Abstract
The use of agitated saline contrast (ASC) during echocardiographic examinations is a well-established practice, most commonly performed to identify atrial septal abnormalities in the context of stroke. In the intensive care unit, this technique may be employed to identify anatomic right-to-left shunts (either intracardiac or transpulmonary) that may be contributing to hypoxemic respiratory failure. This narrative review will describe the technique of ASC injection, summarize clinical scenarios where it may be useful, and review the strengths and limitations of the tool.
Keywords: intensive care, hypoxemia, agitated saline contrast, bubble study
Introduction
Hypoxemic respiratory failure is common in the intensive care unit (ICU). While the primary mechanism causing hypoxemia is usually well understood (with conditions such as the adult respiratory distress syndrome [ARDS], pneumonia, atelectasis, and cardiogenic pulmonary edema being among the most common), 1 in some cases the physiology is less straightforward or dual physiological abnormalities may co-exist. When the degree of hypoxemia is unexpectedly severe and out of keeping with the radiological appearance, an abnormal anatomical connection with right-to-left shunting of deoxygenated blood may be an exacerbating factor. Much less commonly a patient may have no apparent cause to explain their hypoxemia, and anatomical shunting may be considered as the primary explanation. In either case, the use of intravenous agitated saline contrast (ASC) as part of an echocardiographic examination should be considered as the next step in the diagnostic workup.
ASC injection is traditionally performed by sonographers and cardiologists, but given its ease of use, excellent safety profile, and good diagnostic performance characteristics it is well within the reach of all physicians with basic point-of-care ultrasound training. Indeed, international guidelines currently suggest ASC injection as a core skill for intensivists who use ultrasound for the management of acutely unwell patients. 2 In this article, we provide a detailed review of the use of ASC from the perspective of the acute care clinician with an emphasis on the technique itself, the clinical scenarios where it may be useful, and the potential pitfalls of the procedure. We focus on the identification of a patent foramen ovale (PFO) as the most common atrial septal abnormality, although other causes of intra-cardiac and extra-cardiac right-to-left shunting will be discussed briefly.
Historical Background and Relevant Anatomy
Injection of ASC was first described in 1968, 3 and by 1984 the American Society of Echocardiography had reported on over 27 000 ASC studies with no serious complications. 4 The technique has since become ubiquitous in the cardiology community and is supported by several comprehensive position statements,5,6 primarily as part of the evaluation for stroke. Cardiology-performed ASC studies are generally undertaken in stable patients on an elective basis in the echocardiography lab, where scanning conditions are optimal and where the patient can perform provocative measures designed to transiently accentuate a potential shunt.
There are essentially two conditions where aberrant anatomy may allow passage of deoxygenated blood to the systemic circulation: intracardiac and transpulmonary shunting. Intracardiac shunts are much more common, 7 and frequently related to the presence of a PFO. 8 A right-to-left shunt is necessary for the maintenance of fetal life as it allows blood to pass directly from the right atrium (RA) to the left atrium (LA), bypassing the non-aerated fetal lung. Following birth, pulmonary blood flow increases through the newly aerated lung, resulting in lower RA and higher LA pressure and thereafter closure of the foramen ovale. Permanent fusion occurs by 2 years of age in approximately 75% of individuals, with incomplete fusion of the septum primum and secundum resulting in a PFO in the remaining 25%. 9 Atrial septal defects (ASD) are a much less common, 9 but the ultrasound diagnosis of an ASD using ASC is made in the same manner as for a PFO. 10
Terms like “transpulmonary shunt” may have several meanings, and the details here are important. There is a small amount of normal right-to-left physiological shunting due to the bronchial circulation and cardiac veins. Pathological intrapulmonary shunting (sometimes referred to as capillary shunting, with a low or zero V/Q ratio) is the most common cause of hypoxemia in acutely unwell patients and is typically caused by pathology at the level of the alveoli such as atelectasis, pulmonary edema, ARDS, or pneumonia. 11 In the context of this article, however, we are referring to a different (and unexpected) type of shunting at the level of the lungs caused by an abnormal anatomic right-to-left connection, most commonly the result of an arteriovenous malformation (AVM). The presence of an AVM allows deoxygenated blood to transit from the RV to the LA without first being oxygenated. It can cause or aggravate hypoxemia and may be detected by injection of ASC or using computerized tomography (CT). 12 Hereditary hemorrhagic telangiectasia (also called Osler-Weber-Rendu syndrome) is the classic condition associated with this phenomenon. 13
Significant liver disease may be associated with hypoxemia (as well as dyspnea, platypnea, and orthodeoxia), in a phenomenon typically referred to as a hepatopulmonary syndrome. 14 While poorly understood, it may be related to an increase in circulating nitric oxide, resulting in dilatation of the pulmonary capillary bed. 15 This dilatation interferes with normal oxygenation across the pulmonary microcirculation, allowing deoxygenated blood to transit to the LA. While the causative pathology is at the level of the liver, it is not a “transhepatic” shunt that causes hypoxemia, but rather a form of transpulmonary shunting. As such, the echocardiographic appearance and diagnosis is identical to that of other causes of transpulmonary shunting. Confusingly, orthodeoxia has also been associated with intracardiac anatomical shunts as well as pulmonary AVMs, as positional changes may result in more or less shunting depending on the specific anatomy of these abnormalities. 16
There are other, less well-understood scenarios where transpulmonary shunting may occur. It has been described in healthy subjects during exercise and also in septic patients, leading to the hypothesis that low pulmonary transit time due to high cardiac output may be responsible. 17 It has also been speculated that patients with ARDS may have an increased degree of transpulmonary shunting, but this has not been a consistent finding. 15
Physics and Safety
Using a standard preparation technique, ASC injection creates microbubbles of an average size of 16 to 38 µm. 18 Given a pulmonary capillary diameter of approximately 9 µm, 19 such microbubbles cannot transverse to the systemic circulation. While they will transiently obstruct tiny branches of the pulmonary microcirculation, the volume of air injected is much too small to cause any degree of hemodynamic disturbance. A tiny proportion of microbubbles may be smaller than 9 µm, however due to their surface tension characteristics they have a half-life that is less than pulmonary circulation time. The finding of microbubbles in the left atrium following ASC injection, therefore, indicates a pathological intracardiac or transpulmonary shunt.
The primary safety concern with ASC injection involves the risk of cerebral air embolism in the context of a right-to-left shunt, where injected microbubbles cross over to the systemic circulation and occlude a small cerebral vessel. While this risk is extremely low, there are indeed anecdotal reports of stroke. 20 Properly mixed ASC results in microbubbles that are sufficiently small and short lived that cerebral embolism should not occur, even with passage into the left-sided circulation. As such, and given the extremely reassuring safety profile of the test over hundreds of thousands of exams performed, 4 any resulting strokes are both vanishingly rare and likely to be the result of technical errors, with improperly prepared and injected ASC. Encouragingly, the rare ischemic events described in case reports are mild and short lived. 4
An alternate ASC technique, not discussed in detail here, sheds further light on the safety of the procedure. To prove the presence of left-sided microbubbles (and thus a right-to-left shunt), bubbles can be detected by ultrasound within the middle cerebral artery using transcranial Doppler imaging. 21 Visualization of microbubbles within the cerebral circulation following intravenous ASC injection is highly specific and sensitive for right-to-left shunt, 21 and yet not associated with cerebral air embolism causing stroke. 22
Technique of ACS injection
Injection site selection
Recommend practice 6 involves using a peripheral intravenous (IV) (PIV) inserted in the region of the antecubital fossa, and this setup will be adequate for most situations. In real life practice, however, acute care providers can generally use, with good success, any well-functioning IV they have access to. Very small PIVs (smaller than 20 gauge) are not recommended. The IV site should be carefully inspected and tested prior to use, with a vigorous practice injection undertaken to ensure that the IV can withstand high pressure without becoming dislodged or “blowing” the vein.
There are rare scenarios where a specific IV site can be theoretically advantageous. A proximal lower extremity injection site, such as the femoral vein, connected to the RA via the inferior vena cava (IVC), has a hypothetical advantage in that the IVC directs blood (and ASC) more directly toward the inter-atrial septum (IAS).23,24 Lower body injection is also better where there are concerns about a residual inferior sinus venosus ASD after a closure procedure. A left arm site is favored when trying to diagnose a persistent left-sided SVC 25 ; these are all ultra-rare scenarios. More commonly, a critically ill patient may have only a central venous catheter (CVC) for IV access; while there is a paucity of evidence here, in the authors experience CVCs can just as easily be used for ASC injection.
Small details surrounding injection site selection for stable patients with cryptogenic strokes, where minor and intermittent shunting may be important, are not as applicable here. Acute care providers are generally looking for large shunts with significant and continuous right-to-left shunting as an explanation for significant ongoing hypoxemia, and so absolutely optimal ASC injection is less likely to be a crucial factor.
Creating bubbles
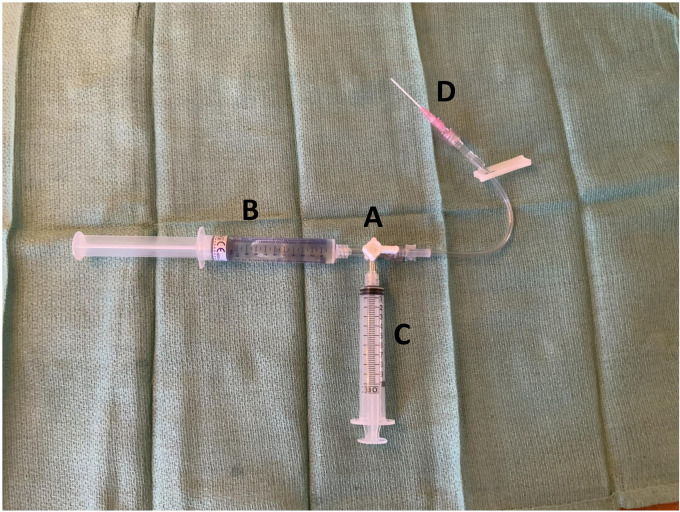
There is some debate as to the best “formula” for making bubbles, but two main options generally prevail. In either case a 3-way stopcock is prepared and purged of air. One port is connected to the patient's IV line, and a second port to an empty 10cc syringe with the plunger fully depressed. The first formula (simpler, more commonly used, and here recommended by the authors) calls for the third port to be connected to a syringe filled with 9ccs of saline and 1cc of air, accomplished by ejecting 1cc of saline from the syringe of normal saline (NS) and replacing it with air (see Figure 1). The second formula calls for a small quantity of the patient's blood to be added, accomplished by withdrawing it from the IV catheter to be used for eventual injection just prior to the procedure. While there is some suggestion that adding blood to the mixture may result in easier to visualize bubbles, 10 most centers use the simpler NS-only recipe. 26 An agitated saline and propofol mixture produced greater contrast intensity during pediatric transesophageal echocardiogram (TEE) in one study, 27 suggesting other options are also possible.
Figure 1.
Basic equipment for injection of agitated saline contrast. (A) The 3-way stopcock. (B) A 10cc syringe with 9cc sterile 0.9% normal saline, and 1cc of air. (C) Empty 10cc syringe. (D) Intravenous catheter inserted into patient.
With the equipment connected to the patient's IV, bubbles are created by turning the stopcock valve to “off” toward the patient and alternately depressing the plungers on the 2 syringes to send the air/NS mixture back-and-forth between them (see Video 1). This should be done forcefully to create an ASC mixture with very small microbubbles, which is the key safety step in the process. As discussed above, vigorous mixing and a properly purged system will create tiny bubbles which are either too small to pass through the pulmonary microcirculation, or so small that they have an extremely short half-life. At the appropriate time, the ASC is pushed into one of the syringes and the stopcock quickly turned to “off” toward the other, allowing the microbubbles to be forcefully injected into the patient.
Echocardiographic views
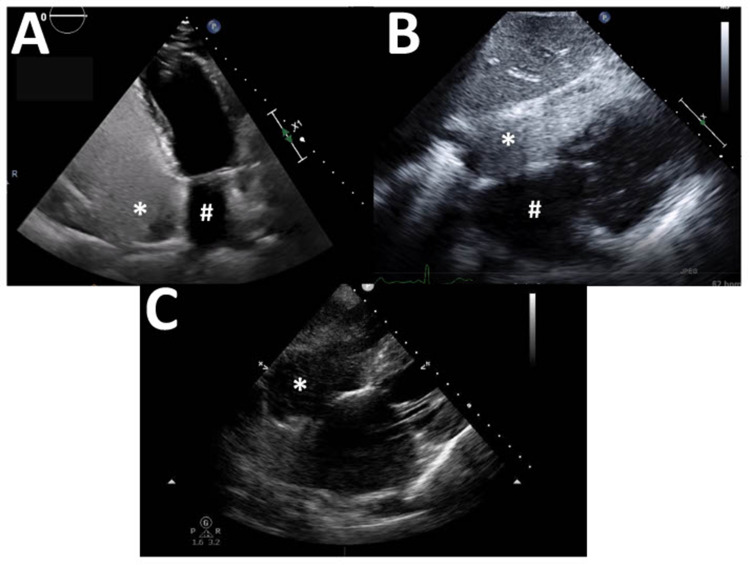
As one operator is preparing the IV equipment, a second can set up the ultrasound machine and determine the best echocardiographic views for the procedure. For a transthoracic exam (TTE), any view where the RA, LA, and IAS can be seen will suffice (Figure 2). Commonly an apical 4-chamber view is favored, with a focus on the bilateral atria. For critically ill patients where views can often be challenging to acquire, the operator may have to settle for the best view available, which is often a sub-xiphoid 4-chamber view.
Figure 2.
Common echocardiography views for a transthoracic study. (A) Apical 4-chamber view with left atrium (#) and right atrium (*; densely opacified by agitated saline contrast) in view. (B) Subcostal 4-chamber view with left atrium (#) and right atrium (*; densely opacified by agitated saline contrast) in view. (C) Subcostal 4-chamber view demonstrating inadequate opacification of the right atrium (*); a few scattered bubbles are seen.
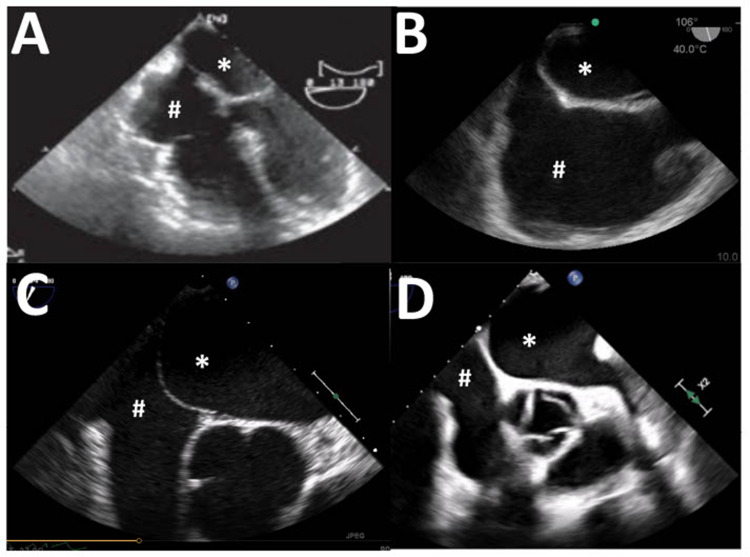
Once the best possible view has been achieved, the ultrasound machine should be set to record a lengthy (approximately 20 s) clip. ASC can then be injected by the second operator, and the recording started at the time of injection in order that the arrival of bubbles in the RA and their potential appearance in the left heart is captured. A vigorous injection should result in dense opacification of the RA (Figure 2B), which is an important quality-control check. If the chamber is not densely opacified (Figure 2C) then a technical problem has occurred, and the exam should not be interpreted. For a TEE exam the procedure is similar, and an array of views which capture the RA, LA, and IAS may be used (Figure 3). Common choices include a mid-esophageal 4-chamber view, mid-esophageal bicaval view, mid-esophageal aortic valve short-axis view, or a mid-esophageal right ventricular inflow-outflow view.
Figure 3.
Common echocardiography views for a transesophageal study. (A) Mid-esophageal 4-chamber view. (B) Bicaval view. (C) Mid-esophageal aortic valve short-axis view. (D) Mid-esophageal right ventricular inflow-outflow view. In all view the right atrium (#) and left atrium (*) are seen.
Exam interpretation
Assuming an injection resulting in the dense opacification of the RA, the exam may be thereafter interpreted. The LA should be examined for a period of at least 10 full beats (electrocardiogram leads are helpful, but not indispensable here), monitoring for the pathological appearance of ASC in the LA or left ventricle (LV).
Where microbubbles are seen in the LA, the first consideration is the timing of their appearance; here the goal is to determine what kind of right-to-left shunt exists. If microbubbles appear immediately (within 3-6 beats is a typically used cutoff), 10 then the shunt is likely to be intracardiac, and statistically most likely due to the presence of a PFO. Under ideal circumstances, and with a clear ultrasound view of the IAS, bubbles can be seen to transit across the septum in real time, confirming the hypothesis. Bubbles arriving after the 3-6 beat cutoff are considered more likely to be due to a transpulmonary shunt, either an AVM or hepatopulmonary syndrome, depending on the clinical circumstances. Further workup here might include a CT angiogram of the chest or workup for cirrhosis. Importantly, the 3-6 beat cutoff is somewhat arbitrary, and its accuracy has been called into question. 26
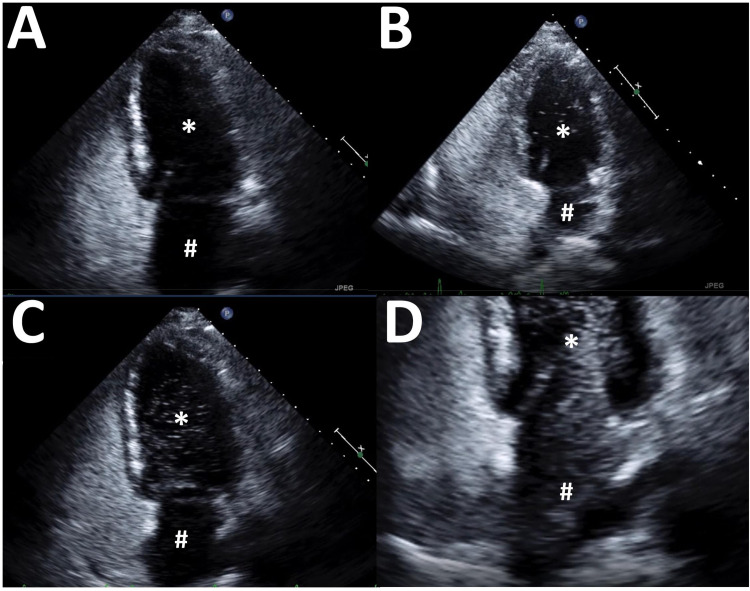
The second element to consider for a positive test is the quantity of microbubbles detected in the LA. While a single bubble is pathological, semiquantitative grading scales have been proposed, for example: grade 0 (no bubbles detected), grade 1 (1-5 bubbles), grade 2 (6-20 bubbles), grade 3 (21-50 bubbles), and grade 4 (>50 bubbles). 28 The counting of microbubbles, however, is laborious and likely to be inaccurate; it depends, for example, on which specific ultrasound frame is chosen for analysis, the quality of the image, and the many subjective elements surrounding the preparation and injection of the ASC. The authors recommend a rough qualitative interpretation: either no bubbles, a small number (roughly <10), a large number (roughly >10), or enough to completely opacifying the LA (Figure 4). While this may seem an oversimplification at first glance, it should be remembered here that the goal is to explain hypoxemia, not find a theoretical small passage to explain a cryptic stroke. Significant continuous hypoxemia requires significant continuous right-to-left shunting, and thus the ongoing passage of many ASC bubbles.
Figure 4.
Varying results from injection of agitated saline contrast. (A) Negative study, no bubbles detected. (B) Positive study, a small number of bubbles detected (<10). (C) Positive study, a large number of bubbles detected. (D) Positive study, a very large number of bubbles detected with opacification of the left atrium. In all view the left atrium (#) and left ventricle (*) are seen.
Valsalva maneuver
The purpose of the Valsalva maneuver is to elicit a transient increase in RA pressure to unmask a subtle right-to-left intracardiac shunt. Even if a pathological connection exists between the RA and LA, there will be no movement of blood if the pressures are equal in both chambers. With LA pressure higher than RA pressure, as is typically the case, blood may move from left-to-right but this phenomenon will not be visualized by injection of ASC into the RA. During the release phase of the Valsalva maneuver, RA pressure transiently rises above that of the LA. The patient is instructed to release the active phase once contrast is visualized in the RA 29 ; this is not feasible in critically ill patients. It is important to confirm elevation of RA pressure above LA pressure by visualizing a deviation in the interatrial septum toward the LA during the release phase. 30 The addition of positive end expiratory pressure has been shown to increase right-to-left shunting in mechanically ventilated patients with PFO, and can be used a provocative manuever in this population. 31 A small connection, especially in the context of an otherwise anatomically normal heart (with normal RA pressure), may only demonstrate right-to-left shunting under specific and transient loading conditions where RA pressure is high and/or LA pressure is low. Such small shunts are typically more relevant in the investigation of stroke; while they can be considered in specific circumstances, they are not typically useful in the ICU and are well covered elsewhere. 32
Performance characteristics
Data regarding the performance characteristics of ASC exams is derived from the stroke literature, and generally involves searching for PFOs with relatively small amounts of shunting. It is therefore difficult to apply to critically ill patients, but nonetheless the data is helpful to understand. TTE is generally more specific (99% in one large meta-analysis) than sensitive (46%) 33 ; this is not ideal as the high rate of potential false negative exams is suboptimal for what is essentially a screening test. TEE seems to perform better (mean sensitivity of 89% and specificity of 91% in one large meta-analysis). 34 Both TTE and TEE perform better with larger amounts of shunting, which is very encouraging for acute care providers.
Pitfalls and Pearls
In the search for the cause or aggravating factor for severe hypoxemia in an acutely unwell patient, most potential problems occur when the ASC study is negative, but the clinical suspicion of an anatomic shunt remains high. Here the clinician should follow the physiology: if the patient is hypoxemic with normal chest imaging, then the pre-test probability of a pathological shunt is high, and a negative ASC test might well be a false negative due to technical errors. Alternatively, areas of low V/Q ratios might not be evident on chest x-ray, and additional radiologic evaluation may be needed (lung ultrasound or CT scan).
Overall exam quality
TTE image acquisition can be notoriously difficult in ICU patients, and the LA can be particularly difficult to visualize and keep in view during the entire lengthy image capture process. Other TTE views can be attempted here, the patient repositioned, or a different echocardiographer can attempt to acquire better images. Otherwise, consideration should be given to TEE, where image quality is almost always excellent.
Bubble quality
The quality of the microbubbles should be evaluated. Did they arrive quickly and in bulk? Did they densely opacify the RA? Were the LA and LV in view as the bubbles arrived and for at least 5-10 beats thereafter? If not, then re-injection should be undertaken after evaluating the IV site and quality of saline agitation.
Patient positioning
Patient position can have an effect on the amount of shunting due to specific anatomical conditions, with some examples given in the next section. Unusual anatomical shunts with important positional dependence have been documented, 25 although they are much less likely to be relevant in severely hypoxemic patients. If the clinical suspicion remains high, then the exam should be repeated in alternate positions which may include right or left deep lateral decubitus, deep Trendelenburg, or with the patient sitting upright.
Unusual anatomy
There are several anatomic variants which can misdirect the flow of blood entering the RA and cause it to interact differently with the IAS. Examples include a Chiari network (caused by incomplete resorption of the embryological sinus venosus) 35 or a Eustachian valve (a remnant of a fetal IVC valve). 36
Summary: False negatives
Particularly for TTE, essentially a screening test in this context, a false negative test can cause significant harm by delaying the eventual diagnosis. Due to the relatively lower sensitivity of this exam, at least when extrapolating from the stroke literature, this possibility should be kept in mind. False negatives can occur if the LA pressure is the same or higher than the RA pressure, with a small shunt, with poor image quality, with poor bubble generation or injection, with IV access problems, or in the presence of a Eustachian valve. A negative test in the context of high pre-test probability argues for the consideration of a TEE.
Summary: False positives
False positives are relatively much less likely due to the relatively good specificity of the test. They can occur if a Eustachian valve is mistaken for the IAS, or in cases where a prolonged Valsalva hold created hyperechoic “Rouleau” in the pulmonary vasculature due to stagnant blood. 37
Clinical Scenarios
Case 1
A 70-year-old man with a history of smoking and a recent diagnosis of probable idiopathic pulmonary fibrosis presents with worsening dyspnea and is subsequently admitted to the ICU due to high oxygen requirements. The rapid increase in symptoms, on the background of very slowly progressive dyspnea on exertion, prompted a workup for other causes. Pulmonary embolism (PE) was excluded by CT chest, which also demonstrated a stable appearance of his radiological usual interstitial pneumonia pattern. A bubble study was conducted via TTE approach, and a strongly positive test was observed from both an apical 4-chamber and subcostal 4-chamber view (Videos 2.1 and 2.2).
Case 2
A 45-year-old morbidly obesity male was intubated and admitted to the ICU for altered mental status in the setting of acute hypercapnic respiratory failure. He became severely hypoxemic after intubation. His chest x-ray was difficult to interpret, and his CT chest and lung ultrasound were normal apart from significant bibasilar atelectasis. There was no deep vein thrombus or PE detected. A bubble study via TTE approach was attempted and interpreted as negative but impeded by poor image quality (Video 3.1). ASC injection was thereafter observed via TEE. Contrast was seen to appear in the LA immediately on a good-quality mid-esophageal 4-chamber view (Video 3.2), confirming a left-to-right intracardiac shunt. However, given the modest number of bubbles this was not felt to be a complete explanation for the patient's hypoxemia, but likely served as an exacerbating factor in the presence of significant atelectasis.
Case 3
A 50-year-old female with a history of injection drug use was admitted to the ICU after having been found down and intubated in the field by paramedics. She was hypoxemic, more so when placed in the right lateral decubitus position. Aspiration was suspected, but chest imaging was not consistent with that diagnosis. ASC injection was performed, initially from the traditional left lateral decubitus position, and was interpreted as negative (Video 4.1). The patient was turned onto her right side and the exam repeated; this time the exam was strongly positive, with immediate opacification of the LA and LV (Video 4.2). A TEE was performed, confirming the presence of an atrial septal abnormality with visible right-to-left shunt by color Doppler (Video 4.3).
Conclusion
Given that hypoxemic respiratory failure is one of the most commonly encountered problems in acutely unwell patients, it is important that physicians caring for them be equipped with the full array of tools to thoroughly investigate and understand the aberrant physiology. While traditionally the purview of cardiologists and sonographers, the injection of ASC is a safe and straightforward procedure, easily within the reach of all acute care providers with basic ultrasound skills. Careful attention to safety concerns and an awareness of common pitfalls will unlock this important technique, to the benefit of patients with hypoxemic respiratory failure.
Supplemental Material
Video 1.
Video 2.
Video 3.
Video 4.
Video 5.
Video 6.
Video 7.
Video 8.
Supplemental material, sj-docx-1-jic-10.1177_08850666231159019 for Agitated Saline Contrast Injection in Patients with Severe Hypoxemia by Scott J. Millington, Henry Mayo-Malasky and Seth Koenig in Journal of Intensive Care Medicine
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Scott J. Millington https://orcid.org/0000-0002-2962-5771
Supplemental Material: Supplemental material for this article is available online.
References
- 1.SRLF Trial Group. Hypoxemia in the ICU: Prevalence, treatment, and outcome. Ann Intensive Care. 2018;13(8):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Expert Round Table on Echocardiography in ICU. International consensus statement on training standards for advanced critical care echocardiography. Intensive Care Med. 2014;40(5):654-666. [DOI] [PubMed] [Google Scholar]
- 3.Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol. 1968;3(5):356-366. [DOI] [PubMed] [Google Scholar]
- 4.Bommer WJ, Shah PM, Allen H, Meltzer R, Kisslo J. The safety of contrast echocardiography: Report of the committee on contrast echocardiography for the American Society of Echocardiography. J Am Coll Cardiol. 1984;3(1):6-13. [DOI] [PubMed] [Google Scholar]
- 5.Waggoner AD, Ehler D, Adams Det al. et al. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: Recommendations of the American Society of Echocardiography Council on Cardiac Sonography. J Am Soc Echocardiogr. 2001;14(5):417-420. [DOI] [PubMed] [Google Scholar]
- 6.Porter TR, Abdelmoneim S, Belcik JT. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: A focused update from the American Society of Echocardiography. J Am Soc Echocardiogr. 2014;27(8):797-810. [DOI] [PubMed] [Google Scholar]
- 7.Pacca R, Maddukuri P, Pandian NG, Kuvin JT. Echocardiographic detection of intrapulmonary shunting in a patient with hepatopulmonary syndrome: Case report and review of the literature. Echocardiography. 2006;23(1):56-59. [DOI] [PubMed] [Google Scholar]
- 8.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59(1):17-20. [DOI] [PubMed] [Google Scholar]
- 9.Sommer RJ, Hijazi ZM, Rhodes JF. Pathophysiology of congenital heart disease in the adult: Part I: Shunt lesions. Circulation. 2008;117(8):1090-1099. [DOI] [PubMed] [Google Scholar]
- 10.Silvestry FE, Cohen MS, Armsby LB. American Society of Echocardiography; Society for Cardiac Angiography and Interventions. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale. J Am Soc Echocardiogr. 2015;28(8):910-958. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Roisin R, Roca J. Mechanisms of hypoxemia. Intensive Care Med. 2005;31(8):1017-1019. [DOI] [PubMed] [Google Scholar]
- 12.Saboo SS, Chamarthy M, Bhalla S. Pulmonary arteriovenous malformations: Diagnosis. Cardiovasc Diagn Ther. 2018;8(3):325-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shovlin CL, Letarte M. Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations: Issues in clinical management and review of pathogenic mechanisms. Thorax. 1999;54(8):714-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas E, Aktas A, Parikh H, Khawaja US, Pergament K. Platypnea-orthodeoxia syndrome in a patient with cryptogenic liver cirrhosis: An elusive cause of hypoxemia. Cureus. 2019;11(1):e3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boissier F, Razazi K, Thille AW. Echocardiographic detection of transpulmonary bubble transit during acute respiratory distress syndrome. Ann Intensive Care. 2015;5(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henkin S, Negrotto S, Pollak PM. Platypnea-Orthodeoxia syndrome: Diagnostic challenge and the importance of heightened clinical suspicion. Tex Heart Inst J. 2015;42(5):498-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eldridge MW, Dempsey JA, Haverkamp HC, Lovering AT, Hokanson JS. Exercise-induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol. 2004;97(3):797-805. [DOI] [PubMed] [Google Scholar]
- 18.Feinstein SB, Ten Cate FJ, Zwehl W. Two-dimensional contrast echocardiography. I. In vitro development and quantitative analysis of echo contrast agents. J Am Coll Cardiol. 1984;3(1):14-20. [DOI] [PubMed] [Google Scholar]
- 19.Butler BD, Hills BA. The lung as a filter for microbubbles. J Appl Physiol Respir Environ Exerc Physio. 1979;47(3):537-543. [DOI] [PubMed] [Google Scholar]
- 20.Romero JR, Frey JL, Schwamm LH. Cerebral ischemic events associated with ‘bubble study’ for identification of right to left shunts. Stroke. 2009;40(7):2343-2348. [DOI] [PubMed] [Google Scholar]
- 21.Komar M, Olszowska M, Przewłocki T. Transcranial Doppler ultrasonography should it be the first choice for persistent foramen ovale screening? Cardiovasc Ultrasound. 2014;1(16):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsivgoulis G, Stamboulis E, Sharma VK. Safety of transcranial Doppler ‘bubble study’ for identification of right to left shunts: An international multicentre study. J. Neurol Neurosurg Psychiatry. 2011;82(11):1206-1208. [DOI] [PubMed] [Google Scholar]
- 23.Cai S, Ratano D, Douflé G. Bubble study from the upper limb? Watch out for eustachius!. Intensive Care Med. 2019;45(11):1649-1650. [DOI] [PubMed] [Google Scholar]
- 24.Gin KG, Huckell V, Pollick C. Femoral vein delivery of contrast medium enhances transthoracic echocardiographic detection of patent foramen ovale. J Am Coll Cardiol. 1993;22(7):1994-2000. [DOI] [PubMed] [Google Scholar]
- 25.Stewart JA, Fraker TD, Slosky DA. Detection of persistent left superior vena cava by two-dimensional contrast echocardiography. J Clin Ultrasound. 1979;7(5):357-360. [DOI] [PubMed] [Google Scholar]
- 26.Bernard S, Churchill TW, Namasivayam M. Agitated saline contrast echocardiography in the identification of intra- and extracardiac shunts: Connecting the dots. J Am Soc Echocardiogr. 2021;34(1):1-12 [DOI] [PubMed] [Google Scholar]
- 27.Dewhirst E. The use of a propofol-saline mixture for enhanced contrast in bubble studies during echocardiographic examinations. Society of Pediatric Anesthesia Meeting. 2012. [Google Scholar]
- 28.Rana BS, Thomas MR, Calvert PA. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc Imaging. 2010;3(7):749-760. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell C, Rahko PS, Blauwet LA. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32(1):1-64. [DOI] [PubMed] [Google Scholar]
- 30.Saric M, Armour AC, Arnaout MS. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr. 2016;29(1):1-42. [DOI] [PubMed] [Google Scholar]
- 31.Cujec B, Polasek P, Mayers I. Positive end-expiratory pressure increases the right-to-left shunt in mechanically ventilated patients with patent foramen ovale. Ann Intern Med. 1993;119(9):887-894. [DOI] [PubMed] [Google Scholar]
- 32.Pinto FJ. When and how to diagnose patent foramen ovale. Heart. 2005;91(4):438-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mojadidi MK, Winoker JS, Roberts SC. Accuracy of conventional transthoracic echocardiography for the diagnosis of intracardiac right-to-left shunt: A meta-analysis of prospective studies. Echocardiography. 2014;31(9):1036-1048. [DOI] [PubMed] [Google Scholar]
- 34.Mojadidi MK, Bogush N, Caceres JD. Diagnostic accuracy of transesophageal echocardiogram for the detection of patent foramen ovale: A meta-analysis. Echocardiography. 2014;31(6):752-758. [DOI] [PubMed] [Google Scholar]
- 35.Schneider B, Hofmann T, Justen MH, Meinertz T. Chiari's network: Normal anatomic variant or risk factor for arterial embolic events? J Am Coll Cardiol. 1995;26(1):203-210. [DOI] [PubMed] [Google Scholar]
- 36.Schuchlenz HW, Saurer G, Weihs W, Rehak P. Persisting eustachian valve in adults: Relation to patent foramen ovale and cerebrovascular events. J Am Soc Echocardiogr. 2004;17(3):231-233. [DOI] [PubMed] [Google Scholar]
- 37.Van Camp G, Cosyns B, Vandenbossche JL. Non-smoke spontaneous contrast in left atrium intensified by respiratory manoeuvres: A new transoesophageal echocardiographic observation. Br Heart J. 1994;72(5):446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1.
Video 2.
Video 3.
Video 4.
Video 5.
Video 6.
Video 7.
Video 8.
Supplemental material, sj-docx-1-jic-10.1177_08850666231159019 for Agitated Saline Contrast Injection in Patients with Severe Hypoxemia by Scott J. Millington, Henry Mayo-Malasky and Seth Koenig in Journal of Intensive Care Medicine