Abstract
Background:
Cotesting with the Papanicolaou (Pap) and human papillomavirus tests detects most precancerous and cancerous lesions and increases the sensitivity for detecting high-grade precancerous and invasive cervical cancers compared with human papillomavirus testing alone.
Objective:
To compare the use of the Papette brush (hereafter Papette) to the traditional spatula with endocervical brush (cytobrush) for cervical cancer screening.
Design:
Pragmatic observational study.
Methods:
Adult women aged 21–64 years who were eligible for a Papanicolaou test at a Midwest Community Internal Medicine practice underwent cervical cancer screening using the Papette or spatula with cytobrush from 18 August 2021 through 1 February 2022. Cluster sampling was used across the practice. Pathology reports were then analyzed to compare the number of satisfactory versus unsatisfactory results between the two collection techniques.
Results:
We collected results for 756 Pap tests. The test results were satisfactory with the Papette 93.8% of the time compared with 93.0% for the spatula with cytobrush.
Conclusion:
The Papette is not inferior to a spatula with cytobrush as a collection method for Pap tests.
Keywords: cytobrush, Pap smear, Papanicolaou test, Papette, spatula
Introduction
In 1940, cervical cancer was the leading cause of death for women of childbearing age. 1 The development of the Papanicolaou (Pap) test by G. N. Papanicolaou in the late 1920s and publication of his landmark paper with H. Traut in 1941 2 led to widespread use of the Pap test in the 1950s, which dramatically reduced the incidence of cervical cancer deaths in the United States by more than 60%. 1 Used to detect abnormal or atypical cervical cellular changes suggestive of an actual or precancerous lesion, the Pap test has remained a standard, routinely used tool for cervical cancer screening.
The Pap test, which assesses cervical cytology on collected samples, can be performed alone or in combination with human papillomavirus (HPV) testing. HPV testing can also be performed alone (currently collected by a practitioner), is now an accepted screening test for cervical cancer for average-risk women aged 30 or older, and has the potential for future self-collection by patients.3–5 Persistent infection with HPV has been associated with almost all causes of cervical cancer, and nearly 39.9% of adult American women aged 18–59 are infected with genital HPV.3,4 If left untreated, approximately 30% of HPV-related precancerous cervical cellular abnormalities become invasive cancers. 3 Cotesting with the Pap test and HPV detects more than 90% of precancerous and cancerous lesions and increases the sensitivity for detecting high-grade precancerous lesions and invasive cervical cancers compared with HPV testing alone. 3 At our institution, the standard practice is cotesting with the Pap and HPV tests in women aged 30–65 years.
The Pap and HPV tests can be performed using either a conventional method with ethyl alcohol or spray fixative on a pathology slide or by using a liquid-based cytology method such as the ThinPrep Pap test (Hologic). Liquid-based cytology is used more often because it is simpler than the conventional method. 6 Approved collection devices include the Papette brush (hereafter Papette), the spatula, and the cytobrush. 6 These devices can be used either alone (Papette) or in combination (spatula with cytobrush). Both the Papette and spatula with cytobrush have advantages and disadvantages. The Papette allows for collection with a single device. However, at our institution, the Papette costs more than other devices, and there was a concern that the softer design might not sample a postmenopausal os as well, which kept it from being widely used. In comparison, the spatula with cytobrush is more affordable and used more widely, although requests for the Papette have increased its use. Whether one collection method is better than the other is unclear, and only limited and dated literature exist for the superiority of collection devices based on sample adequacy and results.1,7,8 In this pragmatic study, we aimed to determine whether the Papette or spatula with cytobrush was a superior collection method for obtaining cervical samples for Pap and HPV tests.
Methods
Study design
Study setting, population, and design
This study was approved by the Mayo Clinic Institutional Review Board (ID 21-006131). Patients were excluded from the study if they did not give consent to have medical records accessed for research purposes or they declined Pap testing. Data were stored in a secured REDCap (Research Electronic Data Capture) database with only team members approved by the institutional review board able to access the data.9,10
The study was performed in the division of Community Internal Medicine (CIM) between 18 August 2021 and 1 February 2022. The target population was adult women aged 21–64 years who were eligible for a Pap test during an appointment with a CIM practitioner. We aimed to compare satisfactory versus unsatisfactory Pap test results for the Papette and the spatula with cytobrush.
To reduce bias, we used cluster sampling for the study. The CIM practice has four distinct building locations. Among these locations are seven care teams with 72 practitioners. The Papette was used to obtain cervical samples from women on three care teams, and the spatula with cytobrush was used to obtain samples from women on four care teams. Care teams using the Papette versus the spatula with cytobrush were split so that approximately equal practitioners were using both collection methods for the same number of patients of various eligible ages. Both collection techniques used liquid-based cytology. The sample size needed for the two treatment groups was determined with a power analysis accounting for a mean difference of 0.2 between groups, an intraclass correlation of 0.1, and an alpha of 0.05. To detect 80% power, a sample size of 706 was needed. Additional patients were included to account for attrition. The findings were analyzed for the primary outcome of adequacy, including the rate of satisfactory versus unsatisfactory Pap tests for the two collection techniques.
Data collected
The following demographic and clinical characteristics were collected: age, race, menopausal status, and presence of an intrauterine contraceptive (IUC).
Outcomes
The cytology reports for cellular adequacy and HPV results (outcomes) were collected. The cytology samples were compared for satisfactory versus unsatisfactory tests with the Papette versus the traditional spatula with cytobrush methods. An unsatisfactory test was defined as having one of the three following results: (1) satisfactory for evaluation, negative for intraepithelial lesion or malignancy, high-risk HPV test results invalid; (2) specimen processed and examined but unsatisfactory for evaluation because of epithelial abnormality due to inadequate squamous cellularity, high-risk HPV test results negative; or (3) specimen unable to be processed because of low cellularity, negative HPV test. A satisfactory test was defined as any result showing a satisfactory evaluation (with either negative for intraepithelial lesion or malignancy, atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesion (LGSIL), high-grade squamous intraepithelial lesion (HGSIL), or atypical glandular cells) and positive or negative HPV results.
Statistical analyses
Patient characteristics were reported as frequencies and percentages for categorical variables and mean (standard deviation (SD)) for continuous variables. Comparisons between collection methods were analyzed using Kruskal–Wallis tests for continuous variables and χ2 tests for categorical variables. All pathology results were available and categorized by chart review in groups from 0 to 13, with those in groups 0, 1, or 2 considered unsatisfactory (0: invalid HPV; 1: epithelial abnormality–inadequate squamous cellularity; 3: low cellularity). In contrast, groups 3 through 13 were considered satisfactory (3: no endocervical or transformation zone component, 4: normal, 5: ASC-US, 6: ASC-US and HPV, 7: ASC-US and endometrial cells, 8: HPV+, 9: LGSIL, 10: LGSIL and HPV, 11: HGSIL or atypical glandular cells, 12: HGSIL and HPV or atypical glandular cells and HPV, 13: atypical endometrial cells). The outcome of satisfactory versus unsatisfactory test results was analyzed with a logistic regression model. Covariates included in the model were collection method, IUC, and menopausal status. Results are given as odds ratios (OR) with 95% confidence intervals (CIs) and p values. All p values less than 0.05 were considered significant. Analyses were computed using SAS software (SAS Institute Inc., version 9.4).
Results
The study had a final sample size of 756 women who came to CIM for routine screening Pap tests. The Papette collection method was used for 357 Pap tests, and the traditional spatula with cytobrush was used for 399 Pap tests. As shown in Table 1, most of the 756 women were White (85.6%); Asian women comprised 6.0% and Black women comprised 3.9% of the study population. The mean age was older in the spatula with cytobrush group than in the Papette group (46.8 years vs 43.9 years; p = 0.004). Menopausal status was classified as premenopausal for 55.8% (n = 422) and postmenopausal for 35.8% (n = 271). Most women did not have an IUC (83.7%, n = 633).
Table 1.
Demographic and clinical characteristics and outcome by type of collection method.
| Characteristic | No. (%) a | p value | ||
|---|---|---|---|---|
| Papette (n = 357) | Spatula with cytobrush (n = 399) | Total (N = 756) | ||
| Age | 0.004 b | |||
| No. | 357 | 399 | 756 | |
| Mean (SD) | 43.9 (13.64) | 46.8 (12.34) | 45.4 (13.04) | |
| Race | 0.56 c | |||
| American Indian/Alaskan Native | 0 (0.0) | 1 (0.3) | 1 (0.1) | |
| Asian | 17 (4.8) | 28 (7.1) | 45 (6.0) | |
| Black | 13 (3.7) | 16 (4.1) | 29 (3.9) | |
| Other | 17 (4.8) | 16 (4.1) | 33 (4.4) | |
| White | 309 (87.0) | 334 (84.6) | 643 (85.7) | |
| Missing | 2 | 4 | 6 | |
| Menopausal status | 0.24 c | |||
| Perimenopausal | 36 (10.1) | 28 (7.0) | 64 (8.5) | |
| Postmenopausal (no periods in the past 1 year) | 121 (33.9) | 150 (37.6) | 271 (35.8) | |
| Premenopausal | 201 (56.3) | 221 (55.4) | 422 (55.8) | |
| IUC | 0.10 c | |||
| No | 291 (81.5) | 342 (85.7) | 633 (83.7) | |
| Yes | 67 (18.8) | 57 (14.3) | 124 (16.4) | |
| HPV+ 3 | 0.75 c | |||
| No | 341 (95.5) | 382 (95.7) | 723 (95.6) | |
| Yes | 17 (4.8) | 17 (4.3) | 34 (4.5) | |
| Pap test results | 0.75 c | |||
| Unsatisfactory d | 23 (6.4) | 28 (7.0) | 51 (6.7) | |
| Satisfactory | 335 (93.8) | 371 (93.0) | 706 (93.4) | |
HPV+ 3 : presence of human papilloma virus; IUC: intrauterine contraceptive; Pap: Papanicolaou.
Data are shown as No. (%) unless noted otherwise.
Kruskal–Wallis p value.
χ2p value.
Results in groups 3–13 (see section “Methods” for definitions).
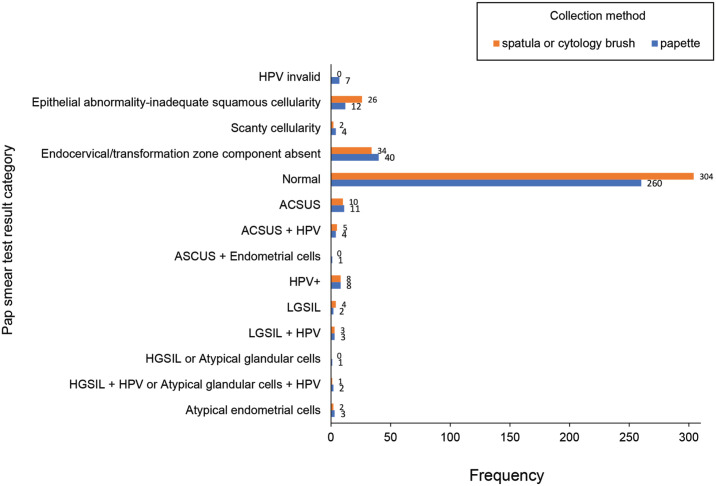
On analysis, 34 (4.5%) samples were positive for high-grade HPV (Figure 1). Of these, nine had ASC-US: six with LGSIL and three with HGSIL or atypical glandular cells. Of the collected samples, 93.4% (n = 706) were satisfactory and 6.7% (n = 51) were unsatisfactory. The number of satisfactory and unsatisfactory results were not significant between the Papette and the spatula with cytobrush collection groups (p = 0.75). There were seven invalid HPV results, which meant that the HPV portion of the test could not be completed; all these samples were collected using the Papette. Of the HPV results, 40 Papette-collected samples had absent endocervical or transformation zone component compared with 34 spatula and cytobrush–collected samples.
Figure 1.
Individual Pap test results by type of collection method. Of the seven patients who had an invalid HPV result (group 0), four were premenopausal, two were perimenopausal, and one was postmenopausal. A sample below group 3 was deemed unviable (see section “Methods” for group definitions). ASC-US: atypical squamous cells of undetermined significance; HIV: human immunodeficiency virus; HPV: human papillomavirus; HGSIL: high-grade squamous intraepithelial lesion; LGSIL: low-grade squamous intraepithelial lesion; Pap: Papanicolaou.
From logistic regression analysis, only menopausal status was significantly associated with having an unsatisfactory or unviable test result (OR: 2.30; 95% CI: 1.26–4.18) (Table 2). Women with postmenopausal status were 2.3 times more likely to have an unsatisfactory result than women with premenopausal status.
Table 2.
Logistic regression for the outcome of satisfactory versus unsatisfactory sample.
| Odds ratio (95% CI) | p value | |
|---|---|---|
| Papette versus spatula with cytobrush | 0.96 (0.54–1.70) | 0.88 |
| IUC: no versus yes | 1.67 (0.57–4.93) | 0.35 |
| Postmenopausal versus premenopausal a | 2.30 (1.26–4.18) | 0.007 |
CI: confidence interval; IUC: intrauterine contraceptive; IUD: intrauterine device.
Premenopausal and perimenopausal are both included in the premenopausal group. Postmenopausal women were 2.3 times more likely to have an unviable test result than the premenopausal women.
Discussion
In this study, we compared the Papette and the spatula with cytobrush collection methods to learn whether one method was superior to the other for yielding satisfactory Pap test results. We did not find any significant difference between the results of the two methods for a satisfactory versus unsatisfactory Pap test. In addition, the study showed that the presence or absence of an IUC did not affect whether a Pap test was satisfactory or unsatisfactory. The only factor in the study that had a significant impact on the likelihood of an unsatisfactory Pap test was a woman’s postmenopausal status. Women who were postmenopausal were 2.3 times more likely to have an unsatisfactory test result than women who were premenopausal.
The most common causes of unsatisfactory Pap tests include insufficient endocervical epithelial cells, excessive smear thickness, cells obscured by inflammatory cells and erythrocytes, the presence of foreign material, poor fixation or staining, insufficient material for analysis, faulty equipment, and poor preparation technique. 11 Alsharif et al. 12 found that low cellularity was the primary cause of unsatisfactory SurePath results (Becton, Dickinson and Company) in a sample of 243,006 Pap tests. They also found that women with unsatisfactory results were older and were more likely to have had a hysterectomy or be menopausal. 12 After menopause or hysterectomy, women are estrogen-deficient and at high risk of developing genitourinary syndrome of menopause. This syndrome includes conditions previously known as vulvovaginal atrophy, atrophic vaginitis, or urogenital atrophy. 13 The atrophy or degeneration of cells results in less material (cells) for analysis, which may be the reason for the unsatisfactory Pap test results.
A notable finding in this study was that seven Pap tests had invalid HPV results. All seven of these samples came from Papette collection. The package insert for the cobas HPV test (Roche Diagnostics) used in the laboratory for this study lists various reasons why the test could be invalid: use of the over-the-counter product Replens vaginal moisturizer (Church & Dwight Co), use of the cobas HPV assay with media types other than PreservCyt solution (Hologic) and SurePath preservation fluid, presence of polymerase chain reaction inhibitors, or too much mucus in the sample. 14 All samples collected in this study were evaluated with compatible media types and did not include any known polymerase chain reaction inhibitors. We did not know whether any patients in either the Papette or spatula with cytobrush groups used an over-the-counter moisturizing product, such as Replens. However, assuming these unknown causes for invalid test results were equally distributed, too much mucus in the sample is the only remaining reason for an invalid HPV test. The Papette may have collected more mucus in the sample than the spatula with cytobrush, resulting in the invalid HPV test results.
Women who were postmenopausal had more unsatisfactory results than women who were premenopausal, possibly due to low cellularity from genitourinary syndrome of menopause. To determine whether low cellularity resulted in unsatisfactory results, a study comparing unsatisfactory versus satisfactory Pap tests should be undertaken for two groups of postmenopausal women: those who were and were not treated with vaginal estradiol, which may be used to manage genitourinary syndrome of menopause. If treating genitourinary syndrome of menopause decreases the rate of unsatisfactory Pap tests, the theory that this syndrome is a causative factor in the increase in unsatisfactory Pap tests in postmenopausal women could be confirmed.
Our study had strengths and limitations. It was a pragmatic practice-based study with a large sample size; therefore, we believe the results can be readily adopted. The study was limited by its observational design and homogeneous population: a Midwest practice with mostly White women; thus, results may not be generalized to other more heterogeneous population groups.
Conclusion
This study showed no significant difference between the Papette and the spatula with cytobrush for obtaining a satisfactory versus an unsatisfactory Pap test, after adjusting for age, race, and IUC status. The Papette was associated with more invalid HPV tests, but this result was not significant. The presence of an IUC did not significantly affect results. Women who were postmenopausal were 2.3 times more likely to have an unsatisfactory test result than women who were premenopausal, and more research is needed to determine the cause of these unsatisfactory tests. We believe the cause may be low cellularity from genitourinary syndrome of menopause. Based on the results of this study, practitioners and institutions can safely choose either the Papette or spatula with cytobrush to collect specimens for Pap tests according to cost and availability without concern for inferiority of the collection tool.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057231170975 for Papanicolaou test collection with the Papette brush or the spatula with cytobrush: A pragmatic study by Danielle J O’Laughlin, Brittany A Strelow, Nicole A Fellows, Joy N Stevens, Elizabeth A Kelsey, Stephanie R Fink, Sonya M Peters, Jennifer A Johnson, Jaclyn P Houghton, Anne M Stolp, Karen M Fischer, Johanna M Tweedy and Ramona S DeJesus in Women's Health
Acknowledgments
We thank our respective division at Mayo Clinic for their support of this study. Marianne Mallia, ELS, MWC, senior scientific/medical editor, Mayo Clinic, substantively edited the manuscript. The Scientific Publications staff at Mayo Clinic provided proofreading, administrative, and clerical support.
Footnotes
ORCID iDs: Brittany A Strelow  https://orcid.org/0000-0003-4858-3279
https://orcid.org/0000-0003-4858-3279
Ramona S DeJesus  https://orcid.org/0000-0003-0216-5223
https://orcid.org/0000-0003-0216-5223
Supplemental material: Supplemental material for this article is available online.
Declarations
Ethics approval and consent to participate: This study was approved by the Mayo Clinic Institutional Review Board (ID: 21-006131).
Consent for publication: Only patients who gave written permission for use of their health records for research were included.
Author contribution(s): Danielle J O’Laughlin: Conceptualization; Methodology; Supervision; Writing—original draft; Writing—review & editing.
Brittany A Strelow: Data curation; Methodology; Writing—original draft; Writing—review & editing.
Nicole A Fellows: Conceptualization; Data curation; Writing—original draft; Writing—review & editing.
Joy N Stevens: Data curation; Writing—original draft.
Elizabeth A Kelsey: Data curation; Writing—original draft.
Stephanie R Fink: Data curation; Writing—original draft; Writing—review & editing.
Sonya M Peters: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Validation; Writing—original draft.
Jennifer A Johnson: Conceptualization; Data curation; Project administration; Writing—original draft.
Jaclyn P Houghton: Project administration; Supervision; Writing —original draft; Writing—review & editing.
Anne M Stolp: Methodology; Writing—original draft; Writing —review & editing.
Karen M Fischer: Formal analysis; Writing—review & editing.
Johanna M Tweedy: Conceptualization; Data curation; Investigation; Methodology; Project administration; Writing—original draft; Writing—review & editing.
Ramona S DeJesus: Project administration; Supervision; Writing—original draft; Writing—review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: All relevant, deidentified data supporting the findings of this study are reported within the article.
References
- 1.Ferenczy A, Robitaille J, Guralnick M, et al. Cervical cytology with the Papette sampler. J Reprod Med 1994; 39(4): 304–310. [PubMed] [Google Scholar]
- 2.Papanicolaou GN, Traut HF.The diagnostic value of vaginal smears in carcinoma of the uterus. Am J Obstetr Gynecol 1941; 42(2): 193–206. [PubMed] [Google Scholar]
- 3.Eun TJ, Perkins RB.Screening for cervical cancer. Med Clin North Am. Nov 2020; 104(6): 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McQuillan G, Kruszon-Moran D, Markowitz LE, et al. Prevalence of HPV in adults aged 18-69: United States, 2011-2014. NCHS Data Brief 2017(280): 1–8. [PubMed] [Google Scholar]
- 5.The American College of Obstetricians Gynecologists. Updated cervical cancer screening guidelines, https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines (accessed 10 October 2022).
- 6.Martin-Hirsch P, Jarvis G, Kitchener H, et al. Collection devices for obtaining cervical cytology samples. Cochrane Database Syst Rev 2000; 2: CD001036. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker CJ, Stamp EC, Young W, et al. Comparison of the efficacy of the Cervex brush and the extended-tip wooden spatula with conventional cytology: a longitudinal study. Cytojournal 2009; 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnippel K, Michelow P, Chibwesha CJ, et al. Cost-effectiveness of using the Cervex-Brush (broom) compared to the elongated spatula for collection of conventional cervical cytology samples within a high-burden HIV setting: a model-based analysis. BMC Health Serv Res 2015; 15: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42(2): 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavranović L, Novak SR, Bolanca IK.[Causes and frequency of unsatisfactory cervicovaginal smears]. Acta Med Croatica 2011; 65(Suppl. 1): 115–119. [PubMed] [Google Scholar]
- 12.Alsharif M, McKeon DM, Gulbahce HE, et al. Unsatisfactory SurePath liquid-based Papanicolaou tests: causes and significance. Cancer 2009; 117(1): 15–26. [DOI] [PubMed] [Google Scholar]
- 13.Angelou K, Grigoriadis T, Diakosavvas M, et al. The genitourinary syndrome of menopause: an overview of the recent data. Cureus 2020; 12(4): e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roche Molecular Systems Inc. cobas® HPV test [package insert]. Branchburg, NJ: Roche Molecular Systems, Inc, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057231170975 for Papanicolaou test collection with the Papette brush or the spatula with cytobrush: A pragmatic study by Danielle J O’Laughlin, Brittany A Strelow, Nicole A Fellows, Joy N Stevens, Elizabeth A Kelsey, Stephanie R Fink, Sonya M Peters, Jennifer A Johnson, Jaclyn P Houghton, Anne M Stolp, Karen M Fischer, Johanna M Tweedy and Ramona S DeJesus in Women's Health