Abstract
Background and Hypothesis
Antipsychotics (APs), the cornerstone of schizophrenia treatment, confer a relatively high risk of constipation. However, the mechanisms underpinning AP-induced constipation are poorly understood. Thus, we hypothesized that (1) schizophrenia patients with AP-induced constipation have distinct metabolic patterns; (2) there is more than one mechanism at play in producing this adverse drug effect; and (3) AP-associated changes in the gut microbiome are related to the altered metabolic profiles.
Study Design
Eighty-eight schizophrenia patients, including 44 with constipation (C) and 44 matched patients without constipation (NC), were enrolled in this study. Constipation was diagnosed by Rome IV criteria for constipation and colonic transit time using radiopaque markers (ROMs) while severity was evaluated with the Bristol Stool Form Scale (BSS) and Constipation Assessment Scale (CAS). Fasting blood samples were drawn from all participants and were subjected to non-targeted liquid chromatography-mass spectrometry (LC-MS) metabolomic analysis.
Study Results
Eleven metabolites were significantly altered in AP-induced constipation which primarily disturbed sphingolipid metabolism, choline metabolism, and sphingolipid signaling pathway (P value < .05, FDR < 0.05). In the C group, changes in the gut bacteria showed a certain degree of correlation with 2 of the significantly altered serum metabolites and were associated with alterations in choline metabolism.
Conclusions
Our findings indicated that there were disturbances in distinct metabolic pathways that were associated with AP-induced constipation. In addition, this study presents evidence of a link between alterations in the gut microbiome and host metabolism which provides additional mechanistic insights on AP-induced constipation.
Keywords: antipsychotics, constipation, gastrointestinal hypomotility, mechanism, metabolomics, schizophrenia
Introduction
Antipsychotics (APs), the gold-standard treatment for schizophrenia, are frequently associated with a high risk of constipation.1 AP-induced constipation has a prevalence rate as high as 60% in schizophrenia patients.2 Among conventional APs, trifluoperazine (~40%), loxapine (~40%), fluphenazine (~30%), thioridazine (~20%), and pimozide (~20%) confer the greatest constipation risk while atypical APs, including clozapine (~80%), paliperidone (~30%), quetiapine (~25%), ziprasidone (~15%), and risperidone (~12%), have the highest probability of inducing constipation.3 Both the physical and mental states of schizophrenia patients are worsened by constipation which can potentially develop into more serious conditions and result in a fatal outcome if it is not detected and treated on time.4,5
Despite its relatively high incidence and life-threatening potentiality, the underlying molecular mechanisms responsible for AP-induced constipation are not entirely known. Neurotransmitter mechanisms have been implicated in AP-induced constipation via the modulation of neurotransmission.3,6–12 The anticholinergic activity of some AP drugs or the concurrent administration of anticholinergic agents can suppress duodenal contraction and delay gastrointestinal transit time. APs additionally have antagonistic effects on several receptors. Antagonism of 5-hydroxytryptamine (5-HT) receptors, H1 receptors, α-noradrenergic receptors, and dopaminergic receptors can inhibit gastrointestinal tract motility and prolong intestinal peristalsis. The activation of δ-opioid receptor can also lead to the impediment of colonic transit time. Thus, the activity of APs at a synaptic level has the potential ability of inducing constipation. However, there might be other mechanisms at play in mediating constipation in patients undergoing AP treatment.
Over the last few years, considerable attention has been given to the relationship of the gut microbiome with neuropsychiatric diseases and psychiatric medication due to the communication and connection of the gut with the central nervous system.13 Although there is accumulating evidence for the presence of gut microbiome alterations in schizophrenia, the current data on the gut microbiome’s diversity, including alpha and beta diversity, are highly heterogeneous and conflicting, regardless of medication status.14–17 A few studies documented alterations in either or both diversity indices while other investigations reported no significant differences in one or both diversity indices.18–23 However, since these experiments were performed in patients who were under AP therapy, it is not possible to distinguish whether the differences in the gut microbiome were related to the disease itself or occurred as a result of AP administration. Interestingly, research that investigated changes in the gut microbiome of drug-naïve schizophrenia patients and those that explored the impact of AP on the gut microbiome in schizophrenia demonstrated similar discrepancies in diversity as with studies which did not factor in AP therapy.24–29 These findings illustrate that the interplay between host and gut microbiome is far more intricate than can be modeled by microbial diversity. Nonetheless, the literature identified substantial richness changes in an inexhaustible list of diverse taxa in schizophrenia, irrespective of AP administration and with little consistency across investigations.14–16 The contrasting picture presented by the current literature prompts further analyses employing metabolomics which can provide better insight into these differences.
While APs exert a strong effect on microbial composition in the gastrointestinal tract, the gut microbiome can also reciprocally influence the effectiveness, tolerability, and toxicity of APs.9,30 These interactions suggest the presence of a bidirectional interaction between the gut microbiome and APs, which can potentially exacerbate AP-induced side effects. It was demonstrated that the gut microbiota partially mediated the metabolic side effects attributable to APs.31 In our previously published article, it was reported that AP-induced constipation exhibited an increase in alpha diversity; lower abundance of phyla Bacteriodetes and Fusobacteria; higher abundance of phyla Firmicutes, Verrucomicrobia, and Synergistetes; the increased richness of genus Christensenella and Desulfovibrio in contrast to the nonconstipation group which is somewhat inconsistent with previous findings. Furthermore, functional predictions of the gut microbiome revealed significant alterations in the propionate and vitamin B6 metabolism in patients with constipation.32 Nonetheless, the precise etiology and interaction of gut microbiome pertaining to AP-induced constipation have not yet been elucidated.
Psychiatric patients under AP therapy have an elevated risk of metabolic disturbances, including alterations in cholesterol, triglycerides, glucose, and insulin levels.33–38 Some studies reported that these disruptions were associated with a reduction in defecation and constipation.39–41 In addition, there is mounting evidence indicating the presence of metabolic dysfunctions, and gut microbiome alterations in association with AP prescription.28,32,42 It was previously revealed that AP agents induced metabolic abnormalities, such as altered glucose and lipid metabolism,43,44 which have been associated with alterations in the intestinal epithelial barrier and a higher risk of constipation.39,45,46 In addition, it was shown that the anti-commensal effects of some APs on the gut microbiota altered microbial diversity and mediated metabolic disturbances.9,28,47–50 These abnormalities in metabolism could influence the secretory and motor functions of the gut and eventually lead to constipation.51 The underlying mechanisms of these metabolic dysfunctions could be explained by the effects of direct receptor binding action of antipsychotics, altered expressions of microbial products, production of alternative metabolites, or changes in metabolite levels.52–54 However, the precise pathophysiological basis through which these disturbances resulted in constipation has not yet been elucidated.
Metabolomics, which is the study of metabolomes of body fluids and tissues, has the ability to identify aberrant metabolic processes involved in disease pathophysiology and their association with disease risk. Furthermore, metabolomics can expand the knowledge on differential metabolites and altered metabolic pathways caused by certain drugs in addition to detecting biomarkers of adverse drug effects.55 Non-targeted liquid chromatography (LC), in combination with mass spectrometry (MS), is a common and sensitive analytical approach which has the ability to characterize, identify, and quantify metabolites at physiological concentrations. Along with multivariate statistical analyses and data visualization, the results obtained from LC-MS can be effectively interpreted. While numerous studies have investigated the metabolic profiles associated with neuropsychiatric diseases, there is a paucity of reports pertaining to the metabolic processes related to AP side effects.
In this study, we hypothesized that schizophrenia patients with AP-induced constipation have distinct metabolic patterns compared to patients without constipation and that there is more than one mechanism at play in producing this adverse drug effect. Using the same dataset from our previous research,32 serum metabolomics were scrutinized whilst correlative gut metagenomic and serum metabolomic were further investigated in order to uncover the potential mechanistic pathways by which the altered gut microbiome might modulate intestinal hypomotility, thereby leading to constipation. Since APs are the cornerstone of schizophrenia treatment and there is insufficient data on the pathophysiological basis of AP-induced constipation, the goals of the present study were to offer deeper insights into the serum metabolic profiles and potential molecular mechanisms of AP-induced constipation. The investigation of metabolites in addition to their relationship with the gut microbiome can expand our understanding of the pathophysiological changes associated with AP-induced constipation as well as identify potential metabolic targets for the prevention and treatment of constipation in addition to novel drug development.
Methods
Participants
While the same dataset, including 90 schizophrenia patients, as our previous article was utilized for the current article, 2 patients were excluded due to their refusal for blood sample collection. As a result, a total of 88 patients were recruited in this study. All participants were native Han Chinese, aged 18–60 years and were recruited from the in-patient department of Psychiatry at the Jiangning District Second People’s Hospital, Nanjing, China from January 2020 to January 2021.
The inclusion and exclusion criteria were the same as our previous article32 and are described in detail in Supplementary Materials (see Supplementary Methods, Participants). Participants were categorized into 2 groups, namely, patients with constipation (C) group and patients without constipation (NC) group based on ROME IV constipation criteria and colonic transit time via radiopaque marker (ROM). The NC group was matched for age, diet, and AP type and dosage. All participants were provided a normal-fat diet by the hospital while the dose equivalence of antipsychotics for each patient was estimated by the equivalent doses of clozapine based on the defined daily doses (see Supplementary Materials, supplementary methods, Antipsychotic medication).56,57
Constipation diagnosis and evaluation are described in detail in Supplementary Materials (see supplementary methods, Rome IV criteria for constipation, Colonic transit test, and Constipation severity evaluation). After diagnosis and evaluation of constipation, 10 ml of fasting blood samples were collected from all subjects.
This study was approved by the Institutional Review Board of Jiangning District Second People’s Hospital in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki.58 All participants signed a written informed consent form prior to the start of the study.
Sample Collection and Pretreatment
A total of 10 ml of fasting blood sample was collected from all participants on the next morning following stool collection for the first study. Blood was drawn into vacutainer tubes containing sodium heparin and the tubes were immediately placed on ice. The blood samples were then centrifuged at 1500g for 15 min and the serum obtained was transferred into polypropylene tubes and stored in a −80°C freezer. The procedures for sample pretreatment are described in Supplementary Materials (see supplementary methods, Sample pretreatment).
Serum Metabolites Profiling via LC-MS-Based Untargeted Analysis
A comprehensive assessment of serum metabolites profiles was obtained using liquid chromatography-mass spectrometry (LC-MS) system composed of ACQUITY UPLC I-Class ultra-high performance liquid phase coupled with Vion IMS QTOF high-resolution mass spectrometer. Conditions for LC analysis are shown in Supplementary Materials (see supplementary methods, LC-MS analysis conditions).
Data Preprocessing and Analysis
Prior to pattern recognition, the acquired LC-MS raw data were analyzed and are described in Supplementary Materials (see supplementary methods, Data preprocessing). Following preprocessing, the identification of metabolites was based on accurate mass, secondary fragments, and isotopic distribution, using the Human Metabolome Database (HMDB), Lipidmaps (v2.3), METLIN database, and self-built libraries.
In order to identify potential metabolic profiles of AP-induced constipation, univariate and multivariate analyses were performed on the normalized data using MetaboAnalyst 3.0 software. Detailed analyses are depicted in Supplementary Materials (see supplementary methods, Data Analysis, and Metabolic Profile Screening). The results were visualized by a principal component analysis (PCA) score plot and orthogonal-partial-least-squares-discriminant analysis (OPLS-DA) s-plot for differential metabolites patterns identification. Hierarchical clustering was performed on the expression levels of the top 50 differential metabolites based on VIP (variable importance of projection) values in order to demonstrate the relationship and expression differences of the metabolites between the 2 groups.
Correlation Analysis and Metabolic Pathway Enrichment Analysis
Correlation analysis between the metabolites was computed by Pearson’s correlation coefficient to measure the degree of linear correlation between 2 metabolites so as to determine the repeatability and to further understand the relationship between metabolites associated with AP-induced constipation. The top 50 significant metabolites alterations of the VIP value were chosen for visual analysis. Nonsignificantly different metabolites between the 2 groups were removed while those with significant alterations were mapped in Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.kegg.jp/kegg/pathway.html) database for metabolic pathway enrichment analysis. Significantly enriched pathways of significantly and differentially expressed metabolites were identified with a hypergeometric test’s P value. The pathway was considered as an enriched pathway when the P value was ≤.05 and was thus retained. Significant enrichment pathway was selected for the bubble plot.
Statistical Analyses
An independent sample t-test was used to analyze the differences in clinical characteristics between the 2 groups using SPSS software, version 19.0 (SPSS Inc., USA), with 2-tailed P < .05 set as significant. The statistical power of the current sample size was analyzed by the power.prop.test script written in R software (http://r-project.org). Data were presented as the mean ± standard deviation (SD). Metabolites were identified according to accurate mass, secondary fragments, and isotopic distribution by Progenesis QI (Waters Corporation, Milford, USA) Data Processing Software, based on public databases including HMBD (http://www.hmdb.ca/); LIPID MAPS (http://www.lipidmaps.org/), and self-built databases. The potentially altered metabolites were selected based on the combination of a statistically significant threshold of VIP values obtained from all the peaks of the 7-fold cross-validated OPLS-DA model, P values from a 2-tailed Student’s t-test on normalized peak areas and adjusted P value for correction obtained by Benjamini and Hochberg false discovery rate (FDR), where VIP values >1 and P < .05 represented potentially altered metabolites. To account for multiple comparisons, P value was adjusted by the Benjamini and Hochberg correction to allow for a maximum 5% probability (q = 0.05) of a false positive detection. Heat maps and hierarchical cluster analyses were conducted using MeV version 4.9.0. Pearson correlation test coefficient was computed in R statistical software for correlation analysis with a P value of <.05 considered as significant between each comparison. The KEGG database (https://www.kegg.jp/kegg/pathway.html) was utilized to annotate significantly different metabolites to the metabolic pathway enrichment analysis. Pearson’s correlation analysis was employed for the correlation analysis of significantly altered serum metabolites and gut microbials in R psych package.
Results
The expected statistical power of the sample size in the present study was 0.9797. Demographic characteristics of the participants are shown in table 1. The results are presented as mean ± SD for continuous variables and as a percentage for categorical variables. Data were compared by independent t-tests where appropriate. Colonic transit time along with BSS and CAS scores were significantly different between the 2 groups (P < .05). Patients with constipation had slower intestinal transit, harder stools, and more problems on defecation compared to the NC group. Patients with constipation had a longer course of schizophrenia and took higher doses of an equivalent to clozapine but these were not significant compared to the NC group. Age, BMI, weight, PANSS scores, and duration of drug use were not significantly different between the 2 groups. A higher proportion of patients in the C group had diabetes compared to the NC group while more patients from the NC group had hypertension and were smokers, albeit nonsignificantly.
Table 1.
Demographic Characteristics of Participants
| Characteristics | C group (n = 44) | NC group (n = 44) | P value |
|---|---|---|---|
| Age (y) | 51.02 ± 9.11 | 50.68 ± 9.01 | .861 |
| Gender | 28M, 16F | 28M, 16F | 1 |
| Sex ratio (M:F) | 1.6:1 | 1.6:1 | 1 |
| BMI (kg/m2) | 23.66 ± 2.94 | 24.39 ± 2.45 | .213 |
| Weight (kg) | 65.84 ± 10.14 | 66.92 ± 9.32 | .606 |
| PANSS | 67.55 ± 5.27 | 67.69 ± 2.69 | .960 |
| CD (y) | 24.89 ± 9.43 | 22.27 ± 9.93 | .212 |
| CAS | 2.32 ± 2.59 | 0.61 ± 0.95 | <.05* |
| BSS | 2.45 ± 1.37 | 4.02 ± 1.09 | <.05* |
| ROM | 0.55 ± 0.50 | 0.25 ± 0.44 | <.05* |
| EDC (mg) | 480.45 ± 248.50 | 434.89 ± 247.98 | .394 |
| Duration of drug use (mo) | 20.20 ± 21.51 | 26.80 ± 28.62 | .229 |
| Diabetes | 31.8% | 20.5% | .229 |
| Hypertension | 22.7% | 29.5% | .472 |
| Smokers | 31.8% | 45.5% | .193 |
Note: Data are presented as mean ± standard deviation. C, patients with constipation; NC, patients without constipation; M, male; F, female; BMI, Body Mass Index; CAS, Constipation Assessment Scale; BSS, Bristol Stool Form Scale;ROM, radiopaque marker; EDC, equivalent dose of clozapine; PANSS, Positive and Negative Syndrome Scale; CD, course of the disease.
Serum Metabolites Profiling
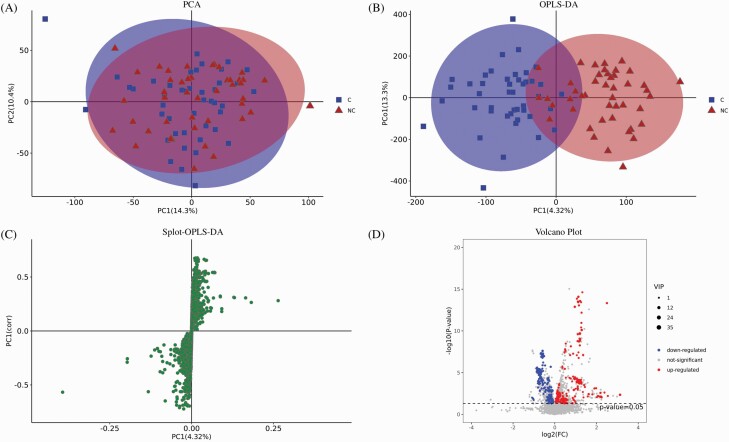
The untargeted LC-MS analysis yielded a total of 8804 peaks and 1065 metabolites from all samples in both positive and negative ion modes. PCA analysis was applied to the serum samples from 44 C and 44 NC patients in addition to QC samples in order to assess the discriminating power of the 8804 peaks, detect outliers and evaluate repeatability. The 2-dimensional PCA score plot provided visual information on the distribution of the metabolic profiles, which could discriminate metabolites between the AP-induced constipation group and the nonconstipation group. The PCA score plot depicting the intrinsic variation between the C and NC groups is shown in figure 1A. It demonstrates the possible presence of outliers, clusters, and other patterns in the data. The sample distribution in the score plot was homogeneous in each group which implied that there were no significant variations within the groups. The QC samples were closely clustered, indicating good detection stability and reproducibility (Supplementary figure 1). The PCA score plot corresponding to the first 2 principal components showed that component 1 and component 2 explained 14% and 10.3% of the total variance in the dataset, respectively. The clustered dots had similar metabolomic compositions while scattered dots exhibited different metabolic components.
Fig. 1.
Results of serum metabolites multivariate analysis. (A) Principal component analysis (PCA) score plot. C: patients with constipation; NC: patients without constipation; PC: principal component. Each blue square represents a serum sample from a patient with constipation while each red triangle depicts nonconstipation patients. (B) Orthogonal-Partial-Least-Squares-Discriminant-Analysis (OPLS-DA) score plot. OPLS-DA allows the differentiation between the two groups. (C) OPLS-DA S-plot of serum samples. Each dot denotes one metabolite. The closer the metabolites in the upper right corner and the lower left corner, the more significant the difference. (D) Volcano plot of differential metabolites in the 2 groups. The x-axis denotes 2-fold up- and downregulation and the y-axis shows the P values. The red dots represent the significantly upregulated differential metabolites while the blue dots depict significantly downregulated differential metabolites and the gray dots indicate no significantly different metabolites.
Supervised OPLS-DA score plot was constructed in order to further evaluate the differential serum metabolites patterns between the 2 groups as observed in PCA. The OPLS-DA score plot is shown in figure 1B. The 2 groups were separated in the OPLS-DA score plot (R2(X) = 0.229; R2(Y) = 0.724; Q2 = 0.287), suggesting that there were significant differences in the metabolic patterns between the 2 groups. A total of 200 permutation tests demonstrated that the method was reliable without overfitting (R2 = 0.545, Q2 = −0.519) (see Supplementary figure 2). Most of the serum samples presented in both PCA and OPLS-DA were located within the elliptical region which represented a 95% confidence interval. An OPLS-DA s-plot of serum samples was constructed so as to screen for significantly differential metabolites between the 2 groups (figure 1C). Metabolites that were farther from the point of origin had significantly greater influence and higher VIP values while those in the middle of the figure did not show any relevance in the model. Potentially altered concentrations of serum metabolites associated with AP-induced constipation were screened and selected for further analyses when the VIP scores of the metabolites were >1.0 and P value was <.05. A volcano plot (figure 1D) was constructed with combined statistical tests, including the P value and fold-change (FC) value, so as to visualize and identify these metabolites.
VIP values >1 and P values <.05 yielded a total of 63 serum metabolites (Supplementary table 2) which were significantly and differentially expressed between the 2 groups and could be classified into the following categories with the number of metabolites in brackets: glycerophospholipids (13); fatty acyls (8); sphingolipids (5); prenol lipids (5); carboxylic acids and derivatives (4); organooxygen compounds (4); steroids and steroid derivatives (4); organic sulfuric acids and derivatives (3); organonitrogen compounds (2); polyketides (2); sterol lipids (2); benzene and substituted derivatives (1); carboximidic acids and derivatives (1); carboximidic acids and derivatives (1); flavonoids (1); phenols (1); phenylpropanoic acids (1); pyrrolines (1); tetrapyrroles and derivatives (1); and unclassified (3). The levels of 45 metabolites were significantly upregulated (FC > 1) while the levels of 18 metabolites were significantly downregulated (FC < 1) in the constipated group.
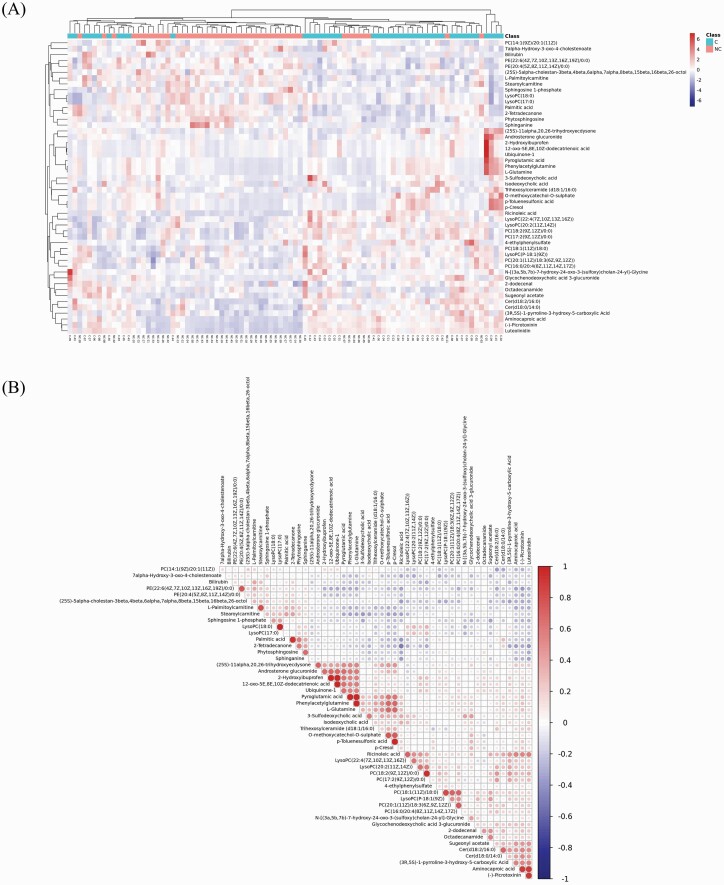
Hierarchical cluster analysis demonstrated the specific expression patterns of the significantly and differentially expressed metabolites among the 2 groups. A heat map was constructed with the top 50 differential metabolites based on VIP values and is shown in figure 2A. The heat map showed distinct separation and the presence of significantly different metabolites’ expression between the 2 groups. Only a few metabolites displayed similarities in expression levels. The metabolites with a higher degree of similar alteration patterns were close on the heat map, ie, the metabolites of patients from the C group were clustered closely and separated from NC patients which further demonstrated distinct changes in the metabolites’ levels between the 2 groups.
Fig. 2.
Correlation analysis of top 50 significantly altered metabolites. (A) Heat map of the top 50 metabolites with significant level alterations between the 2 groups constructed from hierarchical clustering analysis. The x-axis depicts the samples where C is a patient with constipation and NC represents a nonconstipation patient, and the y-axis denotes the differentially expressed metabolites separated by hierarchical clustering. The color scale shows the relative expression levels of the metabolites across all samples; blue represents decreased metabolite level while red represents an increased metabolite level. (B) Correlation of the top 50 differentially expressed metabolites. The color scale depicts the degree of correlation where 1 and −1 imply the strongest positive and negative correlations, respectively. The color red indicates positive correlation and the blue color represents negative correlation. The different dots’ sizes signify the correlation of Pearson’s coefficients.
Correlation analysis was computed so as to investigate the associations of significantly and differentially expressed metabolites between the 2 groups using the top 50 differential metabolites based on VIP values. Differential metabolite correlation analysis is shown in figure 2B. Similar variation trends among the metabolites suggested positive correlations and different variation trends implied negative correlations. The metabolites were mostly positively correlated with each other while only 2 metabolites showed negative associations. The correlation details are given in Supplementary Materials (see supplementary results, correlation analysis between metabolites).
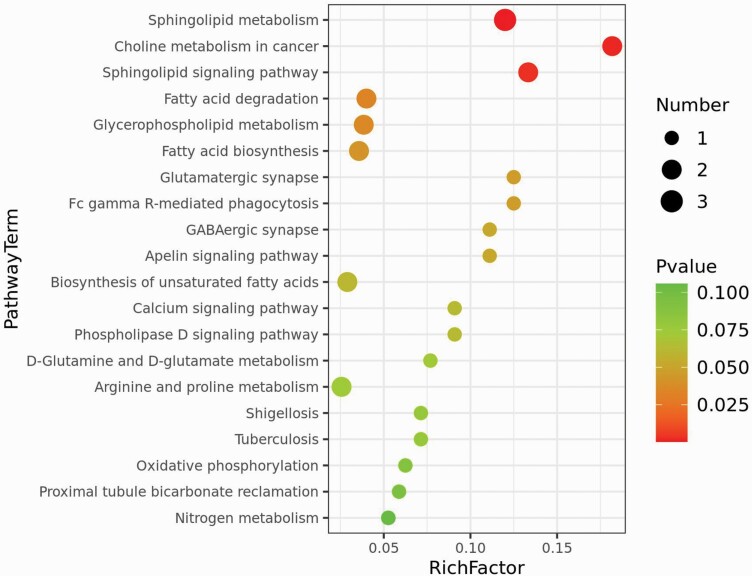
The 63 identified serum metabolites with altered levels were subjected to metabolic pathway enrichment analysis in order to identify affected metabolic pathways. The top 20 metabolic pathways were selected for a bubble map (see figure 3). Eight metabolic pathways with a hypergeometric P value <.05 were significantly altered between the 2 groups and included sphingolipid metabolism, choline metabolism in cancer, sphingolipid signaling pathway, fatty acid degradation, glycerophospholipid metabolism, fatty acid biosynthesis, glutamatergic synapse, and Fc gamma R-mediated phagocytosis (see table 2). However, after FDR correction, sphingolipid metabolism, choline metabolism in cancer and sphingolipid signaling pathway were the most important pathways with lower P values and higher number of altered metabolites level. The concentrations of 11 serum metabolites in these impaired pathways were significantly different in the C group in contrast to those in the NC group (see table 3).
Fig. 3.
Bubble map of metabolic enrichment pathways. The y-axis depicts the metabolic pathways and the x-axis shows the rich factor. Rich factors are presented as enrichment degrees. The larger the rich factor, the greater the enrichment degree. The color scale ranges from green to red and represents a higher significance to a lower significance, respectively. The area of each node denotes the number of enriched metabolites. The larger the node, the greater the number of metabolites is enriched in the pathway.
Table 2.
Impaired Metabolic Pathways in AP-Induced Constipation.
| Metabolic pathway | Total number of altered metabolites | Rich factor | P value | -lg(P value) | FDR correction |
|---|---|---|---|---|---|
| Sphingolipid metabolism | 3 | 0.12 | .0004 | 3.444 | 0.015 |
| Choline metabolism in cancer | 2 | 0.182 | .0017 | 2.762 | 0.037 |
| Sphingolipid signaling pathway | 2 | 0.133 | .0032 | 2.488 | 0.047 |
| Fatty acid degradation | 2 | 0.04 | .0336 | 1.474 | 0.199 |
| Glycerophospholipid metabolism | 2 | 0.038 | .0361 | 1.443 | 0.199 |
| Fatty acid biosynthesis | 2 | 0.036 | .0413 | 1.383 | 0.199 |
| Glutamatergic synapse | 1 | 0.125 | .0459 | 1.339 | 0.199 |
| Fc gamma R-mediated phagocytosis | 1 | 0.125 | .0459 | 1.339 | 0.199 |
Table 3.
Altered Metabolites and Associated Metabolic Pathways in AP-Induced Constipation
| Metabolites | Metabolic pathway | Retention time/min | m/z | VIP | P value | FC value |
|---|---|---|---|---|---|---|
| Sphingosine-1-phosphate | Sphingolipid metabolism, sphingolipid signaling pathway | 7.01 | 378.24 | 1.61 | .001 | 0.83 ↓ |
| Phytosphingosine | Sphingolipid metabolism | 6.28 | 318.3 | 1.79 | .01 | 0.90 ↓ |
| Sphinganine | Sphingolipid metabolism, sphingolipid signaling pathway | 6.77 | 302.31 | 1.32 | .0001 | 0.80 ↓ |
| Phosphatidylcholine | ||||||
| PC(14:1(9Z)/20:1(11Z)) | Choline metabolism in cancer | 9.72 | 780.55 | 4.54 | 0.04 | 0.90 ↓ |
| PC(18:1(11Z)/18:0) | 11.57 | 810.60 | 7.92 | 0.004 | 1.15 ↑ | |
| PC(16:0/20:4(8Z,11Z,14Z,17Z)) | 8.26 | 826.56 | 7.38 | 0.01 | 1.10 ↑ | |
| PC(20:1(11Z)/18:3(6Z,9Z,12Z)) | 8.28 | 854.59 | 8.64 | 0.01 | 1.10 ↑ | |
| Lysophosphatidylcholines | ||||||
| LysoPC(18:0) | Choline metabolism in cancer | 8.34 | 568.36 | 11.6 | .04 | 0.93 ↓ |
| LysoPC(17:0) | 8.34 | 508.34 | 7.89 | .04 | 0.93 ↓ | |
| LysoPC(22:4(7Z,10Z,13Z,16Z)) | 7.84 | 616.36 | 1.24 | .03 | 1.21 ↑ | |
| LysoPC(20:2(11Z,14Z)) | 8.45 | 548.37 | 2.23 | .03 | 1.15 ↑ | |
Note: ↑ and ↓ signs indicate upregulated and downregulated metabolites, respectively.
Correlation Analysis Between Significantly Altered Serum Metabolites and Gut Microbials
The potential correlations between the altered gut microbiome composition at the genus level, obtained from our previous study,32 and the significantly different serum metabolites from the present study, were further investigated. Overall, the analyses revealed that the significant changes in the abundances of bacterial genus had a mild correlation with the significantly altered serum metabolites in AP-induced constipation (r = 0.08, P = .01). In particular, Ruminococcus was positively correlated with serum PC(18:1(11Z)/18:0) (P < .05, r = 0.38), and PC(20:1(11Z)/18:3(6Z,9Z,12Z)) (P < .05, r = 0.30) while Clostridium_XlVb was negatively correlated to serum PC(20:1(11Z)/18:3(6Z,9Z,12Z)) (P < .05, r = −0.32).
Discussion
In the present study, 88 serum samples from 44 schizophrenia patients with constipation and 44 schizophrenia patients without constipation underwent non-targeted LC-MS-based metabolomics analysis in order to investigate the alterations of serum metabolic profiles associated with AP-induced constipation and identify the potential underlying molecular mechanisms. Moreover, the relationship of the significantly altered serum metabolites and altered gut microbiome was further investigated. Comprehensive metabolomics examinations together with statistical analyses demonstrated that patients with AP-induced constipation had marked differences in serum metabolite levels which were associated with an imbalance in distinct metabolic processes that are potentially implicated in the occurrence of constipation in these subjects. In addition, changes in the gut microbiome composition were related to some of the altered serum metabolites, demonstrating an interaction of the gut microbiome and metabolism which might produce AP-induced constipation. Altogether, the impaired metabolic pathways observed in these patients provide additional mechanistic insights underlying AP-induced constipation. To the best of our knowledge, the present study is the first to explore serum metabolic profiles of AP-induced constipation in addition to the relationship of gut metagenomics and serum metabolomics in patients with constipation related to APs.
Patients with AP-induced constipation had lower levels of sphingosine-1-phosphate (S1P), sphinganine, phytosphingosine, lysophosphatidylcholines LysoPC(18:0) and LysoPC(17:0) and lecithin (phosphatidylcholine PC(14:1(9Z)/20:1(11Z))) in addition to higher levels of lysophosphatidylcholines LysoPC(22:4(7Z,10Z,13Z,16Z)) and LysoPC(20:2(11Z,14Z)), phosphatidylcholines PC(18:1(11Z)/18:0), PC(16:0/20:4(8Z,11Z,14Z,17Z)) and PC(20:1(11Z)/18:3(6Z,9Z,12Z)) compared to the NC group. The related significantly disturbed metabolic pathways comprised of sphingolipid metabolism, choline metabolism in cancer, sphingolipid signaling pathway, which distinguished patients with AP-induced constipation from the NC group.
In our study, 3 sphingolipids were significantly reduced in the C group in contrast to the NC group, with impaired sphingolipid metabolism and sphingolipid signaling pathway. Sphingolipids are synthesized in the human body via the de novo pathway, salvage pathway, or intestinal microbiota and could also be obtained through dietary supplementation.59,60 Sphingolipid metabolism produces several bioactive metabolites, including S1P (generated from sphingosine), phytosphingosine, and sphinganine, which also act as signaling molecules and play a role in cell survival, proliferation, migration, differentiation, and apoptosis in addition to the modulation of cardiovascular functions and contraction of smooth muscles.61,62 It was previously demonstrated that S1P induced modulation of intestinal smooth muscle contractility and gastrointestinal motility via interstitial cells of Cajal (ICC).63,64 Since the ICC regulates gastrointestinal motility, lower levels of S1P might disrupt the normal functioning of ICC by lowering or inhibiting colonic motor activity and lead to constipation.65 Altered levels of sphinganine in aged mice were also associated with gastrointestinal contractile dysfunction.66 Disturbed sphingolipid metabolism and sphingolipid-mediated signal transduction via alterations in phytosphingosine and sphinganine were associated with contractile dysfunctions in the heart, airway passage, uterus, and porcine heart,67–70 indicating their role in muscle contraction. Dietary supplementation of both phytosphingosine and sphinganine promoted gastrointestinal motility71 and thus, lower levels might potentially result in hypomotility. Moreover, metabolic disturbances in lipid and glucose metabolism, including prediabetic state, diabetes mellitus, and insulin resistance, were associated with alterations in S1P, sphinganine, and phytosphingosine.72–75
In this study, patients with AP-induced constipation had altered levels of glycerophospholipids involved in abnormal choline metabolism. Lysophosphatidylcholines (LPCs), also termed lysolecithins, are major serum lipids that are generated by the cleavage of phosphatidylcholines (PCs), also known as lecithins. Both LPCs and PCs are the most abundant phospholipids in cell membranes and serum.76 While both are involved in cell survival, migration, proliferation and signaling, lipid metabolism, glucose homeostasis, gene regulation, immunity, intestinal fatty acid uptake, and intestinal mucosal barrier, LPCs are also implicated in glycerophospholipids transport and contraction of gastrointestinal muscles.77–84 LPCs induced alterations in Ca(2+), Na(+), and K(+) concentrations85 which might affect the contractility of muscles in the gut. In an experiment involving mice, LPCs inhibited gastrointestinal contractions and reduced transit time.79 Circulating LPCs also impaired the intestinal barrier by altering intestinal permeability.80 It was reported that ulcerative colitis (UC) patients had reduced PCs and LPCs in their intestinal mucosa86 and nearly half of those affected with UC exhibited proximal constipation.87 These findings suggested that the impaired intestinal mucosal barrier, as a result of alterations in PCs and LPCs levels, could mediate constipation. Moreover, constipation was reported as one of the side effects of dietary lecithin in infant formula.88 The alterations in both LPCs and PCs levels were associated with abnormalities in lipid and glucose metabolism as well as insulin resistance.77,89,90 These results indicated that LPCs and PCs are also involved in metabolic disturbances.
Another notable finding of the current article is the relationship between significantly altered serum metabolites and gut microbiome composition in patients with AP-induced constipation. Out of the 11 significantly altered serum metabolites in the C group, PC(18:1(11Z)/18:0), and PC(20:1(11Z)/18:3(6Z,9Z,12Z)) were substantially correlated with changes in bacterial gut composition. In our previously published article,32 there was a higher abundance of Ruminococcus and Clostridium_XlVb amongst others. In the present study, we found that a higher level of Ruminococcus and Clostridium_XlVb was associated with disturbances in choline metabolism. It was previously demonstrated that Ruminococcus, a constipation-associated gram-positive bacteria with mucolytic activity,91,92 was involved in glycerophospholipid metabolism and was positively associated with circulating glycerophospholipid.93 The Clostridium genus has the ability to produce lecithinase which hydrolyzes lecithin, thereby affecting the absorption of lecithin and inducing metabolic disturbances and mucosal barrier impairment.94 Therefore, a greater abundance of Ruminococcus and Clostridium_XlVb could lead to increased gut permeability into the circulation via loss of epithelial cells and mucosal barrier integrity of the gut. Furthermore, the changes generated by APs in the abundance of these bacteria might induce metabolic disturbances which ultimately lead to constipation.
It is noteworthy that the perturbations detected in the top-hit pathways were previously associated with schizophrenia. However, some of these investigations did not take into account the potential confounding effects of APs,95–100 while others reported that disruptions in these pathways emerged from AP administration,101–104 which is in line with our findings. Nonetheless, a handful of studies have shown disturbances in these pathways in drug-free schizophrenia patients,105–107 suggesting that they might be implicated in the pathophysiology of the disease itself. Due to the disparity in the literature, the notion that the top-hit pathways were altered as a result of AP therapy cannot be excluded. Further analyses are warranted with schizophrenia participants with and without AP administration in order to validate these findings.
Several strengths of this study are worth mentioning. First, the type and dose of antipsychotic drugs were matched in the constipation and nonconstipation groups. These factors produce greater homogeneity between the 2 groups. In addition, a combination of subjective ROME IV constipation criteria and objective colonic transit test using radiopaque markers allowed for more precise discrimination between constipation and nonconstipation patients. Altogether, the rigorous study design provides good credibility and improves the scientific value of this study. Furthermore, serum metabolomics is an indispensable method that provides deeper insights into the interaction of the gut microbiome and the metabolism of the body.
There are also a few limitations in the present study that should be considered. The sample size was relatively small and could have influenced the power of the study. Thus, the current findings should be seen as preliminary and need to be validated in independent and larger samples. Both groups under investigation were taking antipsychotics. As such, we did not investigate the patients before and after receiving medication which could have provided additional information. Other variables could influence the occurrence of constipation and should also be taken into consideration. The databases employed for the identification of metabolites based on the LC-MS approach are less developed than those for gas chromatography and therefore, could have been less effective at identifying all the metabolites. The current study is cross-sectional and employs untargeted metabolomics. Future longitudinal studies utilizing targeted metabolomics and including a larger population with well-defined antipsychotic agents, especially clozapine, are warranted for validation and further analysis.
Conclusion
The results of LC-MS metabolomic analyses on serum samples from patients with and without constipation under AP therapy demonstrated that there were significant differences in the metabolic profiles between the 2 patients’ groups and could be considered as specific to the condition. Overall, the presence of disruptions in organonitrogen compound metabolism, sphingolipid signal transduction, and phospholipid metabolism provide further insights on the potential mechanistic basis of AP-induced constipation. In our previous study, APs instigated propionate and vitamin B6 metabolism alterations in the gut which influenced the gut microbiome composition and function. In the present study, the integrative analysis of fecal metagenomics and serum metabolomics revealed that the interaction between the altered gut microbiome and serum metabolites was associated with an imbalance in choline metabolism which is potentially involved in AP-induced constipation. The administration of APs potentially impedes the normal gut microbiome composition and function that produce metabolic disturbances, intestinal barrier dysfunctions, muscle contractility impairment, and cells dysmotility leading to gut hypomotility and constipation. Our findings suggested that there are multiple mechanisms synergistically at play in AP-induced constipation and could provide a basis for future studies for better optimization of AP treatment in view of mitigating constipation.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Acknowledgments
We would like to express our gratitude to our patients and patient’s family for their generous support, cooperation, and participation. The authors have no conflict of interest to declare.
Contributor Information
Nousayhah Amdanee, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China.
Miaomiao Shao, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China; Department of Psychiatry, The Second People’s Hospital of Jiangning District, Nanjing, Jiangsu, China.
Xiuxiu Hu, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China; Department of Psychiatry, The Second People’s Hospital of Jiangning District, Nanjing, Jiangsu, China.
Xinyu Fang, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China.
Chao Zhou, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China.
Jiu Chen, Institute of Neuropsychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China.
Mohammad Ridwan Chattun, Department of Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China.
Lu Wen, Department of Psychiatry, The Second People’s Hospital of Jiangning District, Nanjing, Jiangsu, China.
Xinming Pan, Department of Psychiatry, The Second People’s Hospital of Jiangning District, Nanjing, Jiangsu, China.
Xiangrong Zhang, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China; The Affiliated Xuzhou Oriental Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China.
Yue Xu, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2018YFC1314300); National Natural Science Foundation of China (Nos. 81971255, 82101572); Social Development Foundation of Jiangsu Province, China (No. BE2019610); Jiangsu Provincial Medical Talent Project of China (No. ZDRCA2016075); Special Project of Basic Research on Frontier Leading Technology of Jiangsu Province, China (No. BK20192004D); Key Project supported by Medical Science and Technology Development Foundation, Nanjing Department of Health (No. YKK20090); and the Science and Technology Development Program of Nanjing Medical University (No. NMUB2019107).
References
- 1. Ozbilen M, Adams CE.. Systematic overview of Cochrane reviews for anticholinergic effects of antipsychotic drugs. J Clin Psychopharmacol. 2009;29(2):141–146. [DOI] [PubMed] [Google Scholar]
- 2. Severance EG, Prandovszky E, Castiglione J, Yolken RH.. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep. 2015;17(5):27–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu Y, Amdanee N, Zhang X.. Antipsychotic-induced constipation: a review of the pathogenesis, clinical diagnosis, and treatment. CNS Drugs. 2021;35:1265–1274. [DOI] [PubMed] [Google Scholar]
- 4. Thomas N, Jain N, Connally F, Yeung JM, Pantelis C.. Prucalopride in clozapine-induced constipation. Aust N Z J Psychiatry. 2018;52(8):804–804. [DOI] [PubMed] [Google Scholar]
- 5. Chen H-K, Hsieh C-J.. Risk of gastrointestinal Hypomotility in schizophrenia and schizoaffective disorder treated with antipsychotics: a retrospective cohort study. Schizophr Res. 2018;195:237–244. [DOI] [PubMed] [Google Scholar]
- 6. Hert MD, Hudyana H, Dockx L, et al. Second-generation antipsychotics and constipation: a review of the literature. Eur Psychiatry. 2011;26(1):34–44. [DOI] [PubMed] [Google Scholar]
- 7. Lechin F, Gomez F, van der Dijs B, Lechin E.. Distal colon motility in schizophrenic patients. J Clin Pharmacol. 1980;20(7):459–464. [DOI] [PubMed] [Google Scholar]
- 8. Dhasmana K, Villalon C, Zhu Y, Parmar S.. The role of dopamine (D2), α and β-adrenoceptor receptors in the decrease in gastrointestinal transit induced by dopamine and dopamine-related drugs in the rat. Pharmacol Res. 1993;27(4):335–348. [DOI] [PubMed] [Google Scholar]
- 9. Cussotto S, Clarke G, Dinan TG, Cryan JF.. Psychotropics and the microbiome: a chamber of secrets…. Psychopharmacology. 2019;236(5):1411–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Every-Palmer S, Lentle RG, Reynolds G, et al. Spatiotemporal mapping techniques show clozapine impairs neurogenic and myogenic patterns of activity in the colon of the rabbit in a dose-dependent manner. Front Pharmacol. 2017;8:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bymaster FP, Nelson DL, DeLapp NW, et al. Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and α1-adrenergic receptors in vitro. Schizophr Res. 1999;37(1):107–122. [DOI] [PubMed] [Google Scholar]
- 12. Sahyoun H, Costall B, Naylor R.. On the ability of domperidone to selectively inhibit catecholamine-induced relaxation of circular smooth muscle of guinea-pig stomach. J Pharm Pharmacol. 1982;34(1):27–33. [DOI] [PubMed] [Google Scholar]
- 13. Dicks LMT, Hurn D, Hermanus D.. Gut bacteria and neuropsychiatric disorders. Microorganisms. 2021;9(12):25832583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borkent J, Ioannou M, Laman JD, Haarman BCM, Sommer IEC.. Role of the gut microbiome in three major psychiatric disorders. Psychol Med. 2022;52(7):1222–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Szeligowski T, Yun AL, Lennox BR, Burnet PWJ.. The gut microbiome and schizophrenia: the current state of the field and clinical applications. Front Psychiatry. 2020;11:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh R, Stogios N, Smith E, et al. Gut microbiome in schizophrenia and antipsychotic-induced metabolic alterations: a scoping review. Ther Adv Psychopharmacol. 2022;12:20451253221096525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGuinness AJ, Davis JA, Dawson SL, et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol Psychiatry. 2022;27(4):1920–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen Y, Xu J, Li Z, et al. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: a cross-sectional study. Schizophr Res. 2018;197:470–477. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olde Loohuis LM, Mangul S, Ori APS, et al. Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Transl Psychiatry. 2018;8(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li S, Song J, Ke P, et al. The gut microbiome is associated with brain structure and function in schizophrenia. Sci Rep. 2021;11(1):9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thirion F, Speyer H, Hansen TH, et al. Alteration of gut microbiome in patients with schizophrenia indicates links between bacterial tyrosine biosynthesis and cognitive dysfunction. Biol Psychiatry Glob Open Sci. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu R, Wu B, Liang J, et al. Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain Behav Immun. 2020;85:120–127. [DOI] [PubMed] [Google Scholar]
- 24. Nguyen TT, Kosciolek T, Daly RE, et al. Gut microbiome in Schizophrenia: altered functional pathways related to immune modulation and atherosclerotic risk. Brain Behav Immun. 2021;91:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu F, Ju Y, Wang W, et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat Commun. 2020;11(1):1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma X, Asif H, Dai L, et al. Alteration of the gut microbiome in first-episode drug-naïve and chronic medicated schizophrenia correlate with regional brain volumes. J Psychiatr Res. 2020;123:136–144. [DOI] [PubMed] [Google Scholar]
- 27. Xiang M, Zheng L, Pu D, et al. Intestinal microbes in patients with schizophrenia undergoing short-term treatment: core species identification based on co-occurrence networks and regression analysis. Front Microbiol. 2022;13:909729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299–306. [DOI] [PubMed] [Google Scholar]
- 29. Yuan X, Wang Y, Li X, et al. Gut microbial biomarkers for the treatment response in first-episode, drug-naïve schizophrenia: a 24-week follow-up study. Transl Psychiatry. 2021;11(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seeman MV. The gut microbiome and antipsychotic treatment response. Behav Brain Res. 2021;396:112886. [DOI] [PubMed] [Google Scholar]
- 31. Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG.. The role of the gut microbiome in the development of schizophrenia. Schizophr Res. 2021;234:4–23. [DOI] [PubMed] [Google Scholar]
- 32. Xu Y, Shao M, Fang X, et al. Antipsychotic-induced gastrointestinal hypomotility and the alteration in gut microbiota in patients with schizophrenia. Brain Behav Immun. 2022;99:119–129. [DOI] [PubMed] [Google Scholar]
- 33. Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang S-C, Goh KK, Lu M-L.. Metabolic disturbances associated with antipsychotic drug treatment in patients with schizophrenia: State-of-the-art and future perspectives. World J Psychiatry. 2021;11(10):696696–6966710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Himmerich H, Minkwitz J, Kirkby K C.. Weight gain and metabolic changes during treatment with antipsychotics and antidepressants. Endocr Metab Immune Disord Drug Targets. 2015;15(4):252–260. [DOI] [PubMed] [Google Scholar]
- 36. Nasrallah HA, Newcomer JW.. Atypical antipsychotics and metabolic dysregulation: evaluating the risk/benefit equation and improving the standard of care. J Clin Psychopharmacol. 2004;24(5):S7–14. [DOI] [PubMed] [Google Scholar]
- 37. Penninx BW, Lange SM.. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues Clin Neurosci. 2018;20(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang C-j, Zhang Z-j, Sun J, et al. Serum free fatty acids and glucose metabolism, insulin resistance in schizophrenia with chronic antipsychotics. Biol Psychiatry. 2006;60(12):1309–1313. [DOI] [PubMed] [Google Scholar]
- 39. D’Antongiovanni V, Pellegrini C, Fornai M, et al. Intestinal epithelial barrier and neuromuscular compartment in health and disease. World J Gastroenterol. 2020;26(14):1564–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abdul Wahab P, Mohd Yusoff D, Abdul Kadir A, Ali SH, Yeong Yeh L.. Prevalence, symptoms, and associated factors of chronic constipation among older adults in north-east of peninsular Malaysia. Clin Nurs Res. 2022;31(2):348–355. [DOI] [PubMed] [Google Scholar]
- 41. Ihana-Sugiyama N, Nagata N, Yamamoto-Honda R, et al. Constipation, hard stools, fecal urgency, and incomplete evacuation, but not diarrhea is associated with diabetes and its related factors. World J Gastroenterol. 2016;22(11):32523252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Skonieczna-Żydecka K, Łoniewski I, Misera A, et al. Second-generation antipsychotics and metabolism alterations: a systematic review of the role of the gut microbiome. Psychopharmacology. 2019;236(5):1491–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Canfrán-Duque A, Casado ME, Pastor O, et al. Atypical antipsychotics alter cholesterol and fatty acid metabolism in vitro [S]. J Lipid Res. 2013;54(2):310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kowalchuk C, Castellani LN, Chintoh A, Remington G, Giacca A, Hahn MK.. Antipsychotics and glucose metabolism: how brain and body collide. Am J Physiol Endocrinol Metab. 2019;316(1):E1–E15. [DOI] [PubMed] [Google Scholar]
- 45. Krishnan B, Babu S, Walker J, Walker AB, Pappachan JM.. Gastrointestinal complications of diabetes mellitus. World J Diabetes. 2013;4(3):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim JE, Choi YJ, Lee SJ, et al. Molecular characterization of constipation disease as novel phenotypes in CRISPR-Cas9-generated leptin knockout mice with obesity. Int J Mol Sci . 2020;21(24):9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dinan TG, Cryan JF.. Schizophrenia and the microbiome: time to focus on the impact of antipsychotic treatment on the gut microbiota. World J Biol Psychiatry. 2018;19(8):568–570. [DOI] [PubMed] [Google Scholar]
- 48. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morgan AP, Crowley JJ, Nonneman RJ, et al. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One. 2014;9(12):e115225e115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bahr S, Tyler B, Wooldridge N, et al. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry. 2015;5(10):e652–e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Borriello SP. Bacteria and gastrointestinal secretion and motility. Scand J Gastroenterol Suppl. 1984;93:115–121. [PubMed] [Google Scholar]
- 52. Starrenburg F, Bogers J.. How can antipsychotics cause diabetes mellitus? Insights based on receptor-binding profiles, humoral factors and transporter proteins. Eur Psychiatry. 2009;24(3):164–170. [DOI] [PubMed] [Google Scholar]
- 53. Chen A, Park TY, Li KJ, DeLisi LE.. Antipsychotics and the microbiota. Curr Opin Psychiatry. 2020;33(3):225–230. [DOI] [PubMed] [Google Scholar]
- 54. Sousa T, Paterson R, Moore V, Carlsson A, Abrahamsson B, Basit AW.. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm. 2008;363(1-2):1–25. [DOI] [PubMed] [Google Scholar]
- 55. Quinones MP, Kaddurah-Daouk R.. Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol Dis. 2009;35(2):165–176. [DOI] [PubMed] [Google Scholar]
- 56. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B-C.. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leucht S, Samara M, Heres S, Davis JM.. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 2016;42(suppl_1):S90–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morris K. Revising the declaration of helsinki. Lancet. 2013;381(9881):1889–1890. [DOI] [PubMed] [Google Scholar]
- 59. Quinville BM, Deschenes NM, Ryckman AE, Walia JS.. A comprehensive review: sphingolipid metabolism and implications of disruption in sphingolipid homeostasis. Int J Mol Sci . 2021;22(11):5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Johnson EL, Heaver SL, Waters JL, et al. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat Commun. 2020;11(1):2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Watterson KR, Ratz PH, Spiegel S.. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell Signal. 2005;17(3):289–298. [DOI] [PubMed] [Google Scholar]
- 62. Maceyka M, Harikumar KB, Milstien S, Spiegel S.. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dragusin M, Wehner S, Kelly S, et al. Effects of sphingosine-1-phosphate and ceramide-1-phosphate on rat intestinal smooth muscle cells: implications for postoperative ileus. FASEB J. 2006;20(11):1930–1932. [DOI] [PubMed] [Google Scholar]
- 64. Kim YD, Han KT, Lee J, et al. Effects of sphingosine-1-phosphate on pacemaker activity of interstitial cells of Cajal from mouse small intestine. Mol Cells. 2013;35(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mostafa R-M, Moustafa YM, Hamdy H.. Interstitial cells of Cajal, the Maestro in health and disease. World J Gastroenterol. 2010;16(26):3239–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Choi S, Kim JA, Kim TH, et al. Altering sphingolipid composition with aging induces contractile dysfunction of gastric smooth muscle via K(Ca) 1.1 upregulation. Aging Cell. 2015;14(6):982–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Qi Y, Gu H, Song Y, et al. Metabolomics study of resina draconis on myocardial ischemia rats using ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry combined with pattern recognition methods and metabolic pathway analysis. Evid Based Complement Alternat Med. 2013;2013:1438680–1438610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Constable PD, Smith GW, Rottinghaus GE, Tumbleson ME, Haschek WM.. Fumonisin-induced blockade of ceramide synthase in sphingolipid biosynthetic pathway alters aortic input impedance spectrum of pigs. Am J Physiol Heart Circ Physiol. 2003;284(6):H2034–H2044. [DOI] [PubMed] [Google Scholar]
- 69. Wills-Karp M. At last—linking ORMDL3 polymorphisms, decreased sphingolipid synthesis, and asthma susceptibility. J Clin Invest. 2020;130(2):604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fang L, Dai N, Wang L, et al. Urine metabolomic study of primary dysmenorrhea patients during menstrual period using an ultra performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry (UPLC-Q-TOF-MS). RSC Adv. 2014;4(83):44208–44213. [Google Scholar]
- 71. Wang S, Bao Y-R, Li T-J, et al. Mechanism of fructus aurantii flavonoids promoting gastrointestinal motility: from organic and inorganic endogenous substances combination point of view. Pharmacogn Mag. 2017;13(51):372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sui J, He M, Wang Y, Zhao X, He Y, Shi B.. Sphingolipid metabolism in type 2 diabetes and associated cardiovascular complications. Exp Ther Med. 2019;18(5):3603–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Samad F, Badeanlou L, Shah C, Yang G.. Adipose tissue and ceramide biosynthesis in the pathogenesis of obesity. Sphingolipids Metab Dis. 2011;721:67–86. [DOI] [PubMed] [Google Scholar]
- 74. Duivenvoorden I, Voshol PJ, Rensen PCN, et al. Dietary sphingolipids lower plasma cholesterol and triacylglycerol and prevent liver steatosis in APOE*3Leiden mice. Am J Clin Nutr. 2006;84(2):312–321. [DOI] [PubMed] [Google Scholar]
- 75. Murakami I, Wakasa Y, Yamashita S, et al. Phytoceramide and sphingoid bases derived from brewer’s yeast Saccharomyces pastorianus activate peroxisome proliferator-activated receptors. Lipids Health Dis. 2011;10(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ridgway N, McLeod R.. Biochemistry of lipids, lipoproteins and membranes. Amsterdam, Netherlands: Elsevier; 2008. [Google Scholar]
- 77. Law S-H, Chan M-L, Marathe GK, Parveen F, Chen C-H, Ke L-Y.. An updated review of lysophosphatidylcholine metabolism in human diseases. Int J Mol Sci . 2019;20(5):1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rogausch H. The effect of lysolecithin on contractile force of isolated gastric smooth muscle. Res Exp Med (Berl). 1978;173(1):9–15. [DOI] [PubMed] [Google Scholar]
- 79. Mihara H, Boudaka A, Shibasaki K, Yamanaka A, Sugiyama T, Tominaga M.. Involvement of TRPV2 activation in intestinal movement through nitric oxide production in mice. J Neurosci. 2010;30(49):16536–16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bolin T, Franzen L, Sjödahl R, Tagesson C.. Passage of molecules through the wall of the gastrointestinal tract. Scand J Gastroenterol. 1986;21(4):441–448. [DOI] [PubMed] [Google Scholar]
- 81. Yea K, Kim J, Yoon JH, et al. Lysophosphatidylcholine activates adipocyte glucose uptake and lowers blood glucose levels in murine models of diabetes. J Biol Chem. 2009;284(49):33833–33840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nakano T, Inoue I, Katayama S, et al. Lysophosphatidylcholine for efficient intestinal lipid absorption and lipoprotein secretion in caco-2 cells. J Clin Biochem Nutr. 2009;45(2):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Furse S, de Kroon AIPM.. Phosphatidylcholine’s functions beyond that of a membrane brick. Mol Membr Biol. 2015;32(4):117–119. [DOI] [PubMed] [Google Scholar]
- 84. van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL.. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 2017;1859(9, Part B):1558–1572. [DOI] [PubMed] [Google Scholar]
- 85. Liu P, Zhu W, Chen C, et al. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020;247:117443. [DOI] [PubMed] [Google Scholar]
- 86. Ehehalt R, Wagenblast J, Erben G, et al. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nanoelectrospray-tandem mass spectrometry. Scand J Gastroenterol. 2004;39(8):737–742. [DOI] [PubMed] [Google Scholar]
- 87. James SL, van Langenberg DR, Taylor KM, Gibson PR.. Characterization of ulcerative colitis-associated constipation syndrome (proximal constipation). JGH Open. 2018;2(5):217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Younes M, Aquilina G, Castle L, et al. Opinion on the re-evaluation of lecithins (E 322) as a food additive in foods for infants below 16 weeks of age and follow-up of its re-evaluation as food additive for uses in foods for all population groups. EFSA J. 2020;18(11):e06266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Han MS, Lim Y-M, Quan W, et al. Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance. J Lipid Res. 2011;52(6):1234–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Meikle PJ, Summers SA.. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol. 2017;13(2):79–91. [DOI] [PubMed] [Google Scholar]
- 91. Zhu L, Liu W, Alkhouri R, et al. Structural changes in the gut microbiome of constipated patients. Physiol Genomics. 2014;46(18):679–686. [DOI] [PubMed] [Google Scholar]
- 92. Paone P, Cani PD.. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang Z, Usyk M, Sollecito C, et al. Altered gut microbiota and host metabolite profiles in HIV-infected women. Clin Infect Dis. 2019;71(9):2345–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Luo D, Chen K, Li J, et al. Gut microbiota combined with metabolomics reveals the metabolic profile of the normal aging process and the anti-aging effect of FuFang Zhenshu TiaoZhi(FTZ) in mice. Biomed Pharmacother. 2020;121:109550. [DOI] [PubMed] [Google Scholar]
- 95. Narayan S, Head SR, Gilmartin TJ, Dean B, Thomas EA.. Evidence for disruption of sphingolipid metabolism in schizophrenia. J Neurosci Res. 2009;87(1):278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wood PL. Targeted lipidomics and metabolomics evaluations of cortical neuronal stress in schizophrenia. Schizophr Res. 2019;212:107–112. [DOI] [PubMed] [Google Scholar]
- 97. MacDonald K, Jiang Y, Krishnan A, et al. Patient stratification using metabolomics to address the heterogeneity of psychosis. Schizophr Bull Open. 2020;1(1):sgaa032. [Google Scholar]
- 98. Altaf-Ul-Amin M, Hirose K, Nani JV, et al. A system biology approach based on metabolic biomarkers and protein–protein interactions for identifying pathways underlying schizophrenia and bipolar disorder. Sci Rep. 2021;11(1):14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhao Z, Xu J, Chen J, et al. Transcriptome sequencing and genome-wide association analyses reveal lysosomal function and actin cytoskeleton remodeling in schizophrenia and bipolar disorder. Mol Psychiatry. 2015;20(5):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kaddurah-Daouk R, McEvoy J, Baillie R, et al. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol Psychiatry. 2007;12(10):934–945. [DOI] [PubMed] [Google Scholar]
- 102. Suvitaival T, Mantere O, Kieseppä T, et al. Serum metabolite profile associates with the development of metabolic co-morbidities in first-episode psychosis. Transl Psychiatry. 2016;6(11):e951–e951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Canfrán-Duque A, Pastor O, García-Seisdedos D, et al. The antipsychotic risperidone alters dihydroceramide and ceramide composition and plasma membrane function in leukocytes in vitro and in vivo. Int J Mol Sci . 2021;22(8):3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Petty A, Glass LJ, Rothmond DA, et al. Increased levels of a pro-inflammatory IgG receptor in the midbrain of people with schizophrenia. J Neuroinflamm. 2022;19(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Du Y, Chen L, Li X-S, et al. Metabolomic identification of exosome-derived biomarkers for schizophrenia: a large multicenter study. Schizophr Bull. 2021;47(3):615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bustillo JR, Rowland LM, Lauriello J, et al. High choline concentrations in the caudate nucleus in antipsychotic-naive patients with schizophrenia. Am J Psychiatry. 2002;159(1):130–133. [DOI] [PubMed] [Google Scholar]
- 107. Smesny S, Schmelzer CE, Hinder A, et al. Skin ceramide alterations in first-episode schizophrenia indicate abnormal sphingolipid metabolism. Schizophr Bull. 2013;39(4):933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.