Abstract
Myocardium generates power to perform external work on the circulation; yet, many questions regarding intermolecular mechanisms regulating power output remain unresolved. Power output equals force × shortening velocity, and some interesting new observations regarding control of these two factors have arisen. While it is well established that sarcomere length tightly controls myocyte force, sarcomere length-tension relationships also appear to be markedly modulated by PKA-mediated phosphorylation of myofibrillar proteins. Concerning loaded shortening, historical models predict independent cross-bridge mechanics; however, it seems that the mechanical state of one population of cross-bridges affects the activity of other cross-bridges by, for example, recruitment of cross-bridges from the non-cycling pool to the cycling force-generating pool during submaximal Ca2+ activation. This is supported by the findings that Ca2+ activation levels, myofilament phosphorylation, and sarcomere length are all modulators of loaded shortening and power output independent of their effects on force. This fine tuning of power output probably helps optimize myocardial energetics and to match ventricular supply with peripheral demand; yet, the discernment of the chemo-mechanical signals that modulate loaded shortening needs further clarification since power output may be a key convergent point and feedback regulator of cytoskeleton and cellular signals that control myocyte growth and survival.
Keywords: Muscle, Muscle activity, Muscle contraction, Muscle mechanics, Muscle mechanisms, Cardiac muscle, Cardiac myocytes, Cardiac sarcomere, Cardiac function, Cardiac cell
Cardiac muscle generates power to propel blood through the pulmonary and systemic circulation. The muscle’s capacity to perform work is highly tuned by a number of acute modulators including second messengers (e.g., Ca2+), post-translational modifications (e.g., phosphorylation of myofilament proteins), and sarcomere strain. The change in cardiac myocyte power by sarcomere strain is a major factor in defining ventricular function curves, also known as Frank–Starling relationships whereby increased end-diastolic volume increases stroke volume. It follows that changes in myocyte power by intracellular signals such as activator Ca2+ and myofilament phosphorylation shift ventricular function curves upward or downward, which yields a family of curves that have provided a basic science framework for clinical manifestations and drug therapy to treat heart disease for over 50 years. In keeping with this line of reasoning, there needs to be precise clarity in the sub-cellular factors that determine and modulate myocyte power output for any transformative advancement in clinical treatments and outcomes related to ventricular function.
Cardiac myocyte power output is determined by two mechanical properties: force generation and shortening rate. For a given cardiac myocyte, there exists a distinct relationship between force and velocity where shortening velocity rises in a hyperbolic fashion as force is reduced. Power output is the product of force × velocity; thus, power is zero at the two extremes of the force–velocity relationship (i.e., isometric force (no shortening) and maximum velocity of shortening (no force)) and rises to a maximum at intermediate loads (~30% of isometric force). The relative load that a cardiac sarcomere works against during systole is virtually impossible to resolve since it depends on the complex interplay between filling volume (preload), Ca2+ activation of myofilaments, arterial impedance (a determinant of afterload), and wall stress (afterload), all of which are in constant flux during systole. It stands to reason, though, that the force produced by the cardiac myofilaments must exceed the load on the sarcomeres for shortening and ejection to occur. During myocyte activation, the number of force-generating cross-bridges (to work against loads opposing shortening) is largely determined by filling volume and the degree of Ca2+ activation of the myofilaments. In an ideal system, the number of cycling force-generating cross-bridges will be tuned to yield sarcomere shortening velocities that produce both peak power and efficiency (i.e., work per ATPase). In actuality, a regulatory system that tends to increase the number of force-generating cross-bridges will always increase the rate that sarcomeres move a given load, and, in the heart, this will increase ventricular power since in vivo afterloads are most likely always enough to exceed ~30% of maximal myocyte force. So how is the number of force-generating cross-bridges determined in a cardiac myocyte? This has been a long-standing area of interest of muscle research. For the context of this review, consider three main factors that determine the number of force-generating cross-bridges: (i) activator [Ca2+], (ii) strong binding myosin cross-bridges, and (iii) sarcomere length. Activator [Ca2+] acts as both a trigger and rheostat for muscle activation, which is defined mechanistically as making thin filament sites available for strong binding myosin cross-bridges [11]. Ca2+ acts as a trigger by binding to the N-terminal region of cardiac troponin C, which initiates a mechanical signal transduction pathway that results in the azimuthal movement of tropomyosin on actin filament. This allows for energized myosin cross-bridges to bind strongly to actin monomers. Strongly bound myosin cross-bridges move tropomyosin further, which allows cross-bridges to make the transition from strongly bound non-force-generating to strongly bound force-generating [44]. Activator [Ca2+] also acts as a rheostat by adjusting the number of regulatory units (one troponin, one tropomyosin molecule, and seven actin monomers) that switch from a blocked to a closed state, which permits subsequent binding of strongly attached cross-bridges to further move tropomyosin, shifting the equilibrium in favor of regulatory units in the open state [16, 32, 41, 46]. The formation of strongly bound cross-bridges also is thought to provide axial propagation of the mechano-activation signal, which is likely transduced, in part, by the physical overlap of neighboring tropomyosin molecules. This mechanical cooperation is thought to yield activation of ~3 regulatory units per strongly bound myosin cross-bridge [34, 43].
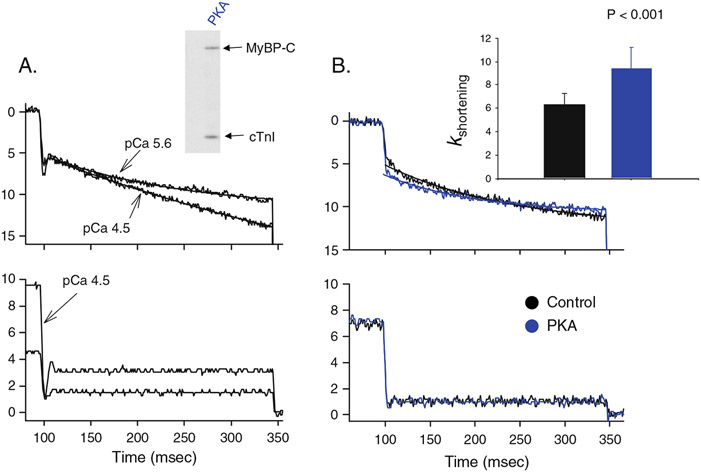
Sarcomere strain is another primary regulator of force production. As sarcomere length increases, there is an exponential rise in force in intact myocardial preparations [40], skinned myocardium [24], and myofilaments [10]. Sarcomere length–tension relationships change with the level of Ca2+ activation, which elicits a family of sarcomere length–tension relationships (similar to ventricular function curves). Sarcomere length–tension relationships shift to the left and from concave upward to concave downward as activator Ca2+ increases; this yields shallower length- tension relationships. More recent studies have found that sarcomere length–tension relationships also vary between individual myocyte preparations [1, 2, 20]. This distribution in length–tension relationships have been shown to be due, in part, to localization in the ventricular wall with the endocardial preparations yielding a greater length dependence of Ca2+ sensitivity of force than epicardial preparations [1, 2]. Recent work from our laboratory found that individual rat left ventricular cardiac myocytes exhibited a nearly bimodal distribution of length–tension relationships, which appeared to be due to the level of PKA-mediated phosphorylation of myosin binding protein-C (MyBP-C) and cardiac troponin I (cTnI) [20]. These findings raise an interesting situation with regards to the physiology of cardiac myocytes in response to β-adrenergic stimulation and its downstream signaling molecule PKA. β1-adrenergic agonists clearly increase the Ca2+ transient [9, 45], which would tend to shallow length–tension relationships (as mentioned above), but PKA-mediated phosphorylation of myofibrillar proteins markedly steepens myocyte length–tension relationships, which is consistent with the fact that β-adrenergic stimulation steepens ventricular function curves [18, 38]. Thus, it appears that, in response to β-adrenergic stimulation, the myofilaments have an inherent mechanism by which to take advantage of the greater activator Ca2+ to increase cross-bridges for more power generation but also become exquisitely sensitive to length such that the myofilaments more readily deactivate in response to shortening. This is consistent with our recent finding that PKA-mediated phosphorylation increased the curvature of length traces during lightly loaded contractions (Fig. 1). In addition, several labs have found that PKA-mediated phosphorylation increased the steepness (i.e., Hill coefficients) of tension–pCa relationships [21, 25, 42]. Taken together, these findings imply that β-adrenergic stimulation yields a myofibrillar apparatus that is able to activate more explosively (by more cooperative activation) but is also more length sensitive so that during shortening, which inevitably decreases the number of cross-bridges, the myofibrillar system more readily inactivates to assist in relaxation; this is especially important to allow for optimal filling during a shortened cardiac cycle because of the increased heart rate.
Fig. 1.
PKA-mediated phosphorylation of myofilament proteins increases the curvature of length traces during isotonic contractions. a During lightly loaded contractions, length traces are nearly linear during maximal Ca2+ activation and become curvilinear during submaximal Ca2+ activation. This implies shortening-induced thin filament cooperative deactivation. b PKA caused increased curvature of length traces in submaximally Ca2+ activated cardiac myocyte preparations undergoing lightly loaded contractions, implicating greater shortening-induced cooperative deactivation following PKA-mediated phosphorylation of MyBP-C and cTnI
The other factor in the power output equation is velocity of shortening. The control of striated muscle shortening has been studied for generations and has engaged several of the finest muscle physiologists. In 1957, A.F. Huxley provided a framework for the study of mechanisms that regulate muscle shortening with his cross-bridge theory of contraction [22]. A central hypothesis of the 1957 model is that filament sliding causes cross-bridges to move through their working range quicker and detach sooner. This elicits two important consequences on the cross-bridge cycle: (i) fewer force-generating cross-bridges since detachment occurs sooner and (ii) less force per attached cross-bridge since the average position of the cross-bridge would shift to a position where its spring is less stretched [11]. Muscle shortening thus decreases force in two ways; it decreases the number of attached cross-bridges, and it decreases the average force per cross-bridge, and in addition, the higher the velocity, the greater will be these two effects. Consistent with this theory, Ford [12] performed sophisticated mechanical measurements in intact frog tibialis anterior skeletal muscle fiber preparations, and results implied decreased force per cross-bridge since the fall in force was greater than the fall in fiber stiffness (an index of number of force-generating cross-bridges) at least during shortening at loads less than 50% of isometric force. More recently, Piazzesi et al. [35] combined X-ray interference and mechanical measurements and observed very little change in force per cross-bridge and stroke size but a precipitous fall in cross-bridge number when frog skeletal muscle fibers shortened against loads from isometric to 40% of isometric force. At loads between 40% and 20% isometric force, the number of cross-bridges continued to fall, and force per bridge started to decrease modestly. At loads less than 20%, isometric force per bridge dropped considerably, and there was a small increase in cross-bridge stroke distance. Overall, it appears that several of the key aspects of the 1957 model (i.e., decrease in the number of cross-bridges, less force per cross-bridge, increased detachment rate) have held up against the test of time but with the caveat that there is probably a load- and shortening velocity-dependence in the quantitative changes of these parameters.
Another interesting aspect of the 1957 model is that at one extreme of the force–velocity relationship where force is zero, and muscle shortening is maximal (i.e., Vmax), filament sliding occurs at speeds where the positive force exerted by cycling cross-bridges is exactly balanced by cross-bridges that have translated past their working range and are exerting compressive forces that resist shortening. According to this concept, maximum velocity of shortening is predicted to be dependent on the detachment rate of compressed cross-bridges and independent of the number of cycling cross-bridges acting in parallel. One way to test this prediction is to change the number of cycling cross-bridges by changing sarcomere length. The number of cross-bridges will progressively decrease along the descending limb of the length–tension relationship due to less filament overlap and, along the ascending limb, due to fewer numbers of cross-bridges producing force in the positive vector because of overlap with thin filaments from the opposite side of the sarcomere. Consistent with the 1957 model, speed of shortening against a very light load stayed nearly constant over the sarcomere length range of ~3.1 μm to 2.2 μm [15]. Consistent with this, velocity of unloaded shortening (Vo) was found to be independent of sarcomere length over the sarcomere length range of 1.8 μm to 2.7 μm based upon slack test analysis of frog skeletal muscle fibers [8]. In these fibers, there was an exponential rise in Vo at sarcomere lengths above 2.7 μm, which was likely due to recoil of passive elastic elements of the prestretched fiber. At sarcomere lengths less than 1.8 μm, Vo decreased linearly as sarcomere length decreased; this was ostensibly due to increased passive internal loads perhaps due to titin compression and/or thick filaments abutting against Z disks. In cardiac muscle, the velocity of shortening during lightly loaded contractions showed a similar sarcomere length dependence (as seen in skeletal muscle fibers) during near maximal Ca2+ activations, but during submaximal Ca2+ activations, shortening velocities were highly dependent on sarcomere length over the SL range of 1.7–2.2 μm as measured directly by calibrated laser light diffraction [6, 7]. A similar sarcomere length of shortening velocity during lightly loaded contractions also was observed in ferret papillary muscle preparations using an electronic feedback system to track muscle segment length [29]. In summary, it seems while Vmax sometimes appears to arise independent of cross-bridge number, there are some situations such as more extreme sarcomere lengths and during submaximal Ca2+ activations in cardiac muscle where Vmax is highly sarcomere length dependent.
Translating the 1957 model to loaded contractions (i.e., the entire force–velocity curve), in theory, altering the number of cross-bridges in parallel (by changing Ca2+ or sarcomere length) should yield force–velocity relationships that superimpose when normalized for changes in isometric force (i.e., relative force velocity relationships). This is predicted if the activity of one population of cross-bridges is not influenced by the activity of other cross-bridge population states. There have been mixed results in this regard for skeletal muscle experiments; for example, some studies have observed nearly superimposable force–velocity curves [13, 37], while others reported a clear downward shift in normalized force–velocity relationships when Ca2+ activation levels were reduced in glycerinated muscle fibers [23]. In these latter experiments, the peak normalized power output fell ~60% due to slower loaded shortening at relative loads around 30% isometric force. Two excellent reviews [33, 36] speculate about possible reasons underlying the varied results regarding how Ca2+ activation levels affect force–velocity relationships in skeletal muscle fiber preparations, but the mechanisms remain unresolved.
Several studies have investigated how force–velocity curves are affected by Ca2+ activation levels and sarcomere length in cardiac muscle. Ford and co-workers [3] reported that force–velocity curves and power–load curves shifted nearly coincidentally with the rise and fall of twitch force in intact cat cardiac muscle preparations. Interestingly, Vmax was nearly constant over much of the twitch time, which the authors interpreted as Vmax being an inaccurate indicator of activation state. In this study, the change in power was due primarily to the change in force; in fact, it appeared that loaded shortening velocity was faster at lower levels of twitch force. This may have arisen from extracellular viscoelastic elements contributing to shortening, especially early in the low load clamp when series elastic recoil would be most prominent and early in the twitch when force-generating cross-bridges are low (see discussion on this issue by Sweitzer and Moss [39]). Overall, the results from this study were argued by the authors to be consistent with the 1957 model, i.e., force–velocity curves are independent of the level of Ca2+ activation, and cycling cross-bridges are not influenced by the state of other cross-bridges. In this case, changes in muscle power output would be due entirely to variations in muscle force. Contrary to this idea, loaded sarcomere shortening velocities were found to be highly dependent upon the level of Ca2+ activation even after normalization for changes in isometric force in intact rat trabeculae preparations [6]. Consistent with this, rat permeabilized cardiac myocyte preparations were observed to yield force–velocity curves that also were tightly regulated by Ca2+ activation levels even after normalization for changes in isometric force [30]. In this study, peak absolute power output increased ~6.5-fold when Ca2+ activation levels were increased from ~25% isometric force to maximal force; this was due to both an increase in force and loaded shortening as normalized force–velocity curves shifted upward with increased Ca2+ activation levels. These results are more consistent with results from Julian [23] in skeletal muscle fibers and suggest that the activity of one pool of cross-bridges is affected by the actions of other cross-bridges. Consistent with this, the addition of a strongly binding myosin analog (NEM-S1) that cooperatively enhances thin filament activation eliminated any Ca2+ activation dependence of normalized power output; this implies that thin filament activation, mediated by the number of strongly bound cross-bridges, is a prominent regulator of cardiac myocyte power output [31].
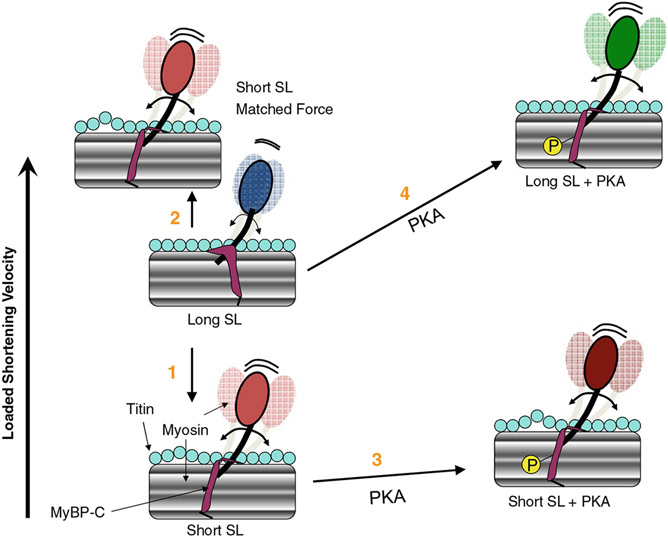
Last, with regards to the effects of sarcomere length on force–velocity relationships, Granzier et al. [17] reported that force–velocity relationships were shifted upward as sarcomere length was decreased from 3.1 to 2.4 μm in intact skeletal muscle fibers, which suggests that the mechanical properties of myosin cross-bridges change with sarcomere length. A significant sarcomere length dependence of force–velocity and power–load curves also has been reported in rat skinned cardiac myocyte preparations [26]. In this study, loaded shortening velocity was slower (i.e., there was downward shift in relative force–velocity and power–load curves) at loads less than ~40% isometric force [26]. A plausible mechanism to explain slower loaded shortening and decreased power output at short sarcomere length is the coincident decrease in thin filament activation levels at short sarcomere length given that force was lower due to the well-described length dependence of Ca2+ sensitivity of force [14]. In fact, when Ca2+ activated force and presumably thin filament activation levels were matched at short sarcomere length to those at long sarcomere length (by increasing the activator [Ca2+]), short sarcomere length actually yielded faster loaded shortening velocities and greater peak normalized power output [26]. This suggests a myofibrillar mechanism that tends to speed loaded cross-bridge cycling to minimize the fall of power at short sarcomere length. Interestingly, treatment of myocytes with 2% dextran to ostensibly compress the myofilament lattice also caused faster loaded shortening at short sarcomere length compared to long sarcomere lengths, again implicating a myofibrillar mechanism that leads to faster loaded cross-bridge cycling at short sarcomere length [26]. This finding is consistent with sarcomere length per se modulating the mechanical properties of the individual cross-bridges and implicates a myofibrillar mechanism that may help sustain ventricular power during periods of lower preloads, and perhaps a breakdown of this mechanism may be causative of impaired function of failing hearts. A potential mechanism for faster loaded shortening at short sarcomere length is that as sarcomere length is shortened, titin becomes less taut which reduces the impedance of cross-bridges, a process that may be mediated by titin’s interaction with MyBP-C on the thick filament (Fig. 2). In other words, a slackened titin leads to greater myosin head radial and azimuthal mobility. This yields faster cross-bridge cycling by creating more flexible cross-bridges that are more likely to maintain thin filament activation, allowing more force-generating cross-bridges to work against a fixed load. Importantly, though, this mechanism alone cannot overcome the decrease in the number of cross-bridges perhaps induced by increased lattice spacing that normally occurs with short sarcomere length, which is why loaded shortening is slower at short sarcomere length when the activator [Ca2+] is the same, and force is greater at long sarcomere length compared to short sarcomere length. These mechanistic ideas are consistent with findings that titin binds C-terminal domains of MyBP-C [28], and ablation of MyBP-C causes both radial displacement of cross-bridges away from the thick filaments [4] to faster loaded shortening in MyBP-C knock-out cardiac myocyte preparations [27]. Another mechanical feature consistent with this model is that PKA increases the sarcomere length dependence of power in skinned cardiac myocytes [19]. This resulted from a greater increase in power at long sarcomere length than short sarcomere length after PKA. This mechanical result fits the conceptual model that cross-bridge flexibility is already elevated at short sarcomere length, thus limiting a PKA-mediated increase in cross-bridge mobility; such an increase in mobility has been shown in response to phosphorylation of MyBP-C [5]. Figure 2 summarizes the interplay between sarcomere length, titin compliance, PKA-mediated MyBP-C phosphorylation, and cross-bridge flexibility on loaded shortening. Assessment of this model is elusive, but animal models are available to test aspects of it including the role of titin compliance and exclusive phosphorylation of MyBP-C on changes in cross-bridge flexibility, loaded shortening, and power output. Alternatively, it is possible that Ca2+ binding, sarcomere length, and phosphorylation of myofibrillar proteins could modulate the activation state of thin filaments. For example, changing the stiffness or persistence length of the thin filaments could markedly effect cooperative activation and increase the span of a regulatory unit, which could have a profound effect on the number of cross-bridges involved in generating power. Just how such factors modulate thin filament dynamics is an area of active research, and clear answers to these issues should be soon forthcoming.
Fig. 2.
Conceptual model of interplay between sarcomere length (SL), PKA-mediated phosphorylation (P) and loaded shortening in cardiac myofilaments. (1) Loaded shortening is decreased at short SL due to decreased numbers of strongly bound cross-bridges to maintain activation of the thin filament due, in part, to increased lattice spacing. (2) If force and, presumably, thin filament activation levels are matched, loaded shortening is actually faster at short SL. Faster loaded shortening at short SL (at matched force) is illustrated to result from titin becoming less taut, which may reduce the mechanical impedance on the myosin cross-bridge (perhaps mediated by titin’s interaction with MyBP-C on the thick filament, which in turn increases myosin head radial and azimuthal mobility). This may lead to faster cross-bridge cycling by the more flexible cross-bridges being better able to maintain thin filament activation and thus allowing more cross-bridges to work against a fixed load. (3) With PKA, loaded shortening appears to be minimally affected at short SL but is faster at long SL (4), perhaps via phosphorylation of MyBP-C, which may increase the flexibility of cross-bridges resulting in more cooperative activation of the thin filament so more cross-bridges are available to work against a given load especially early in the cardiac twitch contraction. The PKA effect is greater at long SL than short SL because cross-bridge flexibility is already increased at short SL due to reduced titin strain
In summary, there are a number of factors that modulate power output in striated muscle. Suffice it to say any factor that increases the number of force-generating cross-bridges will virtually always augment power. Also, it appears that (especially during submaximal Ca2+ activations) cross-bridges do not act as independent entities, but rather, they communicate to one another by mechano-sensitive signals that, in some cases, may sustain thin filament activation to increase the probability of a neighboring cross-bridge to be recruited to actively engage in loaded shortening. This would in effect yield faster loaded shortening at a given relative load consistent with the findings that increased Ca2+ activation, loss of MyBP-C, PKA-mediated myofibrillar phosphorylation, and short sarcomere length have all been shown to speed loaded shortening and increase myocyte power output.
Acknowledgments
Special acknowledgment is given to Dr. Laurin M. Hanft for helpful comments regarding all aspects of the manuscript and with preparation of the figures.
References
- 1.Ait Mou Y, le Guennac J-Y, Mosca E, de Tombe PP, Cazorla O (2008) Differential contribution of cardiac sarcomeric proteins in the myofibrillar force response to stretch. Pflugers Arch 457:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cazorla O, Le Guennec J-Y, White E (2000) Length-tension relationships of subepicardial and sub-endocardial single ventricular myocytes from rat to ferret hearts. J Mol Cell Cardiol 32:735–744 [DOI] [PubMed] [Google Scholar]
- 3.Chiu YC, Ballou EW, Ford LE (1987) Force, velocity, and power changes during normal and potentiated contractions of cat papillary muscle. Circ Res 60:446–458 [DOI] [PubMed] [Google Scholar]
- 4.Colson BA, Bekyarova T, Fitzsimons DP, Irving TC, Moss RL (2007) Radial displacement of myosin cross-bridges in mouse myocardium due to ablation of myosin binding-C. J Mol Biol 367:36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colson BA, Bekyarova T, Locher MR, Fitzsimons DP, Irving TC, Moss RL (2008) Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium. Circulation Research 103:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels M, Noble MIM, Ter Keurs HEDJ, Wohlfart B (1984) Velocity of sarcomere shortening in rat cardiac muscle: relationship to force, sarcomere length, calcium and time. J Physiol 355:367–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Tombe PP, ter Keurs EDJ (1992) An internal viscous element limits unloaded velocity of sarcomere shortening in rat cardiac trabeculae. J Physiol 454:619–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edman KAP (1979) The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibers. J Physiol 291:143–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endoh M, Blinks JR (1988) Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through- and β-adrenoceptors. Circ Res 62:247–265 [DOI] [PubMed] [Google Scholar]
- 10.Fabiato A, Fabiato F (1975) Dependence of the contractile activation of skinned cardiac cells on the sarcomere length. Nature 256:54–56 [DOI] [PubMed] [Google Scholar]
- 11.Ford LE (1991) Mechanical manifestations of activation of cardiac muscle. Circ Res 68:621–637 [DOI] [PubMed] [Google Scholar]
- 12.Ford LE, Huxley AF, Simmons RM (1985) Tension transients during steady shortening of frog muscle fibres. J Physiol 361:131–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford LE, Nakagawa K, Desper J, Seow CY (1991) Effect of osmotic compression on force-velocity properties of glycerinated rabbit skeletal muscle cells. J Gen Physiol 97:73–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs F, Martyn DA (2005) Length-dependent Ca2+ activation in cardiac muscle: some remaining questions. J Muscle Res Cell Motil 26:199–212 [DOI] [PubMed] [Google Scholar]
- 15.Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibers. J Physiol 184:170–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon AM, Homsher E, Regnier M (2000) Regulation of contraction in striated muscle. Physiol Rev 80:853–924 [DOI] [PubMed] [Google Scholar]
- 17.Granzier HLM, Burns DH, Pollack GH (1989) Sarcomere length dependence of the force-velocity relation in single frog muscle fibers. Biophys J 55:499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyton AC, Jones CE, Coleman TG (1973) Circulatory physiology: cardiac output and its regulation. Saunders, Philadelphia [Google Scholar]
- 19.Hanft LM, McDonald KS (2009) Sarcomere length dependence of power output is increased after PKA treatment in rat cardiac myocytes. Am J Physiol 296:H1524–H1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanft LM, McDonald KS (2010) Length dependence of force generation exhibit similarities between rat cardiac myocytes and skeletal muscle fibres. J Physiol 588:2891–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanft LM, McDonald KS (2010) Determinants of loaded shortening in rat cardiac myocytes, 2010 biophysical society meetings abstract [Google Scholar]
- 22.Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318 [PubMed] [Google Scholar]
- 23.Julian FJ (1971) The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol 218:117–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kentish JC, ter Keurs HEDJ, Noble MI, Ricciardi I, Schouten VJA (1983) The relationships between force, [Ca2+] and sarcomere length in skinned trabeculae from rat ventricle. J Physiol 345:24P [Google Scholar]
- 25.Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, deTombe PP (2003) Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol 547:951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korte FS, McDonald KS (2007) Sarcomere length dependence of rat skinned cardiac myocyte mechanical properties: dependence on myosin heavy chain. J Physiol 581:725–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korte FS, McDonald KS, Harris SP, Moss RL (2003) Loaded shortening, power output, and rate of force redevelopment are increased with knockout of cardiac myosin binding protein-C. Circ Res 93:752–758 [DOI] [PubMed] [Google Scholar]
- 28.Luther PK, Bennett PM, Knupp C, Craig R, Padron R, Harris SP, Patel J, Moss RL (2008) Understanding the organisation and role of myosin binding protein C in normal striated muscle by comparison with MyBP-C knockout cardiac muscle. J Mol Biol 384:60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martyn DA, Rondinone JF, Huntsman LL (1983) Myocardial segment velocity at a low load: time, length, and calcium dependence. Am J Physiol 13:H708–H714 [DOI] [PubMed] [Google Scholar]
- 30.McDonald KS (2000) Ca2+ dependence of loaded shortening in rat skinned cardiac myocytes and skeletal muscle fibers. J Physiol 525:169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald KS, Moss RL (2000) Strongly binding myosin cross-bridges regulate loaded shortening and power output in cardiac myocytes. Circ Res 87:768–773 [DOI] [PubMed] [Google Scholar]
- 32.McKillop DF, Geeves MA (1993) Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J 65:693–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moss RL (1992) Ca2+ regulation of mechanical properties of striated muscle: mechanistic studies using extraction and replacement of regulatory proteins. Circ Res 70:865–884 [DOI] [PubMed] [Google Scholar]
- 34.Moss RL, Allen JD, Greaser ML (1986) Effects of partial extraction of troponin complex upon the tension-pCa relation in rabbit skeletal muscle. Further evidence that tension development involves cooperative effects within the thin filament. J Gen Physiol 87:761–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piazzesi G, Reconditi M, Linari M, Lucii L, Bianco P, Brunello E, Decostre V, Stewart A, Gore DB, Irving TC, Irving M, Lombardi V (2007) Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 131:784–795 [DOI] [PubMed] [Google Scholar]
- 36.Podolin RA, Ford LE (1983) The influence of calcium on shortening velocity of skinned frog muscle cells. J Muscle Res Cell Motil 4:263–282 [DOI] [PubMed] [Google Scholar]
- 37.Podolsky RJ, Teichholz LE (1970) The relation between calcium and contraction kinetics in skinned muscle fibres. J Physiol 211:19–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarnoff SJ (1955) Myocardial contractility as described by ventricular function curves; observations on Starling’s law of the heart. Physiol Rev 35:107–122 [DOI] [PubMed] [Google Scholar]
- 39.Sweitzer NK, Moss RL (1993) Determinants of loaded shortening velocity in single cardiac myocytes permeabilized with alpha-hemolysin. Circ Res 73:1150–1162 [DOI] [PubMed] [Google Scholar]
- 40.ter Keurs HE, Rijnsburger WH, van Heuningen R, Nagelsmit MJ (1980) Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ Res 46:703–714 [DOI] [PubMed] [Google Scholar]
- 41.Tobacman L (1996) Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol 58:447–481 [DOI] [PubMed] [Google Scholar]
- 42.van der Velden J, de Jong JW, Owen VJ, Burton PBJ, Stienen GJM (2000) Effect of protein kinase A on calcium sensitivity of force and its sarcomere length dependence in human cardiomyo-cytes. Cardiovasc Res 46:487–495 [DOI] [PubMed] [Google Scholar]
- 43.Vibert P, Craig R, Lehman W (1997) Steric-model for activation of muscle thin filaments. J Mol Biol 266:8–14 [DOI] [PubMed] [Google Scholar]
- 44.Wahr PA, Metzger JM (1999) Role of Ca2+ and cross-bridges in skeletal muscle thin filament activation probed by Ca2+ sensitizers. Biophys J 76:2166–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolska BM, Stojanovic MO, Luo W, Kranias EG, Solaro RJ (1996) Effect of ablation of phospholamban on dynamics of cardiac myocyte contraction and intracellular calcium. Am J Physiol 271:C391–C397 [DOI] [PubMed] [Google Scholar]
- 46.Xu C, Craig R, Tobacman L, Horowitz R, Lehman W (1999) Tropomyosin positions in regulated thin filaments revealed by cryoelectron microscopy. Biophys J 77:985–992 [DOI] [PMC free article] [PubMed] [Google Scholar]