Abstract
In this review, we focused on four topics, namely, minimally invasive esophagectomy (MIE), robot‐assisted minimally invasive esophagectomy (RAMIE), conversion and salvage surgery, and neoadjuvant and adjuvant therapy, based on notable reports published in the years 2020 and 2021. It seems that while the short‐term outcomes of minimally invasive Ivor Lewis esophagectomy (MIE‐IL) were better than those of open Ivor Lewis esophagectomy (OE‐IL), there were no significant differences in the long‐term outcomes between MIE‐IL and OE‐IL. Similarly, the short‐term outcomes of minimally invasive McKeown esophagectomy (MIE‐MK) were better than those of open McKeown esophagectomy (OE‐MK), while there were no significant differences in the long‐term outcomes between MIE‐MK and OE‐MK. Furthermore, the short‐term outcomes of robot‐assisted minimally invasive Ivor Lewis esophagectomy (RAMIE‐IL) were superior to those of completely minimally invasive Ivor Lewis esophagectomy (CMIE‐IL). On the other hand, there were advantages and disadvantages in relation to the short‐term outcomes of robot‐assisted minimally invasive McKeown esophagectomy (RAMIE‐MK) as compared with completely minimally invasive McKeown esophagectomy (CMIE‐MK). However, there were no significant differences in the long‐term outcomes between RAMIE‐MK and CMIE‐MK. Further research is needed to evaluate of short‐term and long‐term outcomes of transmediastinal esophagectomy with and without robotic assistance. Both induction chemotherapy and induction chemoradiotherapy appear to be promising to secure a higher rate of conversion surgery. Neoadjuvant chemoimmunotherapy and chemoimmunoradiotherapy have shown promising results and are expected as new powerful therapies.
Keywords: conversion and salvage surgery, minimally invasive esophagectomy, neoadjuvant and adjuvant therapy, robot‐assisted esophagectomy
There were advantages and disadvantages in relation to the short‐term outcomes of robot‐assisted minimally invasive McKeown esophagectomy (RAMIE‐MK) as compared with completely minimally invasive McKeown esophagectomy (CMIE‐MK). However, there were no significant differences in the long‐term outcomes between RAMIE‐MK and CMIE‐MK. The short‐term and mid‐term outcomes between robot‐assisted minimally invasive transmediastinal esophagectomy (RATME) and open transmediastinal esophagectomy (OTME) were also compared. Although RATME has some disadvantages, such as a higher rate of pulmonary complications as compared with OTME, these are expected to be overcome in the future.
1. INTRODUCTION
Esophageal cancer is one of the most malignant diseases that should be well‐controlled because it ranks seventh in incidence (604 000 new cases) and sixth in terms of overall mortality (544 000 deaths) in 2020 according to Global Cancer Statistics 2020. 1 There are several treatment modalities for esophageal cancer, including surgery, chemotherapy, radiotherapy, immunotherapy, and multidisciplinary treatment. In regard to surgery, the surgical techniques have advanced greatly since Torek first reported the first successful esophagectomy in 1913. 2 Advances in the surgical techniques and perioperative management methods have resulted in better short‐term and long‐term postoperative outcomes. 3 Recently, minimally invasive surgery for esophageal cancer has been widely adopted all over the world since Cuschieri et al. 4 reported the first case in 1992, contributing to an improved quality of life of patients undergoing esophagectomy, which is one of the most invasive surgeries performed for gastroenterological diseases. Moreover, surgical robotic systems for minimally invasive surgery have been developed and widely used around the world, and there are many reports of their usefulness and feasibility. 5 There are two minimally invasive surgical approaches: a thoracoscopic method and a combined method involving mediastinoscopy + laparoscopy. Advances have also been made in the techniques for the latter. 6 The surgical indications have been changing. Unresectable esophageal cancer could be made resectable with preoperative chemoradiotherapy, chemotherapy, immunotherapy, or a combination of these. 7 , 8 The concepts of conversion surgery and salvage surgery are now recognized, and much evidence has been accumulated that attests to confirm their significance. Multidisciplinary cancer treatment is necessary to improve the treatment outcomes, and neoadjuvant therapy and immunotherapy using immune checkpoint inhibitor are big concerns. Therefore, this review focuses on four topics, namely, minimally invasive esophagectomy (MIE), robot‐assisted minimally invasive esophagectomy (RAMIE), conversion and salvage surgery, and neoadjuvant/adjuvant therapy, based on notable reports published in the years 2020 and 2021.
2. MINIMALLY INVASIVE ESOPHAGECTOMY OTHER THAN ROBOT‐ASSISTED SURGERY
Three types of minimally invasive surgical procedures have been used to perform esophagectomy: thoracoscopic esophagectomy with cervical anastomosis (McKeown), 9 thoracoscopic esophagectomy with intrathoracic anastomosis (Ivor Lewis), 10 and transcervical and transhiatal esophagectomy (transmediastinal esophagectomy, TME). 6 Comparison of surgical outcomes should also take into account the type of surgical procedure employed. Therefore, we discuss the surgical outcomes separately for each type of surgical procedure.
2.1. Ivor Lewis esophagectomy
Ivor Lewis esophagectomy has been classified into three types according to the degree of surgical invasiveness: completely minimally invasive (thoracoscopy + laparoscopy) Ivor Lewis esophagectomy (CMIE‐IL), hybrid minimally invasive Ivor Lewis esophagectomy (right thoracotomy + laparoscopy) (HMIE‐IL), and open Ivor Lewis esophagectomy (OE‐IL). Comparisons between any two of the three types in terms of the short‐term and long‐term outcomes are summarized in Table 1. 11 , 12 , 13 , 14 , 15 , 16
TABLE 1.
Minimally invasive Ivor Lewis esophagectomy other than robot‐assisted surgery
| Author | Reference | Study type | Compared outcome | Type of surgery | Number of patients | Advantage |
|---|---|---|---|---|---|---|
| van Workum | 11 | Systematic review and meta‐analyses | Short‐term outcomes | CMIE‐IL | 723 | Lower rate of wound infection, lower amount of blood loss |
| HMIE‐IL | Lower rate of anastomotic leakage, shorter operating time | |||||
| Worrell | 12 | Propensity score matching analysis | Short‐term outcomes | HMIE‐IL | 3659 | Lower rate of positive surgical margins, higher number of harvested LNs, shorter LOS |
| OE‐IL | 3659 | |||||
| Holscher | 13 | Propensity score matching analysis | Short‐term outcomes | HMIE‐IL | T3, 210 | Similar: R0‐resection rate, number of harvested LNs, postoperative mortality |
| OE‐IL | T3, 105 | |||||
| Mariette | 14 | Randomized controlled trial | Short‐term HRQOL | HMIE‐IL | 103 | Smaller reduction in the 30 days role functioning and the social functioning |
| OE‐IL | 104 | |||||
| Patel | 15 | Systematic review and meta‐analyses | Long‐term outcomes | CMIE‐IL | 422 | Similar: 5‐year OS, 5‐year DFS |
| OE‐IL | 527 | |||||
| Nuytens | 16 | Randomized controlled trial | Long‐term outcomes | HMIE‐IL | 103 | Similar: 5‐year OS, 5‐year DFS |
| OE‐IL | 104 | |||||
| Holscher | 13 | Propensity score matching analysis | Long‐term outcomes | HMIE‐IL | T3, 210 | Similar: 5‐year OS |
| OE‐IL | T3, 105 | |||||
| Mariette | 14 | Randomized controlled trial | Long‐term HRQOL | HMIE‐IL | 103 | Two‐year improvement of social functioning and reduction of increased pain |
| OE‐IL | 104 | |||||
| Worrell | 12 | Propensity score matching analysis | Long‐term outcomes | HMIE‐IL | 3659 | OS |
| OE‐IL | 3659 | |||||
Abbreviations: CMIE‐IL, completely minimally invasive Ivor Lewis esophagectomy; DFS, disease‐free survival; HMIE‐IL, hybrid minimally invasive Ivor Lewis esophagectomy; HRQOL, health‐related quality of life; LN, lymph node; LOS, length of stay; OE‐IL, open Ivor Lewis esophagectomy; OS, overall survival.
Van Workum et al. 11 reported that CMIE‐IL had advantages such as a lower rate of wound infection and lower amount of blood loss, but also disadvantages, including a higher rate of anastomotic leakage and longer operating time as compared to HMIE‐IL. The anastomotic leakage is the most feared complication. This result may be due to the difficulty of minimally invasive intrathoracic anastomosis. As they discussed, the learning curve might also influence this outcome, since the learning curve for CMIE‐IL seems to be longer than that for HMIE‐IL. In the study examining the learning curve for minimally invasive Ivor Lewis esophagectomy, the mean incidence of anastomotic leakage reportedly decreased from 18.8% during the learning phase to 4.5% after a learning plateau had been reached. 17 Therefore, anastomotic leakage can be avoided when the procedure is performed by proficient surgeons. Worrell et al. 12 reported that HMIE‐IL had advantages, such as a lower rate of positive surgical margins, a higher number of harvested lymph nodes, and a shorter length of stay (LOS) over OE‐IL. This may be due to the more meticulous procedures and smaller incisions that are required using a laparoscopic approach. These oncologic benefits may result in better overall survival. Holscher et al. 13 reported that there were no significant differences in the R0‐resection rate, the number of harvested lymph nodes, postoperative mortality, and the 5‐year survival rate between HMIE‐IL and OE‐IL in patients with clinical and pathological T3 cancer. This study was performed in a single high‐volume center where the surgical technique was very standardized and did not change over the study period. Therefore, the oncologic radicality of HMIE‐IL seems to be comparable to that of OE‐IL. The results of this study are somewhat different from those of other reports. Mariette et al. 14 reported that when compared with OE‐IL, HMIE‐IL had advantages, such as smaller reductions in 30‐day role functioning and social functioning, and an improvement in social functioning and a reduction in the frequency of increased pain at 2 years. The mechanism responsible for the health‐related quality of life (HRQOL) differences was explained by the reduction in surgical invasiveness and the reduced rate of major complications from HMIE‐IL. Although the oncological aspects of the surgical approach are the most important, HMIE‐IL might be preferable compared with OE‐IL, from the viewpoint of postoperative HRQOL. Patel et al. 15 reported a systematic review and meta‐analysis that was the first study to examine both the 5‐year overall survival (OS) and the 5‐year disease‐free survival (DFS) following CMIE‐IL and OE‐IL. The results were that CMIE‐IL had a 5‐year OS and a 5‐year DFS that were equivalent to those for OE‐IL. This suggests a long‐term oncological equivalence between the two operative approaches. Nuytens et al. 16 reported the results of an MIRO randomized clinical trial to evaluate the long‐term, 5‐year outcomes of HMIE‐IL and OE‐IL. This study also showed no difference in the 5‐year OS and the 5‐year DFS between HMIE‐IL and OE‐IL.
Based on these reports, with particular emphasis on the results of randomized controlled trial, there seems to be no differences in long‐term outcomes among CMIE‐IL, HMIE‐IL, and OE‐IL. As for the short‐term outcomes, although CMIE‐IL is more likely to be associated with anastomotic leakage because the intrathoracic anastomosis is difficult to perform, this problem can be overcome by appropriately skilled surgeons. Moreover, CMIE‐IL or HMIE‐IL may be preferable for securing a better HRQOL.
In addition to comparing the outcomes of the three types of Ivor Lewis esophagectomy, van Workum et al. 18 compared the short‐term outcomes of minimally invasive Ivor Lewis esophagectomy (MIE‐IL) with those of minimally invasive McKeown esophagectomy (MIE‐MK) in patients with cancer of the distal esophagus or gastroesophageal junction, using propensity score matching analysis. They reported that as compared with MIE‐MK, MIE‐IL was associated with a lower incidence of anastomotic leakage, pulmonary complications, recurrent laryngeal nerve palsy, and reoperations, a lower 90‐day mortality, a shorter median length of stay in the intensive care unit, shorter median length of stay in the hospital, and a higher number of harvested lymph nodes. The location of the tumor, learning curve for performing the procedures, and heterogeneity of anastomotic techniques should be taken into account while interpreting these results.
2.2. McKeown esophagectomy
Although the systematic review and meta‐analysis reported by van Workum et al. 11 included both McKeown esophagectomy (92%) and Ivor Lewis esophagectomy (8%), it appears that completely minimally invasive McKeown esophagectomy (CMIE‐MK) has more advantages, that is, a lower rate of pulmonary complications, shorter length of stay, lower amount of blood loss, than hybrid minimally invasive McKeown esophagectomy (thoracoscopy + laparotomy or thoracotomy + laparoscopy) (HMIE‐MK). This may be due to totally smaller incisions that are required using a thoracoscopic and laparoscopic approach (Table 2). 11 , 19 , 20
TABLE 2.
Minimally invasive McKeown esophagectomy other than robot‐assisted surgery
| Author | Reference | Study type | Compared outcome | Type of surgery | Number of patients | Advantage | |
|---|---|---|---|---|---|---|---|
| van Workum | 11 | Systematic reviews and meta‐analyses | Short‐term outcomes | CMIE‐MK a | 2142 | Lower rate of pulmonary complications, shorter LOS, lower amount of blood loss | |
| HMIE‐MK a | |||||||
| Sakamoto | 19 | Propensity score matching analysis | Short‐term outcomes | MIE | 4572 | Lower incidences of in‐hospital mortality, SSI, anastomotic leakage, blood transfusion, reoperation, tracheotomy, and unplanned intubation; shorter LOS | |
| OE | 4572 | Lower incidences of RLNP, shorter intubation period, shorter duration of anesthesia | |||||
| Sugita | 20 | Propensity score matching analysis | Short‐and long‐term outcomes | Elderly (≥75 y/o) | 29 | Similar: Intraoperative data, postoperative complications, in‐hospital mortality rate, CSS, OS | |
| Non‐elderly (<75 y/o) | 29 |
Abbreviations: CMIE‐MK, completely minimally invasive McKeown esophagectomy; CSS, cancer‐specific survival; HMIE‐MK, hybrid minimally invasive McKeown esophagectomy; LOS, length of stay; MIE, minimally invasive esophagectomy (thoracoscopy + laparoscopy or thoracoscopy + laparotomy); OE, open esophagectomy; OS, overall survival; RLNP, recurrent laryngeal nerve palsy; SSI, surgical site infection.
McKeown 92%, Ivor Lewis 8%.
Sakamoto et al. reported that MIE (thoracoscopy + laparoscopy or thoracoscopy + laparotomy) had the advantages of being associated with lower incidences of in‐hospital mortality, surgical site infection, anastomotic leakage, blood transfusion, reoperation, tracheotomy, and unplanned intubation, and a shorter length of stay over OE (thoracotomy + laparotomy) when compared using a propensity score matching analysis of a Japanese inpatient database. On the other hand, the disadvantages of MIE include a higher incidence of recurrent laryngeal nerve palsy, a longer mean intubation period, and a longer duration of anesthesia as compared with open esophagectomy (OE). 19 A previous study examining MIE and OE by Takeuchi et al. 21 using a propensity score matching analysis of data from the Japanese National Clinical Database (NCD) showed similar results, such as lower incidences of surgical site infection and a higher incidence of recurrent laryngeal nerve palsy. To understand the benefits of MIE, the subjects of the analysis and the method of the analysis must be considered. Nevertheless, the items listed here are potential advantages and should be validated in prospective randomized controlled trials. The issue of these surgeries performed in elderly patients was also investigated, and no difference in the short‐term or long‐term outcomes have been reported between elderly patients (≥75 years old) and non‐elderly patients (<75 years old). 20 Therefore, provided appropriate selection criteria are applied, MIE can be selected even in elderly patients.
New interesting reports about preoperative diagnostic imaging examinations in patients undergoing MIE have been published. The effects of esophageal position as diagnosed by preoperative computed tomography (CT) on the short‐term outcomes after MIE‐MK were examined by Uchihara et al. 22 They found that a left‐sided esophagus was associated with an increased incidence of cardiovascular comorbidity and prolonged operation time, and was an independent risk factor for the development of pneumonia. The usefulness of prone‐position CT for preoperative simulation prior to MIE‐MK was investigated by Higuchi et al. 23 Preoperative CT imaging was performed with the patient in both the supine and prone positions, and the calculated magnitude of change in the VE (distance between the ventral aspect of the vertebral body and the midpoint of the esophagus) showed a negative correlation with the thoracic operation time and volume of blood loss during the thoracic procedure. A change in VE by ≥9 mm was identified as an independent risk factor for postoperative pneumonia. Therefore, information on the esophageal location diagnosed by preoperative CT imaging seems to be useful not only for predicting procedural difficulties, but also for predicting postoperative morbidity in patients scheduled to undergo MIE.
2.3. Transmediastinal esophagectomy
Two new techniques of TME have been reported. Daiko et al. 24 developed a new technique for performing bilateral transcervical mediastinoscope‐assisted transhiatal laparoscopic esophagectomy (BTC‐MATLE). After lymph node dissection along the recurrent laryngeal nerves of both sides through bilateral cervical skin incisions, bilateral transcervical mediastinoscopic esophagectomy was performed while avoiding collision of the scope, forceps, and other instruments outside in the cervical region and ensuring a good field for surgery in the mediastinum. The procedure yielded good results, with a reported R0 resection rate of 94% and in‐hospital mortality of 0%. Wu et al. 25 developed a unique technique of esophagectomy using a flexible mediastinoscope that was originally intended for esophagogastroduodenoscopy. An articulatory hook or IT knife was used for dissection of the esophagus under endoscopic guidance. A small amount of bleeding was controlled using electrocoagulation snares. Water could be flushed to provide a clear surgical field. Articulator grasper blunt dissection and an IT knife were used for lymphadenectomy. This procedure was also reported to yield good results, with the use of a flexible mediastinoscope being associated with a shorter operation time and higher number of harvested lymph nodes than use of a rigid mediastinoscope group; the mortality rate was 0%. As shown in these reports, TME has the potential to develop greatly in the future by combining various surgical instruments including surgical assistance robots.
3. ROBOT‐ASSISTED MINIMALLY INVASIVE ESOPHAGECTOMY
3.1. Robot‐assisted esophagectomy (type of procedure undescribed)
Reports that do not describe the types of surgical procedures used for esophagectomy are presented first (Table 3). 26 , 27 Manigrasso et al. 26 reported the advantages of RAMIE over OE and laparoscopic minimally invasive esophagectomy (LMIE) in terms of intraoperative outcomes (a lower volume of blood loss), postoperative complications (a lower rate of wound infection, a lower rate of pneumonia), and oncologic outcomes (a higher number of harvested lymph nodes, a higher R0 resection rate). The possible reasons for these better results may be the magnification of the images and the meticulous dissection that is enabled by robotic technology. In contrast, a disadvantage of RAMIE was the longer operation time; however, the operation time could be shortened if the following points were standardized: the robot settings, the instrument exchange methods, the actual procedures, and communication between the operator and other surgical staff members. Li et al. 27 compared the short‐term outcomes of RAMIE and completely minimally invasive esophagectomy (CMIE) using a systematic review and meta‐analyses. Importantly, RAMIE was associated with several advantages, such as intraoperative outcomes (a lower volume of blood loss), postoperative complications (a lower rate of recurrent laryngeal nerve palsy), and oncologic outcomes (a higher number of total harvested lymph nodes, abdominal lymph nodes, and lymph nodes along the recurrent laryngeal nerves), compared with CMIE. Again, the reasons for these preferable results may be the magnification of the images and the meticulous dissection enabled by the robotic technology. RAMIE could become the standard surgery for esophageal cancer in the near future.
TABLE 3.
Robot‐assisted minimally invasive esophagectomy (procedure undescribed and Ivor Lewis esophagectomy)
| Author | Reference | Study type | Compared outcome | Type of surgery | Number of patients | Advantage |
|---|---|---|---|---|---|---|
| Procedure undescribed esophagectomy | ||||||
| Manigrasso | 26 | Systematic review and meta‐analyses | Short‐term outcomes | RAMIE | 3832 | Higher number of harvested LNs, lower rate of pneumonia |
| LMIE | 7947 | Shorter operation time | ||||
| RAMIE | 1919 | Lower volume of blood loss, lower rate of wound infection and pneumonia, higher number of harvested LNs, higher R0 resection rate | ||||
| OE | 2566 | Shorter operation time | ||||
| Li | 27 | Systematic review and meta‐analyses | Short‐term outcomes | RAMIE | 866 | Higher number of total harvested LNs, abdominal LNs, and LNs along RLN, less blood loss, less incidence of RLNP |
| CMIE | 883 | |||||
| Ivor Lewis esophagectomy | ||||||
| Angeramo | 28 | Systematic review and meta‐analyses | Short‐term outcomes | RAMIE‐IL | 974 | Lower volume of blood loss, lower rate of pneumonia, lower overall morbidity, higher rate of R0 resection |
| CMIE‐IL | 5275 | Shorter operation time | ||||
| Tagkalos | 29 | Propensity score matching analysis | Short‐term outcomes | RAMIE‐IL | 40 | Relatively higher number of harvested LNs, shorter ICU stay |
| CMIE‐IL | 40 | |||||
Abbreviations: CMIE, completely minimally invasive esophagectomy; CMIE‐IL, completely minimally invasive Ivor Lewis esophagectomy; ICU, intensive care unit; LMIE, laparoscopic minimally invasive esophagectomy; LN, lymph node; OE, open esophagectomy; RAMIE, robot‐assisted minimally invasive esophagectomy; RAMIE‐IL, robot‐assisted minimally invasive Ivor Lewis esophagectomy; RLN, recurrent laryngeal nerve; RLNP, recurrent laryngeal nerve palsy.
3.2. Robot‐assisted Ivor Lewis esophagectomy
Angeramo et al. reported the results of a systematic review and meta‐analyses, and Tagkalos et al. reported the results of a propensity score matching analysis comparing robot‐assisted minimally invasive Ivor Lewis esophagectomy (RAMIE‐IL) and CMIE‐IL (Table 3). 28 , 29 RAMIE‐IL had advantages in terms of the intraoperative outcomes (a lower volume of blood loss), postoperative complications (a lower rate of pneumonia, a lower rate of overall morbidity, a shorter intensive care unit stay), and oncologic outcomes (a higher number of harvested lymph nodes, a higher R0 resection rate) compared with CMIE. The usefulness of robotic surgery was also demonstrated in Ivor Lewis esophagectomy.
3.3. Robot‐assisted McKeown esophagectomy
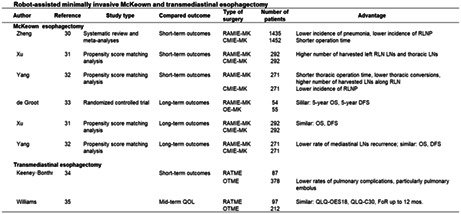
Zheng et al. reported the results of a systematic review and meta‐analyses on short‐term and long‐term outcomes, while Xu et al. and Yang et al. reported the results of propensity score matching analyses comparing robot‐assisted minimally invasive McKeown esophagectomy (RAMIE‐MK) and completely minimally invasive (thoracoscopic + laparoscopic) McKeown esophagectomy (CMIE‐MK). 30 , 31 , 32 De Groot also reported the results of a randomized controlled trial on long‐term outcomes. 33 RAMIE‐MK had the advantages of being associated with the intraoperative outcomes (a shorter thoracic operation time, a lower rate of thoracic conversions), postoperative complications (a lower rate of pneumonia, a lower rate of recurrent laryngeal nerve palsy), and oncologic outcomes (a higher number of harvested lymph nodes along the left recurrent laryngeal nerve and thoracic lymph nodes) than CMIE‐MK (Table 4). 30 , 31 , 32 , 33
TABLE 4.
Robot‐assisted minimally invasive McKeown and transmediastinal esophagectomy
| Author | Reference | Study type | Compared outcome | Type of surgery | Number of patients | Advantage |
|---|---|---|---|---|---|---|
| McKeown esophagectomy | ||||||
| Zheng | 30 | Systematic review and meta‐analyses | Short‐term outcomes | RAMIE‐MK | 1435 | Lower incidence of pneumonia, lower incidence of RLNP |
| CMIE‐MK | 1452 | Shorter operation time | ||||
| Xu | 31 | Propensity score matching analysis | Short‐term outcomes | RAMIE‐MK | 292 | Higher number of harvested left RLN LNs and thoracic LNs |
| CMIE‐MK | 292 | |||||
| Yang | 32 | Propensity score matching analysis | Short‐term outcomes | RAMIE‐MK | 271 | Shorter thoracic operation time, lower thoracic conversions, higher number of harvested LNs along RLN |
| CMIE‐MK | 271 | lower incidence of RLNP | ||||
| de Groot | 33 | Randomized controlled trial | Long‐term outcomes | RAMIE‐MK | 54 | Similar: 5‐year OS, 5‐year DFS |
| OE‐MK | 55 | |||||
| Xu | 31 | Propensity score matching analysis | Long‐term outcomes | RAMIE‐MK | 292 | Similar: OS, DFS |
| CMIE‐MK | 292 | |||||
| Yang | 32 | Propensity score matching analysis | Long‐term outcomes | RAMIE‐MK | 271 | Lower rate of mediastinal LNs recurrence; similar: OS, DFS |
| CMIE‐MK | 271 | |||||
| Transmediastinal esophagectomy | ||||||
| Keeney‐Bonthrone | 34 | Short‐term outcomes | RATME | 87 | ||
| OTME | 378 | Lower rates of pulmonary complications, particularly pulmonary embolus | ||||
| Williams | 35 | Mid‐term QOL | RATME | 97 | Similar: QLQ‐OES18, QLQ‐C30, FoR up to 12 mos. | |
| OTME | 212 | |||||
Abbreviations: CMIE‐MK, completely minimally invasive McKeown esophagectomy; DFS, disease‐free survival; FoR, fear of recurrence; LN, lymph node; OE‐MK, open McKeown esophagectomy; OS, overall survival; OTME, open transmediastinal esophagectomy; RAMIE‐MK, robot‐assisted minimally invasive McKeown esophagectomy; RATME, robot‐assisted minimally invasive transmediastinal esophagectomy; RLN, recurrent laryngeal nerve; RLNP, recurrent laryngeal nerve palsy; QLQ, quality of life questionnaire; QOL, quality of life.
A longer operation time and higher incidence of recurrent laryngeal nerve palsy have also been reported, and the surgical risk of postoperative recurrent laryngeal nerve palsy is a controversial issue. Precise surgical manipulation with the robot improved short‐term outcomes of McKeown esophagectomy, and it is worth noting the improved accuracy of lymph node dissection around the recurrent laryngeal nerve, which is particularly important in McKeown esophagectomy. Although it is reported to both increase and decrease the incidence of recurrent nerve palsy, it is expected to eventually decrease once a gentler surgical technique is standardized. RAMIE‐MK was associated with similar long‐term outcomes, that is, OS and DFS, as OE‐MK and CMIE‐MK, except for a lower rate of mediastinal lymph node recurrence after RAMIE‐MK. Therefore, RAMIE‐MK could be established as a standard procedure for esophageal cancer, especially after several of its disadvantages have been overcome.
3.4. Robot‐assisted transmediastinal esophagectomy
Keeney‐Bonthrone et al. and Williams et al., respectively, compared the short‐term and mid‐term outcomes between robot‐assisted minimally invasive transmediastinal esophagectomy (RATME) and open transmediastinal esophagectomy (OTME). Although RATME has some disadvantages, such as a higher rate of pulmonary complications, especially of pulmonary embolism, as compared with OTME, this disadvantage could be overcome, because most of all the problems encountered in the introduction phase of these new surgical procedures have been successfully resolved, in general (Table 4). 34 , 35 The absence of any significant differences in the scores on the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire in Esophageal Cancer (QLQ‐OES18), the EORTC Quality of Life Questionnaire (QLQ‐C30), and fear of recurrence (FoR) up to 12 months after surgery between RATME and OTME encourage us to expect beneficial effects of using robotic assistance for TME in the near future. A new robotic system, the high‐performance, single‐port robotic surgical system known as da Vinci SP Surgical System, seems to be suitable for RATME, because Chiu et al. 36 reported that TME was technically feasible and could be completed with da Vinci SP Surgical System without additional ports or assistance in the preclinical study. Better surgical outcomes of RATME using the single‐port robotic surgical system are highly anticipated.
4. CONVERSION SURGERY AND SALVAGE SURGERY
Recently, multidisciplinary treatment strategies for esophageal cancer have greatly advanced. Induction treatment, including chemotherapy or chemoradiotherapy, can make locally advanced unresectable esophageal cancer resectable. In regard to the terminology, conversion surgery is defined as surgery for cancer that was judged as being unresectable at the time of initial diagnosis, but has become resectable after treatment. 37 Salvage surgery is defined as surgery for residual or recurrent cancer after definitive (chemo‐) radiotherapy using a radiation dose of 50 Gy or more.
In this section, the terms used by the author have been categorized and described in order of priority because conversion surgery includes salvage surgery. First, we describe reports of conversion surgery for patients with locally advanced unresectable esophageal cancer. A multicenter phase II trial was conducted by Yokota et al. 38 to analyze chemoselection of induction chemotherapy (ICT) with docetaxel, cisplatin, and 5‐fluorouracil (DCF) prior to conversion surgery for locally advanced unresectable esophageal cancer. The treatment started with DCF‐ICT, followed by conversion surgery if the cancer became resectable, or by concurrent chemoradiation if it remained unresectable. The 3‐year OS and 3‐year progression‐free survival (PFS) rates were 46.6% and 39.6%, respectively. The OS and the PFS durations in the patients in whom R0 resection was achieved were longer than in those in whom it was not. Abe et al. 39 investigated the short‐term and long‐term outcomes of conversion surgery after ICT with DCF in patients with locally advanced clinically unresectable esophageal cancer. The response rate to DCF‐ICT was 67%. The R0 resection rate was 81%, with no serious postoperative complications. The pathological CR rate was 17%, and the 3‐ and 5‐year survival rates after conversion surgery were 61% and 54%, respectively. Therefore, DCF‐ICT followed by conversion surgery is feasible and promising in terms of the survival outcomes.
Which should be selected as the initial induction treatment, chemoradiotherapy or chemotherapy? Sugimura et al. 40 conducted a multicenter randomized phase II trial to compare the short‐term and long‐term outcomes of chemoradiotherapy and chemotherapy (DCF) as initial induction therapy prior to conversion surgery in patients with locally advanced unresectable esophageal cancer. The conversion surgery rates were 83% and 84% in the initial induction chemoradiotherapy (ICRT) group and initial ICT group, respectively. The R0 resection rates were also similar in the two groups (78% and 76%). Adverse events (AEs), including hematological toxicity, were more frequent in the ICT group. The pathological complete response (pCR) rate of the primary tumor and histological nodal status were better in the ICRT group than in the ICT group. Therefore, ICRT seems superior to ICT in terms of the pathological effects and adverse events as induction therapy prior to conversion surgery.
A phase II trial to assess the safety and efficacy of ICT (paclitaxel, cisplatin, and 5‐fluorouracil) followed by conversion surgery (CS) in patients with borderline‐resectable esophageal cancer was conducted by Wang et al. 41 The conversion surgery rate was 57%, R0 resection rate was 53.2%, and pathologic CR rate was 8.5%. The OS was more favorable in the conversion surgery group than in chemoradiotherapy group or chemotherapy alone group. No serious postoperative complications were observed. Therefore, ICT followed by conversion surgery seems promising not only for locally advanced unresectable esophageal cancer, but also for locally advanced borderline‐resectable esophageal cancer.
Appropriate candidates for salvage esophagectomy after definitive chemoradiotherapy (dCRT) for locally advanced unresectable esophageal cancer were examined by two study groups. Booka et al. 42 reported that the complete response (CR) rate after dCRT was 22.4% and the rate of salvage esophagectomy was 5.7%. Salvage esophagectomy recipients had a worse OS rate than CR patients, but a better OS rate as compared with the chemotherapy or best supportive care group. Incomplete resection was the only significant variable associated with a poor OS. It was concluded that patients in whom R0 resection is achieved might be good candidates for salvage esophagectomy, regardless of the response status to dCRT. Okamura et al. 43 reported an R0 resection rate of 54.3%, a severe postoperative complication rate of 22.9%, and a surgery‐related mortality rate of 8.6%. The OS rates were 45.7% and 5.7% at 1 and 5 years, respectively. Residual or relapsed tumor limited to T2 or less was identified as an independent prognostic factor for better survival. Postoperative pneumonia and incomplete resection were identified as negative prognostic factors. Therefore, appropriate candidates, in terms of long‐term survival, for salvage esophagectomy seem to be patients with ≤T2 residual tumors, R0 resection, and no postoperative pneumonia.
Robot‐assisted minimally invasive esophagectomy for locally advanced unresectable esophageal cancer after dCRT was successfully performed by Defize et al. 44 The R0 resection rate was 92%, pathologic CR rate was 54%, and the postoperative complication rate was 83%. The DFS at 24 months was 68% in the patients in whom R0 was achieved. These results imply that RAMIE could be adopted for both conversion surgery and salvage surgery.
For patients with esophageal cancer spanning across all stages, Mitchell et al. 45 investigated the outcomes of salvage esophagectomy after chemoradiotherapy with a combination of a fluoropyrimidine and a platinum or taxane with concurrent radiotherapy at 50.4 Gy for squamous cell carcinoma (cTanyN1M0, cT3–4N0M0, high‐risk cT2N0M0). Major postoperative complications occurred in 71% of patients. The 30‐ and 90‐day mortality rates were 8.6% and 17.1%, respectively. Salvage esophagectomy was associated with a high rate of postoperative morbidity and considerably high rates of mortality. The safety and efficacy of conversion surgery and salvage surgery vary depending on the surgical indication, surgical procedure employed, perioperative management method adopted, etc. Patient selection criteria at each hospital are very important.
5. NEOADJUVANT AND ADJUVANT THERAPY
5.1. Neoadjuvant chemoradiotherapy and chemotherapy
There are two reports of neoadjuvant chemoradiotherapy (NACRT) and one report of neoadjuvant chemotherapy (NAC) for esophageal cancer. The significance of pathologic lymph node metastasis after NACRT was examined. Leng et al. 46 conducted an exploratory second study using data from a randomized controlled trial (NEOCRTEC5010) that had already concluded that NACRT plus surgery yielded improved survival over surgery alone in patients with locally advanced esophageal squamous cell carcinoma (ESCC). Patients of the NACRT group received vinorelbine and cisplatin with concurrent radiation (40.0 Gy). This study concluded that persistent pathologic lymph node metastasis after NACRT is a strong poor prognostic factor in patients with ESCC and pCR does not guarantee cure.
The timing of surgery after completion of NACRT was also investigated. Nilsson et al. 47 conducted a multicenter randomized controlled trial (NeoRes II Trial) to compare the short‐term postoperative outcomes associated with a standard time to surgery (TTS) of 4 to 6 weeks versus a prolonged TTS of 10 to 12 weeks. They concluded that TTS after completion of NACRT is not of major importance from the point of view of the short‐term postoperative outcomes; that is, there is no benefit to prolonging the TTS.
The number of chemotherapy courses was investigated. Shiraishi et al. 48 conducted a multicenter randomized controlled phase II trial to compare the short‐term outcomes between two courses and three courses of NAC with DCF in patients with locally advanced ESCC. The study demonstrated that both two‐course and three‐course NAC‐DCF are equally feasible for patients with locally advanced ESCC, and that additional courses of DCF yielded a better response to NAC, without increasing the incidence of adverse events or the postoperative morbidity rate. Although a preferable short‐term outcome was reported for a three‐course NAC‐DCF group, it was recently reported by Makino et al. 49 that the 2‐year progression‐free survival (PFS), which was the primary endpoint of the subsequent study, was comparable between the two‐course and three‐course groups. Therefore, it was concluded that two courses of NAC‐DCF had potential as an optional NAC treatment for locally advanced ESCC. The JCOG 1109 trial examining preoperative treatment for stage II and III ESCC, the conclusions of which were adopted by the Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus 2022 edited by the Japan Esophageal Society, 50 used a three‐course DCF regimen. Based on these results, two courses of DCF may be acceptable in actual practice.
5.2. Neoadjuvant chemoimmunotherapy and chemoimmunoradiotherapy
Therapy with immune checkpoint inhibitors (ICIs), including programmed death 1 (PD‐1) inhibitor, has greatly changed the treatment of many malignant diseases, including gastrointestinal cancers. 51 Although neoadjuvant treatments have been used for many years, more intensive and effective treatments are needed to improve the outcomes in patients with locally advanced resectable esophageal cancer. Several studies have evaluated the treatment response to and safety of neoadjuvant therapy using a PD‐1 inhibitor with chemotherapy or chemoradiotherapy. There are six reports concerning neoadjuvant chemoimmunotherapy and one report concerning chemoimmunoradiotherapy for patients with esophageal cancer (Table 5). 52 , 53 , 54 , 55 , 56 , 57 Nivolumab, pembrolizumab, sintilimab, camrelizumab, and toripalimab are used as PD‐1 inhibitors. In regard to the efficacy of these agents, the objective response rate (ORR) ranged from 58% to 86%, the pCR rate from 21% to 36%, the major pathological response (MPR) rate from 42% to 53%, and the R0 rate from 94% to 100%. The rate of adverse events equal to or greater than Grade III was below 7%, except in the SIN‐ICE study which reported a rate of 30%, and the mortality rate was 0%, except in the NCT03985670 study conducted by Xing et al., 56 which reported a mortality rate of 4%. As comparing to the results of a phase II study of NAC using DCF which reported a pCR rate of 17%, 58 neoadjuvant chemoimmunotherapy seems to be more effective than the current NAC regimen. The safety of neoadjuvant chemoimmunotherapy is also acceptable. Although there is just one report on chemoimmunoradiotherapy for esophageal cancer, both the high pCR rate and zero mortality rate are worthy of note, but the high rate of adverse events equal to or greater than Grade III of 65% is a concern that needs to be addressed. 59
TABLE 5.
Neoadjuvant chemoimmunotherapy and chemoimmunoradiotherapy
| Author | Reference | Trial name /trial number | Phase | Number of patients | Type of histology | Immune checkpoint inhibitor | Chemotherapeutic agent (+radiation dose) | ORR | pCR | MPR | R0 | AE grade III≦ | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemoimmunotherapy | |||||||||||||
| Zhang | 52 | ESONICT‐1 | II | 30 | ESCC | Sintilimab | Nab‐paclitaxel and cisplatin | 67% | 22% | 52% | 100% | 3% | 30 Day, 0% |
| Duan | 53 | SIN‐ICE | II | 23 | ESCC | Sintilimab | Docetaxel, nab‐paclitaxel and nedaplatin | 35% | 53% | 94% | 30% | 90 Day, 0% | |
| Yang, P | 54 | ChiCTR2100051903 | II | 16 | ESCC | Camrelizumab | Paclitaxel and carboplatin | 81% | 31% | 94% | 90 Day, 0% | ||
| Yang, G | 55 | retrospective | 12 | ESCC | Camrelizumab | Nab‐paclitaxel and S1 | 58% | 33% | 42% | 100% | 0% | 0% | |
| 56 | NCT03985670 | II | 30 | ESCC | Toripalimab | Paclitaxel and cisplatin | 67% | 21% (max.36%) | 100% | 3% | 30 Day, 4% | ||
| Shen | 57 | II | 28 | ESCC | Nivolumab, pembrolizumab and camrelizumab | Nab‐paclitaxel and carboplatin | 86% | 33% | 96% | 7% | Hospital, 0% | ||
| Chemoimmunoradiotherapy | |||||||||||||
| Li | 59 | PALACE‐1 | I | 20 | ESCC | Pembrolizumab | Paclitaxel and carboplatin (+41.4 Gy) | 56% | 89% | 94% | 65% | 90 Day, 0% | |
Abbreviations: AE, adverse event; ESCC, esophageal squamous cell carcinoma; MPR, major pathological response; ORR, objective response rate; pCR, pathological complete response; R0, no residual tumor.
5.3. Adjuvant immunotherapy
There is one very important report of adjuvant therapy with an ICI. Kelly et al. 60 conducted a global multicenter randomized controlled phase III trial (CheckMate 577) to evaluate the usefulness of ICIs as adjuvant therapy in patients with esophageal or gastroesophageal junction cancer. Patients with resected (R0) cancer who had received NACRT and had residual pathological disease were assigned to a nivolumab treatment group or a placebo group. The results of this trial revealed a longer DFS duration in the nivolumab group as compared with the placebo group. This represents useful information, and a similar study is needed in patients who have received NAC.
6. CONCLUSION
Four treatment modalities for esophageal cancer from notable reports published in 2020 and 2021 were reviewed. The short‐term outcomes were mostly better in patients treated by MIE, including Ivor Lewis esophagectomy and McKeown esophagectomy, than in those treated by OE, while no significant differences in the long‐term outcomes were observed between MIE and OE. RAMIE showed both advantages and disadvantages from the point of view of the short‐term outcomes, as compared with CMIE. There were, however, no significant differences in the long‐term outcomes between RAMIE and CMIE. Further research is needed to evaluate the short‐term outcomes and long‐term outcomes of TME with or without robotic assistance. Both ICT and ICRT are promising to secure a higher rate of conversion surgery. Neoadjuvant chemoimmunotherapy and chemoimmunoradiotherapy showed promising results and are expected as new powerful therapies for patients with esophageal cancer. It is hoped that the continued efforts of professionals from various medical fields will improve the results of treatment, including surgery, of esophageal cancer in the near future.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
FUNDING INFORMATION
This study was not funded by any organization.
ETHICAL APPROVAL
Approval of the study protocol was not applicable.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Motohide Shimazu (Department of Surgery, Tamakyuryo Hospital) for his advice on the manuscript. The authors used an English Language Service (International Medical Information Center, Tokyo, Japan) for editing the language in the manuscript.
Ozawa S, Uchi Y, Ando T, Hayashi K, Aoki T. Essential updates 2020/2021: Recent topics in surgery and perioperative therapy for esophageal cancer. Ann Gastroenterol Surg. 2023;7:346–357. 10.1002/ags3.12657
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Torek F. The first successful case of resection of the thoracic portion of the oesophagus for carcinoma. Surg Gynecol Obstet. 1913;16:614–7. [Google Scholar]
- 3. Lerut T, Wiesel O. History of esophagectomy for cancer of the esophagus and the gastroesophageal junction. Ann Transl Med. 2021;9(10):897. 10.21037/atm-21-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb. 1992;37(1):7–11. [PubMed] [Google Scholar]
- 5. van Boxel GI, Kingma BF, Voskens FJ, Ruurda JP, van Hillegersberg R. Robotic‐assisted minimally invasive esophagectomy: past, present and future. J Thorac Dis. 2020;12(2):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fujiwara H, Shiozaki A, Konishi H, Otsuji E. Transmediastinal approach for esophageal cancer: a new trend toward radical surgery. Asian J Endosc Surg. 2019;12(1):30–6. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe M, Otake R, Kozuki R, Toihata T, Takahashi K, Okamura A, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. 2020;50(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kakeji Y, Oshikiri T, Takiguchi G, Kanaji S, Matsuda T, Nakamura T, et al. Multimodality approaches to control esophageal cancer: development of chemoradiotherapy, chemotherapy, and immunotherapy. Esophagus. 2021;18(1):25–32. [DOI] [PubMed] [Google Scholar]
- 9. McKeown KC. Total three‐stage oesophagectomy for cancer of the oesophagus. Br J Surg. 1976;63(4):259–62. [DOI] [PubMed] [Google Scholar]
- 10. Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg. 1946;34:18–31. [DOI] [PubMed] [Google Scholar]
- 11. van Workum F, Klarenbeek BR, Baranov N, Rovers MM, Rosman C. Totally minimally invasive esophagectomy versus hybrid minimally invasive esophagectomy: systematic review and meta‐analysis. Dis Esophagus. 2020;33(8):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Worrell SG, Bachman KC, Sarode AL, Perry Y, Linden PA, Towe CW. Minimally invasive esophagectomy is associated with superior survival, lymphadenectomy and surgical margins: propensity matched analysis of the National Cancer Database. Dis Esophagus. 2020;33(10):1–6. [DOI] [PubMed] [Google Scholar]
- 13. Holscher AH, DeMeester TR, Schmidt H, Berlth F, Bollschweiler E. Propensity score‐matched comparison between open and minimal invasive hybrid esophagectomy for esophageal adenocarcinoma. Langenbeck's Arch Surg. 2020;405(4):521–32. [DOI] [PubMed] [Google Scholar]
- 14. Mariette C, Markar S, Dabakuyo‐Yonli TS, Meunier B, Pezet D, Collet D, et al. Health‐related quality of life following hybrid minimally invasive versus open Esophagectomy for patients with esophageal cancer, analysis of a multicenter, open‐label, randomized phase III controlled trial: the MIRO trial. Ann Surg. 2020;271(6):1023–9. [DOI] [PubMed] [Google Scholar]
- 15. Patel K, Askari A, Moorthy K. Long‐term oncological outcomes following completely minimally invasive esophagectomy versus open esophagectomy. Dis Esophagus. 2020;33(6):1–10. [DOI] [PubMed] [Google Scholar]
- 16. Nuytens F, Dabakuyo‐Yonli TS, Meunier B, Gagniere J, Collet D, D'Journo XB, et al. Five‐year survival outcomes of hybrid minimally invasive Esophagectomy in esophageal cancer: results of the MIRO randomized clinical trial. JAMA Surg. 2021;156(4):323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Workum F, Stenstra M, Berkelmans GHK, Slaman AE, van Berge Henegouwen MI, Gisbertz SS, et al. Learning curve and associated morbidity of minimally invasive Esophagectomy: a retrospective multicenter study. Ann Surg. 2019;269(1):88–94. [DOI] [PubMed] [Google Scholar]
- 18. van Workum F, Slaman AE, van Berge Henegouwen MI, Gisbertz SS, Kouwenhoven EA, van Det MJ, et al. Propensity score‐matched analysis comparing minimally invasive Ivor Lewis versus minimally invasive Mckeown Esophagectomy. Ann Surg. 2020;271(1):128–33. [DOI] [PubMed] [Google Scholar]
- 19. Sakamoto T, Fujiogi M, Matsui H, Fushimi K, Yasunaga H. Comparing perioperative mortality and morbidity of minimally invasive Esophagectomy versus open Esophagectomy for esophageal cancer: a Nationwide retrospective analysis. Ann Surg. 2021;274(2):324–30. [DOI] [PubMed] [Google Scholar]
- 20. Sugita Y, Nakamura T, Sawada R, Takiguchi G, Urakawa N, Hasegawa H, et al. Safety and feasibility of minimally invasive esophagectomy for elderly esophageal cancer patients. Dis Esophagus. 2021;34(3):1–7. [DOI] [PubMed] [Google Scholar]
- 21. Takeuchi H, Miyata H, Ozawa S, Udagawa H, Osugi H, Matsubara H, et al. Comparison of short‐term outcomes between open and minimally invasive Esophagectomy for esophageal cancer using a Nationwide database in Japan. Ann Surg Oncol. 2017;24(7):1821–7. [DOI] [PubMed] [Google Scholar]
- 22. Uchihara T, Yoshida N, Baba Y, Nakashima Y, Kimura Y, Saeki H, et al. Esophageal position affects short‐term outcomes after minimally invasive Esophagectomy: a retrospective multicenter study. World J Surg. 2020;44(3):831–7. [DOI] [PubMed] [Google Scholar]
- 23. Higuchi T, Ozawa S, Koyanagi K, Ninomiya Y, Yatabe K, Yamamoto M, et al. Usefulness of prone‐position computed tomography as preoperative simulation prior to thoracoscopic esophagectomy for thoracic esophageal cancer. Esophagus. 2021;18(4):764–72. [DOI] [PubMed] [Google Scholar]
- 24. Daiko H, Oguma J, Fujiwara H, Ishiyama K, Kurita D, Sato T, et al. Novel universally applicable technique for performing bilateral transcervical mediastinoscopic‐assisted transhiatal laparoscopic esophagectomy: a truly minimally invasive procedure. Surg Endosc. 2021;35(9):5186–92. [DOI] [PubMed] [Google Scholar]
- 25. Wu CL, Dong B, Wu B, Li SH, Qi Y. The application of rigid and flexible mediastinoscopy in esophagectomy: our experience and a new technology. World J Surg Oncol. 2021;19(1):234. 10.1186/s12957-021-02352-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manigrasso M, Vertaldi S, Marello A, Antoniou SA, Francis NK, De Palma GD, et al. Robotic Esophagectomy. A systematic review with meta‐analysis of clinical outcomes. J Pers Med. 2021;11(7):234. 10.3390/jpm11070640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li XK, Xu Y, Zhou H, Cong ZZ, Wu WJ, Qiang Y, et al. Does robot‐assisted minimally invasive oesophagectomy have superiority over thoraco‐laparoscopic minimally invasive oesophagectomy in lymph node dissection? Dis Esophagus. 2021;34(2):1–12. [DOI] [PubMed] [Google Scholar]
- 28. Angeramo CA, Bras Harriott C, Casas MA, Schlottmann F. Minimally invasive Ivor Lewis esophagectomy: robot‐assisted versus laparoscopic‐thoracoscopic technique. Systematic review and meta‐analysis. Surgery. 2021;170(6):1692–701. [DOI] [PubMed] [Google Scholar]
- 29. Tagkalos E, Goense L, Hoppe‐Lotichius M, Ruurda JP, Babic B, Hadzijusufovic E, et al. Robot‐assisted minimally invasive esophagectomy (RAMIE) compared to conventional minimally invasive esophagectomy (MIE) for esophageal cancer: a propensity‐matched analysis. Dis Esophagus. 2020;33(4):1–6. [DOI] [PubMed] [Google Scholar]
- 30. Zheng C, Li XK, Zhang C, Zhou H, Ji SG, Zhong JH, et al. Comparison of short‐term clinical outcomes between robot‐assisted minimally invasive esophagectomy and video‐assisted minimally invasive esophagectomy: a systematic review and meta‐analysis. J Thorac Dis. 2021;13(2):708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu Y, Li XK, Cong ZZ, Zhou H, Wu WJ, Qiang Y, et al. Long‐term outcomes of robotic‐assisted versus thoraco‐laparoscopic McKeown esophagectomy for esophageal cancer: a propensity score‐matched study. Dis Esophagus. 2021;34(9):1–9. [DOI] [PubMed] [Google Scholar]
- 32. Yang Y, Zhang X, Li B, Hua R, Yang Y, He Y, et al. Short‐ and mid‐term outcomes of robotic versus thoraco‐laparoscopic McKeown esophagectomy for squamous cell esophageal cancer: a propensity score‐matched study. Dis Esophagus. 2020;33(6):1–9. [DOI] [PubMed] [Google Scholar]
- 33. de Groot EM, van der Horst S, Kingma BF, Goense L, van der Sluis PC, Ruurda JP, et al. Robot‐assisted minimally invasive thoracolaparoscopic esophagectomy versus open esophagectomy: long‐term follow‐up of a randomized clinical trial. Dis Esophagus. 2020;33(Supplement_2):1–6. [DOI] [PubMed] [Google Scholar]
- 34. Keeney‐Bonthrone TP, Abbott KL, Haley C, Karmakar M, Hawes AM, Chang AC, et al. Transhiatal robot‐assisted minimally invasive esophagectomy: unclear benefits compared to traditional transhiatal esophagectomy. J Robot Surg. 2022;16(4):883–91. [DOI] [PubMed] [Google Scholar]
- 35. Williams AM, Kathawate RG, Zhao L, Grenda TR, Bergquist CS, Brescia AA, et al. Similar quality of life after conventional and robotic Transhiatal Esophagectomy. Ann Thorac Surg. 2022;113(2):399–405. [DOI] [PubMed] [Google Scholar]
- 36. Chiu PWY, Ng SSM, Au SKW. Transcervical minimally invasive esophagectomy using da Vinci(R) SP surgical system: a feasibility study in cadaveric model. Surg Endosc. 2019;33(5):1683–6. [DOI] [PubMed] [Google Scholar]
- 37. The Japan Esophageal Society , editor. Japanese Classification of Esophageal Cancer. 12th ed. Tokyo: Kanehara Shuppan; 2022. (In Japanese). [Google Scholar]
- 38. Yokota T, Kato K, Hamamoto Y, Tsubosa Y, Ogawa H, Ito Y, et al. A 3‐year overall survival update from a phase 2 study of chemoselection with DCF and subsequent conversion surgery for locally advanced unresectable esophageal cancer. Ann Surg Oncol. 2020;27(2):460–7. [DOI] [PubMed] [Google Scholar]
- 39. Abe T, Higaki E, Hosoi T, Nagao T, Bando H, Kadowaki S, et al. Long‐term outcome of patients with locally advanced clinically Unresectable esophageal cancer undergoing conversion surgery after induction chemotherapy with docetaxel plus cisplatin and 5‐fluorouracil. Ann Surg Oncol. 2021;28(2):712–21. [DOI] [PubMed] [Google Scholar]
- 40. Sugimura K, Miyata H, Tanaka K, Makino T, Takeno A, Shiraishi O, et al. Multicenter randomized phase 2 trial comparing chemoradiotherapy and docetaxel plus 5‐fluorouracil and cisplatin chemotherapy as initial induction therapy for subsequent conversion surgery in patients with clinical T4b esophageal cancer: short‐term results. Ann Surg. 2021;274(6):e465–e72. [DOI] [PubMed] [Google Scholar]
- 41. Wang Z, Hu M, Hu Y, Li Q, Wu J, Fong WP, et al. Paclitaxel plus cisplatin and 5‐fluorouracil induction chemotherapy for locally advanced borderline‐resectable esophageal squamous cell carcinoma: a phase II clinical trial. Esophagus. 2022;19(1):120–8. [DOI] [PubMed] [Google Scholar]
- 42. Booka E, Haneda R, Ishii K, Kawakami T, Tsushima T, Yasui H, et al. Appropriate candidates for salvage esophagectomy of initially unresectable locally advanced T4 esophageal squamous cell carcinoma. Ann Surg Oncol. 2020;27(9):3163–70. [DOI] [PubMed] [Google Scholar]
- 43. Okamura A, Hayami M, Kozuki R, Takahashi K, Toihata T, Imamura Y, et al. Salvage esophagectomy for initially unresectable locally advanced T4 esophageal squamous cell carcinoma. Esophagus. 2020;17(1):59–66. [DOI] [PubMed] [Google Scholar]
- 44. Defize IL, van der Horst S, Bulbul M, Haj Mohammad N, Mook S, Meijer GJ, et al. Salvage robot‐assisted minimally invasive Esophagectomy (RAMIE) for T4b esophageal cancer after definitive chemoradiotherapy. Ann Surg Oncol. 2021;28(5):2730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mitchell KG, Nelson DB, Corsini EM, Vaporciyan AA, Antonoff MB, Mehran RJ, et al. Morbidity following salvage esophagectomy for squamous cell carcinoma: the MD Anderson experience. Dis Esophagus. 2020;33(3):1–8. [DOI] [PubMed] [Google Scholar]
- 46. Leng X, He W, Yang H, Chen Y, Zhu C, Fang W, et al. Prognostic impact of postoperative lymph node metastases after Neoadjuvant chemoradiotherapy for locally advanced squamous cell carcinoma of esophagus: from the results of NEOCRTEC5010, a randomized multicenter study. Ann Surg. 2021;274(6):e1022–e9. [DOI] [PubMed] [Google Scholar]
- 47. Nilsson K, Klevebro F, Rouvelas I, Lindblad M, Szabo E, Halldestam I, et al. Surgical morbidity and mortality from the multicenter randomized controlled NeoRes II trial: standard versus prolonged time to surgery after Neoadjuvant chemoradiotherapy for esophageal cancer. Ann Surg. 2020;272(5):684–9. [DOI] [PubMed] [Google Scholar]
- 48. Shiraishi O, Makino T, Yamasaki M, Tanaka K, Yamashita K, Ishida T, et al. Two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for treating locally advanced esophageal cancer: short‐term outcomes of a multicenter randomized phase II trial. Esophagus. 2021;18(4):825–34. [DOI] [PubMed] [Google Scholar]
- 49. Makino T, Yamasaki M, Tanaka K, Yamashita K, Urakawa S, Ishida T, et al. Multicenter randomised trial of two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for locally advanced oesophageal squamous cell carcinoma. Br J Cancer. 2022;126(11):1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. The Japan Esophageal Society , editor. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus 2022. Tokyo: Kanehara Shuppan; 2022. (In Japanese). [Google Scholar]
- 51. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Z, Hong ZN, Xie S, Lin W, Lin Y, Zhu J, et al. Neoadjuvant sintilimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: a single‐arm, single‐center, phase 2 trial (ESONICT‐1). Ann Transl Med. 2021;9(21):1623. 10.21037/atm-21-5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Duan H, Wang T, Luo Z, Wang X, Liu H, Tong L, et al. A multicenter single‐arm trial of sintilimab in combination with chemotherapy for neoadjuvant treatment of resectable esophageal cancer (SIN‐ICE study). Ann Transl Med. 2021;9(22):17000. 10.21037/atm-21-6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang P, Zhou X, Yang X, Wang Y, Sun T, Feng S, et al. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study. World J Surg Oncol. 2021;19(1):333. 10.1186/s12957-021-02446-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang G, Su X, Yang H, Luo G, Gao C, Zheng Y, et al. Neoadjuvant programmed death‐1 blockade plus chemotherapy in locally advanced esophageal squamous cell carcinoma. Ann Transl Med. 2021;9(15):1254. 10.21037/atm-21-3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xing W, Zhao L, Zheng Y, Liu B, Liu X, Li T, et al. The sequence of chemotherapy and Toripalimab might influence the efficacy of Neoadjuvant chemoimmunotherapy in locally advanced esophageal squamous cell cancer‐a phase II study. Front Immunol. 2021;12:772450. 10.3389/fimmu.2021.772450 Ecollection 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shen D, Chen Q, Wu J, Li J, Tao K, Jiang Y. The safety and efficacy of neoadjuvant PD‐1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol. 2021;12(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hara H, Tahara M, Daiko H, Kato K, Igaki H, Kadowaki S, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104(11):1455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE‐1). Eur J Cancer. 2021;144:232–41. [DOI] [PubMed] [Google Scholar]
- 60. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant Nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–203. [DOI] [PubMed] [Google Scholar]


