Abstract
Background and Hypothesis
Gut microbiota alterations have been reported in severe mental illness (SMI) but fewer studies have probed for signs of gut barrier disruption and inflammation. We hypothesized that gut leakage of microbial products due to intestinal inflammation could contribute to systemic inflammasome activation in SMI.
Study Design
We measured plasma levels of the chemokine CCL25 and soluble mucosal vascular addressin cell adhesion molecule-1 (sMAdCAM-1) as markers of T cell homing, adhesion and inflammation in the gut, lipopolysaccharide binding protein (LBP) and intestinal fatty acid binding protein (I-FABP) as markers of bacterial translocation and gut barrier dysfunction, in a large SMI cohort (n = 567) including schizophrenia (SCZ, n = 389) and affective disorder (AFF, n = 178), relative to healthy controls (HC, n = 418). We assessed associations with plasma IL-18 and IL-18BPa and leukocyte mRNA expression of NLRP3 and NLRC4 as markers of inflammasome activation.
Study Results
Our main findings were: (1) higher levels of sMAdCAM-1 (P = .002), I-FABP (P = 7.6E−11), CCL25 (P = 9.6E−05) and LBP (P = 2.6E−04) in SMI compared to HC in age, sex, BMI, CRP and freezer storage time adjusted analysis; (2) the highest levels of sMAdCAM-1 and CCL25 (both P = 2.6E−04) were observed in SCZ and I-FABP (P = 2.5E−10) and LBP (3) in AFF; and (3), I-FABP correlated with IL-18BPa levels and LBP correlated with NLRC4.
Conclusions
Our findings support that intestinal barrier inflammation and dysfunction in SMI could contribute to systemic inflammation through inflammasome activation.
Keywords: schizophrenia, affective disorder, inflammasome, mucosal vascular addressin cell adhesion molecule-1, intestinal fatty acid binding protein, lipopolysaccharide binding protein
Introduction
Genetic and epidemiological evidence implicate immune activation and inflammation in the development and progression of severe mental illness (SMI) including schizophrenia (SCZ)1,2 and affective disorders (AFF).3–5 Systemic activation of immune and vascular cells with enhanced secretion of inflammatory mediators has been demonstrated preceding6 and following diagnosis of SCZ and AFF.7–9 Molecular neuroscience studies suggest that inflammation and immune activation may influence neuronal functioning and plasticity, dysregulate neuron-glia cross-talk, and predispose immunocompetent glia to a pro-inflammatory state associated with neurodegeneration.10–13
Comorbid cardio-metabolic conditions such as dyslipidemia, diabetes, and increased fat mass may contribute to systemic inflammation, and over time enhance cardiovascular (CV) mortality, which is two to three-fold higher in SMI.14,15 Accumulating evidence suggests that the gut microbiota composition and disrupted gut-blood barrier leading to gut wall inflammation and leakage of microbial products also may promote systemic inflammation, contributing to the pathogenesis of disorders like diabetes, obesity, and CV disease.16 Altered gut microbiota profiles have also been described in SMI and linked to brain structure,17 symptoms,18,19 and cognitive performance.20 Conversely, stress related behavior may influence the microbiome, potentially representing a vicious circle in SMI.20 This bidirectional communication has been termed the gut–brain axis.21 While many studies have investigated gut microbiome in SMI,22,23 fewer studies have probed for signs of gut barrier disruption and inflammation or linked these to systemic inflammasome activation. Nod-like Receptor Protein (NLRP) 3 inflammasome activation has been suggested as an important link between altered gut microbiota composition, impaired gut barrier and systemic inflammation,24 through interactions between lipopolysaccharide (LPS) and toll-like receptor 4,enhancing the release of the inflammatory cytokines interleukin (IL)-1β and IL-18.25 We have recently demonstrated increased leukocyte mRNA expression of NLRP3 and NLRC4 which are core components of the inflammasome, as well plasma IL-18 levels in SMI.26
Both loss of integrity in the epithelial barrier and bacterial translocation may enhance inflammatory processes in the intestinal mucosa where different lymphocyte subsets are important regulators of immune responses.27 CCR9/CCL25 interaction seems to regulate the inflammatory immune response of the intestinal mucosa by balancing different dendritic and T cell subsets.28 CCL25 is exclusively expressed by thymic cells and intestinal epithelial cells, and enhanced intestinal levels are seen during gut inflammation.29–31 Markedly higher circulating CCL25 has been demonstrated in patients with inflammatory bowel disease (IBD)32 and mucosal levels correlate with the Mayo endoscopic sub-score and mucosal TNF levels, as markers of mucosal inflammation, in ulcerative colitis patients.31 Furthermore, CCR9/CCL25 interactions induce pro-migratory responses, including the activation of integrins and binding to Mucosal addressin cell adhesion molecule-1 (MAdCAM-1), a homing receptor preferentially expressed on gut-associated endothelial cells and lymphoid tissues,33,34 which plays a central role in leukocyte traffic into the mucosal immune compartment.35,36 Elevated MAdCAM-1 has been observed in Crohn’s disease at sites of active inflammation,37 and increased
levels correlate with disease activity in IBD.38 Damage to the intestinal mucosa may lead to leakage of intestinal fatty acid binding protein (I-FABP), a small (15 kD) cytosolic protein exclusively expressed by mature epithelial cells of the mucosal layer of the large and in particular the small intestines.39 Upon damage to the intestinal barrier, I-FABP is released into the bloodstream and considered a marker of epithelial integrity with a causal relationship to permeability and innate barrier function.40,41 Increased levels are seen in patients with enhanced mucosal inflammation and gut barrier dysfunction due to intestinal epithelial cell damage such as celiac disease, intestinal ischemia, and necrotizing colitis.42–45 Leakage of microbial products such as lipopolysaccharides (LPS) to the circulation will stimulate hepatic production of its binding protein, LPS-binding protein (LBP), and is considered a potential surrogate marker of microbial translocation.46
To investigate if gut leakage mechanisms due to intestinal barrier inflammation and dysfunction could contribute to systemic inflammasome activation, being an important component of the innate immune system, we measured plasma levels of CCL25 and sMAdCAM-1 as markers of leukocyte homing, adhesion, and inflammation in the gut, LBP, and I-FABP as markers of bacterial translocation and gut barrier dysfunction, in a large SMI cohort including SCZ (n = 389) and AFF (n = 178), relative to healthy controls (HC, n = 418). Furthermore, we assessed associations with plasma levels of IL-18 and IL-18BPa and leukocyte mRNA expression of NLRP3 and NLRC4 as markers reflecting systemic inflammasome activation. Finally, as MAdCAM-1 is also expressed in the brain47 and potentially could promote leukocyte trafficking across the BBB, we also assessed MADCAM1 mRNA levels in RNA-seq data of dorsolateral prefrontal cortex (DLPFC) post mortem samples from the CommonMind Consortium (CMC, n = 474), to assess if potential systemic dysregulation was reflected in brain tissue.
Materials and Methods
Setting and Participants
The current study is a naturalistic, cross-sectional study which is part of the ongoing Thematically Organized Psychosis (TOP) Study at the NORMENT Centre which includes patients from psychiatric clinics of hospitals in the Oslo region. The clinical participants were recruited to the current study consecutively from 2003 through 2017 mainly from outpatient clinics, but also from intermediate and long term units. Inclusion of patients admitted to acute treatment units was awaited until they were stabilized and able to consent and participate in interviews and assessments. This project studies the underlying mechanisms of SMI, amongst others to assess specific research questions on the role of inflammation and immune activation in SMI, and the current study is part of this aim. The main patient criterion of inclusion in the TOP Study is a diagnosis of schizophrenia (SCZ) spectrum disorder, bipolar spectrum disorder or major depressive disorder with psychotic features according to DSM-IV. All participants were between 18 and 65 years and able to give a written informed consent. Patients recruited after an acute episode (post-acute episode) were only included when they were clinically stable enough to provide informed consent. Participants were excluded if they did not speak Scandinavian and/or demonstrated pronounced cognitive deficits (IQ < 70) and/or severe brain damage/illness in order to ascertain that all participants were able to complete the protocol and fully understand the meaning of participating in the study. The HC participants were randomly invited from statistical records (www.ssb.no) from the same catchment area as the patients. HC were between 18 and 65 years old apparently healthy individuals with none reporting any history of SMI, significant head injury, neurological disorders, illicit drug use, first-degree relatives with SMI, or neurological disorders or other medical problems that could interfere with brain function (eg, severe uncontrolled hypothyroidism, hypertension, or diabetes). All participants were weighed on calibrated digital weights under equal conditions, height was measured with standard methods and body mass index (BMI) (kg/m²) calculated. All participants have given written informed consent and the study was approved by the Regional Committees for Medical and Health Research Ethics (REC) in Norway and the Norwegian Data Protection Agency.
Sample
A total of 567 patients with SMI and 418 HC were included in the current study. In the sample, a total of 389 patients had schizophrenia spectrum disorder (schizophrenia, schizoaffective disorder, schizophreniform disorder, delusional disorder, brief psychotic disorder, and psychosis NOS) and were included in the diagnostic group “Schizophrenia” (SCZ), while 178 patients had affective disorder (bipolar I, bipolar II, bipolar NOS, and major depressive disorder with psychotic features) and were included in the diagnostic group “Affective” (AFF). To avoid individuals with ongoing/intermittent severe infection or inflammation, participants with C-reactive protein (CRP) >10 mg/L for any reason were excluded from the study (n = 103).48
Clinical Assessments
Sociodemographic history, medical history, substance use, psychiatric symptoms, medication, and potential side effects were recorded by interviews and reviewing medical records. They all underwent diagnostic interviews based on Structured Clinical Interview of DSM-IV axis I Disorders (SCID-1), and symptom assessments with Positive and Negative Syndrome Scale (PANSS),49 Young Mania Rating Scale (YMRS)50 and the symptom score of the split version of the Global Assessment of the Functioning Scale (GAF-S).51 Diagnostic evaluation was performed by trained psychologists and physicians supervised by senior researchers and the inter-rater reliability of diagnostic and symptom assessments was satisfactory.52 The HC were interviewed for current or previous history of SMI themselves or in their family and assessed with Primary Care Evaluation of Mental Disorders (PRIME MD).
Biochemistry
EDTA plasma was obtained and processed as described26,53 and levels of sMAdCAM-1, I-FABP, CCL25, and LBP were measured in duplicates by enzyme immunoassays (EIA) using commercially available antibodies (R&D Systems, Minneapolis, MN, USA) in a 384 format using a combination of a SELMA (Jena, Germany) pipetting robot and a BioTek (Winooski, VT, USA) dispenser/washer. Absorption was read at 450 nm with wavelength correction set to 540 nm using an ELISA plate reader (Bio-Rad, Hercules, CA, USA). Plasma levels of IL-18 and IL-18-BPa and leukocyte mRNA expression of NLRP3 and NLRC4 in this population have been reported previously.26,53 Intra- and inter-assay coefficients of variation were <10% for all EIAs.
Medication
The information regarding prescribed antipsychotics (AP), antiepileptic’s (AE) and antidepressants (AD) used by patients were obtained by clinical interviews and hospital records. We calculated “defined daily doses” (DDD) according to the World Health Organization (WHO) principles, as described previously.54 The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults and provide a fixed unit of measurement independent of dosage (https://www.whocc.no/atc_ddd_index/).
RNA-seq of Brain Samples from the CommonMind Consortium (CMC)
See the Supplementary file for details.
Statistical Analyses
Statistical analyses were performed in Stata. Missing values were generated with multiple imputation (with chained equations) to avoid any bias in the association of interest introduced by excluding individuals with missing data. Differences in demographics were assessed using one-way ANOVA, Chi-square test and Scheffe’s posthoc tests. Associations between gut inflammation markers, BMI, CRP, markers of inflammasome (IL-18 and IL-18BPa) and T cell activation (sCD25) inflammatory markers were assessed with Spearman rank-order correlations. Additionally, we assessed pair-wise correlations stratified by use of medications and CRP levels to further assess the pattern of findings (see Supplementary File 2).
Associations between diagnosis (HC, SCZ+AFF, SCZ, AFF) and the gut inflammation markers CCL25, sMAdCAM-1, LBP andI-FABP were assessed using ordinary least square (OLS) regression models, both with and without adjusting for age, sex, BMI, CRP, and freezer storage time. Some previous studies suggest that administration of AP drugs could affect gut permeability markers55,56; therefore, we performed sensitivity analysis by assessing the pattern of associations among those not using APs. Associations between gut inflammation markers and use of medications and between symptom/functionality scores and inflammatory gut markers in patient groups were also assessed using OLS regression with these same covariates. Additionally, we assessed the potential moderating role of age, sex, BMI, CRP, AP use, AD use, and AE use in the association between diagnosis and inflammatory markers with linear and fractional polynomial models.
Results
Demographics and Clinical Characteristics
As shown in Table 1, patients with SCZ (mean 29.2 years, SD 9.4) were younger than HC and AFF (mean 33 years and 31.9 years, respectively). SCZ and HC had a higher proportion of males compared to AFF. SCZ patients had a higher BMI compared to AFF and HC, while AFF had a higher BMI and more females compared to HC. As expected, SCZ patients had more severe symptoms as reflected by PANSS, and lower levels of functioning as reflected by GAF-S, compared to AFF. CRP levels were higher in SCZ compared to HC. As expected, use of AP was more frequent in SCZ compared to AFF, with a higher DDD of APs, and use of AEs was higher in AFF with a higher DDD of AEs.
Table 1.
Demographics
| SCZ (n = 389) | AFF (n = 178) | HC (418) | P | SCZ vs HC | AFF vs HC | SCZ vs AFF | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | P | P | P | ||
| Age, yrs | 389 | 29.2 (9.4) | 178 | 33 (12.2) | 418 | 31.9 (8.6) | 7.3E−081 | 1.7E−053 | 0.2443 | 6.6E−053 |
| Sex (male) | 241 | 61.9 % | 80 | 44.9 % | 244 | 58.4 % | .0012 | .2992 | .0032 | 1.5E−042 |
| PANSS total | 376 | 54.8 (17) | 177 | 41.2 (12) | – | – | – | – | – | 2.7E−073 |
| YMRS | 278 | 10.8 (10.3) | 167 | 10.3 (9.5) | – | – | – | – | – | .6093 |
| GAF-S | 388 | 28.6 (11.5) | 178 | 39.4 (12.7) | – | – | – | – | – | <2E−163 |
| BMI, kg/m2 | 357 | 26.4 (5.1) | 168 | 25.2 (4.2) | 368 | 24.5 (3.5) | 2.1E−121 | 3E−093 | .0333 | .0083 |
| CRP, mg/L | 389 | 3.2 (2.7) | 178 | 2.6 (2.5) | 418 | 2.3 (2.2) | .0011 | 5.4E−073 | .2533 | .0083 |
| Antipsychotics use | 321 | 82.5% | 105 | 58.9% | 0 | 0.0% | – | – | – | 1.8E−092 |
| DDD antipsychotics | 317 | 1.3 (0.9) | 105 | 0.9 (0.8) | – | – | – | – | – | .0013 |
| Anti-epileptics use | 40 | 10.3% | 73 | 41% | 0 | 0.0% | – | – | – | 1.9E−172 |
| DDD anti-epileptics | 40 | 0.6 (0.4) | 72 | 0.9 (0.5) | – | – | – | – | – | .0033 |
| Antidepressants use | 114 | 29.3% | 66 | 37.1% | 0 | 0.0% | – | – | – | .0642 |
| DDD antidepressants use | 106 | 1.7 (0.9) | 59 | 1.4 (0.8) | – | – | – | – | – | .0513 |
Categorical data are given as percentage while continuous data are given as mean (SD).
HC, healthy controls; SCZ, Schizophrenia; AFF, affective disorders; BMI, body mass index; PANSS, Positive and Negative Syndrome Scale; YMRS, Young Mania Rating Scale; GAF-S, Global Assessment of Functioning Scale; CRP, C-reactive protein.
1One-way ANOVA.
2Chi-square test.
3Scheffe’s post hoc test.
Circulating Markers of Gut Inflammation in Severe Mental Illnesses
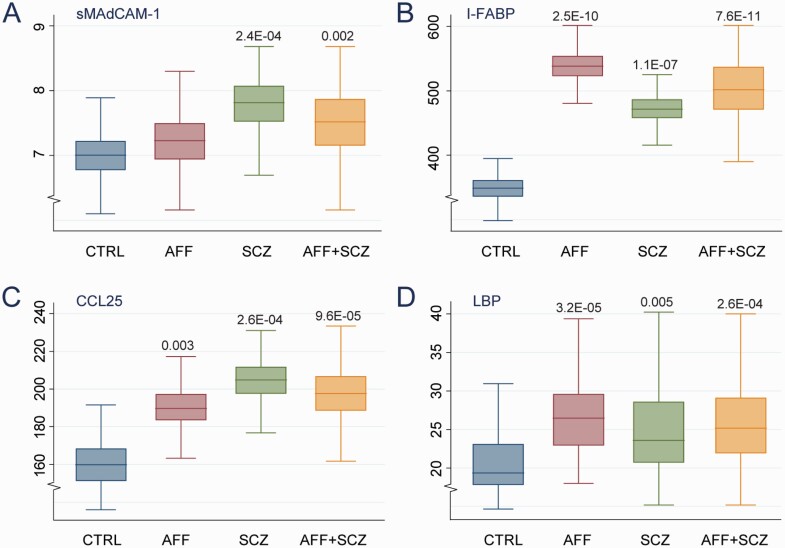
Distributions of plasma markers among SMI patients and HC and within diagnostic groups, with P-values adjusted for age, sex, BMI, CRP, and freezer storage time are shown in Figure 1, with coefficient estimates for different levels of adjustment shown in Table 2. Patients with SMI showed significantly higher plasma sMAdCAM-1 (P = .006), I-FABP (P = 7.3E−11), CCL25 (P = 3.8E−05) and LBP (P = 1.6E−07) than HC in age- and sex-adjusted analysis, with the highest levels in SCZ for sMAdCAM-1 (P = .001) and CCL25 (P = 8.0E−05), while I-FABP was highest (P = 3.5E−10) and sMAdCAM-1 was not regulated differently in AFF. The pattern of findings remained the same among patients not using APs (Supplementary Figure 1). For all markers, these differences remained significant when including BMI, CRP, and freezer storage time in the models, although LBP correlated positively with CRP (r = .42, P < .001) in the SMI group. Data analysis, not using imputed data is given in Supplementary Table 1 showing a similar pattern as with imputed data. We did not have information on GI disorders or antibiotic use in HC, but found 4 patients with GI disorders (2 with IBD, 2 with celiac disease) and 5 using antibiotics. Exclusion of these patients did not change the results as shown in the fully adjusted models in Supplementary Table 2
Figure 1.
Circulating gut and inflammatory proteins by group. (A) Soluble mucosal vascular addressin cell adhesion molecule1 (MAdCAM-1) (B) Intestinal fatty acid binding protein (I-FABP) (C) Chemokine (C–C motif) ligand 25 (CCL25) (D) Lipopolysaccharide binding protein (LBP) levels between SMI patients (AFF+SCZ, n = 567) and HC (n = 418) and within SCZ (n = 389) and AFF (n = 178) groups. Data are shown as adjusted (age, sex, BMI, CRP, and freezer storage time) marginal means.
Table 2.
Associations between diagnosis and inflammatory markers with different levels of adjustment
| Unadjusted | Age + sex + FST | Age + sex + FST + BMI | Age + sex + FST + BMI+ CRP | |||||
|---|---|---|---|---|---|---|---|---|
| β | P-value | β | P-value | β | P-value | β | P-value | |
| sMAdCAM-1 | ||||||||
| SCZ+AFF | 0.60 | .005 | 0.60 | .006 | 0.68 | .002 | 0.68 | .002 |
| SCZ | 0.79 | .001 | 0.80 | .001 | 0.94 | 1.8e−04 | 0.93 | 2.4e−04 |
| AFF | 0.18 | .489 | 0.12 | .664 | 0.12 | .658 | 0.12 | .669 |
| I-FABP | ||||||||
| SCZ+AFF | 141.77 | 7.3e−11 | 143.22 | 1.6e−10 | 149.06 | 6.9e−11 | 149.17 | 7.6e−11 |
| SCZ | 121.66 | 4.9e−08 | 121.01 | 2.0e−07 | 129.43 | 6.0e−08 | 127.56 | 1.1e−07 |
| AFF | 185.74 | 2.8e−10 | 188.74 | 3.5e−10 | 191.85 | 2.2e−10 | 191.54 | 2.5e−10 |
| CCL25 | ||||||||
| SCZ+AFF | 38.81 | 7.3e−05 | 41.46 | 3.8e−05 | 39.85 | 1.0e−04 | 40.01 | 9.6e−05 |
| SCZ | 42.86 | 7.8e−05 | 44.66 | 8.0e−05 | 42.31 | 2.7e−04 | 42.58 | 2.6e−04 |
| AFF | 29.94 | .003 | 30.48 | .003 | 31.11 | .003 | 31.05 | .003 |
| LBP | ||||||||
| SCZ+AFF | 3.68 | 3.6e−07 | 3.86 | 1.6e−07 | 2.97 | 5.0e−05 | 2.56 | 2.6e−04 |
| SCZ | 3.51 | 4.9e−06 | 3.80 | 1.6e−06 | 2.72 | 6.5e−04 | 2.15 | .005 |
| AFF | 4.03 | 1.9e−05 | 4.07 | 2.1e−05 | 3.61 | 1.3e−04 | 3.70 | 3.2e−05 |
Participants with CRP > 10mg/L were excluded.
FST, freezer storage time; BMI, body mass index.
As shown in Supplementary Table 3, none of the markers were associated with symptom/functionality scores except a positive association between LBP and PANSS total in SCZ (β = 0.24, P = .001).
Circulating Markers of Gut Inflammation in Relation to Treatment Modalities
Supplementary Table 4 show the associations between treatments and inflammatory gut markers evaluated in all patients. AP treatment or use of AE and AD were not independently associated with sMAdCAM-1 or CCL25 in our study and there were no significant associations with DDD for the medication groups within users. However, I-FABP was associated positively with DDD AP (β = 48.62, P = .035) and with AE use (β = 145.65, P = .001). On the contrary, LBP was associated negatively with AP use (β = −2.34, P = .048) and DDD AP (β = −1.34, P = .033).
Supplementary Table 5 shows the associations between lithium use and DDD lithium, and inflammatory gut markers. Lithium use was associated positively with LBP (β = 4.39, P = .028) but no significant associations were observed for any of the other inflammatory gut markers.
We next assessed treatment modalities within diagnostic groups. As shown in Supplementary Figure 2, within the SCZ group, AP and AD users had lower and higher levels of LBP, respectively. However, the biggest difference with regard to treatment was found for AE use, where SCZ users of AE had higher levels of I-FABP (P = 3.6E−04, Supplementary Figure 2C), driving the difference seen for the whole group in Supplementary Table 4.
Correlation Between Markers of Gut Inflammation and Inflammasome Activation
Presented in Table 3, we next assessed correlates between the gut leakage/inflammation markers and markers of inflammasome activation. sMAdCAM-1 levels correlated positively with IL-18 in SMI but not HC and with IL-18BPa in all groups. I-FABP correlated positively IL-18 in HC and with IL-18BPa in SM and within diagnostic groups. We adjusted for the number of gut*inflammasome markers (ie, 4 * 4 = 16). Thus, our adjusted threshold is 0.05/16 = 0.0031 for these correlations.
Table 3.
Associations between gut inflammation markers and markers of inflammasome activation (Spearman correlations)
| Patients | MAdCAM-1 | I-FABP | CCL25 | LBP | ||||
|---|---|---|---|---|---|---|---|---|
| rho | P | rho | P | rho | P | rho | P | |
| IL-18 | ||||||||
| HC | 0.01 | .791 | 0.06 | .250 | 0.01 | .821 | 0.10 | .050 |
| SCZ+AFF | 0.07 | .077 | 0.03 | .539 | 0.05 | .229 | 0.02 | .708 |
| SCZ | 0.03 | .559 | −0.02 | .729 | 0.01 | .853 | 0.03 | .597 |
| AFF | 0.15 | .050 | 0.16 | .039 | 0.14 | .068 | −0.01 | .896 |
| IL-18BPa | ||||||||
| HC | 0.05 | .285 | 0.01 | .859 | 0.01 | .866 | −0.00 | .985 |
| SCZ+AFF | 0.11 | .008 | 0.17 | <.001 | 0.04 | .421 | 0.02 | .556 |
| SCZ | 0.12 | .021 | 0.16 | .002 | 0.07 | .204 | 0.02 | .704 |
| AFF | 0.06 | .434 | 0.24 | .001 | −0.04 | .594 | 0.05 | .498 |
| NLRP3 | ||||||||
| HC | 0.07 | .294 | 0.04 | .553 | −0.06 | .408 | −0.01 | .875 |
| SCZ+AFF | 0.02 | .736 | −0.02 | .734 | 0.00 | .994 | 0.15 | .005 |
| SCZ | 0.01 | .850 | −0.07 | .295 | −0.07 | .305 | 0.15 | .020 |
| AFF | 0.03 | .729 | 0.07 | .439 | 0.12 | .188 | 0.14 | .112 |
| NLRC4 | ||||||||
| HC | 0.03 | .607 | −0.10 | .125 | −0.04 | .577 | 0.12 | .053 |
| SCZ+AFF | 0.06 | .284 | −0.05 | .377 | 0.02 | .662 | 0.19 | <.001 |
| SCZ | 0.03 | .660 | −0.09 | .178 | 0.00 | .993 | 0.22 | <.001 |
| AFF | 0.10 | .247 | 0.02 | .839 | 0.05 | .584 | 0.14 | .132 |
Bold = P < .0031.
A weak positive correlation between CCL25 and IL-18BPa was observed in SMI. LBP correlated positively with IL-18 in HC and with IL-18BPa in SMI and within diagnostic groups. No associations were detected between sMAdCAM-1, I-FABP, and CCL25 with NLRP3 or NLRC4 mRNA expression in leukocytes, but LBP correlated positively with NLRP3 in SCZ and with NLRC4 in all groups.
These correlations were also performed in subgroups based on treatment as well as above or below 5 mg/L CRP, as a high and low inflammation group, respectively. As shown in Supplementary Table 6 coefficients were in general similar in these subgroups. The association between LBP and NLRP3 or NLRC4 was not present in patients not using AP.
Expression of MADCAM1 in the Brain
In contrast to the other measured mediators of gut leakage/inflammation (see “Methods”), MADCAM1 is also expressed in the brain. We therefore next assessed MADCAM1 expression in RNA-seq data from a large sample (n = 474) of dorsolateral prefrontal cortex (DLPFC) postmortem tissues from the CMC. Differential expression analyses of the whole brain region (bulk RNA-seq) showed, however, no regulation of MADCAM1 in SMI (Supplementary Figure 3).
Discussion
In the present study, we hypothesized that gut leakage of microbial products due to intestinal inflammation could contribute to systemic inflammation, at least partly due to activation of innate immunity that involve inflammasome activation, in SMI. Our major findings were: (1) higher levels of sMAdCAM-1, I-FABP, and LBP in SMI compared to HC, with the highest levels of sMAdCAM-1 in SCZ and I-FABP in AFF, (2) higher sMAdCAM-1 and I-FABP were independent of BMI and CRP while differences in LBP were mitigated by these factors, (3) modestly elevated CCL25 in SMI, and (4) sMAdCAM-1, I-FABP and LBP correlated with inflammasome activation as reflected by levels of IL-18 and IL-18BPa and leukocyte mRNA expression of NLRP3 and NLRC4. Our findings support that intestinal barrier inflammation and dysfunction in SMI could contribute to systemic inflammation through inflammasome activation in these patients.
Gut microbiota alterations have been reported in SMI over the last years17–20 and there are indications of increased levels of microbial products in the circulation of patients with SCZ57,58 and AFF,59 as summarized in a recent meta-analysis.60 Leakage of microbial products such as LPS to the circulation will stimulate LBP production in the liver and while our finding of increased LBP levels in SMI in age- and sex-adjusted analysis supports bacterial translocation in these patients, the attenuation of these differences upon adjustment with BMI and CRP merit further consideration. First, adipose tissue derived LBP has been shown to promote systemic inflammation and metabolic deterioration in clinical and experimental models of obesity.61,62 Second, although CRP and LBP both are acute phase proteins mainly produced in the liver and a strong correlation between them is expected, we would anticipate a significant leakage of microbial products to induce circulating LBP levels beyond those explained by BMI and CRP. However, gut dysbiosis and endotoxin levels correlate strongly with CRP in other patients with metabolic disease and intestinal inflammation.63,64 Severance et al. demonstrated higher LBP only in SCZ patients with gut and endocrine disturbances, mainly driven by obesity.65 Bacterial translocation from the gut into the circulation has been shown to correlate with negative symptoms, neurocognitive impairments, and aggression in SCZ,57,58 however, we observed only a modest association between LBP level and PANSS total score in SCZ. Finally, the lower LBP in AP users in SCZ could suggest a beneficial effect of these drugs, however, only in AP users we observed a positive correlation between LBP and inflammasome mRNA expression making these results hard to interpret. In contrast, LBP was positively weakly associated with lithium use. However, for effects of treatment modalities, these are best assessed by a temporal design, adjusting for changes in relevant demographics (eg, BMI). Taken together, while LBP seems to reflect the enhanced systemic inflammation in SMI, it is unclear in what degree it also reflect gut leakage of microbial products such as LPS.
Leakage of microbial products to the systemic is dependent on gut barrier disruption, which has been evaluated in SMI through zonulin, an established modulator and marker of intestinal permeability, and increased levels have been demonstrated in SCZ66,67 and AFF.68,69 In addition, Maes et al. demonstrated zonulin mediated breakdown of the gut barrier and linked bacterial translocation to indices of BBB breakdown, negative symptoms and cognitive impairments in SCZ patients.57,70,71 A major finding in our study was the markedly higher levels of I-FABP in AFF and SCZ. I-FABP is a cytosolic protein exclusively expressed in epithelial cells in the small and large intestine and conditions with enhanced mucosal inflammation and gut barrier dysfunction due to intestinal epithelial cell damage show increased systemic levels.45 Thus, while zonulin may reflect increased intestinal permeability, our finding of increased I-FABP, further suggest there may be significant intestinal epithelial cell damage in SMI.42 In our patients, AE use was associated with higher I-FABP levels in SCZ, possibly reflecting some adverse GI effect which has been reported with AE treatment.72,73
MAdCAM-1 seem to play a major role in leukocyte homing into intestinal mucosa, and for B and T cells, CCL9 could also play a role.35,36 These mechanisms are shown to contribute to the chronically inflamed intestine and barrier dysfunction in inflammatory bowel disease.74–76 Our finding of increased sMAdCAM-1 levels in SCZ thus support enhanced leukocyte homing into the intestine in these patients. However, MAdCAM-1 expression has also been reported in the brain47 suggesting it could promote leukocyte trafficking across the BBB. In experimental autoimmune encephalomyelitis, MAdCAM-1 has been suggested to have a role in CNS immune surveillance.77,78 However, an experimental study in mice evaluating the regulation of MAdCAM-1 in acute and chronic inflammation, demonstrated substantial constitutive expression in colonic tissues, that were enhanced by TNF, while no expression was observed in the brain.79 Moreover, in patients with multiple sclerosis, MAdCAM immunoreactivity could not be detected in brain tissue.80 While we detected MADCAM1 mRNA expression in brain necropsies, we observed no differences between patients and controls suggesting the higher systemic levels of sMAdCAM-1 in SCZ probably reflect dysregulated levels in gut-associated endothelial cells or lymphoid tissues, the main sources of MAdCAM-1.33,34 We speculate that the enhanced CCL25 and sMAdCAM-1 levels, at least in SCZ, could reflect increased homing of T cells to the gut and reflect intestinal inflammation. Increased CCL25 levels have been reported in bipolar disorder previously81,82 and increased CCR9 mRNA has been detected in PBMC from patients with SCZ83 which could further enhance T and B cell homing to the gut.
Experimental studies suggest that LPS directly may cause infiltration of leukocytes in the brain,84 and through systemic inflammasome activation, promote neuroinflammation.85 Even chronic exposure of LPS at physiological doses, insufficient to cause acute behavioral alterations, enhances sickness behavior and neural responses over time.86 We recently demonstrated increased plasma levels of IL-18 and IL-18BPa, linked to a higher expression of inflammasome-related genes (NLRP3 and NLRC4) in blood leukocytes in SMI,26 supporting systemic inflammasome activation in these patients. Our finding that I-FABP, sMAdCAM-1, and LBP correlated with IL-18BPa, sMAdCAM-1 correlated with IL-18 and LBP correlated with expression of NLRP3 and NLRC4, with stronger associations in SMI than HC, could suggest that gut inflammation and leakage of microbial products may contribute to chronic dysregulation of innate immune responses through systemic inflammasome activation in SMI. However, as stress related behavior may influence inflammasome activation87,88 and the microbiome,20 enhanced systemic inflammation could augment gut inflammation and leakage of microbial products, further promoting a vicious circle in SMI.
Limitations to our study include (1) despite adjustments for a comprehensive range of variables, residual confounding factors cannot be ruled out, (2) the cross-sectional nature of the study imply we cannot explore causal relationships. The cross-sectional design, after initiating therapy, will in particular hamper the analyses related to the effects of medications on inflammatory markers, (3) the study was not designed to look at intestinal inflammation and leakage of microbial products and lacks relevant fecal biomarkers (eg, fecal calprotectin) or microbiota. Also, the TOP study was not specifically designed to look at GI related issues, and lacked a detailed questionnaire for GI related comorbidities, antibiotic use and dietary information in the whole population. (4) While the biomarkers in our study are relatively specific with regards to tissue, their ability to specifically reflect intestinal inflammation is not firmly established and are currently not used in clinical practice.89,90 (5) Although statistically significant, the coefficients for correlations between inflammatory markers and IL-18 and IL-18BPa were modest. However, systemic inflammation in SMI is longstanding and subtle with multiple sources that also could contribute to inflammasome activation such as smoking,91 adiposity and dyslipidemia,92 in addition to the potential effects of bacterial translocation and gut inflammation.
In conclusion, our data show that, compared to healthy controls, patients with SMI have elevated markers of gut inflammation as reflected by CCL25 and sMAdCAM-1 and leakage as reflected by I-FABP and LBP that correlate with systemic inflammasome activation. To which degree these markers reflect mechanisms that contribute to CNS pathology and represent targets for intervention in these patients should be pursued in future studies.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Acknowledgments
We would like to thank the participants of the study.
Contributor Information
Søren B Jensen, Research Institute of Internal Medicine, Oslo University Hospital, Oslo, Norway.
Mashhood A Sheikh, Research Institute of Internal Medicine, Oslo University Hospital, Oslo, Norway.
Ibrahim A Akkouh, Division of Mental Health and Addiction, Norwegian Centre for Mental Disorders Research, NORMENT, Oslo University Hospital, Oslo, Norway; Department of Medical Genetics, Oslo University Hospital, Oslo, Norway.
Attila Szabo, Division of Mental Health and Addiction, Norwegian Centre for Mental Disorders Research, NORMENT, Oslo University Hospital, Oslo, Norway; Department of Medical Genetics, Oslo University Hospital, Oslo, Norway; K.G. Jebsen Center for Neurodevelopmental disorders, Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Kevin S O’Connell, Division of Mental Health and Addiction, Norwegian Centre for Mental Disorders Research, NORMENT, Oslo University Hospital, Oslo, Norway.
Tove Lekva, Research Institute of Internal Medicine, Oslo University Hospital, Oslo, Norway.
John A Engh, Division of Mental Health and Addiction, Norwegian Centre for Mental Disorders Research, NORMENT, Oslo University Hospital, Oslo, Norway; Division of Mental health and Addiction, Vestfold Hospital Trust, Tønsberg, Norway.
Ingrid Agartz, K.G. Jebsen Center for Neurodevelopmental disorders, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Clinical Neuroscience, Centre for Psychiatry Research, Karolinska Institutet and Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden; Department of Psychiatric Research, Diakonhjemmet Hospital, Oslo, Norway; Norwegian Centre for Mental Disorders Research, NORMENT, Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Torbjørn Elvsåshagen, Division of Mental Health and Addiction, Norwegian Centre for Mental Disorders Research, NORMENT, Oslo University Hospital, Oslo, Norway.
Monica B E G Ormerod, Division of Mental Health and Addiction, Norwegian Centre for Mental Disorders Research, NORMENT, Oslo University Hospital, Oslo, Norway; Norwegian Centre for Mental Disorders Research, NORMENT, Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Melissa A Weibell, Division of Psychiatry, Network for Clinical Psychosis Research, Stavanger University Hospital, Stavanger, Norway; Network for Medical Sciences, Faculty of Health, University of Stavanger, Stavanger, Norway.
Erik Johnsen, Division of Psychiatry, Haukeland University Hospital, Bergen, Norway; Department of Clinical Medicine, University of Bergen, Bergen, Norway; NORMENT Center of Excellence, University of Bergen and Haukeland University Hospital, Bergen, Norway.
Rune A Kroken, Division of Psychiatry, Haukeland University Hospital, Bergen, Norway; Department of Clinical Medicine, University of Bergen, Bergen, Norway; NORMENT Center of Excellence, University of Bergen and Haukeland University Hospital, Bergen, Norway.
Ingrid Melle, Division of Mental Health and Addiction, Norwegian Centre for Mental Disorders Research, NORMENT, Oslo University Hospital, Oslo, Norway; Norwegian Centre for Mental Disorders Research, NORMENT, Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Ole K Drange, Department of Mental Health, Norwegian University of Science and Technology, Trondheim, Norway; Department of Østmarka, Division of Mental Health, St. Olavs University Hospital, Trondheim, Norway; Department of Psychiatry, Sørlandet Hospital, Kristiansand, Norway.
Terje Nærland, K.G. Jebsen Center for Neurodevelopmental disorders, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Rare Disorders, Division of Child and Adolescent medicine, Oslo University Hospital, Oslo, Norway.
Arne E Vaaler, Department of Mental Health, Norwegian University of Science and Technology, Trondheim, Norway; Department of Østmarka, Division of Mental Health, St. Olavs University Hospital, Trondheim, Norway.
Lars T Westlye, Division of Mental Health and Addiction, Norwegian Centre for Mental Disorders Research, NORMENT, Oslo University Hospital, Oslo, Norway; K.G. Jebsen Center for Neurodevelopmental disorders, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Psychology, University of Oslo, Oslo, Norway.
Pål Aukrust, Research Institute of Internal Medicine, Oslo University Hospital, Oslo, Norway; Section of Clinical Immunology and Infectious Diseases, Oslo University Hospital Rikshospitalet, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Srdjan Djurovic, Division of Mental Health and Addiction, Norwegian Centre for Mental Disorders Research, NORMENT, Oslo University Hospital, Oslo, Norway; Department of Medical Genetics, Oslo University Hospital, Oslo, Norway; K.G. Jebsen Center for Neurodevelopmental disorders, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Psychology, University of Oslo, Oslo, Norway.
Nils Eiel Steen, Division of Mental Health and Addiction, Norwegian Centre for Mental Disorders Research, NORMENT, Oslo University Hospital, Oslo, Norway; Norwegian Centre for Mental Disorders Research, NORMENT, Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Ole A Andreassen, Division of Mental Health and Addiction, Norwegian Centre for Mental Disorders Research, NORMENT, Oslo University Hospital, Oslo, Norway; K.G. Jebsen Center for Neurodevelopmental disorders, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Norwegian Centre for Mental Disorders Research, NORMENT, Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Thor Ueland, Research Institute of Internal Medicine, Oslo University Hospital, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway; K.G. Jebsen Thrombosis Research and Expertise Center, University of Tromsø, Tromsø, Norway.
Funding
We are also grateful for the financial support provided by South East Norway Health Authority (project 2018089) for this study.
Conflicts of interest
OAA is a consultant to HealthLytix and has received speaker’s honorarium from Sunovion and Lundbeck. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1. Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sekar A, Bialas AR, de Rivera H, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530(7589):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dieset I, Djurovic S, Tesli M, et al. Up-regulation of NOTCH4 gene expression in bipolar disorder. Am J Psychiatry. 2012;169(12):1292–1300. [DOI] [PubMed] [Google Scholar]
- 4. Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB.. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168(12):1303–1310. [DOI] [PubMed] [Google Scholar]
- 5. Kohler O, Petersen L, Mors O, et al. Infections and exposure to anti-infective agents and the risk of severe mental disorders: a nationwide study. Acta Psychiatr Scand. 2017;135(2):97–105. [DOI] [PubMed] [Google Scholar]
- 6. Weber NS, Gressitt KL, Cowan DN, Niebuhr DW, Yolken RH, Severance EG.. Monocyte activation detected prior to a diagnosis of schizophrenia in the US Military New Onset Psychosis Project (MNOPP). Schizophr Res. 2018;197:465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hope S, Melle I, Aukrust P, et al. Similar immune profile in bipolar disorder and schizophrenia: selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disord. 2009;11(7):726–734. [DOI] [PubMed] [Google Scholar]
- 8. Drexhage RC, Knijff EM, Padmos RC, et al. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010;10(1):59–76. [DOI] [PubMed] [Google Scholar]
- 9. Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A.. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2013;73(10):993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Picker LJ, Morrens M, Chance SA, Boche D.. Microglia and brain plasticity in acute psychosis and schizophrenia illness course: a meta-review. Front Psychiatry. 2017;8:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Najjar S, Pahlajani S, De Sanctis V, Stern JNH, Najjar A, Chong D.. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mondelli V, Vernon AC, Turkheimer F, Dazzan P, Pariante CM.. Brain microglia in psychiatric disorders. Lancet Psychiatry. 2017;4(7):563–572. [DOI] [PubMed] [Google Scholar]
- 13. Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB.. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2(3):258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown S, Kim M, Mitchell C, Inskip H.. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry. 2010;196(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wahlbeck K, Westman J, Nordentoft M, Gissler M, Laursen TM.. Outcomes of Nordic mental health systems: life expectancy of patients with mental disorders. Br J Psychiatry. 2011;199(6):453–458. [DOI] [PubMed] [Google Scholar]
- 16. Troseid M, Andersen GO, Broch K, Hov JR.. The gut microbiome in coronary artery disease and heart failure: current knowledge and future directions. EBioMedicine. 2020;52:102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li S, Song J, Ke P, et al. The gut microbiome is associated with brain structure and function in schizophrenia. Sci Rep. 2021;11(1):9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwarz E, Maukonen J, Hyytiainen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398–403. [DOI] [PubMed] [Google Scholar]
- 19. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu F, Ju Y, Wang W, et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat Commun. 2020;11(1):1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S.. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21(6):738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vindegaard N, Speyer H, Nordentoft M, Rasmussen S, Benros ME.. Gut microbial changes of patients with psychotic and affective disorders: a systematic review. Schizophr Res. 2021;234:1–10. [DOI] [PubMed] [Google Scholar]
- 23. Shoubridge AP, Choo JM, Martin AM, et al. The gut microbiome and mental health: advances in research and emerging priorities. Mol Psychiatry. 2022;27(4):1908–1919. [DOI] [PubMed] [Google Scholar]
- 24. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. [DOI] [PubMed] [Google Scholar]
- 25. Sahoo M, Ceballos-Olvera I, del Barrio L, Re F.. Role of the inflammasome, IL-1beta, and IL-18 in bacterial infections. ScientificWorldJournal. 2011;11:2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szabo A, O’Connell KS, Ueland T, et al. Increased circulating IL-18 levels in severe mental disorders indicate systemic inflammasome activation. Brain Behav Immun. 2021;99:299–306. [DOI] [PubMed] [Google Scholar]
- 27. van Wijk F, Cheroutre H.. Mucosal T cells in gut homeostasis and inflammation. Expert Rev Clin Immunol. 2010;6(4):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wurbel MA, McIntire MG, Dwyer P, Fiebiger E.. CCL25/CCR9 interactions regulate large intestinal inflammation in a murine model of acute colitis. PLoS One. 2011;6(1):e16442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wendland M, Czeloth N, Mach N, et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci USA. 2007;104(15):6347–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wurbel MA, Philippe JM, Nguyen C, et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30(1):262–271. [DOI] [PubMed] [Google Scholar]
- 31. Trivedi PJ, Bruns T, Ward S, et al. Intestinal CCL25 expression is increased in colitis and correlates with inflammatory activity. J Autoimmun. 2016;68:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh UP, Singh NP, Murphy EA, et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine. 2016;77:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kraal G, Schornagel K, Streeter PR, Holzmann B, Butcher EC.. Expression of the mucosal vascular addressin, MAdCAM-1, on sinus-lining cells in the spleen. Am J Pathol. 1995;147(3):763–771. [PMC free article] [PubMed] [Google Scholar]
- 34. Nakache M, Berg EL, Streeter PR, Butcher EC.. The mucosal vascular addressin is a tissue-specific endothelial cell adhesion molecule for circulating lymphocytes. Nature. 1989;337(6203):179–181. [DOI] [PubMed] [Google Scholar]
- 35. Miles A, Liaskou E, Eksteen B, Lalor PF, Adams DH.. CCL25 and CCL28 promote alpha4 beta7-integrin-dependent adhesion of lymphocytes to MAdCAM-1 under shear flow. Am J Physiol Gastrointest Liver Physiol. 2008;294(5):G1257–G1267. [DOI] [PubMed] [Google Scholar]
- 36. Wendt E, White GE, Ferry H, Huhn M, Greaves DR, Keshav S.. Glucocorticoids suppress CCR9-mediated chemotaxis, calcium flux, and adhesion to MAdCAM-1 in human T cells. J Immunol. 2016;196(9):3910–3919. [DOI] [PubMed] [Google Scholar]
- 37. Arihiro S, Ohtani H, Suzuki M, et al. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn’s disease. Pathol Int. 2002;52(5–6):367–374. [DOI] [PubMed] [Google Scholar]
- 38. Menon TS MD. Soluble MAdCAM-1: a potential predictor of clinical outcome in Inflammatory Bowel Disease (IBD). Am J Gastroenterol. 2018;113:392–394. [Google Scholar]
- 39. Pelsers MM, Namiot Z, Kisielewski W, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36(7):529–535. [DOI] [PubMed] [Google Scholar]
- 40. Wells JM, Brummer RJ, Derrien M, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312(3):G171–G193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pelsers MM, Hermens WT, Glatz JF.. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352(1–2):15–35. [DOI] [PubMed] [Google Scholar]
- 42. Adriaanse MP, Tack GJ, Passos VL, et al. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment Pharmacol Ther. 2013;37(4):482–490. [DOI] [PubMed] [Google Scholar]
- 43. Reisinger KW, Derikx JP, Thuijls G, et al. Noninvasive measurement of intestinal epithelial damage at time of refeeding can predict clinical outcome after necrotizing enterocolitis. Pediatr Res. 2013;73(2):209–213. [DOI] [PubMed] [Google Scholar]
- 44. Relja B, Szermutzky M, Henrich D, et al. Intestinal-FABP and liver-FABP: novel markers for severe abdominal injury. Acad Emerg Med. 2010;17(7):729–735. [DOI] [PubMed] [Google Scholar]
- 45. Funaoka H, Kanda T, Fujii H.. Intestinal fatty acid-binding protein (I-FABP) as a new biomarker for intestinal diseases. Rinsho Byori. 2010;58(2):162–168. [PubMed] [Google Scholar]
- 46. Pastor Rojo O, Lopez San Roman A, Albeniz Arbizu E, de la Hera Martinez A, Ripoll Sevillano E, Albillos Martinez A.. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(3):269–277. [DOI] [PubMed] [Google Scholar]
- 47. Leung E, Greene J, Ni J, et al. Cloning of the mucosal addressin MAdCAM-1 from human brain: identification of novel alternatively spliced transcripts. Immunol Cell Biol. 1996;74(6):490–496. [DOI] [PubMed] [Google Scholar]
- 48. Mac Giollabhui N, Ellman LM, Coe CL, Byrne ML, Abramson LY, Alloy LB.. To exclude or not to exclude: considerations and recommendations for C-reactive protein values higher than 10 mg/L. Brain Behav Immun. 2020;87:898–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kay SR, Fiszbein A, Opler LA.. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 50. Young RC, Biggs JT, Ziegler VE, Meyer DA.. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 51. Pedersen G, Hagtvet KA, Karterud S.. Generalizability studies of the Global Assessment of Functioning-Split version. Compr Psychiatry. 2007;48(1):88–94. [DOI] [PubMed] [Google Scholar]
- 52. Simonsen C, Sundet K, Vaskinn A, et al. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr Bull. 2011;37(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morch RH, Dieset I, Faerden A, et al. Inflammatory markers are altered in severe mental disorders independent of comorbid cardiometabolic disease risk factors. Psychol Med. 2019;49(10):1749–1757. [DOI] [PubMed] [Google Scholar]
- 54. Morch RH, Dieset I, Faerden A, et al. Inflammatory evidence for the psychosis continuum model. Psychoneuroendocrinology. 2016;67:189–197. [DOI] [PubMed] [Google Scholar]
- 55. Ishida I, Ogura J, Aizawa E, et al. Gut permeability and its clinical relevance in schizophrenia. Neuropsychopharmacol Rep. 2022;42(1):70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Severance EG, Alaedini A, Yang S, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012;138(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maes M, Sirivichayakul S, Kanchanatawan B, Vodjani A.. Breakdown of the paracellular tight and adherens junctions in the gut and blood brain barrier and damage to the vascular barrier in patients with deficit schizophrenia. Neurotox Res. 2019;36(2):306–322. [DOI] [PubMed] [Google Scholar]
- 58. Wang C, Zhang T, He L, et al. Bacterial translocation associates with aggression in schizophrenia inpatients. Front Syst Neurosci. 2021;15:704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Simeonova D, Stoyanov D, Leunis JC, et al. Increased serum immunoglobulin responses to gut commensal Gram-negative bacteria in unipolar major depression and bipolar disorder type 1, especially when melancholia is present. Neurotox Res. 2020;37(2):338–348. [DOI] [PubMed] [Google Scholar]
- 60. Safadi JM, Quinton AMG, Lennox BR, Burnet PWJ, Minichino A.. Gut dysbiosis in severe mental illness and chronic fatigue: a novel trans-diagnostic construct? A systematic review and meta-analysis. Mol Psychiatry. 2021;27:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim KE, Cho YS, Baek KS, et al. Lipopolysaccharide-binding protein plasma levels as a biomarker of obesity-related insulin resistance in adolescents. Korean J Pediatr. 2016;59(5):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moreno-Navarrete JM, Escote X, Ortega F, et al. A role for adipocyte-derived lipopolysaccharide-binding protein in inflammation- and obesity-associated adipose tissue dysfunction. Diabetologia. 2013;56(11):2524–2537. [DOI] [PubMed] [Google Scholar]
- 63. Salguero MV, Al-Obaide MAI, Singh R, Siepmann T, Vasylyeva TL.. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med. 2019;18(5):3461–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ishida N, Higuchi T, Miyazu T, et al. C-reactive protein is superior to fecal biomarkers for evaluating colon-wide active inflammation in ulcerative colitis. Sci Rep. 2021;11(1):12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Severance EG, Dickerson F, Yolken RH.. Complex gastrointestinal and endocrine sources of inflammation in schizophrenia. Front Psychiatry. 2020;11:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Usta A, Kilic F, Demirdas A, Isik U, Doguc DK, Bozkurt M.. Serum zonulin and claudin-5 levels in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2021;271(4):767–773. [DOI] [PubMed] [Google Scholar]
- 67. Barber GS, Sturgeon C, Fasano A, et al. Elevated zonulin, a measure of tight-junction permeability, may be implicated in schizophrenia. Schizophr Res. 2019;211:111–112. [DOI] [PubMed] [Google Scholar]
- 68. Maget A, Dalkner N, Hamm C, et al. Sex differences in zonulin in affective disorders and associations with current mood symptoms. J Affect Disord. 2021;294:441–446. [DOI] [PubMed] [Google Scholar]
- 69. Kilic F, Isik U, Demirdas A, Doguc DK, Bozkurt M.. Serum zonulin and claudin-5 levels in patients with bipolar disorder. J Affect Disord. 2020;266:37–42. [DOI] [PubMed] [Google Scholar]
- 70. Maes M, Kanchanatawan B, Sirivichayakul S, Carvalho AF.. In schizophrenia, increased plasma IgM/IgA responses to gut commensal bacteria are associated with negative symptoms, neurocognitive impairments, and the deficit phenotype. Neurotox Res. 2019;35(3):684–698. [DOI] [PubMed] [Google Scholar]
- 71. Maes M, Sirivichayakul S, Kanchanatawan B, Vodjani A.. Upregulation of the intestinal paracellular pathway with breakdown of tight and adherens junctions in deficit schizophrenia. Mol Neurobiol. 2019;56(10):7056–7073. [DOI] [PubMed] [Google Scholar]
- 72. Jahromi SR, Togha M, Fesharaki SH, et al. Gastrointestinal adverse effects of antiepileptic drugs in intractable epileptic patients. Seizure. 2011;20(4):343–346. [DOI] [PubMed] [Google Scholar]
- 73. De Hert M, Dockx L, Bernagie C, et al. Prevalence and severity of antipsychotic related constipation in patients with schizophrenia: a retrospective descriptive study. BMC Gastroenterol. 2011;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mann ER, McCarthy NE, Peake ST, et al. Skin- and gut-homing molecules on human circulating gammadelta T cells and their dysregulation in inflammatory bowel disease. Clin Exp Immunol. 2012;170(2):122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wendt E, Keshav S.. CCR9 antagonism: potential in the treatment of inflammatory bowel disease. Clin Exp Gastroenterol. 2015;8:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Trivedi PJ, Adams DH.. Gut-liver immunity. J Hepatol. 2016;64(5):1187–1189. [DOI] [PubMed] [Google Scholar]
- 77. Kanwar JR, Kanwar RK, Krissansen GW.. Simultaneous neuroprotection and blockade of inflammation reverses autoimmune encephalomyelitis. Brain. 2004;127(Pt 6):1313–1331. [DOI] [PubMed] [Google Scholar]
- 78. Engelhardt B, Wolburg-Buchholz K, Wolburg H.. Involvement of the choroid plexus in central nervous system inflammation. Microsc Res Tech. 2001;52(1):112–129. [DOI] [PubMed] [Google Scholar]
- 79. Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB.. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol. 1999;65(3):349–355. [DOI] [PubMed] [Google Scholar]
- 80. Allavena R, Noy S, Andrews M, Pullen N.. CNS elevation of vascular and not mucosal addressin cell adhesion molecules in patients with multiple sclerosis. Am J Pathol. 2010;176(2):556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Poletti S, Vai B, Mazza MG, et al. A peripheral inflammatory signature discriminates bipolar from unipolar depression: a machine learning approach. Prog Neuropsychopharmacol Biol Psychiatry. 2021;105:110136. [DOI] [PubMed] [Google Scholar]
- 82. Goteson A, Isgren A, Sparding T, et al. A serum proteomic study of two case-control cohorts identifies novel biomarkers for bipolar disorder. Transl Psychiatry. 2022;12(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hu WJ. Schizophrenia is a TH2 dominant autoimmune disease possibly against acetylcholine receptors of CNS. Nature Precedings. 2012. doi: 10.1038/npre.2012.6978.1. [DOI]
- 84. Thomson CA, McColl A, Graham GJ, Cavanagh J.. Sustained exposure to systemic endotoxin triggers chemokine induction in the brain followed by a rapid influx of leukocytes. J Neuroinflamm. 2020;17(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhu W, Cao FS, Feng J, et al. NLRP3 inflammasome activation contributes to long-term behavioral alterations in mice injected with lipopolysaccharide. Neuroscience. 2017;343:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tarr AJ, Chen Q, Wang Y, Sheridan JF, Quan N.. Neural and behavioral responses to low-grade inflammation. Behav Brain Res. 2012;235(2):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Maslanik T, Mahaffey L, Tannura K, Beninson L, Greenwood BN, Fleshner M.. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun. 2013;28:54–62. [DOI] [PubMed] [Google Scholar]
- 88. Iwata M, Ota KT, Duman RS.. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dragoni G, Innocenti T, Galli A.. Biomarkers of inflammation in inflammatory bowel disease: How long before abandoning single-marker approaches? Dig Dis. 2021;39(3):190–203. [DOI] [PubMed] [Google Scholar]
- 90. Bennike T, Birkelund S, Stensballe A, Andersen V.. Biomarkers in inflammatory bowel diseases: current status and proteomics identification strategies. World J Gastroenterol. 2014;20(12):3231–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ma Y, Long Y, Chen Y.. Roles of inflammasome in cigarette smoke-related diseases and physiopathological disorders: mechanisms and therapeutic opportunities. Front Immunol. 2021;12:720049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Litwiniuk A, Bik W, Kalisz M, Baranowska-Bik A.. Inflammasome NLRP3 potentially links obesity-associated low-grade systemic inflammation and insulin resistance with Alzheimer’s disease. Int J Mol Sci . 2021;22(11):5603. doi: 10.3390/ijms22115603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.