This cohort study evaluates tumor mutational burden and outcomes in patients with diverse advanced cancers treated with immunotherapy.
Key Points
Question
Is a tumor mutational burden (TMB) biomarker associated with clinical benefit in a cohort of patients with advanced cancer from diverse clinical settings treated with first- or second-line immune checkpoint inhibitors (ICI)?
Findings
In this cohort study of 674 patients with 8 distinct advanced cancer diagnoses, TMB-high cancers were significantly associated with longer overall survival than TMB-low cancers. These findings were robust to the ICI administered and remained significant after adjustment for programmed cell death-ligand 1 and microsatellite instability status.
Meaning
These findings suggest that patients identified as TMB high by the biomarker showed a significant outcome benefit to ICI therapy and should be considered for such therapy.
Abstract
Importance
There are few studies assessing the association of tumor mutational burden (TMB) and clinical outcomes in a large cohort of patients with diverse advanced cancers.
Objective
To clinically validate a TMB biomarker from a next-generation sequencing targeted gene panel assay.
Design, Setting, and Participants
A prespecified cohort study using the deidentified clinicogenomic Tempus database of patients sequenced between 2018 and 2022, which contained retrospective, observational data originating from 300 cancer sites including 199 community sites and 101 academic sites. Patients with advanced solid tumors across 8 cancer types and more than 20 histologies, sequenced with Tempus xT who were treated with immune checkpoint inhibitors (ICIs) in the first-line or second-line setting were included. Data were analyzed from September 2018 to August 2022.
Exposure
Treatment with US Food and Drug Administration (FDA)–approved antiprogrammed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) ICI and/or in combination with a cytotoxic T-lymphocyte-associated protein-4 ICI.
Main Outcomes and Measures
The primary outcome was the association of tumor mutational burden (TMB) binary category (high [≥10 mut/mb] vs low) with overall survival (OS) in patients treated with ICIs. Secondary outcomes were progression-free survival (PFS), and time to progression (TTP).
Results
In the evaluable cohort of 674 patients, the median (IQR) age was 69.4 (28.6-89.8) years, 271 patients (40.2%) were female, and 435 patients (64.5%) were White. The most common advanced cancers were non–small cell lung cancer (330 patients [49.0%]), followed by bladder cancer (148 patients [22.0%]), and head and neck squamous cell carcinoma (96 patients [14.8%]). Median (IQR) follow-up was 7.2 (3.2-14.1) months. High TMB (TMB-H) cancers (206 patients [30.6%]) were significantly associated with longer OS than low TMB (TMB-L) cancers (hazard ratio [HR], 0.72; upper confidence bound [UCB], 0.91; P = .01). In a prospective subset of 403 patients treated with ICIs after TMB testing, TMB-H cancers (135 patients [33.5%]) were significantly associated with longer OS (HR, 0.61; UCB, 0.84; P = .005), PFS (HR, 0.62; UCB, 0.82; P = .003), and TTP (HR, 0.67; UCB, 0.92; P = .02) than TMB-L cancers. An overall survival benefit was seen regardless of the type of ICI used (pembrolizumab, 339 patients; HR, 0.67; UCB, 0.94; P = .03), other ICIs (64 patients; HR, 0.37; UCB, 0.85; P = .03), and after adjusting for PD-L1 and microsatellite stability status (403 patients; HR = 0.67; UCB, 0.92; P = .02).
Conclusions and Relevance
In this cohort study of patients with advanced solid tumors treated with ICIs in diverse clinics, TMB-H cancers were significantly associated with improved clinical outcomes compared with TMB-L cancers.
Introduction
Tumor mutational burden (TMB), defined as the total number of somatic variations per defined region of a tumor genome, is a pantumor biomarker for immune checkpoint inhibitor (ICI) response in patients with advanced cancer. The potential clinical benefit of this biomarker is founded upon the hypothesis that highly mutated tumors produce high-quality neoantigens that increase T-cell reactivity, which in turn leads to improved response to immune checkpoint blockade treatment.1,2 TMB has been associated with mixed clinical results in both prospective and retrospective studies,3,4,5,6 and a lack of demonstration of consistent overall survival benefit has impeded the widespread application of TMB in routine clinical care. In the Checkmate-227 study,7,8 a prospective phase 3 trial assessing patients with advanced non–small cell lung cancer (NSCLC) treated with nivolumab plus ipilimumab vs chemotherapy, efficacy of TMB varied by clinical end point. Among patients treated with nivolumab plus ipilimumab vs chemotherapy, improved progression-free survival (PFS) was seen in high TMB (TMB-H) cancers but not in low TMB (TMB-L) cancers.8 However, this trend was not seen when evaluating overall survival (OS).7 In the phase 2 KEYNOTE-158 study of advanced solid tumors previously treated with pembrolizumab, TMB-H cancers were associated with higher overall response rates (ORRs) than TMB-L cancers; however, minimal differences were observed in PFS and OS according to TMB.9 These results, primarily based on ORR improvement based on TMB stratification,10 led to the 2020 US Food and Drug Administration (FDA) approval of pembrolizumab for treatment of patients with unresectable or metastatic solid tumors with high TMB,11 as defined as 10 or more mut/Mb by the FoundationOne CDx assay.
Retrospective studies have shown variable association of TMB and clinical benefit depending on the cancer type and clinical end points assessed.3,4,5,12 Heterogeneity may arise from the variability in the type of panels used and lack of standardization of TMB thresholds. Although whole exome sequencing is considered the reference standard for estimating TMB, Friends of Cancer Research provides guidelines for harmonizing TMB estimates from different panels with specifics on minimum panel size needed, germline filtering, and removing synonymous alterations,13 all incorporated in the assay. Additionally, a recent study14 performing an in silico analysis comparing several laboratories, including Tempus, with TMB determined by whole exome sequencing, demonstrated concordance of Tempus TMB high/low classification in 95.8% of cases, consistent with the range of other laboratories.
Retrospective studies have focused on different cancer types, variable lines of therapy, and assessment of a wide range of efficacy end points, making it difficult to interpret and standardize these results. Using the commonly adopted TMB threshold of 10 and focusing on patients with clinically relevant first- and second-line FDA-approved ICIs, we performed a prespecified cohort study of the association of TMB using the Tempus xT next-generation sequencing (NGS) targeted gene panel assay with multiple clinical outcomes in patients with advanced solid tumors using the Tempus clinical clinicogenomic database that is reflective of clinical care across multiple institutions.
Methods
Cohort Selection
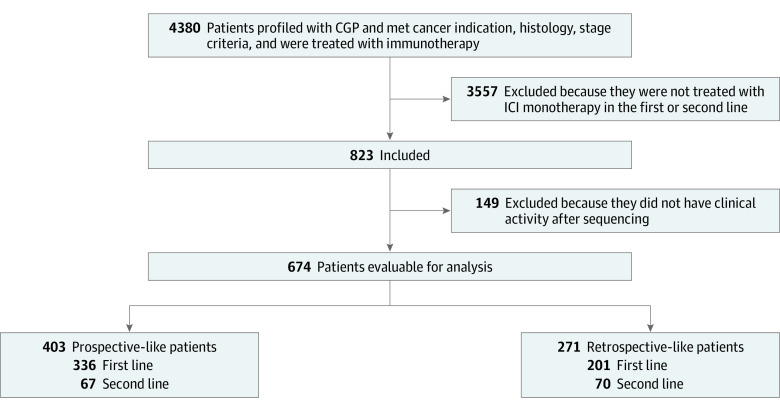
The pancancer cohort consists of patients from the deidentified Tempus clinicogenomic clinical database who received tissue-based next-generation sequencing between 2018 and 2022. Figure 1 delineates the study CONSORT diagram; patients included in the study were tested with Tempus xT.v2 or xT.v4, with diagnoses of metastatic or stage IV disease in 1 of 8 cancer types (Table 1), well-characterized histology listed in eTable 1 in Supplement 1, and treated with FDA-approved ICIs in the first- or second-line setting (eTable 2 in Supplement 1). Evaluable patients included those who had at least 1 follow-up medical record after the later of the first record of ICI administration or the date that the assay was ordered. The prospective cohort was defined as patients treated with an ICI after the date that the assay was ordered, whereas in the retrospective cohort the ICI was given before the date that the assay was ordered. Analyses were performed using deidentified data under an exemption granted from the Advarra, Inc, institutional review board on April 15, 2020. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Figure 1. Study Flow Diagram Showing Cohort Selection.
The final evaluable 674 patient cohort was further stratified into prospective (immune checkpoint inhibitors [ICIs] received after tumor mutational burden testing) and retrospective patients (ICIs received before tumor mutational burden testing; see Methods section). CGP indicates comprehensive genomic profiling.
Table 1. Demographic and Clinical Characteristics of Patients in the Evaluable and the Prospective Cohort by TMB High and Low Groups.
| Clinical characteristic | No. (%) | P value | |||
|---|---|---|---|---|---|
| Evaluable (n = 674) | Prospective (n = 403) | TMB-H prospective (n = 135) | TMB-L prospective (n = 268) | ||
| Cancers | |||||
| NSCLC | 330 (49.0) | 242 (60.1) | 79 (58.5) | 163 (60.8) | <.001 |
| Bladder | 148 (22.0) | 77 (19.1) | 22 (16.3) | 55 (20.5) | |
| HNSCC | 96 (14.2) | 43 (10.7) | 8 (5.9) | 35 (13.1) | |
| Melanoma | 48 (7.1) | 14 (3.5) | 7 (5.2) | 7 (2.6) | |
| CRC | 39 (5.8) | 23 (5.7) | 17 (12.6) | 6 (2.2) | |
| Gastric | 7 (1.0) | 1 (0.2) | 0 | 1 (0.4) | |
| Endometrial | 5 (0.7) | 3 (0.7) | 2 (1.5) | 1 (0.4) | |
| Cervical | 1 (0.1) | 0 | 0 | 0 | |
| Age at start of IO, median (range), y | 69.4 (28.6-89.8) | 70.8 (34.0-89.8) | 69.7 (34.8-89.7) | 71.8 (34.0-89.8) | .24a |
| Sex | |||||
| Female | 271 (40.2) | 180 (44.7) | 61 (45.2) | 119 (44.4) | .97 |
| Male | 403 (59.8) | 223 (55.3) | 74 (54.8) | 149 (55.6) | |
| Race and ethnicity | |||||
| Asian | 14 (2.1) | 11 (2.7) | 3 (2.2) | 8 (3.0) | .56 |
| Black or African American | 47 (7.0) | 32 (7.9) | 13 (9.6) | 19 (7.1) | |
| Hispanic or Latino | 15 (2.2) | 11 (2.7) | 5 (3.7) | 6 (2.2) | |
| White | 435 (64.5) | 268 (66.5) | 83 (61.5) | 185 (69.0) | |
| Unknown | 149 (22.1) | 71 (17.6) | 26 (19.3) | 45 (16.8) | |
| Otherb | 14 (2.1) | 10 (2.5) | 5 (3.7) | 5 (1.9) | |
| Smoking status | |||||
| Smoking data available | 593 (88.0) | 373 (92.6) | 125 (92.6) | 248 (92.5) | |
| Current or former smoker | 472 (79.6) | 314 (84.2) | 104 (83.2) | 212 (85.5) | .67 |
| Histology | |||||
| Adenocarcinoma | 290 (43.0) | 207 (51.4) | 81 (60.0) | 126 (47.0) | .02 |
| Transitional cell carcinoma | 103 (15.3) | 56 (13.9) | 17 (12.6) | 39 (14.6) | |
| Squamous cell carcinoma | 157 (23.3) | 80 (19.9) | 16 (11.9) | 64 (23.9) | |
| Other | 124 (18.4) | 60 (14.9) | 21 (15.6) | 39 (14.6) | |
| Brain metastases | 124 (18.4) | 86 (21.3) | 36 (26.7) | 50 (18.7) | .09 |
| IO medication | |||||
| First-line | 537 (79.7) | 336 (83.4) | 112 (83.0) | 224 (83.6) | .99 |
| Pembrolizumab | 560 (83.1) | 339 (84.1) | 111 (82.2) | 228 (85.1) | .55 |
| Practice setting | |||||
| No. of sites | 300 | 187 | 92 | 143 | |
| AMCs | 101 (33.7) | 56 (30.0) | 25 (27.2) | 43 (30.1) | .74 |
| Community clinic | 199 (66.3) | 131 (70.0) | 67 (72.8) | 100 (69.9) | |
| Biomarker testing | |||||
| PD-L1 assessed | 427 (63.4) | 296 (73.4) | 101 (74.8) | 195 (72.8) | |
| PD-L1 positive | 293 (68.6) | 221 (74.7) | 70 (69.3) | 151 (77.4) | .17 |
| PD-L1 negative | 134 (31.4) | 75 (25.3) | 31 (30.7) | 44 (22.6) | |
| MSI-H | 31 (4.6) | 20 (5.0) | 18 (13.3) | 2 (0.7) | <.001 |
Abbreviations: AMC, academic medical center; CRC, colorectal cancer; HNSCC, head and neck squamous cell carcinoma; IO, immuno-oncology; MSI-H, microsatellite instability-high; NSCLC, non–small cell lung cancer; PD-L1, programmed cell death-ligand 1; TMB, tumor mutational burden; TMB-H, tumor mutational burden-high; TMB-L, tumor mutational burden-low.
Wilcoxon rank-sum test used. All other P values were obtained using Pearson χ2 test.
Other includes American Indian, Alaska Native, or self-identified other race.
Biomarker Assessment
The Assay
NGS was performed via the assay (Tempus Labs), as previously described.15,16,17 Briefly, the assay is a tumor and matched normal NGS targeted panel that detects variants in 596 or 648 genes (xT.v2 and xT.v4, respectively), with high sensitivity and specificity.15,16
TMB Calculation
TMB was calculated by dividing the number of nonsynonymous variations by the size of the panel (2.4 Mb for the panel size of xT.v2 and 1.9Mb for the panel coding region of xT.v4). All nonsilent somatic coding variations such as missense, indel, and stop-loss variants with coverage greater than × 100 and an allelic fraction greater than 5% are included in the count of nonsynonymous variations. TMB calculated using the assay is highly correlated with TMB calculated from whole exome TCGA data (R = 0.986, P < 2.2 × 10-16).16 The xT.v2 TMB score is adjusted for differences in denominators between the versions to be directly comparable to xT.v4. All analyses are completed incorporating both assays, with tumors considered TMB-H if they have an adjusted TMB score of 10 mut/Mb or more.
Microsatellite Instability Calculation
The assay panels include probes for loci that are frequently unstable in tumors with mismatch repair deficiencies to assess microsatellite instability (MSI) and classifies tumors into microsatellite instability-high (MSI-H), and microsatellite stable (MSS) categories.15
PD-L1 Immunohistochemistry
PD-L1 status was determined by clinical Tempus testing with the 22C3 anti-PD-L1 pharmDx assay.17 Slides were scored by a pathologist using the tumor proportion score, which is the percentage of tumor cells with complete or partial membrane staining. PD-L1 positive is defined as a tumor proportion score of 1% or more.
Outcomes
Clinical data were abstracted from the Tempus clinicogenomic database of longitudinal structured and unstructured physician progress notes from diverse oncology practices including both academic medical centers and community practices. Dates of death were captured either from retrospectively abstracted patient medical records or from third-party data sources that come from obituary documentation that is augmented to the death master file from the Social Security Administration. Finally, the resulting death information was integrated with Tempus data via an encrypted token system. Progression dates were retrospectively abstracted from patient medical records.
End Points
End point definitions for overall survival (OS), progression-free survival (PFS), and time to progression (TTP) are described in eTable 3 in Supplement 1. Censoring definitions for all end points are described in eTable 4 in Supplement 1. For OS analysis, patients were censored at the last known clinical record. For PFS and TTP, patients were censored according to the earliest record of the end of ICI treatment, the start of new therapy, or the time on ICI treatment according to the line of therapy (eTable 4 in Supplement 1).
Statistical Analysis
The analyses performed in this study were prespecified in a prospectively declared statistical analysis plan. The primary objective of the study was to evaluate whether high TMB cancers are associated with longer survival in patients treated with ICI than low TMB cancers in the evaluable cohort. A stratified Cox proportional hazards model was fit with stratification by line of therapy (first-line or second-line) with study entry defined as the start of ICI treatment. To account for immortal time bias in OS, corrected study entry was defined as the assay order date if the assay order date occurred after the start of ICI treatment. Risk set adjustment was implemented, in which patients were only considered to be at risk for the OS end point according to the corrected study entry time. The test for significance of the hazard ratio (HR) was performed using a 1-sided Wald test at a 5% significance level. Mathematically, this translates to assessing if the one-sided upper 95% confidence bound on the HR was less than 1. Therefore, the one sided upper 95% confidence bound is provided for all survival analyses. Similarly, stratified Cox models were fit for the PFS and TTP end points. For an initial anticipated cohort size of 850 patients, simulation studies demonstrated more than 80% power for the primary end point. All secondary analyses were conducted in the prospective cohort only as prespecified for the following reasons: the prospective cohort mirrors the design of a prospective clinical trial, it is reflective of how TMB would be used clinically for ICI decision-making, and finally, it limits potential confounders of the tumor environment impacted by early initiation of ICI treatment without TMB result and immortal time bias.
For multivariable analyses, other covariates such as PD-L1 immunohistochemistry status and MSI status were added to the model as main variables. Similarly, stratification by line of therapy was included in all multivariable Cox proportional hazards models which included patients for whom first-line and second-line ICI were indicated. All comparisons of TMB-H with TMB-L cancers were performed using the Wald test at an unadjusted 5% significance level. In individual cancer types, 1-year survival probabilities were compared for TMB-H vs TMB-L cancers. Comparisons of demographic and clinical characteristics between patients who were TMB-H and TMB-L were performed using Wilcoxon rank-sum test or Pearson χ2 test. Statistical analyses were completed using Python version 3.7.13 (Python Software Foundation) and R version 4.0.5 (R Project for Statistical Computing). Data were analyzed from September 2018 to August 2022.
Results
In the evaluable pancancer cohort of 674 patientsv with advanced cancer, the median (IQR) age was 69.4 (61.9-76.6) years, 271 patients (40.2%) were female, and 435 patients (64.5%) were White (Table 1). The most common cancer type was NSCLC (330 patients [49.0%]), followed by bladder (148 patients [22.0%]), HNSCC (96 patients [14.2%]), and other cancer types (100 patients [14.8%]) (Table 1). More than 20 histologies were reported, with the most common being adenocarcinoma (290 patients [43.0%]) (eTable 1 and eTable 5 in Supplement 1). Patients were treated with 1 of 7 FDA-approved ICIs (eTable 2 in Supplement 1), with the majority of the patients treated with pembrolizumab (560 patients [83.1%]) across all cancer types (eTables 6 and 7 in Supplement 1). Self-reported race and ethnicity distribution included Asian (14 patients [2.1%]), Black or African American (47 patients [7.0%]), Hispanic or Latino (15 patients [2.2%]), and White (435 patients [64.5%]) (Table 1). Self-reported race was included to assess the racial diversity of the patient cohort. The majority of the patients were treated in community clinics (199 patients [66.3%]) (Table 1). Two-hundred and six patients had TMB-H cancers (30.6%), which were significantly associated with longer OS than low TMB-L cancers (HR, 0.72; upper confidence bound [UCB], 0.91; P = .01) (eTable 8 in Supplement 1). Among those that had PD-L1 biomarker testing (427 patients [63.4%]), 293 (68.6%) were found to be PD-L1 positive and 134 (31.4%) were PD-L1 negative (Table 1). MSI-H was observed in 31 patients (4.6%) of the total cohort (Table 1).
Similar demographic and clinical characteristics were seen in the prospective cohort, defined as patients treated with ICI following the assay order date (403 patients) (see Methods section). In the prospective cohort, 135 patients were TMB-H (33.5%) (Table 1). In this cohort, demographic and clinical characteristics were well-balanced across the TMB-H and TMB-L patients, with the exception of histology distribution and percentage of patients with brain metastases, and percentage of patients that were MSI-H (Table 1). Adenocarcinomas, brain metastases, and MSI-H status were more frequent in TMB-H patients compared with TMB-L patients (Table 1). Similar well-balanced characteristics across TMB category were seen in the evaluable cohort (eTable 8 in Supplement 1) and by cancer indication (eTable 9 and eTable 10 in Supplement 1).
Association of TMB With Clinical Outcomes
Median (IQR) follow-up was 7.2 ( 3.2 -14.1) months in the evaluable cohort, 9.4 (5.1-15.4) months in the prospective cohort, and 4.7 (1.3-10.5) months in the retrospective cohort. Median OS of the evaluable patient cohort was 13.9 (95% CI, 12.1-16.1) months. In the evaluable patient cohort (674 patients), TMB-H cancers (median OS of 17.3 months; 95% CI, 17.4 months to not reached) were significantly associated with longer overall survival than TMB-L cancers (median OS of 12.7 months; 95% CI, 11.0-14.7; P = .01) (Table 2), meeting the prespecified primary objective of the study. Kaplan-Meier curves are shown in Figure 2A, further stratified by line of therapy in eFigure 1 in Supplement 1.
Table 2. Statistical Summary of the Association of TMB With Clinical Outcomes.
| Cohort | Model | End point | TMB, HR | 1-Sided UCB | P value |
|---|---|---|---|---|---|
| All evaluable | Univariate | OS | 0.72 | 0.91 | .01 |
| Prospective | Univariate | OS | 0.61 | 0.84 | .005 |
| Prospective | Univariate | PFS | 0.62 | 0.82 | .003 |
| Prospective | Univariate | TTP | 0.67 | 0.92 | .02 |
| Prospective pembrolizumab treated | Univariate | OS | 0.67 | 0.94 | .03 |
| Prospective nonpembrolizumab treated | Univariate | OS | 0.37 | 0.85 | .03 |
| Prospective | Multivariablea | OS | 0.67 | 0.92 | .02 |
Abbreviations: OS, overall survival; PFS, progression-free survival; TMB, tumor mutational burden; TTP, time to progression; UCB, upper confidence bound.
In the multivariable model, the variables tested for association with OS are TMB, programmed cell death-ligand 1 status (positive/negative/unknown) and microsatellite instability status (high vs low).
Figure 2. Kaplan-Meier Analysis of Clinical End Points by TMB Status.
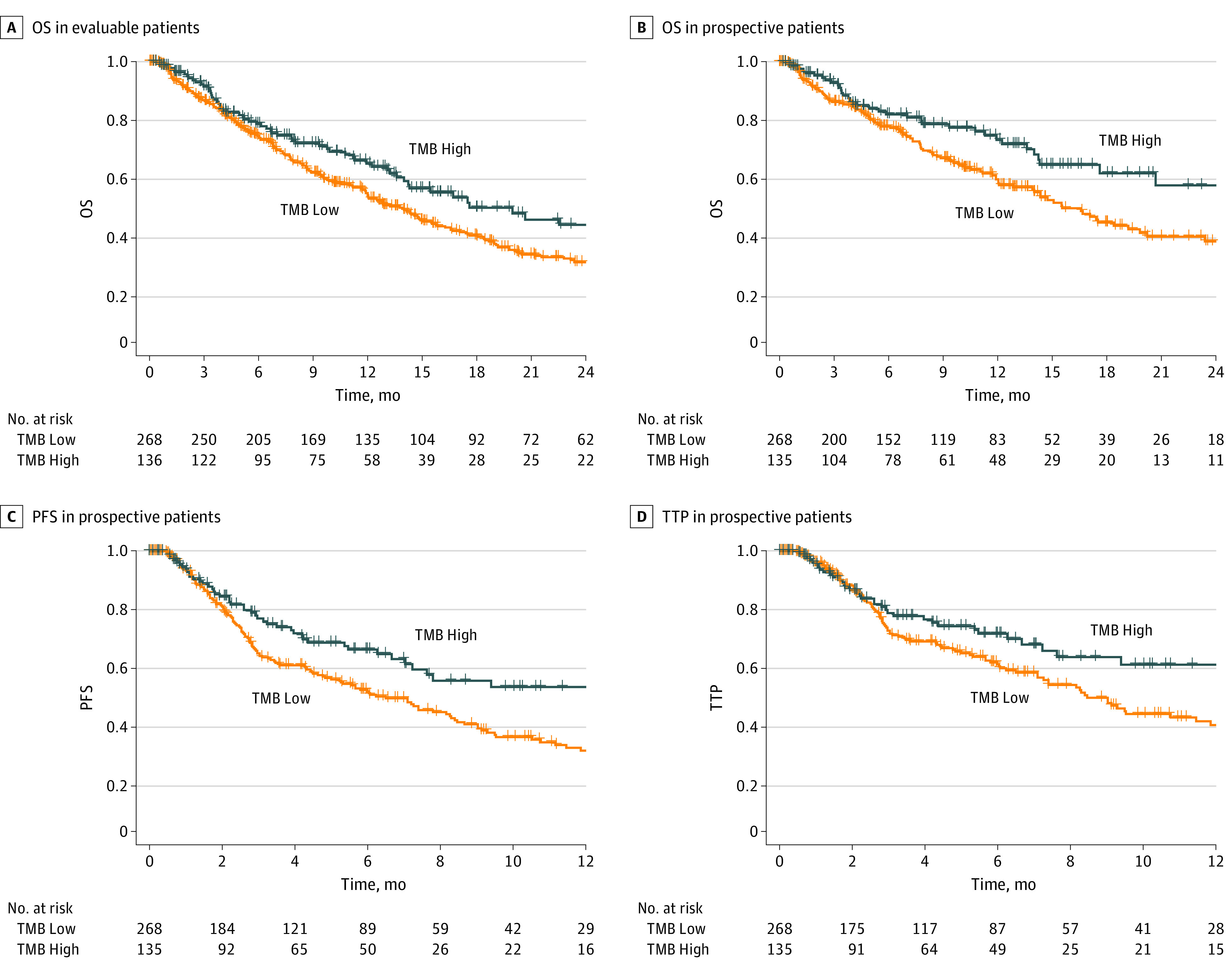
Panel A, Kaplan-Meier plot of overall survival (OS) in evaluable patients treated with immune checkpoint inhibitors. The No. at risk only includes patients with corrected study entry to account for immortal time bias (see Methods section). Panel B, Kaplan-Meier plot of OS in prospective patients treated with immune checkpoint inhibitors. Panel C, Kaplan-Meier plot of progression-free survival (PFS) in prospective patients treated with immune checkpoint inhibitors. Panel D, Kaplan-Meier plot of time to progression (TTP) in prospective patients treated with immune checkpoint inhibitors. OS indicates overall survival; PFS, progression-free survival; TTP, time to progression
Similar to the evaluable cohort, in the prospective cohort, TMB-H cancers (median OS of 32.8 months; 95% CI, 17.4 months to not reached) were also significantly associated with longer overall survival than TMB-L cancers (median OS of 14.9 months; 95% CI, 11.9 months to 18.9 months), with a 39% risk reduction in death (HR, 0.61; UCB, 0.84; P = .005) (Table 2 and Figure 2B). The association between TMB and clinical benefit was also observed in additional secondary clinical outcomes (Figures 2C and D). TMB-H cancers were associated with significantly longer PFS (HR, 0.62; P = .003) (Table 2) and longer TTP (HR, 0.67; P = .02) (Table 2) than TMB-L cancers. Kaplan-Meier curves stratified by line of therapy are shown in eFigure 2 in Supplement 1.
Evaluation of TMB in Individual Cancer Types
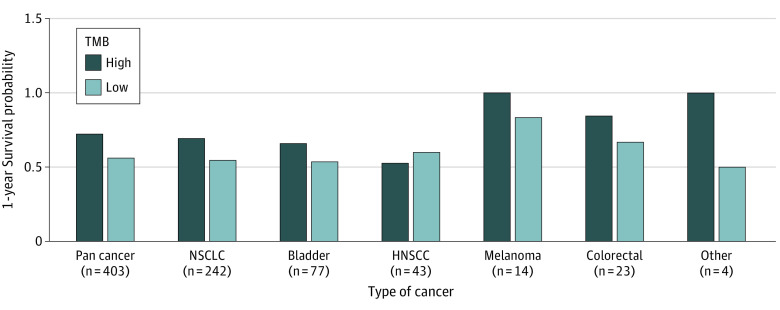
In the largest cohort, NSCLC (242 patients), TMB-H cancers were associated with significantly longer OS than TMB-L cancers (P = .05) (eTable 11 in Supplement 1). Our study was powered to assess the association between TMB and OS from the pancancer cohort, but not in individual cancer types due to the limitation of small sample sizes in some individual cohorts. Despite this limitation, we compared the 1-year survival probabilities between TMB-H vs TMB-L in these individual cancer types who had at least 10 patients in the prospective cohort (Figure 3). Our results were generally consistent with our pancancer cohort, with the 1-year survival probability of TMB-H patients being higher than that of TMB-L patients with NSCLC, bladder, melanoma, and CRC (Figure 3). HNSCC was the only cancer indication in which patients with high TMB (8 patients) had a numerically lower 1-year survival probability than patients with low TMB (35 patients) (52% vs 60%, respectively) (Figure 3). Similar results were seen in the evaluable cohort (eFigure 3 in Supplement 1).
Figure 3. Assessment of Overall Survival by Tumor Mutational Burden (TMB)-Status in Each Cancer Indication.
One-year survival probability in individual cancer types by TMB status estimated from Kaplan-Meier analysis. HNSCC indicates head and neck squamous cell cancer; NSCLC, non–small cell lung cancer.
Evaluation of TMB by ICI Administered
Next, we evaluated if the association between TMB and OS was robust to the type of ICI administered in the prospective cohort, given that the TMB FDA approval was only limited to pembrolizumab therapy. In both the pembrolizumab-treated (339 patients) and nonpembrolizumab-ICI treated (64 patients) cohorts, TMB was significantly associated with OS (HR, 0.67; UCB, 0.94; P = .03; and HR, 0.37; UCB, 0.85; P = .03, respectively) (Table 2), demonstrating TMB clinical benefit regardless of which ICI therapy was used for treatment. Kaplan-Meier curves are shown in eFigure 4 in Supplement 1.
Evaluation of TMB in the Context of Other Biomarkers
Finally, we evaluated if the association between TMB and OS was independent of PD-L1 status and MSI status. In a multivariable analysis of the prospective cohort, TMB-H remained significantly associated with OS after adjusting for PD-L1 status and MSI status (HR, 0.67; UCB, 0.92; P = .02) (Table 2; eTable 12 in Supplement 1). We next focused on our largest NSCLC cancer cohort where PD-L1 status is commonly used for ICI treatment decisions. NSCLC patients were further stratified by clinically relevant PD-L1 expression subgroups: less than 1% (25 patients), 1% to 49% (45 patients), and 50% or higher (119 patients). As expected, NSCLC cancers with PD-L1 expression higher than 50% were significantly associated with longer OS than NSCLC cancers with PD-L1 expression less than 1% (HR, 0.55; UCB, 0.96; P = .04) (eTable 11 in Supplement 1). However, in a multivariable analysis of NSCLC patients, TMB-H remained significantly and independently associated with longer OS even after adjusting for PD-L1 classification (HR, 0.65; UCB, 0.97; P = .04) (eTable 12 in Supplement 1).
Discussion
Although the Friends of Cancer Research TMB harmonization project has established industry standards for defining and analyzing the analytical validity of TMB,18,19 heterogeneity in patient populations, differences in ICIs administered, and various surrogate clinical end points evaluated have made it difficult to assess if TMB is a robust clinical biomarker that can be used widely in routine clinical care for ICI treatment decisions. Many clinicians have contextualized the efficacy evidence for TMB according to the results of the pivotal pancancer KEYNOTE-158 study that led to the FDA approval of pembrolizumab in TMB-H advanced solid cancers, which demonstrated higher ORR in TMB-H vs TMB-L cancers, but no difference in 1-year overall survival, and heterogeneous ORR benefit in individual cancers.9
We believe that our study fills in a critical clinical evidence gap that is urgently needed to demonstrate the value of the TMB biomarker from the xT NGS targeted gene panel assay in a diverse clinical setting. We found that TMB-H cancers are significantly associated with longer OS than TMB-L cancers, consistent with a large pancancer, pan-ICI retrospective study of 1662 patients with diagnoses of 1 of 10 distinct tumor types in which TMB-H cancers were determined by the FDA-authorized Memorial Sloan Kettering IMPACT assay.4 However, unlike the Memorial Sloan Kettering study that included all-comers receiving at least 1 dose of ICI therapy at any time point, our study focused only on patients that received FDA-approved ICIs in the first-line and second-line setting. The value of TMB in the first-line ICI therapy setting is of particular importance clinically since the FDA TMB-based pembrolizumab approval remains limited to the pretreated patient population. Furthermore, as the frontline immunotherapy treatment landscape evolves, for example with the recent FDA-approval of tremelimumab in combination with durvalumab and platinum-based chemotherapy for patients with metastatic NSCLC according to data from the phase III POSEIDON study demonstrating OS benefit in these patients compared with chemotherapy-treated patients,20 robust biomarkers such as TMB will be important for assessing who may benefit most from these new ICI treatments.
Additional strengths of our study include the following: first, the patient cohort was ethnically diverse, with 11.3% of the patient cohort with self-reported race and ethnicity other than White, much more than what is typically reported on clinical trials.21,22 Second, the prospective-only cohort mirrors a prospective clinical trial design and the use of the TMB test as intended. Third, additional secondary analyses demonstrated the robustness of our primary outcome findings. Furthermore, our results were significant in both pembrolizumab and nonpembrolizumab cohorts, demonstrating the efficacy of TMB beyond the FDA-approved TMB label for pembrolizumab in the second-line setting. Finally, TMB was independently associated with ICI clinical benefit after adjusting for PD-L1 and MSI status.
Limitations
This study had limitations. These include its retrospective nature, limited analyzable follow-up for patients treated with ICIs before testing, effectiveness end points that could represent surrogacy to other more established, prospective FDA-grade efficacy end points, heterogeneity in non-immunotherapy treatment patterns that were not prespecified, data missingness due to expected attrition rates within heterogeneous data sets from multiple clinics, the lack of sample size to examine the statistical association of TMB and clinical outcomes in some individual cancer subtypes.
Conclusions
The study findings demonstrate that the TMB category identified from the xT NGS targeted gene panel assay is a robust, reproducible biomarker of clinical benefit to ICIs in a diverse, advanced pancancer cohort treated in multiple clinics. More broadly, this study demonstrates the value of clinical genomic data sets for the assessment of evolving molecular biomarkers and clinical outcomes in diverse settings which are more representative of clinical practice patterns than clinical trials.
eTable 1. Histologies by Cancer Type in the Evaluable Cohort
eTable 2. FDA-Approved Medications Approved by Cancer Type and Line of Therapy Used for Cohort Definition
eTable 3. End Point Event Definitions
eTable 4. End Point Censoring Definitions
eTable 5. Histologies by Cancer Type in the Prospective Cohort
eTable 6. Medications by Cancer Type in the Evaluable Cohort
eTable 7. Medications by Cancer Type in the Prospective Cohort
eTable 8. Patient Summary in the Evaluable Cohort
eTable 9. Patient Summary in the Evaluable Cohort by Cancer Type
eTable 10. Patient Summary in the Prospective Cohort by Cancer Type
eTable 11. Univariate Cox-Proportional Hazards Models
eTable 12. Multivariable Cox-Proportional Hazards Models
eFigure 1. Kaplan-Meier (KM) Analysis of OS by TMB Status in the Evaluable Cohort in First-Line and Second-Line Treated Patients
eFigure 2. Kaplan-Meier (KM) Analysis of Clinical Outcomes by TMB Status in the Prospective Cohort in First-Line and Second-Line Treated Patients
eFigure 3. Assessment of Overall Survival by TMB-Status in Each Cancer Indication in the Evaluable Cohort
eFigure 4. Kaplan-Meier (KM) Analysis of Clinical Outcomes by TMB Status in the Prospective Cohort in Pembrolizumab and Non-pembrolizumab Treated Patients
Data Sharing Statement
References
- 1.Efremova M, Finotello F, Rieder D, Trajanoski Z. Neoantigens generated by individual mutations and their role in cancer immunity and immunotherapy. Front Immunol. 2017;8:1679. doi: 10.3389/fimmu.2017.01679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yarchoan M, Johnson BA III, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17(4):209-222. doi: 10.1038/nrc.2016.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598-2608. doi: 10.1158/1535-7163.MCT-17-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202-206. doi: 10.1038/s41588-018-0312-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGrail DJ, Pilié PG, Rashid NU, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32(5):661-672. doi: 10.1016/j.annonc.2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricciuti B, Wang X, Alessi JV, et al. Association of high tumor mutation burden in non–small cell lung cancers with increased immune infiltration and improved clinical outcomes of PD-L1 blockade across PD-L1 expression Levels. JAMA Oncol. 2022;8(8):1160-1168. doi: 10.1001/jamaoncol.2022.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi: 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 8.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi: 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353-1365. doi: 10.1016/S1470-2045(20)30445-9 [DOI] [PubMed] [Google Scholar]
- 10.Marcus L, Fashoyin-Aje LA, Donoghue M, et al. FDA Approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin Cancer Res. 2021;27(17):4685-4689. doi: 10.1158/1078-0432.CCR-21-0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration . FDA approves pembrolizumab for adults and children with TMB-H solid tumors. June 17, 2020. Accessed October 5, 2022. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors
- 12.Herbst RS, Lopes G, Kowalski DM, et al. Association between tissue TMB (tTMB) and clinical outcomes with pembrolizumab monotherapy (pembro) in PD-L1-positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Ann Oncol. 2019;30(suppl 5):v916-v917. doi: 10.1093/annonc/mdz394.077 [DOI] [Google Scholar]
- 13.Vega DM, Yee LM, McShane LM, et al. ; TMB Consortium . Aligning tumor mutational burden (TMB) quantification across diagnostic platforms: phase II of the Friends of Cancer Research TMB Harmonization Project. Ann Oncol. 2021;32(12):1626-1636. doi: 10.1016/j.annonc.2021.09.016 [DOI] [PubMed] [Google Scholar]
- 14.Mankor JM, Paats MS, Groenendijk FH, et al. ; CPCT Consortium . Impact of panel design and cut-off on tumour mutational burden assessment in metastatic solid tumour samples. Br J Cancer. 2020;122(7):953-956. doi: 10.1038/s41416-020-0762-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaubier N, Tell R, Lau D, et al. Clinical validation of the tempus xT next-generation targeted oncology sequencing assay. Oncotarget. 2019;10(24):2384-2396. doi: 10.18632/oncotarget.26797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaubier N, Bontrager M, Huether R, et al. Integrated genomic profiling expands clinical options for patients with cancer. Nat Biotechnol. 2019;37(11):1351-1360. doi: 10.1038/s41587-019-0259-z [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Stein MM, Kase M, et al. Comparison of the tumor immune microenvironment and checkpoint blockade biomarkers between stage III and IV non-small cell lung cancer. Cancer Immunol Immunother. 2023;72(2):339-350. doi: 10.1007/s00262-022-03252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merino DM, McShane LM, Fabrizio D, et al. ; TMB Harmonization Consortium . Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer. 2020;8(1):e000147. doi: 10.1136/jitc-2019-000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenzinger A, Allen JD, Maas J, et al. Tumor mutational burden standardization initiatives: recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosomes Cancer. 2019;58(8):578-588. doi: 10.1002/gcc.22733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson M. PL02.01 durvalumab +/ tremelimumab + chemotherapy as first-line treatment for mNSCLC: results from the phase 3 POSEIDON study. Presented at: 2021 World Conference on Lung Cancer; September 9, 2021; Virtual. [Google Scholar]
- 21.Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5(10):e191870. doi: 10.1001/jamaoncol.2019.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong YR, Alishahi Tabriz A, Turner K. Racial and ethnic disparities in clinical trial recruitment in the U.S. Am J Prev Med. 2021;61(5):e245-e250. doi: 10.1016/j.amepre.2021.05.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Histologies by Cancer Type in the Evaluable Cohort
eTable 2. FDA-Approved Medications Approved by Cancer Type and Line of Therapy Used for Cohort Definition
eTable 3. End Point Event Definitions
eTable 4. End Point Censoring Definitions
eTable 5. Histologies by Cancer Type in the Prospective Cohort
eTable 6. Medications by Cancer Type in the Evaluable Cohort
eTable 7. Medications by Cancer Type in the Prospective Cohort
eTable 8. Patient Summary in the Evaluable Cohort
eTable 9. Patient Summary in the Evaluable Cohort by Cancer Type
eTable 10. Patient Summary in the Prospective Cohort by Cancer Type
eTable 11. Univariate Cox-Proportional Hazards Models
eTable 12. Multivariable Cox-Proportional Hazards Models
eFigure 1. Kaplan-Meier (KM) Analysis of OS by TMB Status in the Evaluable Cohort in First-Line and Second-Line Treated Patients
eFigure 2. Kaplan-Meier (KM) Analysis of Clinical Outcomes by TMB Status in the Prospective Cohort in First-Line and Second-Line Treated Patients
eFigure 3. Assessment of Overall Survival by TMB-Status in Each Cancer Indication in the Evaluable Cohort
eFigure 4. Kaplan-Meier (KM) Analysis of Clinical Outcomes by TMB Status in the Prospective Cohort in Pembrolizumab and Non-pembrolizumab Treated Patients
Data Sharing Statement