Abstract
Fimbrial adhesins that mediate attachment to host cells are produced by most virulent Escherichia coli isolates. These virulence factors play an important role in the initial stages of bacterial colonization and also in determination of the host and tissue specificity. Isolates belonging to serotype O78 are known to cause a large variety of clinical syndromes in farm animals and humans and have been shown to produce several types of adherence fimbriae. We studied the fimbrial adhesin from an avian septicemic E. coli isolate of serotype O78. Analysis of the genetic organization of the fac (fimbria of avian E. coli) gene cluster indicates that it belongs to the S-fimbrial adhesin family. Seven open reading frames coding for major and minor structural subunits were identified, and most of them showed a high degree of homology to the corresponding Sfa and Foc determinants. The least-conserved open reading frame was facS, encoding a protein known to play an important role in determining adherence specificity in other S-fimbrial gene clusters.
Avian colisepticemia is a major disease of poultry caused by virulent strains of Escherichia coli. This septicemic disease of chickens and turkeys starts as a respiratory tract infection that extends to the air sacs and then to vital organs. The disease is economically important as it brings about heavy economic losses due to mortality and morbidity. In addition, there are also massive indirect losses due to intensification of other respiratory diseases, such as Newcastle disease and mycoplasma infections (35, 36).
Avian colisepticemia is similar in many respects to human extraintestinal diseases (urinary tract infections [UTI] and newborn meningitis [NBM]) caused by virulent E. coli strains (2). These diseases can be contained within the initially infected organ (such as the trachea in poultry or urinary tract in humans) but can develop into septicemia, usually under conditions of stress or immune deficiency. The bacteria involved in both kinds of diseases are similar in terms of the virulence factors that they contain and the organization of the genes coding for them. Bacteria isolated from persons with these diseases are usually nontoxigenic but carry genes encoding serum resistance and an iron-binding protein (6, 10, 28, 47).
One important virulence factor that has been intensively studied in pathogenic extraintestinal E. coli strains is adherence fimbriae. Fimbrial adhesins are fiber-like structures, visible by electron microscopy on the bacterial cell surface, that are composed of major and minor subunit proteins. These fimbriae enable attachment of pathogenic bacteria to moieties of eukaryotic cells, therefore mediating host colonization, which is often an essential step in bacterial infection. Furthermore, the adhesion properties determine the host and tissue specificity (9, 14, 41, 42). In the case of fimbrial adhesins produced by extraintestinal E. coli strains, the minor subunits that facilitate adhesion are preferentially located at the tip of the fimbriae (10, 14, 21, 25, 46). Fimbrial adhesins are classified by their receptor specificity. Strains associated with UTI usually produce P-fimbrial adhesins, which interact with glycolipids containing α-d-Gal-1,4-β-d-Gal (7, 20, 22), and F1C fimbriae, which were recently shown to bind β-GalNac-1,4-β-Gal (13, 15, 34, 44, 45). E. coli strains causing sepsis or NBM in most cases produce S-fimbrial adhesins, which interact with glycoproteins containing sialic acid (12, 25, 30).
We have been studying the adherence fimbriae of an avian E. coli O78 strain (36) that is frequently involved in severe epidemics of colisepticemia. These fimbriae do not hemagglutinate red blood cells but were shown to adhere to epithelial cells, preferentially avian tracheal cells. The fimbriae were purified and named by us AC/I (avian E. coli I) fimbriae (27, 48). Preliminary experiments using long-range mapping with specific DNA probes suggested that AC/I fimbriae may be related to S fimbriae (2).
In this study we describe the molecular cloning and characterization of the fac (fimbriae of avian E. coli strains) gene cluster coding for AC/I fimbriae in avian septicemic E. coli O78. Molecular analysis of this gene cluster reveals a high degree of homology to other S-fimbriae gene clusters in the nature of the subunits and their organization and indicates that AC/I fimbriae constitute a new member of the group of S-fimbriae.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Wild-type E. coli O78 strain 781 was isolated from a chicken with acute septicemia (2, 48). E. coli K-12 strains VCS257 (ton53 dapD8 lacY1 glnV44) (Stratagene Cloning Systems, San Diego, Calif.) and VL584 [Δ(lac-pro) strA thi ara] (kind gift from B. I. Eisenstein) were used for construction and screening of the genomic DNA libraries. E. coli K-12 strain DH5α was used for plasmid manipulation and propagation. Agglutination assays were performed with the E. coli K-12 host strain HB101 (24). The plasmids used are listed in Table 1. Luria-Bertani medium (liquid or solid) was used for bacterial cultivation, and antibiotics were added when required, as described by Sambrook et al. (37).
TABLE 1.
Recombinant DNAs used in this study
| Recombinant DNA | Relevant characteristics | Reference |
|---|---|---|
| pMMB33 | Cosmid vector, carries kanamycin resistance | 8 |
| pRBS15 | pMMB33 genomic library clone of 781 containing the fac determinant | This study |
| pBR322 | Plasmid vector, carries ampicillin and tetracycline resistance | 4 |
| pBRAC3-3 | pBR322 containing the 5′ part of fac (cloned EcoRI-BamHI 5-kb fragment from pRBS15) | This study |
| pRAC221 | pBRAC3-3 containing fac determinant (addition of 6.6-kb EcoRI fragment from pRBS15) | This study |
| pANN801-13 | pBR322 based, containing sfaI gene cluster | 12 |
| pAZZ50 | pBR322 based, containing sfaII gene cluster | 11 |
Recombinant DNA techniques.
Agarose gel electrophoresis and bacterial transformations (CaCl2 method) were performed as previously described (37). The QIAprep spin miniprep kit (Qiagen GmbH) was used for plasmid and cosmid preparations. QIAquick gel extraction kit (Qiagen GmbH) was used for isolation of DNA fragments from the agarose gel. Restriction endonucleases and DNA ligase were obtained from Boehringer Mannheim GmbH and used according to the manufacturer's recommendations.
Construction of genomic library.
Total genomic DNA from strain 781 was isolated and partially digested with Sau3A. DNA fragments of 30 to 40 kb were isolated from a 10 to 40% sucrose gradient and ligated into the BamHI sites of the cosmid vector pMMB33 (8, 37). After in vitro packaging using the Gigapack Gold kit (Stratagene Cloning Systems), 5,000 recombinant E. coli K-12 VCS257 clones were selected. E. coli K-12 strain VL584, which has no fimbriae, was later used as a recipient to detect the positive AC/I-fimbriae clone by DNA-DNA dot-blot colony hybridization and agglutination with monoclonal antibodies.
Preparation of monoclonal antibodies.
The preparation and characterization of the monoclonal antibodies have been described previously (48).
Colony dot-blot and Southern hybridization.
Rapid detection of DNA-DNA hybridization by colony dot-blot and Southern analysis with specific probes were performed as previously described (2).
Subcloning of fac determinant.
Cloning of the fac cluster was done in two steps. Cosmid pRBS15 was cleaved with restriction enzymes EcoRI and both EcoRI and BamHI. The EcoRI-BamHI 5-kb fragment was cloned into vector pBR322 cut with EcoRI and BamHI to obtain plasmid pBRAC3-3. The 6.6-kb EcoRI fragment from pRBS15 was then ligated to pBRAC3-3 linearized by EcoRI. E. coli DH5α cells transformed with the resulting plasmid, pRAC221, were agglutinated by monoclonal antibody 875 (see Results), while these transformed with pBRAC3-3 were not.
Agglutination tests.
Agglutination tests with monoclonal antifimbria antibodies were performed in 24-well microtiter plates as a rapid indication for the production of fimbriae. Cells carrying the recombinant plasmids listed in Table 1 were grown in liquid medium at 37°C for 18 h with agitation, 200 μl of the cell suspension was incubated with the antiserum, and agglutination was determined after 20 min.
DNA sequencing.
The sequence of the DNA region coding for the AC/I fimbrial genes facA, facD, facE, facF, facG, facS, and facH in strain 781 was determined by using the ABI Prism 377XL sequencer and the BigDye terminator cycle sequencing kit (PE Biosystems).
Computerized sequence analysis.
Comparison of different subunit proteins with their related proteins was performed by the multiple sequence alignment program CLUSTAL W (43) at the Network Protein Sequence @nalysis net server (http://pbil.ibcp.fr/NPSA). Pretty printing and shading of the multiple alignment sequences were done by BOXSHADE program, version 3.21 (http://www.ch.embnet.org). Sequence similarity search was performed with the BLAST program at the NCBI server (http://www.ncbi.nlm.nih.gov) (1) or FASTA program at the European Bioinformatics Institute server (http://www2.ebi.ac.uk) (32, 33).
RESULTS
Characterization of fimbriae of E. coli 781.
Most characterized fimbrial adhesins are able to agglutinate red blood cells of different organisms; this phenotype allows the characterization and identification of different fimbriae. Cells of E. coli strain 781 (serotype O78) expressing AC/I fimbriae adhere preferentially to avian epithelial cells. However, the bacteria failed to agglutinate red blood cells from calves, chickens, turkeys, rabbits, sheep, horses, mice, guinea pigs, and humans. In order to further characterize the AC/I fimbriae and study their genetic organization, monoclonal antibodies were raised. Several monoclonal clones were examined (clones 82, 92, 863, 921, and 1421), and all of them agglutinated the piliated 781 strain but failed to agglutinate the negetive control strains E. coli K-12 DH5α and HB101.
Cloning and subcloning of fac gene cluster of E. coli 781.
The fac gene cluster coding for AC/I of septicemic avian E. coli O78 was obtained from a cosmid genomic DNA library. To identify clones that contain the fac determinants, the library was screened by the use of monoclonal antibody 875 (48). Several positively reacting clones were obtained, and one of them (pRBS15) was studied further.
Since preliminary results suggested that the AC/I fimbriae are closely related to other S-fimbriae, we examined the homology between the cosmid pRBS15 and other S-fimbrial adhesin clusters by DNA-DNA hybridization. Southern colony dot-blot analysis of pRBS15 was performed using specific sfa gene probes. Positive hybridization under stringent conditions was observed for sfaAII (the major subunit gene of the S-fimbrial adhesin II complex) and sfaGII, but that with the sfaS gene probe was seen only under less stringent conditions (data not shown). To further characterize cosmid pRBS15, it was cleaved with restriction enzymes EcoRI and both EcoRI and BamHI. Following Southern hybridization with the sfaAII gene probe, an EcoRI-BamHI fragment of 5 kb was observed. When sfaS was used as a probe, a 6.6-kb positive EcoRI fragment was obtained. The sfaA- and sfaS-specific gene probes are located at the two ends of the sfa determinant; therefore, we speculated that the DNA stretch containing the two positively hybridizing fragments will include the whole fac determinant. The EcoRI-BamHI 5-kb fragment was cloned in vector pBR322 to obtain plasmid pBRAC3-3. After cleavage of pBRAC3-3 with EcoRI, the 6.6-kb EcoRI fragment from pRBS15 was inserted to form plasmid pRAC221. As expected, recipient cells transformed with plasmid pRAC221 were agglutinated with monoclonal antibody 875.
Physical maps and serological properties of fac and other S-related determinants.
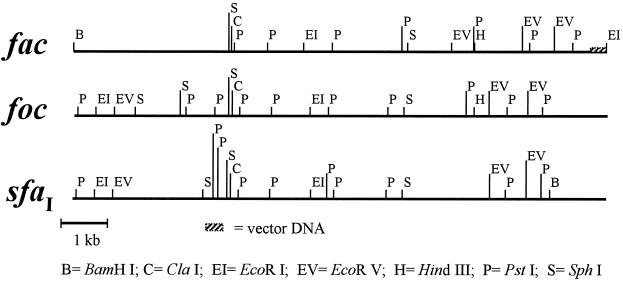
To assess the degree of physical similarity of the fac determinant to other S-related fimbrial clusters, restriction analysis was performed using several enzymes. A restriction map of the 11.6-kb insert was obtained and compared to the restriction pattern of other known S-related clusters (Fig. 1). Although the comparison revealed restriction pattern similarity, the differences found suggest that the AC/I fimbrial cluster is not identical to any of the other members of the S-fimbrial family. The fac restriction pattern was also related but not identical to the one observed for the sfr cluster (31).
FIG. 1.
Physical maps of fac, foc, and sfaI determinants (29).
To estimate the degree of similarity between AC/I fimbriae and other clusters belonging to the S-fimbrial family, the agglutination of several monoclonal antibodies raised against AC/I fimbriae was determined. All the antibodies agglutinated strains producing AC/I and Sfa-II. This observation further supported our view of close relatedness of the fac cluster to genes for S-fimbrial adhesins.
DNA sequence of genes coding for major and minor subunits of fac determinant.
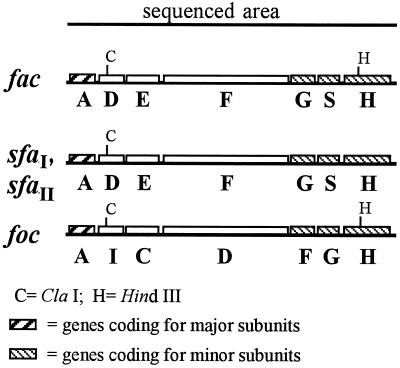
In order to identify the genes involved in the biogenesis of AC/I, the DNA sequence of most of the insert was determined. Sequence analysis performed on a fragment of 6.9 kb revealed seven open reading frames in the same orientation. The cluster was named fac (fimbriae of avian E. coli), and the organization of these genes, named facA, facD, facE, facF, facG, facS, and facH, resembled the gene organization found for fimbriae belonging to the S-fimbrial family (Fig. 2).
FIG. 2.
Genetic organization of fac, sfa, and foc gene clusters (11, 19, 34).
A similarity search using the BLAST program and multiple sequence alignments using the CLUSTAL W program were performed on these open reading frames. The results are depicted in Table 2 and Fig. 3.
TABLE 2.
Identity and similarity of amino acid sequences of the seven open reading frames found in the AC/I fimbrial gene cluster compared with other clusters in the S-fimbrial family (11, 13, 18, 38–40, 44, 45)
| Fac protein | Coding sequence (nucleotides) | Calculated size (kDa) | % Identity (% similarity) to Fac-specific proteins
|
||
|---|---|---|---|---|---|
| Sfa-I homologous | Sfa-II homologous | Foc/F1652 homologous | |||
| FacA | 14–559 | 18.4 | 66 (78) | 100 (100) | 69 (78) |
| FacD | 630–1169 | 19.7 | 98 (98) | ? | ? |
| FacE | 1210–1905 | 25.7 | 99 (99) | ? | 99 (99) |
| FacF | 1975–4605 | 96.3 | 98 (98) | ? | 97 (97) |
| FacG | 4618–5145 | 18.6 | 100 (100) | 99 (99) | 98 (98) |
| FacS | 5167–5658 | 17.1 | 72 (81) | 71 (80) | 60 (68) |
| FacH | 5794–6693 | 30.0 | 80 (89) | 82 (91) | 96 (96) |
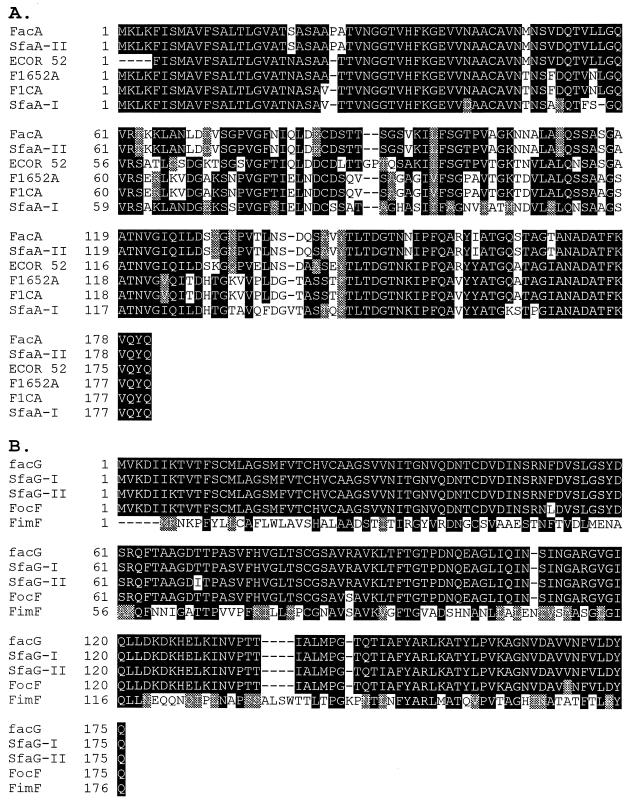
FIG. 3.
Multiple sequence alignment to different proteins encoded by the fac gene cluster. (A) FacA (major subunit). (B) FacG (minor subunit). (C) FacS (minor subunit). (D) FacH (minor subunit) (5, 11, 13, 17, 38, 39, 44, 45). Black shading, identity; grey shading, similarity.
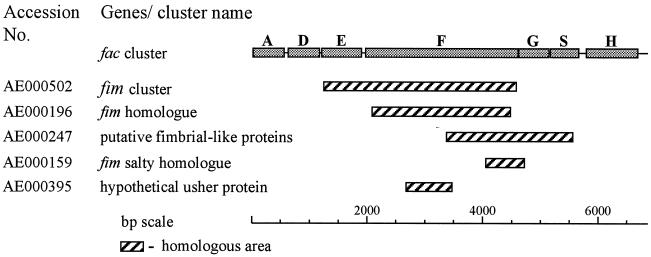
In order to trace fac-related sequences in the E. coli genome, we performed a FASTA search with the 6.9-kb DNA sequence. Sequences that showed more than 50% identity to fragments of the fac cluster are presented in Fig. 4. None of these sequences showed homology to the whole AC/I 6.9-kb fragment sequenced.
FIG. 4.
E. coli genomic DNA sequences homologous to fac gene cluster (3).
DISCUSSION
Although classified as enteric microorganisms, many strains of E. coli are capable of colonizing extraintestinal tissues. In contrast to intestinal colonization, the E. coli strains that colonize other organs often generate systemic infection. The strains involved in extraintestinal diseases do not carry genes for production of enterotoxins but usually express polysaccharide capsules such as K1. Another difference between intestinal and extraintestinal strains involves adherence to epithelial cells. Usually, adherence of intestinal E. coli is mediated via adherence fimbriae, such as K99 and K88, that are coded for by plasmid genes, and adhesion is mediated by the major subunit. In strains involved in human extraintestinal infection, the fimbriae are encoded by chromosomal genes and composed of a major subunit and several minor subunits, which influence binding abilities and are responsible for specific adhesion.
The best-studied extraintestinal human E. coli strains are those involved in UTI, and the majority of them produce P-fimbriae, which bind to the digalactoside part of glycolipids (7, 11, 22, 26). Other extraintestinal strains were shown to produce fimbriae that do not have the same adhesin specificity as P-fimbriae. This includes type F1C fimbriae, Sfr fimbriae, and Sfa-I fimbriae, which recognize receptors containing α-sialyl-2,3-β-Gal (11, 22, 25, 29–31, 34). Recently, a new type of adherence fimbriae was identified in E. coli strains isolated from NBM (11). These fimbriae, named Sfa-II fimbriae, differ from Sfa-I fimbriae in the major subunit and in two minor subunits. However, they also bind sialylgalactosides, and their gene coding for the fimbrial adhesin, sfaS, is identical to the equivalent gene of the sfaI cluster from UTI bacteria (11, 16).
We have studied the AC/I fimbriae produced by an avian E. coli O78 strain. These fimbriae are an important virulence factor, as they mediate preferential binding of this extraintestinal pathogen to avian epithelial tissues. The results presented here indicate that the AC/I fimbriae constitute a new member of the S-fimbrial family.
Several observations demonstrate the relatedness between AC/I and other S-fimbrial gene clusters. (i) The various gene clusters coding for S-fimbriae (fac, sfr, sfa, and foc) have related restriction patterns. (ii) Monoclonal antibodies that were raised against AC/I were also able to bind to another S-fimbrial group member, showing immunological cross-reactivity. (iii) The genetic organization of the fac gene cluster, as determined by DNA sequencing, was in good agreement with that of other clusters belonging to the S-fimbrial family (Fig. 2 and Table 2).
Comparison of the predicted AC/I protein subunits to the corresponding subunits belonging to the S-fimbrial family revealed an interesting insight into the evolution and function of the fimbriae—the AC/I major subunit protein, FacA, is 100% similar to the major subunit protein SfaA-II of the sfaII gene cluster. This finding is unexpected, since previous studies comparing genes from different adhesin clusters have shown that the major subunit genes are the most heterogeneous, probably reflecting better adaptation to different environments encountered by the bacteria in their host (5, 11). The unexpected high degree of identity found in the major subunit proteins SfaA-II and FacA may be a result of the evolutionary process of cluster formation and could perhaps be due to horizontal gene transfer in recent times. This high degree of homology can also explain the immunological cross-reactivity of the monoclonal antibodies that were raised against the AC/I fimbriae.
Despite the similarity between the major subunits of the sfaII and fac clusters, the minor subunit proteins of AC/I show a higher degree of homology to other fimbrial gene clusters, like sfaI and foc. These proteins are preferentially found at the fimbrial tip, and they play an important role in determining adhesin specificity (16, 46).
The most conserved minor subunit is FacG, which is identical to SfaG-I and closely related to SfaG-II and FocF (99 and 98% identity, respectively). It was shown that sfaG contributes to the attachment of the bacteria to tubular cells as well as to plasminogen. The conservation of the FacG protein found in this study supports previous suggestions that this subunit might contribute to the adherence process (11, 23). Based on this conservation and the complete identity to SfaG-I, we propose a similar function for this protein in the avian-virulent strain.
The least conserved minor subunit was FacS, in agreement with its adhesion properties, which are different from those of other fimbriae of the S-group. For example, AC/I fimbriae do not agglutinate red blood cells, in contrast to SfaS-I and SfaS-II, which are identical and responsible for the sialic acid-specific binding of the S-fimbriae. Furthermore, while AC/I fimbriae preferentially bind avian tracheal cells, F1C fimbriae attach to uroepithelial cells of human origin (16, 29).
The data presented indicate that the AC/I cluster contains one gene that is highly homologous to the Sfa-II cluster (the sfaAII gene), while other genes are more homologous to other gene clusters. FacG and FacS are homologues of SfaG-I and SfaS-I, respectively, while FacH is a homologue of the FocH protein. This is also shown at the DNA level, as depicted in Table 3. We propose that the observed phenomenon constitutes a combinatorial shuffling of fimbrial genes, which could be of ecological and functional importance. This gene shuffling could account for the diversity seen in the fimbrial loci and enable the bacteria to adapt better to the changing environment encountered in the host, as well as to the selection pressure imposed by the immune system of the host. This gene shuffling could be achieved by horizontal gene transfer or by recombination events with other genomic fimbrial clusters, such as the fim-like genes (Fig. 4) (3). Evidence for the existence of horizontal gene transfer was obtained from the finding that in the 3′ end of the fac cluster, there is an insertion of 74 nucleotides in a stretch of 100 nucleotides between the end of facS and the start of facH. These 100 nucleotides have a GC content of 30%, compared to the 47.8% GC found in the whole 6.9 kb of the fac sequence, and are homologous to open reading frames of two bacteriophages.
TABLE 3.
Identity of major and minor subunit open reading frames of AC/I to other clusters that belong to the S-fimbrial family (11, 13, 38, 39, 44, 45)
| fac gene | % Identity to fac-specific open reading frames
|
||
|---|---|---|---|
| sfaI homologous | sfaII homologous | foc/f1652 homologous | |
| facA | 72.8 | 100 | 73.1 |
| facG | 100 | 99.8 | 99.2 |
| facS | 76.2 | 76 | 63 |
| facH | 79.4 | 82.6 | 98.4 |
The group of S-fimbriae is interesting because it contains several types of fimbriae with different adherence specificities that are produced by bacteria that cause a variety of clinical symptoms in mammals and poultry. The molecular evolution of the gene clusters coding for these fimbriae can serve as a model system for studying combinatorial gene shuffling as an adaptive process in host-pathogen interactions.
ACKNOWLEDGMENTS
We thank Uri Gophna for critical reading of the manuscript.
This work was supported by the Manja and Morris Leigh Chair for Biophysics and Biotechnology, grants from the German-Israeli Science Foundation (GIF) and the Deutsche Forschungsgemeinschaft (grant SFB 479), and an EMBO short-term fellowship (ASTF 7889) to R.B.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babai R, Blum Oehler G, Stern B E, Hacker J, Ron E Z. Virulence patterns from septicemic Escherichia coli O78 strains. FEMS Microbiol Lett. 1997;149:99–105. doi: 10.1111/j.1574-6968.1997.tb10315.x. [DOI] [PubMed] [Google Scholar]
- 3.Blattner F R, Plunkett G, 3rd, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 5.Boyd E F, Hartl D L. Diversifying selection governs sequence polymorphism in the major adhesin proteins fimA, papA, and sfaA of Escherichia coli. J Mol Evol. 1998;47:258–267. doi: 10.1007/pl00006383. [DOI] [PubMed] [Google Scholar]
- 6.Cherifi A, Contrepois M, Picard B, Goullet P, Orskov I, Orskov F. Clonal relationships among Escherichia coli serogroup O78 isolates from human and animal infections. J Clin Microbiol. 1994;32:1197–1202. doi: 10.1128/jcm.32.5.1197-1202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Man P, Cedergren B, Enerback S, Larsson A C, Leffler H, Lundell A L, Nilsson B, Svanborg-Eden C. Receptor-specific agglutination tests for detection of bacteria that bind globoseries glycolipids. J Clin Microbiol. 1987;25:401–406. doi: 10.1128/jcm.25.2.401-406.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey J, Bagdasarian M, Feiss D, Franklin F C H, Deshusses J. Stable cosmid vectors that enable the introduction of cloned fragments into a wide range of gram negative bacteria. Gene. 1983;24:290–308. doi: 10.1016/0378-1119(83)90090-2. [DOI] [PubMed] [Google Scholar]
- 9.Hacker J. Genetic determinants coding for fimbriae and adhesins of extraintestinal Escherichia coli. Curr Top Microbiol Immunol. 1990;151:1–27. doi: 10.1007/978-3-642-74703-8_1. [DOI] [PubMed] [Google Scholar]
- 10.Hacker J. Role of fimbrial adhesins in the pathogenesis of Escherichia coli infections. Can J Microbiol. 1992;38:720–727. doi: 10.1139/m92-118. [DOI] [PubMed] [Google Scholar]
- 11.Hacker J, Kestler H, Hoschutzky H, Jann K, Lottspeich F, Korhonen T K. Cloning and characterization of the S fimbrial adhesin II complex of an Escherichia coli O18:K1 meningitis isolate. Infect Immun. 1993;61:544–550. doi: 10.1128/iai.61.2.544-550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacker J, Schmidt G, Hughes C, Knapp S, Marget M, Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985;47:434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harel J, Jacques M, Fairbrother J M, Bosse M, Forget C. Cloning of determinants encoding F165(2) fimbriae from porcine septicaemic Escherichia coli confirms their identity as F1C fimbriae. Microbiology. 1995;141:221–228. doi: 10.1099/00221287-141-1-221. [DOI] [PubMed] [Google Scholar]
- 14.Jann K, Hoschutzky H. Nature and organization of adhesins. Curr Top Microbiol Immunol. 1990;151:55–70. doi: 10.1007/978-3-642-74703-8_3. [DOI] [PubMed] [Google Scholar]
- 15.Khan S, Kniep B, Oelschlaeger T A, Van Die I, Korhonen T, Hacker J. Receptor structure for F1C fimbriae of uropathogenic Escherichia coli. Infect Immun. 2000;68:3541–3547. doi: 10.1128/iai.68.6.3541-3547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan A S, Muhldorfer I, Demuth V, Wallner U, Korhonen T K, Hacker J. Functional analysis of the minor subunits of S fimbrial adhesion (SfaI) in pathogenic Escherichia coli. Mol Gen Genet. 2000;263:96–105. doi: 10.1007/pl00008680. [DOI] [PubMed] [Google Scholar]
- 17.Klemm P, Christiansen G. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1987;208:439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- 18.Klemm P, Jorgensen B J, Kreft B, Christiansen G. The export systems of type 1 and F1C fimbriae are interchangeable but work in parental pairs. J Bacteriol. 1995;177:621–627. doi: 10.1128/jb.177.3.621-627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knudsen T B, Klemm P. Probing the receptor recognition site of the FimH adhesin by fimbriae-displayed FimH-FocH hybrids. Microbiology. 1998;144:1919–1929. doi: 10.1099/00221287-144-7-1919. [DOI] [PubMed] [Google Scholar]
- 20.Korhonen T K, Vaisanen V, Saxen H, Hultberg H, Svenson S B. P-antigen-recognizing fimbriae from human uropathogenic Escherichia coli strains. Infect Immun. 1982;37:286–291. doi: 10.1128/iai.37.1.286-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindberg F, Lund B, Johansson L, Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987;328:84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- 22.Lund B, Marklund B I, Stromberg N, Lindberg F, Karlsson K A, Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988;2:255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 23.Marre R, Kreft B, Hacker J. Genetically engineered S and F1C fimbriae differ in their contribution to adherence of Escherichia coli to cultured renal tubular cells. Infect Immun. 1990;58:3434–3437. doi: 10.1128/iai.58.10.3434-3437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 25.Moch T, Hoschutzky H, Hacker J, Kroncke K D, Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci USA. 1987;84:3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morschhauser J, Vetter V, Emody L, Hacker J. Adhesin regulatory genes within large, unstable DNA regions of pathogenic Escherichia coli: cross-talk between different adhesin gene clusters. Mol Microbiol. 1994;11:555–566. doi: 10.1111/j.1365-2958.1994.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 27.Naveh M, Zusman T, Skutelsky E, Ron E Z. Adherence pili in avian strains of Escherichia coli: effect on pathogenicity. Avian Dis. 1984;28:651–661. [PubMed] [Google Scholar]
- 28.Orskov I, Orskov F. Escherichia coli in extra-intestinal infections. J Hyg (London) 1985;95:551–575. doi: 10.1017/s0022172400060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ott M, Hoschutzky H, Jann K, Van Die I, Hacker J. Gene clusters for S-fimbrial adhesin (sfa) and F1C fimbriae (foc) of Escherichia coli: comparative aspects of structure and function. J Bacteriol. 1988;170:3983–3990. doi: 10.1128/jb.170.9.3983-3990.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkkinen J, Rogers G N, Korhonen T, Dahr W, Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986;54:37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawelzik M, Heesemann J, Hacker J, Opferkuch W. Cloning and characterization of a new type of fimbria (S/F1C-related fimbria) expressed by an Escherichia coli O75:K1:H7 blood culture isolate. Infect Immun. 1988;56:2918–2924. doi: 10.1128/iai.56.11.2918-2924.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson W R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- 33.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riegman N, Kusters R, Van Veggel H, Bergmans H, Van Bergen en Henegouwen P, Hacker J, Van Die I. F1C fimbriae of a uropathogenic Escherichia coli strain: genetic and functional organization of the foc gene cluster and identification of minor subunits. J Bacteriol. 1990;172:1114–1120. doi: 10.1128/jb.172.2.1114-1120.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ron E Z. Pathogenicity and potential control programs for Escherichia coli. Poultry Digest. 1987;46:90–94. [Google Scholar]
- 36.Ron E Z, Yerushalmi Z, Naveh M W. Adherence pili of Escherichia coli O78 determine host and tissue specificity. In: Ron E Z, Rottem S, editors. Microbial surface components and toxins in relation to pathogenesis. London, England: Plenum Press; 1990. pp. 61–68. [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 38.Schmoll T, Hacker J, Goebel W. Nucleotide sequence of the sfaA gene coding for the S-fimbrial protein subunit of Escherichia coli. FEMS Microbiol Lett. 1987;41:229–233. [Google Scholar]
- 39.Schmoll T, Hoschutzky H, Morschhauser J, Lottspeich F, Jann K, Hacker J. Analysis of genes coding for the sialic acid-binding adhesin and two other minor fimbrial subunits of the S-fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1989;3:1735–1744. doi: 10.1111/j.1365-2958.1989.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 40.Schmoll T, Morschhauser J, Ott M, Ludwig B, Van Die I, Hacker J. Complete genetic organization and functional aspects of the Escherichia coli S fimbrial adhesion determinant: nucleotide sequence of the genes sfa B, C, D, E, F. Microb Pathog. 1990;9:331–343. doi: 10.1016/0882-4010(90)90067-z. [DOI] [PubMed] [Google Scholar]
- 41.Smyth C J, Marron M B, Twohig J M, Smith S G. Fimbrial adhesins: similarities and variations in structure and biogenesis. FEMS Immunol Med Microbiol. 1996;16:127–139. doi: 10.1111/j.1574-695X.1996.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 42.Soto G E, Hultgren S J. Bacterial adhesins: common themes and variations in architecture and assembly. J Bacteriol. 1999;181:1059–1071. doi: 10.1128/jb.181.4.1059-1071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Die I, Kramer C, Hacker J, Bergmans H, Jongen W, Hoekstra W. Nucleotide sequence of the genes coding for minor fimbrial subunits of the F1C fimbriae of Escherichia coli. Res Microbiol. 1991;142:653–658. doi: 10.1016/0923-2508(91)90078-o. [DOI] [PubMed] [Google Scholar]
- 45.Van Die I, van Geffen B, Hoekstra W, Bergmans H. Type 1C fimbriae of a uropathogenic Escherichia coli strain: cloning and characterization of the genes involved in the expression of the 1C antigen and nucleotide sequence of the subunit gene. Gene. 1985;34:187–196. doi: 10.1016/0378-1119(85)90127-1. [DOI] [PubMed] [Google Scholar]
- 46.Vetter V, Hacker J. Strategies for employing molecular genetics to study tip adhesions. Methods Enzymol. 1995;253:229–241. doi: 10.1016/s0076-6879(95)53022-3. [DOI] [PubMed] [Google Scholar]
- 47.White D G, Dho-Moulin M, Wilson R A, Whittam T S. Clonal relationships and variation in virulence among Escherichia coli strains of avian origin. Microb Pathog. 1993;14:399–409. doi: 10.1006/mpat.1993.1039. [DOI] [PubMed] [Google Scholar]
- 48.Yerushalmi Z, Smorodinsky N I, Naveh M W, Ron E Z. Adherence pili of avian strains of Escherichia coli O78. Infect Immun. 1990;58:1129–1131. doi: 10.1128/iai.58.4.1129-1131.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]