Abstract
Pet toxin is a serine protease from enteroaggregative Escherichia coli which has been described as causing enterotoxic and cytotoxic effects. In this paper we show that Pet produces spectrin and fodrin (nonerythroid spectrin) disruption. Using purified erythrocyte membranes treated with Pet toxin, we observed degradation of α- and β-spectrin chains; this effect was dose and time dependent, and a 120-kDa protein fraction was observed as a breakdown product. Spectrin degradation and production of the 120-kDa subproduct were confirmed using specific antibodies against the α- and β-spectrin chains. The same degradation effect was observed in α-fodrin from epithelial HEp-2 cells, both in purified cell membranes and in cultured cells which had been held in suspension for 36 h; these effects were confirmed using antifodrin rabbit antibodies. The spectrin and fodrin degradation caused by Pet is related to the Pet serine protease motif. Fluorescence and light microscopy of HEp-2 Pet-treated cells showed morphological alterations, which were associated with irregular distribution of fodrin in situ. Spectrin and fodrin degradation by Pet toxin were inhibited by anti-Pet antibodies and by phenylmethylsulfonyl fluoride. A site-directed Pet mutant, which had been shown to abolish the enterotoxic and cytotoxic effects of Pet, was unable to degrade spectrin in erythrocyte membranes or purified spectrin or fodrin in epithelial cell assays. This is a new system of cellular damage identified in bacterial toxins which includes the internalization of the protease, induction of some unknown intermediate signaling steps, and finally the fodrin degradation to destroy the cell.
Enteroaggregative Escherichia coli (EAEC) is a group of bacteria characterized by the ability to adhere to cultured cell monolayers in a “stacked brick” adhesion phenotype (27). There is increasing evidence that EAEC is strongly associated with persistent diarrheal disease in children in India, Brazil, Mexico, Bangladesh, and other areas in the developing world (4, 9, 14, 18, 26). The participation of EAEC strains in several outbreaks of diarrhea in children and adults has also been reported in developing and developed countries such as Serbia (8), Mexico (C. Eslava, J. Villaseca, R. Morales, A. Navarro, and A. Cravioto, Abstr. 93rd Gen. Meet. Am. Soc. Microbiol. 1993, abstr. B-105, 1993), Japan (21), the United Kingdom (36), and Germany (20). In addition, the participation of EAEC as the causative agent of diarrheal disease in human immunodeficiency virus-infected adults in the developed world has also been suggested (24).
The pathogenesis of EAEC infection is not completely understood, although histopathologic alterations of intestinal epithelium from patients and animal models infected with EAEC have been reported. Formation of a thick mucous gel on the intestinal epithelium mucosa was observed in gnotobiotic piglets inoculated with EAEC (38). Hicks et al. (19), using an in vitro organ culture model, observed that EAEC strains were embedded within a mucus-containing biofilm and exfoliation of enterocytes from the mucosal surface of intestinal biopsies. Vial et al. (39), using the rabbit and rat ileal loop models inoculated with EAEC strains, observed lesions characterized by shortening of the villi, hemorrhagic necrosis of the villous tip, and a mild inflammatory response with edema and mononuclear infiltration of the submucosa. Similar histological alterations were observed in autopsy samples of the ileum from children who died as a consequence of persistent diarrhea associated with EAEC infection (Eslava et al., Abstr. 93rd Gen. Meet. Am. Soc. Microbiol. 1993), as well as in rat jejunal preparation mounted in Ussing chambers and treated with a supernatant from EAEC (29). All these observations suggested that some of the alterations caused during EAEC infection were associated with the production of a cytotoxin.
Eslava et al. (Abstr. 93rd Gen. Meet. Am. Soc. Microbiol. 1993) identified two high-molecular-weight proteins from EAEC strains isolated from children who died as a consequence of persistent diarrhea caused by EAEC. These proteins were tested in the rat ileal loop model and were observed to cause shortening of the villi, hemorrhagic and necrotic alterations, and ulceration of the upper epithelium. The gene for one of these two high-molecular-weight proteins located on the 65-MDa EAEC virulence plasmid was cloned, and the protein was named Pet, for plasmid-encoded toxin (13). Pet sequence shows a high homology with the type IV class autotransporter-secreted proteins, including the subfamily that has been called SPATE (Tsh, EspC, and EspP from E. coli and ShMu and SepA from Shigella) (17). It has also been shown that Pet induces cytopathic effects on HEp-2 and HT29 C1 culture cells, characterized by release of the cellular focal contact from glass substratum and rounding and detachment of cells, as well as cytoskeleton contraction and loss of actin stress fibers (30). Navarro-García et al. showed with the Ussing chamber model that Pet induces enterotoxic and cytotoxic effects (29) and that these activities depend upon the serine protease motif (30). However, the specific action mechanism of Pet toxin on epithelial cells has not yet been elucidated. This study shows that Pet toxin causes disruption of spectrin and fodrin (nonerythroid spectrin, which is distributed among the majority of cell types, including epithelial cells), proteins of the membrane skeleton that are connected with the cytoplasmic actin network. Fodrin degradation could explain the previously mentioned cellular alterations and the diarrheal pathogenesis caused by EAEC.
(Preliminary work containing portions of this paper was presented at the 99th General Meeting of the American Society for Microbiology, Chicago, Ill., May 1999.)
MATERIALS AND METHODS
Strains and plasmids.
The minimal Pet clone pCEFN1 (previously described) was constructed by cloning the pet gene of EAEC strain 042 into the BamHI/KpnI site of pSPORT1 and is expressed in E. coli HB101 (13). HB101(pCEFN1) was used to obtain Pet protein, and HB101(pSPORT1) was used as a control for cell experiments. Site-directed mutagenesis was performed to obtain the Pet serine motif mutant (Pet S260I), using the QuikChange site-directed mutagenesis kit from Stratagene exactly as described (30) and cloned in the same vector, HB101(pCEFN2). The strains were maintained on L agar or L broth containing 100 μg of ampicillin/ml.
Protein purification.
Pet protein was obtained from a culture supernatant of pet clone E. coli HB101(pCEFN1), precipitated with 75% ammonium sulfate, and further precipitated with 1.15 and 1.75 M potassium phosphate buffer, eluted from a Q-Sepharose column and then from fast-protein liquid chromatography (FPLC) Mono S HR 5/5 columns. The protein fractions were determined by the Bradford method (5), and the purified protein was analyzed by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis (PAGE) (22).
N-terminal sequence.
The N-terminal sequence was determined by automated Edman degradation on a gas-phase protein sequencer (LF 3000; Beckman Instruments) equipped with an online Beckman System Gold high-performance liquid chromatography (HPLC) system. The HPLC equipment included a model 126 pump and a 168-diode array detector set at 268 and 293 nm for signal and reference, respectively. The HPLC column used was the Beckman Spherogel Micro PTH (2 by 150). The standard Beckman sequencing reagents were used for the analysis.
Protein samples for sequencing were prepared by SDS-PAGE and electroblotting on polyvinylidene difluoride membranes (Millipore Co.), as described by Towbin et al. (37), as well as by direct application of desalted purified protein on Beckman protein supports.
Spectrin assay.
Sheep red blood cells (SRBC; Microlab, Mexico City, Mexico) and HEp-2 cells suspended in phosphate buffer (310 mosM) were centrifuged at 1,000 × g for 10 min (three times), and the pellet was washed with the same buffer and then incubated in a phosphate buffer (20 mosM). The lysed cells were centrifuged at 20,000 × g for 40 min and the pellet obtained was washed by resuspension in hypotonic phosphate buffer followed by centrifugation at 20,000 × g for 20 min (three times) to obtain erythrocyte and HEp-2 cell membranes, which are spectrin or fodrin enrichment fractions, respectively.
These membrane preparations were incubated with different Pet protein concentrations or with E. coli HB101 culture supernatants from 3 to 24 h at 37°C. Reaction mixture samples of 100 μl containing 10 μg of SRBC or 100 μg of HEp-2 cells and 0.1 to 10 μg of Pet were analyzed by SDS-6% PAGE (22). In some experiments purified spectrin (from Sigma Chemical Co., St. Louis, Mo.) also was used.
For antibody inhibition experiments, Pet protein (5 μg) was incubated for 3 h at 37°C with 10 μg of antibodies against Pet protein in 100 μl of RPMI medium (29). To analyze the participation of the serine protease motif, a reaction was performed in the presence of 2 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma Chemical Co.). To further confirm the role of the serine protease motif, similar concentrations of the Pet S260I protein mutant were used instead of Pet protein (30).
Pet effects on HEp-2 cells in suspension.
Cultures from HEp-2 cells were detached with Puck's solution (Gibco BRL) and washed three times with phosphate-buffered saline (PBS). HEp-2 cells held in suspension (3 × 105/ml) in Dulbecco's modified Eagle medium with glucose but free of serum and antibiotics were incubated with 10, 50, or 100 μg per ml of Pet protein for 3, 6, 12, 18, 24, or 36 h. After incubation, the cells were washed three times (15 min each) by centrifugation with PBS and were lysed with SDS-PAGE Laemmli sample buffer. The HEp-2 cell proteins were adjusted to a final concentration of 30 μg of total protein and were separated by SDS-PAGE (22).
Western immunoblot.
Untreated and Pet-treated erythrocyte and HEp-2 cell membrane preparations separated by SDS-6% PAGE were transferred to nitrocellulose sheets (Schleicher & Schuell, Keene, N.H.) as described by Towbin et al. (37). Rabbit anti-alpha and anti-beta spectrin chain antibodies (Sigma Chemical Co.) were used (10−2) to analyze Pet activity on spectrin from cell membranes. The reaction was visualized using goat anti-rabbit antibodies (10−3) conjugated with alkaline phosphatase (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). To detect the α-fodrin of HEp-2 cells in suspension, rabbit antibodies against brain α-fodrin (rabbit antifodrin antibody 9053, kindly proportionated by R. Bloch) in a concentration of 100 ng/ml were used. The reaction was visualized using goat anti-rabbit antibodies (10−4) conjugated with horseradish peroxidase (Kirkegaard & Perry Laboratories) and developed using Western-light chemiluminescent reagent (Du Pont, NEN).
Detection of Pet effects on spectrin in situ.
HEp-2 cell suspensions were incubated during 3 h with 5 μg of Pet/ml. The cell preparations were fixed with glutaraldehyde (3% in PBS, pH 7.4) and permeabilized with Triton X-100 (0.1% in PBS, pH 7.4). The permeabilized cells were incubated with anti-alpha and anti-beta spectrin chain (Sigma Chemical Co.) rabbit antibodies or were stained with Coomassie blue (Sigma Chemical Co.) for 10 min. For immunofluorescence, the reaction was visualized using goat anti-rabbit IgG antibodies labeled with fluorescein (Kirkegaard & Perry Laboratories). The slides were observed by epifluorescence or light microscopy (Karl Zeiss).
RESULTS
Purification of Pet protein.
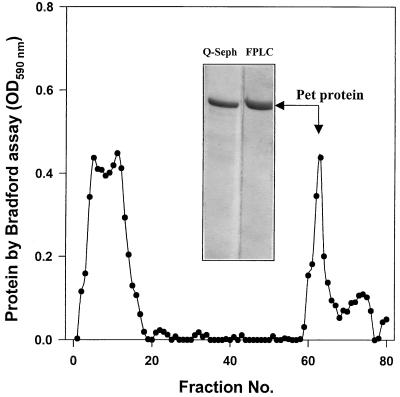
Pet protein was purified from the minimal clone HB101(pCEFN1); the process of purification included ammonium sulfate precipitation, and passing through a Q-Sepharose column and then an HPLC Mono S HR 5/5 column (Fig. 1). The recuperation efficiency rate of Pet protein was about 3.14%, which corresponds to 3.3 mg from 10 liters (105 mg) of overnight culture. The N-terminal amino sequence of the purified protein was determined and the sequence found (ANMDISKAWARDYLDLAQN) was the same as the previously predicted product from the pet gene of the 042 EAEC strain (13).
FIG. 1.
Purification of Pet protein by FPLC. Pet protein was purified from supernatant of the minimal clone HB101(pCEFN1). The supernatant was precipitated by ammonium sulfate and reprecipitated with 1.15 and 1.75 M phosphate buffer. This fraction was passed through a Q-Sepharose column and finally an FPLC column. Ten milliliters from the Q-Sepharose fraction enriched in Pet protein was applied to the Mono S HR 5/5 columns. The fractions eluted (60 to 67) were analyzed by Bradford protein assay and SDS-PAGE. The FPLC profile is shown, and the insert shows the fraction applied (Q-Sepharose) and the purified Pet protein (FPLC). OD590, optical density at 590 nm.
Effects of the Pet protein on erythrocyte membranes.
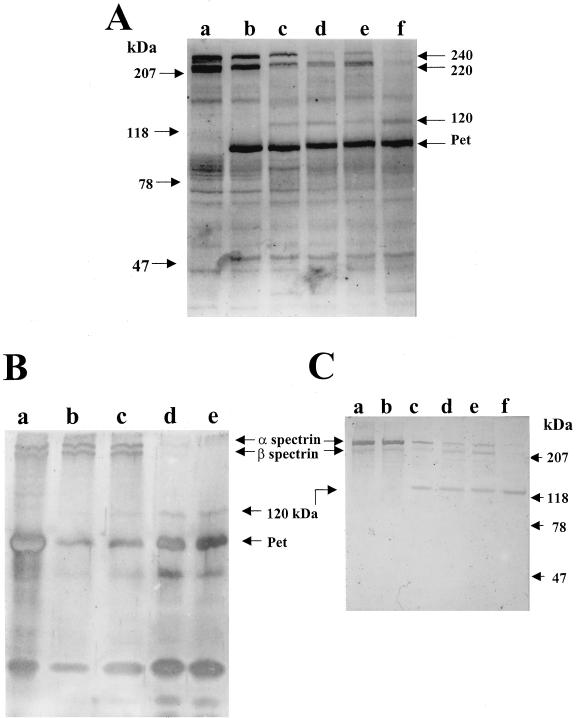
In order to explore the possible effects of Pet on the cell membrane, purified erythrocyte membranes were used. After 6, 12, 18, and 24 h of incubation of 10 μg of membrane proteins with 5 μg of Pet protein in a total volume of 100 μl, Pet induced a change on the normal SDS-PAGE profile of erythrocyte membrane proteins. This was characterized by the degradation of two protein fractions of 240 and 220 kDa, molecular masses that corresponded to the α- and β-spectrin chains. In addition, a new protein fraction of 120 kDa was observed which corresponds to a possible main subproduct of degraded spectrin bands (Fig. 2A). A similar effect was found when a sample of 2 μg of purified erythrocyte spectrin was treated with 1 μg of Pet protein in 20 μl of reaction mixtures, showing degradation of the same protein fractions of 240 and 220 kDa and the production of a 120-kDa breakdown product (Fig. 2B).
FIG. 2.
Spectrin degradation by Pet protein. (A) SDS-PAGE profile of 10 μg of erythrocyte membranes treated with 5 μg of Pet protein (total volume, 100 μl) for 6, 12, 18, and 24 h. Lanes: a, untreated erythrocyte membranes; b, Pet-treated erythrocyte membrane at the start (time 0); c, at 6 h; d, at 12 h; e, at 18 h; and f, at 24 h. (B) Purified spectrin treated with Pet protein. Reaction mixture samples of 20 μl containing purified α- and β-spectrin (2 μg) were treated with purified Pet protein (1 μg) at time 0 (lane a), 6 h (b), 12 h (c), 18 h (d), and 24 h (e). The proteins were detected with silver stain. (C) Western blot of erythrocyte membranes treated with Pet protein and developed with antibodies against α- and β-spectrin. The erythrocyte membranes were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The reaction was visualized using anti-rabbit antibodies conjugated with alkaline phosphatase. The sample was placed as in Fig. 2A.
In order to verify if the two protein fractions degraded by Pet protein correspond to α- and β-spectrin chains, a Western blot assay of Pet-treated erythrocyte membrane proteins was performed using specific antibodies against α- and β-spectrin chains. The results confirmed that the degraded 240- and 220-kDa protein fractions correspond to α- and β-spectrin chains and that the 120-kDa subproduct appeared to come from spectrin (Fig. 2C). It was also seen that the α-spectrin chain was more sensitive to Pet and that the effect was dose and time dependent. Erythrocyte membrane proteins (10 μg) treated with different doses of Pet protein (ranging from 10 ng to 5 μg of Pet in 100 μl) for 3 h of incubation showed that whereas the α- and β-spectrin bands were decreasing, the 120-kDa subproduct band was increasing. Similar results were found when the erythrocyte membrane proteins were incubated with 5 μg of Pet for different lengths of time (data not shown).
To know if the Pet effects on spectrin were specific, antibodies against Pet were used to inhibit them. These antibodies have been shown to neutralize the enterotoxic and cytotoxic activity of Pet (29, 30). Pet protein was preincubated with polyclonal anti-Pet antibodies and then incubated with erythrocyte membranes. These experiments showed that α- and β-spectrin bands were partially degraded, with some subproducts appearing; however, the 120-kDa subproduct was not seen (Fig. 3), suggesting that this partial degradation occurred on another site.
FIG. 3.
Inhibition of Pet activity on erythrocyte membranes. The erythrocyte membrane proteins were subjected to a spectrin degradation assay, treated with inhibitors of Pet activity, and analyzed by SDS-PAGE. Lanes: a, untreated erythrocyte membranes; b, purified Pet protein; c, erythrocyte membranes treated with Pet protein; d, erythrocyte membranes treated with Pet protein in the presence of 2 mM PMSF serine protease inhibitor; e, Pet S260I mutant protein; f, erythrocyte membranes treated with Pet S260I; g, erythrocyte membranes treated with Pet protein; h, erythrocyte membranes treated with Pet protein previously incubated with anti-Pet protein antibodies (the band around 50 kDa corresponds to heavy chains of immunoglobulins).
Role of the serine protease motif on spectrin degradation.
In order to evaluate the role of the serine protease activity of Pet on spectrin, the serine protease inhibitor PMSF was used. Pet protein, previously incubated with 2 mM PMSF, was then used in the spectrin degradation assay. PMSF inhibited the effects of Pet on spectrin (Fig. 3). In order to confirm the role of the serine protease motif on spectrin degradation by Pet, a culture supernatant partially purified from the previously described serine protease mutant, Pet S260I, was used to incubate with erythrocyte membranes. This mutant protein was unable to produce spectrin degradation (Fig. 3).
To establish the cleavage site of spectrin by Pet, the 120-kDa subproduct obtained from purified spectrin and erythrocyte spectrin degradation was analyzed to determine its N-terminal sequence. The results from the N-terminal sequence showed it to be the same as that of mature α-spectrin, which suggested that the cleavage site occurred at the C-terminal site.
Effects of Pet on epithelial cell membranes.
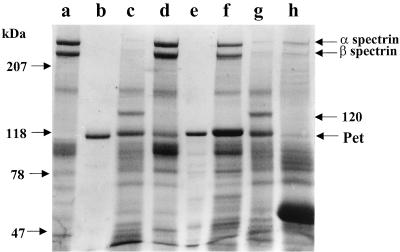
To determine if Pet produces the same alteration on epithelial cells as previously seen with erythrocyte membranes, purified HEp-2 cell membranes were incubated with Pet protein for 3 h. After incubation, the SDS-PAGE protein profile showed a degradation zone around the 240- and 220-kDa protein fractions. However, a fodrin subproduct of 120 kDa was not seen, and no other subproduct was seen (Fig. 4). Similar assays, using precipitated supernatants from E. coli HB101, which lacks Pet protein, were unable to produce alteration in the SDS-PAGE protein profile (Fig. 4).
FIG. 4.
Effects of Pet protein on HEp-2 epithelial cell membranes. Purified epithelial cell membrane proteins (100 μg) were used in the fodrin degradation assay and analyzed by SDS-PAGE. Lanes: a, untreated HEp-2 cell membranes; b, HEp-2 cell membranes treated with HB101 culture supernatant precipitated with ammonium sulfate; c to e, HEp-2 cell membranes treated with 2.5 (c), 5 (d), and 10 (e) μg of Pet protein.
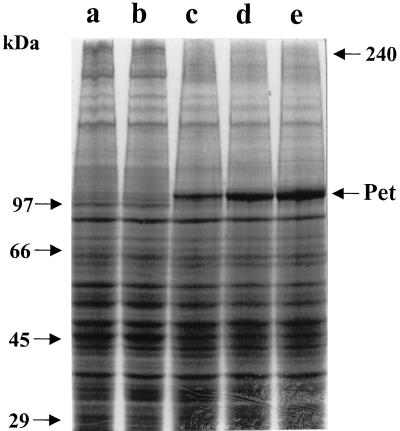
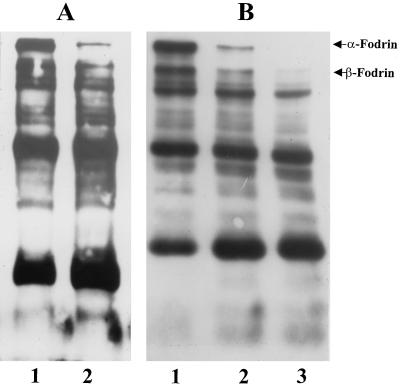
In order to identify Pet activity on live HEp-2 cells, cultured cells in suspension were exposed to Pet protein (10, 50, and 100 μg per ml) for 3, 6, 12, 18, 24, and 36 h and then analyzed for α- and β-fodrin degradation by Western blotting using polyclonal antibodies against brain fodrin. Using this methodology, it was seen that Pet protein caused α-fodrin degradation after 18 h of incubation (Fig. 5A) and degradation of both α-fodrin and another protein of 220 kDa (which probably corresponds to β-fodrin) at 36 h of incubation (Fig. 5B). Although the anti-brain fodrin polyclonal antibodies were unable to detect some specific subproducts, a fraction of approximately 83 kDa was increasing at the same rate as the α- and β-fodrin were degrading (Fig. 5B). The effects of Pet on HEp-2 cell fodrin also were time and dose dependent.
FIG. 5.
Degradation of fodrin from epithelial cells by Pet. HEp-2 cells maintained in suspension were incubated with either 10, 50, or 100 μg per ml of Pet protein for 3, 6, 12, 18, 24, or 36 h. The HEp-2 cell membranes were separated by SDS-PAGE and transferred to a nitrocellulose membrane. To detect the α- and β-fodrin chains, rabbit antibodies prepared against brain fodrin at a concentration of 100 ng/ml were used. The reaction was visualized using goat anti-rabbit antibodies labeled with horseradish and developed using Western-light chemiluminescent reagent. (A) Epithelial cells incubated for 18 h with Pet protein. Lanes: 1, untreated HEp-2 cells; 2, HEp-2 cells treated with Pet (100 μg/ml). (B) HEp-2 cells incubated for 36 h with Pet. Lanes: 1, untreated HEp-2 cells; 2 and 3, HEp-2 cells treated with 50 (2) or 100 (3) μg of Pet protein/ml.
Effects of Pet on HEp-2 cells in situ.
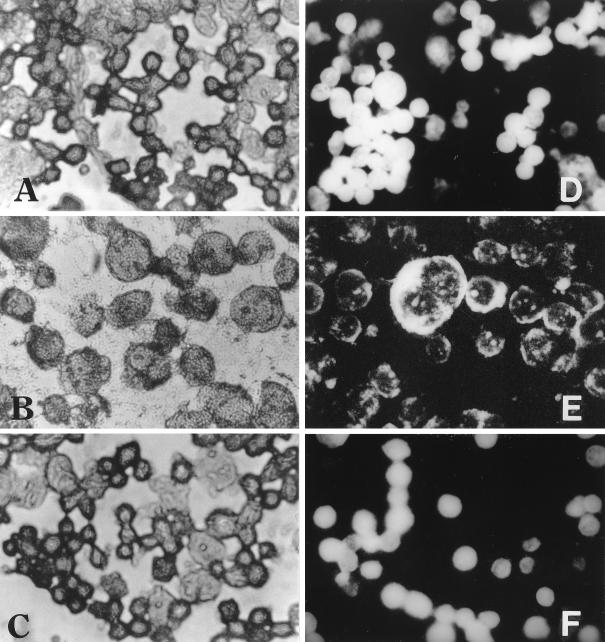
In order to detect the Pet effects on fodrin in HEp-2 cells, HEp-2 cells in suspension were treated with Pet toxin for 3 h at 37°C, stained with Coomassie blue, and observed by light microscopy. These cells showed morphological alterations characterized by damage of the cell membrane in the form of cell swelling (Fig. 6B). In contrast, the untreated cells (Fig. 6A) and those treated with Pet S260I (Fig. 6C) did not show morphological alterations and maintained their normal structure.
FIG. 6.
Effects of Pet protein on HEp-2 epithelial cells in situ. (A and D) Untreated HEp-2 cells. (B and E) HEp-2 cells treated with 5 μg of Pet protein/ml for 3 h. (C and F) HEp-2 cells treated with 5 μg of Pet S260I protein/ml for 3 h. (A, B, and C) Preparations were stained with Coomassie blue. (D, E, and F) Preparations were incubated with anti-α- and anti-β-spectrin antibodies and were developed with anti-rabbit antibodies labeled with fluorescein. The slides were observed under light (A, B, and C) and fluorescence (D, E, and F) microscopy. Magnification, ×40.
To determine if the morphological alterations of the HEp-2 cell membrane caused by Pet were related to fodrin disruption, the HEp-2 cells held in suspension were treated with Pet protein and visualized by immunofluorescence using anti-α- and β-spectrin antibodies. The control slides from untreated cells showed a homogeneous distribution of fluorescence, indicating that fodrin was not modified (Fig. 6D). On the other hand, the Pet-treated cell preparations showed cellular modification characterized by cellular swelling and irregular distribution of fluorescence, indicating fragmentation of fodrin (Fig. 6E) and, as a consequence, a disarrangement of the cell membrane. On the other hand, when the HEp-2 cells were treated with precipitated supernatant from the mutant, Pet S260I, the cells appeared to be normal, as seen in the control cells (Fig. 6F).
DISCUSSION
The cytoskeleton is a target for many intracellular microorganisms, and in some bacterial and parasite pathogens this effect is accomplished by triggering a rearrangement of the membrane skeleton (33, 34). Recently it was shown that Pet EAEC toxin elicits cytopathic effects characterized by release of the cellular focal contact from glass substratum, as well as rounding and detachment of cells, and that these effects were associated with damage to the actin cytoskeleton (30).
The present study shows that cytoskeletal effects by Pet on epithelial cells are associated with the degradation of fodrin, an analog of spectrin. The spectrin protein accounts for 75% of the membrane skeleton protein mass in erythrocytes, and spectrin analogs (such as fodrin) are widely distributed among the majority of cell types. The spectrin-based membrane skeleton is a submembranous, spatially limited, two-dimensional lattice that binds a subset of membrane proteins (2).
The results obtained showed that one of the action mechanisms of Pet is the degradation of both α- and β-spectrin chains (from erythrocyte membranes) and α- and β-fodrin chains (from epithelial HEp-2 cell membranes). These data may explain previous observations from many other investigators who showed cell damage by EAEC. Hicks et al. (19), using the in vitro organ culture model, showed that EAEC strains induced exfoliation of enterocytes from the mucosal surface of intestinal biopsies from children. Nataro et al. (25), utilizing T84 cultured cells, observed that EAEC induced vesiculation of the microvillar membrane followed by exfoliation of cells from the monolayer. On the other hand, intestinal necropsy of Mexican children who died as a consequence of EAEC infection showed alteration on villi morphology and epithelial necrosis (Eslava et al., Abstr. 93rd Gen. Meet. Am. Soc. Microbiol. 1993). Recently, Pet has been found to be involved in the damage of epithelial cells, since Pet produces contraction of the cytoskeleton and cell detachment (30). All these effects may occur and as seen here, degradation of spectrin leads to a disarrangement of the membrane skeleton, since spectrin maintains the connection of the plasma membrane to the cytoskeleton as a mechanism for the generation of cell shape and mechanical stability. Therefore, the cytoskeletal alterations produced by Pet are a consequence of the disruption of spectrin and actin filament connection (2). The vesiculation of the microvillar membrane by EAEC may occur because the lower portion of the actin filament bundle in the microvillus core is anchored in the specialized cortex at the apex of the intestinal epithelial cell, which contains a dense network of spectrin molecules that overlies a layer of intermediate filaments (12).
Pet has also been shown to produce enterotoxic effects on rat jejunal mucosa mounted in an Ussing chamber (29), which may also be explained by the disruption of the membrane skeleton. This is due to the fact that ankyrins have high spectrin affinity (2), which is important for their intermediate role as an adapter between spectrin and the plasma membrane. Na+/K+ pumps and Na+ channels are integral proteins that colocalize with ankyrin; this feature is part of the organization that the spectrin cytoskeleton indirectly supports (32).
Both enterotoxic and cytotoxic effects depend upon the serine protease motif of Pet (30). The present study shows that the use of a mutation at this site (Pet S260I) has no effect on spectrin degradation and that pretreatment of Pet with a serine protease inhibitor prevented spectrin degradation. These data indicate that spectrin is the target of Pet and that the catalytic site is the serine protease motif.
It is interesting to note that spectrin is an intracellular protein, which together with other proteins forms a net-like meshwork of fibrous proteins just beneath the surface membrane. This observation suggests that Pet has to be internalized to cause spectrin degradation. The results obtained in the assay performed with HEp-2 cells in suspension support this proposition and show that the α- and β-fodrin chains from Pet-treated cells, but not from the untreated cells, were degraded (Fig. 5).
Another characteristic of EAEC infection involves enhanced mucus secretion from the mucosa with trapping of the bacteria in a bacterium-mucus biofilm (19, 38). The possible explanation of this effect may also be associated with the disruption of spectrin analogs into goblet cells, allowing delivery of secretory granules containing mucins and forming the bacterium-mucus biofilm (31).
It has been reported that the disruption of spectrin can be caused by other pathogens such as Trichomonas vaginalis, which produces a cysteine protease that is able to degrade spectrin. In contrast to Pet protein, this 30-kDa spectrin protease appears to be nonsecreted, having the ability to degrade spectrin faster and produce smaller subproducts. The intimate contact that occurs between parasite and host cells suggests that the effector delivery may take place through a membrane fusion event or by release through exocytotic microvesicles (15). However, the morphologic effects on erythrocytes and epithelial cells as a consequence of spectrin degradation by this spectrin protease and Pet toxin are the same: cell rounding, detachment, and cell death (1, 30). Interestingly, the most common targets for microbial pathogens, which interact with the host cells' cytoskeleton, are in fact components of the cytoplasmic network, mostly actin, although the ability to target spectrin in the host cell has been reported for the intracellular protozoans Plasmodium falciparum and Plasmodium bergei (10).
Spectrin proteases are involved in many other mechanisms of substrate degradation on erythrocytes (spectrin) and nonerythroid cells (fodrin), such as those that occur under normal and pathophysiological conditions. Under normal conditions, there are membrane-bound proteinases that preferentially degrade oxidatively damaged erythrocyte membrane proteins as a secondary antioxidant defense (3). This secondary antioxidant defense mechanism for the removal of the oxidatively damaged cell membrane proteins by proteinases includes degradation of spectrin by a membrane-bound serine protease of 80 kDa (16), which produces a spectrin breakdown product of around 120 kDa, while the proteolytic activity is inhibited by the serine protease inhibitor diisopropylfluorophosphate (3). These last reports suggest that Pet toxin, the serine protease secreted by EAEC, could use the same cell pathway to degrade spectrin and fodrin from erythrocytes and epithelial cells. On the other hand, under pathophysiological conditions, calcium-activated proteases, such as the calpains, are important intermediaries connecting [intracellular Ca2+] with cell death (11) through degradation of the preferred calpain substrate α-spectrin (6).
Cleavage α-fodrin (nonerythroid spectrin) has been detected during apoptosis in a variety of cell lines of murine and human origin and is inhibited under conditions where apoptosis is inhibited. Interestingly, in cell cultures that have undergone extensive apoptosis, fodrin is cleaved to a single detectable fragment of 120 kDa. However, in cultures containing fewer apoptotic cells, a large fragment of 150 kDa was observed (23), suggesting that the 120-kDa fragment is a further breakdown product of the 150-kDa fodrin fragment. In addition, the formation of these apoptotic nuclei in JURKAT T cells, after Fas antigen ligation, was blocked by the serine protease inhibitors TPCK (tolylsulfonyl phenylalanyl chloromethyl ketone) and DCI and by the interleukin 1β-converting-enzyme (ICE) inhibitor, VAD-FMK; but chromatin degradation and morphological changes were inhibited only by TPCK (7, 35). Nath et al. (28) have found that in cell necrosis (e.g., maitotoxin-treated neuroblastoma SH-SY5Y cells), the α-fodrin breakdown product of 150 kDa was produced by cellular calpains, whereas in neuronal cells undergoing apoptosis an additional breakdown product of 120 kDa was observed. The formation of the 120-kDa fragment was insensitive to calpain inhibitors but was completely blocked by ICE-like protease inhibitors. Furthermore, the authors propose that calpain and ICE can each cleave α-fodrin at two sites; one is VY↓GMMP for a 150-kDa fragment, which is located within a sequence in repeat 11 and just N terminal of the calmodulin-binding domain, whereas ICE cleavage for a 120-kDa fragment must be C terminal to the PEST sequence located between repeats 12 and 13 (28). Interestingly, Pet toxin produced the 120-kDa breakdown product, and its N-terminal sequence was the same as the mature α-spectrin, indicating that the cleavage site must be C terminal and similar to the ICE cleavage site.
In summary, many autotransporter proteins have been implicated as important or putative virulence factors in many gram-negative pathogens (17); however, none of them have been as well characterized as Pet protein from enteroaggregative E. coli, which is part of the SPATE (serine protease autotransporters of Enterobacteriaceae) subfamily. Pet toxin caused enterotoxic and cytotoxic activity involving its serine protease motif. Cytoskeleton contraction and loss of actin stress fibers were also observed, suggesting that one or more components of this cellular structure were the Pet target (30). This study showed that Pet toxin produces damage to the epithelial cells through a novel mechanism of the bacterial toxin involving internalization of the serine protease and α-fodrin degradation. Such alterations of the membrane skeleton could explain previous observations in Pet-intoxicated intestinal segments, HEp-2 and HT29 C1 cells, which showed an induction of a net secretory state, a cytoskeleton contraction, and a loss of actin stress fibers (29, 30). The proteolytic demolition of spectrin within these cells may induce a disaggregation of the membrane skeleton and of its connections with the cytoplasmic actin network, leading to membrane alteration and finally to cell death. The α-fodrin degradation by Pet toxin may occur by following the normal or pathophysiological pathway shown above, and the death of enterocytes may occur due to apoptosis, as suggested by the production of the 120-kDa spectrin breakdown product.
ACKNOWLEDGMENTS
This work was supported by Consejo Nacional de Ciencia y Tecnología de México (CONACYT) grant 25846M to C.E.
We thank Ruth García for her help with HEp-2 cell cultures, Ulises Hernandez for Pet purification experiments, Wendy Resneck, Renato Capello, Rocío Huerta, and Gabriel Pérez for their technical assistance, and Robert Bloch for providing the antifodrin antibodies.
REFERENCES
- 1.Alderete J F, Pearlman E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis. 1984;60:99–105. doi: 10.1136/sti.60.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett V, Gilligan D M. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- 3.Beppu M, Inoue M, Ishikawa T, Kikugawa K. Presence of membrane-bound proteinases that preferentially degrade oxidatively damaged erythrocyte membrane proteins as secondary antioxidant defense. Biochim Biophys Acta. 1994;1196:81–87. doi: 10.1016/0005-2736(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 4.Bhan M K, Raj P, Levine M M, Kaper J B, Bhandari N, Srivastava R, Kumar R, Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989;159:1061–1064. doi: 10.1093/infdis/159.6.1061. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Chard P S, Bleakman D, Savidge J R, Miller R J. Capsaicin-induced neurotoxicity in cultured dorsal root ganglion neurons: involvement of calcium-activated proteases. Neuroscience. 1995;65:1099–1108. doi: 10.1016/0306-4522(94)00548-j. [DOI] [PubMed] [Google Scholar]
- 7.Chow S C, Weis M, Kass G E, Holmstrom T H, Eriksson J E, Orrenius S. Involvement of multiple proteases during Fas-mediated apoptosis in T lymphocytes. FEBS Lett. 1995;364:134–138. doi: 10.1016/0014-5793(95)00370-o. [DOI] [PubMed] [Google Scholar]
- 8.Cobeljic M, Miljkovic-Selimovic B, Paunovic-Todosijevic D, Velickovic Z, Lepsanovic Z, Zec N, Savic D, Ilic R, Konstantinovic S, Jovanovic B, Kostic V. Enteroaggregative Escherichia coli associated with an outbreak of diarrhoea in a neonatal nursery ward. Epidemiol Infect. 1996;117:11–16. doi: 10.1017/s0950268800001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 10.Deguercy A, Hommel M, Schrevel J. Purification and characterization of 37-kilodalton proteases from Plasmodium falciparum and Plasmodium berghei which cleave erythrocyte cytoskeletal components. Mol Biochem Parasitol. 1990;38:233–244. doi: 10.1016/0166-6851(90)90026-i. [DOI] [PubMed] [Google Scholar]
- 11.del Cerro S, Arai A, Kessler M, Bahr B A, Vanderklish P, Rivera S, Lynch G. Stimulation of NMDA receptors activates calpain in cultured hippocampal slices. Neurosci Lett. 1994;167:149–152. doi: 10.1016/0304-3940(94)91049-9. [DOI] [PubMed] [Google Scholar]
- 12.Djabali K. Cytoskeletal proteins connecting intermediate filaments to cytoplasmic and nuclear periphery. Histol Histopathol. 1999;14:501–509. doi: 10.14670/HH-14.501. [DOI] [PubMed] [Google Scholar]
- 13.Eslava C, Navarro-Garcia F, Czeczulin J R, Henderson I R, Cravioto A, Nataro J P. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3155–3163. doi: 10.1128/iai.66.7.3155-3163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang G D, Lima A A, Martins C V, Nataro J P, Guerrant R L. Etiology and epidemiology of persistent diarrhea in northeastern Brazil: a hospital-based, prospective, case-control study. J Pediatr Gastroenterol Nutr. 1995;21:137–144. doi: 10.1097/00005176-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Fiori P L, Rappelli P, Addis M F, Mannu F, Cappuccinelli P. Contact-dependent disruption of the host cell membrane skeleton induced by Trichomonas vaginalis. Infect Immun. 1997;65:5142–5148. doi: 10.1128/iai.65.12.5142-5148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujino T, Ishikawa T, Inoue M, Beppu M, Kikugawa K. Characterization of membrane-bound serine protease related to degradation of oxidatively damaged erythrocyte membrane proteins. Biochim Biophys Acta. 1998;1374:47–55. doi: 10.1016/s0005-2736(98)00131-x. [DOI] [PubMed] [Google Scholar]
- 17.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 18.Henry F, Udoy A, Wanke C, Aziz K. Epidemiology of persistent diarrhea and etiologic agents in Mirzapur, Bangladesh. Acta Paediatr Suppl. 1996;381:27–31. doi: 10.1111/j.1651-2227.1992.tb12368.x. [DOI] [PubMed] [Google Scholar]
- 19.Hicks S, Candy D C, Phillips A D. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun. 1996;64:4751–4760. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huppertz H I, Rutkowski S, Aleksic S, Karch H. Acute and chronic diarrhoea and abdominal colic associated with enteroaggregative Escherichia coli in young children living in western Europe. Lancet. 1997;349:1660–1662. doi: 10.1016/S0140-6736(96)12485-5. [DOI] [PubMed] [Google Scholar]
- 21.Itoh Y, Nagano I, Kunishima M, Ezaki T. Laboratory investigation of enteroaggregative Escherichia coli O untypeable:H10 associated with a massive outbreak of gastrointestinal illness. J Clin Microbiol. 1997;35:2546–2550. doi: 10.1128/jcm.35.10.2546-2550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Martin S J, O'Brien G A, Nishioka W K, McGahon A J, Mahboubi A, Saido T C, Green D R. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem. 1995;270:6425–6428. doi: 10.1074/jbc.270.12.6425. [DOI] [PubMed] [Google Scholar]
- 24.Mayer H B, Wanke C A. Enteroaggregative Escherichia coli as a possible cause of diarrhea in an HIV-infected patient. N Engl J Med. 1995;332:273–274. doi: 10.1056/NEJM199501263320417. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 25.Nataro J P, Hicks S, Phillips A D, Vial P A, Sears C L. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun. 1996;64:4761–4768. doi: 10.1128/iai.64.11.4761-4768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nataro J P, Kaper J B, Robins-Browne R, Prado V, Vial P, Levine M M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987;6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Nath R, Raser K J, Stafford D, Hajimohammadreza I, Posner A, Allen H, Talanian R V, Yuen P, Gilbertsen R B, Wang K K. Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319:683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarro-García F, Eslava C, Villaseca J M, Lopez-Revilla R, Czeczulin J R, Srinivas S, Nataro J P, Cravioto A. In vitro effects of a high-molecular-weight heat-labile enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3149–3154. doi: 10.1128/iai.66.7.3149-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro-Garcia F, Sears C, Eslava C, Cravioto A, Nataro J P. Cytoskeletal effects induced by Pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect Immun. 1999;67:2184–2192. doi: 10.1128/iai.67.5.2184-2192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver M G, Specian R D. Cytoskeleton of intestinal goblet cells: role of microtubules in baseline secretion. Am J Physiol. 1991;260:G850–G857. doi: 10.1152/ajpgi.1991.260.6.G850. [DOI] [PubMed] [Google Scholar]
- 32.Piepenhagen P A, Nelson W J. Biogenesis of polarized epithelial cells during kidney development in situ: roles of E-cadherin-mediated cell-cell adhesion and membrane cytoskeleton organization. Mol Biol Cell. 1998;9:3161–3177. doi: 10.1091/mbc.9.11.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez A, Rioult M G, Ora A, Andrews N W. A trypanosome-soluble factor induces IP3 formation, intracellular Ca2+ mobilization and microfilament rearrangement in host cells. J Cell Biol. 1995;129:1263–1273. doi: 10.1083/jcb.129.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlegel J, Peters I, Orrenius S. Isolation and partial characterization of a protease involved in Fas-induced apoptosis. FEBS Lett. 1995;364:139–142. doi: 10.1016/0014-5793(95)00374-i. [DOI] [PubMed] [Google Scholar]
- 36.Smith H R, Cheasty T, Rowe B. Enteroaggregative Escherichia coli and outbreaks of gastroenteritis in UK. Lancet. 1997;350:814–815. doi: 10.1016/s0140-6736(05)62611-6. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 37.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzipori S, Montanaro J, Robins-Browne R M, Vial P, Gibson R, Levine M M. Studies with enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect Immun. 1992;60:5302–5306. doi: 10.1128/iai.60.12.5302-5306.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vial P A, Robins-Browne R, Lior H, Prado V, Kaper J B, Nataro J P, Maneval D, Elsayed A, Levine M M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988;158:70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]