Summary
Background
Synergistic antitumor effects of immunotherapy and chemotherapy have been demonstrated in several solid tumors. However, this combination strategy has not been addressed in gestational trophoblastic neoplasia (GTN) cases. We therefore compared the safety and therapeutic effect of anti-programmed cell death 1 (PD-1) therapy combined with chemotherapy versus anti-PD-1 monotherapy among high-risk chemorefractory or relapsed GTN patients.
Methods
This retrospective cohort study was conducted at three teaching hospitals in China. Chemorefractory or relapsed GTN cases receiving anti-PD-1 therapy combined with chemotherapy or anti-PD-1 monotherapy were selected from each center between August 2018 and March 2022. Study endpoints included objective response rate (ORR), treatment duration, overall survival (OS) and progression-free survival (PFS). The nature, prevalence and severity of treatment-related adverse events (TRAEs) were evaluated.
Findings
This work enrolled 66 cases. Thirty-five and 31 patients received anti-PD-1 therapy alone and combined with chemotherapy, respectively. The combined treatment dramatically increased the objective response rate from 62.9% (22/35) to 96.8% (30/31) (p < 0.001). The median durations until complete response were 2.2 (interquartile range [IQR], 1.4–4.2) and 2.8 (IQR, 1.8–2.8) months in the anti-PD-1 monotherapy and combined treatment cohorts, respectively (P = 0.299). The complete response rate (CRR) for anti-PD-1-refractory patients to salvage chemotherapy was 84.6% (11/13). No significant difference in OS [HR 0.50 (95% CI 0.07–3.24), p = 0.499] was detected between anti-PD-1 cohort and anti-PD-1 plus chemotherapy cohort. The PFS in combined group was significantly longer than in anti-PD-1 group [HR 0.06 (95% CI 0.02–0.16), p < 0.001]. TRAEs were observed in 27 (77.1%) and 25 (80.6%) patients receiving anti-PD-1 therapy monotherapy and combined therapy, respectively (p = 0.729).
Interpretation
Anti-PD-1 therapy combined with chemotherapy exhibits sustainably improved antitumor effect and tolerable toxic effects among high-risk chemorefractory or relapsed GTN cases. Patients not responding to PD-1 inhibitors can be effectively rescued with salvage chemotherapy.
Funding
The study was supported by National Natural Science Foundation of China (81971475 and 81972451), and the National High Level Hospital Clinical Research Funding (2022-PUMCH-B-083 and 2022-PUMCH-B-084).
Keywords: PD-1 inhibitors, Gestational trophoblastic neoplasia, Combination therapy, Chemotherapy
Research in context.
Evidence before this study
We searched PubMed on October 1st 2022 for all clinical trials or investigations using the term related to “gestational trophoblastic neoplasia (GTN)”, “chemotherapy”, “immunotherapy” and “anti-PD-1 therapy”. No language restrictions were applied. Two ongoing clinical trials on immunotherapy/chemotherapy in GTN patients were found (NCT05139095 and NCT04396223). There were no studies concerning about chemotherapy synergizing with PD-1 inhibitors offering improved anti-tumor effect in gestational trophoblastic neoplasia compared with anti-PD-1 alone. Only limited investigations of small sample size about anti-PD-1 alone showed favorable clinical benefit for patients with chemotherapy-resistant or refractory GTN.
Added value of this study
To our knowledge, this is the first cohort study to investigate the effect of anti-PD-1 therapy plus chemotherapy in patients with chemotherapy-resistant or refractory GTN. The combination of PD-1 inhibitor and chemotherapy provides a more significant improvement in antitumor activity compared with PD-1 inhibitor alone. Patients with no response to anti-PD-1 treatment could have a complete response to salvage chemotherapy regimens, even those which had previously been unsuccessful.
Similar incidences of grade 3–4 treatment-related adverse events were observed in patients with anti-PD-1 monotherapy or combined therapy.
Implications of all the available evidence
This multicenter retrospective study showed anti-PD-1 therapy plus chemotherapy can significantly improve the complete and overall response rates compared with anti-PD-1 therapy alone for patients with high-risk chemorefractory or relapsed GTN. PD-1 inhibitors could also restore sensitivity to following salvage chemotherapy. However, these data should be further confirmed with prospective clinical trials.
Introduction
Gestational trophoblastic neoplasia (GTN) represents a group of gynecological malignancies related to pregnancy, which originates from placental trophoblasts, including choriocarcinoma, malignant invasive mole, epithelioid trophoblastic tumor (ETT) as well as placental site trophoblastic tumor (PSTT). For effective chemotherapeutics have been applied, the overall response rate was over 90% in GTN patients, and a large proportion of cases achieving complete remission are treated by chemotherapy alone.1, 2, 3 Nonetheless, some patients develop chemorefractory tumors or have multiple recurrences after a period of chemotherapy. Subsequently, 0.5–5.0% of deaths occur because of multiple-drug resistance (MDR), resulting in the dismal prognostic outcome in patients with GTN.4 Therefore, it is urgently needed to search for novel treatments for such cases.
Programmed cell death 1/programmed cell death ligand 1 (PD-1/PD-L1) inhibitors have been developed for reinvigorating T cells and interrupting immunosuppression through PD-1/PD-L1 pathway within tumor microenvironment (TME),5 and they exerted anticancer effect on different cancers. Previous studies have demonstrated high PD-L1 expression within healthy placentas and diverse GTN subtypes, independent of International Federation of Gynecology and Obstetrics (FIGO) score, chemoresistance, or poorer clinical outcomes.6,7 Inhibitors targeting PD-1/PD-L1 among chemorefractory GTN cases have been quite popular recently, with successful therapeutic outcomes being attained in several series.8, 9, 10 A prospective study assessing avelumab, a PD-L1 inhibitor, treatment in GTN cases not responding to single-agent chemotherapy reported a complete response rate of 53.3% (8/15).11 As a new therapeutic strategy, the clinical practice guidelines released by the National Comprehensive Cancer Network (NCCN) recommend PD-1/PD-L1 inhibitors as an option in the treatment of patients with GTN resistant to chemotherapy.12
Evidence shows that treatment effectiveness is approximately 50% among chemorefractory or relapsed GTN cases after PD-1/PD-L1 inhibitor monotherapy.13 A combination strategy has recently been investigated to achieve optimal treatment effects. One phase-2 open-label, open-label, prospective trial conducted at Peking Union Medical College Hospital assessed the effectiveness of camrelizumab (PD-1 inhibitor) combined with apatinib (vascular endothelial growth factor receptor inhibitor) on chemorefractory or relapsed GTN.14 In total, 50% (10/20) of the included cases attained complete remission after combined treatment. Notably, five of the 7 (71.4%) patients who subsequently received salvage chemotherapy after discontinuation from treatment for disease progression had a complete remission without recurrence, which offered another potential instructive combination of chemotherapy and anti-PD-1 therapy. Preclinical and clinical studies suggest the synergistic antitumor effects of immunotherapy plus conventional chemotherapy in several tumors.15, 16, 17 However, only few studies assess immunotherapy combined with chemotherapy among GTN cases. Therefore, we conducted a retrospective analysis to describe whether immunotherapy combined with chemotherapy was effective and safe in patients with high-risk chemorefractory or relapsed GTN from three study centers in China and aimed to provide a novel treatment option for these patients.
Methods
Study design and subjects
We carried out the present retrospective cohort study in three tertiary teaching hospitals: Peking Union Medical College Hospital (PUMCH), China Medical University Affiliated to Shengjing Hospital, and Obstetrics and Gynecology Hospital of Fudan University. Patient inclusion criteria were shown below: (1) aged 18–60 years, (2) having chemorefractory (<50% reduction or elevation of serum human chorionic gonadotrophin [hCG] content after 2 or more continuous multidrug chemotherapy cycles) or relapsed (elevation of serum hCG content twice without pregnancy following normal hCG content [< 5 IU/L] for 4 weeks or longer after multidrug chemotherapy) GTN, and (3) having the FIGO score ≥7. Patients conforming to the standards below were excluded: prior or active inflammatory or autoimmune diseases; underlying or out-of-control diseases; or prior application of systemic immune-stimulator, immune checkpoint blockade, and/or systemic immunosuppressive agents and active infections.
Procedures
To determine the stage and FIGO risk factor score, all patients were evaluated comprehensively before study by performing medical history assessment, physical examination, blood test evaluation, thoracic CT, abdominal-pelvic contrast-enhanced CT or MRI scans. Patients with lung metastasis or neurological symptoms would also have brain contrast-enhanced CT or MRI scans. The pathologic slides of PSTT and ETT were reviewed and confirmed by at least two experienced pathologists. PD-1 inhibitor was administered intravenously with the following doses: 200 mg camrelizumab at 2-week intervals, 200 mg pembrolizumab at 3-week intervals, 240 mg toripalimab at 3-week intervals, and 200 mg sintilimab at 3-week intervals. In addition to immunotherapy, 20 patients received 250 mg apatinib through oral administration once a day, which was reported in our previous study.14
The patients from combined treatment group received chemotherapy during immunotherapy. Chemotherapy regimens included floxuridine, dactinomycin, and vincristine; etoposide, methotrexate, dactinomycin/cyclophosphamide, and vincristine (EMA/CO); floxuridine, dactinomycin, etoposide, and vincristine (FAEV); paclitaxel and cisplatin (TP); and paclitaxel, cisplatin/paclitaxel, and etoposide (TP/TE). Prophylactic application of G-CSF was not routinely given. When neutrophil count was <1.0 × 109/L at routine checkup, G-CSF was administered at the discretion of the treating physician. After a complete response was achieved, patients were recommended to receive at least two cycles of combined treatment for consolidation. At least six months of anti-PD-1 treatment after combined treatment was suggested to be a maintenance therapy until progressive disease or intolerable toxicity events occurred.
Serum hCG contents were determined weekly in the key laboratory. Radiographic evaluation was conducted by CT at intervals of 2 treatment cycles. Routine blood, renal and hepatic functions were tested at 2-week intervals. Additional hematological, endocrinological, biochemical, urine, and fecal tests were performed in each cycle. TRAEs were scored in line with National Cancer Institute Common Terminology Criteria or Adverse Events version 5.0.
Endpoints
Serum hCG content was determined to evaluate disease remission at intervals of 2 cycles (four or six weeks depending on the type of PD-1 antibodies) by a researcher. This study deemed complete remission (CR) as the normal hCG level detected 3 weeks consecutively, whereas partial remission (PR) as ≥50% reduction of hCG content at 2 cycles later compared with baseline level. Non-responders were patients with elevation of serum hCG content at 2 cycles later compared with baseline level or with novel metastases. For the hCG response profile with anti-PD-1 treatment remains poorly understood, experimental therapy could be continued when patients had reduction of hCG content at first two cycles of treatment. Non-responders would switch to chemotherapy approximately 2 weeks later due to severe disease conditions. The response definition was previously reported in our clinical trial.14 Primary outcome was the ORR, which indicated CR or PR patient percentage based on the serum hCG content. The secondary outcomes included treatment duration, which indicated the duration between immunotherapy initiation and CR or time of discontinuation from PD-1-based treatment due to the absence of response; overall survival (OS), which suggested the duration between the experimental treatment start and death or last follow-up; and safety outcomes; progression-free survival (PFS), which indicated the duration between treatment start and disease progression in line with serum hCG content or death.
Ethics statement
This work gained approval from ethics committees from each institution involved. Due to the retrospective nature of the study, written informed consent was waived.
Statistical analyses
Categorical data were represented by frequencies and percentages, while continuous data by median (interquartile range [IQR]). Shapiro–Wilk test was used to confirm the normality. Wilcoxon rank-sum test was utilized to compare continuous data, whereas categorical data were compared by chi. When the expected count was less than 5, categorical data were analyzed by Fisher's exact test. CR, ORR, and safety outcomes were assessed as the proportion in each treatment group, and the difference between the two groups was tested using Fisher's exact or Pearson's χ2 test.
Kaplan–Meier (KM) analysis stratified by treatment was conducted for survival analysis. The log-rank test was performed to compare patient survival between different groups. The Cox proportional hazards regression model was used to analyze the variables that may influence the survival of patients. A subgroup analysis to compare the 1-year progression-free survival between anti-PD-1 monotherapy and anti-PD-1 plus chemotherapy was planned for clinically relevant subgroups. Hazard ratios were estimated using univariable Cox regression model. The test of interaction (Wald test) between each variable and treatment was done. Statistical analyses were completed with R version 4.2.2 and SPSS22.0.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
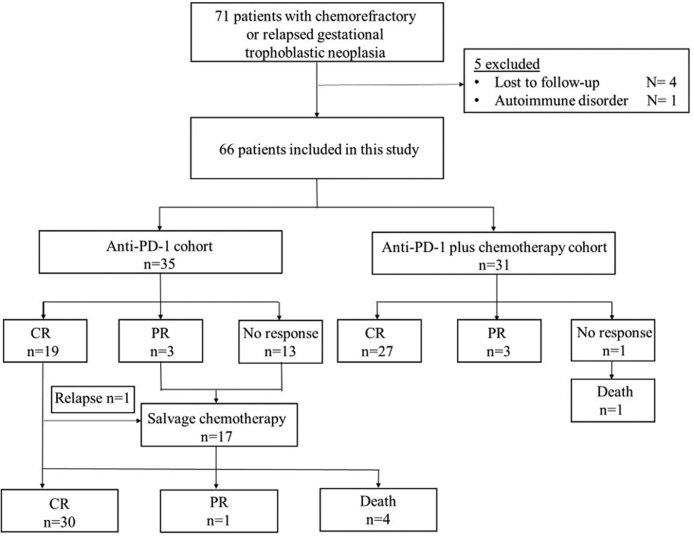
Between August 2018 and March 2022, the present work included 66 cases developing chemorefractory or relapsed GTN receiving anti-PD-1 monotherapy (n = 35) or combined treatment (n = 31) (Fig. 1). For anti-PD-1 therapy alone cohort, 15 (42.9%) and 20 (57.1%) patients received anti-PD-1 monotherapy and anti-PD-1 combined with apatinib, respectively. The majority had choriocarcinoma (59/66, 89.4%), and seven had intermediate trophoblastic tumors (three, ETT; four, PSTT). The 7 patients with PSTT/ETT were all occasionally found on a D&C, hysterectomy or thoracic metastasectomy due to the low hCG level and atypical clinical symptoms. Choriocarcinoma was occasionally seen on a D&C in eleven patients and metastasectomy in eleven patients. Twenty-one patients had surgeries when hCG was controlled after chemotherapy and twelve of them had histological confirmation for choriocarcinoma. Eight patients had pervious pathological diagnosis of hydatidiform mole. Only seven patients did not receive any surgery. Table 1 shows basic patient features. The median hCG concentrations before treatment in anti-PD-1 monotherapy and combined treatment cohorts were 255.67 (IQR, 74.44–3022.97) and 290 (IQR, 58.96–5454.00) IU/L, respectively (p = 0.954). Seven patients had a serum hCG concentration higher than 10,000 IU/L. The details of previous chemotherapies including regimens and courses each patient received are listed in Table S1. There was no significant difference in most characteristics, such as age, FIGO stage, FIGO score, Eastern Cooperative Oncology Group performance status, prior surgical treatment, previous multidrug chemotherapy, or metastasis sites, at the start of subsequent anti-PD-1 treatment with or without chemotherapy, only with discrepancy for type of anti-PD-1 antibodies (p = 0.008). The most common chemotherapy regimens used in patients combined with chemotherapy were FAEV (11 [35.5%] patients), TP/TE (9 [29.0%] patients), and EMA/CO (4 [12.9%] patients).
Fig. 1.
Flow diagram of the study.
Table 1.
Baseline characteristics stratified by treatment.
| Characteristics | Anti-PD-1 (n = 35) | Anti-PD-1 plus chemotherapy (n = 31) | P value |
|---|---|---|---|
| Age (y) | |||
| Median | 34 (28–55) | 32.5 (23–53) | 0.521 |
| Pathological type | 0.999 | ||
| Choriocarcinoma | 31 | 28 | |
| ETT + PSTT | 4 | 3 | |
| ECOG performance status | 0.517 | ||
| 0 | 14 | 14 | |
| 1 | 21 | 17 | |
| FIGO stage | 0.530 | ||
| I-II | 1 | 3 | |
| III | 24 | 15 | |
| IV | 10 | 13 | |
| FIGO score | 0.852 | ||
| 7 to 12 | 20 | 17 | |
| >12 | 15 | 14 | |
| Site of metastases | |||
| Lung | 30 | 22 | 0.234 |
| Brain | 5 | 6 | 0.743 |
| Liver | 3 | 4 | 0.999 |
| Antecedent pregnancy | 0.784 | ||
| Hydatidiform mole | 10 | 7 | |
| Abortion | 10 | 12 | |
| Term | 15 | 12 | |
| Pretreatment hCG concentration, IU/L | 0.480 | ||
| <10³ | 24 | 18 | |
| 10³ to 10⁴ | 7 | 10 | |
| >10⁴ | 4 | 3 | |
| Interval from antecedent pregnancy to anti-PD-1 treatment, years | 0.765 | ||
| <1 | 2 | 3 | |
| 1 to <3 | 13 | 11 | |
| 3 to <6 | 14 | 14 | |
| ≥6 | 6 | 3 | |
| Lines of previous multidrug chemotherapy | 0.165 | ||
| 1 | 2 | 8 | |
| 2 | 14 | 7 | |
| 3 | 10 | 10 | |
| 4 | 5 | 4 | |
| 5 | 4 | 2 | |
| Previous surgery | |||
| Hysterectomy | 19 | 12 | 0.253 |
| Thoracic metastasectomy | 12 | 9 | 0.715 |
| Other metastasectomy | 5 | 8 | 0.244 |
| Type of anti-PD-1 antibodies | 0.008 | ||
| Camrelizumab | 21 | 16 | |
| Sintilimab | 3 | 5 | |
| Toripalimab | 2 | 9 | |
| Pembrolizumab | 9 | 1 |
PD-1 = programmed death-1. ETT = epithelioid trophoblastic tumor. PSTT = placental site trophoblastic tumor. ECOG = Eastern Cooperative Oncology Group. FIGO = International Federation of Gynecology and Obstetrics. hCG = human chorionic gonadotrophin.
As of October 20, 2022, the median follow-up durations were 37.6 (IQR, 31.7–39.0) and 14.4 (IQR, 11.4–26.0) months for patients receiving anti-PD-1 monotherapy and combined treatment, separately. CR rate of combined treatment group (27 [87.1%] of 31 patients) was higher than anti-PD-1 monotherapy group (19 [54.3%] of 35 patients) (p = 0.007, Table 2). Combined treatment dramatically increased the ORR from 62.9% (22/35) to 96.8% (30/31) (p < 0.001, Table 2). The median durations until complete response were 2.2 (IQR, 1.4–4.2) and 2.8 (IQR, 1.8–2.8) months in the anti-PD-1 monotherapy and combined treatment cohorts, respectively (p = 0.299).
Table 2.
Response to treatment in two cohorts.
| Response to treatment | Anti-PD-1 (n = 35) |
Anti-PD-1 plus chemotherapy (n = 31) |
P value | ||
|---|---|---|---|---|---|
| Patients | Treatment duration (months) | Patients | Treatment duration (months) | ||
| Complete response | 19 (54.3%) | 2.2 (IQR, 1.4–4.2) | 27 (87.1%) | 2.8 (IQR, 1.4–2.8) | 0.007 |
| Partial response | 3 (8.6%) | 3 (9.7%) | |||
| Objective response rate | 22 (62.9%, 95%CI 39.4–95.2) | 30 (96.8%, 95%CI 65.3–138.2) | <0.001 | ||
| No response | 13 (37.1%) | 1 (3.2%) | |||
∗P value indicates the comparison of response rates.
PD-1 = Programmed death-1. IQR = interquartile range. CI = confidence interval.
As for anti-PD-1 therapy alone cohort, 19 (54.3%) cases attained CR, followed with consolidation of PD-1 antibodies (median: 6.5 courses, IQR 5.0–7.8). 3 (8.6%) patients attained PR, while 13 (37.1%) discontinued anti-PD-1 treatment due to absence of response. All 13 patients subsequently received salvage chemotherapy. The median interval between previous chemotherapy and salvage chemotherapy was 6.1 months (IQR, 3.1–9.2). Eleven (84.6%) of them attained CR, including 7 (63.6%) with CR to previously unresponsive chemotherapy regimens. Only one case who achieved a complete response via salvage chemotherapy experienced a recurrence 6 months later, and she died 3 years after withdrawing anti-PD-1 treatment. Those two other patients failed to respond to subsequent multiline chemotherapy and died 7 and 16 months after withdrawing anti-PD-1 treatment, respectively. Three patients achieving PR to anti-PD-1 therapy also received salvage chemotherapy. One patient showed complete response after FAEV and lobectomy. One death was reported due to progressive disease (PD) at 8 months following drug withdrawal. The remaining patient was still in a partial response status and received anti-PD-1 combined with carboplatin in last follow-up.
For combined treatment group, 27 cases achieved CR following the median 2.8-month treatment and proceeded with several courses for consolidation (median: 4 courses, IQR 3.5–4). After completion of the combined therapy, these patients subsequently received anti-PD-1 monotherapy as maintenance treatment, and the median duration was 7.6 (IQR, 4.8–10.2) months. The hCG concentrations of the three patients with partial response did not fall to the normal range by the end of follow-up. Only one patient showed disease progression after five cycles of FAEV plus anti-PD-1 treatment and died 6 months after treatment initiation.
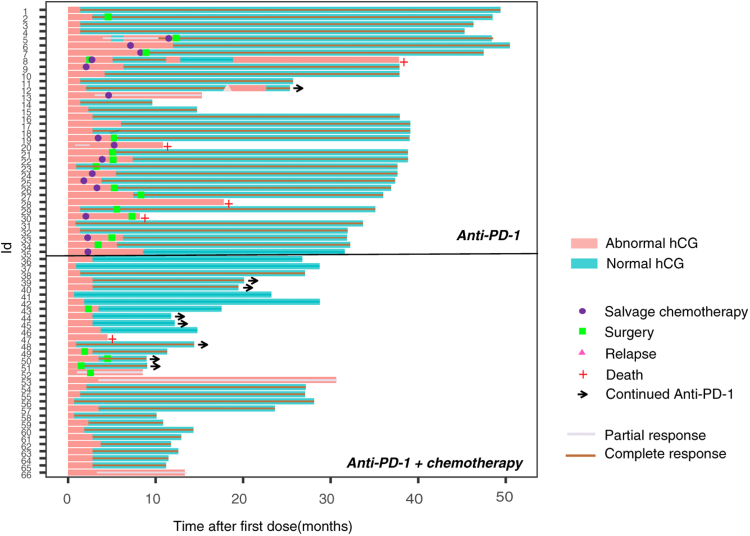
A swimmer plot showing the survival of each patient is shown in Fig. 2. At the time of analysis, only one patient in anti-PD-1 cohort was under treatment of anti-PD-1 combined with chemotherapy. Seven patients in combined group were still receiving anti-PD-1 monotherapy for maintaining by the cutoff date. The PFS and OS data were not mature in the two cohorts. In a post hoc analysis, the 1-year OS rates were 88.6% (95% confidence interval [CI], 74.1–95.5) and 96.8% (95% CI, 83.8–99.4) in the anti-PD-1 monotherapy and combined treatment cohorts, respectively. The 1-year PFS rate was 54.3% (95% CI, 38.2–69.5) of anti-PD-1 monotherapy group, while 96.8% (95% CI, 83.8–99.4) in combined treatment group, respectively. No significant difference in OS [HR 0.50 (95% CI 0.07–3.24), p = 0.499] was detected between anti-PD-1 cohort and anti-PD-1 plus chemotherapy cohort. The PFS in combined group was significantly longer than in anti-PD-1 group [HR 0.06 (95% CI 0.02–0.16), p < 0.001] (Fig. S1). Multivariable analysis adjusting for potential confounders clarified that treatment with anti-PD-1 plus chemotherapy was significantly associated with longer PFS than anti-PD-1 monotherapy (Table S2). In most clinical subgroups, consistent longer PFS with anti-PD-1 plus chemotherapy compared with anti-PD-1 monotherapy was confirmed (Table 3).
Fig. 2.
Swimmer plot for patients treated with anti-PD-1 with or without chemotherapy.
Table 3.
Subgroup analysis of progression-free survival.
| Variable | 1-year progression-free survival (95% CI) |
HR for progression (95% CI)a | Pinteraction | |
|---|---|---|---|---|
| Anti-PD-1 (n = 35) | Anti-PD-1 plus chemotherapy (n = 31) | |||
| Age (y) | 0.941 | |||
| <40 | 52.2 (31.1–72.6) | 95.7 (76.0–99.8) | 0.07 (0.01–0.55) | |
| ≥40 | 50.0 (22.2–77.7) | 100.0 (59.8–100.0) | 0.02 (0.00–13.30) | |
| FIGO score | 0.914 | |||
| 7 to 12 | 55.0 (32.1–76.2) | 100.0 (77.1–100.0) | 0.02 (0.00–3.34) | |
| >12 | 50.0 (22.3–72.6) | 92.9 (64.2–99.6) | 0.10 (0.01–0.83) | |
| Pretreatment hCG concentration, IU/L | 0.414 | |||
| <10³ | 50.0 (29.7–70.4) | 100.0 (78.1–100.0) | 0.02 (0.00–1.70) | |
| 10³ to 10⁴ | 42.9 (11.8–80.0) | 90.0 (54.1–99.5) | 0.02 (0.01–1.06) | |
| >10⁴ | 75.0 (21.9–98.7) | 100.0 (31.0–100.0) | 0.10 (0.01-) | |
| Lines of previous multidrug chemotherapy | 0.927 | |||
| ≤2 | 62.5 (35.9–83.7) | 100.0 (74.7–100.0) | 0.02 (0.00–11.11) | |
| >2 | 42.1 (21.1–66.0) | 93.8 (67.7–99.7) | 0.08 (0.01–0.61) | |
| Type of anti-PD-1 antibodies | 0.875 | |||
| Camrelizumab | 52.4 (30.3–73.6) | 100.0 (75.9–100.0) | 0.02 (0.00–2.49) | |
| Others | 50.0 (24.0–76.0) | 93.3 (66.0–99.7) | 0.14 (0.02–0.94) | |
| All patients | 51.4 (34.3–68.3) | 96.8 (81.5–99.8) | 0.05 (0.01–0.39) | |
Subgroup analysis of progression-free survival. An unstratified univariable Cox model was used to estimate HR.
A Wald test of interaction was done between variables and treatment groups.
PD-1 = programmed death-1. FIGO=International Federation of Gynecology and Obstetrics. hCG = human chorionic gonadotrophin.
HR = hazard ratio. CI = confidence interval.
Reference category: Anti-PD-1.
TRAEs occurred in 27 (77.1%) and 25 (80.6%) patients receiving anti-PD-1 monotherapy and combined treatment, respectively (Table 4). Grade 3–4 TRAEs with higher occurrence frequency included leukopenia (25.8%, combined treatment; 11.4%, anti-PD-1 monotherapy) and increased alanine aminotransferase level (9.7%, combined treatment; 5.7%, anti-PD-1 monotherapy) in all cohorts. One patient experienced anaphylactic shock during the fourth cycle of combined treatment. The patient was administered dexamethasone (5 mg) and adrenaline (0.3 mg) with rapid correction of shock. Cetirizine was administered to resolve allergic events, and treatment was resumed. Drug-related death was not reported in any groups.
Table 4.
Treatment-related adverse events.
| Number of patients with adverse events | Anti-PD-1 (n = 35) |
Anti-PD-1 plus chemotherapy (n = 31) |
||
|---|---|---|---|---|
| Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | |
| Fever | 12 (33.3%) | 0 | 10 (32.3%) | 0 |
| Fatigue | 3 (8.6%) | 0 | 3 (9.7%) | 0 |
| Hyperthyroidism | 6 (17.1%) | 0 | 0 | 0 |
| Hypothyroidism | 4 (11.4%) | 0 | 4 (12.9%) | 0 |
| Gastrointestinal system | ||||
| Diarrhea | 5 (14.3%) | 0 | 4 (12.9%) | 0 |
| Nausea or vomiting | 6 (17.1%) | 0 | 7 (22.6%) | 0 |
| Increased alanine aminotransferase | 8 (22.8%) | 2 (5.7%) | 8 (25.8%) | 3 (9.7%) |
| Increased aspartate aminotransferase | 6 (17.1%) | 4 (11.4%) | 7 (22.6%) | 1 (3.2%) |
| Increased amylase or lipase | 0 | 0 | 1 (3.2%) | 0 |
| Skin | ||||
| Rash or pruritus | 7 (20.0%) | 0 | 4 (12.9%) | 2 (6.5%) |
| RCCEP | 7 (20.0%) | 0 | 8 (25.8%) | 0 |
| Blood suppression | ||||
| Anaemia | 4 (11.4%) | 0 | 3 (9.7%) | 1 (3.2%) |
| Thrombocytopenia | 4 (11.4%) | 0 | 2 (6.5%) | 0 |
| Leukopenia | 12 (34.3%) | 4 (11.4%) | 6 (19.4%) | 8 (25.8%) |
PD-1 = programmed death −1. RCCEP = Reactive cutaneous capillary endothelial proliferation.
Discussion
This retrospective study examined whether PD-1 inhibitor combined with chemotherapy and anti-PD-1 monotherapy were effective and safe in high-risk chemorefractory or relapsed GTN cases. The combined treatment had a significantly higher CR rate and ORR than anti-PD-1 therapy alone. The combined treatment induced more grade 3–4 TRAEs, but all of them could be managed.
PD-L1 expression maintains gestational tolerance during pregnancy.18 PD-L1 is strongly expressed in GTN.7,19 Targeting programmed cell PD-1 inhibitory signaling is considered a potential strategy for chemorefractory or relapsed GTN. This is practical in patients with GTN with chemoresistance under several small series.8,9,20 Although the response rate was relatively higher than that in other solid tumors, exploratory studies on alternative treatments to complement the PD-1 inhibitor attribute are required. A preclinical study has demonstrated that conventional chemotherapeutic drugs, such as oxaliplatin, could cause immunogenic cell death and promote the anticancer immunity.21 Moreover, chemotherapy can also directly enhance the endogenous immune response by strengthening the functions of effector immune cells and inhibiting immunosuppressive cells to recover immunosurveillance.22,23 It is confirmed through clinical trials where immune checkpoint inhibitors administered with chemotherapy show great anticancer effect and controllable toxicity in different cancers including non-small cell lung cancer (NSCLC), esophageal and breast cancers.15,24,25 According to clinical trial results, pembrolizumab combined with chemotherapy was approved for squamous NSCLC in 2018 by the Food and Drug Administration (FDA). In 2021, nivolumab in combination with chemotherapy was approved to treat cases with metastatic or advanced gastric cancer (GC).26 Chemotherapy combined with PD-1 inhibitors has become a first-line therapy in certain cancers. However, research on anti-PD-1 plus chemotherapy in GTN is lacking.
In our work, 27 (87.1%) of 31 refractory cases receiving anti-PD-1 therapy combined with chemotherapy achieved a complete response with no evidence of relapse during follow-up. In anti-PD-1 therapy group, the complete response rate was 54.3% (19/35). Patients with or without apatinib administration presented similar baseline characteristics, response rates and TRAEs (Tables S3−S5). It is worth noting that 11 (84.6%) of 13 patients not responding to anti-PD-1 therapy achieved CR to salvage chemotherapy regimens. Seven (63.6%) of the 11 cases attained CR to previously unresponsive chemotherapy regimens. Consequently, anti-PD-1 may modulate the immune system and restore sensitivity to chemotherapy. Several retrospective studies have shown a promising response rate of salvage chemotherapy after PD-1/PD-L1 inhibitor treatment, which was almost up to that of first-line chemotherapy.27,28 This indicates that patients with no response to PD-1 inhibitors should be treated with caution, as anti-PD-1-refractory patients showed a remarkable response to salvage chemotherapy again. Further studies with larger cohorts are required to investigate how these responders can be selected. However, the sustainability of complete remission after anti-PD-1 therapy plus chemotherapy treatment merits attention. Data from PUMCH showed that 80% of high-risk chemorefractory or relapsed GTN cases relapsed in 6 months following salvage chemotherapy.29 According to our results, cases attaining CR to combined treatment exhibited durable complete remission to the cutoff date. Hence, chemotherapy might be compatible with PD-1 inhibitors to achieve both rapid and long-term tumor control. However, we cannot completely rule out the possibility that the resensitisation was caused by re-retreatment after an interval between 1st and 2nd chemotherapy treatment lines. Further basic researches to dive in to the tumor microenvironments are warranted and much more prospective clinical studies are needed for understanding the exact reason behind this phenomenon before we can really draw firm conclusions.
ETT and PSTT are histologically defined as intermediate trophoblastic tumors that respond poorly to conventional chemotherapy regimens.30 There were three patients with ETT and one with PSTT in anti-PD-1 therapy alone group, whereas three patients with PSTT in combined treatment group. Complete response rates were 50% (2/4) and 66.6% (2/3), respectively. According to a previous study, pembrolizumab showed effectiveness in advanced-stage PSTTs.8 Hence, intermediate trophoblastic tumors may benefit from antibodies blocking PD-1, with or without chemotherapy. However, our results must be confirmed with a large sample size.
As suggested by data regarding whether anti-PD-1 therapy combined with chemotherapy was safe, AEs conformed to those reported in previous publications on other cancers.26,31 Grade 3–4 TRAEs with the highest frequency included leukopenia and increased alanine aminotransferase level. The combined treatment group had a slightly increased incidence of certain TRAEs compared with anti-PD-1 therapy alone, but most of the immune-associated AEs were manageable.
This study has some limitations. First, the results needed to be carefully interpreted, given the retrospective and nonrandomized nature of this study. The selection of combined or single-agent treatment was determined by doctors in charge after comprehensive consideration, which varied between professors in the absence of a recognized criteria for treatment options, thus it was hard to define the standard for case selection. Second, our study had a small sample size because the disease is rare. Besides, sample counts in the two cohorts yielded unavoidable selection bias, as indicated by the difference in types of PD-1 antibodies between the treatment groups. However, the baseline characteristics were generally similar between the two groups, which made the two treatment modalities generally comparable. Additionally, the possibility of an initial increase in hCG concentration (may be pseudoprogression) before the hCG decline and subsequent normalization could not be fully ruled out. Nevertheless, pseudoprogression was not observed in previous reports concerning GTN.11,14 Finally, confirmation of GTN was mainly based on serum hCG concentration and clinical features, whereas pathological diagnosis was not mandatory. The evaluation of tumor tissue focusing on immune characteristics was difficult to conduct in this study. Despite these limitations, this is the largest reported analysis of patients with GTN receiving anti-PD-1 treatment with or without chemotherapy. A phase-II trial conducted in our institutions is currently recruiting patients for the assessment of the combination of anti-PD-1 therapy (NCT05139095). Another ongoing clinical trial aims to compare the efficacy of PD-L1 antibody Avelumab plus methotrexate in low-risk GTN patients (NCT04396223). In the future, they might be able to provide evidence with higher level for this patient population.
To sum up, our results suggest that the combined treatment significantly improves anticancer effect relative to PD-1 inhibitor monotherapy. Considering its acceptable tolerability, the combined treatment is favored over anti-PD-1 therapy alone in high-risk chemorefractory or relapsed GTN cases. Further prospective studies should be conducted for evaluating long-time survival benefits of combined treatment in heavily treated high-risk GTN cases.
Contributors
JJY, XL, and YX had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: JJY, XL, and YX
Acquisition, analysis, or interpretation of data: All authors (WC, XYW, XML, YC, XRW, FZF, TR, JZ, FJ, HYC, YG, LHC, CL, XQL, JJY, XL, and YX)
Drafting of the manuscript: WC, XYW, JJY, and YX
Statistical analysis and draft of manuscript: WC and XYW.
Obtained funding: JJY and YX.
Administrative, technical, or material support: XML and YC.
Supervision: JJY, XL, and YX
Data sharing statement
Data are available on reasonable request. The corresponding author could be contacted with requests.
Declaration of interests
No competing interests were declared.
Acknowledgements
We thank the patients and staff involved in the study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101974.
Contributor Information
Junjun Yang, Email: yangjunjun@pumch.cn.
Xin Lu, Email: xin_lu@fudan.edu.cn.
Yang Xiang, Email: xiangy@pumch.cn.
Appendix A. Supplementary data
References
- 1.Ngan H.Y.S., Seckl M.J., Berkowitz R.S., et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86–93. doi: 10.1002/ijgo.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolze P.A., Riedl C., Massardier J., et al. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of >/=13. Am J Obstet Gynecol. 2016;214(3):390.e1–390.e8. doi: 10.1016/j.ajog.2015.09.083. [DOI] [PubMed] [Google Scholar]
- 3.Freitas F., Braga A., Viggiano M., et al. Gestational trophoblastic neoplasia lethality among Brazilian women: a retrospective national cohort study. Gynecol Oncol. 2020;158(2):452–459. doi: 10.1016/j.ygyno.2020.04.704. [DOI] [PubMed] [Google Scholar]
- 4.Frijstein M.M., Lok C.A.R., Coulter J., et al. Is there uniformity in definitions and treatment of gestational trophoblastic disease in Europe? Int J Gynecol Cancer. 2019;29(1):108–112. doi: 10.1136/ijgc-2018-000028. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolze P.A., Patrier S., Massardier J., et al. PD-L1 expression in premalignant and malignant trophoblasts from gestational trophoblastic diseases is ubiquitous and independent of clinical outcomes. Int J Gynecol Cancer. 2017;27(3):554–561. doi: 10.1097/IGC.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 7.Veras E., Kurman R.J., Wang T.L., et al. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int J Gynecol Pathol. 2017;36(2):146–153. doi: 10.1097/PGP.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghorani E., Kaur B., Fisher R.A., et al. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet. 2017;390(10110):2343–2345. doi: 10.1016/S0140-6736(17)32894-5. [DOI] [PubMed] [Google Scholar]
- 9.Choi M.C., Oh J., Lee C. Effective anti-programmed cell death 1 treatment for chemoresistant gestational trophoblastic neoplasia. Eur J Cancer. 2019;121:94–97. doi: 10.1016/j.ejca.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Goldfarb J.A., Dinoi G., Mariani A., et al. A case of multi-agent drug resistant choriocarcinoma treated with Pembrolizumab. Gynecol Oncol Rep. 2020;32 doi: 10.1016/j.gore.2020.100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You B., Bolze P.A., Lotz J.P., et al. Avelumab in patients with gestational trophoblastic tumors with resistance to single-agent chemotherapy: cohort A of the TROPHIMMUN phase II trial. J Clin Oncol. 2020;38(27):3129–3137. doi: 10.1200/JCO.20.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Rustum N.R., Yashar C.M., Bean S., et al. Gestational trophoblastic neoplasia, version 2.2019, NCCN clinical practice guidelines in Oncology. J Natl Compr Cancer Netw. 2019;17(11):1374–1391. doi: 10.6004/jnccn.2019.0053. [DOI] [PubMed] [Google Scholar]
- 13.Salman L., Bouchard-Fortier G., Covens A. Immune checkpoint inhibitors for the treatment of gestational trophoblastic neoplasia: rationale, effectiveness, and future fertility. Curr Treat Options Oncol. 2022;23(7):1035–1043. doi: 10.1007/s11864-022-00988-8. [DOI] [PubMed] [Google Scholar]
- 14.Cheng H., Zong L., Kong Y., et al. Camrelizumab plus apatinib in patients with high-risk chemorefractory or relapsed gestational trophoblastic neoplasia (CAP 01): a single-arm, open-label, phase 2 trial. Lancet Oncol. 2021;22(11):1609–1617. doi: 10.1016/S1470-2045(21)00460-5. [DOI] [PubMed] [Google Scholar]
- 15.Sun J.M., Shen L., Shah M.A., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 16.Janjigian Y.Y., Shitara K., Moehler M., et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 18.Guleria I., Khosroshahi A., Ansari M.J., et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202(2):231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zong L., Zhang M., Wang W., et al. PD-L1, B7-H3 and VISTA are highly expressed in gestational trophoblastic neoplasia. Histopathology. 2019;75(3):421–430. doi: 10.1111/his.13882. [DOI] [PubMed] [Google Scholar]
- 20.Huang M., Pinto A., Castillo R.P., et al. Complete serologic response to pembrolizumab in a woman with chemoresistant metastatic choriocarcinoma. J Clin Oncol. 2017;35(27):3172–3174. doi: 10.1200/JCO.2017.74.4052. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H., Shan Y., Ge K., et al. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol (Dordr) 2020;43(6):1203–1214. doi: 10.1007/s13402-020-00552-2. [DOI] [PubMed] [Google Scholar]
- 22.Mathew M., Enzler T., Shu C.A., et al. Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther. 2018;186:130–137. doi: 10.1016/j.pharmthera.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Wesolowski R., Duggan M.C., Stiff A., et al. Circulating myeloid-derived suppressor cells increase in patients undergoing neo-adjuvant chemotherapy for breast cancer. Cancer Immunol Immunother. 2017;66(11):1437–1447. doi: 10.1007/s00262-017-2038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer C.J., Gadgeel S.M., Borghaei H., et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes J., Cescon D.W., Rugo H.S., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 26.Hindson J. Nivolumab plus chemotherapy for advanced gastric cancer and oesophageal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8):523. doi: 10.1038/s41575-021-00484-8. [DOI] [PubMed] [Google Scholar]
- 27.Schvartsman G., Peng S.A., Bis G., et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2017;112:90–95. doi: 10.1016/j.lungcan.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Park S.E., Lee S.H., Ahn J.S., et al. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol. 2018;13(1):106–111. doi: 10.1016/j.jtho.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Feng F., Xiang Y., Wan X., et al. Prognosis of patients with relapsed and chemoresistant gestational trophoblastic neoplasia transferred to the Peking Union Medical College Hospital. BJOG. 2010;117(1):47–52. doi: 10.1111/j.1471-0528.2009.02420.x. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz N.S., Goldstein D.P., Berkowitz R.S. Placental site trophoblastic tumors and epithelioid trophoblastic tumors: biology, natural history, and treatment modalities. Gynecol Oncol. 2017;144(1):208–214. doi: 10.1016/j.ygyno.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi L., Rodriguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.