Abstract
Background:
Irritable bowel syndrome (IBS) is a common gastrointestinal condition which entails a high burden in the quality of life (QoL) of patients. Nutritional interventions have been proposed to alleviate symptoms, since still no effective treatments exist for IBS.
Objectives:
Our aim is to analyse the feasibility of the use of starch- and sucrose-reduced diet (SSRD).
Design:
In this study, we used a SSRD accompanied by nutritional and culinary recommendations to measure the effects in IBS patients with diarrhoea.
Methods:
In all, 34 participants completed a 4-week nutritional intervention based on SSRD. Symptoms, QoL and dietary habits were assessed by several questionnaires that were completed at the beginning, daily, after 2 weeks, at the end, and after 2 months.
Results:
85.29% of the participants reached the primary endpoint [reduction of 50 points or more in IBS-symptom severity scale (SSS)], and 58.82% the secondary endpoint (reduction of 50% or more in IBS-SSS). The relief of symptoms and improvement of the QoL were significant after 2 weeks of intervention, at the end and after 2 months. Dietary habits were consistent with the diet and high adherence was achieved.
Conclusions:
SSRD and individualized nutritional and culinary guidance improved symptoms and QoL of IBS patients with diarrhoea, with a high adherence.
Keywords: irritable bowel syndrome, nutritional intervention, starch- and sucrose-reduced diet
Introduction
Irritable bowel syndrome (IBS) is a common gastrointestinal (GI) condition that is developed by the 5–20% of the general population, depending on the characteristics of the population, the region analysed and the diagnosis criteria.1,2 Since there is not any reliable biomarker, the diagnosis of IBS is based on the presence of symptoms, such as abdominal pain and altered bowel habits. Although IBS does not decrease the life expectancy, it entails a high burden in the quality of life (QoL) of the patients and a substantial cost for the health system.1,2 In addition, from the pathogenesis and the clinical management point of view, IBS is defined as an heterogeneous syndrome since its aetiology is unknown. 3 As a consequence, the development of effective treatments in IBS is challenging.
In the absence of effective treatments for IBS, a diet low in FODMAPs (fermentable, oligosaccharides, disaccharides, monosaccharides and polyols) has been used to alleviate the symptoms of the patients. 4 Although the use of a low FODMAP diet seems to improve the symptoms of IBS patients,5,6 it has been observed that the efficacy of this diet is limited in some IBS patients with mutations in sucrase-isomaltase gene, since low FODMAP diet does not limit the consumption of starch and partially limits the intake of sucrose. 7 Previously, a higher prevalence of SI gene variants that could have functional consequences have been detected in IBS patients compared to healthy individuals.8–10 Furthermore, an overlap of symptoms such as diarrhoea, abdominal pain and bloating exists between IBS associated with diarrhoea (IBS-D) and patients who present sucrase-isomaltase deficiency. In addition, starch- and sucrose-reduced diet (SSRD) has been successfully used in IBS patients11–13 but, as far as we know, no previous studies have used culinary and personalized nutritional recommendations for a SSRD-based dietary intervention in a population that presents a high gastronomic culture. Considering that two out three patients associate the onset of their symptoms to the intake of specific foods 14 and the difficulty to adhere to restricted diets in IBS, 15 it was hypothesized that an approach based on a personalized nutritional and culinary advice may facilitate the adherence to a SSRD. Indeed, it should be noted that adherence to carbohydrate-restricted diets is particularly difficult since these nutrients are the main contributors to energy in most people’s diets. 16 Thus, adapting dietary interventions to an individual’s preferences may help to improve adherence to restrictive dietary prescriptions.
In this context, to analyse the feasibility of the use of SSRD in our population, we have carried out an SSRD-based intervention in a pilot study for the treatment of symptoms of diarrhoea predominant IBS patients. For this purpose, a SSRD-based dietary intervention was conducted for 4 weeks and during the intervention, specific nutritional recommendations and culinary recommendations were provided through a trained nutritionist to facilitate the adoption of the diet by participants. In this sense, participants received a menu plan and a recipe book that was adapted with the nutritional criteria demanded by the SSRD. The impact of the SSRD on the symptoms, QoL and nutritional habits of patients was assessed at four time points, as well as, on a daily basis, through questionnaires.
Material and methods
Patient population
Patients were recruited through the Gastroenterology service of Hospital Universitario Donostia (San Sebastian, Spain). In the standard clinical practice, patients that were compatible with diarrhoea predominant IBS diagnosis were informed about the present study and the possibility of participating in it. In addition, inclusion criteria included the following: age between 18 and 70, at least weekly abdominal pain related to bowel habit in the past 3 months, altered bowel habit and more than 175 points in the Spanish version of irritable bowel syndrome-symptom severity scale (IBS-SSS) questionnaire. 17 Exclusion criteria include individuals without enough IBS symptoms, diagnosis of GI diseases, serious diseases or psychiatric conditions, or patients who followed a dietary therapy with a vegan diet, gluten-free diet or low-FODMAP diet. An informed consent from the eligible patients was obtained before the beginning of the trial.
Design of study
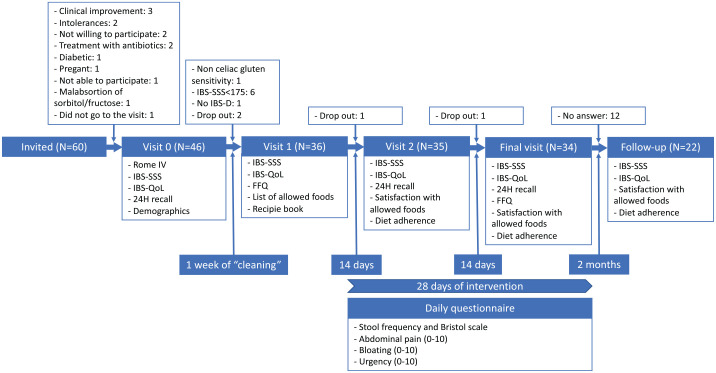
Patients were asked to participate in a study that would test the efficacy of a SSRD to improve IBS symptoms. The study consisted of four visits and lasted 5 weeks (1 week of ‘cleaning’ and 4 weeks of nutritional intervention). The general design of the study is shown in Figure 1.
Figure 1.
Design of the study and the participants in each step.
FFQ, food frequency questionnaire; IBS-SSS, irritable bowel syndrome-symptom severity scale questionnaire; IBS-QoL, irritable bowel syndrome quality of life.
In visit 0, the evaluation of symptoms severity and QoL was assessed. For this purpose, patients fulfilled the following questionnaires: Rome IV – IBS module of Rome Foundation, 18 the Spanish version of IBS-SSS 17 and the Spanish version of Irritable Bowel Syndrome QoL (IBS-QoL). 19 Furthermore, information about patients’ lifestyle habits (tobacco and alcohol consumption) and food intolerances and food allergies were also registered.
By 1 week before starting the intervention (‘cleaning week’), participants received indications to continue with their usual diet, but they were not allowed to take any medication, unless the symptoms were unbearable. In visit 1, patients completed the IBS-SSS, IBS-QoL and food frequency questionnaire (FFQ). 20 Moreover, a trained research nutritionist instructed the patients on the list of foods they would be allowed to consume during the intervention and participants were provided with a recipe book and a menu planning.
Participants were encouraged to start a 4-week dietary intervention. Every day, participants completed a questionnaire about stool frequency and type (Bristol scale), abdominal pain (0–10 score), bloating (0–10 score) and urgency (0–10 score).
Two weeks after visit 1, in the second visit, the participants completed IBS-SSS, IBS-QoL Questionnaires, the questionnaire related with the satisfaction with allowed foods (3: good, 2: regular; 1: bad), and questions about dietary compliance (0–10 score) and the difficulty to follow the dietary protocol (0–10 score).
At the end of the intervention, in the final visit, after 4 weeks of the intervention, the participants completed the IBS-SSS questionnaire, IBS-QoL questionnaire, FFQ and the questionnaire related with the satisfaction with allowed foods (3: good, 2: regular; 1: bad), and questions about dietary compliance (0–10 score) and the difficulty to follow the dietary protocol (0–10 score).
The clinical endpoint of the study was the reduction of 50 points of IBS-SSS score after the intervention. In addition, the reduction of 50% of IBS-SSS score after the intervention was established as a secondary endpoint.
Moreover, 2 months after the end of the intervention follow-up questionnaires were sent by post to the participants: IBS-SSS and IBS-QoL questionnaires, the satisfaction with allowed foods (3: good, 2: regular; 1: bad), and questions about dietary compliance (0–10 score) and the difficulty to follow the dietary protocol (0–10 score).
Starch- and sucrose-reduced diet
Participants were instructed by a nutritionist to follow a SSRD, according to the recommendations given to patients with the congenital sucrase-isomaltase deficiency (https://www.sucroseintolerance.com/choosing-your-foods). At visit 1, they received written material about the dietary protocol with information about sucrose and starch, general recommendations and a list with a variety of foods categorized in three groups: high, medium and low content in sucrose and/or starch. Participants were advised to consume only foods categorized in the third group, those defined as low in sucrose and/or starch. Moreover, participants were recommended to restrict the intake of cereals, and preferably to ingest whole cereals, to a maximum of two servings (approximately 30 g) per week. No specific advice was given regarding overall caloric intake. Indeed, to facilitate the adherence to the dietary protocol, participants received a recipe book, and recipes were developed including foods with low sucrose and/or starch content only (Supplemental Table S1). For the development of the book of recipes, research nutritionists collaborated with chefs from the Technology Center specialized in Gastronomy from Basque Culinary Center (BCCInnovation – https://innovation.bculinary.com/). The book of recipes included 28 recipes for lunch and dinner, healthy options for breakfast and healthy snacking, desserts, vegetarian recipes and a 2-week menu planning with a shopping list.
During the study, two nutritionists were available between visits by email and telephone to answer questions about the diet.
Dietary assessment
Dietary intake was analysed by a validated FFQ 20 at visit 0 and visit 3. The data collection was conducted through a trained nutritionist who asked about the consumption in the last month (questions refer to the food consumption daily, weekly, monthly or never/rarely) of different food groups. Diet was also assessed by a 24-h dietary recall. Participants completed three 24-h recalls (two on weekdays and one recall on a weekend) during the intervention: prior the beginning of the study (visit 0), in the middle of the intervention (visit 2) and at the end of the intervention (visit 3). To guide the participants to complete the recalls, they received a list with domestic measurement units and a food atlas with the most frequently consumed foods with different portion sizes. Energy and nutrient intake, including total sugars, sucrose and starch, were calculated using Diet Creator© software.
Statistical analyses
For all the questionnaires, a paired t-test was used to compare each visit with baseline visit; and the effect size of the paired t-test was calculated using Cohen’s d using the package rstatix (https://CRAN.R-project.org/package=rstatix) of R language. 21 In the case of IBS-SSS and IBS-QoL questionnaires the responses of visits 1, 2 and final visit were compared with the response of visit 0; in the case of FFQ, final visit was compared with visit 1; in the case of energy intake, visit 2 and final visit were compared with visit 0; and in the case of the satisfaction with allowed foods, and the adherence and difficulty of the diet, the final visit was compared with visit 2.
For daily questions, days 28 and 1 were compared, only for the individuals who completed the daily questions both days; and a paired t-test was used to compare days 28 and 1.
All the statistical analyses and graphics were done using R language. 21
All methods were performed in accordance with relevant guidelines and regulations including the Declarations of Helsinki.
Results
In all, 60 subjects were invited to participate in the study and 46 agreed to attend visit 0. In total, 36 of them fulfilled the inclusion criteria and two of them withdraw the study once it was started. In total, IBS-SSS, IBS-QoL and evaluation of dietary adherence were obtained from 34 participants; and FFQ and daily questionnaires were obtained from 26 participants, although only 21 participants gave a response in day 1 and 28 in the daily questionnaires (Figure 1).
The majority of the participants were females (62.76%); 1 out of 3 were current smokers; 3 of them had previous food allergies; and 4 participants had food intolerances (Table 1). In addition, the symptoms of all the participants were compatible with IBS-D definition of Rome IV criteria (Table 1).
Table 1.
Demographics of the participants.
| Total | Female | Male | |
|---|---|---|---|
| N | 34 | 21 (62.76%) | 13 (37.24%) |
| Age (SD) | 42.83 (13.94) | 43.38 (15.08) | 40.38 (12.23) |
| Current smokers | 13 (38.23%) | 9 (42.86%) | 4 (30.77%) |
| Allergies | 3 (8.82%) | 1 (4.76%) | 2 (15.38%) |
| Intolerances | 4 (11.76%) | 2 (9.52%) | 2 (15.38%) |
| Rome IV | |||
| IBS-D | 34 (100%) | 21 (100%) | 13 (100%) |
IBS-D, irritable bowel syndrome associated with diarrhoea; SD, standard deviation.
IBS symptoms and QoL
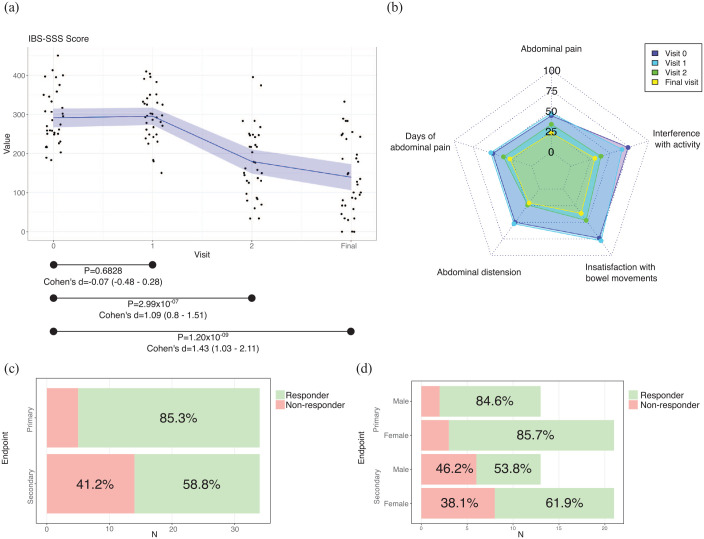
The symptoms of IBS patients, measured by the IBS-SSS questionnaire, showed that there were no differences between visit 0 and visit 1 (p = 0.6828), while in visit 2 there was a significant improvement of the symptoms (visit 2 versus visit 0, p = 2.99 × 10−07). The improvement was maintained at the end of the intervention (final visit versus visit 0, p = 1.20 × 10−09) (Figure 2(a), Supplemental Table S2). According to Cohen’s d, the effect of the improvement was large (Figure 2(a)). In addition, this trend was observed in all the questions available in the IBS-SSS questionnaire (Figure 2(b), Supplemental Table S2).
Figure 2.
Results of IBS-SSS. (a) Total score values. Black dots, individual results; in blue, the mean value; in shadowed blue, 95% of confidence interval. (b) Results of each question. (c) Results of the main (reduction of 50 points of IBS-SSS score) and secondary (reduction of 50% of IBS-SSS score) endpoints. (d) Results of the main (reduction of 50 points of IBS-SSS score) and secondary (reduction of 50% of IBS-SSS score) endpoints by sex.
95% of confidence interval; <0.2, negligible effect; 0.2–0.5, small effect; 0.5–0.8, moderate effect; >0.8, large effect.
Cohen’s d, effect size of paired t-test; p, p value of paired t-test; IBS-SSS, irritable bowel syndrome-symptom severity scale.
At the end of the dietary intervention, 29 participants (85.29%) had a reduction of 50 points or more in their IBS-SSS score (Figure 2(c)). From those participants who achieved the primary endpoint, 18 were females (85.71%) and 11 males (84.62%) (Figure 2(d)). Moreover, 20 participants (58.82%) reduced their IBS-SSS score in more than 50% their baseline score (Figure 2(c)). From those participants who achieved the secondary endpoint, 13 were females (61.91%) and 7 were males (53.85%) (Figure 2(d)). Although the percentages are higher in females, the differences were not significant (χ2 test p value: 0.9299 in primary outcome; p value: 0.6427 in secondary outcome).
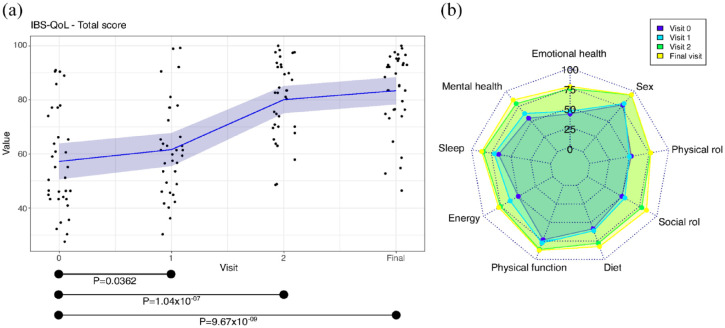
QoL of IBS patients, assessed by IBS-QoL questionnaire, showed a slight improvement between visit 0 and visit 1 (p = 0.0362), and a significant improvement was observed in visit 2 (visit 2 versus visit 0, p = 1.04 × 10−07), which was maintained at the end of the intervention (Final visit versus visit 0, p = 9.67 × 10−09) (Figure 3(a), Supplemental Table S3). According to Cohen’s d, the effect of the improvement at visit 1 was small and it was large at visit 2 and final visit (Figure 3(a)) The improvement was observed in all the dimensions of IBS-QoL questionnaire, especially in the emotional health, mental health, energy, diet and social role (Figure 3(b), Supplemental Table S3).
Figure 3.
Results of IBS-QoL. (a) Total score values. Black dots, individual results; in blue, the mean; in shadowed blue, 95% of confidence interval. (b) Results of each domain.
95% of confidence interval, <0.2, negligible effect; 0.2–0.5, small effect; 0.5–0.8, moderate effect; >0.8, large effect.
Cohen’s d, effect size of paired t-test; p, p value of paired t-test; IBS-QoL, irritable bowel syndrome-quality of life.
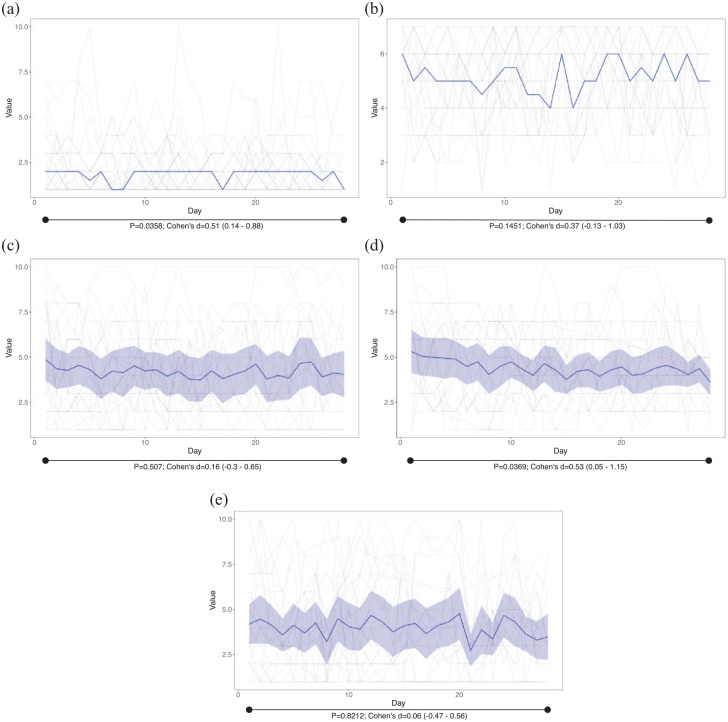
Regarding daily questions, there was an interpersonal and daily variability (Figure 4). We observed a significant decrease between day 1 and day 28 in the stool frequency (25.53% less, p = 0.0358, moderate effect according to Cohen’s d) and bloating (27.48% less, p = 0.0369 moderate effect according to Cohen’s d), while the improvement in Bristol stool scale (from 5.18 to 4.59), abdominal pain (12.16% less) and urgency (5.88% less) was not significant.
Figure 4.
Results of daily answers. In grey, individual responses. (a) Bowel movements; in blue, the median value. (b) Bristol scale; in blue, the median value. (c) Abdominal pain; in blue, the mean; in shadowed blue, 95% of confidence interval. (d) Bloating; in blue, the mean; in shadowed blue, 95% of confidence interval. (e) Urgency; in blue, the mean; in shadowed blue, 95% of confidence interval.
95% of confidence interval, <0.2, negligible effect; 0.2–0.5, small effect; 0.5–0.8, moderate effect; >0.8, large effect.
Cohen’s d, effect size of paired t-test; p, p value of paired t-test.
Dietary habits
As a consequence of the restricted diet, participants consumed significantly less calories 2 weeks after the beginning of the diet (19% less, p = 3.28 × 10−5, large effect according to Cohen’s d, Table 2), a reduction that was maintained at the end of the intervention (12% less comparing with before the intervention, p = 0.0019, moderate effect according to Cohen’s d). Moreover, a significantly reduced consumption of carbohydrates (57% less, p = 4.24 × 10−11, large effect according to Cohen’s d), sugar (49% less, p = 0.0005, moderate effect according to Cohen’s d), sucrose (81% less, p = 2.34 × 10−6, large effect according to Cohen’s d) and starch (93% less, p = 5.13 × 10−08, large effect according to Cohen’s d) was observed after 2 weeks of dietary intervention (Table 2). These results were also observed at the end of the intervention (Table 2): 58% less consumption of carbohydrates (p = 4.04 × 10−11, large effect according to Cohen’s d), 45% less consumption of sugar (p = 0.0018, moderate effect according to Cohen’s d), 79% less of sucrose (p = 4.91 × 10−06, large effect according to Cohen’s d) and 89% less consumption of starch (p = 1.79 × 10−08, large effect according to Cohen’s d).
Table 2.
Energy and nutrient intake of the participants before the beginning of the study (visit 0), after 2 weeks of the study (visit 2) and at the end of the dietary intervention (final visit).
| Food habits | Visit 0 | Visit 2 | Final visit | ||||
|---|---|---|---|---|---|---|---|
| Mean (±SD) | Mean (±SD) | p | Cohen’s d (CI 95%) | Mean (±SD) | p | Cohen’s d (CI 95%) | |
| Energy (kcal/day) | 2063 (±589) | 1664 (±398) | 3.28×10−05 | 0.88 (0.54–1.36) | 1729 (±459) | 0.0019 | 0.65 (0.3–1.13) |
| Protein (g/day) | 93 (±28) | 100 (±31) | 0.4648 | −0.11 (−0.49–0.29) | 102 (±30) | 0.1545 | −0.27 (−0.7–0.1) |
| Fat (g/day) | 107 (±39) | 105 (±28) | 0.4408 | 0.08 (−0.26–0.49) | 115 (±35.22) | 0.2676 | −0.21 (−0.59–0.18) |
| SFA (g/day) | 29 (±12) | 25 (±10) | 0.0872 | 0.27 (−0.14–0.62) | 33 (±15) | 0.1699 | −0.27 (−0.57–0.12) |
| PUFA (g/day) | 14 (±7) | 16 (±6) | 0.5891 | −0.14 (−0.48–0.26) | 16 (±9) | 0.3286 | −0.19 (−0.52–0.25) |
| MUFA (g/day) | 53 (±22) | 50 (±11) | 0.0767 | 0.29 (−0.08–0.72) | 55 (±15) | 0.9449 | −0.01 (−0.42–0.4) |
| Carbohydrates (g/day) | 179 (±51) | 76 (±32) | 4.24×10−11 | 1.93 (1.48–2.76) | 76 (±32) | 4.04×10−11 | 2.00 (−0.45–0.38) |
| Fibre (g/day) | 18 (±9) | 15 (±6) | 0.0547 | 0.33 (−0.03–0.69) | 15 (±7) | 0.1045 | 0.31 (−0.04–0.68) |
| Sugar g/day) | 37 (±24) | 19 (±15) | 0.0005 | 0.70 (0.42–1.1) | 20 (±16) | 0.0018 | 0.65 (0.38–1.11) |
| Sucrose (g/day) | 17 (±13) | 3 (±3) | 2.34×10−06 | 1.07 (0.84–1.6) | 4 (±4) | 4.91×10−06 | 1.07 (0.83–1.65) |
| Starch (g/day) | 64 (±44) | 5 (±7) | 5.13×10−08 | 1.42 (1.19–1.96) | 7 (±9) | 1.79×10−08 | 1.49 (1.22–2.11) |
95% of confidence interval, <0.2, negligible effect; 0.2–0.5, small effect; 0.5–0.8, moderate effect; >0.8, large effect.
Cohen’s d, effect size of paired t-test; MUFA, monounsaturated fatty acids; p, p value of paired t-test; PUFA, polyunsaturated fatty acids; SD, standard deviation; SFA, saturated fatty acids.
Furthermore, a significant modification in the diet of participants was observed. Results showed a significant increase in the intake of fatty fish (60% more, p = 0.0206, small effect according to Cohen’s d), fruits (31% more, p = 0.0202), nuts (217% more, p = 1.03 × 10−07, small effect according to Cohen’s d) and water (16% more, p = 0.0160, small effect according to Cohen’s d); while the consumption of pulses (86% less, p = 1.30 × 10−07, large effect according to Cohen’s d), refined cereals (95% less, p = 3.29 × 10−07, large effect according to Cohen’s d), pastries (88% less, p = 0.0043) and sugar (91% less, p = 9.01 × 10−05, moderate effect according to Cohen’s d) was significantly reduced (Table 3).
Table 3.
Food frequency consumption of different food items by the participants at the beginning (visit 1) and at the end of the dietary intervention (final visit).
| Food groups consumption (servings/month) | Mean visit 1 (±SD) | Mean final visit (±SD) | p | Cohen’s d (95% CI) |
|---|---|---|---|---|
| Whole-fat dairy products | 29 (±36) | 42 (±35) | 0.0573 | −0.33 (−0.66 to 0.00) |
| Low-fat dairy products | 17 (±28) | 20 (±26) | 0.4215 | −0.14 (−0.5 to 0.19) |
| Eggs | 17 (±17) | 22 (±23) | 0.2096 | −0.22 (−0.51 to 0.01) |
| White meat | 12 (±7) | 13 (±6) | 0.4313 | −0.14 (−0.59 to 0.18) |
| Red meat | 8 (±6) | 9 (±5) | 0.4399 | −0.13 (−0.59 to 0.17) |
| Withe fish | 6 (±4) | 8 (±6) | 0.0225 | −0.41 (−0.77 to −0.14) |
| Fatty fish | 4 (±4) | 6 (±5) | 0.0206 | −0.41 (−0.84 to −0.1) |
| Vegetables | 27 (±22) | 33 (±18) | 0.0952 | −0.29 (−0.69 to 0.02) |
| Fruits | 33 (±26) | 44 (±29) | 0.0202 | −0.42 (−0.91 to −0.08) |
| Nuts | 11 (±14) | 34 (±21) | 1.03×10−07 | −1.16 (−1.88 to −0.78) |
| Pulses | 6 (±6) | 1 (±2) | 1.30×10−07 | 1.14 (0.92 to 1.64) |
| Olive oil | 89 (±41) | 89 (±41) | 0.9776 | 0.00 (−0.34 to 0.35) |
| Other fats | 10 (±16) | 9 (±19) | 0.7558 | 0.05 (−0.28 to 0.04) |
| Refined cereals | 64 (±55) | 3 (±6) | 3.29×10−07 | 1.09 (1.47 to 0.82) |
| Whole cereals | 9 (±21) | 2 (±3) | 0.0616 | 0.33 (0.05 to 0.53) |
| Pastries | 10 (±17) | 1 (±5) | 0.0043 | 0.52 (0.74 to 0.36) |
| Sugars | 32 (±37) | 3 (±9) | 9.01×10−05 | 0.76 (1.04 to 0.54) |
| Alcohol | 21 (±47) | 11 (±14) | 0.1714 | 0.24 (−0.2 to 0.43) |
| Water | 128 (±56) | 148 (±34) | 0.0160 | −0.43 (−0.8 to 0.14) |
95% of confidence interval, <0.2, negligible effect; 0.2–0.5, small effect; 0.5–0.8, moderate effect; >0.8, large effect.
Cohen’s d, effect size of paired t-test; p, p value of paired t-test; SD, standard deviation.
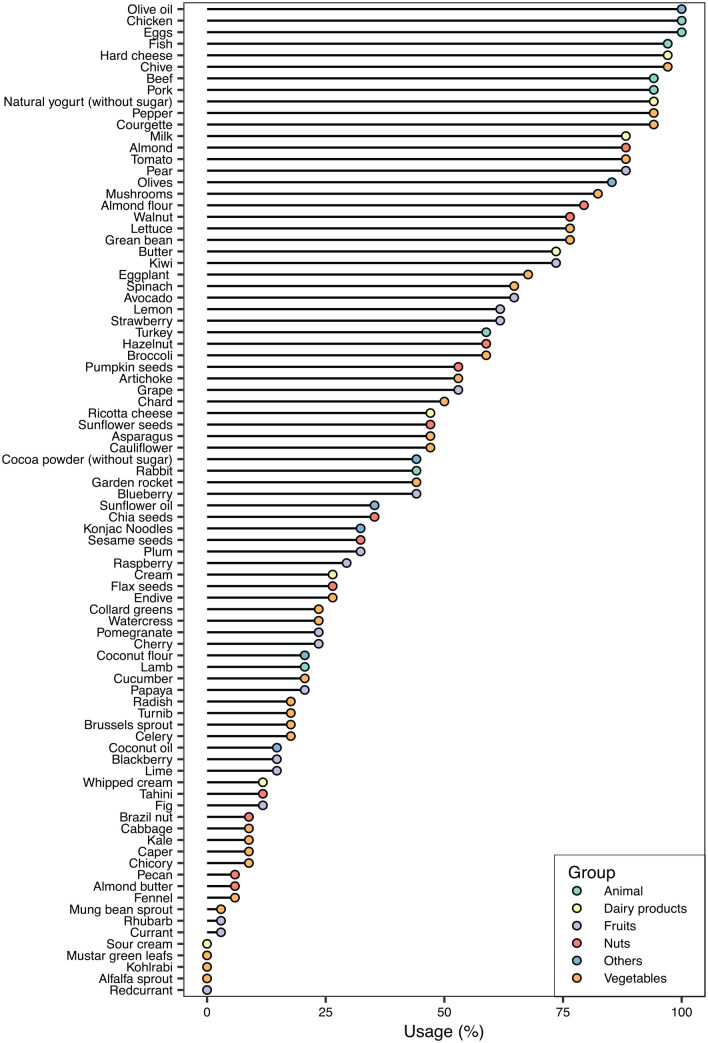
Among the allowed foods, all the participants of the study included olive oil, chicken and eggs in their diet (Figure 5). The evaluation of the food intake did not vary between visit 2 and final visit, and the different foods included in the diet were well accepted (Supplemental Table 4).
Figure 5.
Consumption of allowed foods. The percentage of participants that have consumed a given food is shown.
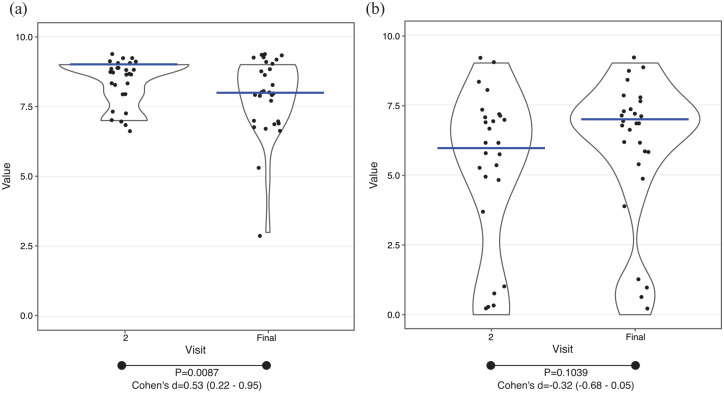
Finally, the adherence to the diet was observed to be high but it was significantly lower at the end of the intervention (visit 2: 8.39 ± 0.83, final visit: 7.86 ± 1.36, p = 0.0087, moderate effect according to Cohen’s d) (Figure 6(a)). Regarding the difficulty to follow the dietary protocol, the participants showed mixed opinions and the change over the diet was not significant (visit 2: 5 ± 3.01, final visit: 5.86 ± 2.71, p = 0.1039) (Figure 6(b)).
Figure 6.
Adherence and difficulty of the diet. Black dots, individual results; blue lines, median value. (a) Adherence to the diet. (b) Difficulty to comply the diet.
95% of confidence interval, <0.2, negligible effect; 0.2–0.5, small effect; 0.5–0.8, moderate effect; >0.8, large effect.
Cohen’s d, effect size of paired t-test; p, p value of paired t-test.
Two-month follow-up
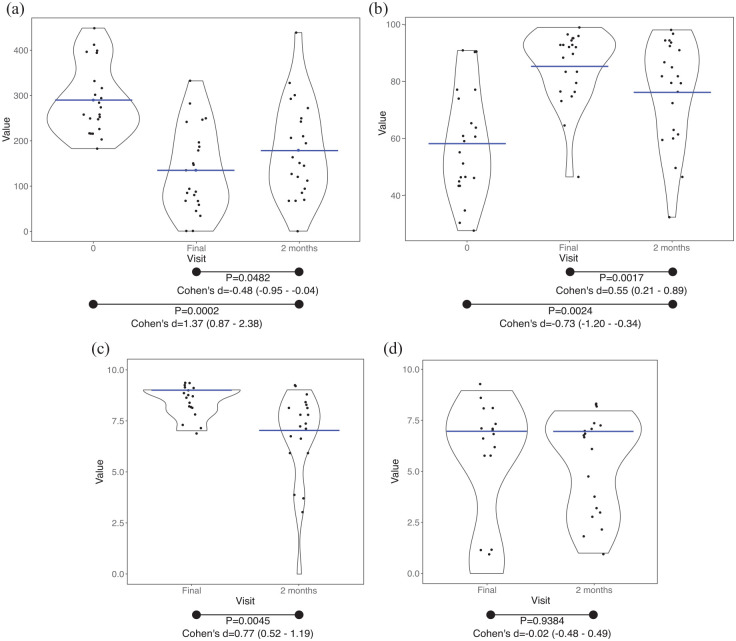
Two months after the end of the intervention, 22 questionnaires were returned by post. After 2 months, the symptoms and QoL deteriorated but they were still better than the situation defined by participants before entering into the dietary intervention (Figure 7(a) and (b)). IBS-SSS score (Figure 7(a)) was higher after 2 months (final visit versus 2-month follow-up, p = 0.0482, small effect according to Cohen’s d) but lower than the score obtained at the beginning of the intervention (visit 0 versus 2-month follow-up, p = 0.0002, large effect according to Cohen’s d). IBS-QoL score (Figure 7(b)) was significantly lower after 2 months (final visit versus 2-month follow-up p = 0.0017, moderate effect according to Cohen’s d), but it was significantly higher than at the beginning of the intervention (visit 0 versus 2-month follow-up, p = 0.0024, moderate effect according to Cohen’s d). In addition, after 2-month follow-up, dietary adherence (Figure 7(c)) was found to be significantly reduced in comparison to the final visit (p = 0.0045, moderate effect according to Cohen’s d) but the difficulty (Figure 7(d)) remained equal (p = 0.9384).
Figure 7.
Two-month follow-up. Black dots, individual results. (a) IBS-SSS score; blue lines, mean value. (b) IBS-QoL total score; blue lines, mean value. (c) Adherence to the diet; blue lines, median value. (d) Difficulty to comply the diet; blue lines, median value.
95% of confidence interval; <0.2, negligible effect; 0.2–0.5, small effect; 0.5–0.8, moderate effect; >0.8, large effect.
Cohen’s d, effect size of paired t-test; IBS-QoL, irritable bowel syndrome-quality of life; IBS-SSS, irritable bowel syndrome-symptom severity scale; p, p value of paired t-test.
Discussion
Finding effective treatments for IBS is difficult and dietary interventions have been shown to be helpful in improving symptoms. One dietary strategy developed by the National Institute for Health and Care Excellence advises individuals to eat smaller portions and more frequently, to eat regularly and in peace, to reduce intake of foods that could potentially stimulate the GI tract and to adapt intake of fibre depending on stool form predominance. Another dietary strategy is the one that recommends a temporary exclusion of fermentable carbohydrates such as FODMAPs since these have been found to trigger symptoms in some patients. Although a low FODMAP diet has been shown to produce clinically meaningful responses in 50–86% of IBS patients, 22 data still suggest that nearly 35% of individuals do not benefit from this management option. 23 There is an increasing evidence that partial sucrase-isomaltase deficiency is associated with a higher risk of IBS in the general population. Indeed, recent investigations are providing evidence that sucrase-isomaltase deficiency is more prevalent and of greater clinical significance than previously suspected.8,24 While the low FODMAP diet has not been linked to a specific mechanism (or at least to a specific carbohydrate digestion pathway), sucrose intolerance offers the opportunity to specifically target the ‘defect’ (possibly genetically determined in some individuals) via dietary restrictions. The restriction in sucrose and starch has been shown to be effective in preliminary studies of IBS patients,8–10 even irrespective of genetics. 25 Hence, the aim of this study was to test a SSRD in a subset of IBS patients with diarrhoea, to provide further evidence of the applicability of such a dietary strategy.
As far as we know, this is the first attempt to use the SSRD in a different population suffering from IBS-D. In this sense, we used this diet accompanied by nutritional and culinary recommendations that might help the participants to comply with the diet and improve the IBS symptoms, as a pilot study to analyse the feasibility of the use of SSRD in our population.
Results from this study showed a significant improvement of the symptoms and a significant amelioration of the patient’s QoL. Even though the dietary intervention lasted 1 month, 2 weeks after the beginning of the SSRD, significant enhancement was observed in symptoms of patients, which was maintained until the intervention was finished. Interestingly, in the 2-month follow-up period, these improvements were also detected. In a study where a low FODMAP diet was followed during 4 weeks, a significant improvement in IBS-SSS score between the baseline and the end of the intervention was reported. 26 Although methodological differences exist between studies, our results are in line with the studies in which low FODMAP diets has demonstrated an amelioration in comparison to the baseline symptoms,5,6 as well as, the improvements observed with the dietary interventions based on SSRD.11–13 In addition, in this study, the participants were more satisfied with their bowel habit and their stool frequency and consistency which improved at the end of the intervention compared to baseline, reducing the main symptoms related to IBS-D.
The mentioned amelioration was observed in almost all participants. However, the success of the intervention cannot be only explained by the reduction in the intake of sucrose and starch. In this study, the dietary pattern of participants was found to be modified towards a healthier diet. Moreover, participants received personalized dietary guidance, together with a recipe book and a menu plan, which could help to improve adherence to the diet.
The changes observed in the intake of calories and food frequency (e.g. the significant reduction of the sugar and starch consumption) were consistent with the characteristics of the recommended SSRD diet. Indeed, these modifications were accompanied with a significant increase in the consumption of healthy foods such as fruits, nuts and fish. These results are in agreement with the hypothesis brought up by other researchers, 11 that suggest that the beneficial effects of SSRD may depend not only on a reduction of the intake of cereals and sweet/soft drinks, but also on a modification to a more healthy dietary pattern. In the case of fibre consumption, there was not significant changes in its intake during the study, which means that fibre intake was not a main factor in decreasing stool frequency. These results are in line with the outcomes obtained in a previous study where the impact of a SSRD on GI symptoms was examined. 12 In the study conducted by Nilholm et al., 13 the effect of a SSRD on GI symptoms was investigated in relation to dietary intake and systemic inflammation. The authors reported that the positive effects on GI symptoms of a SSRD were not mediated by reduced systemic inflammation, and described alternative mechanisms, such as the exhaustion of normal physiological systems, intestinal dysbiosis or sucrase-isomaltase deficiency. These authors reported that there was no correlation between changes in GI symptoms and fibre intake.
Accordingly, in a previous study that investigated the effect of a dietary guidance on the management of the diet and the impact of the diet on the symptoms and QoL of patients, it was concluded that this dietary guidance improved patients’ choice of a healthier diet and QoL and IBS symptoms were also ameliorated. 27 Furthermore, in a more recent study published by the same research group, the relevance of the dietary guidance as a cost-effective option for the management of IBS was suggested. 28
In our study, a high adherence to the diet was observed, as it has been previously demonstrated with a SSRD-based dietary intervention.12,13 Adherence to the diet is an essential parameter for the success of the intervention and the improvement of symptoms has been associated to dietary adherence. 15 In our study, research nutritionists were responsible for providing dietary guidance in each of the visits and were available to answer questions about the diet via email or by telephone between the visits. Therefore, the high adherence and the positive evaluation from patients about the lack of difficulty to follow the diet might be associated with the individualized dietary management guidance provided, as well as to the developed culinary material (recipe book and menu planning) made available for the patients. Taking into consideration the COVID-19 pandemic, during the study, mobility restrictions were established by the government. As a consequence, patients were given the option to conduct a phone call for visit 2 and the final visit, instead of making an in-person visit. In this sense, no relevant issues or limitations were reported by participants regarding the visits that were conducted by phone.
The major limitation of this work is that it was not a randomized trial and also, that the diet was not been compared with other diets, such as the low FODMAP diet. However, patient’s food consumption was controlled by a nutritionist to ensure that the recommendations were successfully completed and dietary intake was analysed by validated FFQs and by a 24-h dietary recall. In this sense, an increase in the consumption of some foods with high FODMAP content was detected, which might indicate that the improvement of symptoms in these patients was not specifically associated with a reduced consumption of FODMAPs. Moreover, patients significantly reduced the intake of refined and whole cereals, pastries and sugars, food components that have been related to GI symptoms in an IBS population. 11 In fact, a change towards a healthier consumption of food items was observed, characterized by an increased intake of white fish, fatty fish, fruits, nuts and vegetables. Therefore, according to previous investigations, 11 the outcomes from this work suggest that the benefits of the intervention might partly depend on the dietary pattern improvement towards the consumption of healthier food items. It should be highlighted that the main aim of this study was to evaluate the impact of a SSRD accompanied by nutritional and culinary recommendations on IBS symptoms. Of note, our results are in line with the results obtained in previous non-randomized and randomized controlled trials in which a low FODMAP diet or SSRD was used for IBS symptoms improvement.5,6,11–13 Considering the results from this study and, as pointed out by other authors, 7 the stratification of IBS patients assessing sucrase-isomaltase function might hold the potential to identify patients who will benefit from a personalized and precision nutrition therapy, and thus, avoid the use of dietary therapies that will not be effective. On the other hand, the accompaniment of a SSRD with nutritional and culinary recommendations seems to be an appropriate approach to help alleviating the symptoms in IBS patients with diarrhoea and, therefore, follow-up studies are needed to validate the results. Those follow-up studies should analyse the impact of sucrose-isomaltase mutations in the success of the diet; compare SSRD with different diets such as low FODMAP diet; include groups of patients with other symptoms (e.g. chronic diarrhoea); and test the use of different limitation and reintroduction phases of food with moderate or high sucrose and starch content.
In conclusion, this pilot study has analysed the effectiveness of an individualized dietary guidance that was accompanied by a culinary guide consisting of a recipe book and a menu planning that was adapted for a SSRD. The conclusions obtained from the conducted intervention suggest that this approach seems to be promising in the improvement of symptoms and QoL of IBS patients with diarrhoea. Furthermore, the present outcomes suggest the potential of this dietary guidance to promote the adherence to the diet to successfully apply a SSRD in our population.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848231156682 for The effect of starch- and sucrose-reduced diet accompanied by nutritional and culinary recommendations on the symptoms of irritable bowel syndrome patients with diarrhoea by Lucia Gayoso, Koldo Garcia-Etxebarria, Teresa Arzallus, Isabel Montalvo, Jacobo Lizasoain, Mauro D’Amato, Usune Etxeberria and Luis Bujanda in Therapeutic Advances in Gastroenterology
Acknowledgments
We would like to thank all participants of the study. The authors wish to acknowledge all the chefs that have participated in the design of the book recipe (Estefanía Simón, Paula Torán and Nahuel Pazos).
Footnotes
ORCID iD: Koldo Garcia-Etxebarria  https://orcid.org/0000-0002-6107-9416
https://orcid.org/0000-0002-6107-9416
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lucia Gayoso, Technology Center in Gastronomy, Basque Culinary Center, BCC Innovation, Donostia-San Sebastián, Spain; Basque Culinary Center, Faculty of Gastronomic Sciences, Mondragon Unibertsitatea, Donostia-San Sebastián, Spain.
Koldo Garcia-Etxebarria, Gastrointestinal Genetics Group, Biodonostia, Dr Beguiristain S/N, San Sebastian 20014, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Barcelona, 08036, Spain.
Teresa Arzallus, Gastrointestinal Diseases Group, Universidad del País Vasco (UPV/EHU), Biodonostia, San Sebastián, Spain.
Isabel Montalvo, Gastrointestinal Diseases Group, Universidad del País Vasco (UPV/EHU), Biodonostia, San Sebastián, Spain.
Jacobo Lizasoain, Gastrointestinal Diseases Group, Universidad del País Vasco (UPV/EHU), Biodonostia, San Sebastián, Spain.
Mauro D’Amato, Gastrointestinal Genetics Lab, Basque Research and Technology Alliance, CIC bioGUNE, Derio, Spain; Ikerbasque, Basque Foundation for Sciences, Bilbao, Spain; Department of Medicine and Surgery, LUM University, Casamassima, Italy.
Usune Etxeberria, Technology Center in Gastronomy, Basque Culinary Center, BCC Innovation, Donostia-San Sebastián, Spain; Basque Culinary Center, Faculty of Gastronomic Sciences, Mondragon Unibertsitatea, Donostia-San Sebastián, Spain.
Luis Bujanda, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Barcelona, Spain; Gastrointestinal Diseases Group, Universidad del País Vasco (UPV/EHU), Biodonostia, San Sebastián, Spain.
Declarations
Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Local Ethics Committee (Comité de Ética del Área Sanitaria de Gipuzkoa, code: BUJ-NUT-2019-01. Approved in 2019/12/17).
Consent for publication: Not applicable.
Author contribution(s): Lucia Gayoso: Conceptualization; Data curation; Investigation; Methodology; Resources; Writing – original draft; Writing – review & editing.
Koldo Garcia-Etxebarria: Conceptualization; Data curation; Formal analysis; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Teresa Arzallus: Resources.
Isabel Montalvo: Resources.
Jacobo Lizasoain: Resources.
Mauro D’Amato: Conceptualization; Methodology; Writing – review & editing.
Usune Etxeberria: Conceptualization; Data curation; Investigation; Methodology; Resources; Writing – review & editing.
Luis Bujanda: Conceptualization; Methodology; Resources; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported in part by funds from the Spanish Ministry of Science and Innovation (MICINN, PID2020-113625RB) to MD.
MD has received unrestricted research grants from QOL Medical. The rest of the authors declare no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1.Camilleri M.Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 2012; 367: 1626–1635. [DOI] [PubMed] [Google Scholar]
- 2.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology 2021; 160: 99–114.e3. [DOI] [PubMed] [Google Scholar]
- 3.Chey WD, Kurlander J, Eswaran S.Irritable bowel syndrome: a clinical review. JAMA 2015; 313: 949–958. [DOI] [PubMed] [Google Scholar]
- 4.Eswaran SL, Chey WD, Han-Markey T, et al. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am J Gastroenterol 2016; 111: 1824–1832. [DOI] [PubMed] [Google Scholar]
- 5.Varjú P, Farkas N, Hegyi P, et al. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: a meta-analysis of clinical studies. PLoS One 2017; 12: e0182942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altobelli E, Del Negro V, Angeletti PM, et al. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients 2017; 9: 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng T, Eswaran S, Photenhauer AL, et al. Reduced efficacy of low FODMAPs diet in patients with IBS-D carrying sucrase-isomaltase (SI) hypomorphic variants. Gut 2020; 69: 397–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henström M, Diekmann L, Bonfiglio F, et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2018; 67: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Etxebarria K, Zheng T, Bonfiglio F, et al. Increased prevalence of rare sucrase-isomaltase pathogenic variants in irritable bowel syndrome patients. Clin Gastroenterol Hepatol 2018; 16: 1673–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng T, Camargo-Tavares L, Bonfiglio F, et al. Rare hypomorphic sucrase isomaltase variants in relation to irritable bowel syndrome risk in UK biobank. Gastroenterology 2021; 161: 1712–1714. [DOI] [PubMed] [Google Scholar]
- 11.Nilholm C, Larsson E, Roth B, et al. Irregular dietary habits with a high intake of cereals and sweets are associated with more severe gastrointestinal symptoms in IBS patients. Nutrients 2019; 11: 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilholm C, Larsson E, Sonestedt E, et al. Assessment of a 4-week starch-and sucrose-reduced diet and its effects on gastrointestinal symptoms and inflammatory parameters among patients with irritable bowel syndrome. Nutrients 2021; 13: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilholm C, Roth B, Ohlsson B.A dietary intervention with reduction of starch and sucrose leads to reduced gastrointestinal and extra-intestinal symptoms in IBS patients. Nutrients 2019; 11: 1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eswaran S, Tack J, Chey WD.Food: the forgotten factor in the irritable bowel syndrome. Gastroenterol Clin North Am 2011; 40: 141–162. [DOI] [PubMed] [Google Scholar]
- 15.De Roest RH, Dobbs BR, Chapman BA, et al. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract 2013; 67: 895–903. [DOI] [PubMed] [Google Scholar]
- 16.Gibson AA, Sainsbury A.Strategies to improve adherence to dietary weight loss interventions in research and real-world settings. Behav Sci (Basel) 2017; 7: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almansa C, García-Sánchez R, Barceló M, et al. Translation, cultural adaptation and validation of a Spanish version of the irritable bowel syndrome severity score. Rev Española Enfermedades Dig Enfermedades Dig 2011; 103: 612–618. [DOI] [PubMed] [Google Scholar]
- 18.Palsson OS, Whitehead WE, van Tilburg MAL, et al. Development and validation of the Rome IV diagnostic questionnaire for adults. Gastroenterology 2016; 150: 1481–1491. [Google Scholar]
- 19.Badia X, Herdman M, Mearin F, et al. Adaptation into Spanish of the IBSQOL questionnaire for the measurement of quality of life in patients with irritable bowel syndrome. Rev Española Enfermedades Dig 2000; 92: 644–650. [Google Scholar]
- 20.Goni Mateos L, Aray Miranda M, Martínez HA, et al. Validation of a food groups frequency questionnaire based in an exchange system. Nutr Hosp 2016; 33: 1391–1399. [DOI] [PubMed] [Google Scholar]
- 21.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2008. [Google Scholar]
- 22.Marsh A, Eslick EM, Eslick GD.Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur J Nutr 2016; 55: 897–906. [DOI] [PubMed] [Google Scholar]
- 23.Gibson PR.The evidence base for efficacy of the low FODMAP diet in irritable bowel syndrome: is it ready for prime time as a first-line therapy? J Gastroenterol Hepatol 2017; 32: 32–35. [DOI] [PubMed] [Google Scholar]
- 24.Chiruvella V, Cheema A, Arshad HMS, et al. Sucrase-isomaltase deficiency causing persistent bloating and diarrhea in an adult female. Cureus 2021; 13: e14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohlsson B.Theories behind the effect of starch‑ and sucrose‑reduced diets on gastrointestinal symptoms in irritable bowel syndrome (review). Mol Med Rep 2021; 24: 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valeur J, Røseth AG, Knudsen T, et al. Fecal fermentation in irritable bowel syndrome: influence of dietary restriction of fermentable oligosaccharides, disaccharides, monosaccharides and polyols. Digestion 2016; 94: 50–56. [DOI] [PubMed] [Google Scholar]
- 27.Østgaard H, Hausken T, Gundersen D, et al. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep 2012; 5: 1382–1390. [DOI] [PubMed] [Google Scholar]
- 28.Mazzawi T, Hausken T, Gundersen D, et al. Effects of dietary guidance on the symptoms, quality of life and habitual dietary intake of patients with irritable bowel syndrome. Mol Med Rep 2013; 8: 845–852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848231156682 for The effect of starch- and sucrose-reduced diet accompanied by nutritional and culinary recommendations on the symptoms of irritable bowel syndrome patients with diarrhoea by Lucia Gayoso, Koldo Garcia-Etxebarria, Teresa Arzallus, Isabel Montalvo, Jacobo Lizasoain, Mauro D’Amato, Usune Etxeberria and Luis Bujanda in Therapeutic Advances in Gastroenterology