Abstract
Self-limited epilepsy with autonomic seizures, formerly known as benign occipital epilepsy of childhood or Panayiotopoulos syndrome is a focal epilepsy that is part of the epileptic syndromes with onset during childhood. The objective of this report is to raise awareness about its importance and describe the clinical manifestations, timely diagnosis, and treatment. A pediatric patient admitted with gastrointestinal manifestations is presented. The autonomic manifestations must be considered as part of the clinical spectrum that includes this disease and the digestive and autonomic manifestations that mask the diagnosis, sometimes even in the absence of motor seizures themselves. Electroencephalographic confirmation was performed, avoiding cataloging it in other differential diagnoses.
Keywords: SeLEAS, benign occipital epilepsy of childhood, autonomic seizures, panayiotopoulos syndrome, pediatrics
Introduction
Self-limited epilepsy with autonomic seizures (SeLEAS) is a focal epilepsy, a part of the epileptic syndromes of childhood according to the classification of the International League Against Epilepsy (ILAE). 1 Due to its varied clinical presentation that includes paroxysmal autonomic symptoms like acute vomiting, paleness, diaphoresis, and altered consciousness, some of the frequent characteristics can be easily confounded with other, mainly gastrointestinal pathologies, becoming a diagnostic challenge for physicians. SeLEAS is not an uncommon syndrome accounting for 5% of childhood epilepsies between 1 and 14 years and 13% of childhood epilepsies between 3 and 6 years. Therefore, it is the second most common focal childhood epilepsy, 2 so the objective of this report is to raise awareness about its importance and describe its particular clinical manifestations, and timely diagnosis that needs to be differentiated from other pathologies in order to establish a proper treatment.
Case presentation
An 8-year-old male patient from Bogotá, Colombia, mestizo, with no significant pathological history, was admitted to the emergency department due to symptoms that began 30 min prior to the consultation, consisting of generalized abdominal pain associated with a single emetic episode of food content, including diaphoresis, mucocutaneous paleness and dizziness, dysarthria, incoherent speech, no eye contact, and no other neurological symptoms (loss of consciousness, abnormal movements, or sphincter relaxation). At the end of the episode, which lasted 10 min, he presented with frontal location headache, at intensity 5/10 with persistent nausea. Family members denied the use of toxic substances or medication; 18 months ago, he had presented a similar event, which was diagnosed as migraine. A comprehensively past and familiar clinical history was interrogated with no family of personal history of epilepsy or any neurological disorders. The patient neurodevelopmental milestones were according to his age. He was attending fourth grade with good school performance.
The patient was admitted to the emergency room (ER); his vital signs were taken which showed normal, except for arterial pressure above 95% percentile for his age. The neurological exam showed altered content of consciousness, confused and disoriented, unable to recognize his mother, and no verbal response. However, he was able to follow orders and visual commands; the motor examination did not show any focal alterations, and no motor or sensory deficit. Approximately 20 min after admission, he showed recovery of consciousness, improvement in orientation, recognized the accompanying persons and responded consistently to questions and commands. During the first hours of hospitalization, he presented 3 consecutive and self-limited emetic events and headache that gradually improved after the administration of Diclofenac 45 mg IV in a single dose (1 mg/kg/dose).
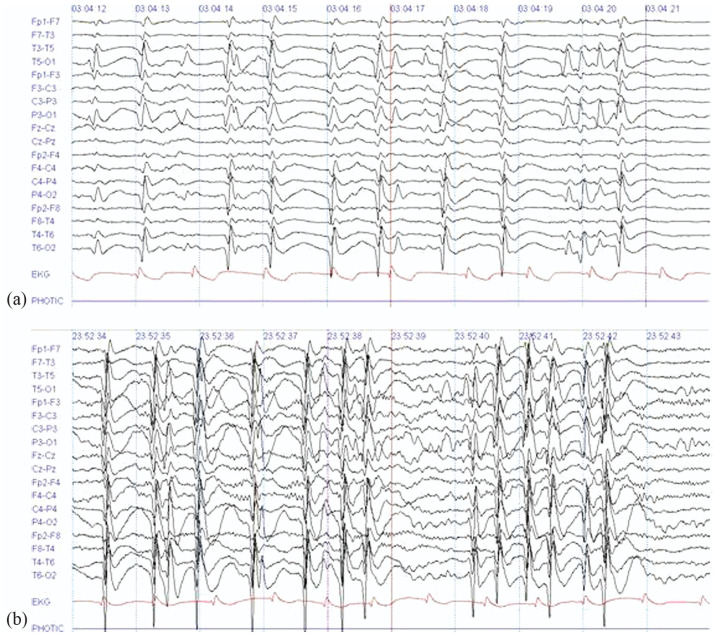
After admission, complementary serum and hematology tests were found within normal range, including electrolytes with sodium, chlorine, potassium, magnesium, and phosphorus. Brain epilepsy protocol magnetic resonance imaging (MRI) was reported within normal limits for age. A 12-h video electroencephalogram (EEG) starting 18 h after admission (Figure 1(a) and (b)) showed spike-wave complexes at 1.5–3 Hz with predominance in bilateral occipital regions without correlation with clinical activity. The findings were suggestive of occipital focal epilepsy.
Figure 1.
(a) Sleep record, (b) awake record: (sensitivity 15 and 7 Mv/mm; speed 30 mm/s; high cut 70 Hz; low cut 1 Hz), Spike-wave complexes at 1.5–3 Hz are shown with a predominance in bilateral occipital regions during both sleep and wakefulness. These are evidenced on multiple occasions along the 12-h video EEG monitoring. Source: original by the authors.
Figure 1(a) and (b): epochs of 12-h video EEG taken during recording awake and sleep states 18 h after admission. Slow spike wave focal complexes of 1.5–3 Hz are shown with predominance in bilateral occipital regions and with extension to bilateral anterior regions, these are evidenced during multiple occasions along the study.
With the clinical findings, the pediatric epilepsy service is consulted, and the clinical history and paraclinical findings diagnose SeLEAS, and management is started with carbamazepine gradually increasing until a dose of 25 mg/kg/day is achieved. The patient was monitored and managed intrahospital for 1 day, given the adequate evolution and good response at the beginning of the medication, and he was released to continue management for epilepsy on an outpatient basis. The patient was contacted 30 days after discharge, with good evolution, good tolerance to the medications, no adverse reactions, and seizure control.
Discussion
SeLEAS is the second most common focal epilepsy in childhood. 3 The ILAE defines four clinical syndromes: self-limited epilepsy with centrotemporal spikes (SeLECTS), formerly Rolandic epilepsy with a frequency of presentation of up to 25% and a peak of incidence of presentation between 5 and 10 years;4–6 SeLEAS (benign occipital epilepsy or Panayiotopoulos syndrome), late onset (benign), occipital epilepsy or idiopathic childhood occipital epilepsy-Gastaut type,7,8 and idiopathic photosensitive occipital lobe epilepsy.
SeLEAS was described in 1988 by Chrysostomos Panayiotopoulos. 9 It is a frequent form of benign epilepsy, characterized mainly by autonomic symptoms of paroxysmal appearance and/or digestive disorders, predominantly nausea, repeated vomiting, and status epilepticus which require high clinical suspicion for diagnosis.4,6,8,10,11 Due to its specific characteristics, there is an underdiagnosis of this entity, despite the good prognosis. 7
The etiopathogenesis is not clear. A genetic component has been proposed, although there are usually no reports of similar familiar seizures.4,9 It has been documented that the mutation in the SCN1A gene 9 can explain those cases with greater severity (early-onset seizures, longer duration of episodes and febrile precipitants); 12 however, it is still a matter of investigation.
This epilepsy syndrome is now considered as multifocal epilepsy in which the epileptic focus tends to localize in the occipital region during early childhood, but shifting their epileptic EEG from occipital regions to more anterior regions along with age, with the most preference to the centrotemporal and frontopolar regions.12,13
The main clinical characteristic is dysautonomic episodes, including paleness, redness, cyanosis, miosis, mydriasis and pupillary hippus, thermoregulatory, gastrointestinal, or cardiorespiratory alterations, hypersalivation, and urinary or fecal incontinence or headache, with sudden onset and termination as seizures do. Among them, emetic episodes are usually the main or only symptom of the attacks.14,15 Ictal emesis implies an intact state of consciousness. These autonomic seizure manifestations are presumably produced by hyperactivation of the autonomic nervous system through the central autonomic network (CAN). This is a circuit that includes the insular cortex, amygdala, hypothalamus, periaqueductal gray matter, nucleus of the tractus solitaries, and ventrolateral medulla. The CAN controls visceromotor and neuroendocrine responses, as well as behavioral and pain functions. Certain immaturity has been postulated in children, assuming a threshold of autonomic activation of the central autonomous network much lower than other sensory or motor areas. This explains why epileptic seizure activates these networks, generating the symptoms classically described in this entity. 9 In motor manifestations, ocular deviation and seizures are described, which can be generalized tonic clonic or unilateral clonic; status epilepticus focal to bilateral tonic-clonic can occur sometimes preceded by recurrent ictal vomiting during sleep. Syncope-like seizures characterized by unresponsive generalized flaccid posture for some time can occur in 20% of cases.4,5,9,15 The presence of visual manifestations is not usual and when they occur, they tend to be of short duration.4,6,7 Almost two-thirds of seizures occur during nighttime sleep and daily naps in which the child wakes up with nausea or emesis.6,8,15 When they occur in wakefulness, they are characterized by nausea, confusion, and fluctuating alteration of consciousness suggesting CNS origin of importance to distinguish from gastrointestinal diseases.6,7,15 Syncope-like seizures can occur in 20% of cases.4,5,9,15 Normally, neurodevelopment and neurological physical examination are normal.6,7
In some case reports, a family or febrile seizures history can happen in 40% and 25% of cases, respectively. 7 Short-term migraine headaches are also common. 9
This report, as described in the literature, highlighted the autonomic symptoms. Likewise, the age of presentation is in the range described, considering that the patient had presented an episode with similar characteristics 18 months ago that had been diagnosed as a migraine episode, which is part of the wide spectrum of differential diagnoses and great simulators of this entity.
There was no personal or family history of seizures. At the EEG level, as usual, the discharges started at the occipital lobes extending to anterior areas of the cerebral cortex, which helped the diagnostic approach.
Diagnosis and treatment
The diagnostic challenge in this entity is to recognize dysautonomic manifestations as forms of seizure.6,14 The EEG usually shows spikes and slow wave at the occipital level, although it is not the only epileptiform abnormalities location, others may also be in the anterior, central, temporal and midline regions.5,7,14,15 Something common is the migration of discharges to the ipsilateral hemisphere or to the contralateral hemisphere. 4 Darkness and hyperventilation can activate epileptiform discharges and subtle visual stimuli can attenuate them.7,15 Two-thirds of the patients have bilateral occipital epileptiform abnormalities. At least 90% of patients have an altered EEG. 9
Usually, its course tends to be benign; there are reports of cases with “atypical” presentation associated with aphasia and neurological compromise. 7 Usually MRI is normal, as well as metabolic studies. Regarding the latter, it is not necessary to carry them out if we are dealing with a neurologically healthy patient and without risk factors for having metabolic alterations. 7 Diagnostic images could be useful for differential diagnosis, especially in those patients with unclear neurocognitive and motor symptoms, alterations on neurological examination, and children under 1 year of age. 9
Genetic testing to date has not been determined and currently, there is no causal association of mutation with the epileptic syndrome.
Symptoms presented as headache and emetic episodes are common in migraine and are part of the clinical spectrum of differential diagnoses.4,14 Other non-epileptic differential diagnoses are cyclic emesis syndrome, gastroenteritis, and gastroesophageal reflux disease.9,14 It is not uncommon for migraine, syncope, or gastroenteritis to be misdiagnosed.4,14
Regarding treatment, an adequate response to drugs such as carbamazepine, phenobarbital, and valproic acid has been described. Cases with adequate clinical evolution without pharmacological management have even been documented.6,7,15 There is no universal consensus on the management or superiority of an anticonvulsant. In the same way, the prognosis does not seem to be subject to the choice of a particular drug, although antiepileptic drugs with focal action would be preferred.6,14,15
Finally, these entities have a good prognosis, hence their name. A third of patients have a single seizure and the rest have 2–5 episodes in their lifetime. Only 5% of patients will have more than 10 seizures in their lifetime. 14 Therefore, there is no specific recommendation in the initiation of medication because it should be determined, weighing the risk of long-term medication (as well as economic burden) and that of recurrent seizures affecting the neurocognitive development of children. Some authors recommend considering it at least after a second attack, according to the duration and associated neurological abnormalities of the seizures. 9
Conclusion
The literature reports a low incidence of SeLEAS, perhaps due to the underdiagnosis in the pediatric population, despite being one of the most frequent benign epilepsies in childhood. The autonomic manifestations must be considered as part of the clinical spectrum that includes this disease and the digestive manifestations that mask the diagnosis, sometimes even in the absence of motor seizures themselves. Suspicion of SeLEAS through the medical history and EEG confirmation facilitates approach and treatment in the ER and avoids time-consuming differential diagnosis.
Acknowledgments
The authors thank Sociedad de Cirugía de Bogotá—Hospital de San José, Fundación Universitaria de Ciencias de la Salud (FUCS), Bogotá, Colombia for their support.
Footnotes
Authors’ Note: All co-authors have read and agreed on the current version of this manuscript.
Contributors: J.D.R., J.C.C., L.P.O., A.M.C., and G.S.G.: full access to all the case-relevant information and responsibility for integrity of the data and accuracy of the report.
J.D.R. and J.C.C.: concept and design of the study.
L.P.O., A.M.C., and G.S.G.: acquisition of the data, and literature review.
All authors drafted the initial manuscript and reviewer’s corrections.
J.D.R.: critical revision of the manuscript for important intellectual content.
J.D.R. and J.C.C.: supervision.
Data availability: Data are available upon reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The resolution of the Colombian Ministry of Health number 8430 of 1993 establishes the scientific, technical, and administrative standards for health research. This work is classified as non-risk. The study was approved by the Research Committee of the School of Medicine at Fundación Universitaria de Ciencias de la Salud (FUCS) and by the Ethics Committee on Human Subjects at Hospital de San Jose.(CEISH) 458-2022. Written informed consent was obtained from the patient’s mother.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from legally authorized representatives before the study. Written informed consent was obtained from the patient’s mother.
ORCID iD: Jhon Camacho-Cruz  https://orcid.org/0000-0003-0898-9223
https://orcid.org/0000-0003-0898-9223
References
- 1.Specchio N, Wirrell EC, Scheffer IE, et al. International league against epilepsy classification and definition of epilepsy syndromes with onset in childhood: position paper by the ILAE task force on nosology and definitions. Epilepsia 2022; 63(6): 1398–1442. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Mehra K, Sharma S. 375 self-limited epilepsy with autonomic seizures (SeLEAS): a case series from a developing country in Asia. Archiv Dis Childhood 2022; 107: A222. [Google Scholar]
- 3.Akca Kalem S, Elmali AD, Demirbilek V, et al. Panayiotopoulos syndrome and Gastaut syndrome are distinct entities in terms of neuropsychological findings. Epilepsy Behav 2019; 99: 106447. [DOI] [PubMed] [Google Scholar]
- 4.Bruni O, Novelli L, Malluci A, et al. Benign Rolandic and occipital epilepsies of childhood. Sleep Med Clin 2012(7): 135–145.23503584 [Google Scholar]
- 5.Hodges SL, Gabriel MT, Perry MS. Neuropsychological findings associated with panayiotopoulos syndrome in three children. Epilepsy Behav 2016; 54: 158–162. [DOI] [PubMed] [Google Scholar]
- 6.Ferrie C, Caraballo R, Covanis A, et al. Panayiotopoulos syndrome: a consensus view. Develop Med Child Neurol 2006; 48: 236–240. [DOI] [PubMed] [Google Scholar]
- 7.Yépez I, Zambrano J, Vásquez E. Epilepsia Occipital Benigna de la Niñez tipo Panayiotopoulos presentación de cinco casos clínicos y revisión de la literatura. Rev Ecuat Neurol 2007; 15(2–3): 2006. [Google Scholar]
- 8.Capovilla G, Striano P, Beccaria F. Changes in Panayiotopoulos syndrome over time. Epilepsia 2009; 50(Suppl. 5): 45–48. [DOI] [PubMed] [Google Scholar]
- 9.Graziosi A, Pellegrino N, Di Stefano V, et al. Misdiagnosis and pitfalls in Panayiotopoulos syndrome. Epilepsy Behav 2019; 98(Pt. A): 124–128. [DOI] [PubMed] [Google Scholar]
- 10.Panayiotopoulos CP. Early-onset benign childhood occipital seizure susceptibility syndrome: a syndrome to recognize. Epilepsia 1999; 40(5): 621–630. [DOI] [PubMed] [Google Scholar]
- 11.Garófalo N, Vargas J, Novoa L, et al. Evolución atípica del síndrome de Panayiotopoulos a síndrome de punta ondas continuas durante el sueño lento. Revista Cubana De Pediatría 2019; 91(3): 1561. [Google Scholar]
- 12.Ferrie CD, Beaumanoir A, Guerrini R, et al. Early -onset benign occipital seizure susceptibility syndrome. Epilepsia 1997; 38: 285–293. [DOI] [PubMed] [Google Scholar]
- 13.Ohtsu M, Oguni H, Hayashi K, et al. EEG in children with early-onset benign occipital seizure susceptibility syndrome: panayiotopoulos syndrome. Epilepsia 2003; 44(3): 435–442. [DOI] [PubMed] [Google Scholar]
- 14.Covanis A. Panayiotopoulos syndrome: a benign childhood autonomic epilepsy frequently imitating encephalitis, syncope, migraine, sleep disorder, or gastroenteritis. Pediatrics 2006; 118: e1237–e1243. [DOI] [PubMed] [Google Scholar]
- 15.Parisi P, Villa MP, Pelliccia A, et al. Panayiotopoulos syndrome: diagnosis and management. Neurol Sci 2007; 28(2): 72–79. [DOI] [PubMed] [Google Scholar]