Summary
Background
We aimed to develop and validate a prognostic model for predicting malignant brain oedema in patients with acute ischaemic stroke in a real-world setting of practice.
Methods
A prospective multicentre study enrolled adult patients with acute ischaemic stroke with brain CT < 24 h of onset of symptoms admitted to nine tertiary-level hospitals in China between September 2017 and December 2019. Malignant brain oedema was defined as any patient who had decompressive craniectomy, discharge in coma, or in-hospital death attributed to symptomatic brain swelling. The derivation cohort was a consecutive cohort of patients from one centre and the validation cohort was non-consecutive patients from the other centres. Multivariable logistic regression was used to define independent predictors from baseline clinical characteristics, imaging features, complications, and management. A web-based nomogram and a risk score were developed based on the final model. Model performance was assessed for discrimination and calibration in both derivation and validation cohorts. The study is registered, NCT03222024.
Findings
Based on the derivation cohort (n = 1627), the model was developed with seven variables including large infarct (adjusted odds ratio [OR] 40.90, 95% CI 20.20–82.80), National Institutes of Health Stroke Scale (NIHSS) score (OR 1.09, 1.06–1.12), thrombolysis (OR 2.11, 1.18–3.78), endovascular treatment (OR 2.87, 1.47–5.59), pneumonia (OR 2.47, 1.53–3.97), brain atrophy (OR 0.57, 0.37–0.86), and recanalisation (OR 0.36, 0.17–0.75). The classification threshold of a predicted probability ≥0.14 showed good discrimination and calibration in both derivation cohort (area under the receiver-operating curve [AUC] 0.90, 0.87–0.92; sensitivity 0.95, 0.92–0.98) and validation cohort (n = 556, AUC 0.88, 0.82–0.95; sensitivity 0.84, 0.73–0.95). The risk score based on this model had a total point that ranged from −1 to 20, with an optimal score of ≥10 showing good discrimination and calibration in both derivation (AUC 0.89, 0.87–0.92; sensitivity 0.95, 0.92–0.98) and validation (AUC 0.88, 0.82–0.95; sensitivity 0.84, 0.73–0.95) cohorts.
Interpretation
The INTEP-AR model (i.e. large Infarct, NIHSS score, Thrombolysis, Endovascular treatment, Pneumonia, brain Atrophy, and Recanalisation) incorporating multiple clinical and radiological characteristics has shown good prognostic value for predicting malignant brain oedema after acute ischaemic stroke.
Funding
National Natural Science Foundation of China; Science and Technology Department of Sichuan Province; West China Hospital.
Keywords: Ischaemic stroke, Malignant brain oedema, Predictive model, Prospective multicentre cohort
Research in context.
Evidence before this study
Malignant brain oedema is a leading cause of death in the acute phase of ischaemic stroke, but there is limited data to inform its early prediction and timely management. In our systematic review in 2018 (CRD42017075701) and the updated search in August 2022 (CRD42022356883) and January 2023, we systematically searched MEDLINE and EMBASE for studies reporting the predictors of the development of malignant brain oedema after acute ischaemic stroke, using the search terms ‘ischaemic stroke’ AND ‘severe OR malignant OR oedema’ AND ‘predict∗’. Nine studies reported the overall discrimination performance of multivariable models for predicting malignant brain oedema. The application of existing models is limited by highly selective inclusion criteria, retrospective data collection, moderate ability for discrimination, lack of validation, and of data derived from studies with small sample size.
Added value of this study
This is the first prospective, multicentre study specifically designed to investigate features of severe ischaemic stroke, where malignant brain oedema was a pre-specified outcome measure (NCT03222024). We have been able to reliably define the frequency, characteristics, management including surgery and reperfusion therapies, and outcomes of malignant brain oedema in a large and relatively unselective cohort of patients with acute ischaemic stroke. We systematically collected variables at different stages of admission to hospital and outcomes over the subsequent 12 months. Following rigorous methodology for prediction modelling, we developed and validated a model with a continuous range of possible probabilities for predicting malignant brain oedema in a broad range of patients, and accordingly developed a web-based tool as well as a risk score as simple and easy-to-use tools for prediction in practice. The model showed good discrimination and calibration for predicting malignant brain oedema in both the derivation and validation cohorts, as well as through sensitivity analysis and in specific subgroups of patients. Our study provides simple algorithms to individualise the early prediction of malignant brain oedema in patients with acute ischaemic stroke.
Implications of all the available evidence
Our findings corroborate those of previous retrospective studies to show that malignant brain oedema occurs in approximately one in 10 patients within the first few days after the onset of acute ischaemic stroke, and carries a high case fatality and odds of unfavourable functional outcome. Severe neurological impairment, large infarct on brain imaging, and pneumonia were predictors for malignant brain oedema, whilst brain atrophy and successful recanalisation were associated with less malignant brain oedema. Our model incorporating these and other clinical variables provides a useful and reliable tool for predicting malignant brain oedema.
Introduction
Acute ischaemic stroke is a leading cause of global disease burden,1 where there is an ongoing need to improve systems of health care and outcomes.2 Cerebral oedema is a common complication that causes early neurological deterioration and death.3,4 Typically occurring after thrombo-embolic occlusion of the middle cerebral artery (MCA), it is described as malignant MCA infarction,5 massive cerebral infarction,6 or large hemispheric infarction.7 The generic term of malignant brain oedema is often defined as a clinical syndrome with rapid increase in the severity of neurological deficits associated with cerebral oedema, characterised by massive swelling of the infarction with or without haemorrhagic transformation, which leads to space-occupying effects, transtentorial herniation, and high likelihood of death or poor functional outcome.8 Despite such devastating consequences, there is little evidence to inform the prevention and treatment of malignant brain oedema other than the use of decompressive craniectomy for patients with symptomatic brain swelling.7,9, 10, 11, 12 Although the increasing availability of endovascular therapy has reduced the need for decompressive craniectomy,13 the procedure remains under-used in practice2 due to uncertainty over whether gains in survival are offset by persistent severe disability.14, 15, 16
Our systematic review of 38 studies involving 3278 patients analysed 24 clinical factors, 7 brain imaging markers, 13 serum biomarkers, and 4 models, found that younger age, severe neurological deficits, large infarct, and unsuccessful revascularisation were predictors for malignant brain oedema.8 However, there was wide variability in the precision of the existing predictive models,17, 18, 19, 20 and their application was limited by having only a moderate ability for discrimination, small sample sizes of individual studies, and a lack of validation.8 Thus, we undertook a large prospective multicentre cohort study to better define the prognostic variables available at different stages after admission to hospital, in order to develop and validate a prognostic model for predicting malignant brain oedema after acute ischaemic stroke.
Methods
Study design and participants
A prospective, multicentre, cohort study was undertaken at nine tertiary-level hospitals in China (Appendix Table S1). The study design is outlined elsewhere,21 and was approved by the Biomedical Research Ethics Committee of West China Hospital, Sichuan University (reference No. 2017[130]) and by local human research ethical committees. The study is registered at ClinicalTrials.gov (NCT03222024). Briefly, patients with acute ischaemic stroke admitted to departments of neurology at participating centres were screened for eligibility between September 2017 and December 2019. Broad inclusion criteria were used that included age ≥18 years, symptoms and signs of clinically definite acute stroke, and time from the onset of symptoms to admission <30 d. Exclusion criteria were the primary intracranial haemorrhage and stroke mimics after brain imaging, the likelihood of a patient being unable or unavailable for follow-up assessments, and refusal to participate. All participants (or appropriate proxies) provided written informed consent before any study procedures. For predictive modelling of malignant brain oedema, only patients who had initial brain CT performed <24 h after the onset of stroke symptoms were included in analyses. This report follows the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) guidelines.22
Procedures
We collected information on patient demographics, medical history, clinical characteristics and imaging features of the stroke event, and subsequent in-hospital complications and management. All patients were treated following the routine medical care. Stroke severity was assessed by the responsible neurologist using the National Institutes of Health Stroke Scale (NIHSS) on admission and at the time of neurological deterioration (defined as an increase of ≥2 points on the NIHSS or a depressed level of consciousness from baseline). Large cerebral infarct was defined as the extent of infarct covering at least half of the territory of MCA, anterior cerebral artery (ACA) or posterior cerebral artery (PCA) on brain CT or MRI, according to a modified infarct scale in the International Stroke Trial.23 Pneumonia was diagnosed for patients with respiratory symptoms confirmed by chest CT. Recanalisation was defined on angiography immediately after endovascular treatment according to the modified Treatment In Cerebral Ischaemia (mTICI) scores of 2b or 3.24
Symptomatic brain swelling was defined as neurological deterioration with imaging signs of compression of lateral ventricle, midline shift, or compression of basal cistern. The chief investigator at each centre made the diagnosis of malignant brain oedema in any patient with symptomatic brain swelling, when the patient required decompressive craniectomy (as assessed by local neurosurgeon), died in hospital, or was discharged in coma. The investigator was blind to information of baseline characteristics and in-hospital management. Trained researchers blind to medical care contacted patients (or an appropriate surrogate) not known to have died by telephone and administered a structured interview to collect vital status and functional outcome on the modified Rankin scale (mRS) at 3 months and 1 year.
Statistical analysis
Data were described in mean (standard deviation [SD]) or median (interquartile range [IQR]) for continuous variables and counts (%) for categorical variables. We used a non-random design to split the dataset,22 with a multivariable prediction model derived in a consecutive cohort of patients from West China Hospital then validated in an independent cohort of non-consecutive patients from the other participating centres. In the derivation cohort, we selected variables available at different stages after admission that were expected to predict malignant brain oedema, based on clinical experience and recent literature.8 In step 1, the initial model was fitted with demographics and stroke characteristics that had a significance level of p < 0.10 in univariate analysis. In step 2, imaging features on the initial brain CT (including parenchymal hypoattenuation, hyperdense artery sign, brain swelling, midline shift, brain atrophy, white matter demyelination, parenchymal haemorrhage, and old infarct25,26; any with p < 0.10 in univariate analysis) were added to the significant variables in step 1. In step 3, hyperacute reperfusion treatment was added to significant variables in step 2. In step 4, variables of in-hospital complications were added to significant variables in step 3. The final model was developed with all significant variables from step 4, in which the interaction variables of large infarct × endovascular treatment and large infarct × pneumonia, were also tested, respectively.
Associations between potential prognostic factors and malignant brain oedema were assessed by odds ratios (OR) and relevant 95% confidence intervals (CI) in multivariable logistic regression model. The predicted probability for the development of malignant brain oedema was calculated for each patient based on the beta coefficient in logistic regression. For a continuous range of predicted probabilities in the model, classification threshold was determined by Youden index. Patients with a predicted probability higher than the classification threshold were classified as the risk group; the sensitivity and specificity were calculated for the classification systems. Based on final model, a web-based dynamic nomogram27 (R Shiny package, version 1.7.4) and a simplified prediction score28 were developed following the established methods, respectively. Discrimination of the model was quantified with the area under the receiver-operating characteristic curve (AUC). The overall fit of the model was assessed with adjusted pseudo R2. Hosmer–Lemeshow goodness-of-fit test was performed and calibration plots were developed using the deciles of the predicted probability of the fitted logistic model. Since large infarct and higher NIHSS scores are recognised predictors for malignant brain oedema,8 we compared overall classification between our model and the model containing only large infarct and NIHSS by net reclassification index (NRI) (R PredictABEL package, version 1.2–4).29 We performed a sensitivity analysis by excluding patients with malignant brain oedema associated with parenchymal haematoma, and conducted subgroup analyses for patients (a) with large infarct, where the variable of large infarct was removed from the model, and (b) who had received endovascular treatment, where the variable of endovascular treatment was removed from the model and the variable of large infarct was replaced with a more restricted definition of large infarct presenting prior to endovascular treatment.
In the validation cohort, we calculated the predicted probability for each patient, based on the logistic algorithm of the final model developed in the derivation cohort. Next, the full range of probabilities and the classification threshold of predicted probabilities, as well as the optimal prediction score (as defined in the derivation cohort) were tested for discrimination and calibration. Following the rule of at least 10 outcome events per variable, we estimated a sample size of 1000 in the derivation cohort to allow about 10 variables in the multivariable model with an estimated prevalence of 10% for malignant brain oedema in patients with acute ischaemic stroke. Patients with missing follow-up interviews were not included for the analysis of mRS. Statistical analyses were performed in Stata Statistical Software (Release 16. College Station, TX: StataCorp LLC) and IBM SPSS Statistics (v25.0. Armonk, NY: IBM Corp.) unless otherwise stated.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Among 4201 patients enrolled within 30 d of stroke onset, 2183 patients (age mean 67.9 ± 13.7 years, female 39.8%; Appendix Fig. S1) had initial brain CT performed within 24 h after onset of stroke symptoms. Overall, 232 (10.6% of 2183) patients developed malignant brain oedema (227 had neurological deterioration in hospital [onset to worsening median 35 h, IQR 18–67 h] and 5 patients were in persistent coma since admission), of whom 65.1% (151/232) died within 30 days (onset to death median 5 d, IQR 3–11 d), and 97.4% (221/227, 5 lost to follow-up) and 95.6% (217/227, 5 lost to follow-up) had unfavourable function outcome (mRS ≥3) at 3 months and 1 year, respectively. The derivation cohort consisted of 74.5% (1627/2183) of the entire cohort; they had more severe neurological deficits (p < 0.001), higher proportion of large infarct (p < 0.001), lower proportion of intravenous thrombolysis (p = 0.50), higher proportions of endovascular treatment (p < 0.001) and subsequent recanalisation (p < 0.001), more in-hospital complications (p < 0.001), and worse functional outcomes (p < 0.001), than patients in the validation cohort (Table 1).
Table 1.
Characteristics, management, and outcomes of patients with malignant brain oedema after acute ischaemic stroke (n = 2183).
| Derivation cohort (n = 1627) | Validation cohort (n = 556) | Malignant brain oedema |
|||
|---|---|---|---|---|---|
| Yes (n = 232) | No (n = 1951) | p value | |||
| Demographics | |||||
| Age | 67.7 ± 14.1 | 68.4 ± 12.4 | 71.3 (13.5) | 67.5 (13.7) | <0.001 |
| Female sex | 653 (40.1) | 216 (38.8) | 114 (49.1) | 755 (38.7) | 0.002 |
| Stroke characteristics | |||||
| Onset to admission, h | 4 (2–7) | 5 (3–14) | 3 (2–5) | 4 (2–8) | 0.01 |
| Duration in hospital, d | 9 (7–14) | 11 (7–15) | 5 (2–15) | 10 (7–14) | 0.003 |
| GCS on admission | 15 (11–15) | 15 (12–15) | 9 (7–13) | 15 (13–15) | <0.001 |
| NIHSS on admission | 8 (3–16) | 5 (2–13) | 19 (16–24) | 6 (2–13) | <0.001 |
| Large infarcta | 432 (26.6) | 78 (14.0) | 216 (93.1) | 294 (15.1) | <0.001 |
| Pathological subtypes | <0.001 | ||||
| LAA | 454 (27.9) | 318 (57.2) | 76 (32.8) | 696 (35.7) | – |
| CE | 640 (39.3) | 112 (20.1) | 134 (57.8) | 618 (31.7) | – |
| SVO | 289 (17.8) | 63 (11.3) | 0 | 352 (18.0) | – |
| Other | 78 (4.8) | 8 (1.4) | 5 (2.2) | 81 (4.2) | – |
| UND | 166 (10.2) | 55 (9.9) | 17 (7.3) | 204 (10.5) | – |
| Medical comorbidities | |||||
| Previous stroke | 298 (18.3) | 104 (18.7) | 33 (14.2) | 369 (18.9) | 0.08 |
| Atrial fibrillation | 549 (33.7) | 103 (18.5) | 117 (50.4) | 535 (27.4) | <0.001 |
| Congestive heart failure | 94 (5.8) | 25 (4.5) | 27 (11.6) | 92 (4.7) | <0.001 |
| Hypertension | 978 (60.1) | 341 (61.3) | 126 (54.3) | 1193 (61.1) | 0.04 |
| Diabetes mellitus | 442 (27.2) | 157 (28.2) | 61 (26.3) | 538 (27.6) | 0.68 |
| Brain imaging features | |||||
| Parenchymal hypoattenuation | 1452 (89.2) | 401 (72.1) | 209 (90.1) | 1644 (84.3) | 0.02 |
| Hyperdense artery sign | 210 (12.9) | 29 (5.2) | 69 (29.7) | 170 (8.7) | <0.001 |
| Brain swelling | 420 (25.8) | 32 (5.8) | 123 (53.0) | 329 (16.9) | <0.001 |
| Midline shift | 19 (1.2) | 13 (2.3) | 17 (7.3) | 15 (0.8) | <0.001 |
| Brain atrophy | 707 (43.5) | 246 (44.2) | 80 (34.5) | 873 (44.7) | 0.003 |
| White matter demyelination | 584 (35.9) | 207 (37.2) | 64 (27.6) | 727 (37.3) | 0.004 |
| Parenchymal haemorrhage | 12 (0.7) | 12 (2.2) | 8 (3.4) | 16 (0.8) | <0.001 |
| Old infarct | 164 (10.1) | 88 (15.8) | 20 (8.6) | 232 (11.9) | 0.14 |
| Management | |||||
| Intravenous thrombolysis | 191 (11.7) | 83 (14.9) | 42 (18.1) | 232 (11.9) | 0.007 |
| EVT | 290 (17.8) | 35 (6.3) | 91 (39.2) | 234 (12.0) | <0.001 |
| Recanalisationb | 228/290 (78.6) | 27/35 (77.1) | 55/91 (60.4) | 200/234 (85.5) | <0.001 |
| Antiplatelet agent(s) | 1423 (87.5) | 499 (89.7) | 128 (55.2) | 1794 (92.0) | <0.001 |
| OAC/CE | 260/640 (40.6) | 36/112 (32.1) | 11/134 (8.2) | 285/618 (46.1) | <0.001 |
| Mannitol | 649 (39.9) | 106 (19.1) | 215 (92.7) | 540 (27.7) | <0.001 |
| Anti-HTN/HTN | 626/978 (64.0) | 178/341 (52.2) | 64/126 (50.8) | 740/1193 (62.0) | 0.02 |
| Anti-DM/DM | 328/442 (74.2) | 96/157 (61.1) | 42/61 (68.9) | 382/538 (71.0) | 0.73 |
| Lipid lowering | 1431 (88.0) | 523 (94.1) | 166 (71.6) | 1788 (91.6) | <0.001 |
| TCM | 1015 (62.4) | 269 (48.4) | 97 (41.8) | 1187 (60.8) | <0.001 |
| Neuroprotective agents | 380 (23.4) | 453 (81.5) | 74 (31.9) | 759 (38.9) | 0.04 |
| Rehabilitation | 623 (38.3) | 253 (45.5) | 61 (26.3) | 815 (41.8) | <0.001 |
| Endotracheal intubation | 152 (9.3) | 26 (4.7) | 100 (43.1) | 78 (4.0) | <0.001 |
| DC | 22 (1.4) | 1 (0.2) | 23 (9.9) | 0 | <0.001 |
| In-hospital complications | |||||
| Seizure | 43 (2.6) | 3 (0.5) | 11 (4.7) | 35 (1.8) | 0.003 |
| Pneumonia | 624 (38.4) | 108 (19.4) | 186 (80.2) | 546 (28.0) | <0.001 |
| Gastrointestinal bleeding | 171 (10.5) | 23 (4.1) | 53 (22.8) | 141 (7.2) | <0.001 |
| Outcomes | |||||
| Death in hospital | 97 (6.0) | 30 (5.4) | 95 (40.9) | 32 (1.6) | <0.001 |
| 3-month death | 246/1578 (15.6) | 83/526 (15.8) | 169/227 (74.4) | 160/1877 (8.5) | <0.001 |
| 3-month mRS 3-6 | 735/1578 (46.6) | 195/526 (37.1) | 221/227 (97.4) | 709/1877 (37.8) | <0.001 |
| 1-year death | 310/1574 (19.7) | 100/488 (20.5) | 178/227 (78.4) | 232/1835 (12.6) | <0.001 |
| 1-year mRS 3-6 | 694/1574 (44.1) | 167/488 (34.2) | 217/227 (95.6) | 644/1835 (35.1) | <0.001 |
Data are mean (SD), median (IQR), n (%) or n/N (%) when the denominator differs from the N value in the column heading, unless otherwise stated. OAC/CE means the proportion of patients with cardioembolism who used oral anticoagulation; the same applies to anti-HTN/HTN and anti-DM/DM.
CE = cardioembolism; DC = decompressive craniectomy; DM = diabetes mellitus; EVT = endovascular treatment; GCS = Glasgow Coma Scale; HTN = hypertension; LAA = large artery atherosclerosis; MBE = malignant brain oedema; mRS = modified Rankin scale; NIHSS=National Institutes of Stroke Scale; OAC = oral anticoagulation; SVO = small vessel occlusion; TCM = traditional Chinese medicine; UND = undetermined subtype.
Large infarct is defined as the extent of infarct covering at least half of the territory of MCA, ACA or PCA on brain CT/MRI.
Recanalisation is defined on angiography immediately after endovascular treatment according to the modified Treatment In Cerebral Ischaemia (mTICI) scores of 2b or 3.
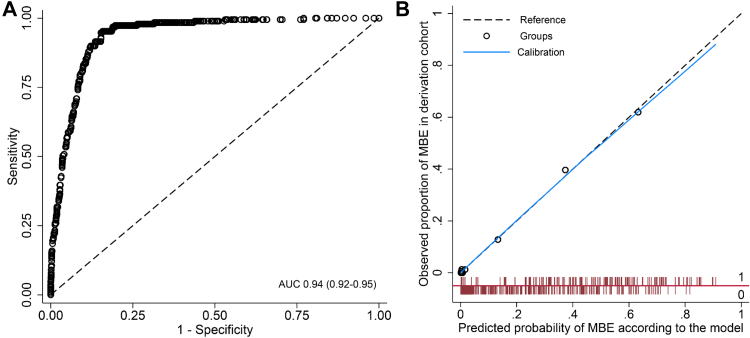
In the derivation cohort (n = 1627; age mean 67.7 ± 14.1 years, female 40.1%), 189 patients (11.6%) had malignant brain oedema. Patients with higher NIHSS scores, large infarct, pneumonia, receiving intravenous thrombolysis or endovascular treatment, were more likely to develop malignant brain oedema, and in those with brain atrophy and where recanalisation was achieved there was a reduced odds of malignant brain oedema (Appendix Table S2). The final model was developed with these seven variables (Table 2); there was no significant interaction of large infarct with either endovascular treatment or pneumonia (Appendix Table S3). The model had good discrimination (AUC 0.94, 95% CI 0.92–0.95; Fig. 1A) and calibration (Hosmer–Lemeshow test χ2 = 6.38, df = 8, p = 0.60; Fig. 1B), with better overall classification than the model with only large infarct and NIHSS (NRI 0.81, 0.67–0.95, p < 0.001). Patients with a predicted probability >0.82 (n = 9, including one patient >0.90) had all developed malignant brain oedema. The predicted probability ≥0.14 had the highest Youden index in c-statistic analysis (AUC 0.90, 0.87–0.92), which was defined as the classification threshold, with a sensitivity of 0.95 (0.92–0.98) and specificity of 0.84 (0.82–0.86) for malignant brain oedema. A web-based nomogram was developed for the model to visualise the predicted probability for individual patients (https://severeischaemicstroke.shinyapps.io/DynNomapp/, Appendix Fig. S2, with an example patient presented). In addition, a scored prediction tool was developed based on the model (Appendix Tables S4 and S5), with a possible total score that ranged from −1 to 20 and the corresponding predicted probability ranged from 0.19% to 94.15% (AUC 0.94, 0.92–0.95). This risk score with a total score ≥10 had the highest Youden index in c-statistic analysis (AUC 0.89, 0.87–0.92), with sensitivity of 0.95 (0.92–0.98) and specificity of 0.84 (0.82–0.86).
Table 2.
The INTEP-AR model for predicting malignant brain oedema in the derivation cohort (n = 1627).
| Variables | Beta | OR | 95% CI | p value |
|---|---|---|---|---|
| Large infarcta | 3.711 | 40.90 | 20.20–82.80 | <0.001 |
| NIHSS | 0.086 | 1.09 | 1.06–1.12 | <0.001 |
| Thrombolysis i.v. | 0.746 | 2.11 | 1.18–3.78 | 0.01 |
| EVT | 1.053 | 2.87 | 1.47–5.59 | 0.002 |
| Pneumonia | 0.902 | 2.47 | 1.53–3.97 | <0.001 |
| Brain atrophy | −0.570 | 0.57 | 0.37–0.86 | 0.007 |
| Recanalisationb | −1.035 | 0.36 | 0.17–0.75 | 0.006 |
| Intercept | −6.208 | |||
| Model performance | ||||
| AUC (95% CI) | 0.94 (0.92–0.95) | |||
| Adjusted pseudo R2 | 0.48 | |||
| Hosmer–Lemeshow test | χ2 = 6.38, df = 8, p = 0.60 |
INTEP-AR represents large Infarct, NIHSS score, intravenous Thrombolysis, Endovascular treatment, Pneumonia, brain Atrophy, and Recanalisation.
AUC = area under the receiver-operating curve, for discrimination of predicted probability against the occurrence of malignant brain oedema; CI = confidence interval; EVT = endovascular treatment; i.v. = intravenous; NIHSS=National Institutes of Stroke Scale.
Large infarct is defined as the extent of infarct covering at least half of the territory of MCA, ACA or PCA on brain CT/MRI.
Recanalisation is defined on angiography immediately after endovascular treatment according to the modified Treatment In Cerebral Ischaemia (mTICI) scores of 2b or 3.
Fig. 1.
Model performance of INTEP-AR model for predicting malignant brain oedema in the derivation cohort (n = 1627). (A) The receiver-operating characteristic curve (AUC = 0.94, 95% CI 0.92–0.95) for discrimination; (B) Calibration plot (Hosmer–Lemeshow test χ2 = 6.38, df = 8, p = 0.60): the x-axis is the predicted probability for MBE based on the model and the y-axis is the observed proportion of MBE in the cohort; the black dotted line represents the reference of y = x; the blue line represents the calibration curve; black circles (Groups) represent the difference between the predicted probability and the observed proportion in each of the 10 groups based on deciles of predicted probabilities; and red bars represent the distribution of patients with (value = 1, above the red horizontal line) and without (value = 0, below the red horizontal line) observed MBE across the range of predicted probabilities from 0 to 1. INTEP-AR represents large Infarct, NIHSS score, intravenous Thrombolysis, Endovascular treatment, Pneumonia, brain Atrophy, and Recanalisation. AUC = area under the receiver-operating characteristic curve; CI = confidence interval; MBE = malignant brain oedema.
In the sensitivity analysis (n = 1567) that excluded patients with malignant brain oedema associated with parenchymal haematoma, the AUC of a continuous range of predicted probabilities (as calculated based on logistic algorithm of the final model in the entire derivation cohort) was 0.95 (0.94–0.96); the classification threshold ≥0.14 had AUC of 0.91 (0.89–0.93), sensitivity of 0.98 (0.95–0.99), and specificity of 0.84 (0.82–0.86); the risk score ≥10 had AUC of 0.91 (0.88–0.93), sensitivity of 0.98 (0.95–0.99), and specificity of 0.83 (0.82–0.85). In the subgroup of patients with large infarct (n = 432), all other variables remained significant in the model after the variable of large infarct had been removed; the model showed moderate discrimination for malignant brain oedema (AUC 0.73, 0.68–0.77; Appendix Table S6). In subgroup of 290 patients who had received endovascular treatment, the variable of large infarct restricted to that presented prior to endovascular treatment remained significant (adjusted OR 3.70, 1.80–7.63) in the model (Appendix Table S6).
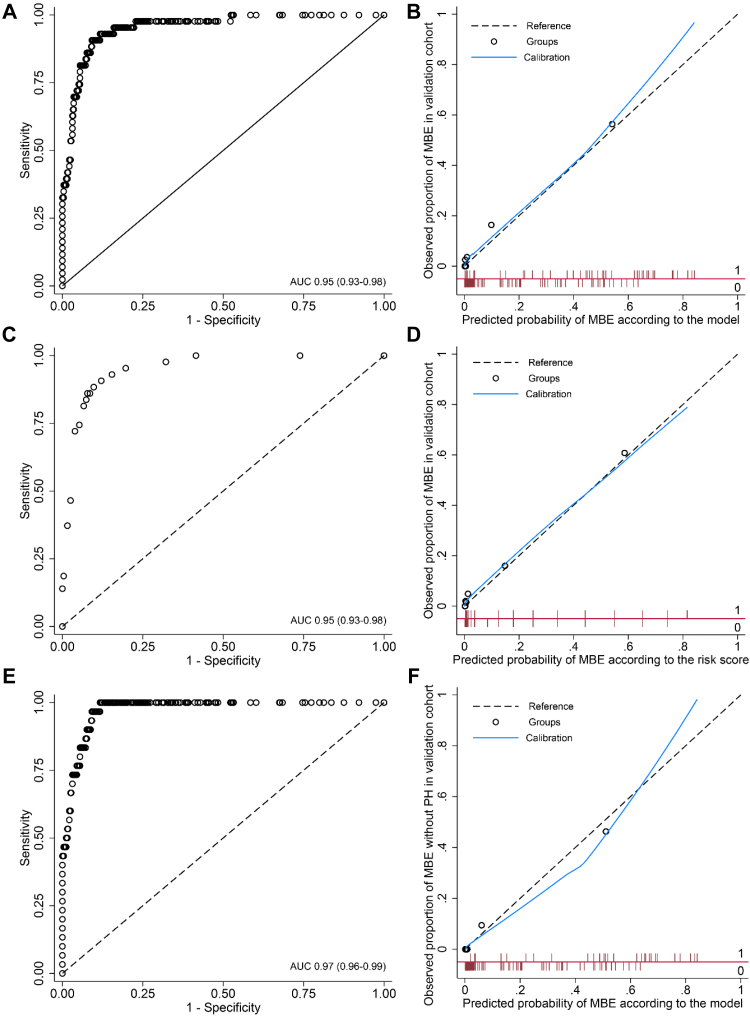
In the validation cohort (n = 556), 43 patients (7.7%) had malignant brain oedema. For each patient, we calculated the predicted probability for malignant brain oedema based on logistic algorithm of the final model in the derivation cohort (Appendix Table S7). The AUC of a continuous range of predicted probabilities of model was 0.95 (0.93–0.98, Fig. 2A) and the calibration slope was 0.90 (Fig. 2B); the classification threshold of a predicted probability ≥0.14 showed AUC of 0.88 (0.82–0.95), sensitivity of 0.84 (0.73–0.95), and specificity of 0.93 (0.90–0.95) for predicting malignant brain oedema. The AUC of a full range of the risk score was 0.95 (0.93–0.98) with a calibration slope of 0.87 (Fig. 2C and D). The total risk score ≥10 had AUC of 0.88 (0.82–0.95), sensitivity of 0.84 (0.73–0.95), and specificity of 0.93 (0.90–0.95). In the sensitivity analysis (n = 543) by excluding patients with malignant brain oedema associated with parenchymal haematoma, the AUC of a continuous range of predicted probabilities was 0.97 (0.96–0.99) with a calibration slope of 1.04 (Fig. 2E and F); the AUC of the classification threshold ≥0.14 was 0.90 (0.82–0.97), with sensitivity of 0.87 (0.75–0.99) and specificity of 0.93 (0.90–0.95).
Fig. 2.
Discrimination and calibration of INTEP-AR model for predicting malignant brain oedema in the validation cohort. (A) The receiver-operating characteristic curve of the model in validation cohort (n = 556; AUC = 0.95, 95% CI 0.93–0.98); (B) calibration plot of the model (slope 0.90); (C) receiver-operating characteristic curve of the risk score in validation cohort (n = 556; AUC = 0.95, 95% CI 0.93–03.98); (D) calibration plot of the risk score (slope 0.87); (E) receiver-operating characteristic curve of the model in validation cohort excluding MBE with PH (n = 543; AUC = 0.97, 95% CI 0.96–0.99); (F) calibration plot of the model in validation cohort excluding MBE with PH (slope 1.04). The x-axis of the calibration plot is the predicted probability for MBE based on the model and the y-axis is the observed proportion of MBE in the cohort; the black dotted line represents the reference of y = x; the blue line represents the calibration curve; black circles (Groups) represent the difference between the predicted probability and the observed proportion in each of the 10 groups based on deciles of predicted probabilities; and red bars represent the distribution of patients with (value = 1, above the red horizontal line) and without (value = 0, below the red horizontal line) observed MBE across the range of predicted probabilities from 0 to 1. INTEP-AR represents large Infarct, NIHSS score, intravenous Thrombolysis, Endovascular treatment, Pneumonia, brain Atrophy, and Recanalisation. AUC = area under the receiver-operating characteristic curve; MBE = malignant brain oedema; PH = parenchymal haematoma.
Discussion
In this large prospective multicentre cohort study, malignant brain oedema occurred in approximately 10% of patients with acute ischaemic stroke who had initial brain CT performed within 24 h of the onset of stroke symptoms, with clinical features evident within the first few days after stroke onset. It is an important complication with high early case fatality and unfavourable functional outcome. Large infarct, greater neurological impairment, receipt of modern reperfusion treatment with either thrombolysis or endovascular therapy, and the occurrence of pneumonia were predictors of malignant brain oedema, whilst brain atrophy and achieving good recanalisation after endovascular treatment were associated with less malignant brain oedema. We developed and validated a prognostic model with these seven simple variables for predicting malignant brain oedema, in a cohort of relatively unselected patients who admitted at an early stage after stroke.
Our INTEP-AR (large Infarct, NIHSS, Thrombolysis, Endovascular treatment, Pneumonia, brain Atrophy, and Recanalisation) model provided good discrimination and calibration for predicting malignant brain oedema. We developed a web-based dynamic nomogram for the model to facilitate the calculation of the predicted probability for individual patient. In addition, we developed a risk score to simplify the use of our model in low-resource settings with poor access to the network, which showed comparable predictive capability to the dynamic nomogram. For the example patient (Appendix Fig. S2), we used variables collected at 7 h after onset of stroke and calculated predicted probability based on the online nomogram (predicted probability 0.57) and the risk score (scored 15), both correctly classified this patient to the risk group. The patient subsequently developed malignant brain oedema and died at 28 h after onset of stroke, for whom the prediction at 7 h is informative to guide timely management at an early stage.
Patients with a large cerebral infarction had the highest weight in the INTEP-AR model for predicting malignant brain oedema. However, as not all patients with large infarct had a malignant course, other clinical features are necessary to strengthen prediction. Our model, incorporating other clinical and imaging features, showed better classification than using a combination of large infarct and NIHSS for predicting malignant brain oedema. In addition, for the subgroup of patients with large infarct, all variables in the model (after excluding the variable of large infarct) remained as significant predictors for malignant brain oedema. Our data confirm the benefits of reperfusion therapy where successful recanalisation is achieved to reduce the likelihood of malignant brain oedema. Consistent with reperfusion trials,23,30,31 we found that the receipt of thrombolysis or endovascular treatment might increase the risk of malignant brain oedema in some patients; however, it should be noted that reperfusion treatment generally improve functional outcomes after acute ischaemic stroke in patients with indications. In addition, the association of intravenous thrombolysis and endovascular treatment with malignant brain oedema is likely to reflect indication bias as patients with more severe stroke were more likely to receive such reperfusion therapies. The INTEP-AR model provides a useful tool to identify patients, including those who had received reperfusion therapies, at a high risk of malignant brain oedema, to inform the implementation of early prevention. Consistent with previous studies,8 we found brain atrophy was associated with reduced odds of malignant brain oedema, possibly by providing a buffer space. We did not include the use of antiplatelet agents, oral anticoagulation, mannitol and rehabilitation, as these interventions are related to the degree of active management. For example, mannitol is commonly used for patients with cerebral oedema but is otherwise avoided in most patients with acute ischaemic stroke.10 We did not exclude parenchymal haematoma from the definition of malignant brain oedema, given it would similarly present mass effect and require for surgical decompression as those without haematoma.32 As approximately one third of patients with malignant brain oedema had parenchymal haematoma, we performed a sensitivity analysis by excluding them and showed good model discrimination and calibration consistent with the entire cohort.
Key strengths of our study were the prospective multicentre design to purposely develop a prognostic model for malignant brain oedema in over 2000 relatively unselected participants from a real-world setting with contemporary management of acute ischaemic stroke. This allowed us to validate the model in a cohort of patients with variations in distribution of the predictor variables in the model as compared to the derivation cohort, whilst existing models for predicting malignant brain oedema (Appendix Table S8) have primarily focused on developing models in highly-selected patients. Yet, studies with specific type of patients may provide more details that are relevant to specific patient groups; for example, recent studies in patients after endovascular treatment found that intervention time window,33 and post-operative NIHSS scores34 and collateral circulation35 were important predictors, which we have been unable to replicate in our study. In addition to the risk scores that were commonly reported in previous studies,17, 18, 19, 20 our study as well as some recent studies33, 34, 35, 36 report nomograms that allow precise prediction with visualisation. Moreover, we developed both a web-tool with a continuous range of predicted probabilities and a risk score, both with cut-off values defined for optimal classification to facilitate use. Furthermore, we directly applied the logistic algorithm of the model from the derivation cohort to the validation cohort, which confirmed good discrimination and calibration of the model and strengthened its generalisability. In general, our model has merit and applicability as it was derived from a large sample size, used more rigorous methodology in prediction modelling, showed better discrimination and calibration, and has readiness for easy-to-use application into practice.
A limitation is that we were only able to recruit consecutive patients in one centre, which accounted for 75% of the total sample, whereas patients were enrolled according to an estimated realistic target in other centres with less experience in research, raising issues of selection bias. Despite the variation between the derivation cohort and the validation cohort in the distribution of variables in the model, the INTEP-AR model showed good discrimination and calibration in the validation cohort. Since the calibration was assessed subjectively based on a visualised plot, we also reported the calibration slope, as well as sensitivity and specificity, to inform the classification ability. Second, although we analysed a wide range of clinical, imaging and treatment factors that may influence the occurrence of malignant brain oedema, some relevant factors may not have been included. For example, some studies have investigated associations of imaging features on brain MRI and malignant brain oedema,37 which we have been unable to replicate due to poor access to such imaging as some patients were unstable or critically unwell. In addition, we were unable to analyse post-interventional factors. Studies specifically designed to malignant brain oedema after endovascular treatment may provide better information for this group of patients.33, 34, 35,38 Finally, our model was derived and validated in a Chinese population, for which further validation in a wider population in the world may broaden its generalisability.
In summary, this large prospective study identified the occurrence of malignant brain oedema in approximately one in 10 patients within the first few days after the onset of acute ischaemic stroke. Large infarct, higher NIHSS score, the receipt of thrombolysis or endovascular treatment, and pneumonia were predictors for the development of malignant brain oedema, whilst brain atrophy and successful recanalisation were associated with less malignant brain oedema. The INTEP-AR model incorporating these factors provides a useful tool for individualised prediction of malignant brain oedema after acute ischaemic stroke.
Contributors
SW designed the study, wrote the protocol, collected, analysed and interpreted the data, wrote the original draft and revised the manuscript. YW and RY contributed to literature search, review of case report forms, and data collection and analysis. FG, DY, ZL, BHW, CW, JD, TL and HZ contributed to collection and verification of the data. SZ and BW designed the study, reviewed the case report forms, and oversaw the study conduct. CSA contributed to the study design, data interpretation, and draft writing. ML conceived and designed the study, obtained the funding, supervised the study, and wrote the draft. All authors reviewed and revised the manuscript for important intellectual content. SW and ML had full access to and verified the data in the study. All authors had final responsibility for the decision to submit for publication.
Data sharing statement
Non-published anonymised data will be shared with qualified investigators on request to the corresponding author.
Declaration of interests
CSA has received grants from National Health and Medical Research Council (NHMRC) of Australia, Medical Research Council (MRC) of the UK, Penumbra, and Takeda. He is Vice-President of the World Stroke Organisation and Editor-in-Chief of Cerebrovascular Diseases. SW is the Associate Editor of Cerebrovascular Diseases.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 82171285, 81974181), the Science and Technology Department of Sichuan Province (2021YJ0433, 2017SZ0007), and the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD18009). We thank Professor Peter Sandercock, University of Edinburgh, who provided advice on the design of this study. We thank Professor Cairong Zhu, Sichuan University, for her advice on statistical analysis. We thank Ms Jinyao He and Ms Xue Li for their work of coordinating research centres. We would like to thank all participants and their families and are grateful to clinical and research staff in participating centres who supported the study, without whom the study would not be possible.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101977.
Contributor Information
Simiao Wu, Email: simiao.wu@hotmail.com.
Ming Liu, Email: wyplmh@hotmail.com.
Appendix A. Supplementary data
References
- 1.Johnson C.O., Nguyen M., Roth G.A., et al. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu S., Wu B., Liu M., et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18:394–405. doi: 10.1016/S1474-4422(18)30500-3. [DOI] [PubMed] [Google Scholar]
- 3.Balami J.S., Chen R.L., Grunwald I.Q., Buchan A.M. Neurological complications of acute ischaemic stroke. Lancet Neurol. 2011;10:357–371. doi: 10.1016/S1474-4422(10)70313-6. [DOI] [PubMed] [Google Scholar]
- 4.White O.B., Norris J.W., Hachinski V.C., Lewis A. Death in early stroke, causes and mechanisms. Stroke. 1979;10:743. doi: 10.1161/01.str.10.6.743. [DOI] [PubMed] [Google Scholar]
- 5.Hacke W., Schwab S., Horn M., Spranger M., De Georgia M., von Kummer R. 'Malignant' middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 6.Robertson S.C., Lennarson P., Hasan D.M., Traynelis V.C. Clinical course and surgical management of massive cerebral infarction. Neurosurgery. 2004;55:55–62. doi: 10.1227/01.neu.0000126875.02630.36. [DOI] [PubMed] [Google Scholar]
- 7.Torbey M.T., Bosel J., Rhoney D.H., et al. Evidence-based guidelines for the management of large hemispheric infarction : a statement for health care professionals from the Neurocritical Care Society and the German Society for Neuro-intensive Care and Emergency Medicine. Neurocritical Care. 2015;22:146–164. doi: 10.1007/s12028-014-0085-6. [DOI] [PubMed] [Google Scholar]
- 8.Wu S., Yuan R., Wang Y., et al. Early prediction of malignant brain edema after ischemic stroke a systematic review and meta-analysis. Stroke. 2018;49:2918–2927. doi: 10.1161/STROKEAHA.118.022001. [DOI] [PubMed] [Google Scholar]
- 9.Wijdicks E.F., Sheth K.N., Carter B.S., et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1222–1238. doi: 10.1161/01.str.0000441965.15164.d6. [DOI] [PubMed] [Google Scholar]
- 10.Powers W.J., Rabinstein A.A., Ackerson T., et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 11.Cook A.M., Morgan Jones G., Hawryluk G.W.J., et al. Guidelines for the acute treatment of cerebral edema in neurocritical care patients. Neurocritical Care. 2020;32:647–666. doi: 10.1007/s12028-020-00959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Worp H.B., Hofmeijer J., Jüttler E., et al. European Stroke Organisation (ESO) guidelines on the management of space-occupying brain infarction. Eur Stroke J. 2021;6 doi: 10.1177/23969873211027001. :XC-CX. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Khattar N.K., Ugiliweneza B., Fortuny E.M., et al. Inverse national trends in decompressive craniectomy versus endovascular thrombectomy for stroke. World Neurosurg. 2020;138:e642–e651. doi: 10.1016/j.wneu.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Hofmeijer J., Kappelle L.J., Algra A., Amelink G.J., van Gijn J., van der Worp H.B. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy after Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009;8:326–333. doi: 10.1016/S1474-4422(09)70047-X. [DOI] [PubMed] [Google Scholar]
- 15.Juttler E., Schwab S., Schmiedek P., et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke. 2007;38:2518–2525. doi: 10.1161/STROKEAHA.107.485649. [DOI] [PubMed] [Google Scholar]
- 16.Vahedi K., Vicaut E., Mateo J., et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial) Stroke. 2007;38:2506–2517. doi: 10.1161/STROKEAHA.107.485235. [DOI] [PubMed] [Google Scholar]
- 17.Kasner S.E., Demchuk A.M., Berrouschot J., et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke. 2001;32:2117–2123. doi: 10.1161/hs0901.095719. [DOI] [PubMed] [Google Scholar]
- 18.Shimoyama T., Kimura K., Uemura J., et al. The DASH score: a simple score to assess risk for development of malignant middle cerebral artery infarction. J Neurol Sci. 2014;338:102–106. doi: 10.1016/j.jns.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Jo K., Bajgur S.S., Kim H., Choi H.A., Huh P.W., Lee K. A simple prediction score system for malignant brain edema progression in large hemispheric infarction. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong C.J., Gluckstein J., Laurido-Soto O., Yan Y., Dhar R., Lee J.M. Enhanced detection of edema in malignant anterior circulation stroke (EDEMA) score: a risk prediction tool. Stroke. 2017;48:1969–1972. doi: 10.1161/STROKEAHA.117.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S., Yuan R., Xiong Y., Zhang S., Wu B., Liu M. Clinical features, management and outcomes of severe ischaemic stroke in tertiary hospitals in China: protocol for a prospective multicentre registry-based observational study. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-024900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moons K.G., Altman D.G., Reitsma J.B., et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–W73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 23.Wu S., Mair G., Cohen G., et al. Hyperdense artery sign, symptomatic infarct swelling and effect of alteplase in acute ischaemic stroke. Stroke Vasc Neurol. 2021;6:238–243. doi: 10.1136/svn-2020-000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaidat O.O., Yoo A.J., Khatri P., et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wardlaw J.M., Sellar R. A simple practical classification of cerebral infarcts on CT and its interobserver reliability. AJNR Am J Neuroradiol. 1994;15:1933–1939. [PMC free article] [PubMed] [Google Scholar]
- 26.Wardlaw J.M., Mielke O. Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment--systematic review. Radiology. 2005;235:444–453. doi: 10.1148/radiol.2352040262. [DOI] [PubMed] [Google Scholar]
- 27.Venema E., Mulder M., Roozenbeek B., et al. Selection of patients for intra-arterial treatment for acute ischaemic stroke: development and validation of a clinical decision tool in two randomised trials. BMJ. 2017;357:j1710. doi: 10.1136/bmj.j1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson P.W., D'Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 29.Pencina M.J., D'Agostino R.B., Sr, D'Agostino R.B., Jr., Vasan R.S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 30.Kimberly W.T., Dutra B.G., Boers A.M.M., et al. Association of reperfusion with brain edema in patients with acute ischemic stroke: a secondary analysis of the MR CLEAN trial. JAMA Neurol. 2018;75:453–461. doi: 10.1001/jamaneurol.2017.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng F.C., Yassi N., Sharma G., et al. Cerebral edema in patients with large hemispheric infarct undergoing reperfusion treatment: a HERMES meta-analysis. Stroke. 2021;52:3450–3458. doi: 10.1161/STROKEAHA.120.033246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaghi S., Willey J.Z., Cucchiara B., et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e343–e361. doi: 10.1161/STR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 33.Guo W., Xu J., Zhao W., et al. A nomogram for predicting malignant cerebral artery infarction in the modern thrombectomy era. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.934051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Q.M., Yu S. Predictors and dynamic nomogram to determine the individual risk of malignant brain edema after endovascular thrombectomy in acute ischemic stroke. J Clin Neurol. 2022;18:298–307. doi: 10.3988/jcn.2022.18.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du M., Huang X. A nomogram model to predict malignant cerebral edema in ischemic stroke patients treated with endovascular thrombectomy: an observational study. Neuropsychiatr Dis Treat. 2020;16:2913–2920. doi: 10.2147/NDT.S279303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun W., Li G., Song Y., et al. A web based dynamic MANA Nomogram for predicting the malignant cerebral edema in patients with large hemispheric infarction. BMC Neurol. 2020;20:360. doi: 10.1186/s12883-020-01935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomalla G., Hartmann F., Juettler E., et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: a prospective multicenter observational study. Ann Neurol. 2010;68:435–445. doi: 10.1002/ana.22125. [DOI] [PubMed] [Google Scholar]
- 38.Bernsen M.L.E., Kauw F., Martens J.M., et al. Malignant infarction after endovascular treatment: incidence and prediction. Int J Stroke. 2022;17:198–206. doi: 10.1177/17474930211006290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.