Abstract
Increasing evidence indicates that angiogenesis plays a pivotal role in tumor progression. Formin‐like 2 (FMNL2) is well‐known for promoting metastasis; however, the molecular mechanisms by which FMNL2 promotes angiogenesis in colorectal cancer (CRC) remain unclear. Here, we found that FMNL2 promotes angiogenesis and metastasis of CRC in vitro and in vivo. The GDB/FH3 domain of FMNL2 directly interacts with epidermal growth factor‐like protein 6 (EGFL6). Formin‐like 2 promotes EGFL6 paracrine signaling by exosomes to regulate angiogenesis in CRC. Cytoskeleton associated protein 4 (CKAP4) is a downstream target of EGFL6 and is involved in CRC angiogenesis. Epidermal growth factor‐like protein 6 binds to the N‐terminus of CKAP4 to promote the migration of HUVECs by activating the ERK/MMP pathway. These findings suggest that FMNL2 promotes the migration of HUVECs and enhances angiogenesis and tumorigenesis in CRC by regulating the EGFL6/CKAP4/ERK axis. Therefore, the EGFL6/CKAP4/ERK axis could be a candidate therapeutic target for CRC treatment.
Keywords: angiogenesis, CKAP4, colorectal cancer, EGFL6, FMNL2
Formin‐like 2 (FMNL2), which promotes tumor metastasis, is well recognized; however, the molecular mechanisms by which FMNL2 promotes angiogenesis in colorectal cancer (CRC) remain largely unclear. Here, we confirmed that FMNL2 promotes angiogenesis and metastasis of CRC via EGFL6/CKAP4/ERK axis.
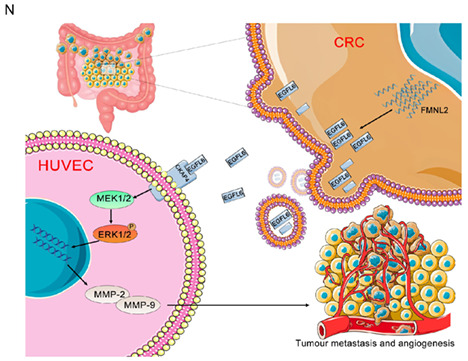
Abbreviations
- CAM
chorioallantoic membrane
- CM
conditioned medium
- Co‐IP
coimmunoprecipitation
- CRC
colorectal cancer
- CKAP4
cytoskeleton‐associated protein 4
- EGF
epidermal growth factor
- EGFL6
epidermal growth factor‐like protein 6
- EMT
epithelial–mesenchymal transition
- EV
extracellular vesicle
- FMNL2
formin‐like 2
- IHC
immunohistochemistry
- miR
microRNA
- TCGA
The Cancer Genome Atlas
- TME
tumor microenvironment
- VEGFA
vascular endothelial growth factor A
- VEGFR2
vascular endothelial growth factor receptor 2
- WB
western blot
1. BACKGROUND
Colorectal cancer is one of the most common malignant tumors worldwide, with the third‐highest incidence and second‐highest mortality rate. 1 , 2 Metastasis is the leading cause of death in most patients with CRC. Thus, determining the molecular mechanisms related to CRC metastasis and finding effective treatment approaches are essential.
Formins are well‐known members of the actin‐nucleating protein family. They are involved in actin remodeling and control different cellular processes. Formins are frequently deregulated in pathological conditions, such as tumorigenesis and metastasis. 3 Formin‐like 2, a member of the diaphanous‐related formins, is upregulated in CRC cell lines and metastatic lymph nodes. FMNL2 promotes CRC progression through the Rho signaling pathway and EMT, 4 but the molecular mechanism by which FMNL2 is involved in angiogenesis in CRC remains unclear. This study found that FMNL2 interacts with epidermal growth factor‐like protein 6 in a yeast two‐hybrid system. First discovered in 1999, EGFL6 is a member of the EGF repeats superfamily. It is located on human chromosome Xp22 and includes the EGF domain repeat and the MAM domain, also named MAEG. Epidermal growth factor‐like protein 6 regulates the cell cycle, proliferation, and differentiation. 5 Recent studies have revealed that the EGFL6/p‐ERK pathway promotes angiogenesis to facilitate bone fracture healing and expedite breast and ovarian cancer progression. 6 However, the relationship between EGFL6 and FMNL2 and the role of FMNL2 in angiogenesis have not yet been thoroughly explored.
Cytoskeleton‐associated protein 4, a surface receptor, plays a key role in alveolar epithelium, bladder epithelium, bladder cancer, and vascular smooth muscle. It regulates cell mitosis and intracellular protein metabolism through palmitoylation and promotes microtubule polymerization. It is transported to the cell membrane through PI3K. 7 , 8 , 9 Mass spectrometry analysis showed that CKAP4 is an EGFL6‐interacting protein. Therefore, we hypothesized that EGFL6 is important in promoting CRC angiogenesis through CKAP4.
Here, we reveal that FMNL2 is involved in CRC angiogenesis through the EGFL6/CKAP4/ERK axis, providing a new therapeutic target for CRC treatment.
2. MATERIALS AND METHODS
2.1. Colorectal cancer tissue samples, cell culture, and transfection
Data from CRC tissue samples are presented in the Document S1. Cell culture and transfection were carried out according to previously described methods. 10 , 11 Human CKAP4‐specific siRNAs were synthesized by GenePharma (Document S2, Table S1). The primers constructed for the overexpression and knockdown of the FMNL2/EGFL6/CKAP4 axis are listed in Table S1.
2.2. Quantitative real‐time RT‐PCR
Total RNA was extracted from CRC cell lines using TRIzol reagent (Invitrogen). Reverse transcription was undertaken using RevertAid Premium Reverse Transcriptase according to the manufacturer's protocol (MBI Fermentas). All primer sequences are listed in Tables S1 and S3.
2.3. Western blot and IHC analyses
Western blot and IHC analyses were carried out according to previously described methods. 12 The reagents used in relevant experiments can be found in Document S3.
2.4. Flow cytometry
Flow cytometry was carried out according to previously described methods. 13 The HUVECs were transfected with overexpression plasmids and/or siRNAs of CKAP4 after 6 h. They were treated with stable EGFL6 or EGFL6‐shRNA CRC supernatant for 36 h, and then fixed in 70% ice‐cold ethanol. The cells were then incubated with propidium iodide (4 mg/ml) in PBS.
2.5. Cell migration and angiogenesis assay
Transwell assay, 14 wound healing assay, 15 tube formation assay, 16 and chicken CAM assay 17 were carried out based on the previously described methods.
2.6. Coimmunoprecipitation
SW620 and LoVo cells were lysed in ice‐cold Co‐IP buffer (Roche) for 30 min. The cells were then centrifuged. The supernatant was collected and incubated with GammaBind Plus Sepharose (GE Healthcare) with gentle shaking overnight at 4°C. The mixture was washed with Co‐IP buffer and analyzed by WB.
2.7. Immunofluorescence
SW620 and LOVO cells were washed with PBS, fixed with methanol for 15 min at room temperature, and then washed in PBST. Cell nuclei were counterstained with DAPI (Beyotime) for 20 min. Confocal images of the stained cells were captured using Zeiss AIM software on a Zeiss LSM 700 confocal microscope system (Carl Zeiss Jena).
2.8. Animal models
The animal model was constructed according to the previously described methods (Document S4). It was approved by the Institutional Animal Ethical Committee of Xinxiang Medical University.
2.9. Mass spectrometry
293T cells with stable Flag‐FMNL2 expression were collected using a scraper and lysed in a weak lysis buffer. The cell lysate was incubated with FLAG magnetic affinity resin (Sigma‐Aldrich). We used SDS‐PAGE to separate proteins, and the gel was stained using Coomassie Brilliant Blue R250. Protein bands were excised from the gel and analyzed using liquid chromatography–mass spectrometry. Data analysis and protein identification were undertaken using the NCBI protein database.
2.10. Pull‐down assay
A GST pull‐down assay was carried out based on previously described methods. 18
2.11. Immunoassay
An ELISA assay was carried out based on previously described methods. 19
2.12. Bioinformatics analysis
We downloaded and organized the RNA sequencing data of TCGA‐COAD and TCGA‐READ from TCGA (https://portal.gdc.cancer.gov/) to extract the paired paracancerous and cancerous samples with the corresponding numbers. We used R software to analyze the paired expression of FMNL2 and EGFL6. Wilcoxon signed‐rank test was used for analysis.
2.13. Statistical analysis
All experiments were repeated at least three times, and the results are summarized as the mean ± SEM. Student's t‐test and one‐way ANOVA were used to analyze the data, and χ2‐tests were used to analyze categorical variables. All statistical tests were undertaken using IBM SPSS (version 25.0; SPSS), and p values <0.05 were regarded as indicating statistically significant differences (*p < 0.05, **p < 0.01, ***p < 0.001).
3. RESULTS
3.1. Formin‐like 2 and EGFL6 are associated with angiogenesis in CRC
Using the yeast two‐hybrid assay, previous studies found that FMNL2 interacts with EGFL6 (data not shown). Bioinformatics analysis indicated that the expression of FMNL2 and EGFL6 in TCGA‐COAD and TCGA‐READ was significantly higher than that in matched paracancerous tissues (Figure 1A). A total of 48 CRC samples were obtained to explore the expression of FMNL2 and EGFL6 in CRC. Similarly, IHC showed that the expression levels of FMNL2 and EGFL6 were significantly higher in CRC tissues (Figure 1B–D). Unexpectedly, the expression level of CD31, a marker of neovascularization, was also significantly increased in CRC tissues (Figure 1E). These results suggest that FMNL2 and EGFL6 could play crucial roles in the angiogenesis of CRC.
FIGURE 1.
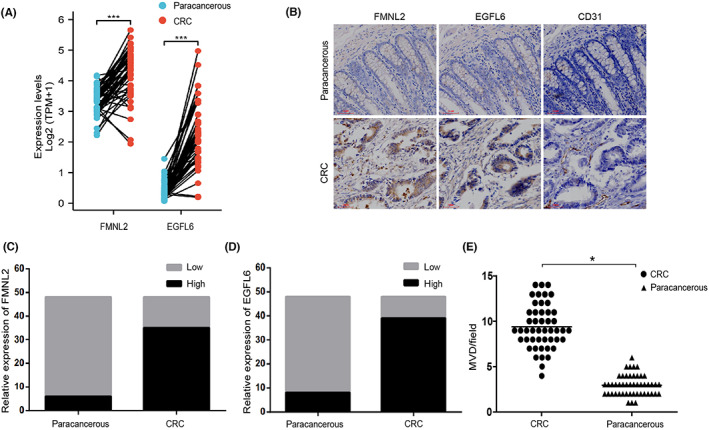
Formin‐like 2 (FMNL2) and epidermal growth factor‐like protein 6 (EGFL6) are associated with angiogenesis in colorectal cancer (CRC). (A) Paired sample expression of FMNL2 and EGFL6 from The Cancer Genome Atlas. (B) Immunohistochemical staining indicated that the expression of FMNL2, CD31, and EGFL6 was in CRC and paracancerous tissues. (C, D) Relative expression levels of FMNL2 and EGFL6 were detected in 48 samples of CRC and paired paracancerous tissues. (E) Quantification of microvessel density (MVD) by evaluation of CD31 expression between CRC tissues and paracancerous tissues. *p < 0.05, ***p < 0.001
3.2. Formin‐like 2 promotes angiogenesis of CRC in vitro
To investigate the potential role of FMNL2 in the angiogenesis of CRC, we first detected the expression level of FMNL2 in different CRC cell lines (Figure S1A,B) and constructed FMNL2‐overexpressed SW480 and DLD‐1 cells (Figure S1F,G), FMNL2‐silenced SW620 cells, and LoVo cells (Figure S1D,E). Next, the supernatants of CRC cells from different treatment groups were collected to treat HUVECs. Transwell assay showed that the number of migrated HUVECs was significantly higher in the FMNL2‐overexpressing CRC cells group compared with the control group (Figure 2A,B). Consistently, the number of tubes in the FMNL2‐overexpressing group was markedly higher than that in the control group (Figure 2E,F). Moreover, the CAM assay showed an increased density of capillaries in the FMNL2 overexpression group compared with the control group (Figure 2I,J). In contrast, FMNL2 knockdown significantly decreased the number of migrated cells and lowered tube formation and capillary density (Figure 2C,D,G,H,K,L). Overall, these findings suggest that FMNL2 enhances angiogenesis in CRC by promoting tube formation of HUVECs in vitro.
FIGURE 2.
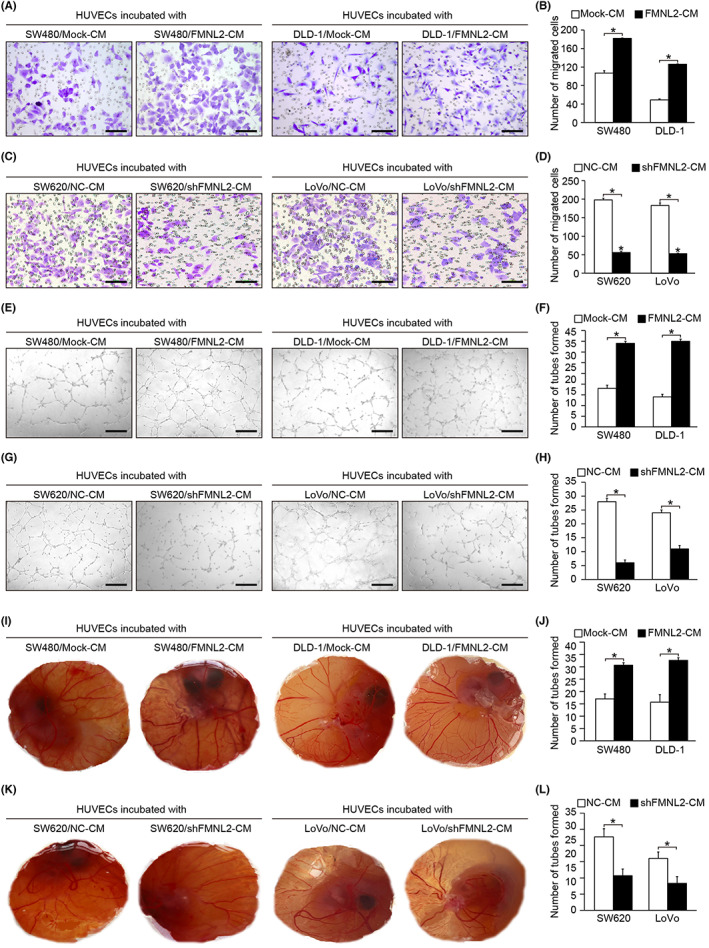
Formin‐like 2 (FMNL2) promotes angiogenesis of colorectal cancer in vitro. (A, B) Effect of conditioned medium (CM) from FMNL2‐overexpressed SW480 and DLD‐1 cells on the migration ability of HUVECs. (C, D) Effect of CM from FMNL2‐silenced SW620 and LoVo cells on the migration ability of HUVECs. (E, F) Effect of CM from FMNL2‐overexpressed SW480 and DLD‐1 cells on the tube formation ability of HUVECs. (G, H) Effect of CM from FMNL2‐silenced SW620 and LoVo cells on the tube formation ability of HUVECs. (I, J) Effect of CM from FMNL2‐overexpressed SW480 and DLD‐1 cells on the angiogenesis of chorioallantoic membrane (CAM) models. (K, L) Effect of CM from FMNL2‐silenced SW620 and LoVo cells on the angiogenesis of CAM models. Data are presented as the mean ± SEM; n = 3 independent experiments. *p < 0.05. NC, negative control
3.3. Formin‐like 2 promotes angiogenesis and metastasis of CRC in vivo
To further assess the effects of FMNL2 on angiogenesis in vivo, CRC cells with different levels of FMNL2 expression were subcutaneously implanted in nude mice. On the 30th day postinjection, the mean tumor weight was significantly greater in the FMNL2‐overexpressing group than in the control group (n = 6 per group, p < 0.01) (Figure 3A,B). Consistently, the mean tumor weight was significantly lower in the FMNL2‐knockdown group than in the control group (n = 5, p < 0.01) (Figure 3C,D). Moreover, IHC showed that microvessel density increased in the FMNL2‐overexpressing group, while it decreased in the FMNL2‐knockdown group (p < 0.01) (Figure 3E–G). Additionally, the orthotopic transplantation assay showed that the number of metastatic liver nodules also increased in the FMNL2‐overexpressing group (Figure 3H,I). These findings show that FMNL2 promoted tumor angiogenesis and metastasis in vivo.
FIGURE 3.
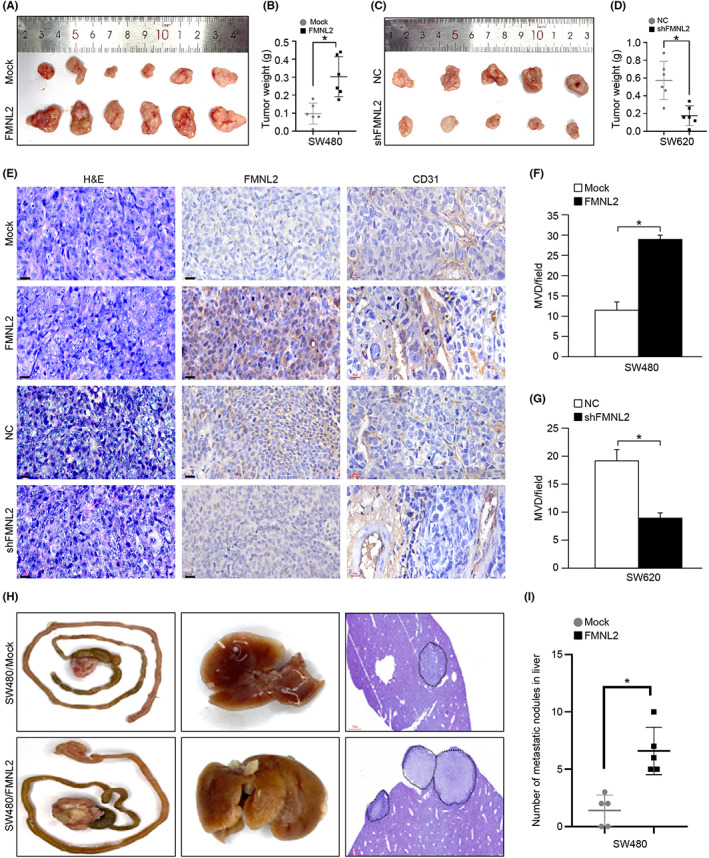
Formin‐like 2 (FMNL2) promotes angiogenesis and metastasis of colorectal cancer (CRC) in vivo. (A, B) Weight of subcutaneous tumor tissues derived from FMNL2‐overexpressed cells and control cells in BALB/c mice. n = 6 for each group. (C, D) Weight of subcutaneous tumor tissues derived from FMNL2‐silenced cells and control cells in BALB/c mice. n = 5 for each group. (E) Representative images of CD31 and FMNL2 expression in an indicated group of paraffin‐embedded subcutaneous tissues by immunohistochemical staining. (F, G) Quantifying microvessel density (MVD) by evaluating CD31 expression in an indicated group of subcutaneous tumor tissues. (H, I) Formation of primary CRC, liver metastases derived from FMNL2‐overexpressed SW480 cells, and control cells in BALB/c mice. Representative gross images of CRC orthotopic tumors and corresponding livers are shown. n = 5 for each group. *p < 0.05. NC, negative control
3.4. Formin‐like 2 regulates the paracrine function of EGFL6
We undertook a yeast two‐hybrid experiment and screened EGFL6 as a potential FMNL2 interacting protein to explore the mechanism by which FMNL2 promotes angiogenesis in CRC. The Co‐IP assay further verified the interaction between FMNL2 and EGFL6 in SW620 and LoVo cells (Figure 4A). Moreover, the immunofluorescence assay revealed the colocalization of FMNL2 and EGFL6 in SW620 and LoVo cells (Figure 4B). The GST pull‐down assay showed that FH3 + FH2 and GBD/FH3 domains of FMNL2 interact with EGFL6, suggesting that the GBD/FH3 domain is required for FMNL2‐EGFL6 interaction (Figure 4C,D).
FIGURE 4.
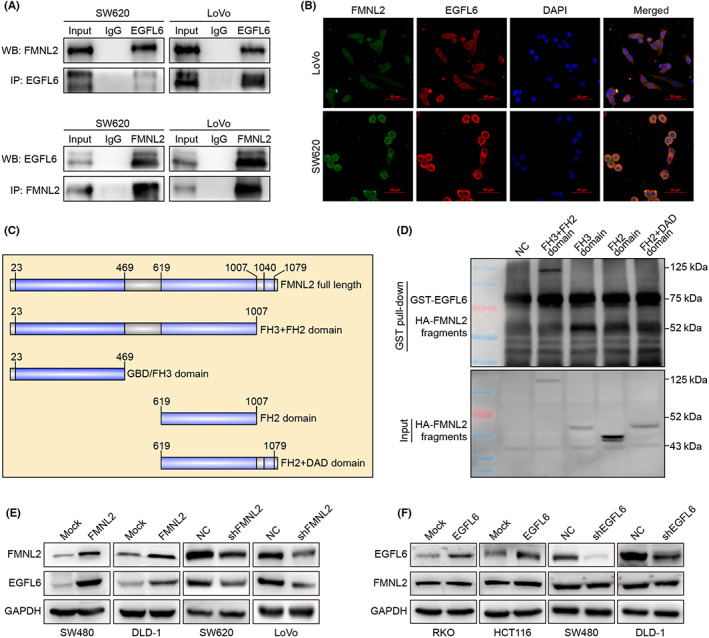
Formin‐like 2 (FMNL2) interacts with epidermal growth factor‐like protein 6 (EGFL6) in colorectal cancer (CRC) cells. (A) Coimmunoprecipitation (IP) assay of the interaction between FMNL2 and EGFL6. (B) Representative images of immunofluorescence colocalization assay of FMNL2 and EGFL6 in CRC cells. (C) Schematic diagram of different truncations of FMNL2. (D) GST pull‐down assay of the interaction between several truncations of FMNL2 and EGFL6. (E) Expression of EGFL6 in FMNL2‐overexpressed SW480 and DLD‐1 cells, FMNL2‐silenced SW620 and LoVo cells, and control cells by western blot analysis (WB). (F) Expression of FMNL2 in EGFL6‐overexpressed RKO and HCT116 cells, EGFL6‐silenced SW480 and DLD‐1 cells, and control cells by WB. Expression of FMNL2 in EGFL6‐overexpressed RKO and HCT116 cells, EGFL6‐silenced SW480 and DLD‐1 cells, and control cells by WB. NC, negative control
Western blot analysis showed that the expression of EGFL6 increased in the FMNL2‐overexpressing group and decreased in the FMNL2‐knockdown group (Figure 4E). However, the expression of FMNL2 had no significant differences between EGFL6‐overexpressing cells and EGFL6‐knockdown cells (Figure 4F). These results indicated that EGFL6 is positively regulated by FMNL2.
We further examined the correlation between FMNL2 and EGFL6 in 16 CRC tissues and found that FMNL2 and EGFL6 were significantly upregulated in CRC tissues compared with paracancerous tissues (Figure S2A). The expression level of FMNL2 was positively correlated with EGFL6 in CRC specimens (Figure S2B,C). The expression of CD31 was also positively correlated with FMNL2 and EGFL6 expression in CRC tissues (Figure S2D,E). Taken together, these findings strongly support that FMNL2 upregulates EGFL6 expression and its subsequent paracrine signaling to promote angiogenesis.
3.5. Formin‐like 2 promotes extracellular secretion of EGFL6 through the vesicular pathway
Using WB and ELISA, we detected the expression level of EGFL6 in the supernatant of CRC lines with stable FMNL2 expression. The results showed that FMNL2 overexpression significantly enhanced EGFL6 expression, while FMNL2 knockdown significantly reduced EGFL6 expression in CRC cells (Figure 5A–C). Additionally, transmission electron microscopy showed that exocytosis occurs in CRC cells as vesicles with a discontinuous plasma membrane. Secreted exosomes had a typical size of 30–100 nm with circular or oval morphology and bilayer membrane (Figure 5D). Western blot analysis confirmed that the transmembrane proteins, CD9 and TSG101, were expressed in both EVs and cells (Figure 5E). Immunofluorescence showed that the expression and secretion of CD9 occurred in both EVs and CRC cells. Furthermore, EGFL6 upregulation in HCT116 cells promoted EV formation (Figure 5F). These results suggested that FMNL2 regulates the secretion of EGFL6 in CRC cells through the vesicular pathway.
FIGURE 5.
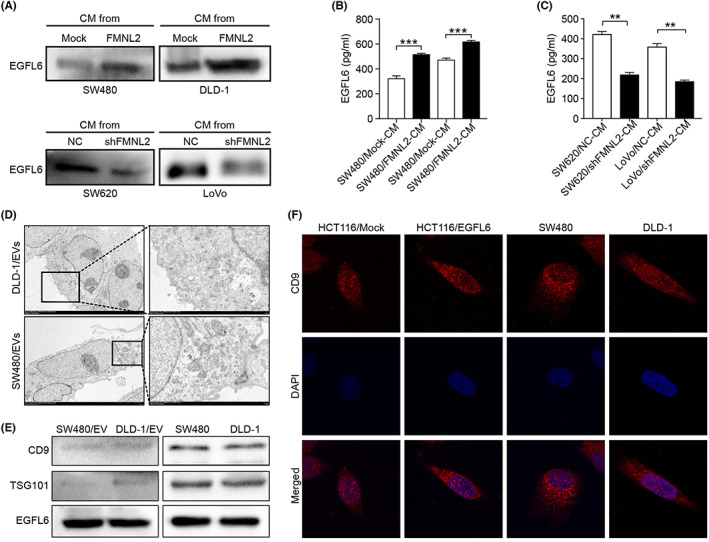
Formin‐like 2 (FMNL2) promotes the paracrine of epidermal growth factor‐like protein 6 (EGFL6) in colorectal cancer (CRC). (A) Expression of EGFL6 protein in the conditioned medium (CM) of FMNL2‐overexpressed SW480 and DLD‐1 cells and FMNL2‐silenced SW620 and LoVo cells by western blot analysis (WB). (B, C) Expression of EGFL6 protein in the CM of FMNL2‐overexpressed SW480 and DLD‐1 cells and FMNL2‐silenced SW620 and LoVo cells by ELISA assay. (D) Morphology of SW480 and DLD‐1 cells was examined by transmission electron microscopy. (E) WB detected the extracellular vesicle (EV) and intracellular expression of the transmembrane protein TSG101 and CD9 in DLD‐1 and SW480 cells, respectively. (F) Immunofluorescence analysis detected the expression and location of CD9 in DLD‐1, SW480, and HCT116 cells with different EGFL6 expressions. Representative images are shown
3.6. Formin‐like 2 promotes tumorigenesis and angiogenesis through EGFL6
To explore the effect of EGFL6 on tumor angiogenesis, the expression level of EGFL6 was determined in different CRC cells (Figure S1A–C). We constructed EGFL6‐overexpressing RKO and HCT116 cells (Figure S1N–O), and EGFL6‐knockdown DLD‐1 and SW480 cells (Figure S1L–M). EGFL6 overexpression in RKO and HCT116 cells significantly promoted migration and tube formation of HUVECs and enhanced the formation of second‐ and third‐order microvessels in HUVECs (Figure 6A,B,E,F,I,J). In contrast, DLD‐1 and SW480 cells with EGFL6 knockdown had significantly decreased migration, tube formation, and second‐ and third‐order microvessel formation in HUVECs (Figure 6C,D,G,H,K,L). Moreover, FMNL2 knockdown weakened the migration and angiogenic capacity of HUVECs (Figure 6A,B,E,F,I,J). Similarly, FMNL2 upregulation partly enhanced weak angiogenesis after EGFL6 knockdown (Figure 6C,D,G,H,K,L).
FIGURE 6.
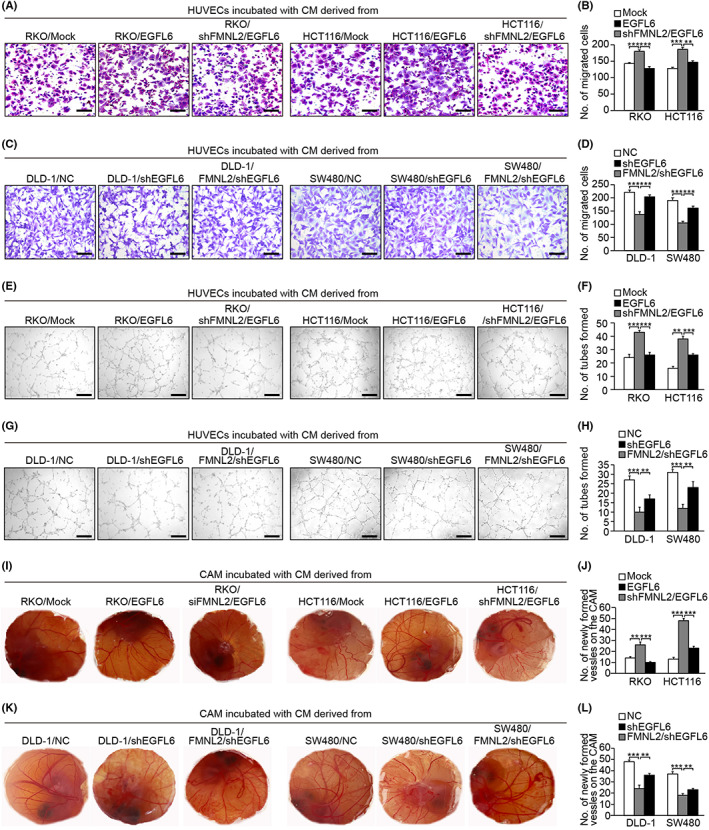
Epidermal growth factor‐like protein 6 (EGFL6) mediates angiogenesis by formin‐like 2 (FMNL2). (A, B) Transwell assay was carried out in HUVECs treated with conditioned medium (CM) from EGFL6‐overexpressing RKO and HCT116 cells, with or without FMNL2 knockdown, to investigate the effect on the migration ability of HUVECs. (C, D) Transwell migration assay to investigate the effect of CM from EGFL6‐knockdown SW480 and DLD‐1 cells, with or without FMNL2 overexpression, on the migration ability of HUVECs. (E, F) Tube formation to investigate the effect of CM from EGFL6‐overexpressing RKO and HCT116 cells, with or without FMNL2 knockdown, on HUVEC tube formation. (G, H) Tube formation to investigate the effect of CM from EGFL6‐knockdown SW480 and DLD‐1 cells, with or without FMNL2 overexpression, on HUVEC tube formation. (I, J) Chorioallantoic membrane (CAM) assay was used to investigate the effect of CM from EGFL6‐overexpressing RKO and HCT116 cells, with or without FMNL2 knockdown, on HUVEC tube formation. (K, L) CAM assay was carried out to investigate the effect of CM from EGFL6‐knockdown SW480 and DLD‐1 cells with or without FMNL2‐overexpression, on HUVEC tube formation. Data are presented as the mean ± SEM; n = 3 independent experiments. **p < 0.01, ***p < 0.001. NC, negative control
To determine the effect of EGFL6 on angiogenesis in vivo, EGFL6 and shFMNL2/EGFL6 HCT116 cells were subcutaneously transplanted into nude mice. Tumor weight was greater in the EGFL6‐overexpressing group than in the shFMNL2/EGFL6 group (Figure 7A,B). Orthotopic transplantation assay showed that the number of metastatic liver lesions was significantly higher in the EGFL6 group than in the shFMNL2/EGFL6 group (Figure 7C,D). These results indicated that FMNL2 promotes tumor growth and metastasis through EGFL6.
FIGURE 7.
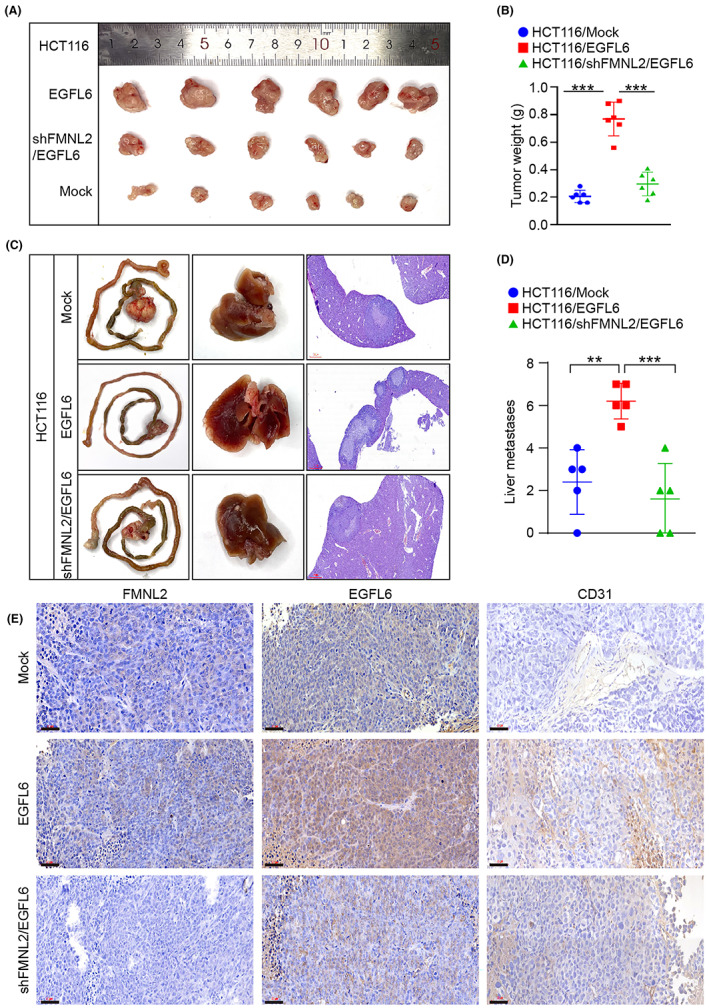
Epidermal growth factor‐like protein 6 (EGFL6) mediates the proangiogenesis of formin‐like 2 (FMNL2) in vivo. (A, B) Mice of the xenograft model were subcutaneously injected with HCT116 cells transfected with EGFL6 overexpressing plasmids, control plasmids, or shFMNL2/EGFL6 plasmids. Tumor weights were monitored after 5 weeks. n = 6 for each group. (C, D) Mice of the metastatic liver model of orthotopic cecal implantation were injected with HCT116 cells transfected with EGFL6 overexpressing plasmids, control plasmids, or shFMNL2/EGFL6 plasmids. n = 5 for each group. (E) Representative images of CD31, EGFL6, and FMNL2 immunohistochemical staining of paraffin‐embedded tumor samples from different mice xenograft model groups. **p < 0.01, ***p < 0.001
Additionally, we assessed vascular density in subcutaneous tumors derived from EGFL6‐overexpressing HCT116 cells and shFMNL2/EGFL6 HCT116 cells. Immunohistochemistry showed that higher expression of CD31, an angiogenesis marker, in the EGFL6‐overexpressing group compared with the shFMNL2/EGFL6 group (Figure 7E). Taken together, these results indicated that EGFL6 plays an essential role in FMNL2‐mediated tumorigenesis and angiogenesis in CRC.
3.7. Cytoskeleton‐associated protein 4 is the critical effector of EGFL6‐induced angiogenesis
To further explore the mechanism by which EGFL6 promotes angiogenesis, human recombinant protein EGFL6 was used to stimulate HUVECs. Mass spectrometry showed a peak of the differential proteins at nearly 70 kDa. Cytoskeleton‐associated protein 4 was identified as a candidate protein (Figure 8A,B). The GST pull‐down assay showed that EGFL6 directly interacted with the cytoplasmic domain (1–127 a.a.) of CKAP4 (Figure 8C,D). Cytoskeleton‐associated protein 4 was selected as the target protein for the follow‐up research.
FIGURE 8.
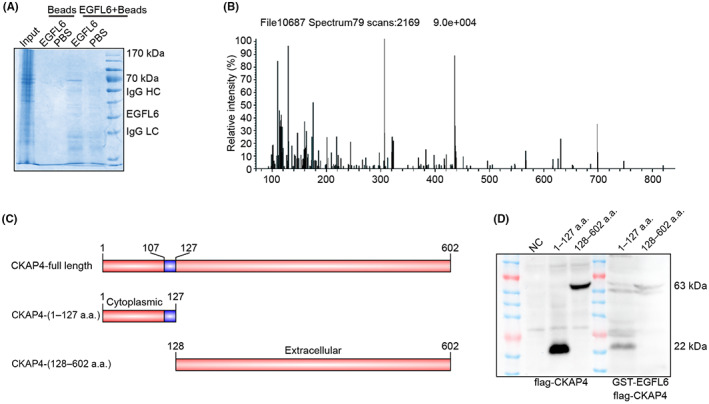
Epidermal growth factor‐like protein 6 (EGFL6) directly interacts with cytoskeleton‐associated protein 4 (CKAP4). (A, B) Mass spectrometry analysis was carried out to investigate the interaction protein of EGFL6. (C) Schematic diagram of different truncations of CKAP4. (D) GST pull‐down assay of the interaction between several truncations of EGFL6 and CKAP4. HC, heavy chain; LC, light chain
Transwell assay revealed that CKAP4 knockdown reduced the migration capacity of HUVECs (Figure 9A–D), whereas CKAP4 overexpression enhanced HUVEC migration (Figure 9E,F). Furthermore, the scratch wound‐healing assay indicated that CKAP4 knockdown markedly decreased the migration ability of HUVECs (Figure 9G–J). Cytoskeleton‐associated protein 4 overexpression had the opposite effect (Figure 9K,L). These results indicated that CKAP4 promoted HUVEC migration.
FIGURE 9.
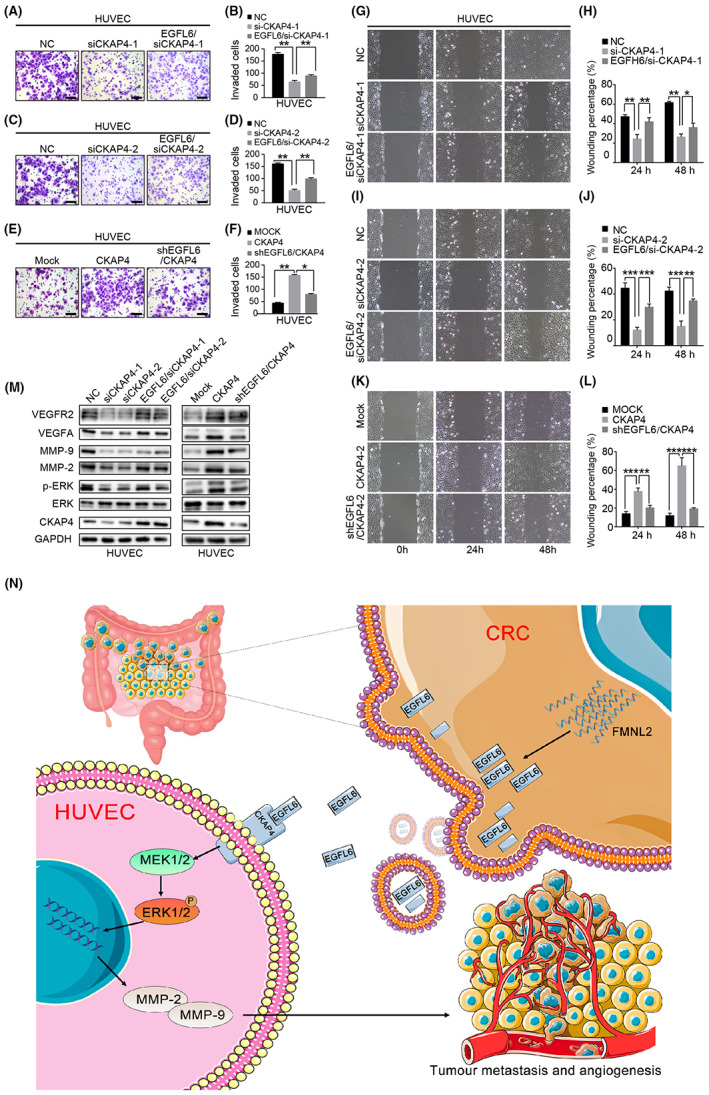
ERK1/2 signaling pathway involved in formin‐like 2 (FMNL2)/epidermal growth factor‐like protein 6 (EGFL6) mediates angiogenesis. (A–D) Transwell migration assay to investigate the effect of cytoskeleton‐associated protein 4 (CKAP4) knockdown, with or without being treated with conditioned medium (CM) from colorectal cancer (CRC) cells, after transfection with EGFL6 overexpression plasmids on the migration ability of HUVECs. (E, F) Transwell migration assay to investigate the effect of CKAP4 overexpression, with or without being treated with CM from CRC cells, after transfection with EGFL6 shRNA plasmids on the migration ability of HUVECs. (G–J) Scratch wound‐healing motility assay was used to observe the changes in the migration of HUVECs after CKAP4 knockdown, with or without stimulation with CM from CRC cells after transfection with EGFL6 overexpression plasmids. (K, L) Scratch wound‐healing motility assay was carried out to observe the changes in the migration of HUVECs after CKAP4 overexpression with or without stimulation with CM from CRC cells after transfection with EGFL6 shRNA plasmids. (M) Western blot assay to detect the expression level of ERK pathway‐related proteins (ERK, p‐ERK, vascular endothelial growth factor A [VEGFA], vascular endothelial growth factor receptor 2 [VEGFR2], MMP2, and MMP9) in HUVECs with different CKAP4 expression, with or without stimulation with CM from CRC cells with different EGFL6 expression. (N) Briefly, FMNL2 overexpression in CRC cells inhibits EGFL6 degradation by binding with EGFL6 and promotes its secretion. Subsequently, EGFL6 integrates with the HUVEC membrane receptor CKAP4 to activate the ERK1/2 signaling pathway, thereby promoting angiogenesis and metastasis. Data are presented as the mean ± SEM; n = 3 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001. NC, negative control
Overexpression of EGFL6 promoted the migration of CKAP4‐knockdown HUVECs. In addition, EGFL6 knockdown inhibited the migration capacity of CKAP4‐overexpressing HUVECs (Figure 9M). However, the overexpression of CKAP4 or EGFL6 did not affect the proliferative capacity of HUVECs (Figure S4). These results suggest that the interaction between EGFL6 and CKAP4 induces HUVEC migration and promotes CRC angiogenesis. In addition, our findings revealed that CKAP4 is involved in EGFL6‐induced angiogenesis in CRC.
3.8. Epidermal growth factor‐like protein 6 promotes CRC angiogenesis through the ERK pathway
To further explore the molecular mechanism of EGFL6‐induced angiogenesis, we treated HUVECs with rEGFL6. The results showed that rEGFL6 enhanced ERK1/2 phosphorylation in a time‐dependent manner peaking at 30 min, while it did not affect STAT3 phosphorylation (Figure S4A). The CM from EGFL6‐overexpressing CRC cells significantly upregulated p‐ERK1/2 in HUVECs. Consistently, CM from EGFL6‐knockdown CRC significantly downregulated p‐ERK1/2 in HUVECs (Figure S4B,C). In addition, CKAP4 knockdown suppressed the expression of p‐ERK, VEGFA, VEGFR2, MMP2, and MMP9 in HUVECs, whereas CKAP4 overexpression upregulated them (Figure 9M). Consistently, EGFL6 enhanced the expression of p‐ERK, VEGFA, VEGFR2, MMP2, and MMP9 in CKAP4‐knockdown HUVECs. Furthermore, CKAP4 overexpression or EGFL6 knockdown downregulated p‐ERK, VEGFA, VEGFR2, MMP2, and MMP9 in HUVECs (Figure 9M). These results indicate that EGFL6 interacts with CKAP4 to activate the downstream proteins of the ERK pathway.
4. DISCUSSION
We showed that FMNL2 promotes tumor angiogenesis through paracrine signaling of EGFL6, and EGFL6 binds to CKAP4 receptor on HUVECs, thereby promoting CRC metastasis. In this study, we found that FMNL2 promotes CRC metastasis by modulating angiogenesis in the TME. Our previous study showed that FMNL2 is markedly upregulated in CRC specimens and is associated with metastasis. We also found that FMNL2 induces EMT in CRC cells through RhoA pathway and promotes motility, invasion, and metastasis. 4 MicroRNA‐137, miR‐206, and miR‐34a can inhibit the invasion and metastasis of CRC by regulating the downstream target FMNL2 gene expression. 20 , 21 , 22 In addition, FMNL2 is a specific downstream effector of RhoC that participates in the invasion and migration of CRC cells. 23 Recent studies have shown that FMNL2 plays a vital role in CRC progression and TME regulation. However, the mechanism by which FMNL2 promotes CRC metastasis remains unclear. During investigating the role of FNML2 in CRC metastasis, it was unexpectedly found that FMNL2 promotes tumor angiogenesis (Figure 1). Here, we identified FMNL2 as a novel angiogenesis regulator in CRC, which promotes the migration and angiogenesis of HUVECs (Figure 2). Animal experiments showed that FMNL2 promotes CRC proliferation and metastasis (Figure 3). We further explored the molecular mechanisms by which FMNL2 promotes CRC metastasis. Both Co‐IP and immunofluorescence assays confirmed that FMNL2 interacts with EGFL6 (Figure 4A,B). The GST pull‐down assay further revealed that the FH3/GBD domain of FMNL2 interacts with EGFL6 (Figure 4D), suggesting that FMNL2 promotes CRC angiogenesis by regulating the paracrine secretion of EGFL6.
Epidermal growth factor‐like protein 6, a member of the EGF‐like superfamily, plays a pivotal role in embryonic development and tumor angiogenesis. Previous studies have revealed that EGFL6 regulates distraction osteogenesis through the Wnt/β‐catenin signaling pathway, 6 promotes breast cancer progression by activating AKT and ERK signaling pathways, 24 and regulates ovarian cancer progression through the FGF‐2/PDGFB pathway. 25 Our results are consistent with previous studies showing that EGFL6 significantly increases HUVEC migration and tube formation in vitro and in vivo and promotes FMNL2‐mediated tumorigenesis and angiogenesis in CRC. Consistently, FMNL2 inhibition reversed the effects of EGFL6 overexpression on CRC angiogenesis (Figures 6 and 7). In addition, FMNL2 activates the paracrine signaling of EGFL6. We hypothesized that EGFL6 regulates CRC angiogenesis through cell surface receptors. Mass spectrometry and GST pull‐down assays confirmed that CKAP4 is a cell surface receptor involved in EGFL6‐mediated angiogenesis in CRC.
Cytoskeleton‐associated protein 4 is involved in the progression of multiple tumors, such as lung cancer, esophageal cancer, hepatocellular carcinoma, and pancreatic cancer. 9 , 24 , 26 , 27 Retinol‐binding protein 1 activates CKAP4 to promote the malignant progression of oral squamous cell carcinoma through autophagy activation. 28 Here, we identified CKAP4 as a HUVEC membrane receptor for EGFL6. The N‐terminal intracellular region of CAKP4 interacts with EGFL6, promoting angiogenesis by activating the ERK pathway. Importantly, we elucidated the involvement of FMNL2 in the regulatory role of EGFL6 in CKAP4 expression.
In conclusion, our study identified FMNL2 as a novel angiogenic factor promoting CRC growth and metastasis through the EGFL6/CKAP4/ERK axis. In addition, this study shed light on the role of FMNL2 in CRC progression, constructing a solid foundation for developing anticancer drugs.
AUTHOR CONTRIBUTIONS
L.L., X.Q., L.Z., G.H., W.L., and W.Z. conceived and designed the study. G.H., W.L., and W.Z. performed most experiments and analyses. H.M. and Q.C. performed experiments and analyses. G.W., Q.C., J. Z., H. Z., W.W., M.D., and Z.X. recruited the study subjects and collected clinical data. J.H. provided assistance and bioinformatic analysis. L.L., G.H., W.L., and L.Z. drafted the manuscript. L.L., L.Z., X.Q., G.H., and W.L. critically revised the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol by an institutional review board: This study was approved by the Medical Ethics Committee of the Xinxiang Medical University.
Informed consent: Informed consent was obtained from all CRC patients.
Animal studies: Animal studies conformed to international principals.
Supporting information
Document S1
Document S2.
Document S3.
Document S4.
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Table S1.
Table S2.
Table S3.
ACKNOWLEDGMENTS
We thank all study subjects for their enthusiastic support in this research. This work was supported by the National Natural Science Foundation of China (81772524, 81903007), Henan Province Key Research and Development and Promotion of Special (Science and Technology) project (222102310613), and the Cultivation Project of Basic Medical College of Xinxiang Medical College (JCYXYKY202101). We would like to acknowledge EditSprings for its linguistic assistance.
He G, Li W, Zhao W, et al. Formin‐like 2 promotes angiogenesis and metastasis of colorectal cancer by regulating the EGFL6/CKAP4/ERK axis. Cancer Sci. 2023;114:2014‐2028. doi: 10.1111/cas.15739
Guoyang He, Wei Li, Wenli Zhao, and Hui Men contributed equally to this work.
Contributor Information
Guoyang He, Email: 071014@xxmu.edu.cn.
Li Liang, Email: lli@smu.edu.cn.
DATA AVAILABILITY STATEMENT
The original contributions of this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Kanth P, Inadomi JM. Screening and prevention of colorectal cancer. BMJ. 2021;374:n1855. [DOI] [PubMed] [Google Scholar]
- 3. Valencia DA, Quinlan ME. Formins. Curr Biol. 2021;31:R517‐R522. [DOI] [PubMed] [Google Scholar]
- 4. Zeng Y, Xie H, Qiao Y, et al. Formin‐like2 regulates rho/ROCK pathway to promote Actin assembly and cell invasion of colorectal cancer. Cancer Sci. 2015;106:1385‐1393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Kang J, Wang J, Tian J, Shi R, Jia H, Wang Y. The emerging role of EGFL6 in angiogenesis and tumor progression. Int J Med Sci. 2020;17:1320‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen J, Sun Y, Liu X, et al. EGFL6 regulates angiogenesis and osteogenesis in distraction osteogenesis via Wnt/beta‐catenin signaling. Stem Cell Res Ther. 2021;12:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kimura H, Fumoto K, Shojima K, et al. CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J Clin Invest. 2016;126:2689‐2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Osugi Y, Fumoto K, Kikuchi A. CKAP4 regulates cell migration via the interaction with and recycling of integrin. Mol Cell Biol. 2019;39(16):e00073‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kimura H, Yamamoto H, Harada T, et al. CKAP4, a DKK1 receptor, is a biomarker in exosomes derived from pancreatic cancer and a molecular target for therapy. Clin Cancer Res. 2019;25:1936‐1947. [DOI] [PubMed] [Google Scholar]
- 10. Dirksmeyer T, Stahl P, Vallet C, et al. Advances towards cell‐specific gene transfection: a small‐molecule approach allows order‐of‐magnitude selectivity. Chemistry. 2022;28:e202202024. [DOI] [PubMed] [Google Scholar]
- 11. Longo PA, Kavran JM, Kim MS, Leahy DJ. Transient mammalian cell transfection with polyethylenimine (PEI). Methods Enzymol. 2013;529:227‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liao WT, Jiang D, Yuan J, et al. HOXB7 as a prognostic factor and mediator of colorectal cancer progression. Clin Cancer Res. 2011;17:3569‐3578. [DOI] [PubMed] [Google Scholar]
- 13. McKinnon KM. Flow cytometry: An overview. Curr Protoc Immunol. 2018;120: 5 1:1‐5 1 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cui YM, Jiao HL, Ye YP, et al. FOXC2 promotes colorectal cancer metastasis by directly targeting MET. Oncogene. 2015;34:4379‐4390. [DOI] [PubMed] [Google Scholar]
- 15. Martinotti S, Ranzato E. Scratch wound healing assay. Methods Mol Biol. 2020;2109:225‐229. [DOI] [PubMed] [Google Scholar]
- 16. Jiao HL, Ye YP, Yang RW, et al. Downregulation of SAFB sustains the NF‐kappaB pathway by targeting TAK1 during the progression of colorectal cancer. Clin Cancer Res. 2017;23:7108‐7118. [DOI] [PubMed] [Google Scholar]
- 17. Celerier J, Cruz A, Lamande N, Gasc JM, Corvol P. Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension. 2002;39:224‐228. [DOI] [PubMed] [Google Scholar]
- 18. Kim SY, Hakoshima T. GST pull‐down assay to measure complex formations. Methods Mol Biol. 2019;1893:273‐280. [DOI] [PubMed] [Google Scholar]
- 19. Tabatabaei MS, Ahmed M. Enzyme‐Linked Immunosorbent Assay (ELISA). Methods Mol Biol. 2022;2508:115‐134. [DOI] [PubMed] [Google Scholar]
- 20. Liang L, Li X, Zhang X, et al. MicroRNA‐137, an HMGA1 target, suppresses colorectal cancer cell invasion and metastasis in mice by directly targeting FMNL2. Gastroenterology. 2013;144(624–635):e624. [DOI] [PubMed] [Google Scholar]
- 21. Lu G, Sun Y, An S, et al. MicroRNA‐34a targets FMNL2 and E2F5 and suppresses the progression of colorectal cancer. Exp Mol Pathol. 2015;99:173‐179. [DOI] [PubMed] [Google Scholar]
- 22. Ren XL, He GY, Li XM, et al. MicroRNA‐206 functions as a tumor suppressor in colorectal cancer by targeting FMNL2. J Cancer Res Clin Oncol. 2016;142:581‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitzing TM, Wang Y, Pertz O, Copeland JW, Grosse R. Formin‐like 2 drives amoeboid invasive cell motility downstream of RhoC. Oncogene. 2010;29:2441‐2448. [DOI] [PubMed] [Google Scholar]
- 24. Shinno N, Kimura H, Sada R, et al. Activation of the Dickkopf1‐CKAP4 pathway is associated with poor prognosis of esophageal cancer and anti‐CKAP4 antibody may be a new therapeutic drug. Oncogene. 2018;37:3471‐3484. [DOI] [PubMed] [Google Scholar]
- 25. Zhu W, Liu C, Lu T, et al. Knockout of EGFL6 by CRISPR/Cas9 mediated inhibition of tumor angiogenesis in ovarian cancer. Front Oncol. 2020;10:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y, Yu H, Xie X, et al. Plasmalemma vesicle‐associated protein promotes angiogenesis in cholangiocarcinoma via the DKK1/CKAP4/PI3K signaling pathway. Oncogene. 2021;40:4324‐4337. [DOI] [PubMed] [Google Scholar]
- 27. Huang X, Chen Q, Li X, et al. CKAP4 antibody‐conjugated Si quantum dot micelles for targeted imaging of lung cancer. Nanoscale Res Lett. 2021;16:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao L, Wang Q, Ren W, et al. The RBP1‐CKAP4 axis activates oncogenic autophagy and promotes cancer progression in oral squamous cell carcinoma. Cell Death Dis. 2020;11:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Document S1
Document S2.
Document S3.
Document S4.
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Table S1.
Table S2.
Table S3.
Data Availability Statement
The original contributions of this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.


