Abstract
Radioresistance remains a major obstacle to efficacious radiotherapy in non–small‐cell lung cancer (NSCLC). DNA replication proteins are novel targets for radiosensitizers. POLQ is a DNA polymerase involved in DNA damage response and repair. We found that POLQ is overexpressed in NSCLC and is clinically correlated with high tumor stage, poor prognosis, increased tumor mutational burden, and ALK and TP5 mutation status; POLQ inhibition impaired lung tumorigenesis. Notably, POLQ expression was higher in radioresistant lung cancer cells than in wild‐type cancer cells. Moreover, POLQ expression was further increased in radioresistant cells after radiation. Enhanced radioresistance is through a prolonged G2/M phase and faster repair of DNA damage, leading to reduced radiation‐induced apoptosis. Novobiocin (NVB), a POLQ inhibitor, specifically targeted cancer cells. Genetic knockdown of POLQ or pharmacological inhibition by NVB decreased radioresistance in lung adenocarcinoma while causing little toxicity to normal pulmonary epithelial cells. In conclusion, POLQ is a promising and practical cancer‐specific target to impair tumorigenesis and enhance radiosensitivity in NSCLC.
Keywords: DNA damage repair, lung cancer, POLQ, radioresistance, tumorigenesis
Polymerase θ is overexpressed in lung cancer and is clinically correlated with advanced tumor stage, worse prognosis, high TMB score, and TP53 and ALK mutation status. POLQ is a promising and practical target to impair tumorigenesis and enhance radiosensitivity in NSCLC.
Abbreviations
- ALK
anaplastic lymphoma kinase
- alt‐EJ
alternative end‐joining
- CI
confidence interval
- DSBs
DNA double‐stranded breaks
- GEO
Gene Expression Omnibus
- GO
Gene Ontology
- GSEA
enrichment and gene set enrichment analysis
- HR
homologous recombination
- ICB
immune checkpoint blockade
- IC50s
half‐maximal inhibitory concentrations
- IHC
immunohistochemical
- IRF1
interferon regulatory factor 1
- LUAD
lung adenocarcinoma
- NSCLC
non–small‐cell lung cancer
- NVB
novobiocin
- OS
overall survival
- PHBE
primary human bronchial epithelial cell
- POLQ
polymerase θ
- SEM
standard error of mean
- TCGA
The Cancer Genome Atlas
- TMB
tumor mutational burden
- γH2AX
phosphorylated histone H2AX
1. INTRODUCTION
Lung cancer is a leading cause of tumor‐related death. Lung adenocarcinoma is the most common subtype of lung cancer. 1 Chest radiotherapy is crucial for patients with thoracic malignancies. 2 However, radioresistance limits the efficacy of radiotherapy. Accordingly, the mechanisms of radioresistance in lung cancer have been a major research topic over the past few years. However, a clinically effective radiosensitizer that overcomes radioresistance has not yet been identified.
DNA replication is a fundamental process in all living organisms. Sustained proliferation in cancer cells induces genomic instability and immune escape from apoptosis. 3 DNA polymerases are enzymes that synthesize DNA during replication and protect cells against DNA damage. 4 POLQ is an error‐prone DNA repair enzyme that is involved in the repair of DNA DSBs through alt‐EJ; this is the main pathway in which POLQ is involved in the genomic toxicity of DNA damaging agents. 5 , 6 Notably, alt‐EJ is considered a mere backup DNA repair pathway due to its high mutagenicity. Normal tissue cells express no or low levels of POLQ. 7 However, cancer cells often gain mutations in DNA repair genes and respond by rewiring their DNA repair network to utilize compensatory pathways for survival, 5 making POLQ essential in certain cancers. POLQ is overexpressed in many cancers, and upregulation is associated with poor prognosis. 8 POLQ overexpression is associated with increased somatic mutation load in LUAD. 9 POLQ knockdown interrupts the development and progression of hepatocellular carcinoma. 10 In addition, POLQ is synthetic lethal with many genes involved in HR, including BRCA1/2. 11 POLQ may be a promising novel target in overcoming drug resistance to poly(ADP‐ribose) polymerase (PARP) inhibitors in BRCA1/2 mutated tumors.
Polymerase θ inhibitors, currently in development, are soon to be tested in clinical trials. 5 Recent studies have shown that the antibiotic NVB, a novel specific POLQ inhibitor, selectively kills HR‐deficient tumor cells in vitro and in vivo. 12 Radiotherapy induces excessive DNA lesions that affect DNA integrity and DNA replication 13 ; therefore, targeting POLQ may enhance the radiosensitivity of lung cancer. A previous study has indicated that the lack of POLQ radiosensitizes bone marrow stromal cells in vitro and increases reticulocyte micronuclei after total‐body irradiation. 14 Another study described how POLQ knockdown resulted in radiosensitization of laryngeal, bladder, pancreas, and cervix cancer cell lines using a siRNA screen. 15 However, the role of POLQ in lung cancer radioresistance has not yet been elucidated.
Thus, this study aimed to explore the association of POLQ with the characteristics and prognosis of patients with lung cancer. We used transcriptomic data derived from TCGA and IHC staining analysis of LUAD tissue microarrays. We showed that POLQ is overexpressed and predicts a poor prognosis in patients with LUAD and that targeting POLQ will significantly hinder lung tumorigenesis. Moreover, we found that POLQ is upregulated in radioresistant lung cancer cells. Genetic or pharmacologic inhibition of POLQ overcomes radioresistance in LUAD while having a minimal effect on normal cells. These indicate that POLQ and its inhibitor NVB are a promising cancer‐specific therapeutic target and radiosensitizer, respectively.
2. MATERIALS AND METHODS
2.1. Data source, cell culture, and antibodies and reagents
mRNA expression and corresponding clinical data for LUAD were collected from TCGA dataset and the GEO dataset (GSE19804). The human lung cancer cell lines A549, H1299, H446, and H69. and the human bronchial epithelial cell line BEAS‐2B were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI‐1640 medium supplemented with 10% fetal bovine serum at 37°C in 5% carbon dioxide. All cell lines were regularly tested for contamination with mycoplasma or other pathogens and authenticated using short tandem repeat profiling. The PHBE cells were isolated and cultured from freshly surgically removed human lung tissue as described previously. 16
The primary antibodies used in this study were anti‐POLQ (Thermo Fisher Scientific; SAB1402530; 1:1000 and Sigma, SAB1402530; 1:1000) and anti‐GAPDH (Abclonal; AC002; 1:5000). The secondary antibodies used as follows: anti‐mouse (Proteintech; SA00001‐1; 1:5000) and anti‐rabbit (Proteintech; SA00001‐2; 1:5000). The reagent used was novobiocin (Selleck; S2492).
2.2. Construction of radioresistant H1299 cell lines
The Varian Vital Beam Linear Accelerator (Varian Medical Systems) delivered a total X‐ray radiation dose of 138 Gy. The cells were irradiated with 6 Gy every 3 days (dose rate of 4 Gy/min, source–surface distance of 100 cm).
2.3. POLQ silencing by small interfering RNA
Transfection of POLQ siRNA and negative control siRNA was performed using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. POLQ siRNA was used for pooling, mixing siRNA‐1 and siRNA‐2 in a 1:1 ratio. Transfection efficacy was detected using western blotting 48 h post‐transfection. The siRNA sequences were as follows: Scramble siRNA, 5′‐UUCUCCGAACGUGUCACGU‐3′; SiPOLQ‐1, 5′‐CGGGCCUCUUUAGAUAUAAAU‐3′; SiPOLQ‐2, 5′‐GCUGACCAAGAUUUGCUAUAU‐3′.
2.4. EdU essay
The EdU Cell Proliferation Kit (Beyotime) was used to detect the proliferation ability of lung cancer cells, following the manufacturer's instructions. Briefly, 48 h after siRNA transfection, EdU was added to the cells and incubated for 2 h; after which, the cells were fixed and washed again. Following permeabilization, click additive solution was added to the cells and incubated for 30 min. Finally, cells were stained with Hoechst 33342 for 10 min, observed, and photographed under a microscope.
2.5. Immunohistochemistry
A LUAD tissue microarray consisting of tumor and paired adjacent nontumor tissue samples from 98 patients with LUAD was purchased from Outdo Biotech (Shanghai, China). Immunohistochemistry assays were performed as previously described. 17 POLQ primary antibody (Thermo Fisher Scientific; PA5‐115130; 1:200) was used. The immunohistochemistry score was defined as the product of the staining intensity and the positively stained area; this was assessed by two independent pathologists. Low and high expression levels were defined as an immunohistochemistry score of <6 and ≥6, respectively. Staining intensity was also categorized based on the immunohistochemistry score as negative, weak, moderate, and strong for scores of 0, 1, 2, and 3, respectively. In addition, positively stained areas were also categorized based on the score as 0%–25%, score 1; 26%–50%, score 2; 51%–75%, score 3; and > 75%, score 4.
2.6. Quantitative reverse transcription‐polymerase chain reaction
Total RNA was extracted from the cells using TRIzol reagent (Invitrogen) according to standard procedures. qRT‐PCR was performed using SYBR Premix Ex Taq reagents (TaKaRa) in two steps according to the manufacturer's instructions. The primer sequences of POLQ were as follows: F: TATCTGCTGGAACTTTTGCTGA; R: CTCACACCATTTCTTTGATGGA.
2.7. RNA sequencing
Irradiated and control A549 cells (n = 3) were lysed with TRIzol reagent and sent for transcriptome sequencing in liquid nitrogen (BGI). Total RNA was extracted from cells. Oligo(dT)‐attached magnetic beads were used to purify the mRNA. Single‐stranded circular DNA was used as the final library. The final library was amplified with phi29 to form a DNA nanoball, which had more than 300 copies of one molecule. DNA nanoballs (DNBs) were loaded into the patterned nanoarray and single‐end 50‐base reads were generated on the BGIseq500 platform (BGI). The original RNA‐seq data generated in the study were uploaded to the GEO under registration number GSE211118.
The subsequent analysis and data mining were performed on Dr. Tom multiomics data‐mining system (https://biosys.bgi.com). Gene expression levels were calculated using RSEM (v1.3.1). 18 The heatmap was drawn using pheatmap (v1.0.8) according to the gene expression difference in different samples. Differential expression analysis was performed using DESeq2 (v1.4.5) with a Q‐value of <0.05. 19 Functional enrichment analyses, including GO 20 enrichment and GSEA, were performed.
2.8. Cell clonogenic survival assay
The same numbers of lung cancer cells with different treatments were implanted in 6‐well plates, and different doses of radiation were delivered the next day. After ~2 weeks, the cells were fixed and stained. The numbers of colonies were then counted and compared between groups. The single‐hit multitarget model was used to calculate the surviving fraction, which was performed as previously described. 21
2.9. Immunofluorescence
Approximately 5000 lung cancer cells were seeded in 24‐well plates coated with sterile glass coverslips. The next day, the cells were irradiated with 6 Gy of X‐rays and then fixed with 4% paraformaldehyde after 24 h. The cells were permeabilized with PBS containing 0.2% Triton X‐100 for 15 min. Next, the cells were blocked with 5% bovine serum albumin and incubated with γH2AX primary antibodies (Abcam; ab81299; 1:500) at 4°C overnight. The next day, the cells were washed and incubated with secondary antibodies. The cells were then stained with DAPI, observed, and photographed under a fluorescence microscope.
2.10. Neutral comet assay
The Comet Assay Kit (Trevigen) was used to detect the level of DSB of lung cancer cells after radiation according to the manufacturer's instructions as previously described. 21
2.11. Cell cycle distribution
The cell cycle distribution of lung cancer cells was examined using the Cell Cycle and Apoptosis Analysis Kit (Beyotime). Briefly, lung cancer cells were collected 24 h after irradiation. Then, the cells were fixed with 70% ethanol overnight. The next day, the cells were stained with propidium iodide staining solution for 30 min. The stained cells were then analyzed by flow cytometry using a FACSCalibur system.
2.12. Measurement of cell apoptosis and in vivo experiments
The lung cancer cells were collected and fixed 48 h after radiation. Cell apoptosis was detected using the Annexin V‐FITC Apoptosis Detection Kit (Beyotime) following the manufacturer's instructions.
For the in vivo experiments, female 6‐week‐old BALB/c nude mice were raised at the Tongji Medical College of Huazhong University of Science and Technology. All animal procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Mice were randomly divided into four groups. A549 cells (5 × 106) were injected subcutaneously. NVB was administered intraperitoneally at 15 mg/kg starting from the day when the tumor volume reached 120 mm3. NVB treatment was given every 3 days and continued for 2 weeks. The tumors were irradiated with a 10‐Gy dose the next day after the first NVB dose. All tumors were measured using Vernier calipers every 3 days, and the tumor volume was calculated as follows: V = (L × W2)/2, where V = volume (mm3), L = length (mm), and W = width (mm).
2.13. Statistical analysis
All experiments were repeated at least three times. Experimental data were expressed as the mean ± SEM for continuous variables. In all experiments, categorical variables were compared between groups using the χ 2 test, while continuous variables were compared using Student's t‐test (two‐tailed) or analysis of variance, as appropriate. All data met the assumptions of the tests, and statistical tests are justified as appropriate. Kaplan–Meier survival curves were plotted to explore the association between POLQ expression and OS. High or low POLQ expression was determined according to the median POLQ expression, and differences between the POLQlow and POLQhigh curves were compared using the log‐rank test. Specifically, for Kaplan–Meier curves, p‐values and hazard ratio with 95% CI were generated by log‐rank tests and univariate Cox proportional hazards regression. The correlation between genes and pathway was analyzed using the R software GSVA package, with the parameter as method = ‘ssgsea’. To calculate single‐sample gene set enrichment, the GSVA program was used to derive the absolute enrichment scores of gene sets from previous publications and previous experimentally validated gene signatures from MsigDB. 22 Statistical difference was analyzed using Spearman correlation. GSEA analysis was performed to demonstrate the primary biological function of POLQ. 23 The C2.cp.kegg.v7.0.symbols.gmt dataset was obtained from the Molecular Signatures Database (MSigDB). NOM p‐values <0.05, |NES| >1, and false discovery rate (FDR) q < 0.25 were considered statistically significant. To determine the association between POLQ expression and gene mutational frequency, the data of mutations were downloaded and visualized using the maftools package in R software (R Foundation for Statistical Computing, Vienna, Austria). Genes with higher mutational frequency in the high and low POLQ expression groups were plotted in a histogram, and significant differences were analyzed using the χ 2 test. Correlation analysis between POLQ gene expression and tumor mutational burden was performed using Spearman's correlation analysis. All statistical analyses were performed using R software v4.0.3 or SPSS 26.0 (IBM Corp). Differences were considered statistically significant at p < 0.05 (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3. RESULTS
3.1. POLQ is overexpressed and predicts poor prognosis in lung cancer
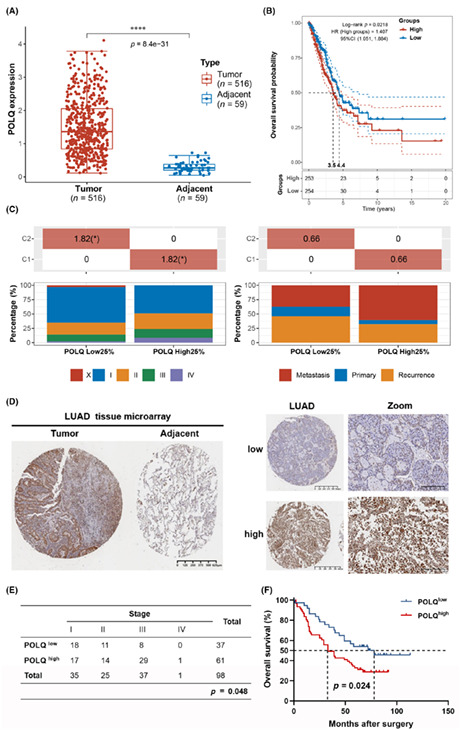
To determine the clinical significance of POLQ, mRNA expression levels were analyzed based on LUAD data from TCGA database. POLQ mRNA expression was markedly higher in LUAD tissues than in adjacent nontumor tissues (Figure 1A). Analysis of the GSE19804 dataset was consistent with that of the TCGA dataset (Figure S1). In addition, high mRNA expression of POLQ was correlated with poor OS (Figure 1B) and high tumor stage. Meanwhile, although the difference was not statistically significant, the metastasis rate was higher in patients with high POLQ mRNA levels (Figure 1C), indicating that POLQ is overexpressed in lung cancer tumors and is related to a worse prognosis. Analysis of the association between mRNA expression of POLQ and gene expressions and mutations in LUAD showed that high POLQ expression was associated with increased expression of immune checkpoint proteins including PDL1, CTLA4, and LAG3 (Figure S2A). It was also associated with increased TMB (Figure S2B), which has been recognized as an immunotherapy biomarker. 24 This indicated that high POLQ expression might be clinically correlated with better outcomes of ICB treatment. p53 is a key tumor suppressor that is encoded by TP53, and loss of p53 function is frequently a prerequisite for cancer development. 25 The results showed that the POLQ mRNA expression level was associated with the TP53 mutation status (Figure S3). Genetic alterations in ALK have been identified to promote tumorigenesis and have been detected in a subset of NSCLC patients. 26 The current study further found that POLQ mRNA expression was associated with the ALK mutation status (Figure S4). Collectively, these data suggested that POLQ was associated with specific clinical characteristics of patients with LUAD.
FIGURE 1.
Polymerase θ is overexpressed and associated with poor prognosis in lung adenocarcinoma. (A) The distribution of POLQ expression in tumor tissues and adjacent normal tissues from the TCGA dataset. (B) Kaplan–Meier survival analysis by POLQ expression in the TCGA dataset. (C) The distribution of clinical characteristics (stage and metastasis) in the samples from different groups. (D) Representative immunohistochemical staining images of POLQ in lung cancer tissue and adjacent nontumor tissue from lung adenocarcinoma tissue microarray. (E) Statistical analysis of the correlation between POLQ expression and tumor stage from lung adenocarcinoma tissue microarray. (F) Kaplan–Meier survival analysis by POLQ expression.
Consistent with these findings from TCGA analysis, results at the protein level showed that POLQ expression was higher in LUAD tissues than in adjacent nontumor tissues (Figure 1D). In addition, POLQ protein expression was correlated with tumor stage and OS (Figure 1E,F). Moreover, consistent with mRNA data from TCGA, high POLQ protein expression was associated with high PDL1 protein expression (Figure S5). These results suggested that POLQ was overexpressed in a majority of patients with LUAD and was associated with advanced stage, poor prognosis, and PDL1 expression.
3.2. POLQ promotes the growth and proliferation of lung cancer cells
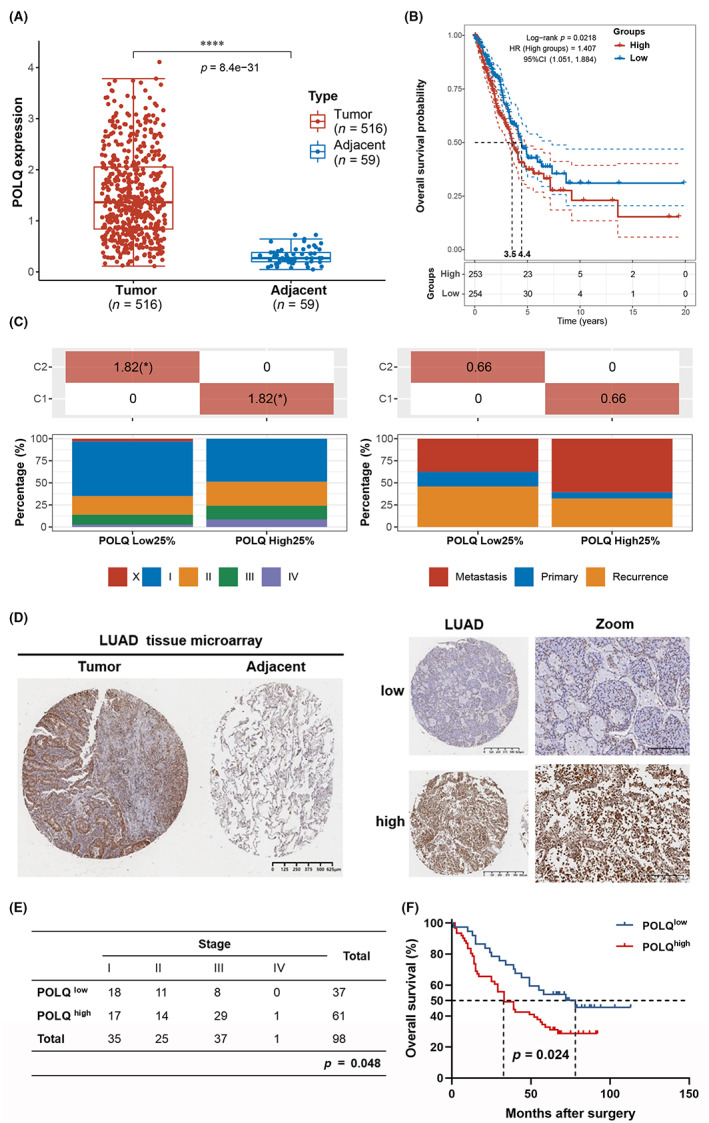
Given its overexpression in patients with LUAD, we hypothesized that POLQ might function as an oncogene and promotes lung tumorigenesis. The results showed that POLQ protein expression was higher in lung cancer cells than in the normal human lung epithelial cell line BEAS‐2B and the primary human lung bronchial epithelial cell PHBE. POLQ protein expression was especially higher in NSCLC cell lines (Figure 2A). To further investigate the correlation between POLQ expression and lung tumorigenesis, POLQ expression was knocked down in lung cancer cells by siRNA silencing. Since the transfection efficiency was not satisfactory using two separate siRNA (Figure S6), siRNA was used as pooling (Figure 2B). POLQ knockdown in A549 and H1299 lung cancer cell lines via siRNA significantly reduced the growth and colony formation ability of H1299 and A549 (Figure 2C,D). EdU experiment revealed that the ratio of EdU+ cells decreased significantly, indicating that POLQ functions as an oncogene and promoter of lung cancer cell proliferation (Figure 2E).
FIGURE 2.
Polymerase θ promotes the proliferation ability of lung adenocarcinoma cell. (A) Protein levels of POLQ in different lung cancer cell lines and normal human lung epithelial cells, with statistical analysis. (B) Western blot for the analysis of siRNA efficacy for POLQ knockdown. (C) POLQ knockdown delays the growth of lung cancer cells. (D) POLQ knockdown impairs the colony formation ability of lung cancer cells. (E) EdU essay showing that POLQ silencing inhibits the proliferation of lung cancer cells.
3.3. POLQ is associated with DNA repair and is upregulated in vitro after radiation in lung cancer
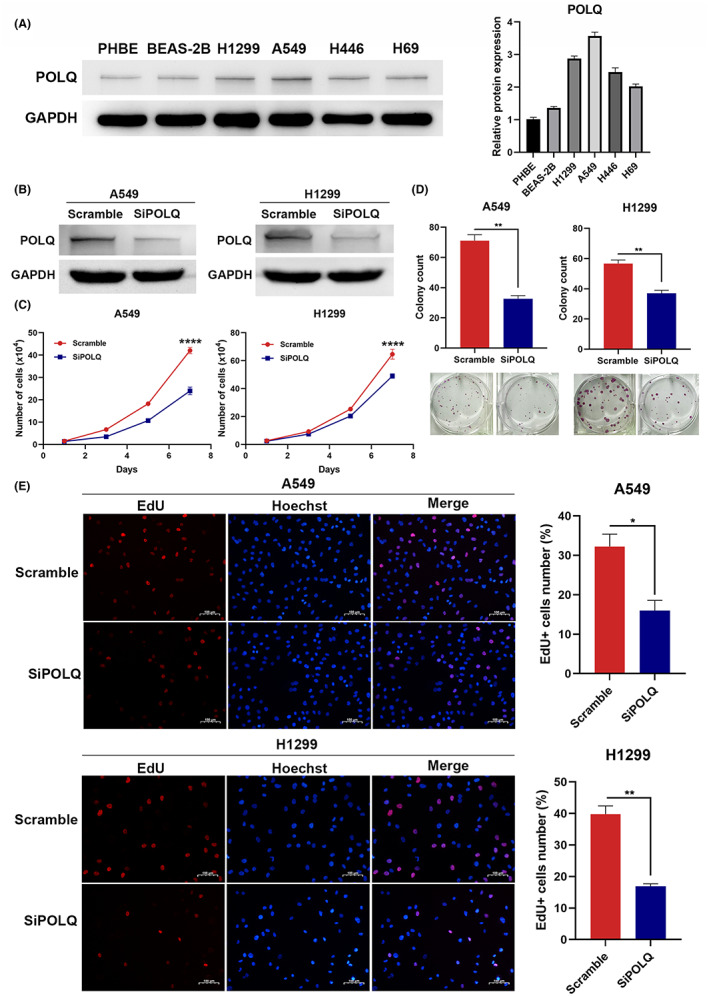
The bioinformatics analysis from TCGA revealed that POLQ function was focused on the DNA repair pathway, G2/M checkpoint pathway, and DNA replication pathway (Figure 3A). GSEA analysis also showed that the G2/M checkpoint pathway and DNA replication pathways were enriched (Figure S7). Therefore, we speculated that POLQ might be involved in lung cancer radioresistance. To determine the response of lung cancer cells after radiation, we treated A549 with 10 Gy radiation, RNA was extracted 24 h later and RNA‐seq was performed. GO analysis showed that lung cancer cells had highly upregulated genes associated with DNA damage repair (Figure 3B). GSEA revealed that irradiated lung cancer cells strongly expressed genes involved in the DNA damage checkpoint and p53 pathways (Figure 3C). The heatmap also showed that irradiated lung cancer cells had upregulated genes that were closely associated with DSB repair, including CDK1, CDK2, POLQ, CDC6, and RAD51 (Figure 3D).
FIGURE 3.
Polymerase θ is upregulated after radiation and is associated with radiosensitivity in lung cancer. (A) The correlations between POLQ and pathway score. (B) GO analysis of signaling pathways affected by radiation in A549 cells. (C) GSEA plots showing enrichment of the DNA damage checkpoint pathway and p53 pathway in the irradiated A549 cells. (D) Heatmap showing the change in genes correlated with DNA damage repair. (E) qRT‐PCR showing that the mRNA level of POLQ expression is increased after irradiation in A549 and H1299 cells. (F) Western blot showing that the protein level of POLQ is increased after irradiation in A549 and H1299 cells (* indicates the predicted POLQ band). (G) Cell clonogenic survival assay confirming the establishment of an H1299 radioresistant cell line. (H) qRT‐PCR showing mRNA levels of POLQ in H1299WT and H1299IRR cells. (I) Western blot showing that the protein level of POLQ is increased after radiation in H1299IRR cells.
To validate these data, mRNA and protein levels of POLQ were measured in A549 and H1299 cell lines at different time points after radiation. The results revealed that POLQ expression was upregulated after radiation both at the mRNA and protein levels (Figure 3E,F). To further determine the role of POLQ in radioresistance, an H1299 radioresistant cell line (H1299IRR) was constructed and confirmed by cell clonogenic survival assay (Figure 3G). A comparison of POLQ mRNA expression between wild‐type cells (H1299WT) and radioresistant cells showed that POLQ was upregulated in radioresistant cells (Figure 3H). Further analysis of POLQ expression at the protein level in H1299WT and H1299IRR cells before and after radiation showed that, after radiation, POLQ expression was higher in H1299IRR cells than in H1299WT cells (Figure 3I). These data suggest that POLQ played a potential role in the radiosensitivity of lung cancer.
3.4. POLQ silencing enhances in vitro radiosensitivity in lung cancer
To evaluate whether POLQ promoted radioresistance, a clonogenic survival assay was performed to examine the proliferation ability after different doses of radiation. It showed that POLQ knockdown enhanced the radiosensitivity of A549, H1299WT, and H1299IRR cells (Figure 4A). The neutral comet assay also showed that POLQ‐deficient cells presented more severe DNA DSBs after radiation (Figure 4B). An immunofluorescence assay was then performed to evaluate the foci of γH2AX, a recognized sensor of DNA strand breaks. It showed that POLQ knockdown cells had more DNA damage‐induced γH2AX foci (Figure 4C).
FIGURE 4.
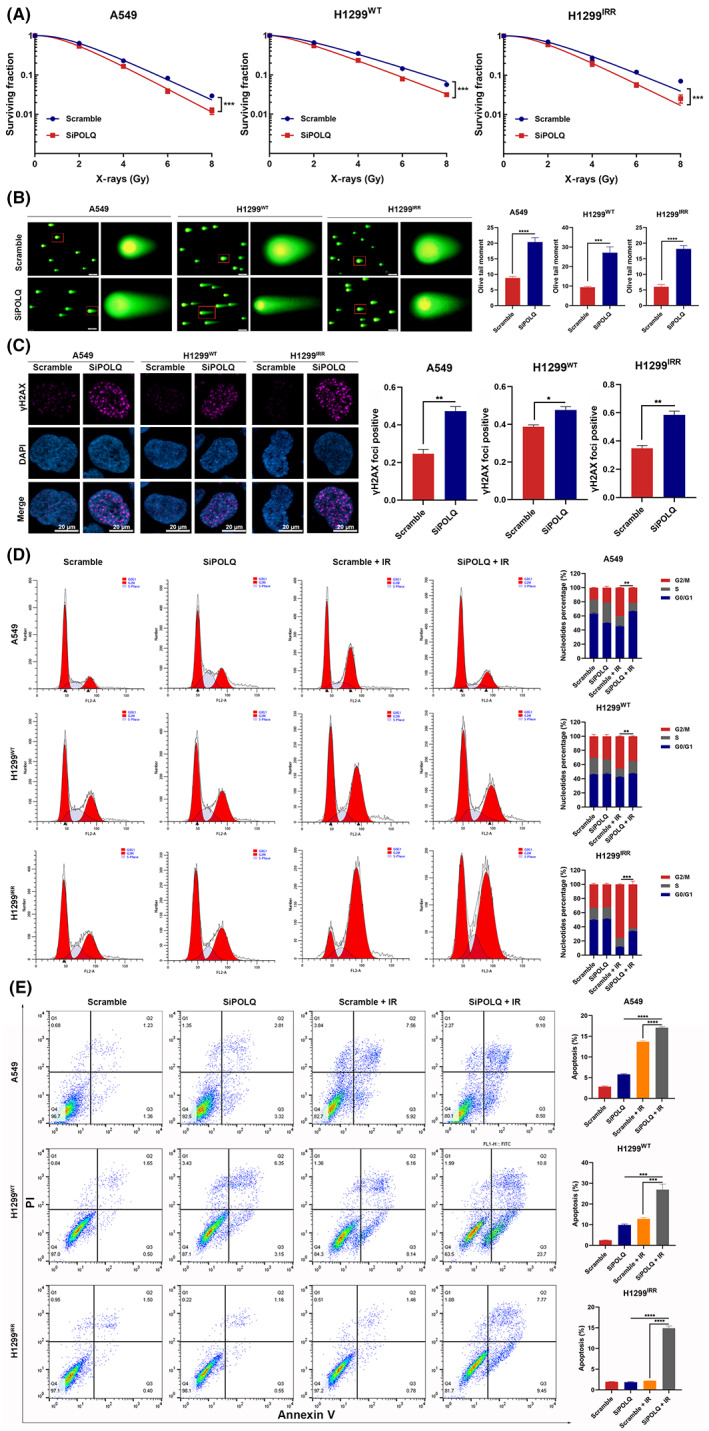
Polymerase θ silencing enhances in vitro radiosensitivity in lung cancer. (A) Clonogenic survival assay showing that POLQ silencing enhances radiosensitivity in A549, H1299WT, and H1299IRR cells. (B) Neutral comet assay showing the effect of POLQ silencing on tail formation. (C) Representative immunostaining images and quantification showing γH2AX foci 24 h after irradiation (6 Gy). A cell containing ≥10 foci is considered foci‐positive. (D) Cell cycle distribution is detected in A549, H1299WT, and H1299IRR cells 24 h after irradiation (6 Gy). (E) POLQ silencing increases radiation‐induced cell apoptosis in A549, H1299WT, and H1299IRR cells 48 h after irradiation (6 Gy).
Activation of apoptosis and cell cycle arrest are critical responses to acute DNA damage. 27 Cell cycle distribution experiment in the current study showed that control cells were arrested in the G2/M phase after radiation. Importantly, G2/M cell cycle arrest after radiation was significantly more attenuated in POLQ‐deficient cells (Figure 4D). To determine why POLQ knockdown induces growth inhibition, flow cytometry was performed to determine the level of apoptosis. The results showed that radiation alone resulted in a higher apoptotic population of A549 and H1299WT cells than in control cells, while the apoptotic population remained nearly unchanged in 1299IRR cells. Notably, POLQ knockdown markedly enhanced the radiation‐induced apoptosis in A549, H1299WT, and 1299IRR cells (Figure 4E). Collectively, these results indicated that POLQ played a critical role in radiosensitivity in lung cancer, and POLQ silencing reversed radioresistance.
3.5. NVB inhibition of POLQ enhances in vitro radiosensitivity in lung cancer
To explore the potential of NVB as a radiosensitizer through POLQ inhibition, we performed an array of experiments to test its efficacy. First, the IC50s of NVB in H1299 and A549 cell lines were determined (Figure S8), and 100 μM NVB was used in the succeeding experiments. Clonogenic survival assay showed that, similar to POLQ silencing, NVB treatment rendered lung cancer cells more susceptible to radiation (Figure 5A). Moreover, neutral comet assay showed that the olive tail moment was higher in NVB‐treated cells than in PBS‐treated cells (Figure 5B). Immunofluorescence staining revealed that DNA damage‐induced γH2AX foci were increased in NVB‐treated cells (Figure 5C). In addition, NVB treatment alleviated the G2/M phase arrest induced by radiation (Figure 5D). The apoptotic population was significantly higher after radiation in NVB‐treated cells than in PBS‐treated cells (Figure 5E). Moreover, additional use of NVB did not significantly change the cell cycle distribution and the apoptotic population in irradiated POLQ‐silenced lung cancer cells, further confirming the consistent effect of NVB and POLQ silencing on lung cancer cells and that these observed effects did not result from off‐target effects (Figure S9). These findings indicated that NVB was a potential radiosensitizer.
FIGURE 5.
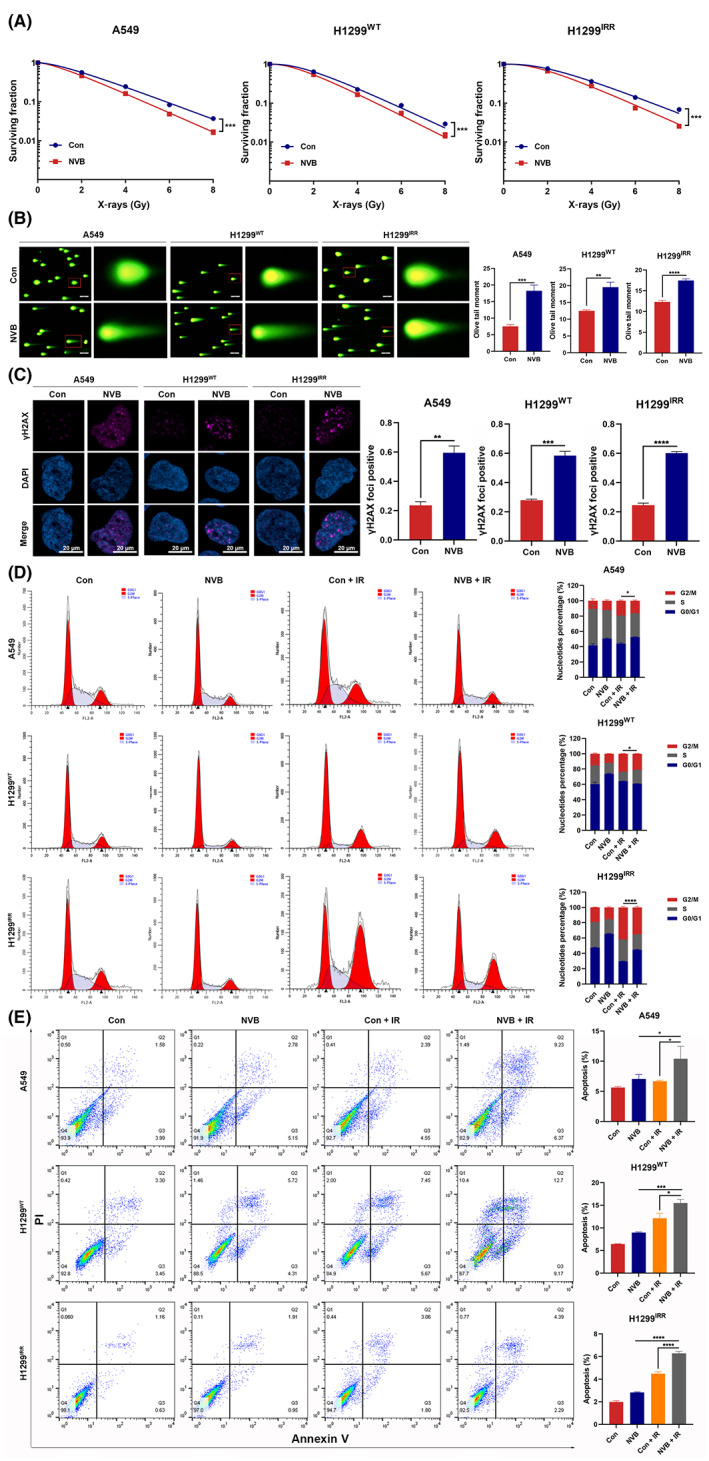
Polymerase θ inhibition with NVB impairs in vitro radioresistance in lung cancer. (A) The effect of NVB on colony formation ability after irradiation in A549, H1299WT, and H1299IRR cells. (B) Representative images of neutral comet assay performed 4 h after radiation in lung cancer cells pretreated with NVB or PBS for 24 h. (C) Representative images and quantification of γH2AX foci in A549, H1299WT, and H1299IRR cells pretreated with NVB. (D) Cell cycle distribution is examined in NVB‐ or PBS‐treated lung cancer cells 24 h after irradiation (6 Gy). (E) NVB treatment increases radiation‐induced cell apoptosis in A549, H1299WT, and H1299IRR cells 48 h after irradiation (6 Gy).
3.6. POLQ inhibition has minimal effect on normal pulmonary epithelial cells
To investigate whether POLQ inhibition increased the toxicity to normal pulmonary epithelial cells, POLQ knockdown was performed using siRNA (Figure 6A). An EdU assay was first performed on BEAS‐2B cells. It showed that POLQ knockdown had little effect on the proliferation ability of pulmonary epithelial cells (Figure 6B). Next, we explored whether POLQ inhibition alters the radiosensitivity of normal cells. Immunofluorescence staining revealed that the DNA damage‐induced γH2AX foci were not significantly increased in POLQ‐silenced or NVB‐treated cells compared with controls after radiation (Figure 6C). In addition, POLQ silencing or NVB treatment did not change the cell cycle distribution or the apoptotic population of normal cells after radiation (Figure 6D,E), strongly supporting that POLQ inhibition led to cancer‐specific radiosensitization.
FIGURE 6.
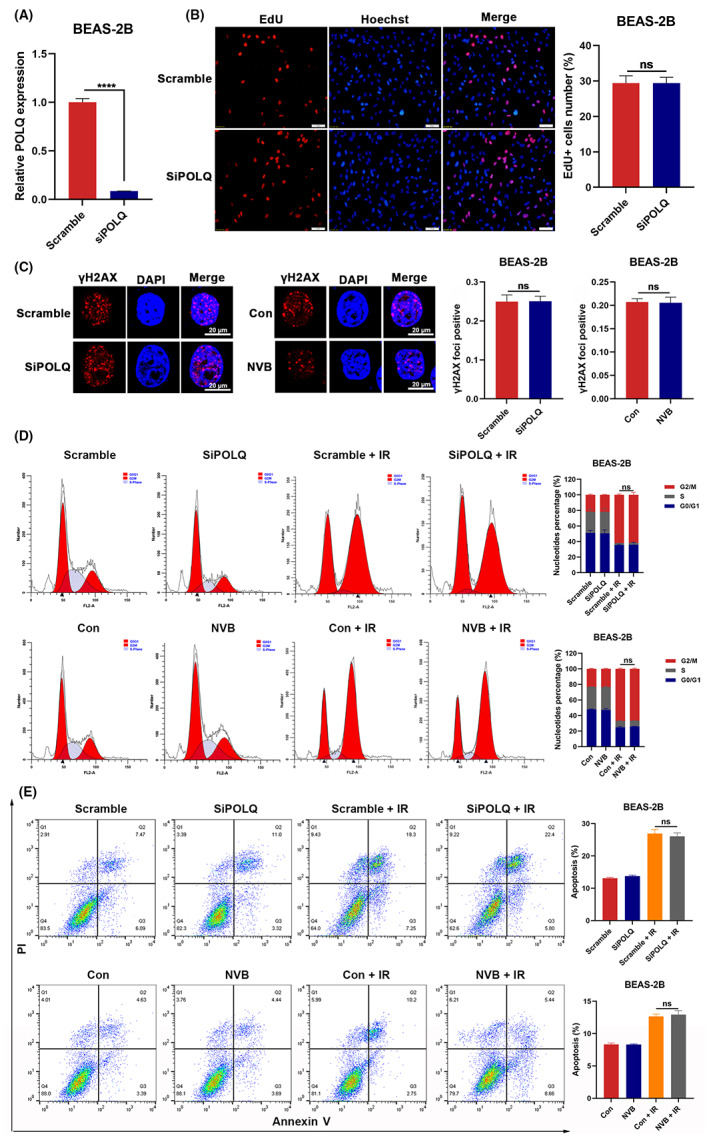
The effect of POLQ knockdown on normal pulmonary epithelial cells. (A) Analysis of the siRNA efficacy for POLQ knockdown using qRT‐PCR. (B) EdU essay showing that POLQ silencing does not inhibit the proliferation of BEAS‐2B cells. (C) Representative images and quantification of γH2AX foci in BEAS‐2B cells pretreated with POLQ siRNA or NVB. (D) Cell cycle distribution is examined in POLQ‐silenced or NVB‐treated BEAS‐2B cells 24 h after irradiation (6 Gy). (E) The apoptotic rate was examined in POLQ‐silenced or NVB‐treated BEAS‐2B cells 48 h after irradiation (6 Gy).
3.7. NVB mitigates lung cancer progression and enhances radiosensitivity in vivo
The experimental workflow of the mouse xenograft is shown in Figure 7A. NVB treatment alone did not significantly reduce tumor growth, but tumor growth was impaired more significantly when NVB and X‐ray radiation were combined than with radiation alone (Figure 7B,C). Immunohistochemistry revealed that Ki67 expression of tumor cells was markedly suppressed when NVB and radiation treatment were combined (Figure 7D). These results suggested that NVB could be a promising drug for tumor regression and radiosensitization in LUAD.
FIGURE 7.
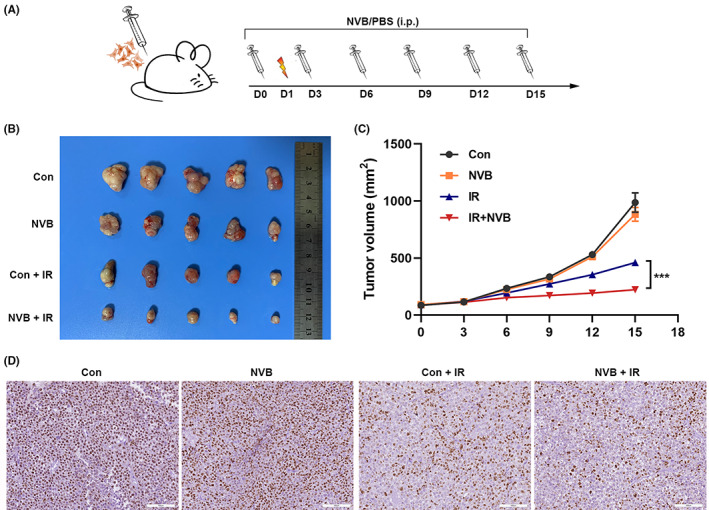
The POLQ inhibitor NVB enhances in vivo radiosensitivity in lung cancer. (A) Schematic graph of the xenograft study design and experimental workflow. (B) Images of xenografts implanted with A549 cells in different groups. (C) Growth curves of xenograft tumors in different groups. (D) Representative images of immunohistochemical staining of Ki67 in xenograft tumors in different groups.
4. DISCUSSION
This study found that POLQ is overexpressed in lung cancer and is clinically correlated with advanced tumor stage, worse prognosis, high TMB score, and TP53 and ALK mutation status. Thus, POLQ expression is a potential biomarker. In addition, POLQ is upregulated in radioresistant lung cancer cells, promoting tumorigenesis and radioresistance. Moreover, this study found that NVB inhibition of POLQ overcame radioresistance. To the best of our knowledge, this study is the first to report that NVB, as a POLQ inhibitor, enhances in vitro and in vivo radiosensitivity in lung cancer.
The DNA repair enzyme POLQ, an error‐prone translesion polymerase, is also involved in DNA DSB repair and is often upregulated in cancer. 5 DSB is the most severe DNA damage, and may lead to genomic rearrangements and cell death. 5 POLQ participates in alt‐EJ, a pathway required for HR‐deficient cancers. 28 , 29 alt‐EJ repair often introduces characteristic sequence alterations including microhomology‐flanked deletions and templated insertions, 30 , 31 which may result in tumor cell survival and gain of new malignant biological capabilities. POLQ overexpression has been reported in several cancers including hepatocellular carcinoma, prostate cancer, and breast cancer. 10 , 32 , 33 The current study found that POLQ was associated with a poor prognosis, high expression of immune checkpoint point proteins, high TMB score, and ALK and TP53 mutation status in LUAD. The abnormalities in DNA damage repair pathways were closely linked with the development of malignancies and the upregulation of these pathways was linked with resistance to treatment. 34 Changes in the DNA repair pathway were correlated with the efficacy of ICB, partially due to immune recognition of neoantigens, formed because of somatic mutation. 35 Another study demonstrated that DSB repair enhanced PDL1 expression dependent on IRF1. 36 However, the precise molecular mechanism of POLQ‐induced upregulation of immune checkpoint proteins and the exact correlation between POLQ expression and ICB efficacy remain to be explored. Our subsequent experiments revealed that POLQ was abnormally upregulated and acted as an oncoprotein in lung cancer cells. Considering its role in genomic instability and its connection with patient characteristics, POLQ expression may be an important biomarker for predicting the efficacy of immunotherapy or targeted therapy of related mutation genes. Overall, POLQ can be a promising target in lung cancer therapy.
Notably, we found a novel role for POLQ in lung cancer radioresistance. In H1299 radioresistant cell lines, POLQ expression was significantly higher after radiation in H1299IRR cells than in wild‐type cells, indicating its crucial role in the repair of radiation‐induced DSB. Further, POLQ inhibition resulted in increased radiation‐induced DNA damage, attenuated G2/M cell cycle arrest, and increased cell apoptosis, therefore making both radioresistant and wild‐type lung cancer cells more susceptible to ionizing radiation. Normal pulmonary epithelial cells barely used alt‐EJ for the repair of DNA damage, and thus, POLQ inhibition has minimal effect on normal tissue as it functions through the inhibition of cancer‐specific alterations. 5 Our data revealed that POLQ contributed to radioresistance in lung cancer and is a promising therapeutic target.
Polymerase θ inhibitors are currently in development. 5 The antibiotic NVB was recently identified as a specific POLQ inhibitor that selectively killed HR‐deficient tumor cells in vitro and in vivo. 12 NVB‐mediated POLQ inhibition impaired POLQ DNA repair function and phenocopied POLQ depletion in human cells. 12 The current study demonstrated for the first time that NVB is a radiosensitizer in lung cancer. POLQ inhibition with NVB enhanced radiosensitivity by augmenting DNA damage, attenuating G2/M cell cycle arrest, and promoting cancer cell apoptosis, but it had a minimal effect on normal pulmonary epithelial cells. Furthermore, the mouse xenograft model showed that NVB significantly increased the efficacy of radiotherapy and inhibited tumor growth. These findings provide baseline evidence for future clinical trials on the combination treatment of NVB and radiotherapy for lung cancer.
In conclusion, POLQ is an oncoprotein that promotes radioresistance in lung cancer. Accordingly, targeting POLQ impairs tumorigenesis and enhances radiosensitivity. In addition, NVB as a POLQ inhibitor is a promising tumor suppressor and radiosensitizer in lung cancer. Clinical trials on POLQ are warranted.
AUTHORS' CONTRIBUTIONS
Rui Zhou and Gang Wu contributed to the conception and design of the current study and ensured that the descriptions were accurate and agreed to by all authors. Xinrui Rao, Biyuan Xing, and Zilong Wu performed most of the experiments, curated the data, and drafted the original manuscript. Geng Wang, Yingzhuo Xu, Yunshang Chen, Dong Zhou, Xiaoshu Zhou, Ye Wang, and Weibin Chen conducted some experiments and confirmed the authenticity of all the raw data. Xinrui Rao, Biyuan Xing, and Wu Gang performed the formal analysis. Xiaorong Dong, Sheng Zhang, and Xiaorong Dong critically revised the manuscript. Rui Zhou and Gang Wu supervised the entire investigation process. All authors read and approved the final manuscript.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (no. 81802287 to R Zhou) and the China Postdoctoral Science Foundation (no. 2022 M721264 to X Rao).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVAL
The use of human samples and the research protocol was approved by the Ethics Review Board of Wuhan Union Hospital, Huazhong University of Science and Technology.
Approval of the research protocol by an Institutional Reviewer Board.
Informed Consent: The participating patients gave their informed consent.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: All animal procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Supporting information
Figures S1–S9.
ACKNOWLEDGMENTS
The authors would like to thank all participants for their cooperation.
Rao X, Xing B, Wu Z, et al. Targeting polymerase θ impairs tumorigenesis and enhances radiosensitivity in lung adenocarcinoma. Cancer Sci. 2023;114:1943‐1957. doi: 10.1111/cas.15727
Xinrui Rao, Biyuan Xing, and Zilong Wu contributed equally to this work.
Contributor Information
Gang Wu, Email: whzlwg@163.com.
Rui Zhou, Email: mimiruirui2@163.com.
DATA AVAILABILITY STATEMENT
The dataset(s) supporting the conclusions of this article is(are) included within the article (and its additional file(s)).
REFERENCES
- 1. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. The Lancet. 2021;398(10299):535‐554. [DOI] [PubMed] [Google Scholar]
- 2. Vinod SKHE. Radiotherapy treatment for lung cancer: current status and future directions. Respirology. 2020;25(Suppl 2):61‐71. [DOI] [PubMed] [Google Scholar]
- 3. Macheret M, Halazonetis TD. DNA replication stress as a hallmark of cancer. Annu Rev Pathol. 2015;10:425‐448. [DOI] [PubMed] [Google Scholar]
- 4. Yan Y, Xu Z, Huang J, et al. The deubiquitinase USP36 regulates DNA replication stress and confers therapeutic resistance through PrimPol stabilization. Nucleic Acids Res. 2020;48(22):12711‐12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schrempf A, Slyskova J, Loizou JI. Targeting the DNA repair enzyme polymerase theta in cancer therapy. Trends Cancer. 2021;7(2):98‐111. [DOI] [PubMed] [Google Scholar]
- 6. Wood RD, Doublie S. DNA polymerase theta (POLQ), double‐strand break repair, and cancer. DNA Repair (Amst). 2016;44:22‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawamura K, Bahar R, Seimiya M, et al. DNA polymerase theta is preferentially expressed in lymphoid tissues and upregulated in human cancers. Int J Cancer. 2004;109(1):9‐16. [DOI] [PubMed] [Google Scholar]
- 8. Lemee F, Bergoglio V, Fernandez‐Vidal A, et al. DNA polymerase theta up‐regulation is associated with poor survival in breast cancer, perturbs DNA replication, and promotes genetic instability. Proc Natl Acad Sci U S A. 2010;107(30):13390‐13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shinmura K, Kato H, Kawanishi Y, et al. POLQ overexpression is associated with an increased somatic mutation load and PLK4 overexpression in lung adenocarcinoma. Cancers (Basel). 2019;11(5):722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pan Q, Wang L, Liu Y, et al. Knockdown of POLQ interferes the development and progression of hepatocellular carcinoma through regulating cell proliferation, apoptosis and migration. Cancer Cell Int. 2021;21(1):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mateos‐Gomez PA, Gong F, Nair N, Miller KM, Lazzerini‐Denchi E, Sfeir A. Mammalian polymerase theta promotes alternative NHEJ and suppresses recombination. Nature. 2015;518(7538):254‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou J, Gelot C, Pantelidou C, et al. A first‐in‐class polymerase theta inhibitor selectively targets homologous‐recombination‐deficient tumors. Nat Cancer. 2021;2(6):598‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y, Wu L, Wang Z, et al. Replication stress: a review of novel targets to enhance Radiosensitivity‐from bench to clinic. Front Oncol. 2022;12:838637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goff JP, Shields DS, Seki M, et al. Lack of DNA polymerase theta (POLQ) radiosensitizes bone marrow stromal cells in vitro and increases reticulocyte micronuclei after total‐body irradiation. Radiat Res. 2009;172(2):165‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins GS, Prevo R, Lee YF, et al. A small interfering RNA screen of genes involved in DNA repair identifies tumor‐specific radiosensitization by POLQ knockdown. Cancer Res. 2010;70(7):2984‐2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yaghi A, Zaman A, Dolovich M. Primary human bronchial epithelial cells grown from explants. J Vis Exp. 2010;37:1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rao X, Zhou X, Wang G, et al. NLRP6 is required for cancer‐derived exosome‐modified macrophage M2 polarization and promotes metastasis in small cell lung cancer. Cell Death Dis. 2022;13(10):891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li B, Dewey CN. RSEM: accurate transcript quantification from RNA‐Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jie X, Fong WP, Zhou R, et al. USP9X‐mediated KDM4C deubiquitination promotes lung cancer radioresistance by epigenetically inducing TGF‐beta2 transcription. Cell Death Differ. 2021;28(7):2095‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739‐1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Subramanian ATP, Mootha VK, Mukherjee S, et al. Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545‐15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jardim DL, Goodman A, de Melo GD, Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. 2021;39(2):154‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang C, Liu J, Xu D, Zhang T, Hu W, Feng Z. Gain‐of‐function mutant p53 in cancer progression and therapy. J Mol Cell Biol. 2020;12(9):674‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Golding B, Luu A, Jones R, Viloria‐Petit AM. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non‐small cell lung cancer (NSCLC). Mol Cancer. 2018;17(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boutelle AM, Attardi LD. p53 and tumor suppression: it takes a network. Trends Cell Biol. 2021;31(4):298‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ceccaldi R, Liu JC, Amunugama R, et al. Homologous‐recombination‐deficient tumours are dependent on Poltheta‐mediated repair. Nature. 2015;518(7538):258‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Z, Song Y, Li S, et al. DNA polymerase θ (POLQ) is important for repair of DNA double‐strand breaks caused by fork collapse. J Biol Chem. 2019;294(11):3909‐3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schimmel J, van Schendel R, den Dunnen JT, Tijsterman M. Templated insertions: a smoking gun for polymerase theta‐mediated end joining. Trends Genet. 2019;35(9):632‐644. [DOI] [PubMed] [Google Scholar]
- 31. Sallmyr A, Tomkinson AE. Repair of DNA double‐strand breaks by mammalian alternative end‐joining pathways. J Biol Chem. 2018;293(27):10536‐10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carvajal‐Maldonado D, Wood RD. Regulating Poltheta in breast cancer. Cancer Res. 2021;81(6):1441‐1442. [DOI] [PubMed] [Google Scholar]
- 33. Ravindran F, Jain A, Desai S, et al. Whole‐exome sequencing of Indian prostate cancer reveals a novel therapeutic target: POLQ. J Cancer Res Clin Oncol. Published online June 23, 2022. doi: 10.1007/s00432-022-04111-0 [DOI] [PubMed] [Google Scholar]
- 34. Crowley F, Park W, O'Reilly EM. Targeting DNA damage repair pathways in pancreas cancer. Cancer Metastasis Rev. 2021;40(3):891‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science. 2015;348(6230):124‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sato H, Niimi A, Yasuhara T, et al. DNA double‐strand break repair pathway regulates PD‐L1 expression in cancer cells. Nat Commun. 2017;8(1):1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S9.
Data Availability Statement
The dataset(s) supporting the conclusions of this article is(are) included within the article (and its additional file(s)).


