Abstract
Background and Hypothesis
Disrupted control of brain state transitions may contribute to the diverse dysfunctions of cognition, emotion, and behavior that are fundamental to schizophrenia. Control theory provides the rationale for evaluating brain state transitions from a controllability perspective, which may help reveal the brain mechanism for clinical features such as cognitive control deficits associated with schizophrenia. We hypothesized that brain controllability would be altered in patients with schizophrenia, and that controllability of brain networks would be related to clinical symptomatology.
Study Design
Controllability measurements of functional brain networks, including average controllability and modal controllability, were calculated and compared between 125 first-episode never-treated patients with schizophrenia and 133 healthy controls (HCs). Associations between controllability metrics and clinical symptoms were evaluated using sparse canonical correlation analysis.
Study Results
Compared to HCs, patients showed significantly increased average controllability (PFDR = .023) and decreased modal controllability (PFDR = .023) in dorsal anterior cingulate cortex (dACC). General psychopathology symptoms and positive symptoms were positively correlated with average controllability in regions of default mode network and negatively associated with average controllability in regions of sensorimotor, dorsal attention, and frontoparietal networks.
Conclusions
Our findings suggest that altered controllability of functional activity in dACC may play a critical role in the pathophysiology of schizophrenia, consistent with the importance of this region in cognitive and brain state control operations. The demonstration of associations of functional controllability with psychosis symptoms suggests that the identified alterations in average controllability of brain function may contribute to the severity of acute psychotic illness in schizophrenia.
Keywords: schizophrenia, resting-state functional magnetic resonance imaging, controllability, sparse canonical correlation analysis
Introduction
Considerable evidence documents alterations of brain connectivity in schizophrenia, including dysfunction of the ability to adaptively switch into and out of brain states.1,2 Consideration of both connectivity and dynamics of brain activity is a promising approach for characterizing brain dysfunction in psychiatric disorders.3 Most previous studies of brain networks focused on the evaluation of the connectome without consideration of dynamics and state transitions in activity states over time.4,5
Brain state transitions are crucial for supporting adaptive cognitive, emotional, and behavioral functions by selectively organizing appropriate brain states in task-relevant brain networks.6 Disrupted control of brain states may contribute to the diverse dysfunctions of cognition, emotion, and behavior that are fundamental to schizophrenia.7 Classical control theory provides the rationale and mathematical procedures for evaluating both brain connectivity and its dynamic change over time. Its model and methods originated from engineering research, and are rooted in the idea that energetic input modulates transitions from a current state of activity to another desired state to improve system function relevant to current demands.8 Human brain regions and the networks they comprise can alter functional states and drive transitions to different states, which corresponds to state transformations in the engineering control process framework.
Average controllability and modal controllability are the most popular controllability metrics. Average controllability represents the inverse of the average impulse response energy (control input) that supports the transition of brain states.9 Impulse response denotes the total magnitude and extent of signal spread which relies on the links of a given node with others in the brain network.9 Functional hub nodes, preferentially located in the default mode network (DMN), tend to have higher average controllability.10 These hub nodes have dense connections with other brain regions, thus can drive the transition of brain states with lower energy input. Modal controllability evaluates the ability of nodes to drive shifts into difficult-to-reach brain states.11 Easy-to-reach states are states where the control system can drive from the current state to another state with relatively little energy, which is close to the resting state of the brain. Hard-to-reach states are the opposite, and include states needed to perform complex cognitive operations. Higher modal controllability of nodes is associated with lower nodal strength.12 Higher modal controllability predominantly resided in weakly connected cognitive control networks such as frontoparietal network (FPN) which plays roles in task-control initiation and adjustment, and cingulo-opercular network (CON) is characterized by task stable maintenance.13
Previous studies of healthy individuals have shown that during performance of tasks requiring activity in specific task networks, average controllability of functional brain networks decreases while modal controllability increases, reflecting increasing energy demand in task states from a control perspective.12 Controllability metrics have been shown to predict performance on cognitive tasks.12 One study of depressed patients by Fang et al.14 found increased global average controllability and decreased global modal controllability, which predicted the effects of drug treatment. From a psychopharmacology perspective, photoswitchable muscarinic agonists have been shown to manipulate the control of state transitions,15 indicating that observation of brain controllability can provide an index of target engagement for drug treatments. Thus, applications of control theory and controllability metrics may represent a promising approach for developing a deeper understanding of brain dysfunction in schizophrenia. However, there has yet to be a study investigating the alteration of brain intrinsic controllability in schizophrenia and its association with clinical symptom severity.
In the present study, we tested for altered brain network controllability in acutely ill, first-episode, never-treated patients with schizophrenia relative to healthy controls (HCs), and examined relations of identified controllability alterations to psychotic symptom severity. We hypothesized that: (1) brain controllability would be altered in patients with schizophrenia, and (2) altered controllability of brain networks would be related to clinical symptomatology.
Methods
Participants
Detailed inclusion and exclusion criteria are provided in supplementary materials, but in summary, participants were 18–60 years old and right-handed. We included 125 first-episode never-treated patients with schizophrenia and 133 HCs in our final analyses. There were no significant between-group differences in age, sex, or education years (table 1).
Table 1.
Demographic and Clinical Characteristics for First-Episode Never-Treated Patients With Schizophrenia and Healthy Controls
| Patients (n = 125) | Controls (n = 133) | P Value | |
|---|---|---|---|
| Age, years | 24.3 ± 7.1 (18–46) | 26.1 ± 7.7 (18–52) | .055 |
| Gender, male/female | 55/70 | 64/69 | .507 |
| Education, years | 12.6 ± 2.9 (3–20) | 13.2 ± 3.0 (2–19) | .079 |
| Illness duration, month | 8.1 ± 9.4 (0.03–36) | — | — |
| PANSS scores | |||
| Total score | 89.6 ± 16.6 (46–127) | — | — |
| Positive symptoms | 25.1 ± 6.5 (7–39) | — | — |
| Negative symptoms | 18.6 ± 7.8 (7–40) | — | — |
| General psychopathology symptoms | 45.9 ± 9.7 (20–71) | — | — |
Note: Values in the table were presented as mean±standard deviation (range). P value of gender was computed by chi-square test and P values of age and education were calculated by two-sample t-test.
Abbreviation: PANSS, Positive and Negative Syndrome Scale.
Data Acquisition and Preprocessing
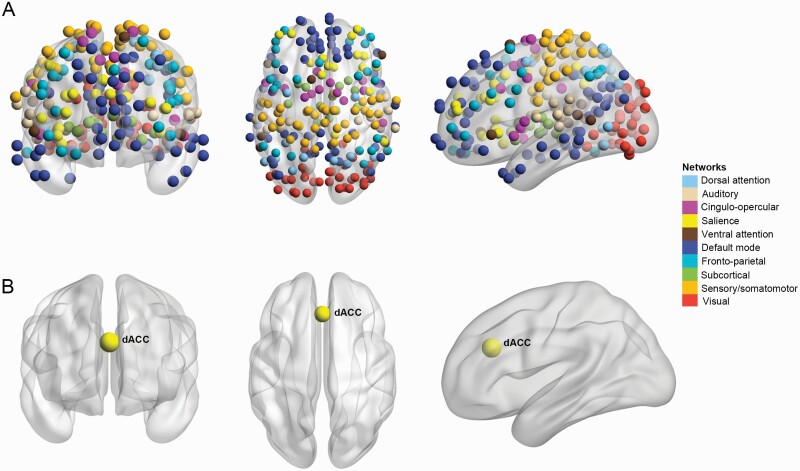
Details of magnetic resonance imaging (MRI) acquisition and data preprocessing are presented in supplementary methods. Time series were extracted from the preprocessed functional MRI images within defined anatomic regions using the Power functional template.16 Each region of interest (ROI) was defined using a sphere with a 3 mm radius centered on the peak coordinates of a functional area provided by Power et al.16 Functional connectivity (FC) between defined ROI was calculated via wavelet coherence rather than Pearson’s correlation. Compared to Pearson’s correlations, wavelet coherence is less sensitive to outliers17 and less affected by interregional differences in hemodynamic response functions.18 With a study focused on nodal controllability in functional brain networks, we excluded data from 33 ROIs in the template not assigned to a specific functional network and 4 cerebellar nodes with poor scan quality or insufficient scan coverage. Finally, 227 ROIs were examined in the present study that belonged to 1 of 10 brain networks including the auditory network, CON, dorsal attention network (DAN), DMN, FPN, salience network (SAN), sensorimotor network (SMN), subcortical network, ventral attention network, and visual network (figure 1A).19
Fig. 1.
The 227 functional brain regions included in our analysis belonged to 10 major networks (A). The altered controllability of dorsal anterior cingulate cortex was found in first-episode never-treated patients with schizophrenia compared to controls (B). The nodes represent brain areas in the Power functional template and the color of the node indicates the brain network to which it belongs.
Controllability Metrics of Nodes
Brain network controllability reflects the possibility of driving a current network state to other desired target states with external control energy input. The most commonly used metrics of network controllability are average controllability and modal controllability.8 Average controllability is a measure of the ability of a node to drive the brain to all possible easily reachable states considering the average input energy cost. Average controllability is equivalent to the trace of the Gramian matrix and it is inversely proportional to the control energy required to drive shifts in brain states. Brain areas with higher average controllability can guide the transition of functional states with lower input energy, indicating that they can more readily be transitioned into desirable network states.
Modal controllability quantifies the ease of a single control node to drive the brain into difficult-to-reach states. Regions with higher modal controllability can more easily drive dynamics of a brain network towards hard-to-reach states, which imposes high energy costs for completing complex goal-specific operations. Details of the derivation of these metrics are provided in supplementary materials. The association between average controllability and modal controllability was explored by Pearson correlation for all participants.
We used a nonparametric permutation method (10 000 iterations) to test for significant differences in controllability metrics between patients and HCs after controlling for age, sex, years of education, and head motion. Pearson correlation analysis was performed to test for associations between the altered controllability of nodes in regions with significant group differences and demographic and clinical characteristics (Positive and Negative Syndrome Scale [PANSS] scores) in patients controlling for age, sex, years of education, and head motion. Permutation tests were performed for each node and the univariate correlational analyses were performed for the nodes with significant differences detected by between-group comparison. The false discovery rate (FDR) correction for multiple comparisons was performed for both between-group comparison (454 corrections) and univariate correlation analysis of PANSS scores with altered controllability in patients (6 corrections).
Sparse Canonical Correlation Analysis
To capture clinical relationships of activity in different brain regions in patients, sparse canonical correlation analysis (sCCA) was used to establish the maximal correlations between linear combinations of variables in 2 multivariate datasets, average and modal controllability of 227 brain nodes, and 3 clinical symptom dimensions (positive, negative, and general psychopathology scores from the PANSS) with applied regularization to achieve sparsity (mathematical details in supplementary materials).20
Permutation testing with 1000 iterations was conducted to evaluate the statistical significance of each canonical variate. As permutation testing shuffled the rows of symptomatology dimensions causing the order of canonical variates or a sign of weights to be changed, we matched the canonical variates deriving from permuted data with the ones resulting from the original data by comparing the loadings of controllability.21 The FDR correction for multiple comparisons was performed for the 3 canonical variates. Finally, bootstrapping procedures with 1000 resamplings were performed to obtain features that stably contributed to each canonical variate. As the number of features of controllability (n = 227) was larger than that of features of clinical symptoms (n = 3), we want to have a more conservative assessment of stable contributions for features of controllability to the sCCA model. Thus, we set confidence intervals of 99% for controllability and 95% for symptomatology dimensions. Features whose confidence intervals of loading value did not cross zero were taken as stable and significant contributions to the association of controllability and illness severity.
Generalized additive model (GAM) that took controllability score (which was calculated by originally scaled controllability value × estimated loading value of controllability by sCCA) for each participant as the dependent variable and took age and sex as independent variables was employed to analyze age-related effects and sex differences in the canonical relationships between controllability and clinical symptoms in patients.
Reliability Analysis
We used repeated measures to assess 3 kinds of reliability including the reliability of controllability measures, findings of between-group comparisons, and findings of sCCA (Details in supplementary materials). Briefly, a bootstrap method with 1000 resamplings was used to assess reliability. In addition, to assess the reliability of estimated u and v vectors, the leave-one-out approach was applied. The correlation between the estimated u and v vectors in each leave-one-out analysis and u and v vectors in the original dataset was examined.
Results
Group Comparison of Controllability
A weak negative correlation between average and modal controllability was observed in all the participants (r = −.124, P = .047), indicating that the 2 controllability metrics characterize different and relatively independent aspects of brain function. Compared to HCs, first-episode never-treated patients with schizophrenia showed increased average controllability (PFDR = .023) and decreased modal controllability (PFDR = .023) of dorsal anterior cingulate cortex (dACC) (table 2 and figure 1B). These findings indicate that it is easier for dACC to drive transitions between easily reachable states but harder to steer the system to difficult-to-reach states in patients.
Table 2.
Differences in Controllability of Functional Brain Networks Between First-Episode Never-Treated Patients With Schizophrenia and Healthy Controls
| Features | Brain Regions | MNI Coordinates | Network | FDR Corrected P Value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Patients > healthy controls | ||||||
| Average controllability | dACC | 0 | 30 | 27 | SAN | .023 |
| Modal controllability | None | |||||
| Patients < with healthy controls | ||||||
| Average controllability | None | |||||
| Modal controllability | dACC | 0 | 30 | 27 | SAN | .023 |
Note: dACC, dorsal anterior cingulate cortex; FDR, false discovery rate; MNI, Montreal Neurological Institute; SAN, salience network.
Correlation Analyses
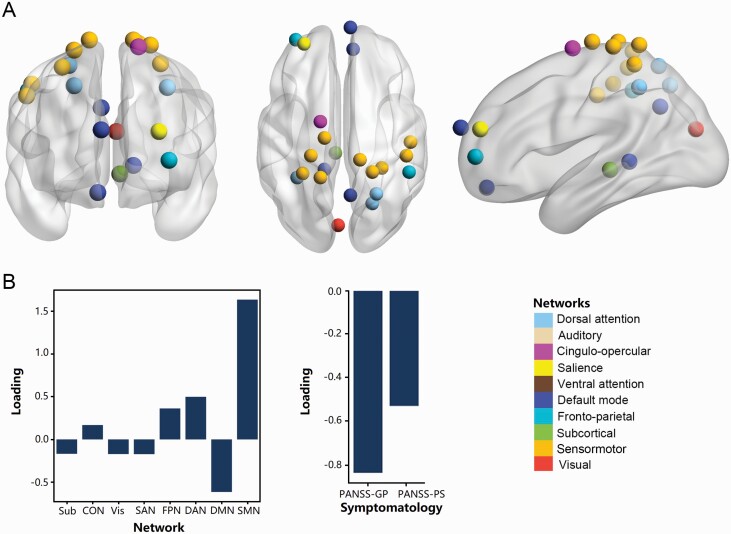
The sCCA of average controllability and clinical symptoms revealed that the first canonical variate was statistically significant in patients with schizophrenia (r = .673, P = .008, PFDR = .024) (supplementary figure S2). To facilitate the presentation of findings, we summarized the significant sCCA loadings of controllability at a network level (figure 2), and the detailed loadings for features that stably contributed to each canonical variate were provided in table 3. The first canonical variate primarily extracted features of general psychopathology symptoms and positive symptoms and average controllability in brain nodes mainly belonging to SMN (including postcentral gyrus), DAN (precuneus and intraparietal sulcus), FPN (supramarginal gyrus and dorsolateral PFC [dlPFC]), and DMN (medial prefrontal cortex [mPFC], orbitofrontal cortex [OFC], post cingulate cortex [PCC], and parahippocampal gyrus) (table 3 and figure 2). General psychopathology symptoms and positive symptoms were positively correlated with average controllability in DMN and negatively associated with average controllability in SMN, DAN, and FPN. The estimated u and v vectors were presented in supplementary table S1.
Fig. 2.
Association patterns between controllability and clinical symptom in patients. The positive symptoms were predominantly positively correlated with average controllability in regions of DMN, while mainly negatively associated with average controllability in regions of FPN, DAN, and SMN. The significant brain regions denoted by nodes were identified by sparse canonical correlation analysis in the first canonical variate of average controllability associated with symptoms (A). Loadings of average controllability at a brain network level and loadings of symptomatology dimension (B). Abbreviations: CON, cingulo-opercular network; DAN, dorsal attention network; DMN, default mode network; FPN, frontoparietal network; GP, general psychopathology; PANSS, Positive and Negative Syndrome Scale; PS, positive symptoms; SAN, salience network; SMN, sensorimotor network; Sub, subcortical network; Vis, visual network.
Table 3.
Loadings of Features that Stably Contributed to the First Canonical Variate in the Sparse Canonical Correlation Analysis of Average Controllability and Clinical Symptoms in Patients
| Network | ROI No. |
Brain Region | MNI Coordinate | Loading | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Average controllability stably contributed to linked clinical symptoms | ||||||
| Sensorimotor network | 7 | R. Paracentral lobule | 13 | −33 | 75 | 0.159 |
| 11 | L. Postcentral gyrus | −23 | −30 | 72 | 0.233 | |
| 13 | R. Parietal sub-gyral | 29 | −39 | 59 | 0.132 | |
| 14 | R. Postcentral gyrus | 50 | −20 | 42 | 0.249 | |
| 18 | L. Parietal sub-gyral | −29 | −43 | 61 | 0.269 | |
| 20 | R. Superior parietal lobule | 22 | −42 | 69 | 0.151 | |
| 23 | L. Precentral gyrus | −13 | −17 | 75 | 0.17 | |
| 26 | L. Postcentral gyrus | −16 | −46 | 73 | 0.139 | |
| 218 | R. Inferior parietal lobule | 47 | −30 | 49 | 0.132 | |
| Dorsal attention network | 219 | R. Precuneus | 22 | −65 | 48 | 0.177 |
| 221 | R. Precuneus | 25 | −58 | 60 | 0.152 | |
| 222 | L. Intraparietal sulcus | −33 | −46 | 47 | 0.169 | |
| Fronto-parietal network | 164 | R. Supramarginal gyrus | 49 | −42 | 45 | 0.159 |
| 171 | L. Dorsolateral prefrontal gyrus | −34 | 55 | 4 | 0.203 | |
| Default mode network | 64 | R. Orbitofrontal cortex | 8 | 48 | −15 | −0.178 |
| 65 | L. Parahippocampal gyrus | −13 | −40 | 1 | −0.149 | |
| 75 | R. Dorsal posterior cingulate cortex | 6 | −59 | 35 | −0.137 | |
| 92 | R. Medial prefrontal gyrus | 6 | 64 | 22 | −0.149 | |
| Cingulo-opercular network | 38 | L. Superior frontal gyrus | −16 | −5 | 71 | 0.168 |
| Visual network | 145 | L. Cuneus | −3 | −81 | 21 | −0.171 |
| Salience network | 188 | L. Middle frontal gyrus | −28 | 52 | 21 | −0.172 |
| Subcortical network | 199 | L. Thalamus | −5 | −28 | −4 | −0.168 |
| The stable clinical symptoms of the first canonical variate | ||||||
| Positive and Negative Syndrome Scale: general psychopathology symptoms | −0.844 | |||||
| Positive and Negative Syndrome Scale: positive symptoms | −0.535 | |||||
Note: L, left; MNI, Montreal Neurological Institute; R, right; SD, standard deviation; ROI No., region-of-interest number (in Power functional template).
Based on GAM, we found that the model was not significant, indicating that age and sex did not have a significant impact on the detected canonical correlations between average controllability metrics and clinical symptoms in patients (P > .05). There was no significant correlation between modal controllability and symptomatology in sCCA (P > .05). Both univariate correlational analysis and examining the salience of such variables within the sCCA framework showed that there were no significant correlations between the controllability of dACC and clinical characteristics in patients (all P > .05).
Reliability Analysis
The reliability analysis indicated that the controllability measures, and our findings of between-group comparison and sCCA analysis were stable. Detailed reliability results were provided in supplementary materials.
Discussion
In the current study, patients with first-episode never-treated schizophrenia showed increased average controllability and decreased modal controllability in dACC relative to HCs. Average controllability of regions in SMN, DAN, FPN, and DMN was associated with general psychopathology and positive symptoms in patients. Our findings in patients suggest that altered controllability of functional activity in dACC may play a critical role in the pathophysiology of schizophrenia, consistent with the importance of this region in cognitive control operations which has been related to disturbances of cognitive control in many schizophrenia studies. Considering control processes from a bioengineering framework may provide a useful biological framework for understanding the causes of widely observed cognitive control deficits in schizophrenia, particularly with regard to the role of ACC in mediating shifts in cognition and behavioral planning. The demonstration of associations of functional controllability with general psychopathology and positive symptoms suggests that the identified alterations in average controllability of brain function may contribute to the severity of acute psychotic illness in schizophrenia.
Altered Controllability
Using metrics derived from control theory, we identified a significant alteration in the ability of dACC to drive alterations of brain states in other brain regions. Patients with schizophrenia exhibited increased average controllability and decreased modal controllability in dACC, suggesting that dACC activity can more readily shift functional brain states with lower energy input, but less readily drive brain states transition to less readily achieved brain states such as those required for high-order task performance requiring cognitive control. Optimal brain function requires an optimal balance between the ability to shift and remain in brain states depending on circumstances. If activity shifts too readily while engaging in activities that require persistent focus for longer periods of time, behavior would be compromised. Similarly, if difficult-to-reach states cannot be reached when needed, higher cognitive functions, particularly those requiring cognitive control, could be compromised. This balance is mediated to a significant degree by dACC,22 so its disturbance could contribute significantly to the complex cognitive and behavioral presentation of schizophrenia as has been suggested previously.23
Cui et al. observed widespread effective connectivity of ACC in HCs but few ACC connections in first-episode patients with schizophrenia.24 As effective connectivity strength is negatively correlated with average controllability,12 our finding of increased average controllability in schizophrenia is consistent with the previous findings. However, while the 2 measures both evaluate brain dynamics and connectivity, significant differences in effective connectivity strength were not detected in group comparison in the study of Cui et al. while we found a significant difference of controllability in schizophrenia relative to HCs. Thus, average controllability may be a more sensitive biomarker than effective connectivity strength for evaluating the ability of a region to shift states of activity in other brain regions. In addition, a study by Li et al. demonstrated that never-treated schizophrenia patients exhibited higher global FC in dACC than HCs, while a significant reduction of global FC in dACC was detected after short-term treatment in schizophrenia,25 suggesting that dACC alterations may vary with the state of illness and be responsive to antipsychotic medication. Global FC was negatively correlated with modal controllability,12 thus the findings of increased global FC in dACC in first-episode patients are consistent with our findings.
At the large-scale network level, dACC is a prominent area of SAN that is involved in integrating external stimuli and internal processing priorities, and engaging in regulating information propagation and transitions between DMN and task-positive networks such as central-executive network (CEN).26 DMN contributes to internally oriented cognition, while CEN is characterized by externally directed cognition. SAN, DMN, and CEN form a classical triple network, with SAN mediating dynamic interactions between DMN and CEN. Aberrant connectivity of the SAN-centered triple network has been identified in schizophrenia,27 characterized by decreased FC between SAN and CEN28 and increased FC between SAN and DMN.29 dACC plays a well-established role in initiating switches between states in DMN and CEN, especially during performance of a complex task requiring cognitive control.26,30 The dysfunction of dACC observed in the present study in managing state transitions is consistent with the notion that dACC alterations may represent a causal factor in the disruption of optimal large-scale network function and cognition in schizophrenia.
Clinical Relevance
Our multivariate correlation analysis revealed that positive and general psychopathology symptoms in patients were related to network controllability in SMN (postcentral gyrus), DAN (intraparietal sulcus and precuneus), FPN (dlPFC and supramarginal gyrus), and DMN (mPFC, OFC, PCC, and parahippocampal gyrus). These associations were present with average but not modal controllability. This pattern of findings suggests that excessive spontaneous switching between easily reachable network states was associated with symptom severity in first-episode schizophrenia, and may be responsible for the heightened distractibility during acute psychosis. Associations of aberrant brain state transitions and psychotic symptoms have been reported previously in schizophrenia.31
A large majority of functional brain states are easily reachable,32 so as to support optimal cognition and behavior. Unmodulated excessive switching between brain states, however, could leave the brain in suboptimal conditions for performing cognitive, perceptual, and behavioral functions that depend on maintaining brain states over time for effective cognition and modulation of emotions. Such abilities are particularly disturbed during acute psychosis. As to hard-to-reach states such as those needed for higher-order cognitive control, these alterations in patients were not significantly related to the severity of acute psychosis, consistent with observations that neuropsychological tests of higher cognitive functions are relatively stable regardless of acute illness severity.33,34 This pattern of findings suggests that distractibility and exaggerated behavioral flexibility seen in acute psychosis may be more state related than the regulation of higher cognitive function in schizophrenia. Neurocognitive studies of the behavioral impact of the identified state transition disturbance are required to confirm this interpretation, as are longitudinal studies aiming to evaluate the state-dependent features of the identified controllability functions.
Analyses of average controllability in SMN demonstrated their association with more severe positive symptoms. Lower controllability in postcentral gyrus reflects a reduced ability to use higher energy inputs to other regions when needed to perform more complex network state shifts. This might be related to reduced activity of postcentral gyrus that has been independently documented in schizophrenia.35 Decreased functional coupling of postcentral gyrus has previously been correlated with positive symptom severity.36
Intraparietal sulcus and precuneus, core regions of DAN, are related to top-down visuospatial attention control processes,37 and dysfunction of these areas may contribute to visuospatial and perceptual deficits reported in schizophrenia patients.38 Our study identified a negative correlation between average controllability of intraparietal sulcus and precuneus with general psychopathology and positive symptoms, indicating that the lower ability of DAN to facilitate executive state shifts, the greater the severity of psychosis symptoms. Increased perfusion of DAN (more energy supply) benefits insight preservation in schizophrenia, which has been associated with treatment outcomes of patients,39 and symptomatic improvement after treatment has been associated with increased regional homogeneity of DAN in schizophrenia.40
A negative association between average controllability of FPN regions including dlPFC and supramarginal gyrus with general psychopathology and positive symptoms was also observed. Dysfunction of dlPFC is known to be associated with executive and working memory disturbances in schizophrenia.41 Consistent with our finding, patients with auditory verbal hallucinations in schizophrenia have been shown to have altered N-AcetyI-Aspartate levels in dlPFC.42 Supramarginal gyrus contributes to language perception and processing which have well-established alterations in patients with schizophrenia.43 Thus, our findings are consistent with a literature suggesting that supramarginal gyrus alterations may be related mechanistically to positive symptoms in schizophrenia, and add a controllability perspective on those alterations in terms of their impact on inducing network state changes for optimized cognitive function.
Higher average controllability in multiple regions of DMN including mPFC, OFC, PCC, and parahippocampal gyrus was positively related to more severe general psychopathology and positive symptoms. Previous studies have also identified increased FC44 and activity45 within DMN to be positively correlated with patients’ positive symptoms. Other studies have reported both structural46 and functional47 abnormalities in DMN in schizophrenia as well.
One important observation from a network-level perspective based on previous research is that network interactions in SMN, DAN, FPN, and DMN are interactive.48 This observation may in part account for observations that dysfunctions of sensory and perceptual processing are related to alterations in high-order cognition in schizophrenia.49 Moreover, those abnormalities were all related to the severity of positive symptoms in our untreated, acutely ill first-episode schizophrenia patients.50,51 Altered controllability in the inter-network interactions, reflected in lower average controllability of SMN, DAN, FPN, and higher average controllability of DMN, may be a contributing factor to the severity of acute psychosis in patients.
Limitations
Some limitations of the current study need to be considered. First, we did not evaluate neuropsychological function in this population, particularly cognitive and affective processes dependent on dynamic control processes, so a direct linkage between observed alterations in brain regions and alterations in behavioral functions they serve is not possible from our study. Evaluating cognitive functions in this population in future studies using tasks demanding cognitive control will be important for understanding the behavioral significance of controllability deficits determined using engineering-based analytic models applied to brain imaging data. Future studies incorporating such information on cognitive assessment and controllability may help parse the cognition-related pathophysiological basis of schizophrenia. Second, the controllability of brain network was built on a linear and time-invariant model, while neural dynamics are nonlinear. Future studies might consider whether nonlinear neural dynamics may provide novel insights, though existing studies have reported that the blood oxygenation level dependent (BOLD) signal could be reasonably well predicted by linear models.52 Third, although associations with general psychopathology and positive symptoms were observed, it is difficult to conclude that deficits of modal controllability are stable over the illness course, or vary with illness state in a cross-sectional study, especially when all patients were acutely ill at the time of scans. Whether our controllability findings vary with the state of illness and whether they are related to dynamic cognitive control task performance remain to be investigated in future studies. Additionally, the assumption of the suitability of the preselected nodes of the networks should also be evaluated. Also, there are limits to the sensitivity of BOLD signals across the brain. Despite denoising and related preprocessing steps, it remains a concern. Finally, there is the possibility that treating schizophrenia as a unified illness may have limitations, as heterogeneity within the clinical population dilutes findings in discrete subgroups.53,54,55
Conclusion
In conclusion, our study documented that altered controllability in the dACC represents a significant alteration of the functional brain connectome in schizophrenia, and that altered average controllability in SMN, DAN, FPN, and DMN is associated with symptoms of acute psychosis. Dysfunction in the ability to switch network states for optimal brain function may be an important mechanistic feature underlying the prominent neurocognitive and neurobehavioral alterations in schizophrenia.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Conflict of interest
Dr. Sweeney has consulted to VeraSci. Other authors declare no biomedical financial interests or potential conflicts of interest.
Contributor Information
Qian Li, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, Sichuan, P.R. China; Functional and Molecular Imaging Key Laboratory, Sichuan University, Chengdu 610041, Sichuan, P.R. China.
Li Yao, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, Sichuan, P.R. China; Functional and Molecular Imaging Key Laboratory, Sichuan University, Chengdu 610041, Sichuan, P.R. China.
Wanfang You, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, Sichuan, P.R. China; Functional and Molecular Imaging Key Laboratory, Sichuan University, Chengdu 610041, Sichuan, P.R. China.
Jiang Liu, Department of Computer Science and Engineering, University of Electronic Science and Technology of China, Chengdu, China.
Shikuang Deng, Department of Computer Science and Engineering, University of Electronic Science and Technology of China, Chengdu, China.
Bin Li, Department of Computer Science and Engineering, University of Electronic Science and Technology of China, Chengdu, China.
Lekai Luo, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, Sichuan, P.R. China; Functional and Molecular Imaging Key Laboratory, Sichuan University, Chengdu 610041, Sichuan, P.R. China.
Youjin Zhao, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, Sichuan, P.R. China; Functional and Molecular Imaging Key Laboratory, Sichuan University, Chengdu 610041, Sichuan, P.R. China.
Yuxia Wang, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, Sichuan, P.R. China; Functional and Molecular Imaging Key Laboratory, Sichuan University, Chengdu 610041, Sichuan, P.R. China.
Yaxuan Wang, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, Sichuan, P.R. China; Functional and Molecular Imaging Key Laboratory, Sichuan University, Chengdu 610041, Sichuan, P.R. China.
Qian Zhang, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, Sichuan, P.R. China; Functional and Molecular Imaging Key Laboratory, Sichuan University, Chengdu 610041, Sichuan, P.R. China.
Fenghua Long, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, Sichuan, P.R. China; Functional and Molecular Imaging Key Laboratory, Sichuan University, Chengdu 610041, Sichuan, P.R. China.
John A Sweeney, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati, Cincinnati, OH 45219, USA.
Shi Gu, Department of Computer Science and Engineering, University of Electronic Science and Technology of China, Chengdu, China.
Fei Li, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, Sichuan, P.R. China; Functional and Molecular Imaging Key Laboratory, Sichuan University, Chengdu 610041, Sichuan, P.R. China.
Qiyong Gong, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, Sichuan, P.R. China; Functional and Molecular Imaging Key Laboratory, Sichuan University, Chengdu 610041, Sichuan, P.R. China.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant Nos. 61876032 to FL and SG, 82102007 to LY, 82001795 to YJZ, 81621003, 81761128023, and 82027808 to QYG, and 81820108018 to JAS and QYG), National Key R&D Program (2022YFC2009900), Sichuan Science and Technology Program (2021YFS0077 to LY and 2022YFS0069 to YJZ). Dr. Qiyong Gong and Dr. Fei Li contributed equally to playing the role of corresponding and senior author.
Data Availability
Code is available at HYPERLINK https://github.com/QianLi9423/Project_SCZ_controllability.
References
- 1. Narr KL, Leaver AM.. Connectome and schizophrenia. Curr Opin Psychiatry. 2015;28(3):229–235. [DOI] [PubMed] [Google Scholar]
- 2. Andalman AS, Burns VM, Lovett-Barron M, et al. Neuronal dynamics regulating brain and behavioral state transitions. Cell. 2019;177(4):970–985.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci. 2014;15(10):683–695. [DOI] [PubMed] [Google Scholar]
- 4. Wang J, Zuo X, He Y.. Graph-based network analysis of resting-state functional MRI. Front Syst Neurosci. 2010;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friston KJ, Harrison L, Penny W.. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. [DOI] [PubMed] [Google Scholar]
- 6. Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298(5596):1191–1194. [DOI] [PubMed] [Google Scholar]
- 7. Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31(4):875–881. [DOI] [PubMed] [Google Scholar]
- 8. Gu S, Pasqualetti F, Cieslak M, et al. Controllability of structural brain networks. Nat Commun. 2015;6:8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeganathan J, Perry A, Bassett DS, Roberts G, Mitchell PB, Breakspear M.. Fronto-limbic dysconnectivity leads to impaired brain network controllability in young people with bipolar disorder and those at high genetic risk. Neuroimage Clin. 2018;19:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deng S, Li J, Thomas Yeo BT, Gu S.. Control theory illustrates the energy efficiency in the dynamic reconfiguration of functional connectivity. Commun Biol. 2022;5(1):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamdan AMA, Nayfeh AH.. Measures of modal controllability and observability for first- and second-order linear systems. J Guid Control Dyn. 1989;12(3):421–428. [Google Scholar]
- 12. Deng S, Gu S.. Controllability analysis of functional brain networks. arXiv preprint arXiv:2003.08278,2020.
- 13. Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104(26):11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang F, Godlewska B, Cho RY, Savitz SI, Selvaraj S, Zhang Y.. Effects of escitalopram therapy on functional brain controllability in major depressive disorder. J Affect Disord. 2022;310:68–74. [DOI] [PubMed] [Google Scholar]
- 15. Barbero-Castillo A, Riefolo F, Matera C, et al. Control of brain state transitions with a photoswitchable muscarinic agonist. Adv Sci (Weinh). 2021;8(14):e2005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Müller K, Lohmann G, Neumann J, Grigutsch M, Mildner T, von Cramon DY.. Investigating the wavelet coherence phase of the BOLD signal. J Magn Reson Imaging. 2004;20(1):145–152. [DOI] [PubMed] [Google Scholar]
- 18. White LB, Boashash B.. Cross spectral analysis of nonstationary processes. IEEE Trans Inf Theory. 1990;36(4):830–835. [Google Scholar]
- 19. Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS.. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16(9):1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Witten DM, Tibshirani R, Hastie T.. A penalized matrix decomposition, with applications to sparse principal components and canonical correlation analysis. Biostatistics. 2009;10(3):515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mišić B, Betzel RF, de Reus MA, et al. Network-level structure-function relationships in human neocortex. Cereb Cortex. 2016;26(7):3285–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D’Cruz AM, Ragozzino ME, Mosconi MW, Pavuluri MN, Sweeney JA.. Human reversal learning under conditions of certain versus uncertain outcomes. Neuroimage. 2011;56(1):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carter CS, Mintun M, Nichols T, Cohen JD.. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154(12):1670–1675. [DOI] [PubMed] [Google Scholar]
- 24. Cui LB, Liu J, Wang LX, et al. Anterior cingulate cortex-related connectivity in first-episode schizophrenia: a spectral dynamic causal modeling study with functional magnetic resonance imaging. Front Hum Neurosci. 2015;9:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li H, Ou Y, Liu F, et al. Reduced connectivity in anterior cingulate cortex as an early predictor for treatment response in drug-naive, first-episode schizophrenia: a global-brain functional connectivity analysis. Schizophr Res. 2020;215:337–343. [DOI] [PubMed] [Google Scholar]
- 26. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. [DOI] [PubMed] [Google Scholar]
- 27. Liang S, Wang Q, Greenshaw AJ, et al. Aberrant triple-network connectivity patterns discriminate biotypes of first-episode medication-naive schizophrenia in two large independent cohorts. Neuropsychopharmacology. 2021;46(8):1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dong D, Wang Y, Chang X, Luo C, Yao D.. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44(1):168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shao J, Meng C, Tahmasian M, et al. Common and distinct changes of default mode and salience network in schizophrenia and major depression. Brain Imaging Behav. 2018;12(6):1708–1719. [DOI] [PubMed] [Google Scholar]
- 30. Chand GB, Dhamala M.. Interactions among the brain default-mode, salience, and central-executive networks during perceptual decision-making of moving dots. Brain Connect. 2016;6(3):249–254. [DOI] [PubMed] [Google Scholar]
- 31. Yang H, Zhang H, Di X, et al. Reproducible coactivation patterns of functional brain networks reveal the aberrant dynamic state transition in schizophrenia. Neuroimage. 2021;237:118193. [DOI] [PubMed] [Google Scholar]
- 32. Bassett DS, Greenfield DL, Meyer-Lindenberg A, Weinberger DR, Moore SW, Bullmore ET.. Efficient physical embedding of topologically complex information processing networks in brains and computer circuits. PLoS Comput Biol. 2010;6(4):e1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hill SK, Reilly JL, Harris MS, Khine T, Sweeney JA.. Oculomotor and neuropsychological effects of antipsychotic treatment for schizophrenia. Schizophr Bull. 2008;34(3):494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA.. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naïve patients with schizophrenia. Schizophr Res. 2004;68(1):49–63. [DOI] [PubMed] [Google Scholar]
- 35. Gong J, Wang J, Luo X, et al. Abnormalities of intrinsic regional brain activity in first-episode and chronic schizophrenia: a meta-analysis of resting-state functional MRI. J Psychiatry Neurosci. 2020;45(1):55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berman RA, Gotts SJ, McAdams HM, et al. Disrupted sensorimotor and social-cognitive networks underlie symptoms in childhood-onset schizophrenia. Brain. 2016;139(Pt 1):276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Power JD, Petersen SE.. Control-related systems in the human brain. Curr Opin Neurobiol. 2013;23(2):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wible CG. Hippocampal temporal-parietal junction interaction in the production of psychotic symptoms: a framework for understanding the schizophrenic syndrome. Front Hum Neurosci. 2012;6:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Faget-Agius C, Boyer L, Padovani R, et al. Schizophrenia with preserved insight is associated with increased perfusion of the precuneus. J Psychiatry Neurosci. 2012;37(5):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shan X, Liao R, Ou Y, et al. Increased regional homogeneity modulated by metacognitive training predicts therapeutic efficacy in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2021;271(4):783–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang M-L, Khoh T-T, Lu S-J, et al. Relationships between dorsolateral prefrontal cortex metabolic change and cognitive impairment in first-episode neuroleptic-naive schizophrenia patients. Medicine. 2017;96(25):e7228–e7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Psomiades M, Mondino M, Fonteneau C, et al. N-Acetyl-Aspartate in the dorsolateral prefrontal cortex in men with schizophrenia and auditory verbal hallucinations: a 1.5 T magnetic resonance spectroscopy study. Sci Rep. 2018;8(1):4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brown M, Kuperberg GR.. A hierarchical generative framework of language processing: linking language perception, interpretation, and production abnormalities in schizophrenia. Front Hum Neurosci. 2015;9:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tang J, Liao Y, Song M, et al. Aberrant default mode functional connectivity in early onset schizophrenia. PLoS One. 2013;8(7):e71061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD.. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164(3):450–457. [DOI] [PubMed] [Google Scholar]
- 46. Liu N, Xiao Y, Zhang W, et al. Characteristics of gray matter alterations in never-treated and treated chronic schizophrenia patients. Transl Psychiatry. 2020;10(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guo S, He N, Liu Z, Linli Z, Tao H, Palaniyappan L.. Brain-wide functional dysconnectivity in schizophrenia: parsing diathesis, resilience, and the effects of clinical expression. Can J Psychiatry. 2020;65(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dixon ML, De La Vega A, Mills C, et al. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Natl Acad Sci USA. 2018;115(7):E1598–E1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Javitt DC. Sensory processing in schizophrenia: neither simple nor intact. Schizophr Bull. 2009;35(6):1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hornix B, Havekes R, Kas M.. Multisensory cortical processing and dysfunction across the neuropsychiatric spectrum. Neurosci Biobehav Rev. 2019;97:138–151. [DOI] [PubMed] [Google Scholar]
- 51. Cassidy CM, Balsam PD, Weinstein JJ, et al. A perceptual inference mechanism for hallucinations linked to striatal dopamine. Curr Biol. 2018;28(4):503–514.e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goñi J, van den Heuvel MP, Avena-Koenigsberger A, et al. Resting-brain functional connectivity predicted by analytic measures of network communication. Proc Natl Acad Sci USA. 2014;111(2):833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xiao Y, Liao W, Long Z, et al. Subtyping schizophrenia patients based on patterns of structural brain alterations. Schizophr Bull. 2022;48(1):241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clementz BA, Parker DA, Trotti RL, et al. Psychosis biotypes: replication and validation from the B-SNIP consortium. Schizophr Bull. 2022;48(1):56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao Y, Zhang Q, Shah C, et al. Cortical thickness abnormalities at different stages of the illness course in Schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(6):560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code is available at HYPERLINK https://github.com/QianLi9423/Project_SCZ_controllability.