Abstract
This study examines the availability, cost, and consumer ratings of blood pressure–measuring devices relative to validation status across 10 countries.
For clinic and home blood pressure (BP) monitoring, hypertension guidelines recommend using automated upper arm cuff BP-measuring devices that have undergone adequate clinical validation. These validated devices have proven accuracy and greater precision than nonvalidated devices.1,2 Patients may obtain a device by online purchase,3 but recent reports detailed a high prevalence of nonvalidated devices on international e-commerce websites.4,5 However, the availability of nonvalidated and validated devices among the most popular brands sold online is unknown. This study sought to determine the availability, cost, and consumer ratings of devices relative to validation status.
Methods
A prospective analysis of the 100 best-selling lists of BP devices sold by Amazon was undertaken every 8 weeks for a year, beginning February 2020, across 10 countries with available data (Australia, Canada, France, Germany, India, Italy, Mexico, Spain, United Kingdom, and United States). The best-selling lists are the “most popular products based on sales.” The precise sales volume and the algorithms that generate the best-selling lists are not disclosed. Validation status of devices was determined from international listings STRIDE-BP and Medaval and country-specific listings where relevant (eg, US Blood Pressure Validated Device Listing for data from the United States only; see Supplement 1). Devices were considered validated if reported as such on any validation listings and nonvalidated if they were not on any lists. Analysis was conducted for automated or semiautomated upper arm or wrist cuff devices. The percentage of nonvalidated and validated devices was assessed from the 100 best-selling lists after excluding manual devices and nonrelevant items, including spare cuffs and stethoscopes (Supplement 1). The cost of each device was recorded in the local currency for each country and converted to US dollars for standardized comparisons. The median costs and difference between median costs of nonvalidated and validated devices were calculated. Consumer ratings were determined from the device rating out of a possible 5 stars (Supplement 1). Data were analyzed using R version 4.1.1 (R Foundation).
Results
The median number of upper arm devices identified from each country at each data collection point among the most popular brands sold online was 41 (IQR, 38-49) nonvalidated and 12 (IQR, 6-16) validated devices, with a median of 79% (IQR, 73%-85%) of available devices being nonvalidated. The median number of wrist cuff devices was 16 (IQR, 12-22) nonvalidated and 2 (IQR, 1-3) validated devices, with a median of 84% (IQR, 78%-94%) of available devices being nonvalidated. The highest percentages of nonvalidated upper arm devices were from India (97%) and Australia (95%), and the lowest were from Canada (69%) and Germany (65%) (Table). The highest percentages of nonvalidated wrist cuff devices were from the United States (100%) and Mexico (93%), and the lowest were from Germany (73%) and India (71%). Nonvalidated upper arm devices were $35.2 cheaper than validated devices (median price of $32.0 [IQR, $26.0-$43.9] vs $67.2 [IQR, $42.7-$89.7] for all countries and time points). Nonvalidated wrist cuff devices were $23.7 cheaper than validated devices wrist devices (median price of $25.7 [IQR, $20.7-$36.5] vs $49.4 [IQR, $37.4-$67.1]). Across all countries, the costs of nonvalidated upper arm and wrist devices were always lower than costs of validated devices (Figure). Nonvalidated and validated upper arm devices received the same median consumer rating (median rating of 4.5 [IQR, 4.3-4.7] vs 4.5 [IQR, 4.2-4.6] of 5 stars). The data were similar for wrist devices (median rating of 4.2 [IQR, 4.0-4.4] vs 4.3 [IQR, 4.1-4.4] of 5 stars).
Table. Number and Percentage of Nonvalidated Upper Arm and Wrist Cuffs Among the 100 Best-Selling Blood Pressure Device Lists From 10 Country-Specific Amazon Websites.
| Country | Median (IQR) | |||||
|---|---|---|---|---|---|---|
| Upper arm cuff devicesa | Wrist cuff devicesa | |||||
| Total No.b | Nonvalidated devices, No.c | Nonvalidated devices, %d | Total No.b | Nonvalidated devices, No.c | Nonvalidated devices, %d | |
| Australia | 44 (42-49) | 42 (40-46) | 95 (94-96) | 23 (22-23) | 20 (19-21) | 87 (86-88) |
| Canada | 54 (53-56) | 38 (34-41) | 70 (64-74) | 15 (14-16) | 12 (11-13) | 81 (80-83) |
| France | 50 (48-54) | 38 (37-40) | 76 (76-77) | 23 (23-27) | 22 (20-23) | 88 (85-92) |
| Germany | 63 (61-64) | 39 (39-43) | 65 (62-67) | 21 (20-22) | 15 (14-16) | 73 (68-79) |
| India | 56 (55-59) | 55 (53-57) | 97 (96-97) | 5 (4-6) | 4 (2-5) | 69 (67-76) |
| Italy | 58 (55-60) | 42 (40-44) | 74 (73-75) | 16 (15-17) | 12 (11-13) | 77 (70-85) |
| Mexico | 39 (36-42) | 33 (30-35) | 84 (82-84) | 30 (29-32) | 28 (26-31) | 93 (92-96) |
| Spain | 50 (50-51) | 39 (37-40) | 75 (71-80) | 22 (18-23) | 18 (17-21) | 91 (88-95) |
| United Kingdom | 66 (62-68) | 52 (50-53) | 78 (76-80) | 14 (14-17) | 13 (11-15) | 85 (79-91) |
| United States | 63 (60-65) | 51 (50-55) | 84 (83-85) | 32 (28-33) | 32 (28-33) | 100 (100-100) |
| Total | 55 (49-61) | 41 (38-49) | 79 (73-85) | 21 (15-25) | 2 (1-3) | 84 (78-94) |
The total number of upper arm and wrist cuff devices does not sum to 100 because there were items meeting prespecified exclusion criteria (eg, manual sphygmomanometers, cuffs, stethoscopes) that were not analyzed.
Data are the median (IQR) total number of devices recorded at each of the 7 discrete data capture dates across the 1-year period between February 2020 and January 2021.
Data are the median (IQR) number of nonvalidated devices identified from the 7 discrete data capture dates across the 1-year period.
The median (IQR) of the percentage of nonvalidated devices recorded at each of the 7 discrete data capture dates across the 1-year period.
Figure. Cost of Blood Pressure–Measuring Devices Among the 100 Best-Selling Lists From Country-Specific Amazon Websites.
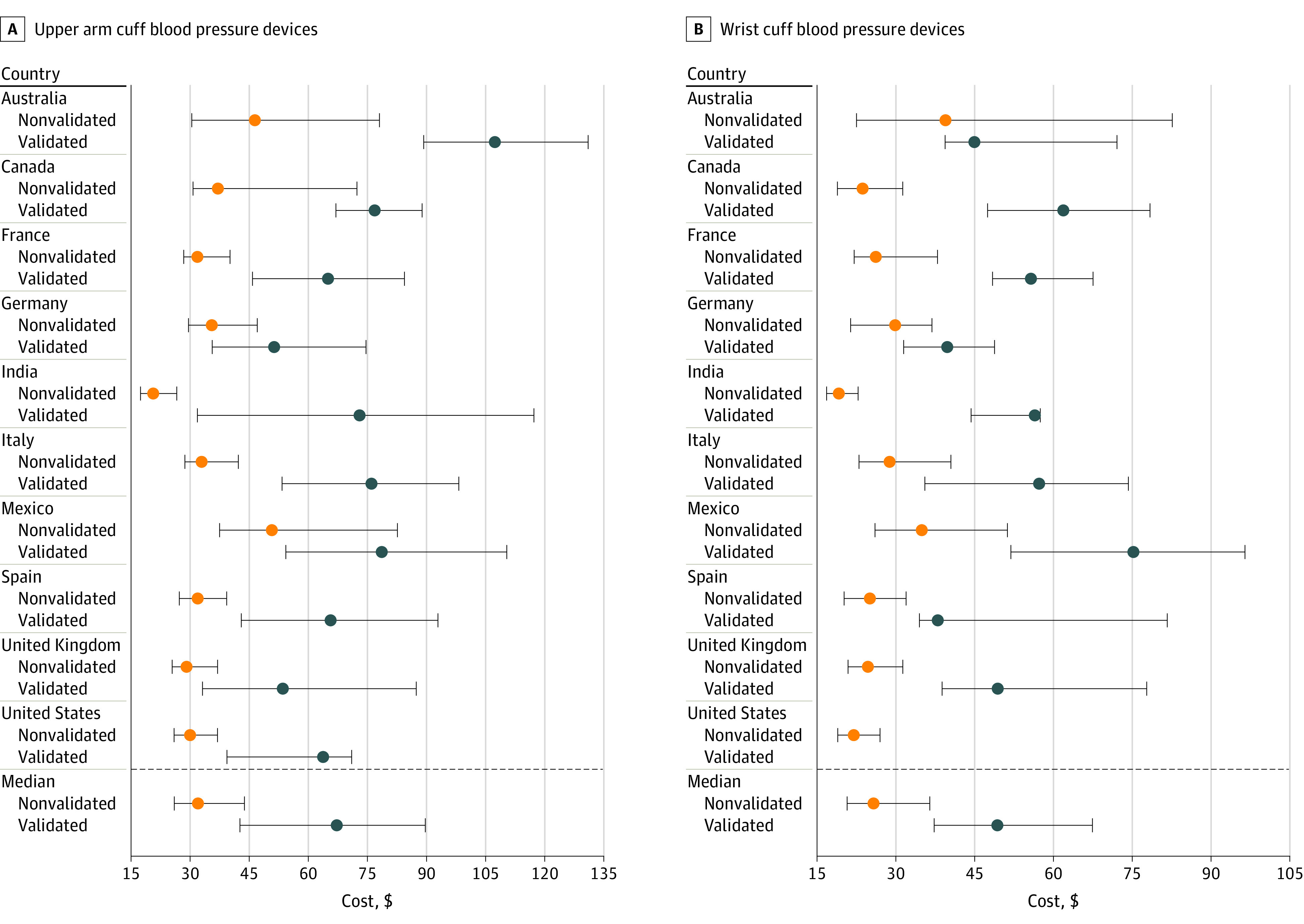
Cost standardized to US dollars is the median and IQR of data merged from 7 data collection points across a 1-year period.
Discussion
Among the top 100 best-selling BP devices on a popular online site, 79% of upper arm and 83% of wrist cuff devices were nonvalidated. The predominance of nonvalidated devices may have adverse consequences for management of hypertension; therefore, clinicians should ensure they are recommending validated devices to their patients.6 Study limitations include that sales volume is unknown, the results are specific only to Amazon and the countries assessed, and it is unknown whether the identified devices are used for making clinical decisions.
Consumers purchasing a device from an international online business cannot have confidence that it has been validated. Stronger public health messaging is required to improve understanding of the need to measure BP using validated devices. Policies should be implemented to require clinical validation of devices.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Senior Editor.
eMethods
Data Sharing Statement
References
- 1.Akpolat T, Dilek M, Aydogdu T, Adibelli Z, Erdem DG, Erdem E. Home sphygmomanometers: validation versus accuracy. Blood Press Monit. 2009;14(1):26-31. doi: 10.1097/MBP.0b013e3283262f31 [DOI] [PubMed] [Google Scholar]
- 2.Jung MH, Kim GH, Kim JH, et al. Reliability of home blood pressure monitoring: in the context of validation and accuracy. Blood Press Monit. 2015;20(4):215-220. doi: 10.1097/MBP.0000000000000121 [DOI] [PubMed] [Google Scholar]
- 3.Blood Pressure Monitoring Devices Market Size, Share & Trends Analysis Report by End-Use (Hospitals, Homecare), by Product (Ambulatory, Aneroid BP Monitors), by Region, and Segment Forecasts 2023-2030. Grand View Research; 2023. Accessed March 27, 2023. https://www.grandviewresearch.com/industry-analysis/blood-pressure-monitoring-devices-market
- 4.Picone DS, Deshpande RA, Schultz MG, et al. Nonvalidated home blood pressure devices dominate the online marketplace in Australia: major implications for cardiovascular risk management. Hypertension. 2020;75(6):1593-1599. doi: 10.1161/HYPERTENSIONAHA.120.14719 [DOI] [PubMed] [Google Scholar]
- 5.Picone DS, Campbell NRC, Schutte AE, et al. Validation status of blood pressure measuring devices sold globally. JAMA. 2022;327(7):680-681. doi: 10.1001/jama.2021.24464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharman JE, Ordunez P, Brady T, et al. The urgency to regulate validation of automated blood pressure measuring devices: a policy statement and call to action from the world hypertension league. J Hum Hypertens. Published online December 7, 2022. doi: 10.1038/s41371-022-00747-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Data Sharing Statement


