Abstract
Ovarian cancer (OC) is characterized by frequent widespread peritoneal metastasis. Cancer‐associated fibroblasts (CAFs) represent a critical stromal component of metastatic niche and promote omentum metastasis in OC patients. However, the role of exosomes derived from omental CAFs in metastasis remains unclear. We isolated exosomes from primary omental normal fibroblasts (NFs) and CAFs from OC patients (NF‐Exo and CAF‐Exo, respectively) and assessed their effect on metastasis. In mice bearing orthotopic OC xenografts, CAF‐Exo treatment led to more rapid intraperitoneal tumor dissemination and shorter animal survival. Similar results were observed in mice undergoing intraperitoneal injection of tumor cells. Among the miRNAs downregulated in CAF‐Exo, miR‐29c‐3p in OC tissues was associated with metastasis and survival in patients. Moreover, increasing miR‐29c‐3p in CAF‐Exo significantly weakened the metastasis‐promoting effect of CAF‐Exo. Based on RNA sequencing, expression assays, and luciferase assays, matrix metalloproteinase 2 (MMP2) was identified as a direct target of miR‐29c‐3p. These results verify the significant contribution of exosomes from omental CAFs to OC peritoneal metastasis, which could be partially due to the relief of MMP2 expression inhibition mediated by low exosomal miR‐29c‐3p.
Keywords: exosome, metastasis, miR‐29c‐3p, myofibroblast, ovarian tumor
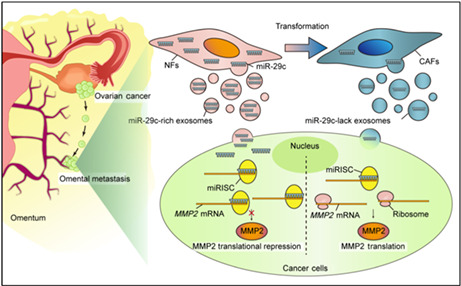
The conversion from NFs to CAFs causes a reduction of miR‐29c‐3p. Subsequently, the low level of miR‐29c‐3p in the CAF‐derived exosomes contributes to the derepression of MMP2, which promotes the aggression of OC cells.
Abbreviations
- α‐SMA
α‐smooth muscle actin
- CAF
cancer‐associated fibroblast
- CAF‐Exo
cancer‐associated fibroblast‐derived exosome
- ExoDP‐CM
exosome‐depleted conditioned medium
- GEO
Gene Expression Omnibus
- IHC
immunohistochemistry
- ISH
in situ hybridization
- miRNA
microRNA
- MUT
mutant
- NC
negative control
- NF
normal fibroblast
- NF‐Exo
normal fibroblast‐derived exosome
- OC
ovarian cancer
- OS
overall survival
- qRT‐PCR
quantitative real‐time PCR
- TME
tumor microenvironment
1. INTRODUCTION
There were more than 207,000 deaths worldwide related to OC in 2020, making OC the fifth most common cause of cancer‐related death for women and the second most common among gynecologic cancers. 1 Ovarian cancers are prone to disseminate intraperitoneally, which is tightly associated with poor prognosis in patients. 2 Thus, molecular mechanisms underlying OC metastasis need to be clarified, which may fuel new strategies to improve the treatment of OC.
In recent years, increasing attention has been given to tumor stromal cells in addition to tumor cells in the field of OC metastasis. 3 The omentum, where OC metastases most often occur, 4 is rich in various stromal cells, including adipocytes, mesenchymal stem cells, fibroblasts, and macrophages. 5 These activated stromal cells can promote omental metastasis of OC cells, and among them, CAFs are the main active components. Increasing evidence shows that CAFs play pivotal roles in tumor metastasis, growth, metabolic reprogramming, immunosuppression, and angiogenesis. 6 In OC, tumor cells can induce the activation of CAFs, and the activated CAFs can also promote tumor cell metastasis by secreting cytokines, growth factors, or exosomes. 7 Cancer‐associated fibroblasts have been reported to secrete prometastatic cytokines in a paracrine manner to promote OC cell metastasis. 8 , 9 In addition to cytokines, exosomes play an important role in the communication between CAFs and tumor cells. 10 It has been reported that exosomes derived from CAFs promote breast cancer and esophageal squamous cell cancer metastasis. 11 , 12 However, the effects of CAF‐Exo on OC metastasis remain poorly understood.
Exosomes are extracellular vesicles measuring between 30 and 150 nm in diameter that can deliver bioactive molecules of different kinds, for example, nucleic acids and proteins, and are reported to be vital mechanisms of communication between the tumor microenvironment (TME) and tumor cells. 13 MicroRNAs are small noncoding regulatory RNAs, 17–25 nt in size, that cause translational repression or target degradation by binding to the 3′‐UTR of genes. 14 As important components in exosomes, miRNAs can be delivered into recipient cells, thereby finetuning the cross‐talk between cancer cells and noncancer cells in the TME. For example, CAF‐derived exosomal miR‐500a‐5p could promote breast cancer metastasis by targeting USP28, and exosomal miR‐34a‐5p derived from CAFs promoted oral cancer growth and metastasis. 11 , 15 In OC, it has been reported that exosomes derived from CAFs could promote OC cells to develop drug resistance by delivering miR‐21 or miR‐98‐5p. 16 , 17 Nevertheless, the mechanisms through which CAF‐Exos promote OC metastasis remain elusive. To clarify whether CAF‐derived exosomal miRNAs can be used as therapeutic targets for OC, their mechanistic role in OC metastasis has to be understood.
In our study, we showed that omental CAF‐Exos promoted OC cell invasion and migration. In orthotopic xenograft mouse models and intraperitoneal transplanted mouse models of OC, CAF‐Exos could promote OC metastasis in vivo. Mechanistically, high‐throughput miRNA sequencing and qRT‐PCR revealed that miR‐29c‐3p was significantly downregulated in CAF‐Exos, and miR‐29c‐3p‐reduced CAF‐Exos facilitated OC metastasis by disinhibiting MMP2 expression in cancer cells both in vivo and in vitro. Our findings indicate that loss of omental CAF‐derived exosomal miR‐29c‐3p promotes OC metastasis, providing a new viewpoint into the mechanism of OC metastasis and new ideas for OC treatment.
2. MATERIALS AND METHODS
2.1. Isolation and culture of primary omental fibroblasts from OC patients
Omental specimens were collected from 18 OC patients who underwent surgery at our hospital from January 2017 to January 2019. No patients received chemotherapy or radiotherapy before surgery. The pathological information of these OC patients and the use of fibroblasts are shown in Table S1. Primary human NFs and CAFs were isolated from metastasis‐negative and metastasis‐positive omental tissues, respectively. 18 In primary fibroblast cultures, DMEM/F12, 10% FBS (Gibco), and 1% penicillin–streptomycin were used in a humidified 37°C incubator (5% CO2). All the primary fibroblasts were passaged fewer than five times.
2.2. Exosome isolation
As previously described, fibroblast exosomes were isolated by serial centrifugation. 19 , 20 In sequence, the supernatant was centrifuged at 300 g for 10 min and 2000 g for 20 min at 4°C. Then 0.22 mm filters (Millipore) were used to filter the supernatant from the centrifuge at 4°C for 70 min. The supernatant was then dissolved in PBS for later use or storage at −80°C.
2.3. Orthotopic xenograft mouse model of OC
Four‐week‐old female nude mice were purchased from Beijing Hua Fukang Biological Polytron Technologies, Inc. All the orthotopic xenograft models of OC were established using a previously described method. 21 For these models, ES2 cells transfected with the lentiviral luciferase reporter gene (ES2‐Luc) were used, which allow bioluminescence in vivo imaging of the tumors. Fifty micrograms of CAF‐Exos, 50 μg NF‐Exos, or an equal volume of PBS were injected intraperitoneally every 3 days. The first bioluminescence in vivo imaging was undertaken once a week from 1 week after modeling until the first nude mouse died naturally. The malignant progression of mice was observed, and the time of death was recorded. To detect the effects of the exosomal miR‐29c‐3p, 50 μg CAF‐Exo, 50 μg miR‐29c‐3p‐Exos, or 50 μg NC‐Exos were injected intraperitoneally every 3 days. The in vivo imaging procedures were consistent with those described above. Mice were killed together when the first mouse was dying.
2.4. Dual luciferase reporter assay
SKOV3 cells were transfected with plasmids containing WT or mutant MMP2 3′‐UTR inserts, and cotransfected with miR‐29c‐3p or NC mimics. Cells were harvested and lysed after transfection for 48 h, and the Dual‐Luciferase Reporter Assay System (Promega) and GloMax 20/20 Luminometer (Promega) were used to detect the luciferase activity under the constructions. The normalized luciferase reporter activity was determined by comparing the activity of the luciferase reporter to that of Renilla luciferase.
2.5. Statistical analysis
GraphPad Prism 8.0 software and SPSS 23.0 statistical software were used for statistical analyses. Numerical data are shown as the mean ± SD. Differences between subgroups were analyzed by Student's t‐test and considered statistically significant at a threshold of p < 0.05. K–M Plotter was used to investigate the prognostic significance of miR‐29c‐3p and MMP2 in pancancer datasets and OC datasets. 22
Additional materials and methods are provided in Appendix S1.
3. RESULTS
3.1. Characterization of omental CAF‐derived exosomes from OC patients
To analyze the role of CAF exosomes in the metastasis of OC, we isolated primary CAFs and NFs from the omentum tissues of OC patients, as the omentum represents the most common metastatic site (Figure 1A). The fibroblasts showed a typical morphology of long spindle shape, arranged in a spiral, and a vimentin‐positive and cytokeratin 8‐negative expression pattern (Figure S1A,B). The CAFs displayed strong α‐SMA staining, whereas the NFs showed only modest α‐SMA staining (Figure S1C), which agreed with the α‐SMA level in the omental tissues from which they were isolated (Figure S1C). Exosomes were isolated from the culture medium of CAFs and NFs and characterized a double‐layer membrane structure (Figure 1B), with a particle size distribution predominantly ranging between 80 and 200 nm (Figure 1C), and their positive markers CD63, CD9, and CD81 were accompanied by negative calnexin (Figure 1D). To determine whether fibroblast‐derived exosomes can be internalized by OC cells, PKH67‐labeled exosomes were incubated with FM4‐64‐labeled SKOV3 and CAOV3 cells for 24 h. After the incubation, both CAF‐Exos and NF‐Exos were observed in most recipient OC cells (Figures 1E,F and S1D).
FIGURE 1.
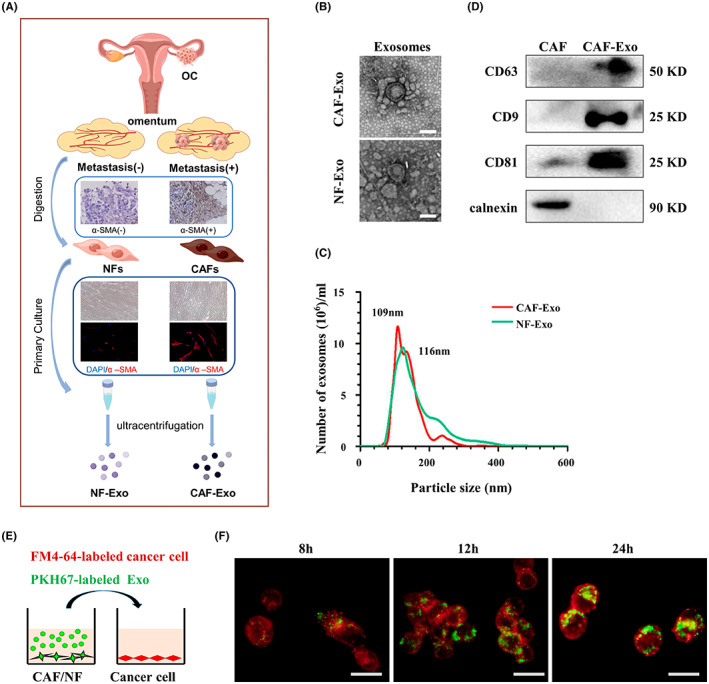
Isolation of exosomes. (A) Schematic illustration of exosome isolation from primary omentum fibroblasts. (B) Electron micrograph of cancer‐associated fibroblast (CAF)‐derived exosome (CAF‐Exo) and normal fibroblast (NF)‐derived exosome (NF‐Exo). Scale bar, 100 nm. (C) Western blot analysis of CD63, CD9, CD81 and calnexin in CAFs and CAF‐Exo. (D) Nanoparticle tracking analysis of CAF‐Exo and NF‐Exo. (E) Schematic illustration of the uptake of PKH67‐labeled exosome by FM4‐64‐labeled SKOV3 cells. (F) Representative immunofluorescence images show the uptake of CAF‐derived exosomes (labeled with PKH67, green) by SKOV3 cells (labeled with FM4‐64, red). Scale bar, 20 μm. α‐SMA, α‐smooth muscle actin; OC, ovarian cancer
3.2. Cancer‐associated fibroblast‐derived exosomes promote OC metastasis
To confirm the impact of CAF‐Exos on OC cell mobility, SKOV3 or CAOV3 cells were incubated with CAF‐Exos, NF‐Exos, or CAF‐ExoDP‐CM, and Transwell assays were used to detect the invasion and migration properties of tumor cells in vitro. Compared to the blank control, the invasion and migration of tumor cells were significantly enhanced by CAF‐Exo treatment but not the NF‐Exo; the promoting effects were largely reduced in the CAF‐ExoDP‐CM group versus the CAF‐Exo group, indicating a predominant role of exosomes in CAF–tumor cell interactions (Figures 2A,B and S2A,B). To further investigate the effects of CAF‐Exos on OC metastasis, nude mice harboring ES2‐luc orthotopic xenografts were intraperitoneally injected with PBS, NF‐Exos, or CAF‐Exos, and luciferase signals were monitored by in vivo imaging. The mice undergoing CAF‐Exo treatment showed significantly increased tumor burden with earlier contralateral metastasis than those in the PBS and NF‐Exo groups 21 days after inoculation, suggesting that CAF‐Exos accelerate tumor growth and spread (Figure 2C,D). Moreover, CAF‐Exo treatment significantly shortened the survival time of nude mice (Figure 2E). Immunohistochemical analysis revealed decreased E‐cad and increased N‐cad, Vimentin, and Ki‐67 in xenografts of the CAF‐Exo group, when compared with the NF‐Exo and PBS groups (Figure 2F,G), suggesting that CAF‐Exos induce an epithelial–mesenchymal transition in cancer cells in vivo. To further confirm the EMT‐inducing effect of CAF‐Exos, OC cells were treated with PBS, NF‐Exos, or CAF‐Exos in vitro. Western blot analysis showed that the E‐Cadherin level was downregulated and N‐Cadherin and Vimentin levels were upregulated in OC cells treated with CAF‐Exos compared to those treated with NF‐Exos (Figure S2C). Moreover, in the mouse models intraperitoneally transplanted with ES2‐luc cells, both the CAF‐Exo group and the NF‐Exo group showed higher fluorescence intensity and a larger range of abdominal metastasis compared to the PBS group, with more intensive metastasis in the CAF‐Exo group (Figure 2H,I). Collectively, these data indicate that CAF‐Exos promote OC metastasis.
FIGURE 2.

Cancer‐associated fibroblast‐derived exosomes (CAF‐Exos) promote metastasis of ovarian cancer in vitro and in vivo. (A, B) SKOV3 cells were treated with normal fibroblast‐derived exosome (NF‐Exo), CAF‐Exo, or CAF‐Exo‐depleted conditioned medium (CAF‐ExoDP‐CM). (A) Representative images from migration and invasion assays of SKOV3 cells. Scale bar, 50 μm. (B) Histograms showing the number of SKOV3 cells that underwent migration and invasion. (C–G) Mice bearing ES2‐luc orthotopic xenotransplants were injected intraperitoneally with PBS, NF‐Exo, or CAF‐Exo. (C) Bioluminescence images of abdominal metastasis in mice bearing ES2‐luc orthotopic xenotransplants. (D) Quantitative radiance values from IVIS imaging of ES2‐luc orthotopic xenotransplants. (E) Survival curve of orthotopic xenograft mouse. (F) Representative images of immunohistochemical staining with anti‐E‐cadherin (E‐cad), anti‐N‐cadherin (N‐cad), anti‐Vimentin, and anti‐Ki‐67 in tumor tissues from orthotopic xenotransplants. Scale bar, 50 μm. (G) Histograms showing the Ki‐67 index and mean density of E‐cad, N‐cad, and Vimentin. (H) Bioluminescence images of abdominal metastasis in mice bearing ES2‐luc intraperitoneal transplanted mouse. (I) Quantitative radiance values from IVIS imaging in metastases on abdominal organs. *p < 0.05; **p < 0.01. ns, no significance
3.3. MicroRNA‐29c‐3p is downregulated in CAF‐Exos and related to poor survival in OC patients
Given that miRNAs are frequently involved in exosome functions, we analyzed the miRNA sequencing datasets of NF‐Exos from normal ovarian fibroblasts and CAF‐Exos from OC CAFs in the GEO public database. The data analysis identified five upregulated miRNAs and six downregulated miRNAs with at least two‐fold changes in CAF‐Exos versus NF‐Exos (Figure 3A,B). Subsequently, we verified the decreased expression of several downregulated miRNAs (miR‐135a, miR‐328, miR‐29c‐3p, miR‐345, miR‐99a, and miR‐203) in the CAF‐Exos compared to the NF‐Exos obtained from three sets of primary CAFs and NFs by using qRT‐PCR (Figure 3C, Table S1). Among these miRNAs, miR‐29c‐3p and miR‐345 expression were positively related to OS in OC patients (Figure 3D). As the downregulation of miR‐29c‐3p in CAF‐Exos was more remarkable, we focused on this miRNA in subsequent experiments. Bioinformatics analysis of GSE83693 from the GEO database showed that, compared with normal ovarian tissue, the miR‐29c‐3p expression level was significantly lower in OC tissue and it was further lower in recurrent OC than in primary OC (Figure 3E). To confirm the correlation between miR‐29c‐3p and the OS of OC patients, we assessed miR‐29c‐3p in 66 frozen OC specimens and 33 normal ovary tissues by ISH (Figure 3F). The ISH staining showed that miR‐29c‐3p was significantly higher in normal ovary tissues than in OC in both epithelial cells and stroma cells (Figure 3G). Twenty‐nine OC specimens showed a high miR‐29c‐3p level, and higher miR‐29c‐3p levels in OC indicated better OS (p = 0.0209) (Figure 3H). Furthermore, a higher miR‐29c‐3p level (ISH score >3) was negatively associated with tumor metastasis in patients (p = 0.0171; Table 1). These results suggest that miR‐29c‐3p could act as a cancer suppressor.
FIGURE 3.
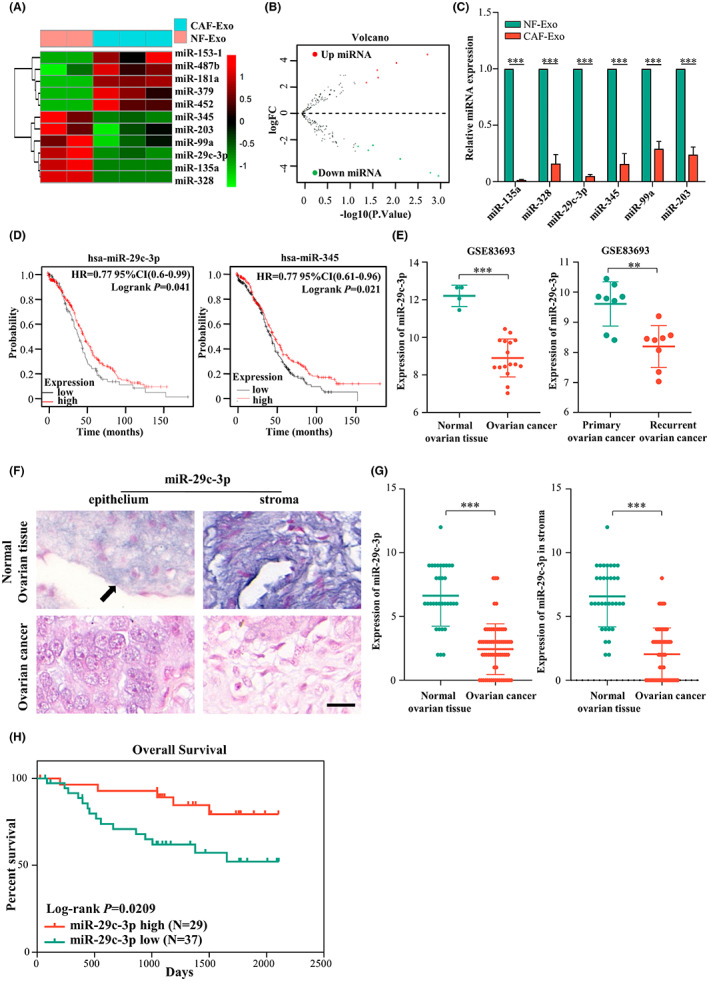
MicroRNA (miR)‐29c‐3p is significantly reduced in cancer‐associated fibroblast‐derived exosomes (CAF‐Exos). (A) Heatmap diagram of most differential miRNA expression profiles between CAF‐Exo and normal fibroblast‐derived exosome (NF‐Exo) from the Gene Expression Omnibus (GEO) public database. (B) Volcano plot showing the differential expression of miRNA between CAF‐Exo and NF‐Exo. (C) Histograms showing the relative expression of the six most downregulated miRNAs in CAF‐Exo and NF‐Exo by real‐time PCR. (D) Kaplan–Meier analysis of miR‐29c‐3p in ovarian cancer (OC) in pancancer analysis with K–M Plotter. (E) Bioinformatics analysis GSE83693 from the GEO database to validate the expression of miR‐29c‐3p in normal ovarian tissues and OC, and primary OC and recurrent OC. (F) In situ hybridization (ISH) of miR‐29c‐3p in normal ovarian tissues and OC tissues (scale bar, 20 μm). Arrow indicates ovarian surface epithelium; violet indicates positive staining. (G) Histograms showing the expression of miR‐29c‐3p in epithelium and stroma of normal ovarian tissues (n = 33) compared to OC (n = 66). (H) Kaplan–Meier survival curves of overall survival in 63 OC patients based on miR‐29c‐3p ISH stains (log–rank test). **p < 0.01; ***p < 0.01. FC, fold change
TABLE 1.
Relationship between microRNA (miR)‐29c‐3p and clinical parameters in ovarian cancer (N = 66)
| Characteristic | miR‐29c‐3p expression | p value † | |
|---|---|---|---|
| Low | High | ||
| Age (years) | |||
| ≤49 | 13 | 12 | 0.6037 |
| 49 | 24 | 17 | |
| FIGO stage | |||
| I–II | 9 | 11 | 0.2326 |
| III–IV | 28 | 18 | |
| Histological type | |||
| Non‐HGSOC | 11 | 10 | 0.6807 |
| HGSOC | 26 | 19 | |
| Intraperitoneal metastasis | |||
| No | 2 | 8 | 0.0171 |
| Yes | 35 | 21 | |
| Lymph node metastasis | |||
| No | 28 | 23 | 0.7266 |
| Yes | 9 | 6 | |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics 2009 staging criteria; HGSOC, high‐grade serous ovarian cancer.
χ2‐test.
3.4. MicroRNA‐29c‐3p downregulation in CAF‐Exos promotes OC metastasis
To detect the role of miR‐29c‐3p in OC metastasis, SKOV3‐GFP cells were cocultured with CAFs transfected with Cy3‐tagged miR‐29c‐3p mimics (CAFs‐miR‐29c‐3p‐cy3) (red), and red fluorescence was observed in SKOV3‐GFP cells (green) (Figure 4A,B). Similarly, SKOV3‐GFP cells were incubated with exosomes isolated from CAFs‐miR‐29c‐3p‐cy3 (CAF‐miR‐29c‐3p‐Exo), and red fluorescence was also observed in the recipient cells (green), indicating that miR‐29c‐3p can be transferred from CAFs to OC cells through exosomes (Figure S3). As expected, miR‐29c‐3p expression was increased in CAF‐miR‐29c‐3p and CAF‐miR‐29c‐3p‐Exos compared to CAFs transfected with NC mimics (CAF‐NC) and their exosomes (CAF‐NC‐Exos) by qRT‐PCR (Figure 4C). In addition, miR‐29c‐3p in SKOV3‐GFP cells was upregulated after indirect cocultured with CAF‐miR‐29c‐3p (Figure 4D).
FIGURE 4.

MicroRNA (miR)‐29c‐3p downregulation in cancer‐associated fibroblast‐derived exosomes (CAF‐Exos) promotes metastasis of ovarian cancer in vitro and in vivo. (A) Schematic illustration of the indirect coculture of CAFs transiently transfected with cy3‐tagged miR‐29c‐3p mimics (CAFs‐miR‐29c‐3p‐cy3) with GFP‐labeled ovarian cancer cells SKOV3 (SKOV3‐GFP). (B) The representative immunofluorescence images show the uptake of cy3‐tagged miR‐29c‐3p (red) by SKOV3‐GFP cells (green). Scale bar, 20 μm. (C) Histograms showing the relative expression of miR‐29c‐3p in CAF transfected with miR‐29c‐3p mimics and their exosomes detected by quantitative RT‐PCR (qRT‐PCR). (D) Histograms showing the relative expression of miR‐29c‐3p in SKOV3 cells indirectly cocultured with CAFs transfected with miR‐29c‐3p mimics or negative control (NC) mimics in detected by qRT‐PCR. (E–G) Mice bearing ES2‐luc orthotopic xenotransplants were injected intraperitoneally with PBS, CAF‐NC‐Exo, or CAF‐miR‐29c‐3p‐Exo. (E) Bioluminescence images of abdominal metastasis in mice bearing ES2‐luc intraperitoneal transplanted mouse model injected with PBS, CAF‐NC‐Exo, or CAF‐miR‐29c‐3p‐Exo. (F) Quantitative radiance values from IVIS imaging in metastases on abdominal organs. (G) Quantitative radiance values from IVIS imaging of ES2‐luc intraperitoneal transplanted mouse. (H, I) Histograms showing the number of migration and invasion SKOV3 cells transfected with miR‐29c‐3p mimics, NC mimics, or miR‐29c‐3p inhibitors and NC inhibitors. *p < 0.05; **p < 0.01; ***p < 0.01. ns, no significance
To confirm the impact of miR‐29c‐3p on OC metastasis, CAF‐Exos, CAF‐NC‐Exos, and CAF‐miR‐29c‐3p‐Exos were injected intraperitoneally into orthotopic xenograft model mice and luciferase signals were monitored by in vivo imaging. The mice undergoing CAF‐miR‐29c‐3p‐Exo treatment every 3 days showed a significantly lower tumor burden with later contralateral metastasis and fewer metastases on abdominal organs than those in the CAF‐Exo and CAF‐NC‐Exo groups 21 days after inoculation, suggesting that CAF‐miR‐29c‐3p‐Exo inhibits tumor spread (Figure 4E–G). SKOV3 and CAOV3 cells were then transiently transfected with miR‐29c‐3p mimics, NC mimics, miR‐29c‐3p inhibitors, or NC inhibitors (Figure S4A,B). Transwell assays indicated that upregulation of miR‐29c‐3p restrained SKOV3 and CAOV3 cell migration and invasion (Figures 4H and S4C–E), whereas inhibition of miR‐29c‐3p significantly promoted OC cell migration and invasion (Figures 4I and S4F–H).
3.5. MMP2 is a target gene of miR‐29c‐3p in OC cells
To explore the mechanism by which miR‐29c‐3p regulates tumor cell metastasis, differentially expressed genes in the SKOV3 and CAOV3 cells transfected with NC mimics or miR‐29c‐3p mimics were analyzed through RNA sequencing. Overall, compared to the NC group, 91 genes were identified as downregulated in both SKOV3 and CAOV3 cells that were transfected with miR‐29c‐3p mimics (Figure 5A,B). The GO enrichment analyses showed that these downregulated genes were mainly enriched in ECM organization, extracellular structure organization, and collagen fibril organization, which are related to tumor metastasis (Figure S5A). To confirm the results of RNA sequencing, we assessed the expression of eight genes with the highest expression multiples (BMF, CD93, COL‐1, COL‐2, LOX, MMP2, NID1, and SPARC) in the SKOV3 and CAOV3 cells transfected with NC or miR‐29c‐3p mimics using qRT‐PCR. We found that all these genes were significantly downregulated in the miR‐29c‐3p mimics groups compared to the NC groups (Figures 5C and S5B). Among these genes, MMP2 has been reported to play an important role in metastasis in various cancers, 23 and high expression of MMP2 in OC indicates poorer OS (p = 0.0015) based on The Cancer Genome Atlas datasets (Figure 5D). Therefore, MMP2 was selected for further investigation. The IHC analysis verified that CAF‐Exo treatment increased MMP2 but not MMP9 in the xenografts from the CAF‐Exo group compared with the NF‐Exo and PBS groups (Figure 5E,F). Western blot analysis indicated a decrease in MMP2 protein levels in SKOV3 cells overexpressing miR‐29c‐3p, whereas MMP2 protein expression increased when miR‐29c‐3p was downregulated (Figure 5G). Gelatin zymography assays showed that MMP2 activities were also attenuated by miR‐29c‐3p in OC cells (Figure 5H). Moreover, miR‐29c‐3p and MMP2 were assessed by ISH and IHC in 66 frozen or paraffin‐embedded OC specimens, respectively, and a negative correlation between miR‐29c‐3p and MMP2 was found (r = −0.4139, p = 0.0006; Figure 5I,J).
FIGURE 5.
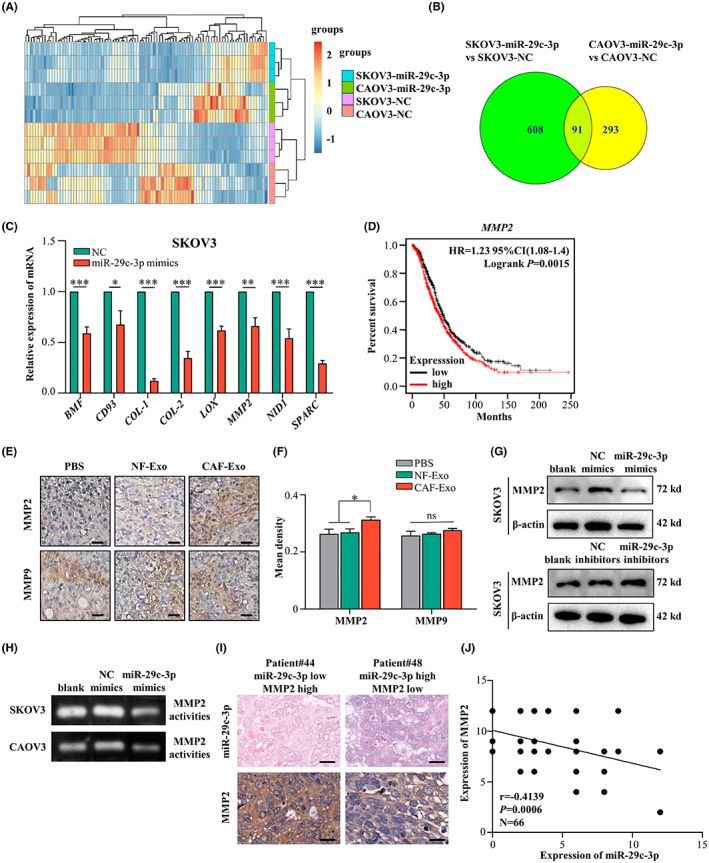
MMP2 is identified as a target gene of microRNA (miR)‐29c‐3p in ovarian cancer cells. (A) Heatmap diagram of differential mRNA expression profiles between CAOV3‐negative control (NC) and CAOV3‐miR‐29c‐3p, and SKOV3‐NC, and SKOV3‐miR‐29c‐3p. (B) Venn diagram illustrating the putative candidate 91 target genes of miR‐29c‐3p in SKOV3 and CAOV3 cells. (C) Histograms showing the relative expression of eight potential target genes of miR‐29c‐3p in SKOV3‐NC and SKOV3‐miR‐29c‐3p cells detected by quantitative RT‐PCR. (D) Kaplan–Meier analysis of MMP2 in ovarian cancer in pancancer analysis with K–M Plotter. (E) Representative images of immunohistochemical staining with anti‐MMP2 and anti‐MMP9 in tumor tissues from orthotopic xenotransplants. Scale bar, 50 μm. (F) Histograms showing the mean density of MMP2 and MMP9 in the three groups of orthotopic xenograft mouse model. (G) Western blot analysis of MMP2 in SKOV3‐NC, SKOV3‐miR‐29c‐3p, and SKOV3‐miR‐29c‐3p inhibitor. (H) Conditioned media from SKOV3‐NC, SKOV3‐miR‐29c‐3p, or CAOV3‐NC, CAOV3‐miR‐29c‐3p were subjected to gelatin zymography, to assess gelatinolytic activities of MMP‐2. (I) Representative images of in situ hybridization (ISH) of miR‐29c‐3p and immunohistochemical staining of MMP2. (J) Scatter diagrams show the negative correlation between miR‐29c‐3p and MMP2 in ovarian cancer based on miR‐29c‐3p ISH and MMP2 stains scores. Scale bar, 20 μm. *p < 0.05; **p < 0.01; ***p < 0.01. ns, no significance
3.6. MicroRNA‐29c‐3p directly targets MMP2 to suppress OC cell invasion
To clarify whether MMP2 is a direct target of miR‐29c‐3p, the binding site was analyzed using TargetScan bioinformatics tools. The predicted interaction between miR‐29c‐3p and the target site in the MMP2 3′‐UTR is illustrated in Figure 6A. To validate the direct targeting, the MUT and WT luciferase reporters were generated by cloning the mutated or WT binding sites in the 3′‐UTR of MMP2 into the luciferase reporter plasmid. After cotransfection with miR‐29c‐3p mimics in SKOV3 cells, a marked decrease in luciferase activity was observed in the WT group but not in the MUT group, indicating that MMP2 is a direct target gene of miR‐29c‐3p (Figure 6B). To further determine whether miR‐29c‐3p exerts its impact by targeting MMP2 in OC cells, MMP2 was knocked down with siRNA in SKOV3 and CAOV3 cells and cotransfected with miR‐29c‐3p inhibitors or NC inhibitors (Figure S6). Western blot analysis showed that inhibition of miR‐29c‐3p could partly rescue the expression of MMP2 in OC cells transfected with siRNA (Figure 6C). Moreover, the enhanced invasion ability of OC cells caused by miR‐29c‐3p inhibition was attenuated by MMP2 downregulation in vitro (Figure 6D,E). Overall, these findings indicated that miR‐29c‐3p can directly target MMP2, which could contribute to the effects of miR‐29c‐3p on OC metastasis.
FIGURE 6.
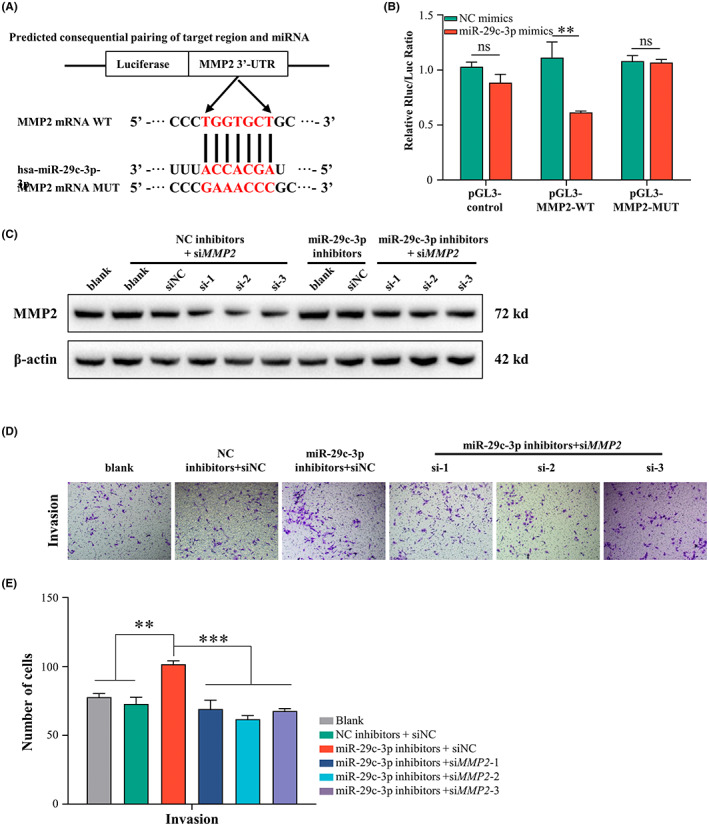
MicroRNA (miR)‐29c‐3p directly targets MMP2 to suppress ovarian cancer metastasis. (A) Schematic diagram of luciferase reporter plasmid constructed containing WT and mutant (MUT) binding sites in the MMP2 3′‐UTR. (B) Luciferase activity of WT or MUT plasmids after cotransfection with miR‐29c‐3p mimics into SKOV3 cells. Each experiment was conducted at least in triplicate. (C–E) SKOV3 cells after silencing MMP2 were transfected with inhibitors of miR‐29c‐3p or negative control (NC). (C) Western blot analysis of MMP2 in SKOV3 cells. (D) Representative images from invasion assays of SKOV3 cells. Scale bar, 50 μm. (E) Histograms showing the number of invasion cells of SKOV3. *p < 0.05; **p < 0.01; ***p < 0.01. ns, no significance
4. DISCUSSION
Implantation metastasis to the peritoneal organs is the most common form of OC metastasis. 24 As the major component of omental stroma cells, CAFs are accountable for tumor progression, including tumor cell growth, invasion, metastasis, and other malignant biological behaviors. 25 Accumulating studies have focused on the cross‐talk between cancer cells and CAFs, which participates in tumor metastasis, but the underlying mechanism for this process remains unclear. Our previous research found that fibroblasts could be activated by OC cells to promote OC metastasis. 18 However, the underlying mechanism by which CAFs promote OC metastasis needs clarification. In this research, we highlighted the role of omental CAF‐Exos in OC metastasis, which harbored significantly less miR‐29c‐3p than NF‐Exos and could increase MMP2 expression in OC cells, indicating that miR‐29c‐3p supplement could inhibit tumor dissemination in the peritoneal cavity.
As the dominating cells within the TME, CAFs play key roles in promoting or suppressing tumor progression and have attracted more and more attention in studies regarding tumor metastasis. In our study, we isolated patient‐derived primary NFs and CAFs and extracted their exosomes. In previous studies, CAFs were shown to communicate with tumor cells through exosomes in addition to classical signals such as cytokine secretion. 26 Therefore, we isolated exosomes from CAFs and NFs and found that CAF‐Exos could significantly promote OC metastasis compared to NF‐derived exosomes. Exosomes usually contain various types of bioactive molecules, such as nucleic acids and proteins, in which miRNAs are abundant and play an important role in cell communication. 27 We further found that lower miR‐29c was present in CAF‐Exos than in NF‐Exos and miR‐29c could be directly transferred to the recipient cancer cells from CAFs. Next, we showed that overexpressed miR‐29c‐3p could inhibit tumor cell migration and invasion by targeting MMP2 both in vitro and in vivo. These findings demonstrated the effects of the loss of miR‐29c in CAF‐Exos on OC cells. Yugawa et al. found that miR‐150‐3p was significantly reduced in CAF‐Exos and inhibited hepatocellular carcinoma migration and invasiveness. 28 In another study, miR‐148b, which inhibits endometrial cancer metastasis by directly binding to DNMT1, was significantly reduced in CAF‐Exos. 29 In addition to miR‐29c‐3p, miRNAs that are aberrantly expressed in CAF‐Exos require further study to elucidate their roles in the cross‐talk between CAFs and OC cells.
The miR‐29 family members have the dual characteristics of being oncogenic and tumor suppressors. 30 There are three mature members in the miR‐29 family, miR‐29a, ‐29b, and ‐29c, which are encoded by two gene clusters and share conserved sequences. In our study, OC tissues showed low levels of miR‐29c‐3p, and poor outcomes were associated with low levels of miR‐29c‐3p. In addition, the overexpression of miR‐29c‐3p restrained OC cell invasion and migration in vivo and in vitro. Furthermore, less miR‐29c‐3p was found in CAF‐Exos, which resulted in less miR‐29c‐3p transfer to target OC cells. MicroRNA‐29c‐3p has been found to play a tumor‐suppressive role in multiple cancers, such as colorectal cancer, 31 lung cancer, 32 breast cancer, 33 gastric cancer, 34 and pancreatic cancer. 35 Recent studies have reported that miR‐29c plays an important role in OC. For example, Deng et al. reported that miR‐29c improved the antitumor efficacy of natural killer cells by directly targeting B7‐H3 in vitro. 36 Researchers found that miR‐29c expression could be used as a biomarker to predict poor outcomes in OC patients in another study. 37 Hu et al. reported that the FOXP1/ATG14 pathway was downregulated when miR‐29c was overexpressed, which inhibited autophagy and cisplatin resistance in part. 38
A precondition of tumor metastasis is to break through the natural barrier of the ECM. In this study, MMP2 was verified as a target of miR‐29c‐3p. By binding to the 3′‐UTR region of MMP2 mRNA, miR‐29c‐3p could directly target MMP2 mRNA, resulting in a decrease in MMP2 protein levels and its enzymatic activities, thus inhibiting OC cell metastasis. Among the MMPs, MMP2 is capable of cleaving ECM molecules and signal transduction molecules, promoting the spread of tumors. It has been reported that MMP2 is involved in the metastasis of various cancers, such as breast cancer, 39 renal cell carcinoma, 40 esophageal squamous cell carcinoma, 41 and lung cancer. 42 In gastric cancer, MMP2 overexpression might be a predictive factor for poor prognosis. 43 Another study reported elevated expression of MMP2 in the placental tissues and serum of gestational trophoblastic disease patients. 44 It is worthy of note that the MMP2 expression in tumor cells was only partially rescued by miR‐29c‐3p, which could be due to multiple mechanisms involved in MMP2 expression regulation. At the same time, MMP2 inhibition could not completely rescue the effects of miR‐29c‐3p inhibitors on cell invasion, suggesting additional targets of miR‐29c‐3p might play a role. For example, Zhang et al.'s study showed that miR‐29c‐3p could inhibit the proliferation and migration of colorectal cancer by targeting SPARC. 31 Based on our findings, we proposed an omentum metastasis model in which the transformation from NFs to CAFs causes a reduction in antitumor factors such as miR‐29c‐3p. In this way, low levels of miR‐29c‐3p promote an aggressive phenotype in OC cells (Figure 7).
FIGURE 7.
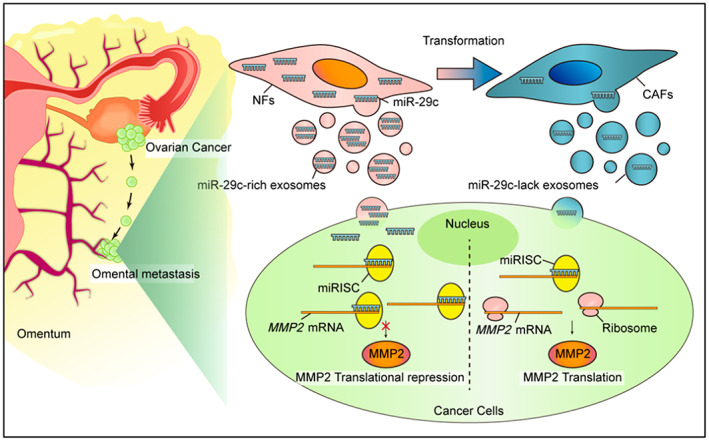
Schematic diagram of proposed mechanism. The conversion from normal fibroblasts (NFs) to cancer‐associated fibroblasts (CAFs) causes a reduction of microRNA (miR)‐29c‐3p. Subsequently, the low level of miR‐29c‐3p in the CAF‐derived exosomes contributes to the derepression of MMP2, which promotes the aggression of ovarian cancer cells. miRISC, miRNA‐containing RNA induced silencing complex
In recent years, the concept of precision medicine has promoted the progress of precise tumor diagnosis and treatment and provided more possibilities for improving the survival rate of tumor patients. In this study, we revealed that CAF Exo‐miR‐29c‐3p significantly inhibited tumor metastasis in orthotopic xenograft mice, and the treatment with CAF‐Exos clearly shortened the survival time of these mice. With their unique characteristics, exosomes are widely used in tumor diagnosis, prognosis prediction, curative effect prediction and dynamic monitoring, and accurate targeted drug delivery. A number of studies have shown that exosome vaccinations stimulate an immune response against tumors, indicating that exosomes can play a significant role in cancer treatment. 45 , 46 Exosomes can also be used to deliver non‐native therapeutics, another exciting application for therapeutic development. 47 Li et al. 48 demonstrated that extracellular vesicles derived from CAF loaded with miR‐195 could reduce tumor size, concentrate within tumors, and improve the survival of treated rats with cholangiocarcinoma.
In conclusion, our findings indicate the significant contribution of exosomes from omental CAFs to OC peritoneal metastasis and the potential role of the miR‐29c‐3p–MMP2 axis in the metastasis‐promoting effect of CAF‐Exo, providing novel insights into the mechanisms underlying OC metastasis by highlighting the roles of stromal‐derived exosomes.
AUTHOR CONTRIBUTIONS
Qing Han and Shuran Tan performed the experiments and wrote the paper. Lanqing Gong, Guoqing Li, and Qiulei Wu gave assistance in immunohistochemistry staining and animal experiments. Le Chen, Shi Du, and Xiaoli Liu collected tissues samples. Wenhan Li gave advice in bioinformatics analysis. Qing Han, Shuran Tan, Jing Cai, and Zehua Wang were responsible for the conception and design of experiments. All authors read and approved the final manuscript.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (NSFC) through Grant Nos. 81772781 and 81902665 and Education Commission of Hubei Province of China (Grant No. D20211206).
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest.
ETHICAL STATEMENT
Approval of the research protocol by an institutional review board: The protocol for the human study was reviewed and approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (IORG NO: IORG0003571).
Informed consent: Informed consent was obtained from all patients.
Registry and registration no. of the study/trial: N/A.
Animal studies: All animal experiments were approved by the Animal Management Committee of Tongji Medical College, Huazhong University of Science and Technology (IACUC Number: 2446).
Supporting information
Figures S1–S6
Tables S1–S4
Appendix S1
ACKNOWLEDGMENT
None.
Han Q, Tan S, Gong L, et al. Omental cancer‐associated fibroblast‐derived exosomes with low microRNA‐29c‐3p promote ovarian cancer peritoneal metastasis. Cancer Sci. 2023;114:1929‐1942. doi: 10.1111/cas.15726
Qing Han and Shuran Tan contributed equally to this work.
Contributor Information
Jing Cai, Email: jingcai@hust.edu.cn.
Zehua Wang, Email: zehuawang@163.net.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet (London, England). 2018;391:1023‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luo Z, Wang Q, Lau WB, et al. Tumor microenvironment: the culprit for ovarian cancer metastasis? Cancer Lett. 2016;377:174‐182. [DOI] [PubMed] [Google Scholar]
- 4. Pascual‐Antón L, Cardeñes B, Sainz de la Cuesta R, et al. Mesothelial‐to‐mesenchymal transition and exosomes in peritoneal metastasis of ovarian cancer. Int J Mol Sci. 2021;22:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bilbao M, Aikins JK, Ostrovsky O. Is routine omentectomy of grossly normal omentum helpful in surgery for ovarian cancer? A look at the tumor microenvironment and its clinical implications. Gynecol Oncol. 2021;161:78‐82. [DOI] [PubMed] [Google Scholar]
- 6. Liu T, Han C, Wang S, et al. Cancer‐associated fibroblasts: an emerging target of anti‐cancer immunotherapy. J Hematol Oncol. 2019;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang M, Chen Z, Wang Y, Zhao H, Du Y. The role of cancer‐associated fibroblasts in ovarian cancer. Cancers (Basel). 2022;14:2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yeung TL, Leung CS, Wong KK, et al. TGF‐β modulates ovarian cancer invasion by upregulating CAF‐derived versican in the tumor microenvironment. Cancer Res. 2013;73:5016‐5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yue H, Li W, Chen R, Wang J, Lu X, Li J. Stromal POSTN induced by TGF‐β1 facilitates the migration and invasion of ovarian cancer. Gynecol Oncol. 2021;160:530‐538. [DOI] [PubMed] [Google Scholar]
- 10. Li C, Teixeira AF, Zhu HJ, Ten Dijke P. Cancer associated‐fibroblast‐derived exosomes in cancer progression. Mol Cancer. 2021;20:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen B, Sang Y, Song X, et al. Exosomal miR‐500a‐5p derived from cancer‐associated fibroblasts promotes breast cancer cell proliferation and metastasis through targeting USP28. Theranostics. 2021;11:3932‐3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao Q, Huang L, Qin G, et al. Cancer‐associated fibroblasts induce monocytic myeloid‐derived suppressor cell generation via IL‐6/exosomal miR‐21‐activated STAT3 signaling to promote cisplatin resistance in esophageal squamous cell carcinoma. Cancer Lett. 2021;518:35‐48. [DOI] [PubMed] [Google Scholar]
- 13. Wortzel I, Dror S, Kenific CM, Lyden D. Exosome‐mediated metastasis: communication from a distance. Dev Cell. 2019;49:347‐360. [DOI] [PubMed] [Google Scholar]
- 14. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203‐222. [DOI] [PubMed] [Google Scholar]
- 15. Li YY, Tao YW, Gao S, et al. Cancer‐associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome‐mediated paracrine miR‐34a‐5p. EBioMedicine. 2018;36:209‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Au Yeung CL, Co NN, Tsuruga T, et al. Exosomal transfer of stroma‐derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo H, Ha C, Dong H, Yang Z, Ma Y, Ding Y. Cancer‐associated fibroblast‐derived exosomal microRNA‐98‐5p promotes cisplatin resistance in ovarian cancer by targeting CDKN1A. Cancer Cell Int. 2019;19:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cai J, Tang H, Xu L, et al. Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis. 2012;33:20‐29. [DOI] [PubMed] [Google Scholar]
- 19. Du S, Qian J, Tan S, et al. Tumor cell‐derived exosomes deliver TIE2 protein to macrophages to promote angiogenesis in cervical cancer. Cancer Lett. 2022;529:168‐179. [DOI] [PubMed] [Google Scholar]
- 20. Cai J, Gong L, Li G, Guo J, Yi X, Wang Z. Exosomes in ovarian cancer ascites promote epithelial‐mesenchymal transition of ovarian cancer cells by delivery of miR‐6780b‐5p. Cell Death Dis. 2021;12:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo J, Cai J, Zhang Y, Zhu Y, Yang P, Wang Z. Establishment of two ovarian cancer orthotopic xenograft mouse models for in vivo imaging: a comparative study. Int J Oncol. 2017;51:1199‐1208. [DOI] [PubMed] [Google Scholar]
- 22. Gyorffy B, Lánczky A, Szállási Z. Implementing an online tool for genome‐wide validation of survival‐associated biomarkers in ovarian‐cancer using microarray data from 1287 patients. Endocr Relat Cancer. 2012;19:197‐208. [DOI] [PubMed] [Google Scholar]
- 23. Micheal Wells J, Amit Gaggar J, Blalock E. MMP generated matrikines. Matrix Biol. 2015;44‐46:122‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biffi G, Tuveson DA. Diversity and biology of cancer‐associated fibroblasts. Physiol Rev. 2021;101:147‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer‐associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mori MA, Ludwig RG, Garcia‐Martin R, Brandão BB, Kahn CR. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019;30:656‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yugawa K, Yoshizumi T, Mano Y, et al. Cancer‐associated fibroblasts promote hepatocellular carcinoma progression through downregulation of exosomal miR‐150‐3p. Eur J Surg Oncol. 2021;47:384‐393. [DOI] [PubMed] [Google Scholar]
- 29. Li BL, Lu W, Qu JJ, Ye L, Du GQ, Wan XP. Loss of exosomal miR‐148b from cancer‐associated fibroblasts promotes endometrial cancer cell invasion and cancer metastasis. J Cell Physiol. 2019;234:2943‐2953. [DOI] [PubMed] [Google Scholar]
- 30. Kwon JJ, Factora TD, Dey S, Kota J. A systematic review of miR‐29 in cancer. Mol Ther Oncolytics. 2019;12:173‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang S, Jin J, Tian X, Wu L. hsa‐miR‐29c‐3p regulates biological function of colorectal cancer by targeting SPARC. Oncotarget. 2017;8:104508‐104524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arechaga‐Ocampo E, Lopez‐Camarillo C, Villegas‐Sepulveda N, et al. Tumor suppressor miR‐29c regulates radioresistance in lung cancer cells. Tumour Biol. 2017;39:1010428317695010. [DOI] [PubMed] [Google Scholar]
- 33. Li W, Yi J, Zheng X, et al. miR‐29c plays a suppressive role in breast cancer by targeting the TIMP3/STAT1/FOXO1 pathway. Clin Epigenetics. 2018;10:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu B, Chen X, Li J, et al. microRNA‐29c inhibits cell proliferation by targeting NASP in human gastric cancer. BMC Cancer. 2017;17:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu Y, Hu J, Sun W, Li S, Deng S, Li M. MiR‐29c inhibits cell growth, invasion, and migration of pancreatic cancer by targeting ITGB1. Onco Targets Ther. 2016;9:99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deng M, Wu D, Zhang Y, Jin Z, Miao J. MiR‐29c downregulates tumor‐expressed B7‐H3 to mediate the antitumor NK‐cell functions in ovarian cancer. Gynecol Oncol. 2021;162:190‐199. [DOI] [PubMed] [Google Scholar]
- 37. Feng S, Luo S, Ji C, Shi J. miR‐29c‐3p regulates proliferation and migration in ovarian cancer by targeting KIF4A. World J Surg Oncol. 2020;18:315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Hu Z, Cai M, Zhang Y, Tao L, Guo R. miR‐29c‐3p inhibits autophagy and cisplatin resistance in ovarian cancer by regulating FOXP1/ATG14 pathway. Cell Cycle (Georgetown, Tex). 2020;19:193‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dofara SG, Chang SL, Diorio C. Gene polymorphisms and circulating levels of MMP‐2 and MMP‐9: a review of their role in breast cancer risk. Anticancer Res. 2020;40:3619‐3631. [DOI] [PubMed] [Google Scholar]
- 40. Fan B, Niu Y, Ren Z, et al. Long noncoding RNA MMP2‐AS1 contributes to progression of renal cell carcinoma by modulating miR‐34c‐5p/MMP2 Axis. J Oncol. 2022;2022:7346460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Z, Wei Y, Li X, et al. IQGAP1 enhances cell invasion and matrix metalloproteinase‐2 expression through upregulating NF‐κB activity in esophageal squamous cell carcinoma cells. Gene. 2022;824:146406. [DOI] [PubMed] [Google Scholar]
- 42. Liu J, Ding D, Liu F, Chen Y. Rhein inhibits the progression of Chemoresistant lung cancer cell lines via the Stat3/snail/MMP2/MMP9 pathway. Biomed Res Int. 2022;2022:7184871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shen W, Xi H, Wei B, Chen L. The prognostic role of matrix metalloproteinase 2 in gastric cancer: a systematic review with meta‐analysis. J Cancer Res Clin Oncol. 2014;140:1003‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weng D, Han T, Dong J, et al. Angiogenin and MMP‐2 as potential biomarkers in the differential diagnosis of gestational trophoblastic diseases. Medicine (Baltimore). 2022;101:e28768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morse MA, Garst J, Osada T, et al. A phase I study of dexosome immunotherapy in patients with advanced non‐small cell lung cancer. J Transl Med. 2005;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Escudier B, Dorval T, Chaput N, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived‐exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940‐948. [DOI] [PubMed] [Google Scholar]
- 48. Li L, Piontek K, Ishida M, et al. Extracellular vesicles carry microRNA‐195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology (Baltimore, Md). 2017;65:501‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S6
Tables S1–S4
Appendix S1


