Abstract
In a previous study, we showed that Staphylococcus aureus supernate (SaS) is a potent agonist for both neutrophils and mononuclear cells. To further investigate the immunomodulating effects of SaS, the effect on different neutrophil receptors was studied. Expression of various neutrophil receptors, before and after treatment with SaS, was quantified by flow cytometry. We found that SaS treatment of neutrophils resulted in a specific and total downregulation of the C5a and the fMLP receptor, both serpentine receptors, while other receptors were totally unaffected. Since these two receptors are both involved in chemotaxis, we tested the effect of SaS in calcium flux and chemotaxis assays. We showed that preincubation with SaS abrogated the rise in intracellular calcium concentration upon triggering with fMLP and C5a. We also showed that SaS is a potent inhibitor of neutrophil chemotaxis towards fMLP and C5a, but does not interfere with chemotaxis towards interleukin-8. These findings indicate that S. aureus produces a virulence factor extracellularly, which impairs chemotaxis towards the infected site.
Staphylococcus aureus is one of the most common causes of bacterial infections in humans and animals. It produces serious wound infections, and in the hospital, it is one of the most important causes of nosocomial infections. S. aureus contains a variety of pathogenic factors that play a role in the disease processes and interfere with host defense. Cell wall components, such as protein A and peptidoglycan, are directly able to hamper humoral and cellular defenses. Protein A is capable of binding specifically to the Fc portion of the immunoglobulin G (IgG) molecule. Once bound to IgG, protein A may sterically block the interaction of IgG with its Fc receptor on phagocytic cells (16). Musher et al. described that both phagocytosis and chemotaxis were suppressed by prior preincubation of neutrophils with 2.5 μg of peptidoglycan of S. aureus/ml (14). The suppression of neutrophil function was independent of the presence of human serum but was abolished by rabbit antiserum to peptidoglycan. Cell wall components of staphylococci are also able to stimulate phagocytes. Peptidoglycan, teichoic acid, and lipoteichoic acid induce the release of cytokines by monocytes as previously described by several authors (1, 5, 9, 11, 12, 20).
S. aureus produces a variety of extracellular products, including cytolytic toxins that affect phagocytes as well as other cells. Most of the staphylococcal exoproducts are synthesized at the end of the logarithmic growth phase and are under the control of regulatory systems. The enterotoxins and toxic shock syndrome toxin 1 are superantigens which activate a subset of T cells by binding to major histocompatibility complex class II molecules on antigen-presenting cells and cross-linking to the T-cell receptor (10, 13). Proinflammatory cytokines are produced by T cells and monocytes following superantigen activation. Panton-Valentine leukocidin is leukotoxic for human and rabbit neutrophils and is able to induce the release of histamine, leukotriene B4, interleukin-8 (IL-8), and oxygen metabolites from human granulocytes. Panton-Valentine leukocidin-producing strains are mostly associated with infections as furuncles and abscesses as well as with severe pyodermic infections, such as dermonecrosis (9).
Delta toxin affects neutrophil leukotriene metabolism and, in conjunction with endotoxin, primes the oxidative burst activity (19). We recently described that additional products in staphylococcal culture supernates (SaS) stimulate phagocytes in whole blood generating tumor necrosis factor and IL-1β. We also showed that the cell-free supernate of S. aureus is able to upregulate CR3 expression on neutrophils, which was not caused by any of the known extracellular products (23).
The aim of this study was to further investigate the influence of staphylococcal cell-free supernates and their effects on neutrophil function. Since staphylococcal infections are often localized infections, like furunculosis, wound infection, arthritis, and endocarditis, we were particularly interested in the effect of extracellular products that interact with migration of neutrophil, a key event of the inflammatory response.
MATERIALS AND METHODS
Materials.
N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP) and recombinant C5a were purchased from Sigma Chemical Co., St. Louis, Mo. 4,4-Difluoro-4-bora-3a,4a-diaza-S-indacene (BODIPY)-labeled fMLP (BODIPY- N-formyl-Nle-Leu-Phe-Nle-Tyr-Lys), BCECF-AM [acetoxymethyl ester of bis-(2-carboxyethyl-5-(and-6)-carboxyfluorescein], and Fluo3-AM were from Molecular Probes (Eugene, Oreg.) Recombinant IL-8 was purchased from Pepro Techno Inc. (Rockey Hill, N.J.). Platelet-activating factor 16 (PAF-16) and ionomycin were purchased from Calbiogem-Novabiochem (San Diego, Calif.).
Monoclonal antibodies.
The following monoclonal antibodies (MAbs) were used: 44a (anti-CD11b; CR3), 541 (anti-CD35; CR1), 60bca (anti-CD14), TS-1/22 (anti-CD11a), IB4 (anti-CD18), IV.3 (anti-CD32; FcRII), and W6/32 (anti-HLA class I) were obtained from the American Type Culture Collection as hybridoma cell lines, and the produced antibodies were purified over protein G-Sepharose (Pharmacia, Uppsala, Sweden); leu8-PE (anti-CD62L; l-selectin) and leuM5-PE (anti-CD11c) were from Becton Dickinson (San Jose, Calif.), and CLB-gran/1 (anti-CD16; FcRIII), CLB-gran/12 (anti-CD63), and CLB-gran/10 (anti-CD66) were from CLB (Amsterdam, The Netherlands). S 5/1 (anti-CD88; C5a receptor) was a gift from Otto Götze (University Göttingen, Göttingen, Germany), and SE-2 (anti-CD128w; I1-8R, Type A) and an anti-PAF receptor were from Alexis Corporation (San Diego, Calif.). Since no MAb for the fMLP receptor was available, the expression of this receptor was determined by binding of fluorescence-labeled fMLP. The secondary flourescein isothiocyanate (FITC)-labeled goat F(ab′)2 anti-mouse Ig was from Dako (Glostrup, Denmark).
SaS.
S. aureus 1690 (a clinical isolate from a patient with a staphylococcal bacteremia) was cultured overnight at 37°C in Iscove's modified Dulbecco's medium (IMDM) synthetic medium (Life Technologies, Breda, The Netherlands), diluted 40-fold in fresh IMDM, and cultured for another 7 h under constant agitation. Bacteria were pelleted and the cell-free supernate was passed over a 0.2-μm-pore-size filter to remove residual bacteria. The supernate was dialyzed against phosphate-buffered saline (PBS) by using a dialysis membrane with a 10-kDa cutoff. After dialysis, SaS was aliquoted and stored at −20°C.
Cells.
Blood obtained from healthy human volunteers was collected into tubes containing sodium heparin (Vacuette, Greiner, Alphen a/d Rijn, The Netherlands) as anticoagulants. Neutrophils were isolated as described by Troelstra et al. (22). For the isolation, heparinized blood was diluted 1:1 (vol/vol) with pyrogen-free PBS and layered onto a gradient of Ficoll (Pharmacia) and Histopaque (density, 1.119 g/ml; Sigma). After centrifugation for 20 min at 320 × g, the neutrophils were collected from the Histopaque phase. Cells were subjected to a brief hypotonic shock with water, washed, and suspended at 5 × 106 cells/ml in RPMI medium containing 0.05% human serum albumin (HSA) (CLB).
Receptor expression.
A sample of 50 μl of either isolated neutrophils (5 × 106 cells/ml) or whole heparinized blood was mixed with different dilutions of SaS in a 1:1 (vol/vol) ratio and incubated for 30 min at 37°C under constant agitation. Samples were put on ice to terminate the interaction of SaS with the cells, and subsequently, the samples were incubated for 30 min on ice with 5 μl of MAbs at optimal concentration or 10 μM BODIPY-fMLP. Thereafter, whole-blood samples were treated for 5 min with flourescent-activated cell sorter (FACS) lysis buffer (Becton Dickinson), centrifuged, and washed once with RPMI-HSA, while isolated neutrophils were washed twice with buffer. Samples were either directly fixed with 0.5% paraformaldehyde in PBS or incubated with F(ab′)2 FITC-labeled goat anti-mouse-Ig for another 30 min on ice. These samples were washed once more and also fixed with 0.5% paraformaldehyde. Controls of unlabeled cells and cells with only the secondary FITC conjugate were included. All samples were analyzed on a FACScan flow cytometer (Becton Dickinson), and the mean fluorescence was determined from 5,000 neutrophils after proper gating of the cells by forward and sideward scatter parameters. The expression of the different antigens was expressed relative to that of cells incubated in control buffer.
Measurement of calcium fluxes.
Cells were loaded with 2 μM Fluo-3-AM in RPMI-HSA for 20 min at 37°C under agitation, washed twice with buffer, and suspended to 106 cells/ml in RPMI-HSA. Preliminary experiments were done to determine the optimal concentrations of the various chemoattractants for an adequate calcium flux. Chemokine-induced calcium fluxes are very rapid and transient, and therefore, from each sample, repetitive measurements of 2,000 events that required an average time of 10 s for sampling were done and saved to disk before the next acquisition. Each sample of 500 μl of cells was first measured for their fluorescence (at 530 nm) to determine the basal calcium level. Next, 5 μl of 100-fold-concentrated reagent was added while vortexing and quickly placed in the sample holder to start the timed series of measurements. For receptor desensitization, the labeled neutrophils were first incubated for 10 min at room temperature with SaS, fMLP, C5a, or buffer and subsequently stimulated with agonist. Samples were analyzed after gating the neutrophil population, thereby excluding cell debris and background noises. The calcium flux response of the cells for each time point was expressed as a relative value compared to the basal unstimulated level of that individual sample, and no conversions to actual calcium concentrations were done.
Chemotaxis.
Chemotaxis of neutrophils towards chemoattractants like C5a, fMLP, and IL-8 was determined by using fluorescent labeled neutrophils that migrated through a membrane fitted into an insert Transwell system (Costar) containing a prewetted 3-μm-pore-size polycarbonate filter. Therefore 5 × 106 neutrophils were first labeled with 3.3 μM BCECF-AM for 20 min at room temperature, washed twice, and resuspended in RPMI-HSA. Labeled neutrophils were incubated with 10% (vol/vol) SaS or RPMI for 30 min at 37°C on a shaker (400 rpm). After incubation, the cells were washed once and suspended in Hanks balanced salt solution (HBSS)–1% HSA at 2.5 × 106 cells/ml. The upper compartment of the Transwell system was filled with 100 μl of cells and placed into a 24-well microtiter plate containing 600 μl of control buffer or chemoattractant (fMLP, 10−7 M; C5a, 1 ng/ml; IL-8, 10−8 M). After incubation in a 37°C, 5% CO2 incubator for 30 min, the Transwell inlays were removed and the fluorescence of the wells was read in the CytofluorII multiwell fluorometer (PerSeptive Biosystems, Framingham, Mass.) with an excitation, (485 nm) and emission (530 nm) filter. Control wells of only BCECF-labeled cells were included for the maximal fluorescence value (100%). The fluorescence values of the samples were expressed relative to the total value, yielding the percentage of cells that migrated across the membrane. The fluorescence signal is linear with cell number as determined by serial dilution of the BCECF-labeled cells with a detection limit of 2,500 cells (equals 1% of the added amount). During the incubation, there was no loss of fluorescent label from the cells.
RESULTS
Receptor profile of neutrophils.
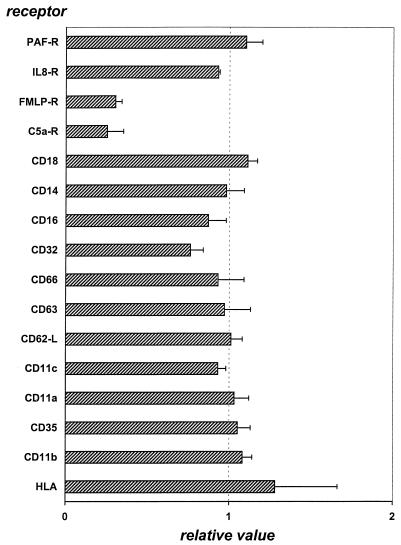
To study the dynamics and conditions for neutrophil receptor modulation by SaS, we used neutrophils isolated by standard Histopaque-Ficoll procedure. A panel of specific MAbs was used to determine the neutrophil receptor profile after incubation with 10% SaS. This panel contained antibodies against receptors which are involved in immunomodulating processes, like the complement receptors (CR1, CR3, and C5aR), the Fcγ receptors II and III (CD32 and CD16), receptors for adhesion molecules like l-selectin (CD62L) and ICAM (CD11a), phagocyte chemoattractant receptors for fMLP, PAF, C5a (CD88), and IL-8 (CD128), and some other receptors which serve as activation markers for inflammation (CD63, CD66b, HLA class1). For simplicity, we will refer to binding of labeled ligand or labeled antibody to its receptor as “receptor expression” hereafter. The expression of most of the receptors tested did not change after SaS treatment except for the expression of the chemokine receptors for fMLP and C5a (CD88) (Fig. 1). The expression of receptors for the chemotactic agents IL-8 and PAF showed no change after incubation with SaS. Previously, we showed that SaS upregulates CR3 (CD11b) on neutrophils in heparinized whole blood. In isolated cells, we did not observe an upregulation of CR3. When whole blood was incubated with 10% (vol/vol) SaS and subsequently analyzed for neutrophil chemokine receptor profile, only the C5a receptor (CD88) showed a 60% reduction in expression, while the fMLP and IL-8 (CD128w) receptors were not changed (not shown).
FIG. 1.
Receptor expression on neutrophils after 30 min of incubation with 10% (vol/vol) SaS. Data are expressed as relative values compared to those of control cells incubated with buffer. The data represent the means and standard errors of the means of five separate experiments.
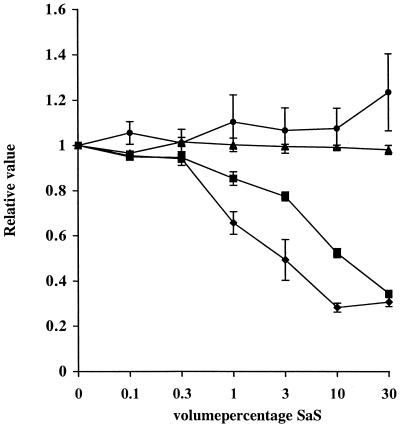
The reduced expression of the receptors for C5a and fMLP on isolated neutrophils was studied in more detail. Neutrophils were incubated with various concentrations of SaS and analyzed for receptor expression. The expression of both the C5a and fMLP receptors was decreased in a concentration-dependent matter (Fig. 2). The fMLP receptor expressed was more sensitive and required only 3% SaS for a 50% reduction. Addition of neither phenylmethylsulfonyl fluoride nor EDTA to SaS changed the receptor expression profile for fMLP, C5a, or IL-8 (not shown). The reduction of fMLP receptor expression was not affected by heating the supernate for 30 min at 56 or 100°C. Treatment of the supernate with trypsin or proteinase K (1 mg/ml) did abolish the effect of SaS on fMLP receptor expression (not shown). Time course experiments showed almost immediate effects of SaS on the receptor expression. SaS was not toxic for the cells, as indicated by the lack of change of other receptors. In addition, viability was directly determined with propidium iodide and trypan blue dye exclusion by flow cytometry and microscopy, respectively. Viability of the cells incubated with 50% SaS for 60 min was not affected as compared with control cells and was >95%.
FIG. 2.
Chemokine receptor expression (●, PAF-R; ▴, IL-8-R; ▪,C5a-R; ♦, fMLP-R) on neutrophils after 30 min of incubation with different concentrations (vol/vol) of SaS. Data are expressed as relative values compared to data of controls when neutrophils were not incubated with SaS. The data represent the means and standard errors of the means of three separate experiments.
Thirteen other blood isolates of S. aureus were tested to see if their supernates were able to downregulate the fMLP receptor on neutrophils as well. About one-third of these strains showed a decreased expression of the fMLP receptor on neutrophils after preincubation with these supernates.
Functional consequences of receptor downmodulation. (i) Calcium flux.
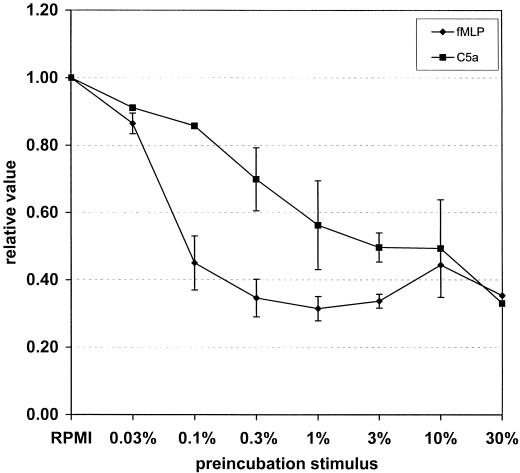
Chemokine receptors are G-protein coupled and induce a rapid and transient calcium flux in neutrophils when stimulated. Firstly, a direct calcium flux by SaS was observed with a concentration of 10% (vol/vol) or higher, which was rapid and back to baseline within 2 min. The maximal response was somewhat lower compared to an optimal dose of fMLP (10−7 M) or C5a (1 ng/ml). Next, cells were incubated with different amounts of SaS for 10 min at room temperature, after which the calcium levels were recuperated to initial basal levels again. These treated cells were subsequently triggered with an optimal concentration chemoattractant and measured over time to determine the calcium fluxes. Buffer-pretreated cells were run in parallel. SaS concentration dependently prevented the fMLP- and C5a-induced calcium fluxes (Fig. 3). These results are in agreement with the previous data concerning receptor expression.
FIG. 3.
Calcium flux in neutrophils stimulated with either 10−7 M fMLP (♦) or 1 ng of C5a per ml (▪) after preincubation for l0 min with different concentrations of SaS. Data are expressed as relative values compared to data of control cells incubated with buffer only.
(ii) Chemotaxis in vitro.
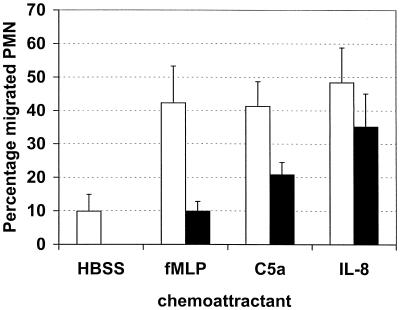
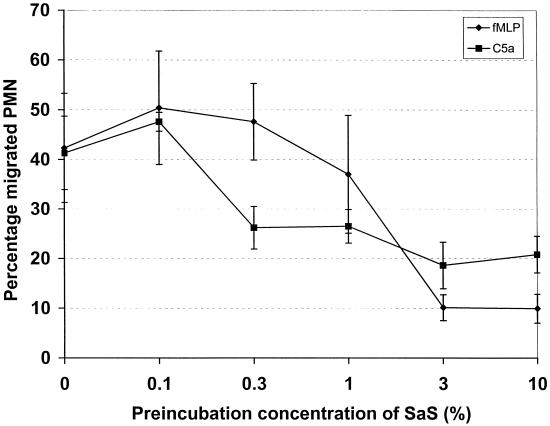
A decrease in receptor expression as measured by fluorescent-antibody binding could be limited to a loss of only the epitope recognized by that specific antibody. That can be the result of masking or conformation change of that specific epitope or loss of the complete antigen. The fMLP receptor expression was actually determined by binding of its (fluorescent) ligand, thereby indicating that its function was affected. Therefore, we not only determined the expression of the receptors but also looked at whether the incubation of the cells with SaS would have consequences for the chemotactic ability of the neutrophils. Mediated C5a chemotaxis was measured by the under-agarose technique and was diminished by SaS in a dose-dependent fashion (not shown). In the Transwell system assay, the chemotactic response of fluorescent-labeled neutrophils incubated with SaS towards fMLP and C5a was abolished, while chemotaxis towards IL-8 was not altered (Fig. 4). These results are in agreement with the data on receptor expression and in addition rule out nonspecific effects of SaS on the neutrophils. In parallel with receptor downmodulation and calcium flux, SaS concentration dependently diminished chemotaxis towards fMLP and C5a (Fig. 5). SaS itself in the lower compartment was slightly chemotactic for neutrophils, but only at a 100% concentration.
FIG. 4.
Chemotaxis of neutrophils towards 10−7 M fMLP, 1 ng of C5a/ml, and 10−8 M IL-8 in a Transwell system after preincubation for 30 min with 10% (vol/vol) SaS (black bars) or only with buffer (white bars). Data are expressed as the percentage of migrated neutrophils off cells added to the upper compartment. The data represent the means and standard errors of the mean of three separate experiments.
FIG. 5.
Concentration-dependent inhibition of chemotaxis of neutrophils towards 10−7 M fMLP (♦) and 1 ng of C5a/ml (▪) in a Transwell system after preincubation for 30 min with different concentrations of SaS. Data are expressed as the percentage of migrated neutrophils off cells added to the upper compartment. The data represent the means and standard errors of the means of three separate experiments.
DISCUSSION
Directed migration of neutrophils to a focus of infection is an important step in the eradication of invading bacteria by phagocytosis and intracellular killing. A variety of chemoattractants are known to react with specific receptors on phagocytes. Ligation of different chemokine receptors not only initiates chemotaxis but also activates or primes other functions of phagocytes.
Several chemoattractants are known to induce directed migration of neutrophils, including lipid mediators like PAF and leukotriene B4, formylated peptides, activated complement components C3a and C5a, and the chemokines IL-8, NAP, and GRO. Chemoattractants can originate from the bacteria themselves, such as the formylated proteins. Rot et al. purified an fMLP-like tetrapeptide with high chemotactic potency from culture fluids of S. aureus (17). Also, bacteria can indirectly generate chemoattractants either via activation of complement resulting in complement fragments with chemotactic activity, such as C3a and C5a, or via activation of cells which in turn produce chemokines. Yao et al. described IL-8 mRNA and protein expression in S. aureus-infected endothelial cells (25).
These chemoattractants react with specific receptors that all belong to the family of seven transmembrane “serpentine” receptors that are coupled to G proteins for signaling. The formylated proteins, exemplified by the synthetic tripeptide fMLP, are long recognized as bacterial derived products that induce neutrophil migration by binding to a specific receptor, the fMLP-R. Ligation of fMLP-R not only induces migration but also triggers the generation of oxygen radicals and causes degranulation (24). In turn, it initiates the production of lipid mediators like PAF and LTB4 that are chemoattractants for neutrophils themselves.
We describe here that the supernate of growing S. aureus contains a factor(s) that specifically reduce(s) binding of fMLP and αC5a to their receptors on neutrophils, resulting in a strongly attenuated chemotaxis. Staphylococci release several products into the extracellular environment that potently modulate immune cells, including neutrophils. As described for several bacteria, low-molecular-weight formylated proteins (or peptides) that have chemotactic potency are found in the supernate of S. aureus. Because fMLP is an efficient agonist for neutrophil activation, screening of bacterial supernates for neutrophil modulation was performed with SaS devoid of low-molecular-weight components by prior dialysis. Incubation of isolated neutrophils with the dialyzed SaS resulted in a strong attenuation of the expression of receptors for fMLP and C5a. The phenotype of cells incubated with SaS did not resemble that of activated cells, meaning that CD11b expression was not upregulated and no concurrent shedding of l-selectin was observed. In general, activation of neutrophils by many agonists leads to a change in these two receptors, as shown, for instance, for tumor necrosis factor or endotoxin. Moreover, the expression of neutrophil activation markers like CD66 and CD63 was also not changed. Direct toxic effects were not likely, since viability of cells treated for 1 h with 50% (vol/vol) SaS was not affected. Thus, SaS induces specific downmodulation of fMLP and C5a receptor expression, both receptors involved in neutrophil migration. SaS did not change the expression of two other chemoattractant receptors on neutrophils, the PAF-R and IL-8-R, as measured with specific MAbs. The fMLP receptor was more sensitive to SaS treatment than the C5a receptor, but for both molecules, expression was dose dependent.
In whole blood, the effect of SaS on fMLP and C5a receptor expression was less pronounced, probably due to neutralizing components in plasma. In vivo migration takes place after leaving the bloodstream, and staphylococcal infections are mainly located in tissues. Here, concentrations of plasma components are far lower.
Because binding of fluorescent ligand, BODIPY-fMLP, monitored expression of the fMLP receptor, we anticipated that the functional response of the treated neutrophils would also be diminished. SaS efficiently attenuated chemotaxis of neutrophils in a modified Boyden system through a 3-μm-pore-size polycarbonate membrane. The dose response for diminished chemotaxis correlated well with receptor expression as measured by flow cytometry. For the screening of C5a receptor expression, an MAb that binds to an epitope on the N terminus of the C5aR that blocks C5a binding and C5a-mediated activation was used. Therefore, a diminished binding of this antibody to the cells is indicative of a diminished reactivity of the receptor for C5a. Treatment of cells with SaS also concomitantly attenuated the chemotaxis towards recombinant C5a in a dose-dependent way. Directed migration towards IL-8 was not affected in cells treated with SaS, indicating that the effects of SaS were highly specific. In addition, this shows that cells do respond normally to other agonists and are not paralyzed in their locomotory machinery by possible toxic compounds present in SaS.
The family of chemokine receptors that includes the fMLP-R, C5aR, and IL-8-R (CXCR1 and CXCR2) are G protein coupled and are characterized by a rapid and transient rise in intracellular calcium concentration ([Ca]i) upon triggering. Incubation of neutrophils with SaS up to a concentration of 10% initiated a small but significant rise in [Ca]i. Pretreatment of cells with SaS and subsequent triggering with an optimal concentration of fMLP or C5a resulted in a dose-dependent abrogation of the rise in [Ca]i. The calcium response of cells to IL-8 was not affected by SaS. Chemokine receptors show the phenomenon of receptor desensitization that can be the result of both homologous and heterologous ligands. It is known that the receptors for fMLP and C5a show mutual cross-desensitization as measured by ligand binding, receptor recycling, and [Ca]i. Tomhave et al. described efficient receptor cross-desensitization that was restricted between several protein chemokine receptors as well as restricted between lipid chemokine receptors. No cross-talk between protein and lipid receptors was shown (21). In that study, it was also shown that triggering the fMLP or C5a receptor also effectively blunted the response to IL-8 and vice versa. Since SaS did not affect the neutrophil response to IL-8, in neither receptor expression, chemotaxis, nor [Ca]i, it is unlikely that SaS acts directly as an agonist of either the fMLP or C5a receptor. Therefore, we believe that in the supernate of S. aureus, one or more products may have an antagonistic effect on both the fMLP and C5a receptors and do not affect the IL-8 receptor on human neutrophils. This results in an abrogated chemotactic response of the cells towards a focus of S. aureus.
Modulation of the chemotactic response of neutrophils has been described for several microbial products. Some important studies in this area include the study of Rozdzinski et al., who found that pertussis toxin subunits S2 and S3 of Bordetella pertussis share amino acid sequence similarity with the lectin domains of the eukaryotic selectin family and competitively inhibited adherence of neutrophils to endothelial cells in vitro. Intravenous administration of these peptides to animals with meningitis disrupted recruitment of leukocytes into the cerebrospinal fluid (18). The pertussis toxin itself is known to inhibit G-protein-linked receptors like the serpentine receptors by ADP-ribosylation of the G protein (8, 15). Porphyromonas gingivalis produces a proteinase which is able to cleave the C5a receptor on neutrophils, thereby inhibiting the chemotactic function towards them (7). Other microorganisms prevent neutrophil influx by interfering with the chemoattractants directly, like most strains of group B streptococci, which elaborate a cell surface-associated C5a-ase that rapidly inactivates the human complement-derived chemoattractants C5a and C5adesarg (2, 6). Glucuronoxylomannan (GXM) of Cryptococcus neoformans has been postulated by several authors to prevent neutrophil migration towards chemokines like fMLP, C5a, and IL-8 (3, 4). Lipovsky et al. suggested that cross-desensitization of the receptor might be the reason for the inhibition of neutrophil migration towards IL-8 in the cerebrospinal fluid of infected brain (M. M. Lipovsky, L. J. van Elden, A. M. Walenkamp, J. Dankert, and A. I. Hoepelman, Letter, J. Infect. Dis. 178:1231–1232, 1998).
The nature of the factor(s) in the staphylococcal supernate responsible for the interference with the fMLP and C5a receptor is subject to further study. Since addition of trypsin as well as proteinase K abolished the effect of SaS on fMLP receptor expression, the responsible factor in SaS is probably a protein which is heat stable. One could speculate that it might be a protease cleaving off (a part of) the receptor, but we blocked serine protease and metalloprotease activity by adding, respectively, phenylmethyl sulfonyl fluoride and EDTA to SaS in the receptor expression assay without changing the receptor downregulation. Of course, these experiments do not exclude a protease, which has to be checked more thoroughly. Another possibility would be a blocking ligand for these two receptors. Nonspecific general toxic effects of SaS on neutrophils are unlikely since the cells still excluded trypan blue and propidium iodide and not all receptors were prone to change by SaS. To our knowledge, there are no data of a bacterial product which is able to specifically downregulate these two receptors involved in chemotaxis.
REFERENCES
- 1.Bhakdi S, Klonisch T, Nuber P, Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohnsack J F, Chang J K, Hill H R. Restricted ability of group B streptococcal C5a-ase to inactivate C5a prepared from different animal species. Infect Immun. 1993;61:1421–1426. doi: 10.1128/iai.61.4.1421-1426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Z M, Murphy J W. Intravascular cryptococcal culture filtrate (CneF) and its major component, glucuronoxylomannan, are potent inhibitors of leukocyte accumulation. Infect Immun. 1995;63:770–778. doi: 10.1128/iai.63.3.770-778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drouhet E, Segretain G. Inhibition de la migration leucocytaire in vitro par un polyoside capsulaire de Torulopsis (Cryptococcus) neoformans. Ann Inst Pasteur (Paris) 1951;81:674–676. [PubMed] [Google Scholar]
- 5.Heumann D, Barras C, Severin A, Glauser M P, Tomasz A. Gram-positive-cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill H R, Bohnsack J F, Morris E Z, Augustine N H, Parker C J, Cleary P P, Wu J T. Group B streptococci inhibit the chemotactic activity of the fifth component of complement. J Immunol. 1988;141:3551–3556. [PubMed] [Google Scholar]
- 7.Jagels M A, Travis J, Potempa J, Pike R, Hugli T E. Proteolytic inactivation of the leukocyte C5a receptor by proteinases derived from Porphyromonas gingivalis. Infect Immun. 1996;64:1984–1991. doi: 10.1128/iai.64.6.1984-1991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katada T, Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci USA. 1982;79:3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konig B, Prevost G, Piemont Y, Konig W. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J Infect Dis. 1995;171:607–613. doi: 10.1093/infdis/171.3.607. [DOI] [PubMed] [Google Scholar]
- 10.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 11.Mattsson E, Rollof J, Verhoef J, Van Dijk H, Fleer A. Serum-induced potentiation of tumor necrosis factor alpha production by human monocytes in response to staphylococcal peptidoglycan: involvement of different serum factors. Infect Immun. 1994;62:3837–3843. doi: 10.1128/iai.62.9.3837-3843.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattsson E, Verhage L, Rollof J, Fleer A, Verhoef J, Van Dijk H. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6. FEMS Immunol Med Microbiol. 1993;7:281–287. doi: 10.1111/j.1574-695X.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 13.Murray D L, Ohlendorf D H, Schlievert P M. Staphylococcal and streptococcal superantigens: their role in human diseases. ASM News. 1995;61:229–235. [Google Scholar]
- 14.Musher D M, Verbrugh H A, Verhoef J. Suppression of phagocytosis and chemotaxis by cell wall components of Staphylococcus aureus. J Immunol. 1981;127:84–88. [PubMed] [Google Scholar]
- 15.Neer E J, Clapham D E. Roles of G protein subunits in transmembrane signalling. Nature. 1988;333:129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- 16.Peterson P K, Verhoef J, Sabath L D, Quie P G. Effect of protein A on staphylococcal opsonization. Infect Immun. 1977;15:760–764. doi: 10.1128/iai.15.3.760-764.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rot A, Henderson L E, Sowder R, Leonard E J. Staphylococcus aureus tetrapeptide with high chemotactic potency and efficacy for human leukocytes. J Leukoc Biol. 1989;45:114–120. doi: 10.1002/jlb.45.2.114. [DOI] [PubMed] [Google Scholar]
- 18.Rozdzinski E, Jones T, Burnette W N, Burroughs M, Tuomanen E. Antiinflammatory effects in experimental meningitis of prokaryotic peptides that mimic selectins. J Infect Dis. 1993;168:1422–1428. doi: 10.1093/infdis/168.6.1422. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz F J, Veldkamp K E, Van Kessel K P M, Verhoef J, Van Strijp J A G. Delta-toxin from Staphylococcus aureus as a costimulator of human neutrophil oxidative burst. J Infect Dis. 1997;176:1531–1537. doi: 10.1086/514152. [DOI] [PubMed] [Google Scholar]
- 20.Timmerman C P, Mattsson E, Martinez Martinez L, De Graaf L, Van Strijp J A G, Verbrugh H A, Verhoef J, Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993;61:4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomhave E D, Richardson R M, Didsbury J R, Menard L, Snyderman R, Ali H. Cross-desensitization of receptors for peptide chemoattractants. J Immunol. 1994;153:3267–3275. [PubMed] [Google Scholar]
- 22.Troelstra A, Giepmans B N, Van Kessel K P, Lichenstein H S, Verhoef J, Van Strijp J A. Dual effects of soluble CD14 on LPS priming of neutrophils. J Leukoc Biol. 1997;61:173–178. doi: 10.1002/jlb.61.2.173. [DOI] [PubMed] [Google Scholar]
- 23.Veldkamp K E, Van Kessel K P M, Verhoef J, Van Strijp J A G. Staphylococcal culture supernates stimulate human phagocytes. Inflammation. 1997;21:541–551. doi: 10.1023/a:1027315814817. [DOI] [PubMed] [Google Scholar]
- 24.Wirthmueller U, Baggiolini M, de Weck A L, Dahinden C A. Receptor-operated activation of polymorphonuclear leukocytes: different effects of NAP-1/IL-8 and fMet-Leu-Phe or C5a. Biochem Biophys Res Commun. 1991;176:972–978. doi: 10.1016/0006-291x(91)90377-j. [DOI] [PubMed] [Google Scholar]
- 25.Yao L, Lowy F D, Betman J W. Interleukin-8 gene expression in Staphylococcus aureus-infected endothelial cells. Infect Immun. 1996;64:3407–3409. doi: 10.1128/iai.64.8.3407-3409.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]