Dear Editor,
We read with great interest that monoclonal antibody (mAb) cocktail may serve as an effective and targeted therapeutic strategy in the treatment of patients with COVID-19.1 Since they can reduce virulence and viral load and enhance prognosis, specific anti-spike monoclonal antibodies, such as casirivimab-imdevimab, are an appealing alternative for treating COVID-19 infection.2
Casirivimab and imdevimab are two recombinant human IgG1 monoclonal antibodies that bind non-competitively to non-overlapping epitopes of the spike protein receptor-binding domain of SARS-CoV-2, thereby blocking the viral entry into host cells.2 Based on a randomized placebo-controlled clinical trial that showed a significant reduction in viral load among patients who received the casirivimab-imdevimab combination, the US Food and Drug Administration- Federal Agency (FDA), European Medical Agency (EMA), and Central Drug Standard Control Organization have authorized the use of imdevimab-casirivimab for emergency purposes.3 Casirivimab and imdevimab's Emergency Use Authorization (EUA) was modified in June 2021 to include subcutaneous delivery as a substitute for patients who cannot undergo intravenous (IV) infusion. Beginning in August 2021 and continuing through the beginning of January 2022, patients received treatment with subcutaneous injections of casirivimab and imdevimab. During this time, the health system had multiple COVID-19 case spikes including the Omicron and Delta varieties of concern.
There are now a number of studies reporting the effect of using casirivimab-imdevimab on patient clinical outcomes, however their findings are inconsistent. There have been no prior meta-analyses describing the association between casirivimab-imdevimab treatment and patient prognosis following COVID-19 infection to the best of our knowledge. Hence, we perform in this study the first meta-analysis in the literature to evaluate the relationship between casirivimab-imdevimab administration and patient outcomes following COVID-19 infection.
An electronic search was carried out between December 1, 2019, and April 1, 2023, in the databases of PubMed, Embase, the Cochrane Library, Scopus, medRxiv, and bioRxiv. There were no restrictions on publishing or language. The following search phrases and MeSH (Medical Subject Heading) terms were employed: (“coronavirus disease 2019 or novel coronavirus or SARS-CoV-2 or 2019-nCoV or COVID-19”) AND (casirivimab or imdevimab or REGN-COV or REGEN-COV2 or Ronapreve or REGN10933 or REGN10987).
The following criteria were used for inclusion: (1) patients that have COVID-19 confirmed; (2) clinical outcomes were evaluated between the casirivimab-imdevimab therapy and control groups (standard of care or placebo). The study excluded publications with identical content, reviews, letters, editorials, conference abstracts, case reports, and other publications. The first author's name, the publication year, the study's design, the participants' age, gender, the use of casirivimab-imdevimab, and the outcomes of interest (mortality and hospitalization) were also collected as data on the studies' initial characteristics.
The statistical analysis was carried out using Review Manager, version 5.2 (Cochrane Collaboration, Oxford). The odds ratio (OR), with a 95% confidence interval, was employed to evaluate dichotomous variables. We assessed the heterogeneity of the data using the I2 statistic and the Cochran's Q test. A P value of 0.05 or less indicates statistical significance. The protocol for this study is registered with PROSPERO (CRD42023418212).
Through a thorough literature search, a total of nine studies, including 84,875 in the casirivimab-imdevimab group and 322,943 in the control group arm, were identified for this meta-analysis.2, 3, 4, 5, 6, 7, 8, 9, 10 Table 1 lists the illness features and demographics of the 407,818 patients who were included in the pooled study. The majority of the included studies were from the United States. The other investigations were retrospective and prospective cohort studies, while one research was a randomized controlled study. Patients with mild-to-moderate COVID-19 were diagnosed in the majority of trials. Three studies included COVID-19 individuals who were all outpatients.4, 5, 6 Casirivimab-imdevimab was given intravenously or subcutaneously in the included studies. The selected studies, which were all published between 2022 and 2023, had various sample patient sizes ranging from 152 to 384,447 patients with COVID-19.
Table 1.
Characteristics of included studies.
| Study | Country | Study type | Sample size | Intervention | Patients included | Key study outcomes |
|---|---|---|---|---|---|---|
| Al-Obaidi 2022 | United States | Retrospective cohort | 8426 | Casirivimab-imdevimab | High-risk patients with COVID-19 | Mortality, hospitalization, ICU admission |
| Bierle 2021 | United States | Retrospective cohort | 630 | Casirivimab-imdevimab | Mild to moderate COVID-19 | Hospitalization, number of patients with hypoxia |
| Hussein 2022 | United States | Retrospective cohort | 384,447 | Casirivimab-imdevimab | Outpatients with COVID-19 | Mortality, composite outcome of all-cause mortality or COVID-19-related hospitalizations |
| Joy 2022 | India | Retro-prospective comparative study | 152 | Casirivimab-imdevimab | Patients amidst and post COVID-19 treatment | Mortality, hospitalization, need for mechanical ventilation, high flow O2 requirement |
| McCreary 2022 | United States | Prospective cohort | 1959 | Casirivimab-imdevimab | Outpatients with mild to moderate COVID-19 | Mortality, hospitalization, emergency department admission or hospitalization |
| Razonable 2021 | United States | Retrospective cohort | 1392 | Casirivimab-imdevimab | Mild to moderate COVID-19 | Mortality, hospitalization, ICU admission |
| RECOVERY 2022 | United Kingdom | RCT | 9785 | Casirivimab-imdevimab vs usual care | Patients admitted to hospital with COVID-19 | Mortality, need for mechanical ventilation, renal replacement therapy |
| Rhudy 2023 | United States | Retrospective cohort | 1170 | Casirivimab-imdevimab | Outpatients with symptomatic COVID-19 | Mortality, hospitalization, emergency department admission |
| Suzuki 2022 | United States | Retrospective cohort | 444 | Casirivimab-imdevimab | Mild to moderate COVID-19 | Mortality, need for mechanical ventilation//ECMO, deterioration during hospitalization |
RCT: randomized controlled trial; ICU: intensive care unit; ECMO, extracorporeal membrane oxygenation.
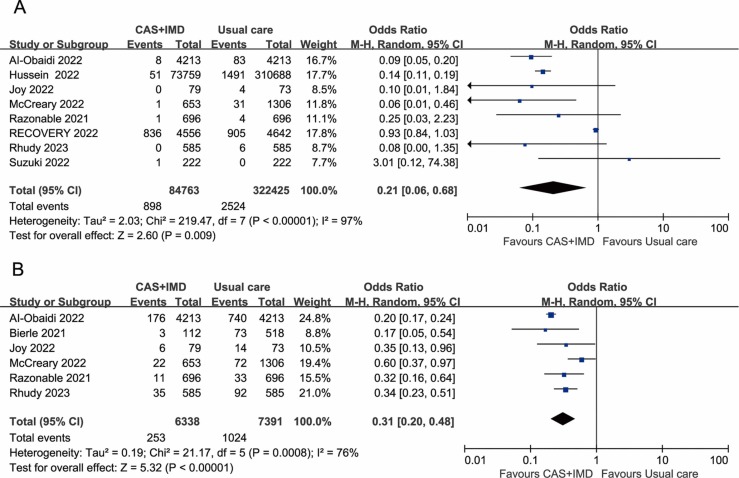
The meta-analysis revealed that the casirivimab-imdevimab treatment was associated with a lower mortality rate than the control group who did not receive casirivimab-imdevimab (OR=0.21, 95%CI: 0.06–0.68, P = 0.03; I2 =97%) ( Fig. 1A). In addition, patients who received casirivimab-imdevimab had significantly lower hospitalization rates (OR=0.31, 95%CI: 0.20–0.48, P<0.00001; I2 =76%) (Fig. 1B).
Fig. 1.
A. Association between casirivimab-imdevimab treatment and mortality, B. Association between casirivimab-imdevimab treatment and hospitalization.
The results demonstrate a significant beneficial effect of casirivimab-imdevimab treatment on mortality and hospitalization in COVID-19 patients.
As a result of a higher risk of severe COVID-19 among those who are not vaccinated, the reduction in outcome risk among patients treated with casirivimab-imdevimab was marginally greater among unvaccinated patients than among vaccinated patients.4 The results demonstrate that treatment with casirivimab-imdevimab can be beneficial not only for people who cannot or do not want to take the COVID-19 vaccine, but also for those who have received the vaccine. The findings show that vaccinated individuals with COVID-19 can also benefit from therapy with casirivimab-imdevimab. This is in addition to the benefits of treatment for those who cannot or do not want to receive the vaccine. In addition, although RECOVRY collaborative group found that therapeutic use of casirivimab and imdevimab combination in the hospital setting would be best restricted to seronegative patients, the development of the variant (omicron), which can evade antibodies raised against previous SARS-CoV-2 variants, the validity of seropositive status as a predictor of treatment non-response to monoclonal antibodies is weakened.7
Our study has several limitations. With nine included literature, the meta-analysis's sample size was relatively small. In terms of mortality and hospitalization, there was also a considerable heterogeneity. Additionally, the patient populations' immunization status, baseline serostatus, type of viral variants, and use of casirivimab-imdevimab in different trials may differ. Despite these limitations, our study has significant importance as the first meta-analysis to examine the outcomes of casirivimab-imdevimab therapy in COVID-19-infected patients.
To sum up, using casirivimab-imdevimab to treat COVID-19 patients has considerable advantages in terms of preventing hospitalization and mortality. These results need to be confirmed by further research.
Funding
None declared.
Declaration of Competing Interest
The authors declare that they have no competing interest.
Acknowledgments
None.
References
- 1.Wang Y., Zheng J., Zhu K., Xu C., Wang D., Ho M. The effect of tixagevimab-cilgavimab on clinical outcomes in patients with COVID-19: a systematic review with meta-analysis. J Infect. 2023;86(1):e15–e17. doi: 10.1016/j.jinf.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razonable R.R., Pawlowski C., O'Horo J.C., Arndt L.L., Richard Arndt R., Bierle D.M., et al. Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. eClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joy A.P., Karattuthodi M.S., Chandrasekher D., Augustine. A.T. The impact of casirivimab-imdevimab antibody cocktail in patients amidst and post COVID 19 treatment: a retro-prospective comparative study in India. J Assoc Physicians India. 2022;70(4):11–12. doi: 10.1016/j.cegh.2022.100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussein M., Wei W., Mastey V., Sanchez R.J., Wang D., Murdock D.J., et al. Real-world effectiveness of casirivimab and imdevimab among patients diagnosed with COVID-19 in the ambulatory setting: a retrospective cohort study using a large claims database. BMJ Open. 2022;12(12) doi: 10.1136/bmjopen-2022-064953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCreary E.K., Bariola J.R., Wadas R.J., Shovel J.A., Wisniewski M.K., Adam M., et al. Association of subcutaneous or intravenous administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in adults with COVID-19. JAMA Netw Open. 2022;5(4) doi: 10.1001/jamanetworkopen.2022.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhudy C., Bochenek S., Thomas J., James G.S., Zeltner M., Platt T., et al. Impact of a subcutaneous casirivimab and imdevimab clinic in outpatients with symptomatic COVID-19: a single-center, propensity-matched cohort study. Am J Health Syst Pharm. 2023;80(3):130–136. doi: 10.1093/ajhp/zxac305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RECOVERY Collaborative Group Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399(10325):665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Obaidi M.M., Gungor A.B., Nematollahi S., Zangeneh T.T., Bedrick B.J., Johnson K.M., et al. Effectiveness of casirivimab-imdevimab monoclonal antibody treatment among high-risk patients with severe acute respiratory syndrome coronavirus 2 B.1.617.2 (Delta variant) infection. Open Forum Infect Dis. 2022;9(7):ofac186. doi: 10.1093/ofid/ofac186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bierle D.M., Ganesh R., Razonable R.R. Breakthrough COVID-19 and casirivimab-imdevimab treatment during a SARS-CoV-2 B1.617.2 (Delta) surge. J Clin Virol. 2021;145 doi: 10.1016/j.jcv.2021.105026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki Y., Shibata Y., Minemura H., Nikaido T., Tanino Y., Fukuhara A., et al. Real-world clinical outcomes of treatment with casirivimab-imdevimab among patients with mild-to-moderate coronavirus disease 2019 during the Delta variant pandemic. Int J Med Sci. 2022;19(5):834–841. doi: 10.7150/ijms.71132. [DOI] [PMC free article] [PubMed] [Google Scholar]