Abstract
Interest in the measurement of circulating tumor DNA (ctDNA) in colorectal cancer (CRC) has increased during the past decade. The analysis of quantitative ctDNA changes as a general response evaluation criterion during systemic treatment is a scientific approach with high clinical potential, and results can be transferred to a pan-cancer concept if relevantly investigated. The purpose of this overview is to discuss the current evidence for ctDNA as a marker of response in metastatic CRC (mCRC) and to propose criteria for definitions of response to systemic therapies applicable in prospective clinical trials. We discuss the literature, which supports a new definition of ctDNA Response Evaluation Criteria in Solid Tumors. Finally, we discuss the challenges in preparations of the optimal trial design to establish the true clinical utility of ctDNA.
Keywords: circulating tumor DNA, clinical trial designs, metastatic colorectal cancer, response evaluation criteria
Introduction
Patients suffering from metastatic disease may undergo several lines of palliative chemotherapy with the hope of prolonging survival and improving quality of life. Currently, the only tool for assessing the efficacy of therapy during treatment is the Response Evaluation Criteria in Solid Tumors (RECIST) which compares imaging measures of lesions at defined treatment intervals (three to four cycles) with baseline status. 1 There is only a poor correlation between objective response by RECIST and oncologic outcome and response is not a valid surrogate endpoint for overall survival (OS). 2 This was demonstrated in a recent meta-analysis, reporting that 91% of 32 clinical trials showed a low correlation with OS. 3 Despite this knowledge, repeated imaging is still used as a key to treatment decisions by most clinicians, and response rates have been used for approval of several new drugs by the Food and Drug Administration. 4
As RECIST is far from optimal, treatment decisions made on this basis are not necessarily the best for the patient. Despite mall lesions, some metastases show highly aggressive behavior, while some patients have an indolent course of the disease despite a larger tumor burden. Better tools for evaluating efficacy are needed to spare the patient ineffective treatments with high toxicity, allowing for an early change in the treatment approach.
It is well known that small fractions of cell-free DNA circulate in the blood and other bodily fluids.5–8 This is a mixture of DNA from both healthy and malignant cells, termed cfDNA. Measurement of circulating-free DNA from tumor cells [circulating tumor DNA (ctDNA)] has gained interest during the past decade, as a less invasive and sensitive method for detection of cancer after curative treatment, and for identification of timely molecular features of the disease, thereby overcoming heterogeneity and the need for repeated tumor biopsies. 9 Quantitative measures of ctDNA hold prognostic information, with high levels indicating a poor prognosis.10,11 However, despite the advantages, and the development over the past decade, ctDNA monitoring has not been implemented in daily clinical practice. Current knowledge is mainly based on retrospective analyses, and it is, therefore, urgent to facilitate progress of prospective clinical trials, and ultimately clinical implementation of ctDNA-guided treatment. ctDNA is a pan-cancer concept12,13 and methods for ctDNA analysis have been developed and validated in many tumor types. These include either aberrant methylation assays or detection of tumor-specific genetic alterations by digital droplet PCR (ddPCR), or next generation sequencing (NGS) methods.14–16
Colorectal cancer (CRC) is the most investigated disease.11,17,18 This malignant tumor contains a high fraction of easily detectable tumor-specific mutations (KRAS, BRAF, NRAS) which are used as a target for ctDNA analysis. Methods can be described as either targeted assays or broad coverage assays, and the most frequently used in CRC are NGS-based platforms and ddPCR methods. Alternative technologies have been developed for quantification of epigenetic changes, most frequently as analysis of aberrant methylations by sensitive ddPCR methods.
This allows for tumor agnostic approaches, with direct analysis of blood samples without any knowledge of the molecular features of the tumor tissue. It also allows for tumor-informed strategies, with primary analysis of the tumor tissue and subsequent development of tailored assays for detection of the identified tumor-specific mutations in the liquid biopsy. Both approaches have advantages and potentially a place in the clinical setting.
The clinical potential of ctDNA can be grouped into different categories, according to the clinical setting. These include ctDNA analysis prior to a given treatment modality, after curative treatment and during systemic palliative therapies. Multiple studies have documented that ctDNA analysis can provide information on pretreatment molecular characteristics and up front prognostic information from ctDNA quantification.11,19 This has not yet been prospectively tested in randomized studies, and is, therefore, not established for clinical use. Detection of ctDNA after surgery has been widely analyzed as a marker of minimal residual disease (MRD). 18 ctDNA has potential as a tool for risk assessment and guidance of adjuvant treatment. Recently, results from the DYNAMICS trial demonstrated that ctDNA-guided adjuvant treatment reduced the use of adjuvant chemotherapy without compromising the recurrence-free survival. 20 These results are likely to take the ctDNA development closer to clinical implementation within the near future, although many aspects still need elucidation. Multiple clinical trials have been initiated in this setting, as reported in a recent overview. 21 Furthermore, at least one prospective randomized trial investigates the value of ctDNA during follow-up after curative surgery for CRC. 22
In the incurable metastatic setting, ctDNA has potential as a tool for monitoring treatment effect by identifying new genetic alterations as a marker of treatment resistance or evaluation of response by quantitative ctDNA changes. Studies have prospectively investigated the utility of ctDNA-based detection of mutations as markers of resistance to epidermal growth factor receptor (EGFR) inhibition, and application as a re-treatment criterion.23–27 ctDNA-based mutational testing is now available in many oncological centers, 28 and although large-scale data are not available, approval as a tool for standard treatment is expected. However, there is an obvious need to establish the value of ctDNA beyond the use of anti-EGFR therapy, and ctDNA precision medicine is on the horizon in other rare subtypes.
The analysis of quantitative ctDNA changes as a general response evaluation criterion during systemic treatment is a fundamentally different scientific approach, but with a potential of wide application. Results could be transferred to a pan-cancer concept if relevantly investigated. The purpose of this overview is to discuss the current evidence for ctDNA as a marker of response in metastatic CRC (mCRC) and to propose criteria for definitions of response to systemic therapies applicable in prospective clinical trials.
Definitions of clinical utility and surrogate endpoints for ctDNA studies
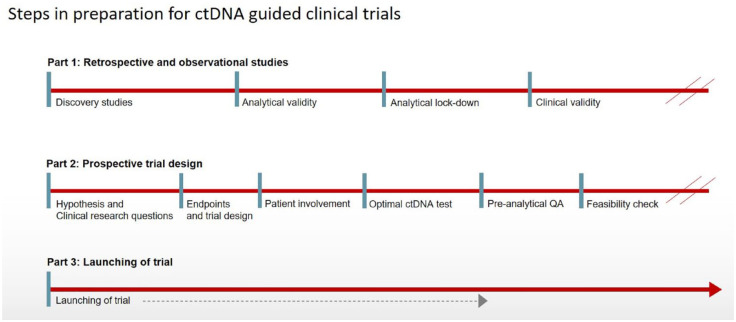
Validation of a potential new biomarker, as a tool in clinical settings, must undergo a number of well-defined steps (Figure 1). The terminology described by the Evaluation of Genomic Applications in Practice and Prevention working group 29 was refined into the ctDNA perspective in a joint review by American Society for Clinical Oncology and American Pathologists in 2018. 14 Analytical validity was defined as the ‘ability of an assay to detect and measure, with statistical significance, the presence of a biomarker of interest accurately, reproducibly, and reliably’. Although large-scale work is ongoing to establish optimal parameters for both pre- and post-analytical steps for ctDNA analysis, 15 the literature clearly demonstrates that analytical lockdown is rarely established due to the constant development of new methodical platforms and high commercial interests. At present, it is mandatory to define the accepted analytical criteria of a proposed method together with the optimal fit to the current purpose (i.e. broadness of assay versus sensitivity and reliable reproducible quantitative measures). Cross method validations and generalized multi-laboratory setups will also become essential for large-scale clinical trials.
Figure 1.
Illustration of the steps toward clinical utility for ctDNA.
ctDNA, circulating tumor DNA.
The optimal ctDNA analysis for ctDNA response evaluation is a method that allows for a reliable quantitative measure and covers the majority of cases. This is in contrast to the requirements for a test for say MRD, where an ultra-high sensitivity can be prioritized over quantification, and suggests a quantitative ddPCR method, as discussed below.
Here the term ‘clinical validity’ refers to the accuracy with which ctDNA can predict different outcomes.14,16 This implies analysis in relation to both efficacy by RECIST and survival. The clinical utility can only be established when there is high-level evidence that ctDNA-guided treatment can improve outcome compared to non-ctDNA-guided treatment. This indirectly implies a validation of ctDNA responses as a stronger surrogate endpoint than current imaging methods. According to the approach described by Buyse et al., 30 validation of ctDNA as surrogate endpoint will need a demonstration of a clear correlation between ctDNA response and survival, and ultimately to the effect of a given treatment. Thus, there are multiple steps to consider in the design of the optimal clinical trial to demonstrate clinical utility of ctDNA response criteria in the metastatic setting. Encouragingly, the most recent development enables us to prepare for randomized trials in the nearby future.
Clinical validity of ctDNA: response in studies with mCRC
Although there is an increasing number of observational studies analyzing the clinical potential of ctDNA in mCRC, 11 surprisingly only a few studies have addressed the value of early quantitative changes of ctDNA in relation to outcome parameters. According to a systematic review and meta-analysis performed by Callesen et al., 11 a total of 22 CRC studies (reported until December 2020) on ctDNA changes in relation to any outcome parameter. Most studies provided a statistically significant relation between ctDNA changes and outcome despite multiple different methods and diversity in the reporting of the results. They generally agreed that a ctDNA decrease during treatment implies a better survival compared to nondecreasing ctDNA but there is no agreement as to a quantitative definition of the decrease to qualify it as ctDNA response. The meta-analysis also supports a correlation between unfavorable quantitative ctDNA increase and shorter progression-free survival (PFS) and OS, but again sufficient data cannot be extracted for a consensus definition of ctDNA progression. Finally, 11 studies also presented a statistically significant association to response according to RECIST, providing a relevant background for a prospective analysis. Several publications indicate a strong signal despite a considerable diversity of methods, clinical settings, and methods for the measurement of ctDNA, but the broad approach hampers direct comparisons and consensus definitions for prospective validations. A tool for clinical decision-making, which is directly comparable to RECIST criteria, is warranted.
The current literature describes both absolute and relative changes. It can be argued that a single measure of detectability, or elimination, is sufficient for defining a favorable response. 31 The material from the German AIO-KRK-0207 ctDNA study was analyzed by qPCR (methylation of hyperplastic polyposis 1; N = 467). The results confirmed that early elimination of methylated cfDNA by 2–3 weeks after first administration of chemotherapy implied a significantly better prognosis compared to nonresponding cases. A later analysis of KRAS mutations with ddPCR in 151 patients from the cohort confirmed the prognostic value of ctDNA elimination. 32 These two studies are published sequentially, but a direct comparison between the two ctDNA targets and responses would have been a valuable contribution to the field, as detected levels are highly dependent on the performance of the individual methods, the volume analyzed, and the pre-analytical factors. More recently, Kim et al. 33 described the prognostic value of any decrease in variant allele frequency (VAF) in patients treated with first-line chemotherapy for mCRC. In that study, the authors also analyzed ctDNA changes together with RECIST. A ctDNA decrease was independently significant when added to a multivariate analysis, also containing the RECIST groups. Furthermore, patients within the same RECIST group showed a different PFS according to ctDNA changes. Patients achieving a ctDNA clearance had a longer PFS.
Other studies have used relative changes, ranging between a 10-fold reduction after the first cycle of chemotherapy presented by Tie et al., 34 a 30% reduction of mutant allele fraction by ddPCR analysis (N = 55), 35 and a 80% variant frequency reduction by targeted sequencing. 36 The latter was a small study cohort of 15 patients. More recently, Ye et al. 37 presented a 50% reduction in BRAF VAF by sequencing, where a ctDNA clearance was associated with a HR = 0.23 for PFS, but was not statistically significance for OS. Nakamura et al. 38 described a clear association between proportional changes in ctDNA fractions and PFS in patients with rare subtypes harboring human epidermal growth factor receptor 2 (HER2) amplifications, treated with HER2 inhibition. Lim et al. 39 used a definition of VAF < 1% as ctDNA clearance and reported a significant association with longer PFS in patients who obtained the ctDNA clearance. In that study, the authors also presented a combined analysis of ctDNA responses and RECIST evaluation, suggesting that ctDNA response could add value to the current criteria. Nevertheless, the most relevant clinical threshold for ctDNA response from each approach has not been defined, and similarly, limited studies have focused on a definition of early ctDNA progression. The clinical need is a nuanced classification of both favorable response, stable condition, and progression, comparable to RECIST.
Recently, Thomsen et al. 40 presented data with a novel approach to the quantitative changes based on the measured values with their inherently produced CIs from the ddPCR. The assay was designed to target aberrant methylations in plasma samples. This approach was based on the assumptions that the distribution of the measurements resembles a Gaussian distribution due to the high number of droplets analyzed (e.g. 20.000) in each sample. A ctDNA change can, therefore, be defined as a change of value where the 95% CI does not overlap the previous values CIs. Using the definition of undetectable ctDNA with the 95% CIs overlapping zero, early responding patients with ctDNA elimination at the first treatment cycle in a first-line setting had a median survival of 25.4 months compared to 13.5 months in the group with detectable ctDNA. Similarly, patients with ctDNA progression, defined as an increase above the 95% CIs of the previous sample, imply a poor prognosis. This was recently illustrated in a study on patients with ovarian cancer. 41 Raunkilde et al., 42 used the same methylation assay in a cohort of patients treated for mCRC with first-line therapy. An early evaluation of ctDNA response after the first cycle of chemotherapy revealed that PFS was 10.1 and 7.6 months, in ctDNA responders and nonresponders, respectively (p = 0.02, HR = 0.43). In comparison, the PFS rates were 10.1 and 7.3 months, in responders and nonresponders, respectively, according to RECIST 1.1., but with a HR = 0.65 and p = 0.17. Finally, Jakobsen et al. 43 presented the combined analysis of ctDNA changes in five different cohorts including a total of 420 patients with lung, ovarian, cholangiocarcinoma, and CRC. The results revealed a strong correlation between ctDNA response and long survival, which also support superiority in comparison to RECIST criteria. This classification was developed from the analysis of aberrant methylations by ddPCR. No such studies have been performed on ddPCR-based results of mutational detection, but could optimally be extracted from already analyzed samples and pooled into a meta-analysis.
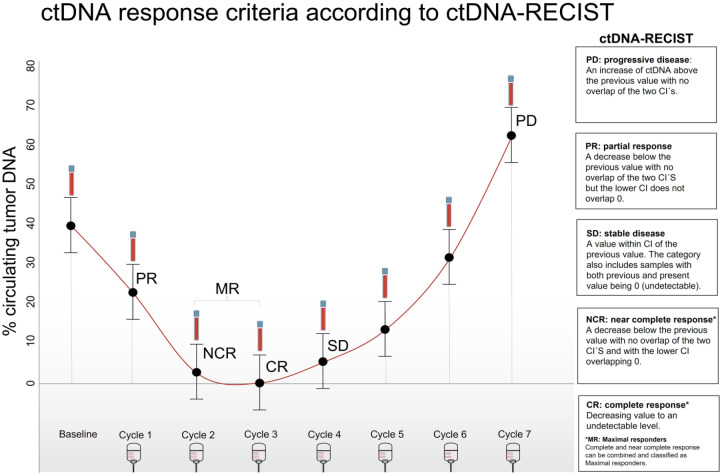
Based on the literature, it is clear that there is a strong clinical perspective in validating ctDNA as a surrogate endpoint in mCRC and directly comparing ctDNA response criteria with the currently used surrogate endpoint by RECIST. The ctDNA development has passed the steps from discovery to analytical validity and several studies have clearly documented its clinical validity in this setting. Prospective validation of the true clinical utility is, therefore, the next critical step, which cannot be initiated unless consensus on methodological aspects is reached and standardized criteria for response are defined. Multiple definitions are used in the literature, of which validations are missing. The use of inherent 95% CI intervals from ddPCR results is a promising approach to define the ctDNA response evaluation criteria (ctDNA-RECIST) as we have recently discussed 44 and presented in Figure 2.
Figure 2.
Definitions of ctDNA-RECIST.
Source: Figure by Garm Spindler and Truelsen.
CR, complete response; ctDNA-RECIST, circulating tumor DNA Response Evaluation Criteria in Solid Tumors; MR, maximal response; NCR, near complete response; PD, progression; PR, partial response.
CtDNA Response Evaluation Criteria in Solid Tumors
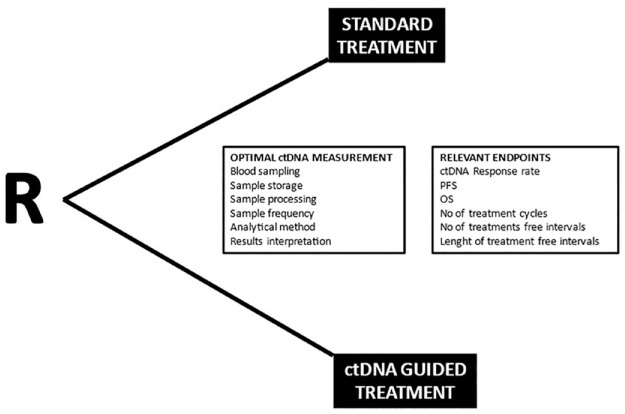
Based on the observed clinical validity of ctDNA response and progression in several diseases, we defined the criteria for ctDNA responses and progression. This allows for direct comparison with the RECIST evaluations. This was recently published elsewhere 44 and illustrated in Figure 3.
Figure 3.
Illustration of a randomised trial design and relevant points for consideration.
A ctDNA-RECIST progression can be defined as an increase in ctDNA above the previous value with no overlap of the two CIs. Stable ctDNA-RECIST is defined as a value within the CI of the previous value. This category also includes situations where both the previous and present values are 0 (undetectable). A ctDNA-RECIST partial response is a decrease in ctDNA below the previous value with no overlap of the two CIs, and the lower CI does not overlap 0. A ctDNA-RECIST complete response implies a decreasing value to an undetectable level, whereas a ‘near complete’ ctDNA-RECIST response can be defined as when the value is below the previous value with the lower CI overlapping 0. These can be combined and termed ctDNA-RECIST maximal response. The ctDNA-RECIST criteria are compared to objective RECIST in Table 1 of Jakobsen et al. 44 The two classification systems are compliant apart from the near complete response which is not included in the standard RECIST criteria. Although it is expected that the major part of this small subgroup will biologically have no true ctDNA (and thus considered clinically complete responders), we find it semantically misleading to classify samples with a low signal as complete responders while acknowledging that the signal should be interpreted with caution in these cases. Consequently, we have defined a combined group of maximum responders.
Table 1.
Presentations of the definitions of ctDNA-RECIST and corresponding RECIST.
| Criteria | Standard RECIST v1.1 (Imaging) | ctDNA-RECIST |
|---|---|---|
| Progression | At least a 20% increase in the sum of diameters of target lesions | An increase in ctDNA above the previous value with no overlap of the two CIs |
| Stable disease | Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD | A value within CI of the previous value. The category also includes samples with both previous and present values being 0 (undetectable) |
| Partial response | At least a 30% decrease in the sum of diameters of target lesions | A decrease below the previous value with no overlap of the two CIs but the lower CI does not overlap 0 |
| Complete response* | Disappearance of all target lesions | Decreasing value to an undetectable level |
| Near complete response* | Has no corresponding standard RECIST value | A decrease below the previous value with no overlap of the two CIs and with the lower CI overlapping 0* |
Complete and near complete response can be combined and classified as maximal responders.
ctDNA-RECIST, circulating tumor DNA Response Evaluation Criteria in Solid Tumors.
Challenges in trial design to analyze the clinical utility of ctDNA-RECIST criteria
A randomized trial comparing ctDNA-RECIST-guided treatment decisions with RECIST is necessary if we aim to establish the true clinical utility of ctDNA response evaluation in mCRC. There are multiple challenging factors in such a trial design. Treatment of mCRC implies several choices in terms of first-line chemotherapy and optional addition of targeted treatment. International guidelines summarize the options for induction chemotherapy, maintenance, and treatment breaks. 45 Re-induction of first-line and second-line regimens is widely used, and there is increasing availability for late-line systemic options. The concept of intensified treatment until progression with immediate shift of regimen is no longer a standard approach. Consequently, there are various clinical situations where ctDNA-RECIST could improve treatment decisions.
Our first hypothesis is that ctDNA-guided treatment decisions will prevent unnecessary ineffective therapy by identifying lack of response at an earlier timepoint than conventional strategies. It can also be hypothesized that ctDNA-guided decisions on treatment breaks will allow for a longer chemotherapy-free interval, and thereby better quality of life. The ultimate hypothesis is that ctDNA-guided treatment decisions will lead to improved survival by ensuring the most effective therapy at the earliest possible time during the course of the disease. Based on these hypotheses, primary endpoints, such as a reduced number of cycles of chemotherapy in first line, chemotherapy-free interval, and PFS at a given timepoint, are all relevant endpoints. Finally, thorough considerations must be given to add a non-inferiority approach.
The most clinically relevant timepoint for ctDNA-RECIST evaluations must also be defined. Studies have shown that ctDNA response can be evaluated already after a single cycle of treatment, but a higher fraction of responses may be expected with longer observation times. 46 Therefore, it can be suggested that ctDNA response evaluation should be performed prior to each cycle, and with clinically relevant intervals during treatment breaks (e.g. monthly samplings).
Once the overall aims and trial design have been defined, the optimal laboratory strategy must be decided. A relevant quality assurance process for sampling and pre-analytical steps should be performed at clinical sites. International consensus guidelines will be useful for providing the relevant standard operating procedure for volume, type of tubes, centrifugation, transportation, and storage. 47 It is important to know that degradation of the DNA due to time issues, as well as contamination with normal DNA from lymphocytes during sampling, can lead to falsely elevated total DNA levels and thereby difficulties in ctDNA detection and quantification. Commercially available DNA preserving tubes allow for storage and transportation for up to 48 h before processing at room temperature, and it is therefore a valuable option for multicenter trials with the need for central analysis and immediate results. The laboratory setup must allow for a clinically relevant time to results, which must fit into the patient’s treatment schedules without major delays.
Finally, the choice of the ctDNA measuring method is essential. A high detection rate together with a reproducible quantitative measure is mandatory. In CRC, data have shown that ctDNA analysis targeting a small number of different aberrant methylations is relevant 48 and will detect ctDNA in up to 80% of cases in localized CRC 49 and >90% in the metastatic disease.32,50 This allows for a direct tumor agnostic approach. Another option is to analyze ctDNA for tumor-specific mutations. The most common tumor-specific mutations in this disease are the RAS mutations, which together with BRAF will represent up to approximately 60% of cases. 51 The rate of ctDNA-positive samples prior to first-line therapy depends on several factors including the tumor mutational status, sensitivity of the ctDNA test, and the amount of total DNA shedding into the blood stream. Shedding of ctDNA varies with the tissue of origin, for example, liver metastases leading to higher levels of ctDNA in the blood samples. 52 Consequently, there will be a high but not complete concordance between mutations detected in the tumor tissue and blood samples, and analysis of the most commonly detected mutations will thus provide a ctDNA measure in approximately 40–50% of cases 51 , but these will not provide the same degree of a reliable quantitative measure. The panel of detected mutations can be broadened by, for example, mass array technology, or targeted or multigene panel NGS-based ctDNA assays but this methodology needs further clarification with respect to precision. It is not clear how the CI of a given value should be calculated when using these methods, thus hampering the evaluation of relative changes. Alternatively, a tumor-informed approach can be added with identification of mutations in the tumor tissue and subsequent design of a tailored ddPCR. With this methodological strategy a similar detection rate can be expected in the metastatic setting, which will allow for a reliable quantification, but on the other hand, this approach is resource demanding and time-consuming.
Investigating the clinical utility of ctDNA-guided treatment can lead to a breakthrough in the way we monitor cancer. There are the obvious advantages of less time spent in hospital for scans, more precise response evaluations, and avoidance of overinterpretation of measures on tumor lesions and the uncertainties. Omitting imaging procedures will provide a more rational use of resources for imaging in the healthcare system, but it will be relevant to add prospective cost–benefit analysis to the first generation of these trials. Changing the paradigm for monitoring palliative treatment will demand changing the culture among oncologists. In addition, ctDNA as a pan-cancer concept will be highly relevant in the education of younger oncologist and trainees. Finally, including patients in the process of developing the trial design will provide valuable learning for physicians as to the preferences and relevant communication necessary for a fruitful process.
Conclusion and perspectives
The current literature holds high hopes for the clinical utility of ctDNA. An obvious precondition is a sharper focus on quantitative monitoring of the treatment course. Another absolute condition is valid definitions of ctDNA progression and response. The latter may serve as a surrogate endpoint for OS but the final proof must come from randomized trials. A prospective validation will have crucial impact in clinical oncology, which may move away from imaging-based monitoring toward blood-based guidance. Such a shift of paradigm will also have a heavy impact on the development of new drugs, thereby changing the measure of effect from dubious changes of tumor volume to an objective parameter in a blood sample.
Acknowledgments
We sincerely thank Dr. Christina Gliesman Truelsen for helping with the design of the figures.
Footnotes
ORCID iD: Anders Jakobsen  https://orcid.org/0000-0003-2110-2615
https://orcid.org/0000-0003-2110-2615
Contributor Information
Karen-Lise Garm Spindler, Department of Oncology, Aarhus University Hospital, Aarhus University, Palle Juul-Jensens Boulevard 99, Aarhus DK-8200, Denmark.
Anders Jakobsen, Department of Oncology, Institute of Regional Health Services, University of Southern Denmark, Vejle University Hospital, Vejle, Denmark.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Karen-Lise Garm Spindler: Conceptualization; Data curation; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Anders Jakobsen: Conceptualization; Investigation; Methodology; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Karen-Lise Garm Spindler is supported by The Research Foundation of The Central Denmark Region.
The authors declare that there is no conflict of interest.
Availability of data and material: Not applicable.
References
- 1.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 2.Sharma MR, Maitland ML, Ratain MJ, et al. RECIST: no longer the sharpest tool in the oncology clinical trials toolbox-point/counterpoint. Cancer Res 2012; 72: 5145–5150. [DOI] [PubMed] [Google Scholar]
- 3.Haslam A, Hey SP, Gill J, et al. A systematic review of trial-level meta-analyses measuring the strength of association between surrogate end-points and overall survival in oncology. Eur J Cancer 2019; 106: 196–211. [DOI] [PubMed] [Google Scholar]
- 4.Chen EY, Raghunathan V, Prasad V. An overview of cancer drugs approved by the US food and drug administration based on the surrogate end point of response rate. JAMA Intern Med 2019; 179: 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouliere F. A hitchhiker’s guide to cell-free DNA biology. Neurooncol Adv 2022; 4: ii6–ii14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kustanovich A, Schwartz R, Peretz T, et al. Life and death of circulating cell-free DNA. Cancer Biol Ther 2019; 20: 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thierry AR, El Messaoudi S, Gahan PB, et al. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev 2016; 35: 347–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diehl F, Li M, Dressman D, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A 2005; 102: 16368–16373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med 2018; 379: 1754–1765. [DOI] [PubMed] [Google Scholar]
- 10.Garm Spindler KL, Pallisgaard N, Andersen RF, et al. Circulating free DNA as biomarker and source for mutation detection in metastatic colorectal cancer. PLoS One 2015; 10: e0108247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callesen LB, Hamfjord J, Boysen AK, et al. Circulating tumour DNA and its clinical utility in predicting treatment response or survival in patients with metastatic colorectal cancer: a systematic review and meta-analysis. Br J Cancer 2022; 127: 500–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kansara M, Bhardwaj N, Thavaneswaran S, et al. Early circulating tumor DNA dynamics as a pan-tumor biomarker for long-term clinical outcome in patients treated with durvalumab and tremelimumab. Mol Oncol 2023; 17: 298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014; 6: 224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merker JD, Oxnard GR, Compton C, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. J Clin Oncol 2018; 36: 1631–1641. [DOI] [PubMed] [Google Scholar]
- 15.Williams PM, Forbes T, Lund SP, et al. Validation of ctDNA quality control materials through a precompetitive collaboration of the Foundation for the National Institutes of Health. JCO Precis Oncol 2021; 5: 910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes DF. Defining clinical utility of tumor biomarker tests: a clinician’s viewpoint. J Clin Oncol 2021; 39: 238–248. [DOI] [PubMed] [Google Scholar]
- 17.Dasari A, Morris VK, Allegra CJ, et al. ctDNA applications and integration in colorectal cancer: an NCI colon and rectal-anal task forces whitepaper. Nat Rev Clin Oncol 2020; 17: 757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faulkner LG, Howells LM, Pepper C, et al. The utility of ctDNA in detecting minimal residual disease following curative surgery in colorectal cancer: a systematic review and meta-analysis. Br J Cancer 2023; 128: 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichert ZR, Morgan TM, Li G, et al. Prognostic value of plasma circulating tumor DNA fraction across four common cancer types: a real-world outcomes study. Ann Oncol 2023; 34: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tie J, Cohen JD, Lahouel K, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med 2022; 386: 2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masfarré L, Vidal J, Fernández-Rodríguez C, et al. ctDNA to guide adjuvant therapy in localized colorectal cancer (CRC). Cancers (Basel) 2021; 13: 2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nors J, Henriksen TV, Gotschalck KA, et al. IMPROVE-IT2: implementing noninvasive circulating tumor DNA analysis to optimize the operative and postoperative treatment for patients with colorectal cancer–intervention trial 2. Study protocol. Acta Oncol 2020; 59: 336–341. [DOI] [PubMed] [Google Scholar]
- 23.Vlachou MS, Mauri D, Zarkavelis G, et al. Plasma ctDNA RAS status selects patients for anti-EGFR treatment rechallenge in metastatic colorectal cancer: a meta-analysis. Exp Oncol 2021; 43: 252–256. [DOI] [PubMed] [Google Scholar]
- 24.Sartore-Bianchi A, Pietrantonio F, Lonardi S, et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat Med 2022; 28: 1612–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aparicio J, Virgili Manrique AC, Capdevila J, et al. Randomized phase II trial of FOLFIRI-panitumumab compared with FOLFIRI alone in patients with RAS wild-type circulating tumor DNA metastatic colorectal cancer beyond progression to first-line FOLFOX-panitumumab: the BEYOND study (GEMCAD 17-01). Clin Transl Oncol 2022; 24: 2155–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manca P, Corallo S, Lonardi S, et al. Variant allele frequency in baseline circulating tumour DNA to measure tumour burden and to stratify outcomes in patients with RAS wild-type metastatic colorectal cancer: a translational objective of the Valentino study. Br J Cancer 2022; 126: 449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima H, Kotani D, Bando H, et al. REMARRY and PURSUIT trials: liquid biopsy-guided rechallenge with anti-epidermal growth factor receptor (EGFR) therapy with panitumumab plus irinotecan for patients with plasma RAS wild-type metastatic colorectal cancer. BMC Cancer 2021; 21: 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basile D, Gallois C, Puglisi F, et al. Practices and expectations on the use of circulating tumor DNA in colorectal cancer patients: a bi-national AGEO/AIOM/GERCOR/FFCD/FRENCH survey. Clin Res Hepatol Gastroenterol 2021; 45: 101681. [DOI] [PubMed] [Google Scholar]
- 29.Teutsch SM, Bradley LA, Palomaki GE, et al. The evaluation of genomic applications in practice and prevention (EGAPP) initiative: methods of the EGAPP working group. Genet Med 2009; 11: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buyse M, Molenberghs G. Criteria for the validation of surrogate endpoints in randomized experiments. Biometrics 1998; 54: 1014–1029. [PubMed] [Google Scholar]
- 31.Herbst A, Vdovin N, Gacesa S, et al. Methylated free-circulating HPP1 DNA is an early response marker in patients with metastatic colorectal cancer. Int J Cancer 2017; 140: 2134–2144. [DOI] [PubMed] [Google Scholar]
- 32.Lueong SS, Herbst A, Liffers ST, et al. Serial circulating tumor DNA mutational status in patients with KRAS-mutant metastatic colorectal cancer from the phase 3 AIO KRK0207 trial. Clin Chem 2020; 66: 1510–1520. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Lim Y, Kang JK, et al. Dynamic changes in longitudinal circulating tumour DNA profile during metastatic colorectal cancer treatment. Br J Cancer 2022; 127: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tie J, Kinde I, Wang Y, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol 2015; 26: 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parikh AR, Mojtahed A, Schneider JL, et al. Serial ctDNA monitoring to predict response to systemic therapy in metastatic gastrointestinal cancers. Clin Cancer Res 2020; 26: 1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu HC, Lapke N, Wang CW, et al. Targeted sequencing of circulating tumor DNA to monitor genetic variants and therapeutic response in metastatic colorectal cancer. Mol Cancer Ther 2018; 17: 2238–2247. [DOI] [PubMed] [Google Scholar]
- 37.Ye LF, Huang ZY, Chen XX, et al. Monitoring tumour resistance to the BRAF inhibitor combination regimen in colorectal cancer patients via circulating tumour DNA. Drug Resist Updat 2022; 65: 100883. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura Y, Okamoto W, Kato T, et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med 2021; 27: 1899–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim Y, Kim S, Kang JK, et al. Circulating tumor DNA sequencing in colorectal cancer patients treated with first-line chemotherapy with anti-EGFR. Sci Rep 2021; 11: 16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomsen CB, Hansen TF, Andersen RF, et al. Early identification of treatment benefit by methylated circulating tumor DNA in metastatic colorectal cancer. Ther Adv Med Oncol 2020; 12: 1758835920918472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faaborg L, Andersen RF, Waldstrøm M, et al. Prognostic impact of circulating methylated homeobox A9 DNA in patients undergoing treatment for recurrent ovarian cancer. Cancers (Basel) 2022; 14: 1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raunkilde L, Hansen TF, Andersen RF, et al. NPY gene methylation in circulating tumor DNA as an early biomarker for treatment effect in metastatic colorectal cancer. Cancers (Basel) 2022; 14: 4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jakobsen A, Andersen RF, Hansen TF, et al. Early ctDNA response to chemotherapy. A potential surrogate marker for overall survival. Eur J Cancer 2021; 149: 128–133. [DOI] [PubMed] [Google Scholar]
- 44.Jakobsen AKM, Spindler KLG. ctDNA- response evaluation criteria in solid tumors–a new measure in medical oncology. Eur J Cancer 2023; 180: 180–183. [DOI] [PubMed] [Google Scholar]
- 45.Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2023; 34: 10–32. [DOI] [PubMed] [Google Scholar]
- 46.Thomsen CB, Hansen TF, Andersen RF, et al. Monitoring the effect of first line treatment in RAS/RAF mutated metastatic colorectal cancer by serial analysis of tumor specific DNA in plasma. J Exp Clin Cancer Res 2018; 37: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meddeb R, Pisareva E, Thierry AR, et al. Guidelines for the preanalytical conditions for analyzing circulating cell-free DNA. Clin Chem 2019; 65: 623–633. [DOI] [PubMed] [Google Scholar]
- 48.Nassar FJ, Msheik ZS, Nasr RR, et al. Methylated circulating tumor DNA as a biomarker for colorectal cancer diagnosis, prognosis, and prediction. Clin Epigenet 2021; 13: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bregni G, Pretta A, Senti C, et al. Circulating DNA in the neoadjuvant setting of early stage colon cancer. Acta Oncol 2022; 61: 1223–1229. [DOI] [PubMed] [Google Scholar]
- 50.Øgaard N, Reinert T, Henriksen TV, et al. Tumour-agnostic circulating tumour DNA analysis for improved recurrence surveillance after resection of colorectal liver metastases: a prospective cohort study. Eur J Cancer 2022; 163: 163–176. [DOI] [PubMed] [Google Scholar]
- 51.van’t Erve I, Greuter MJE, Bolhuis K, et al. Diagnostic strategies toward clinical implementation of liquid biopsy RAS/BRAF circulating tumor DNA analyses in patients with metastatic colorectal cancer. J Mol Diagn 2020; 22: 1430–1437. [DOI] [PubMed] [Google Scholar]
- 52.Hamfjord J, Guren TK, Glimelius B, et al. Clinicopathological factors associated with tumour-specific mutation detection in plasma of patients with RAS-mutated or BRAF-mutated metastatic colorectal cancer. Int J Cancer 2021; 149: 1385–1397. [DOI] [PubMed] [Google Scholar]