H, et al. Evaluation and validation of social and psychological markers in randomised trials of complex interventions in mental health: a methodological research programme. Health Technol Assess. 2015;19(93):1–115, v-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Hout B, Janssen M, Feng Y-S, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–715. [DOI] [PubMed] [Google Scholar]
- 53. Manca A, Hawkins N, Sculpher MJ.. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ. 2005;14(5):487–496. [DOI] [PubMed] [Google Scholar]
- 54. Curtis L, Burns A.. Unit Costs of Health and Social Care 2018. Kent: Personal Social Services Research Unit; 2019. [Google Scholar]
- 55. Gur RE, March M, Calkins ME, et al. Negative symptoms in youths with psychosis spectrum features: complementary scales in relation to neurocognitive performance and function. Schizophr Res. 2015;166(1-3):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nkire N, Scully PJ, Browne DJ, et al. Systematic comparison of duration of untreated illness versus duration of untreated psychosis in relation to psychopathology and dysfunction in the Cavan-Monaghan first episode psychosis study (CAMFEPS). Eur Neuropsychopharmacol. 2021;47:20–30. [DOI] [PubMed] [Google Scholar]
- 57. Leeson VC, Robbins TW, Matheson E, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66(6):586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Birchwood M, Connor C, Lester H, et al. Reducing duration of untreated psychosis: care pathways to early intervention in psychosis services. Br J Psychiatry. 2013;203(1):58–64. [DOI] [PubMed] [Google Scholar]
- 59. Birchwood M, Lester H, McCarthy L, et al. The UK national evaluation of the development and impact of E arly I ntervention S ervices (the N ational EDEN studies): study rationale, design and baseline characteristics. Early Interv Psychiatry. 2014;8(1):59–67. [DOI] [PubMed] [Google Scholar]
- 60. Seccomandi B, Agbedjro D, Bell M, et al. Can IQ moderate the response to cognitive remediation in people with schizophrenia? J Psychiatr Res. 2021;133:38–45. [DOI] [PubMed] [Google Scholar]
- 61. Leucht S, Samara M, Heres S, Davis JM.. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 2016;42(suppl_1):S90–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. National Institute for Health and Care Excellence. How NICE Measures Value for Money in Relation to Public Health Interventions; 2013. https://wwwniceorguk/Media/Default/guidance/LGB10-Briefing-20150126pdf. Accessed 1 November, 2022.
- 63. McGurk SR, Mueser KT, Xie H, et al. Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852–861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.