Abstract
Objective:
Urinary bladder cancer (UBC) is the fourth most common cancer among men and tenth most common cancer in women. This study investigated an association of interleukins -17A promoter region single nucleotide polymorphism (SNP)-rs2275913 with UBC in Pakistani population.
Methods:
Population-based study was designed with 127 UBC patients and 100 healthy individuals. Only UBC Patients were included and other diseases hepatitis or any other malignancy/cancer were excluded from the study. Polymerase chain reaction Restriction fragment length polymorphism technique was used to genotype the rs2275913 SNP in patients and control. Linear regression analysis was performed on the genotype data and allelic frequency data. Online statistical tool was used to calculate ratio of odds.
Results:
Linear regression analysis showed that there was no association between rs2275913 SNP and UBC patients in the dominant model (OR = 0.815, CI = 0.415–1.6), recessive model (OR = 0.389, CI = 0.014–5.565), codominant model (OR = 0.376, CI=0.013–5.420) and (OR = 0.855, CI = 0.427–1.713). Moreover, among the UBC samples, low-grade non-muscle invasive UBC samples dominant model (OR = 0.722, CI = 0.316–1.637), recessive model (OR = 0.000, CI = 0.000–5.864), codominant model (OR = 0.864, CI = 0.030–12.668), and (OR = 0.788, CI = 0.341–1.806) did also not show any association. When same analysis was performed for high-grade muscle invasive UBC, dominant (OR = 0.936, CI = 0.403–2.155), recessive model (OR = 0.875, CI = 0.031–12.696), and codominant model (OR = 0.864, CI = 0.030–12.668,), and (OR = 0.942, CI = 0.394–2.232) did not show any association.
Conclusion:
Results revealed that rs2275913 did not show any associated with the high risk of UBC in Pakistani population. Some limitations of the studies are firstly, the samples size and other are detailed information on UBC and role of inflammation.
Keywords: Urinary bladder cancer, polymerase chain reaction-restriction fragment length polymorphism, interleukins-17A, linear regression, single nucleotide polymorphism
Introduction
Urinary tract is the system in the body that functions to produce, store, and excrete the urine. This system consists of two kidneys, pelvis, and ureter that lead to bladder and ends at urethra. Bladder is a hollow organ in the pelvis with flexible muscular walls. The key function of bladder is storing urine before it is excreted from the body.[1] The whole urinary tract inner surface is covered with a type of stratified epithelium. Majority of urinary bladder cancer (UBCs) develops from stratified epithelium. UBC begins when cells in the urinary bladder grow uncontrollably. These cancer cells when develop, they form a tumor that may spread to other parts of the body such as lymph nodes, bones, lung, liver, and peritoneum.[2] UBC is classified on the basis of the type of cells, grade, and stages. UBC accounts for 3.2% cancer international burden. It is the fourth most common cancer among men and tenth most common cancer in women.[3] UBC is the tenth leading cause cancer associated mortality.[4] Among urinary system cancers, bladder cancer has highest incidence and mortality rate and it is second most common genitourinary malignancy after prostate cancer among men,[5] In Pakistan, UBC is among the five most prevalent cancer.[6] UBC represents 4.7% of new cancer cases in 2017 and results in 2.7% of deaths among mortality caused by cancer.[7] Smoking is the most common risk factor in predisposing a person to bladder cancer and increase the risk by ten folds. It is estimated that 65% of the UBC cases in men are caused due to smoking and 25% in women.[8] The second most common risk factor is the occupation exposure to carcinogens.[9] It is estimated that 22% of all bladder cancer have occupational exposure to some carcinogens.[10] Genetic factors also play convincing role in increasing the susceptibility of cancer. Different studies have been carried out to understand the underlying genetic changes occurring in UBC to identify prognostic markers and molecular targets. Some of the major alternations in cell biology that are involved in UBC are self-sufficient growth signals, apoptosis, sustained angiogenesis, tissue invasion, and metastasis.[11] Interleukins (IL) are important inflammatory components that have a role in cell signaling and enhance the production of other cytokines. ILs are well studied for involvement in the progression of cancer as it favors angiogenesis and cell proliferation. The risk of bladder cancer can be modulated by studying genetic polymorphisms.[12] Moreover, the cytokine IL-17 is a good candidate to study because of its effects on metastasis and angiogenesis. IL-17A, a member of IL-17, is pro-inflammatory cytokine and is produced by T-helper (Th) cells. Main function of IL-17 is induction of immune signaling molecules. IL-17A has been found in providing microenvironment for tumor progression as it induces expression of vascular endothelial growth factor and IL-6. IL-6 further amplifies Th-17 differentiation and results in chronic inflammatory state that aids in tumor growth and metastasis.[13] IL-17A is produced by the IL17A gene. On chromosome 6p12, the rs2275913 single nucleotide polymorphism (SNP) is situated 2KB upstream of the IL17A gene. The IL-17A gene’s promoter region contains the rs2275913 SNP, and the A allele of this SNP is linked to the gene’s increased promoter activity. The rs2275913 SNP of the IL-17A gene is apparently linked to an increased risk of gastrointestinal cancer, gastric cancer, and cervical cancer in people with the AA genotype.[14] SNPs are biallelic point mutations that occur more frequently than 1% in a population. Since SNPs can affect gene expression or function depending on where they are located in the DNA sequence, they are also thought to be the main cause of variation in humans. SNPs are also regarded as one of the greatest biological markers in association or case-control studies since they are reasonably simple to detect. As a result, several SNPs in cytokine loci have been described and investigated in complicated disorders such cancer, autoimmune diseases, and infectious diseases.[15] Various studies demonstrate that a large number of disorders are strongly related with the rs2275913 SNP, which is created when the G nucleotide base in the IL-17A gene promoter is changed to an A nucleotide base 9, 25, 26,27,28,29. Allelic variations of the rs2275913 SNP have been shown to differentially bind the transcription factor NFAT, resulting in variations in IL-17A secretion.[16]
Association between bladder cancer and a number of polymorphism of IL-17A has been reported in various populations of the world. Association between rs2275913 of the promoter region with UBC has been found in Chinese population.[17] The rs2275913 polymorphism is situated in the 2 Kb upstream region of IL-17A gene on chromosome 6 and ancestral A allele is associated with higher promoter activity of IL-17A. The AA genotype is associated with high risk of digestive cancer in Japanese population and cervical cancer in Chinese population.[18] To further investigate the possible association of genetic variants in inflammatory pathways and disease susceptibility, the present study was designed to investigate promoter polymorphism rs2275913 in IL-17A in relation to bladder cancer in Pakistani population. The novelty of the current work is that various studies elaborate the association between bladder cancer and a number of polymorphism of IL-17A have been reported in various populations of the world and our results shows that SNP rs2275913 did not show any association with UBC in Pakistani population.
Materials and Methods
Study type and design
The current research under the title “Association Study of Promoter Region SNP rs2275913 of IL-17A with UBC in Pakistani Population” was conducted in COMSATS University, Islamabad. Samples of 10 uL of venous blood from UBC patients were collected. Moreover, samples were also obtained from human molecular genetics lab of COMSATS Islamabad.
Sampling techniques and sample size calculation
Patients with UBC were randomly selected from human molecular genetics laboratory of COMSATS Islamabad Pakistan from 2010 to 2018 and were histopathologically diagnosed. The medical information (types, grades, and TNM classification) of patients with UBC was obtained from histopathological reports and hospital records. All healthy volunteers were age-matched (1:2) to healthy subjects acquired from the general population. Whole blood (10 uL) was collected from each subject. We used a self-structured questionnaire to acquire along with information about personal demographics, detailed history, and UBC-related risk factors. Patients with other diseases hepatitis and those suffering from any malignancy/cancer were excluded. Without any prior history of major immune-, inflammatory-, or malignant diseases were also excluded from this study. The informed consent was obtained from all the study subjects before their enrolment while maintaining the confidentiality of the data/available record. The study included 127 bladder cancer patients and 100 age and gender matched controls. The sample size was calculated using OpenEpi (http://www.openepi.com/Menu/OE_Menu.htm) with 80% statistical power and 95% confidence intervals (95% CIs).
Ethics-related issues
The current research under the title “Association Study of Promoter Region SNP rs2275913 of IL-17A with UBC in Pakistani Population” was approved by the ethical committee of COMSATS University, Islamabad. Informed written consent was taken from the patients as well as controls recruited in this study. This case–control association study was conducted on 127 bladder cancer cases and 100 age and gender matched controls.
Primer designing
Primers for IL-17A polymorphism rs2275913 were designed using Primer3 tool(http://primer3.ut.e/). The sequence of the SNP was taken from NCBI FASTA sequence (https://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs = 2275913). Keeping primer size minimum of 19bp and maximum of 23bp and temperature kept was minimum of 50 °C and maximum of 60 °C. Forward Primers selected were 5′‘GCTCAGCTTCTAACAAGTAAG 3’ and the reverse primer selected was 5’ AAGAGCATCGCACGTTAGTG 3’. These primers were rechecked in in-silico polymerase chain reaction (PCR) of UCSC genome browser. The PCR product size was 338bp.
Restriction fragment length polymorphism (RFLP) designing
After designing primers, RFLP was designed using online NEB cutter tool (http://nc2.neb.com/NEBcutter2/). The enzyme EarI was selected because it had cutting site in the amplified PCR product. EarI has a recognition sequence CTCTTC. If there is type allele that is “G,” the enzyme will not cut sequence because the cutting site of the enzyme is abolished giving only one band of 338bp. Whereas, in case of variant type allele “A,” the enzyme will cut the amplicon producing two bands of 259bpb and 79bp.
DNA extraction
Genomic DNA was extracted from 2 mL blood by phenol-chloroform method. 2 mL of blood was taken in 15 mL falcon tubes and 6 mL of RBC Lysis buffer was added and mixed gently. It was centrifuged at 4400 rpm for 5 min at 25°C. Afterward, supernatant was discarded while retaining the pellet. The pellet was suspended in 2 mL RBC Lysis Buffer and centrifuged for 5 min at 25°C. Supernatant was discarded while retaining the pellet and 200 uL of Nuclear Lysis Buffer, 15 uL 20% SDS, 10 uL of proteinase K, and then 1.5 mL of TE buffer and 1.5 mL of phenol was added. Moreover, centrifuged for 4400 pm for 10 min at 4°C. The upper layer was separated in separate tubes with cut tips and after adding equal volume of chloroform isoamylalcohol,and centrifuged at 4400 rpm for 10 min at 4°C. The upper was again separated in separate tube and 1/10th volume of sodium acetate and equal volume of chilled isopropanol was added. And then, the tubes were centrifuged at 4400 rpm for 10 min at 4°C and supernatant was discarded. 1 mL of 70% ethanol was added and centrifuged again for 4400 rpm for 10 min at 4°C. The supernatant was again discarded 200 uL of DNase and RNase free water was added to dissolve DNA.
DNA quantification and PCR
A dilution from the stock DNA was prepared in concentration of 1:10 and 50 μL DNA concentrations was obtained. DNA was quantified by running on freshly prepared 1% gel at 70V for 2 h and the quantity was estimated by comparing with λ DNA.
PCR product was checked by loading 5 μL of the PCR product and 1.5 μL loading dye on 2% agarose gel along with 3 uL DNA ladder and was run at 100V for 45 min.
RFLP
Enzyme EarI was used to perform RFLP. 10 uL of PCR product was digested with 1.5 U EarI enzyme by mixing 1× of Tango buffer (50 Mm Potassium Acetate Potatium Acetate, 20 mM Triss Acetate, 10 Mm Magnesium Acetate, 100 ug/mL BSA) and was left at 37°C for 24 h at incubator. RFLP product was run on 2% gel for 40 min at 100V.
Statistical analysis
Quantitative variables in our case-control study are consistently given as mean standard deviation, while categorical variables are shown as absolute frequencies or percentages. Linear regression analysis was performed on the genotype data and allelic frequency data. To investigate the association, online statistical tool (http://statpages.info/ctab2 × 2.html) was used to calculate ratio of odds, and 0.05 and below P-value was considered significant.
Results
In the present study, 127 patients presenting UBC and 100 age- and gender-matched controls were recruited. The UBC patients were further bifurcated in low-grade non-muscle invasive and high-grade muscle invasive UBC patients. Among the controls, 76% had wild type homozygous genotype (GG), 22% had heterozygous genotype (GA), and 2% had homozygous variant genotype (AA). In case of UBC, 79.52% had wild type homozygous genotype (GG), 19.62% had heterozygous genotype (GA), and 0.79% had homozygous variant genotype (AA). Genotype frequency of UBC patients and control is also shown in Figure 1.
Figure 1.
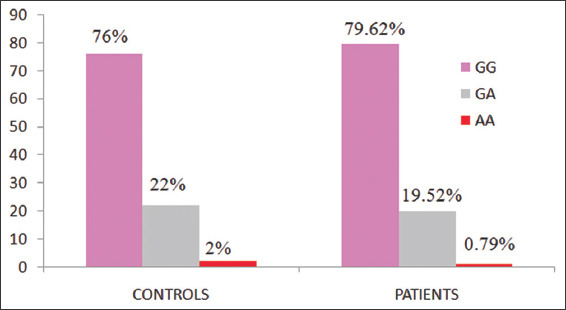
It shows the genotype frequency of UBC patients and control group
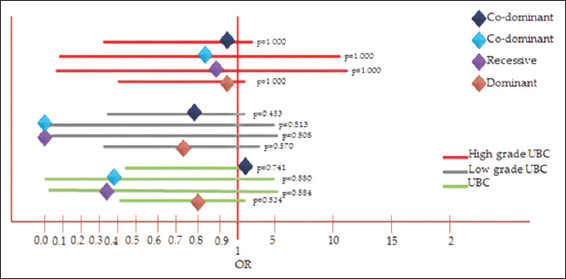
The linear regression analysis for the dominant model did not show any association (OR = 0.815, CI = 0.415–1.613, P = 0.524) of rs2275913 with UBC in Pakistani population. The recessive model also did not show any association (OR = 0.389, CI = 0.014-5.565, P = 0.584) of the SNP with the UBC in Pakistani population. The codominant model as well did not show any association (a) (GG vs. GA = OR = 0.376, CI = 0.013–5.420, P = 0.580) (b) (AA vs. GA = OR = 0.855, CI = 0.427–1.713, P = 0.741) of the rs2275913 with UBC in Pakistani population. Similarly linear regression analysis for allele frequency also did not show any association (OR = 0.796, CI = 0.432–1.467, P = 0.464) which is also shown in Table 1 and Figure 2.
Table 1.
Genotype frequency and allele frequency of rs2275913 among urinary bladder cancer patients and controls in Pakistani population
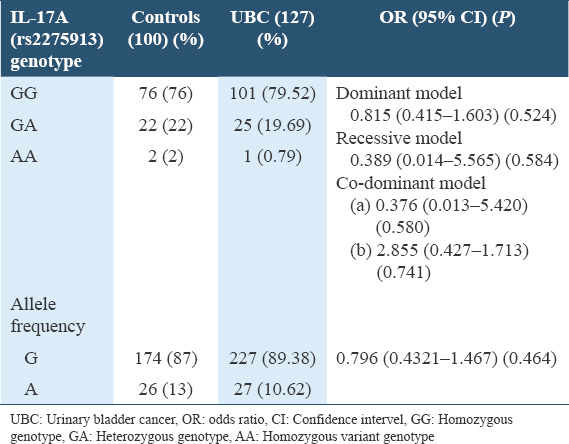
Figure 2.
Linear regression analysis for allele frequency of UBC patients
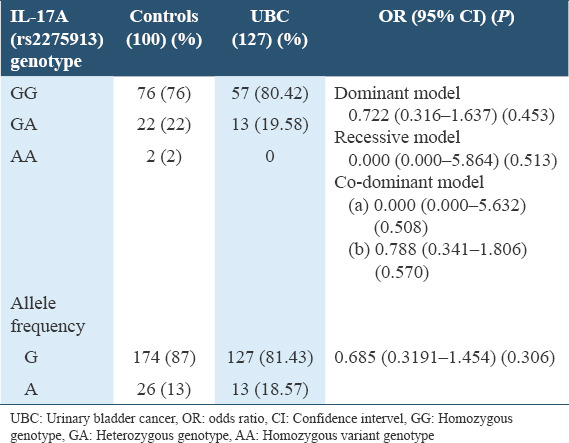
When the linear regression analysis was done separately for the bifurcated data, for low-grade non-muscle invasive UBC samples, the dominant model did not show any association (OR = 0.722, CI = 0.316–1.637, P = 0.453). Recessive model also did not show any association (OR = 0.000, CI = 0.000–5.864, P = 0.513). Codominant model of low-grade non-muscle invasive UBC samples (a) (GG vs. GA = OR = 0.864, CI = 0.030–12.668, P = 1.000) (b) (AA vs. GA = OR = 0.788, CI = 0.341–1.806, P = 0.570) did not show any association of rs2275913 with UBC in Pakistani population. Linear regression analysis of allele frequency of low-grade non-muscle invasive UBC samples also did not show any relationship (OR = 0.685, CI = 0.319–1.454, P = 0.306) of the SNP rs2275913 with the UBC in Pakistani population which is shown Table 2.
Table 2.
Genotype and allelic frequency distribution of rs2275913 among low grade non muscle invasive urinary bladder cancer patients and controls in Pakistani population
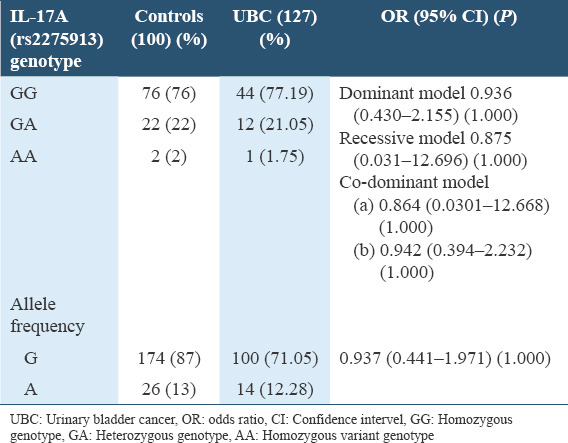
For high-grade muscle invasive UBC, dominant model did not show any association (OR = 0.936, CI = 0.403–2.155, P = 1.000), recessive model also did not show any association (OR = 0.875, CI = 0.031–12.696, P = 1.0000). Codominant model of high-grade muscle invasive UBC samples a) (GG vs. GA = OR = 0.864, CI = 0.030–12.668, P = 1.000) and b) (AA vs. GA = OR = 0.942, CI = 0.394–2.232, P = 0.1000) did not show association which us shown in Table 3.
Table 3.
Genotype and allelic frequency distribution of rs2275913 among high grade non muscle invasive urinary bladder cancer patients and controls in Pakistani population
Discussion
UBC is a type of cancer that arises from urinary bladder tissue. Other than environmental factors, genetic factors also play considerable role in increasing the predisposition of cancer. Genetic factors pathways studied in relation to cancer include apoptosis, metastasis, angiogenesis, and inflammation. Links between cancer and inflammation were first revealed in 19th century, based on the evidence that tumors often arises at the site of chronic inflammation and inflammatory cells were found in the biopsies samples of cancer patients. Inflammatory cells including chemokines and cytokines were found in the microenvironment of all tumors of animal models.[19] IL-17 plays an important role in the pathogenesis of inflammation and is found to be elevated in many types of cancer, one of which in UBC.[20] IL-17A SNP rs2275913 is located in the promoter region and it promotes high production of IL-17 and as a result IL-17A mediated immune response is unregulated in the form of over production of inflammatory chemokines, cytokines, and favors the growth of tumor by angiogenesis.[21] Association between IL-17 SNPs and risk for UBC has been widely studied and IL-17A SNP rs2275913 variant homozygous AA was found significantly higher in UBC patients than controls in Chinese population. According to the studies, rs2275913 was significantly associated with high risk of UBC in Chinese population but was not found associated with high risk in Caucasians population.[22] Similarly, significant association was found between cervical cancer risk and rs2275913 in Chinese population but showed decreased risk in colorectal cancer.[22] One study reported that IL-17A rs2275913 polymorphism was significantly associated with the increased of gastric cancer;[23] however, in another study, it was reported that the rs763780 polymorphism shows association with gastric cancer, whereas the rs2275913 polymorphism did not show any association with gastric cancer.[24] Our study did not shows any association of promoter region SNP rs2275913 in IL-17A in Pakistani population. Linear regression analysis was applied to the data obtained. Results revealed that rs2275913 did not show any associated with the high risk of UBC. When subgroups analysis was performed, no association was found between rs2275913 and risk of low-grade and high-grade UBC in Pakistani population. The rs2275913 polymorphism is located at the promoter region of the IL-17A gene and the presence of allele “A” changes gene transcription and results in over production of IL- 17A which in turns provides microenvironment for the cancer. As the allele frequency of allele A (https://www.pharmgkb.org/variant/PA166157030) is found to very less (0.049) and this could be one of the reasons that rs2275913 did not show any role in the susceptibility and progression of UBC in Pakistani population. Despite our efforts to find association between genetic risk factors and UBC, some limitations of the studies could not be ignored. First, the samples size (127) was very small to be used for association studies. It is possible that increase in the sample size could authenticate our results. Second, this analysis was designed based on literatures which were mostly based on relationship of IL-17A SNP rs2275913 with other populations whereas, detailed information on UBC and role of inflammation was not available which further limited the present study. Despite these limitations, it could be concluded from the results that SNP rs2275913 did not show any association with UBC in Pakistani population. Further studies are required to exclude the mentioned limitations and validate the results.
Authors’ Declaration Statements
Ethical approval and patient’s consent
This study was approved by the ethical committee of COMSATS University, Islamabad. Written informed consents were taken from all participants.
Consent for publication
None.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Competing Interest
None of the authors of this study have disclosed any conflicts of interest.
Funding Statement
None.
Authors’ Contribution
Dr.Humaira Ayub supervised the project. Maryam Anwar, Muhammad Ejaz, Muhammad Ijaz and Gulzar Ahmad perform and analyze the experiments; all the authors have written the manuscript, reviewed the manuscript and agreed to submit it.
Acknowledgment
I would like to thank the Institute of Integrative Biosciences, Cecos University Peshawar and COMSATS University Islamabad for providing us with its facility and all the research materials. I also want to express my gratitude to all of my fellows, whose support allowed us to accomplish this study.
References
- 1.Chu FM, Dmochowski R. Pathophysiology of overactive bladder. Am J Med. 2006;119:3–8. doi: 10.1016/j.amjmed.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Yousef PG, Gabril MY. An update on the molecular pathology of urinary bladder tumors. Pathol Res Pract. 2018;214:1–6. doi: 10.1016/j.prp.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends-an updateglobal cancer rates and trends-an update. Cancer Epidemiol Biomarkers Prevent. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 4.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality:A global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 6.Sarwar MR, Saqib A. Cancer prevalence, incidence and mortality rates in Pakistan in 2012. Cogent Med. 2017;4:1288773. [Google Scholar]
- 7.Chou R, Dana T. Screening adults for bladder cancer:A review of the evidence for the U. S. Preventive services task force. Ann Int Med. 2010;153:461–8. doi: 10.7326/0003-4819-153-7-201010050-00009. [DOI] [PubMed] [Google Scholar]
- 8.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737–45. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiriluk KJ, Prasad SM, Patel AR, Steinberg GD, Smith ND. Bladder cancer risk from occupational and environmental exposures. Urol Oncol. 2012;30:199–211. doi: 10.1016/j.urolonc.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Colt JS, Friesen MC, Stewart PA, Donguk P, Johnson A, Schwenn M, et al. A case-control study of occupational exposure to metalworking fluids and bladder cancer risk among men. Occup Environ Med. 2014;71:667–74. doi: 10.1136/oemed-2013-102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netto GJ, Cheng L. In:Molecular Surgical Pathology. Germany: Springer; 2013. Molecular pathology of urinary bladder cancer; pp. 229–53. [Google Scholar]
- 12.Ferlito A, Shaha AR, Buckley JG, Caruso G, Rinaldo A. Metastatic cervical lymph nodes from urogenital tract carcinoma:A diagnostic and therapeutic challenge. Acta Otolaryngol. 2001;121:556–64. [PubMed] [Google Scholar]
- 13.Gakis G. The role of inflammation in bladder cancer. Adv Exp Med Biol. 2014;816:183–96. doi: 10.1007/978-3-0348-0837-8_8. [DOI] [PubMed] [Google Scholar]
- 14.Ohka S, Nishizawa D, Hasegawa J, Takahashi K, Nakayama K, Ebata Y, et al. Association between rs2275913 single-nucleotide polymorphism of the interleukin-17A gene and perioperative analgesic use in cosmetic orthognathic surgery. Neuropsychopharmacol Rep. 2018;38:67–74. doi: 10.1002/npr2.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolandelli A, del Pino RE, Pellegrini JM, Tateosian NL, Amiano NO, de La Barrera S, et al. The IL-17A rs2275913 single nucleotide polymorphism is associated with protection to tuberculosis but related to higher disease severity in Argentina. Sci Rep. 2017;7:40666. doi: 10.1038/srep40666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espinoza JL, Takami A, Nakata K, Onizuka M, Kawase T, Akiyama H, et al. A genetic variant in the IL-17 promoter is functionally associated with acute graft-versus-host disease after unrelated bone marrow transplantation. PLoS One. 2011;6:e26229. doi: 10.1371/journal.pone.0026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Liu Y, Xie L, Li S, Qin X. Association of IL-17A and IL-17F gene polymorphisms with chronic hepatitis B and hepatitis B virus-related liver cirrhosis in a Chinese population:A case-control study. Clin Res Hepatol Gastroenterol. 2016;40:288–96. doi: 10.1016/j.clinre.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Pandey S, Mittal B, Srivastava M, Singh S, Srivastava K, Lal P, et al. Evaluation of Toll-like receptors 3 (c. 1377C/T) and 9 (G2848A) gene polymorphisms in cervical cancer susceptibility. Mole Biol Rep. 2011;38:4715–21. doi: 10.1007/s11033-010-0607-z. [DOI] [PubMed] [Google Scholar]
- 19.Lyman GH, Giuliano AE, Somerfield MR, Benson AB, 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer:Summary of the 2013 guidelines. Eur Urol. 2014;65:778–92. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka S, Terasaki M, Hayashi N, Oka S, Chayama K. Warning for unprincipled colorectal endoscopic submucosal dissection:Accurate diagnosis and reasonable treatment strategy. Dig Endosc. 2013;25:107–16. doi: 10.1111/den.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He J, Chan-Yeung M, Becker A, Dimich-Ward H, Ferguson A, Manfreda J, et al. Genetic variants of the IL13 and IL4 genes and atopic diseases in at-risk children. Genes Immun. 2003;4:385–9. doi: 10.1038/sj.gene.6363985. [DOI] [PubMed] [Google Scholar]
- 23.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long ZW, Yu HM, Wang YN, Liu D, Chen YZ, Zhao YX, et al. Association of IL-17 polymorphisms with gastric cancer risk in Asian populations. World J Gastroenterol. 2015;21:5707–18. doi: 10.3748/wjg.v21.i18.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


