Summary
Currently approved COVID-19 vaccines administered parenterally induce robust systemic humoral and cellular responses. While highly effective against severe disease, there is reduced effectiveness of these vaccines in preventing breakthrough infection and/or onward transmission, likely due to poor immunity elicited at the respiratory mucosa. As such, there has been considerable interest in developing novel mucosal vaccines that engenders more localised immune responses to provide better protection and recall responses at the site of virus entry, in contrast to traditional vaccine approaches that focus on systemic immunity. In this review, we explore the adaptive components of mucosal immunity, evaluate epidemiological studies to dissect if mucosal immunity conferred by parenteral vaccination or respiratory infection drives differential efficacy against virus acquisition or transmission, discuss mucosal vaccines undergoing clinical trials and assess key challenges and prospects for mucosal vaccine development.
Keywords: Mucosal immunity, Vaccines, Adaptive immunity, Respiratory viruses
Introduction
The essential function of the respiratory tract in oxygen exchange leaves this expansive mucosal surface susceptible to both exposure to and infection with respiratory pathogens. For viruses such as measles, influenza and SARS-CoV-2, the ability to facilitate transmission via exhaled droplets or aerosols can drive rapid spread through susceptible human populations. As such, there is a clear and current interest in rethinking traditional vaccination paradigms, which have focussed on generating strong systemic antibody and cellular immunity, and instead seeding immunity more proximal to the mucosa at risk. So called “mucosal vaccine” strategies, which generally rely upon delivery of replicating viral vectors to the respiratory mucosa, have been utilised for many decades. Nevertheless, the case for optimal usage for such vaccines still remains unclear and comprehensive demonstration of protective superiority to parenteral delivery remains elusive. Here, we review adaptive immunity at mucosal sites, comparative elicitation by parenteral versus mucosal delivery, and the challenges and opportunities for mucosal vaccine development for SARS-CoV-2.
Localised immunity at mucosal surfaces
Mucosal surfaces comprise a physical barrier against exogenous antigens and pathogens and are safeguarded by the mucosal immune system, which consists of innate and adaptive immune components. Innate immune mechanisms provide multiple layers of barrier protection to prevent viral infection. These include physical factors such as mucins, polymeric glycoproteins produced by goblet cells, and a plethora of other anti-microbial compounds that are secreted by epithelial cells including lysozymes, proteolytic enzymes, specific protease inhibitors, reactive oxygen species; all of which contributes to enhanced opsonisation and clearance of exogenous agents.1 In addition, a range of innate immune cells, including macrophages, dendritic cells and natural killer cells can either directly phagocytose pathogens or alternatively recognise conserved structures on viral surfaces through membrane bound and intracellular receptors to initiate signalling cascades that promote anti-viral responses, including cytokines such as interferons, chemokines and the upregulation of co-stimulatory molecules to coordinate adaptive immune responses.2
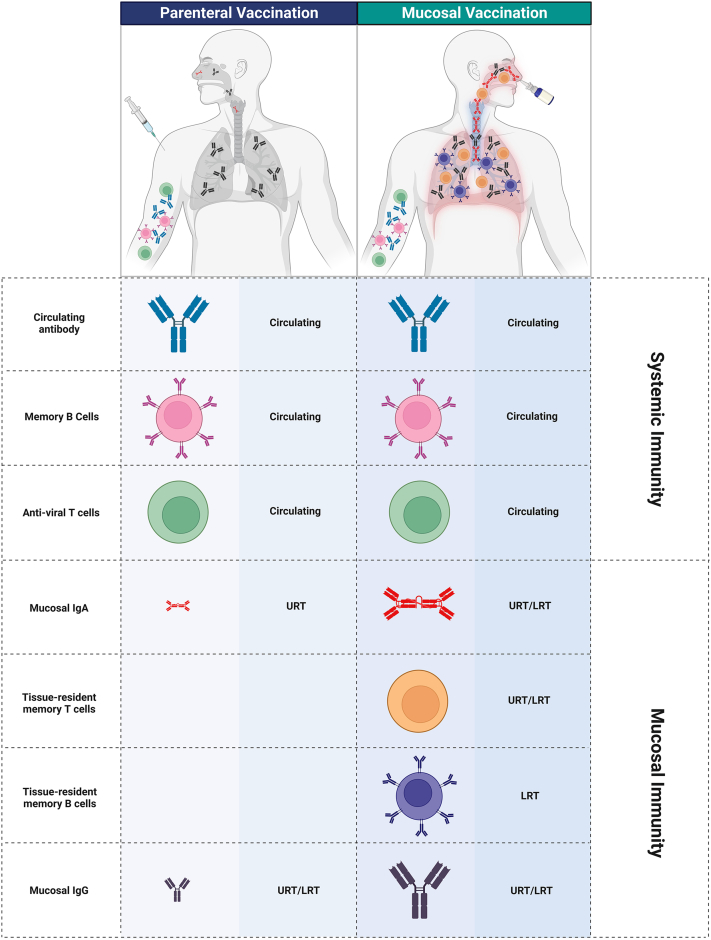
In terms of adaptive immunity, antigen recognition and processing sites can be initiated within proximal lung-draining lymph nodes and non-encapsulated lymphoid follicles, the latter defined as mucosal-associated lymphoid tissue (MALT) embedded in the mucosa and submucosa. MALTs in the upper respiratory tract include nasopharynx associated lymphoid tissues (NALTs), which is the rodent equivalent of Waldeyer's ring that includes the adenoids or nasopharyngeal tonsils, the palatine tonsils, and the bilateral lingual tonsils in humans, and broncho-associated lymphoid tissues (BALT) in the lower respiratory tract (LRT). While these mucosal sites can mount robust local adaptive responses, comprising tissue-resident memory T and B cells, and localised antibodies, the ability of mucosal-targeted vaccines to prime durable and robust mucosal immunity to curb respiratory virus infection and/or transmission remains unclear. We focus upon these adaptive immune arms below to better understand their contributions to mucosal immunity and how these pathways can potentially be elicited by vaccines delivered via the respiratory tract (Fig. 1).
Fig. 1.
Differential immune outcomes of parenteral and mucosal vaccines. Intramuscular administration of vaccines elicits predominantly systemic responses involving high levels of circulating anti-viral T cells, memory B cells and antibodies, with a minor proportion of mucosal secretory IgA detected due to transportation across mucosal epithelia. In contrast, mucosal vaccination induces both systemic and mucosal antibody responses. Mucosal vaccination promotes retention of memory B and T cells within mucosal associated lymphoid tissues in the upper (URT) and lower respiratory tract (LRT), providing niches for local antigen encounter and rapid recall responses. Created withBioRender.com.
Mucosal antibodies
A major effector molecule at mucosal sites is antibody, which has two major sources, translocation of circulating IgG to the mucosa, and local production of IgA. Although IgG is the most abundant isotype in the blood and the lower respiratory tract, this is reversed in the secretions of the upper respiratory tract where IgA can be as much as 3-fold enriched compared to IgG.3,4 Mucosal IgG is typically derived via transudation from the plasma but can be locally produced by mucosal B cells in the lamina propria that constitutively secrete IgG and other immunoglobulin subclasses. In addition to direct neutralisation of viruses, non-neutralising antibodies can also mediate clearance of virus and virally infected cells via interactions of the antibody Fc domain with complement,5 or with Fc-gamma receptors (FcγR) of effector cells enabling antibody-dependent cellular cytotoxicity (ADCC) or antibody-dependent cellular phagocytosis (ADCP).6 Recently, survival following moderate-severe SARS-CoV-2 infection has been correlated to antibody responses with robust Fc effector activity7 suggesting such immunity might contribute to protection against respiratory disease.
IgA can be expressed at mucosal surfaces in both monomeric or dimeric secretory IgA (sIgA) forms, and in humans, is found in two isotypes, with IgA1 present in both systemic and mucosal secretions and IgA2 predominantly in the mucosa.8 IgA displaying B cells arrive at tissues where they differentiate into IgA secreting plasma cells. Monomeric IgA are linked together by J-chains to form polymeric IgA (pIgA), which can be transported to the luminal side of the epithelial cells by the poly-Ig receptor (pIgR) expressed on the basal membrane side of epithelial cells.9 Part of the pIgR is digested on the luminal side, leading to the formation of sIgA. The extracellular secretory component derived from pIgR confers resistance against degradation by proteases in mucosal secretions and aids in release of antibody complexes transported through epithelial cells. Viruses opsonised by sIgA are eliminated from the upper respiratory tract through mucociliary clearance. The higher order polymeric structures of sIgA have been suggested to confer greater avidity and increased neutralisation capacity in comparison to IgG.10
Both parenteral and mucosal vaccination induce robust levels of serum IgG, which in turn can be transported to mucosal surfaces such as the lower respiratory tract.3,11 While elicitation of sIgA at oral and nasal mucosal surfaces following intramuscular vaccination has been reported in both clinical and animal studies for influenza11 and SARS-CoV-2,12 titres tend to be modest and variable.13 In contrast, mucosal immunisation readily elicits robust sIgA responses at the mucosa of the upper and lower respiratory tracts.14,15
Tissue resident memory lymphocytes
A subset of memory lymphocytes (T and B cells), known as tissue-resident memory cells, reside as stable populations within non-lymphoid barrier tissues, such as skin, lungs and intestine, and in non-barrier tissues including the brain and liver. Thought to act as sentinels, tissue-resident memory cells provide rapid recall of localised immunity in response to secondary exposure to pathogens at these tissue sites.
Unlike circulating memory T cells found within the bloodstream and lymphoid sites, tissue-resident memory T cells (TRM) are maintained within peripheral tissues following respiratory infection or mucosal vaccination. Canonical CD8+ TRM are primarily defined by the co-expression of markers CD69, CD103, CXCR3 and downregulation of CCR7 and CD62L, with concurrent downregulation of S1PR1 function that alters cellular chemotaxis and allows for tissue retention.16,17 CD8+ TRM are found throughout the respiratory tract after viral infection, including the airways, parenchyma, and associated lymph nodes.18,19 Due to their anatomical positioning, CD8+ TRM are mobilised more rapidly upon antigen re-exposure compared to circulating CD8 T cells, and facilitate viral clearance via robust IFNγ, TNFα, and IL-2 cytokine production, and cytolytic effector granzyme B molecules.20 Secondary to direct viral clearance, CD8+ TRM can trigger an organ-wide antiviral state by cytokine-mediated activation of local adaptive and innate immunity.
CD4+ TRM cells are less well defined than CD8+ TRM due to the heterogeneity of these cell types, but are more abundantly found as compared to CD8+ TRM.21 Following pulmonary infection, CD4+ TRM expressing CD69 occupies niches around airways and within inducible bronchus-associated lymphoid tissues (iBALT) structures, characterised as clusters of B and T cell areas embedded in a network of stromal cells, follicular dendritic cells, antigen presenting cells and high endothelial venules.22 In murine studies, adoptively transferred lung CD4+ TRM confer protection against influenza challenge23 with accelerated viral clearance potentially linked to IFNγ secretion supporting development of CD8+ TRM within the lung microenvironment.24 Additionally, a subset of resident BCL6+/PSGL1lo/FR4hi CD4 T follicular helper-like cells were recently discovered, which upon their reactivation directly support B cells co-localised in iBALT for differentiation into antibody secreting cells and lung antibody production.25,26
Tissue resident memory B cells (BRM) are long-lived, quiescent cells maintained within mucosal tissues following infection. In the lower respiratory tract, lung BRM can be located within iBALTs or throughout the lung parenchyma within proximity to alveoli, independent of iBALT.27,28 In mice, lung BRM phenotypically express CXCR3, CCR6 and CD69, while downregulating CD62L, and are transcriptionally and functionally distinct to their circulating and splenic counterparts.29 Unlike circulating counterparts, BRM exhibit tissue probing behaviour and differentiate into plasma cells upon antigen encounter, contributing to increased local antibody production to drive accelerated pathogen clearance.28 Adoptive transfer studies show that lung BRM reduces viral titres in the lower respiratory tract compared to memory B cells isolated from spleens.30 In addition to antigen-specific BRM, bystander BRM populations provide a secondary function by retaining and presenting exogenous antigens in the form of immune-complexes.31
Analogous to mucosal antibodies, tissue-resident lymphocytes in the respiratory mucosa are preferentially elicited by mucosal vaccination and are low or absent following parenteral immunisation, demonstrated in animal models for influenza32 and SARS-CoV-2.13 Similarly, SARS-CoV-2 infection in humans robustly induces lung-resident T and B cells, while comparatively little to no lung-resident lymphocytes are detectable in SARS-CoV-2-vaccinated individuals with robust serological responses but no prior history of infection.33,34
The case for mucosal vaccines for SARS-CoV-2
First-generation COVID-19 vaccines have been highly effective in mitigating severe illness, hospitalisations, and deaths. Neutralising antibodies directed against the viral spike are thought to mechanistically underpin observed vaccine protection against acquiring SARS-CoV-2 infection or developing severe disease for COVID-19. However, rapid waning of immunity has been observed after vaccination,35 necessitating the implementation of boosters to maintain or increase immunity. In addition to this, the emergence of novel variants of concern (VOCs) that possess enhanced transmissibility and immune evasion capabilities, has led to significant erosion in the efficacy of currently licensed vaccines to curb viral transmission. Vaccination via mucosal routes has been widely proposed as a pathway to strengthen vaccine protection against viral transmission, with the hypothesis that localised respiratory mucosal immunity mediates stronger protection against acquiring infection, or alternatively, limiting the onward transmission to new hosts. While data from actual mucosal COVID-19 vaccines in humans is currently absent, we can examine immunity induced by primary SARS-CoV-2 infection for indicators that protective immunity induced via mucosal antigen exposure could be qualitatively different to parenterally administered vaccines, and if this drives differential efficacy against acquiring or transmitting SARS-CoV-2.
The biogenesis of adaptive immunity at mucosal surfaces after recovery from SARS-CoV-2 infection has been well established in animal models and human clinical studies. In convalescent individuals, elevated concentrations of airway immunoglobulins, particularly IgA,12,33 and the seeding of tissue resident B and T cells33,36 into the lung have been reported. In contrast, in non-infected but vaccinated individuals, such mucosal responses are poorly elicited or absent.33,34,37 Importantly, while immunity gained by either infection or vaccination provides durable protection against hospitalisation or death following COVID-19, there are epidemiological indicators that previous infection might provide improved protection against viral transmission. In a longitudinal prospective cohort from Qatar, immunity gained from infection over 300 days prior provided effective protection against symptomatic Omicron BA.1 (50.2%) and BA.2 (46.1%) infection comparable to protection after 3 vaccine doses (52.2%), while a 2-dose vaccination regimen show negligible effectiveness by 6 months after the second dose for BA.1 (−4.9%) and BA.2 (−1.1%).38 A recent systematic review by the COVID-19 Forecasting Team also show durable protection from prior infection against pre-omicron variants at 85.2% at 4 weeks with a modest decline to 78.6% at 40 weeks and 55.5% at 80 weeks.39 Similar levels of durable protection have been reported in Denmark40 and Sweden41 cohorts, and prior infection providing durable reductions in the rates of re-infection in health care workers42 or prison populations43 undergoing surveillance testing for SARS-CoV-2 infection.
Neutralising antibodies in the blood are a clear correlate of the protective efficacy of vaccination,35,44 and while respiratory infection clearly seeds mucosal immunity, it remains to be demonstrated that mucosal effectors are analogous correlates of protection against re-infection. In general, serological levels of neutralising antibodies are lower in unvaccinated convalescent individuals than in individuals with 2 or 3 doses of approved COVID-19 vaccines, yet epidemiological protection against symptomatic infection appears similar.38 This observation suggests immunity uniquely elicited by infection augments protection against either acquiring re-infection, or alternatively developing symptoms during reinfection. While the greater magnitude and breadth of memory T cell responses seeded by prior infection likely act to limit disease severity,45 it is also plausible that additional immune effectors at mucosal sites could directly limit acquisition. Two recent studies have suggested that concentrations of serum and mucosal sIgA were inversely associated with the risk of breakthrough infection, suggesting mucosal antibodies are actively contributing to barrier protection.12,46 However, there remains a lack of clarity around (i) which specific mucosal effectors are mediating protection and (ii) if analogous mucosal responses can be elicited by vaccination instead of infection.
Reductions in transmission from mucosal immunity might also be achieved by blockade of onward transmission. Individuals with immunity from either prior infection or vaccination display lower viral load during breakthrough infections, potentially indicative of a reduced capacity to transmit.47 However, individuals previously infected show more durable control of virus when compared for time since vaccination or infection48 In contrast to these differences observed in upper respiratory viral loads, household- or close-contact studies appear to indicate little to no impact on onward transmission from prior immunity. In SARS-CoV-2 surveillance of 35 California state prisons, vaccination or prior-infection alone showed comparable reduction in risk of transmission to close-contacts.49 This was mirrored in a household transmission study showing upon breakthrough infection, incidences of onward transmission were equally as likely from individuals that were previously infected or not.50 Taken together, these epidemiological findings indicate that mucosal responses seeded by prior infection can mediate durable protection against breakthrough infections but are unlikely to impede onward transmission upon acquisition of infection. Moreover, these findings provide a proxy to assess responses by mucosal vaccine candidates.
Mucosal vaccines against SARS-CoV-2 in clinical development
It has been well established that parenteral vaccination is relatively ineffective at establishing or boosting mucosal immunity without prior mucosal priming events34,51,52 Viral-vectored vaccines have traditionally been favoured for mucosal immunisation due to relative ease of production and natural tropism for delivery to the mucosa. Diverse platforms are being explored as mucosal vaccines against SARS-CoV-2, with many showing promising pre-clinical efficacy (reviewed in53, and several advancing to human clinical trials (Table 1).
Table 1.
Mucosal vaccines for SARS-CoV-2 undergoing clinical evaluation or approved for emergency use.
| Vaccine platform | Vaccine candidate name | Vaccine description | Developers | Country | Phase | Identifier |
|---|---|---|---|---|---|---|
| Viral Vector (Replicating) | NDV-HXP-S | Newcastle Disease Virus expressing trimerised spike | Sean Liu, Icahn School of Medicine at Mount Sinai | USA | Phase 2/3 | NCT05354024 |
| Viral Vector (Non-replicating) | BBV154 | Adenoviral vector expressing WA1 spike | Bharat Biotech International Limited | India | Approved (India) | |
| CVXGA1/PIV5-SARS-CoV-2 | Parainfluenza virus 5 expressing WA1 spike | CyanVac LLC | USA | Phase 1 | NCT04954287 | |
| SC-Ad6-1 | Adenoviral vector expressing spike | Tetherex Pharmaceuticals Corporation | USA | Phase 1 | NCT04839042 | |
| ChAdOx1/AZD1222 | Adenoviral vector expressing harbouring DNA encoding spike | University of Oxford and AstraZeneca Biopharmaceuticals | UK | Phase 1 | NCT04816019 | |
| Ad5-nCoV-IH (Convidecia Air) | Adenoviral vector expressing WA1 spike | CanSinoBio | China | Approved (China, Morocco) | ||
| Sputnik V/Gam-COVID-Vac | Adenoviral vector | The Gamaleya Research Institute of Epidemiology and Microbiology | Russia | Approved | ||
| Live attenuated virus | CoviLiv | Attenuated SARS-CoV-2 WA1 strain | Codagenix/Serum Institute of India | India | Phase 3 | ISRCTN15779782 |
| Live attenuated virus-vector | DelNS1-2019-nCoV-RBD-OPT1 | Replication deficient Influenza A (CA4-DelNS1) virus expressing RBD domain of spike protein | University of Hong Kong, Xiamen University and Beijing Wantai Biological Pharmacy | China | Phase 3 |
ChiCTR2100051391 PACTR202110872285345 |
| MV-014-212 | Respiratory Syncytial virus expressing spike | Meissa Vaccines, Inc | USA | Phase 1 | NCT04798001 | |
| hAd5-S-Fusion + N-ETSD | Human adenovirus serotype 5 expressing spike and nucleocapsid | ImmunityBio Inc | USA | Phase 2 | NCT04591717 | |
| Protein subunit | CIGB-669 (RBD + AgnHB) (Mambisa) | RBD adjuvanted with aluminium hydroxide | Center for Genetic Engineering and Biotechnology (CIGB) | Cuba | Phase 2 | RPCEC00000345 |
| ACM-SARS-CoV-2- beta ACM CpG vaccine candidate (ACM-001) | Spike encapsulated by an artificial cell membrane | ACM Biolabs | Singapore | Phase 1 | NCT05385991 | |
| RAZI-COV PARS | Recombinant spike protein | Razi Vaccine and Serum Research Institute | Iran | Approved (Iran) |
Researchers in Beijing developed a live attenuated influenza vector harbouring SARS-CoV-2 RBD (designated CA4-dNS-nCoV-RBD or dNS1-RBD).15 A prime-boost vaccine regimen was shown to elicit lung localised RBD-specific T cell responses, as well as moderate levels of RBD-specific IgA and IgG responses in bronchoalveolar lavage (BAL) fluid of BALB/c mice. Furthermore, dNS1-RBD provided protection following experimental challenge with SARS-CoV-2 Omicron, preventing severe disease and reduction in viral loads in golden Syrian hamsters. In a randomised, double-blind, placebo-controlled phase 2 trial, dNS1-RBD elicited systemic T cell and RBD-specific IgG responses in approximately 40% and <22% of vaccine recipients, respectively, while mucosal responses were relatively weaker with less than 13% of vaccine recipients eliciting mucosal sIgA despite being well tolerated.54
MV-014-212 intranasal COVID-19 vaccine developed by Meissa Vaccines is a live-attenuated chimeric human respiratory syncytial virus expressing the SARS-CoV-2 spike. In pre-clinical non-human primates testing, MV-014-212 elicited approximately 8- and 2-fold increases in nasal IgA and serum IgG anti-spike responses, respectively, compared to vehicle controls. While correlation analysis of mucosal IgA and protection of vaccinated animals were not performed, infectious viral titres were 1000-fold lower in nasal swabs or BAL samples of vaccinated animals following experimental challenge.14 Subsequent Phase I clinical data have reported that a single dose of vaccine elicits a nasal IgA response comparable to that induced by natural infection.55
NDV-HXP-S, also referred to as Patria/ADAPTCOV/COVIVAC, is a Newcastle disease virus expressing Hexapro-stabilised spike protein on the virion surface.56 Similar to influenza virus, NDV-HXP-S is produced in embryonated chicken eggs. The vaccine has been assessed as an inactivated intramuscularly administered or intranasally administered live viral vector in a number of preclinical models.57, 58, 59 The live viral vector has been shown to induce potent serum IgG with cross-neutralisation capabilities. Challenge studies in mice and hamsters have shown that live NDV-HXP-S provides protection by reducing viral titres in the lungs and viral shedding. Clinical trials for NDV-HXP-S are ongoing with interim results from multiple phase I trials showing that the live viral vector is immunogenic and safe.60 Phase I clinical examination contrasting intranasal, intramuscular, or combined intranasal/intramuscular administration routes have also been conducted, although this study did not assess mucosal responses (NCT05181709).61
The widely deployed Oxford/AstraZeneca vaccine (ChAdOx1) based on the chimpanzee adenovirus platform was clinically assessed using intranasal delivery in cohorts of vaccine-naïve, or individuals previously vaccinated twice with intramuscular ChAdOx1 or BNT162b2.62,63 While well tolerated, weak and inconsistent elicitation of mucosal IgA or IgG against spike was observed in both cohorts, with corresponding serum IgA and IgG poorly boosted. A similar Ad5-vectored intranasal SARS-CoV-2 vaccine developed by Altimmune was discontinued after Phase I trials due to poor immunogenicity outcomes.64
Four mucosal vaccines have been approved by regulators and/or deployed, albeit with scarce public data to date supporting efficacy. CanSinoBio Biologics Convidecia Air™ (Ad5-nCoV-IH) consists of the same adenoviral vector delivering SARS-CoV-2 spike approved for intramuscular delivery, however reformulated to be aerosolised using a nebuliser and orally delivered.65 Initial clinical testing for safety and immunogenicity suggested boosting with aerosolised Ad5-nCoV could efficiently recall systemic antibody and cellular immunity. In individuals previously vaccinated with two doses of CoronaVac, boosting with aerosolised Ad5-nCoV resulted in elevated neutralising antibodies when compared to those who received three doses of the intramuscular vaccine.66 While the extent of mucosal immunity and protective efficacy of this platform remain to be demonstrated, rollout commenced into the general population of China in September 2022 and Morocco in November 2022.
Bharat Biotech's iNCOVACC (BBV154) is a recombinant replication-deficient chimpanzee adenovirus vectored vaccine expressing pre-fusion stabilised spike protein67 formulated to be delivered via intranasal drops. In non-human primate studies, the vaccine demonstrated immunogenicity and some capacity for non-sterilising protection via reductions in viral replication post–challenge.13 Phase III trials were conducted in approximately 3100 subjects in direct comparison to whole inactivated virus-based COVID-19 vaccine Covaxin (NCT05522335). A recent preprint study reports detection of spike-specific sIgA in saliva concomitant with significantly elevated IgA secreting plasma blasts 14 days after receiving a second dose of iNCOVACC.68 Beginning January 2023, iNCOVACC has been approved as a booster vaccine via intranasal delivery in India.
Two further vaccines, RAZI-COV PARS, a recombinant SARS-CoV-2 spike protein administered as a nasal spray made by Iranian Razi Vaccine and Serum Research Institute, and an intranasal version of the Russian Sputnik V adenoviral vaccine have also been reported to be approved for human use, however lacking public clinical data on mucosal responses elicited.
Challenges for mucosal vaccine development
There are significant challenges that should temper expectations about the protective utility of mucosal vaccines. Firstly, given rapid global spread, more than half the global population69 have been infected during sequential ancestral, Delta and Omicron waves, meaning a large majority of people should already possess a degree of mucosal immunity. In this background, the usage case for mucosal vaccines becomes unclear, with potential benefits potentially already baked in. Another challenge to effective mucosal vaccines is the ability to elicit durable mucosal responses, with studies showing airway IgA rapidly waning between 3 and 9 months after recovery from hospitalisation for COVID-19.52 These mucosal responses are even less robust and durable with infections within the mild-moderate spectrum,70 thus highlighting a high barrier (i.e., severe infection) imposed for the elicitation of effective and long-lived mucosal responses in the general population.
Some encouragement comes from observations in pre-clinical models, where mucosal vaccination of animals with pre-established immunity from parenteral immunisation was able to induce and redirect SARS-CoV-2 spike immunity into the lungs, leading to superior protection against experimental challenge.71 Similar heterologous immune exposure history in humans (so-called “hybrid immunity”) has also been well established to be highly immunogenic72,73 and drives strong protection in epidemiological studies against acquiring SARS-CoV-2 infection.74 Therefore, mucosal vaccines may provide a tractable pathway to extend systemic immunity to the mucosa without risking poor clinical outcomes from infection, despite the reported mildness of most vaccine breakthrough infections with Omicron.75
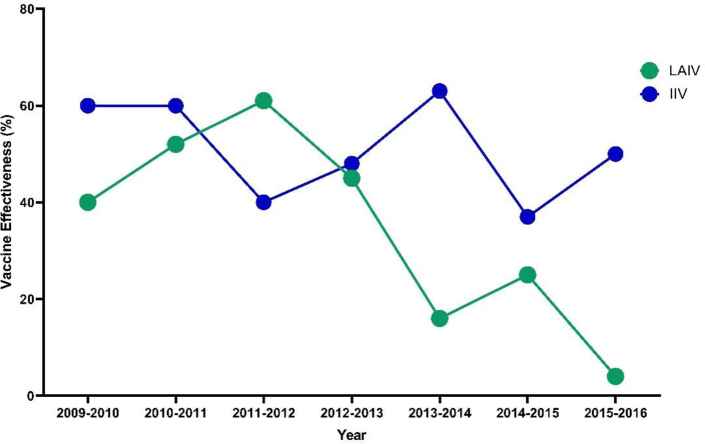
A second consideration is the past experiences with live-attenuated influenza vaccines (LAIV) such as Flumist. Flumist (sold as Fluenz Tetra in Europe) was first licensed in the USA in 2003 and has been used to deliver 116 million doses of seasonal influenza vaccine globally.76 As a nasally delivered replicating viral vector, LAIV can efficiently induce both systemic and mucosal immunity, specifically sIgA77,78 and resident memory T cells.32 However, in terms of vaccine effectiveness, despite some indications of superior protection against influenza B in children79 LAIV generally does not surpass protection observed with comparator inactivated influenza vaccines delivered parenterally, and for many past seasons has been inferior as shown in Fig. 2.81, 82, 83 The drivers of underperformance of LAIV are not well understood but may relate to a combination of viral production issues and impacts from baseline population immunity against the vector. Notably, anti-vector immunity is likely to similarly confound mucosal COVID-19 vaccines based upon LAIV or other viruses with high seroprevalence in humans such as adenovirus serotype 5. To mitigate confounding of viral vector-based vaccines by host immunity, synthetic mRNA, recombinant proteins, naturally occurring polymers such as chitosan, liposomes and emulsions are being explored as alternative mucosal vaccine platforms (reviewed in84).Vaccine viral vectors, such as LAIV or ChAdOx1 nCoV-19, commonly incorporate genetic features that attenuate or render them replication-defective for safety purposes. While vector safety remains a priority, the restricted replication of these vectors in vivo highlights a “Goldilocks” conundrum, whereby robust and durable mucosal immunity may require sufficient vector replication and antigen expression, which correspondingly would increase the potential risk of adverse outcomes with use of more replicative vectors. Given intranasal delivery is a far more practical immunisation approach (e.g., nasal sprays), one way to circumvent poor immunogenicity of these attenuated vectors is to incorporate multi-dose regimens to potentially amplify the immune response. There is an increasing body of work highlighting that continual or escalating antigen delivery within an acute timeframe can significantly improve germinal centre responses but have so far been tested only by traditional parenteral routes of vaccination.85
Fig. 2.
Vaccine effectiveness of licensed influenza vaccines; live attenuated influenza vaccines (LAIV) and inactivated influenza vaccine (IIV) from 2009 to 2016. Data compiled from Centers for Disease Control and Prevention.80
Finally, we need to consider if our expectations of vaccinations are simply too high? It is notable that a vaccine has never been developed that engenders sterilising immunity where recovery from infection fails to. With regards to respiratory infections, human populations remain susceptible to recurrent lifetime infections with the same pathogen. Near universal childhood infection with endemic coronaviruses (HCoV OC43, NL63, HKU1 and 229E) renders most populations seropositive86,87 however this background immunity largely fails to prevent recurrent re-infections during adulthood.88 Similarly, adults experience recurrent periodic infections with seasonal influenza89 and respiratory syncytial virus (RSV),90 with high prevalence and notable asymptomatic infection observed within adults during seasonal outbreaks.91 In all cases, re-infection is generally associated with acute symptoms limited to the upper respiratory tract suggesting prior immunity can efficiently limit disease severity, but not halt acquisition effectively. Nevertheless, it remains possible that mucosal immunity is manifestly incapable of conferring sufficient and long-lasting protection to the extensive respiratory tract to prevent viral infection.
Conclusions and outstanding questions
The emergence of successive SARS-CoV-2 variants and common occurrences of breakthrough infections despite widespread vaccine uptake has underscored the necessity for developing next generation vaccines that improve upon traditional parenteral approaches. While mucosal vaccines have thus far been recognised for their safety, ease of administration and relative cost-effectiveness, their demonstration of augmented mucosal immunity, and more importantly, convincing improvements in protective efficacy against viral acquisition and/or transmission remains elusive.
For mucosal vaccines to be successfully implemented, there is a need to:
-
•
Identify mucosal immune correlates that prevent acquisition and onward transmission of infection. A key challenge is the reliability of mucosal tissue sampling. While nasal/salivary sampling from the upper respiratory tract is practical, sampling the lower respiratory tract (BAL, tracheal aspirates) is challenging. In conjunction, establishing systemic immune correlates that are concordant with robust mucosal responses might further aid our ability to rapidly assess the efficacy of mucosal vaccines.
-
•
Clarify the extent to which anti-vector responses will curtail vaccine immunogenicity, given that the current landscape of mucosal vaccines in development predominantly utilise viral vectors. In conjunction, diversification of mucosal vaccine delivery platforms should be pursued, including advances to lipid formulations to potentially unlock mucosal delivery of next-generation mRNA vaccines.
-
•
Deconvolute the protective contribution of antibodies (IgG, IgA), memory T cells and memory B cells at the mucosa to enable rational vaccine design to maximise protective mechanisms in vivo.
-
•
Assess mucosal vaccination delivery modalities (i.e., nasal sprays, inhalation, nebulisers) and formulation (i.e., dry powder, liquid-jet) in driving the magnitude and distribution of mucosal immune responses elicited by mucosal vaccines.
-
•Incorporate non-surface glycoprotein targets (i.e., nucleocapsid, RdRp) that are less prone to genetic drift to improve vaccine efficacy by broadening humoral and cellular immune responses.
Search strategy and selection criteria.
Data for this Review were identified by searches of PubMed and references from relevant articles using the search terms “mucosal,” “immunity”, “SARS-CoV-2,” “COVID-19,” “intranasal,” and “vaccines”. Data was also collected from submitted but not yet peer-reviewed articles collected from bioRxiv and medRxiv as well as non-peer reviewed data from WHO, CDC, COVID-19 vaccine tracker and ClinicalTrial.Gov websites.
Contributors
D.P wrote the original draft, A.K.W. and H-X.T revised and edited the manuscript. All authors read and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
D.P acknowledges the financial support from Australian Government Research Training Program Scholarship. A.K.W. and H-X.T are supported by NHMRC fellowships. Funders were not involved in the decision to submit this review for publication or in the writing of this review.
References
- 1.Linden S.K., Sutton P., Karlsson N.G., Korolik V., McGuckin M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1(3):183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dwivedy A., Aich P. Importance of innate mucosal immunity and the promises it holds. Int J Gen Med. 2011;4:299–311. doi: 10.2147/IJGM.S17525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Twigg H.L., 3rd Humoral immune defense (antibodies): recent advances. Proc Am Thorac Soc. 2005;2(5):417–421. doi: 10.1513/pats.200508-089JS. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds H.Y. Immunoglobulin G and its function in the human respiratory tract. Mayo Clin Proc. 1988;63(2):161–174. doi: 10.1016/s0025-6196(12)64949-0. [DOI] [PubMed] [Google Scholar]
- 5.Mellors J., Tipton T., Longet S., Carroll M. Viral evasion of the complement system and its importance for vaccines and therapeutics. Front Immunol. 2020;11:1450. doi: 10.3389/fimmu.2020.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang A., Stacey H.D., D'Agostino M.R., Tugg Y., Marzok A., Miller M.S. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat Rev Immunol. 2022:1–16. doi: 10.1038/s41577-022-00813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zohar T., Loos C., Fischinger S., et al. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell. 2020;183(6):1508–1519.e12. doi: 10.1016/j.cell.2020.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steffen U., Koeleman C.A., Sokolova M.V., et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun. 2020;11(1):120. doi: 10.1038/s41467-019-13992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woof J.M., Russell M.W. Structure and function relationships in IgA. Mucosal Immunol. 2011;4(6):590–597. doi: 10.1038/mi.2011.39. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T., Kawaguchi A., Ainai A., et al. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc Natl Acad Sci U S A. 2015;112(25):7809–7814. doi: 10.1073/pnas.1503885112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brokstad K.A., Cox R.J., Olofsson J., Jonsson R., Haaheim L.R. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis. 1995;171(1):198–203. doi: 10.1093/infdis/171.1.198. [DOI] [PubMed] [Google Scholar]
- 12.Sheikh-Mohamed S., Isho B., Chao G.Y.C., et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022;15(5):799–808. doi: 10.1038/s41385-022-00511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan A.O., Feldmann F., Zhao H., et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep Med. 2021;2(4) doi: 10.1016/j.xcrm.2021.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tioni M.F., Jordan R., Pena A.S., et al. Mucosal administration of a live attenuated recombinant COVID-19 vaccine protects nonhuman primates from SARS-CoV-2. NPJ Vaccines. 2022;7(1):85. doi: 10.1038/s41541-022-00509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J., Wang P., Yuan L., et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci Bull. 2022;67(13):1372–1387. doi: 10.1016/j.scib.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skon C.N., Lee J.Y., Anderson K.G., Masopust D., Hogquist K.A., Jameson S.C. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14(12):1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar B.V., Ma W., Miron M., et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 2017;20(12):2921–2934. doi: 10.1016/j.celrep.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim J.M.E., Tan A.T., Le Bert N., Hang S.K., Low J.G.H., Bertoletti A. SARS-CoV-2 breakthrough infection in vaccinees induces virus-specific nasal-resident CD8+ and CD4+ T cells of broad specificity. J Exp Med. 2022;219(10) doi: 10.1084/jem.20220780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pizzolla A., Nguyen T.H.O., Smith J.M., et al. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol. 2017;2(12) doi: 10.1126/sciimmunol.aam6970. [DOI] [PubMed] [Google Scholar]
- 20.Schenkel J.M., Fraser K.A., Beura L.K., Pauken K.E., Vezys V., Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiner D., King C.G. CD4+ memory T cells at home in the tissue: mechanisms for health and disease. Front Immunol. 2018;9:2394. doi: 10.3389/fimmu.2018.02394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva-Sanchez A., Randall T.D. Role of iBALT in respiratory immunity. Curr Top Microbiol Immunol. 2020;426:21–43. doi: 10.1007/82_2019_191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teijaro J.R., Turner D., Pham Q., Wherry E.J., Lefrancois L., Farber D.L. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187(11):5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laidlaw B.J., Zhang N., Marshall H.D., et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014;41(4):633–645. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son Y.M., Cheon I.S., Wu Y., et al. Tissue-resident CD4(+) T helper cells assist the development of protective respiratory B and CD8(+) T cell memory responses. Sci Immunol. 2021;6(55) doi: 10.1126/sciimmunol.abb6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swarnalekha N., Schreiner D., Litzler L.C., et al. T resident helper cells promote humoral responses in the lung. Sci Immunol. 2021;6(55) doi: 10.1126/sciimmunol.abb6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allie S.R., Bradley J.E., Mudunuru U., et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat Immunol. 2019;20(1):97–108. doi: 10.1038/s41590-018-0260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacLean A.J., Richmond N., Koneva L., et al. Secondary influenza challenge triggers resident memory B cell migration and rapid relocation to boost antibody secretion at infected sites. Immunity. 2022;55(4):718–733.e8. doi: 10.1016/j.immuni.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan H.X., Juno J.A., Esterbauer R., et al. Lung-resident memory B cells established after pulmonary influenza infection display distinct transcriptional and phenotypic profiles. Sci Immunol. 2022;7(67) doi: 10.1126/sciimmunol.abf5314. [DOI] [PubMed] [Google Scholar]
- 30.Onodera T., Takahashi Y., Yokoi Y., et al. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc Natl Acad Sci U S A. 2012;109(7):2485–2490. doi: 10.1073/pnas.1115369109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregoire C., Spinelli L., Villazala-Merino S., et al. Viral infection engenders bona fide and bystander subsets of lung-resident memory B cells through a permissive mechanism. Immunity. 2022;55(7):1216–1233.e9. doi: 10.1016/j.immuni.2022.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zens K.D., Chen J.K., Farber D.L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 2016;1(10) doi: 10.1172/jci.insight.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poon M.M.L., Rybkina K., Kato Y., et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci Immunol. 2021;6(65) doi: 10.1126/sciimmunol.abl9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang J., Zeng C., Cox T.M., et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci Immunol. 2022;7(76) doi: 10.1126/sciimmunol.add4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 36.Grau-Exposito J., Sanchez-Gaona N., Massana N., et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat Commun. 2021;12(1):3010. doi: 10.1038/s41467-021-23333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren C., Gao Y., Zhang C., et al. Respiratory mucosal immunity: kinetics of secretory immunoglobulin A in sputum and throat swabs from COVID-19 patients and vaccine recipients. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.782421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altarawneh H.N., Chemaitelly H., Ayoub H.H., et al. Effects of previous infection and vaccination on symptomatic Omicron infections. N Engl J Med. 2022;387(1):21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Covid-19 Forecasting Team Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. 2023;401(10379):833–842. doi: 10.1016/S0140-6736(22)02465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michlmayr D., Hansen C.H., Gubbels S.M., et al. Observed protection against SARS-CoV-2 reinfection following a primary infection: a Danish cohort study among unvaccinated using two years of nationwide PCR-test data. Lancet Reg Health Eur. 2022;20 doi: 10.1016/j.lanepe.2022.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nordstrom P., Ballin M., Nordstrom A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. 2022;22(6):781–790. doi: 10.1016/S1473-3099(22)00143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall V., Foulkes S., Insalata F., et al. Protection against SARS-CoV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chin E.T., Leidner D., Lamson L., et al. Protection against Omicron from vaccination and previous infection in a prison system. N Engl J Med. 2022;387(19):1770–1782. doi: 10.1056/NEJMoa2207082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng S., Phillips D.J., White T., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koutsakos M., Reynaldi A., Lee W.S., et al. SARS-CoV-2 breakthrough infection induces rapid memory and de novo T cell responses. Immunity. 2023;56(4):879–892.e4. doi: 10.1016/j.immuni.2023.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Havervall S., Marking U., Svensson J., et al. Anti-spike mucosal IgA protection against SARS-CoV-2 Omicron infection. N Engl J Med. 2022;387(14):1333–1336. doi: 10.1056/NEJMc2209651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puhach O., Adea K., Hulo N., et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat Med. 2022;28(7):1491–1500. doi: 10.1038/s41591-022-01816-0. [DOI] [PubMed] [Google Scholar]
- 48.Woodbridge Y., Amit S., Huppert A., Kopelman N.M. Viral load dynamics of SARS-CoV-2 Delta and Omicron variants following multiple vaccine doses and previous infection. Nat Commun. 2022;13(1):6706. doi: 10.1038/s41467-022-33096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan S.T., Kwan A.T., Rodriguez-Barraquer I., et al. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the Omicron wave. Nat Med. 2023;29(2):358–365. doi: 10.1038/s41591-022-02138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frutos A.M., Kuan G., Lopez R., et al. Infection-induced immunity is associated with protection against SARS-CoV-2 infection and decreased infectivity. Clin Infect Dis. 2023;074 doi: 10.1093/cid/ciad074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azzi L., Dalla Gasperina D., Veronesi G., et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. eBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liew F., Talwar S., Cross A., et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. EBioMedicine. 2023;87 doi: 10.1016/j.ebiom.2022.104402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alu A., Chen L., Lei H., Wei Y., Tian X., Wei X. Intranasal COVID-19 vaccines: from bench to bed. EBioMedicine. 2022;76 doi: 10.1016/j.ebiom.2022.103841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu F., Zhuang C., Chu K., et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir Med. 2022;10(8):749–760. doi: 10.1016/S2213-2600(22)00131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meissa announces positive preliminary clinical data on safety and immunogenicity of intranasal COVID. 2021. October 28 2021 [press release]. [Google Scholar]
- 56.Sun W., McCroskery S., Liu W.C., et al. A Newcastle disease virus (NDV) expressing a membrane-anchored spike as a cost-effective inactivated SARS-CoV-2 vaccine. Vaccines (Basel) 2020;8(4) doi: 10.3390/vaccines8040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun W., Liu Y., Amanat F., et al. A Newcastle disease virus expressing a stabilized spike protein of SARS-CoV-2 induces protective immune responses. Nat Commun. 2021;12(1):6197. doi: 10.1038/s41467-021-26499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun W., Leist S.R., McCroskery S., et al. Newcastle disease virus (NDV) expressing the spike protein of SARS-CoV-2 as a live virus vaccine candidate. EBioMedicine. 2020;62 doi: 10.1016/j.ebiom.2020.103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tcheou J., Raskin A., Singh G., et al. Safety and immunogenicity analysis of a Newcastle disease virus (NDV-HXP-S) expressing the spike protein of SARS-CoV-2 in sprague dawley rats. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.791764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pitisuttithum P., Luvira V., Lawpoolsri S., et al. Safety and immunogenicity of an inactivated recombinant Newcastle disease virus vaccine expressing SARS-CoV-2 spike: interim results of a randomised, placebo-controlled, phase 1 trial. eClinicalMedicine. 2022;45 doi: 10.1016/j.eclinm.2022.101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ponce-de-Leon S., Torres M., Soto-Ramirez L.E., et al. Safety and immunogenicity of a live recombinant Newcastle disease virus-based COVID-19 vaccine (Patria) administered via the intramuscular or intranasal route: interim results of a non-randomized open label phase I trial in Mexico. medRxiv. 2022 2022.02.08.22270676. [Google Scholar]
- 62.Madhavan M., Ritchie A.J., Aboagye J., et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: an open-label partially-randomised ascending dose phase I trial. EBioMedicine. 2022;85 doi: 10.1016/j.ebiom.2022.104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altimmune announces update on AdCOVID™ phase 1 clinical trial. 2021. Gaithersburg, Maryland USA, June 29 2021 [press release]. [Google Scholar]
- 65.Wu S., Huang J., Zhang Z., et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21(12):1654–1664. doi: 10.1016/S1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J.X., Wu S.P., Guo X.L., et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir Med. 2022;10(8):739–748. doi: 10.1016/S2213-2600(22)00087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hassan A.O., Kafai N.M., Dmitriev I.P., et al. A single-dose intranasal Chad vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1):169–184.e13. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh C., Verma S., Reddy P., et al. Immunogenicity and tolerability of BBV154 (iNCOVACC®), an intranasal SARS-CoV-2 vaccine, compared with intramuscular Covaxin® in healthy adults: a randomised, open-label, Phase 3 Clinical Trial. SSRN. 2023 [Google Scholar]
- 69.Cumulative Infection Collaborators Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: a statistical analysis. Lancet. 2022;399(10344):2351–2380. doi: 10.1016/S0140-6736(22)00484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cagigi A., Yu M., Osterberg B., et al. Airway antibodies emerge according to COVID-19 severity and wane rapidly but reappear after SARS-CoV-2 vaccination. JCI Insight. 2021;6(22) doi: 10.1172/jci.insight.151463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mao T., Israelow B., Pena-Hernandez M.A., et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science. 2022;378(6622) doi: 10.1126/science.abo2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stamatatos L., Czartoski J., Wan Y.H., et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372(6549):1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reynolds C.J., Pade C., Gibbons J.M., et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372(6549):1418–1423. doi: 10.1126/science.abh1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bobrovitz N., Ware H., Ma X., et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23(5):556–567. doi: 10.1016/S1473-3099(22)00801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sigal A. Milder disease with Omicron: is it the virus or the pre-existing immunity? Nat Rev Immunol. 2022;22(2):69–71. doi: 10.1038/s41577-022-00678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.AstraZeneca provides update on Flumist Quadrivalent Vaccine in the US for the 2016-17 influenza season. AstraZeneca Website; 2016. 23 June 2016. [press release] [Google Scholar]
- 77.Clements M.L., Murphy B.R. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986;23(1):66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohn K.G., Smith I., Sjursen H., Cox R.J. Immune responses after live attenuated influenza vaccination. Hum Vaccines Immunother. 2018;14(3):571–578. doi: 10.1080/21645515.2017.1377376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohn K.G., Brokstad K.A., Pathirana R.D., et al. Live attenuated influenza vaccine in children induces B-cell responses in tonsils. J Infect Dis. 2016;214(5):722–731. doi: 10.1093/infdis/jiw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Centers for disease control and prevention past seasons vaccine effectiveness. https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html updated 22 December 2022. Available from:
- 81.Chung J.R., Flannery B., Ambrose C.S., et al. Live attenuated and inactivated influenza vaccine effectiveness. Pediatrics. 2019;143(2) doi: 10.1542/peds.2018-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zimmerman R.K., Nowalk M.P., Chung J., et al. 2014-2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis. 2016;63(12):1564–1573. doi: 10.1093/cid/ciw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.King J.P., McLean H.Q., Meece J.K., et al. Vaccine failure and serologic response to live attenuated and inactivated influenza vaccines in children during the 2013-2014 season. Vaccine. 2018;36(9):1214–1219. doi: 10.1016/j.vaccine.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 84.Huang J., Ding Y., Yao J., et al. Nasal nanovaccines for SARS-CoV-2 to address COVID-19. Vaccines (Basel) 2022;10(3) doi: 10.3390/vaccines10030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee J.H., Sutton H.J., Cottrell C.A., et al. Long-primed germinal centres with enduring affinity maturation and clonal migration. Nature. 2022;609(7929):998–1004. doi: 10.1038/s41586-022-05216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tamminen K., Salminen M., Blazevic V. Seroprevalence and SARS-CoV-2 cross-reactivity of endemic coronavirus OC43 and 229E antibodies in Finnish children and adults. Clin Immunol. 2021;229 doi: 10.1016/j.clim.2021.108782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kolehmainen P., Heroum J., Jalkanen P., et al. Serological follow-up study indicates high seasonal coronavirus infection and reinfection rates in early childhood. Microbiol Spectr. 2022;10(3) doi: 10.1128/spectrum.01967-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galanti M., Shaman J. Direct observation of repeated infections with endemic coronaviruses. J Infect Dis. 2021;223(3):409–415. doi: 10.1093/infdis/jiaa392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Somes M.P., Turner R.M., Dwyer L.J., Newall A.T. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: a systematic review and meta-analysis. Vaccine. 2018;36(23):3199–3207. doi: 10.1016/j.vaccine.2018.04.063. [DOI] [PubMed] [Google Scholar]
- 90.Blunck B.N., Aideyan L., Ye X., et al. A prospective surveillance study on the kinetics of the humoral immune response to the respiratory syncytial virus fusion protein in adults in Houston, Texas. Vaccine. 2021;39(8):1248–1256. doi: 10.1016/j.vaccine.2021.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel M.M., York I.A., Monto A.S., Thompson M.G., Fry A.M. Immune-mediated attenuation of influenza illness after infection: opportunities and challenges. Lancet Microbe. 2021;2(12):e715–e725. doi: 10.1016/S2666-5247(21)00180-4. [DOI] [PubMed] [Google Scholar]