Abstract
Purpose:
To observe the photoreceptor anomalies in cases of ametropic amblyopia.
Methods:
A prospective study with 25 isoametropic amblyopic children in the age group of 5–14 years and 25 age-matched controls was done. Examination included refraction, best-corrected visual acuity (BCVA), and color vision. Adaptive optics (AO) and multifocal electroretinogram (mf-ERG) were done to assess the anatomy and function of photoreceptors. The subgroup analysis of the improved and non-improved groups was done.
Results:
The mean cone density in cases and control in the superior, temporal, and nasal quadrants was respectively as follows (21640 ± 5713, 24040 ± 3386, P = 0.01) (19755 ± 6282, 21832 ± 2911, P = 0.03) (19897 ± 5418, 22171 ± 3660, P = 0.01) (20768 ± 4799, 22819 ± 3241, P = 0.01). The amplitude of N1 wave and P1 wave in cases was significantly low compared to the controls. Cases with subnormal color vision had reduced BCVA (0.55 ± 0.018) in comparison to the children with normal response (0.350 ± 0.014). Cone density was also significantly reduced in children with subnormal color vision. Sixteen out of 25 cases showed BCVA improvement with spectacles. Baseline cone density was found to be significantly higher in the improved group. There was no correlation between BCVA and AO parameters.
Conclusion:
Patients with ametropic amblyopia show subnormal photoreceptor properties than controls. Low cone density may be associated with defective color vision and poor prognosis in these cases.
Keywords: Adaptive optics, amblyopia, ametropic amblyopia
Isoametropic amblyopia, a bilateral diminution of the visual acuity resulting from large, approximately equal, and uncorrected refractive errors in both the eyes of a young child is usually amenable to treatment with just spectacle correction in a majority of cases.[1] But it is intriguing as even after full refractive correction it shows slow recovery, taking months instead of weeks compared to anisometropic amblyopia.[2,3] And in some cases the visual recovery may not be able to reach normal standards.[1–3] The pathopysiology of this amblyopia is poorly understand and there are some questions remain unanswered as why only certain subset of patients donot show improvement with refractive correction. Although this problem may be more commonly seen in patients of hypermetropia, patients with myopia and astigmatism may also have same prognosis. Is it an anomaly of the accommodation ability[2,4] or some inherent retinal pathology that is responsible for incomplete recovery in these cases? Adaptive optics (AO) is a recent advancement in the imaging technique and allows the study of retina at the micro-cellular level. The current devices for retinal imaging have their limitation in the form of wavefront distortions due to the optical irregularity of the structures of the human eye, including the cornea and lens, which induce higher level aberrations. Innovative AO technology enhances the image quality by eliminating wavefront distortions, which improves the resolution at up to 2 mm, and making the visualization of individual cone photoreceptors possible.[5]
In the present study, we examined the retinal structure at the photoreceptor level using AO to understand the role of photoreceptors in pathophysiology as well as recovery in cases of isoametropic amblyopia.
Methods
This prospective case–control study was conducted at a tertiary eye care center from October 2017 to March 2019 after obtaining ethical clearance from the institutional ethics committee and as per the tenets of the Declaration of Helsinki. Fifty eyes of 25 isoametropic amblyopic children in the age group of 5–14 years were selected as per the criteria for refractive error with myopia >−3 D, hyperopia >+4.5, astigmatism >2.00 D according to the American Academy of Ophthalmology guidelines for prescription of glasses[6] and Snellen’s best-corrected visual acuity (BCVA) of 6/36 ≥6/12. Fifty eyes of 25 age-matched controls with no refractive error and a Snellen’s BCVA of 6/6 were selected. Other types of amblyopia, ocular pathologies involving the macula including pathological myopia, ocular media subnormal ities, severe dry eye, poor fixation, and children with attention deficit disorder were excluded from the study. Parents who refused to give consent for their children to participate in the study were also excluded. The demographic details and baseline characteristics were recorded. Detailed history, age at presentation, age of prescription of glasses, similar family history, previous surgeries, previous trauma, if any, and systemic illnesses were asked and thorough clinical examination, including uncorrected visual acuity (UCVA), BCVA on the Snellen chart and was (coverted into logMAR for final analysis), anterior segment and posterior segment examination were performed.
Both the groups underwent a series of structural and functional tests: AO photoreceptor biomarkers, color vision on Ishihara pseudoisochromatic plate (38 plates version), and multifocal electroretinogram (mf-ERG).
Adaptive optics
We acquired a series of images of the retina on each eye using a commercially available flood-illuminated AO retinal camera (rtx1, Imagine Eyes, Orsay, France). A set of 40 raw images of the same retinal area was acquired at a rate of 9.5 frames per second, with an exposure time of 10 ms to form the final image. The final AO image was averaged in a 4° × 4° field (i.e., 1500 × 1500 pixels). Images were acquired at 2° eccentricity along the four meridians (nasal, temporal, superior, and inferior). The subject was asked to fixate on a yellow cross controlled by the operator. Cones are automatically detected by a software provided by the manufacturer (AO detect Mosaic b13; Imagine Eyes). The background of the image was removed, and the histogram was stretched, then adaptive and multiple-scale digital filters were applied to the resulting image. The local maxima of the resulting filtered image were detected. Their spatial distribution was analyzed in terms of inter-cone spacing, local cell density, cone hexagonal morphology, and number of nearest neighbours using Delaunay triangulation and Voronoi diagrams. Cones within the 2° central area (i.e., up to 1° from the center of the fovea) cannot be resolved because of the limit of resolution of the device. Adaptive optics photoreceptor biomarkers (each at 2° eccentricity from fixation) studied were cone density (cells/mm²), cone spacing (μm), cone regularity (%), and cone dispersion index (SD of mean of spacing/mean of spacing).
mfERG
To assess the functional parameter of photoreceptors, mf-ERG (Model MonPackONE, Metrovision, France) was done. Pupillary dilatation was performed using tropicamide (1%). The stimulus field contained 61 hexagons covering a field diameter of 54°. Refractive errors of the subjects were corrected during recording. First-order kernel responses were recorded. The amplitude of P1 and N1 waves and implicit time at 2° and 2°–5° were used for analysis.
Color vision
Color vision was assessed using Ishihara’s pseudoisochromatic plates (38 plate). If a child identified four or more plates as wrong then that was considered an subnormal response as per standard protocol.
Cycloplegic refraction was done and glasses were prescribed to the patient; they were followed-up for a year to assess BCVA. We looked for improvement in BCVA of two or more lines on the Snellen visual acuity (VA) chart and further subdivided our group. At the end of the follow-up (one year) children who improved by ≥2 lines on the Snellen chart were included in subgroup A (n = 16). Those children who showed an improvement of ≤2 lines on the Snellen chart were included in subgroup B (n = 9). In subgroups, baseline data of AO and mf-ERG were then compared to ascertain any differences that pointed to a better VA outcome in subgroup A in relation to subgroup B.
Statistical analysis using unpaired t-test was performed to compare the data between various groups. Correlation analysis using regression tests were done between BCVA and AO and mf-ERG wave parameters in cases to see if poor visual function correlated with changes in the AO biomarkers studied. A statistical value less 0.05 was considered as significant.
Results
The mean age of the patients in the isoametropic amblyopia group was 7.3 ± 2.01 years and the range extended from 5 to 14 years. The mean age of the patients in the control group was 7.6 ± 2.55 years and the range extended from 5 to 14 years. The two groups were comparable in terms of age with P > 0.14 with unpaired t-test. There was a predominance of male gender in both the case and control groups. In the control group, 9 out of 25 were females (36%) and in cases 8 out of 25 were females (32%). Six out of 25 cases were myopes (24%). The mean myopia was − 4.25 ± 0.66 D (−3.75 D to − 5 D). The total number of hypermetropes were 12 (48%) with mean of 6.15 ± 2.53 D (+4.5 D to + 11 D). Seven out 25 (28%) had astigmatism with mean of 3.31 DC ± 0.74 DC (2.25 DC to 3.75 DC).
Comparison between cases and controls
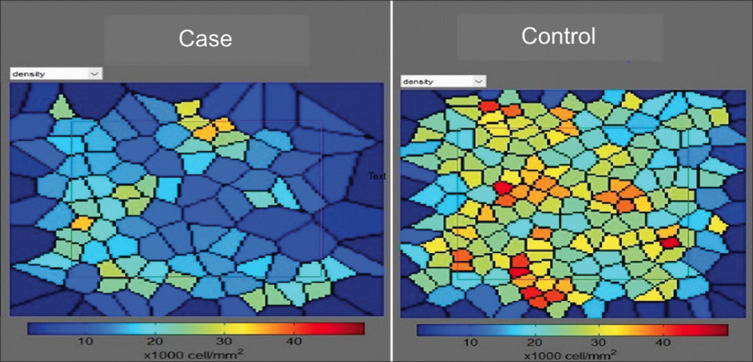
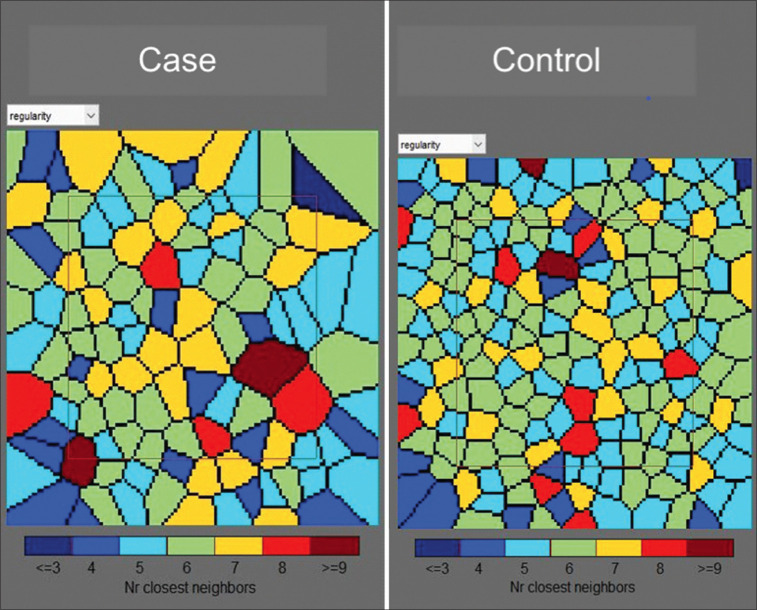
There was a difference in the mosaic of cone between cases and controls. The mosaic in the control group was tightly packed with regular hexagonal pattern of the cones, whereas the cones in the case group showed decreased cone density with enlarged size of cones and loss of regular hexagonal shape [Figs. 1 and 2]. There was a statistically significant difference (P < 0.05) in cone density between the case and the control groups in all the quadrants. Cone spacing was increased and cone regularity was reduced in cases but the differences between the two groups were not statistically significant. Cone dispersion was increased in all the quadrants in cases but the differences was borderline significant only in the inferior quadrant control group. The comparison between various AO parameters is given in Table 1.
Figure 1.
Voronoi tiles showing cone Density distribution in cases and control at 2° eccentricity from fixation. Case is a 7-year-old with a refractive error of +4.5 DS and control is an 8-year-old with no refractive error. Note the tightly packed cones in the control mosaic while in case mosaic the size of the cone seems to enlarge with decreased density
Figure 2.
Voronoi tiles showing cone regularity index in same case and control at 2° eccentricity from fixation. Note the increase in size of the cones and loss of regular hexagonal pattern in the case mosaic
Table 1.
Comparison of AO parameters between cases (n=25) and controls (n=25) at 2° eccentricity in all four quadrants
| Parameter | Nasal | Temporal | Superior | Inferior | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Cases | Controls | P | Cases | Controls | P | Cases | Controls | P | Cases | Controls | P | |
| Cone density (mm2) | 21640±5713 | 24040±3386 | 0.01 | 19755±6282 | 21832±2911 | 0.03 | 19897±5418 | 22171±3660 | 0.01 | 20768±4799 | 22819±3241 | 0.01 |
| Cone spacing | 7.65±1.1 | 7.56±0.96 | 0.6 | 8.11±1.38 | 7.81±0.70 | 0.1 | 7.95±1.2 | 7.86±1.21 | 0.6 | 7.96±1.80 | 7.6±1.06 | 0.2 |
| Cone regularity | 90.1±5.51 | 91.13±4.12 | 0.32 | 89.22±5.35 | 90.73±4.68 | 0.13 | 90.8±10.68 | 92.19±3.95 | 0.41 | 90.1±5.57 | 90.30±5.03 | 0.91 |
In the case group, 12 out of 25 children (48%) had an subnormal color vision response. No colour vision deficiency was found amongst the control group (P < 0.01). Among the cases, children with subnormal color vision had reduced mean BCVA (0.55 ± 0.018 logMAR) in comparison to children with normal response (0.350 ± 0.014 logMAR) (P < 0.01). Cone density was also found to be significantly reduced in children with subnormal color vision in comparison to the children with normal response in cases, in all the four quadrants [Table 2], whereas the rest of the AO parameters did not show any significant difference.
Table 2.
Comparison of cone density values in between cases with subnormal and normal color vision
| Nasal (Mean±SD) | Temporal (Mean±SD) | Superior (Mean±SD) | Inferior (Mean±SD) | |
|---|---|---|---|---|
| Cases with subnormal color vision (n=12) | 21640±5713 | 19755±6282 | 19897±5418 | 20768±4799 |
| Cases with normal color vision (n=13) | 24040±3386 | 21832±2911 | 22171±3660 | 22819±3241 |
| P | 0.01 | 0.03 | 0.01 | 0.01 |
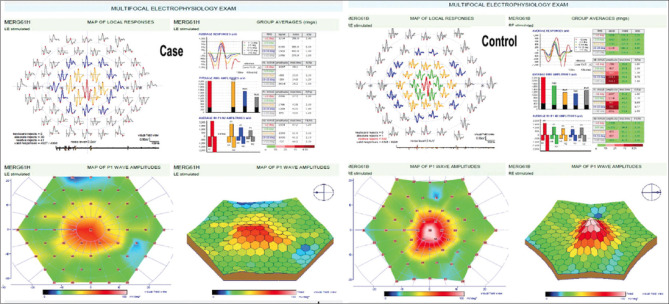
There was significant difference in various parameters on mf-ERG, between cases and controls. The amplitude of N1 wave and P1 wave in cases were significantly low in central 2 °and 2°–5° compared to controls. The implicit time of P1 wave in central < 2° zone was significantly higher than in the control group [Fig. 3]. Table 3 shows the difference in amplitude of N1 and P1 waves in central <2° and 2°–5° between cases and controls.
Figure 3.
Multifocal ERG P1 amplitude at <2° in same case and control showing decrease in foveal peak in case
Table 3.
Comparison of amplitude and implicit time of N1 and P1 waves in central<2° and 2°-5° between cases and controls
| N1 amplitude | N1 implicit time | P1 amplitude | P1 implicit time | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| <2° | 2°-5° | <2° | 2°-5° | <2° | 2°-5° | <2° | 2°-5° | |
| Cases (n=25) | −711.05±199.08 | −389.25±176.83 | 26.47±6.27 | 25.85±2.21 | 905.85±543.66 | 691.44±305.06 | 44.72±5.68 | 44.76±5.18 |
| Controls (n=25) | 751.35±299.08 | −440.15±163.80 | 25.7±3.83 | 26.03±1.51 | 1146.5±613.94 | 874.31±349.47 | 42.08±3.72 | 44.26±1.38 |
| P | 0.008 | 0.07 | 0.4616 | 0.6 | 0.02 | 0.005 | 0.02 | 0.5115 |
Improved versus nonimproved
At the end of the follow-up (one year), cases were divided in to two subgroups—improved and non-improved—on the basis of improvement in their visual acuity. Children who improved by ≥2 lines on the Snellen chart were included in the improved group. Those children who had an improvement of ≤2 lines on the Snellen were included in the non-improved group. Out of 25, 16 (64%) showed improvement while 9 (36%) did not show improvement. Even though the numbers were less in each group, we did a comparative analysis between the groups. There was no difference in the baseline parameters between the groups. The mean BCVA at presentation in the improved and non-improved groups was 0.41 ± 0.23 and 0.45 ± 0.15, respectively, while the final BCVA was 0.22 ± 0.02 and 0.41 ± 0.14 in improved and non-improved groups, respectively. In the improved group, there were four myopes, eight hypermetropes, and four with cylindrical error. In the non-improved group, there were two myopes, four hypermetropes, and three with cylindrical error. There were no differences in the electrophysiological parameters between the groups. Cone density and cone spacing were significantly low in the non-improved group, in the nasal and temporal quadrant, even though mean reduction was seen in all four quadrants. The morphology of cone was found to be altered in the non-improved cases. Cones were found to be enlarged with loss of regular hexagonal pattern [Fig. 2]. This was reflected in reduced cone regularity in non-improved group in all four quadrants; however statistical difference was present only in the superior quadrant. The difference in AO parameters between the groups is shown in Table 4.
Table 4.
Comparison of AO parameters between improved and non improved in sub-group analysis
| Parameter | Nasal | Temporal | Superior | Inferior | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Improved | Non improved | P | Improved | Non Improved | P | Improved | Non improved | P | Improved | Non-improved | P | |
| Cone density (mm2) | 24199±4932 | 18405±4956 | 0.00 | 22498±6000 | 17178±5154 | 0.00 | 21624±4259 | 19184±5816 | 0.11 | 21987±5128 | 20369±3678 | 0.25 |
| Cone spacing | 7.2±0.9 | 8.2±1.11.1 | 0.00 | 7.5±1.1 | 8.5 ± 1.3 | 0.00 | 7.6±0.8 | 8.1±1.3 | 0.1 | 7.6±0.8 | 7.8±0.7 | 0.3 |
| Cone regularity | 91.1±5.2 | 88.8±5.9 | 0.18 | 90.9±5.6 | 88.2±3.6 | 0.06 | 94.6±40 | 87.7±15.3 | 0.03 | 90.8±5.1 | 90.8±4.9 | 0.69 |
Structural and functional correlations
To understand the relationship between visual acuity and photoreceptors in cases of amblyopia, Spearman correlation analysis was done between BCVA and various AO parameters. A negative correlation of logMAR BCVA with cone density and cone regularity, and positive correlation of logMAR BCVA with cone spacing and cone dispersion were found; however none of them could reach a statistically significant level.
Discussion
Isoametropic amblyopia is a poorly understood entity where the pathophysiology is not a cortical rivalry as both the eyes are at a similar disadvantage presumably due to pattern vision deprivation.[7] While some children with uncorrected hyperopia may accommodate to achieve clear retinal images and develop refractive esotropia, other children with uncorrected hypermetropia develop amblyopia. Subnormal accommodative amplitudes have been reported in these children which may not be enough to overcome the refractive error.[2,8] The treatment though possible with full refractive correction is slower than with anisometropic amblyopia and often incomplete. Around 30%–50% of patients may fail to achieve visual acuity better than 20/25.[1,3]
The role of retinal dysfunction in amblyopia is still under evaluation. Researchers have reported changes in macular thickness, macular volume, and retinal nerve fiber layer on optical coherence tomography (OCT).[9–13] However, the results from these studies are variable and inconclusive and are limited to anisometropic and strabismic variety of amblyopia. New technological advances like AO offer the opportunity to look at the retina at the microcellular level. This study was carried out with the aim of understanding the changes at the photoreceptor level which is the seat of visual acuity. We compared the AO parameters between isoametropic amblyopic and emmetropic patients. The cone density was significantly reduced in all the quadrants in adaptive optics done at 2° from the fixation point among isoametropic amblyopes compared to the age-matched normal children. Even cone spacing and dispersion was found to be higher while cone regularity was found to be lesser in isoametropic amblyopes, but this was not significant.
A similar study was conducted by Liao et al.[14] where they compared anisometropic amblyopic eyes with fellow eyes and emmetropic eyes and concluded that cone density in linear units was higher in anisometropic amblyopic eyes as compared to their fellow non-amblyopic eyes and age-matched normal subjects in all the quadrants; however this difference was no longer existent after axial length (AL) and age adjustment in four meridians.
The dysfunction of cones in ametropic amblyopia is further reinforced by the fact that 48% of cases had subnormal color vision. These cases with color deficiency had significantly poorer baseline BCVA compared to those with normal color vision. The cone density was found to be significantly reduced in children with subnormal color vision in comparison to the children with normal response in cases, in all the four quadrants. It may be difficult to interpret that color vision deficit is due to poor visual acuity directly per se or indirectly due to lower cone density. Interestingly a study by Rajavi et al.[15] showed that children with color vision deficiency had lower visual acuity compared to normal color vision children, and amblyopia was observed in 8.3% of patients with color vision deficiency versus 2.1% of children with normal color vision perception. No association between color vision and refractive error was seen in the present study, but our sample size was too small to detect any association, if present.
While AO gives the structural assessment of photoreceptors, functional evaluation via mf-ERG provides the functional assessment of the retina in a topographical method Al-Haddad et al.[16] reported decreased amplitude of P1 waves in the central rings. They found that the amplitudes were reduced in both strabismic and anisometropic amblyopia. They also showed that patients with severe amblyopia had greater reduction compared to mild amblyopia. Reduced amplitudes in anisometropic amblyopia have been reported in few other studies as well.[17,18] In our study on ametropic amblyopia, we found that on mf-ERG the amplitude of N1 and P1 waves in 2° and 2°–5° were significantly reduced in cases when compared to control eyes. Additionally, the implicit time of P1 was significantly increased in central 2° in amblyopic eyes when compared to control eyes.
The results of these studies including ours suggest that there are both functional and anatomical changes in the retina in patients with amblyopia. Functional changes may be a direct reflection of visual acuity, and the anatomical changes in these patients suggest that amblyopia may not be a cortical phenomenon entirely and local structural changes may play role in pathogenesis and prognosis of amblyopia. We performed the correlation analysis between the BCVA and AO parameters and found that the logMAR BCVA showed negative correlation with cone regularity and density and positive correlation with cone dispersion and spacing. However none of the parameters reached to the level of statistical significance, which we feel could be due to the small sample size.
To understand the role of retinal receptors in recovery from amblyopia, we subdivided the cases on the basis of their recovery which was defined as an improvement of 2 or more lines. Sixty-four percent of the patients showed improvement in BCVA of greater than 2 lines on the Snellen chart. Among patients who showed improvement in BCVA, the mean cone density and cone regularity was significantly more in the improved group compared to the non-improved group and the mean cone spacing and mean cone dispersion were significantly low among patients who showed improvement at least in two out of four quadrants. Baraas et al.[19] conducted a study on patients with S-cone dystrophy and showed the presence of distorted cone mosaic. These patients showed realignment and re-organization of the cone mosaic in absence of S-cone type of cone photoreceptors.. Song et al.[20] studied the retinal phenotype at a cellular level in patients with autosomal cone–rod dystrophy. They found that the individual cones appeared enlarged and were characterized as having a “dark” (hypo-reflective) center surrounded by a darker halo. In our study, we found that children who did not improve with spectacle correction had an altered cone mosaic. Cone density was significantly less in the nasal and temporal quadrants and cones were enlarged with a smaller number of hexagonal cones which may explain the poor cone regularity in these children. We hypothesize that these children could represent a forme fruste of cone dystrophy, and further genetic analysis needs to be done to establish it. Thus, the density and the regularity index of the cone in a patient of amblyopia may be a useful tool to prognosticate a visual recovery in cases of ametropic amblyopia. Contrary to AO parameters, we did not find any difference in mf-ERG parameters between the improved and non-improved patients, which may be due to the small sample size.
Studies have shown that with increase in axial length, cone density decreases.[21–23] We did not find any correlation between the refractive error and AO parameters, which is similar to what was reported by Legras et al.[21] We acknowledge that we did not evaluate the axial length, which may have influenced the low cone density in our cases; however it should be noted that the number of myopes in our study were less than half of the number of hypermetropia and astigmatism patients combined. Moreover the machine adjusts for the axial length by its own to a certain extent, so we believe that the reflection of cone density in our study is a true reflection of poor densities in patients of ametropic amblyopia and may be reponsible for poorer outcomes.
The major limitations of our study was the small sample size—particularly in the subgroup analysis—and we did not analyze cone density at various degrees of eccentricity from the fovea, which may have influenced the result.
Conclusion
In conlusion, adaptive optics biomarkers could be used to prognosticate the visual recovery in isoametropic amblyopic children. Also, poor performance in color vision tests and a high cylindrical error points to a poorer prognosis. The children who did not improve with spectacles could represent a forme fruste of cone dystrophy though, further genetic studies need to be done to confirm this hypothesis. Studies with a larger sample size need to be assessed to substantiate our results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wallace DK, Chandler DL, Beck RW, Arnold RW, Bacal DA, Birch EE, et al. Treatment of bilateral refractive amblyopia in children three to less than 10 years of age. Am J Ophthalmol. 2007;144:487–96. doi: 10.1016/j.ajo.2007.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoenleber DB, Crouch ER., Jr Bilateral hypermetropic amblyopia. J Pediatr Ophthalmol Strabismus. 1987;24:75–7. doi: 10.3928/0191-3913-19870301-06. [DOI] [PubMed] [Google Scholar]
- 3.Klimek DL, Cruz OA, Scott WE, Davitt BV. Isoametropic amblyopia due to high hyperopia in children. J AAPOS. 2004;8:310–3. doi: 10.1016/j.jaapos.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Candy TR, Gray KH, Hohenbary CC, Lyon DW. The accommodative lag of the young hyperopic patient. Invest Ophthalmol Vis Sci. 2012;53:143–9. doi: 10.1167/iovs.11-8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Roorda A. Evaluating the lateral resolution of the adaptive optics scanning laser ophthalmoscope. J Biomed Opt. 2006;11:014002. doi: 10.1117/1.2166434. [DOI] [PubMed] [Google Scholar]
- 6.Wallace DK, Morse CL, Melia M, Sprunger DT, Repka MX, Lee KA, et al. Pediatric eye evaluations preferred practice pattern®:I. vision screening in the primary care and community setting;II. comprehensive ophthalmic examination American academy of ophthalmology preferred practice pattern pediatric ophthalmology/strabismus panel. Ophthalmology. 2018;12:P184–227. doi: 10.1016/j.ophtha.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 7.von Noorden GK. Binocular Vision and Ocular Motility:Theory and Management of Strabismus. 5th ed. St Louis: Mosby; 1996. [Google Scholar]
- 8.Werner DB, Scott WE. Amblyopia case reports:Bilateral hypermetropic ametropic amblyopia. J Pediatr Ophthalmol. 1985;22:203–5. doi: 10.3928/0191-3913-19850901-09. [DOI] [PubMed] [Google Scholar]
- 9.Andalib D, Javadzadeh A, Nabai R, Amizadeh Y. Macular and retinal nerve fibre layer thickness in unilateral anisometropic or strabismic amblyopia. J Pediatr Ophthalmol Strabismus. 2013;50:218–21. doi: 10.3928/01913913-20130319-02. [DOI] [PubMed] [Google Scholar]
- 10.Yalcin E, Balci O. Peripapillary retinal nerve fibre layer and foveal thickness in hypermetropic anisometropic amblyopia. Clin Ophthalmol. 2014;12:749–53. doi: 10.2147/OPTH.S58541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Haddad CE, El Mollayess GM, Mahfoud ZR, Jaafar DF, Bashshur ZF. Macular ultrastructural features in amblyopia using high-definition optical coherence tomography. Br J Ophthalmol. 2013;97:318–22. doi: 10.1136/bjophthalmol-2012-302434. [DOI] [PubMed] [Google Scholar]
- 12.Yakar K, Kan E, Alan A, Alp MH, Ceylan T. Retinal nerve fibre layer and macular thicknesses in adults with hyperopic anisometropic amblyopia. J Ophthalmol. 2015;2015:946467. doi: 10.1155/2015/946467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu SQ, Zhu LW, Xu QB, Xu JL, Zhang Y. Macular and peripapillary retinal nerve fibre layer thickness in children with hyperopic anisometropic amblyopia. Int J Ophthalmol. 2013;6:85–9. doi: 10.3980/j.issn.2222-3959.2013.01.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao N, Jiang H, Mao G, Li Y, Xue A, Lan Y, et al. Changes in macular ultrastructural morphology in unilateral anisometropic amblyopia. Am J Transl Res. 2019;11:5086–95. [PMC free article] [PubMed] [Google Scholar]
- 15.Rajavi Z, Sabbaghi H, Baghini AS, Yaseri M, Sheibani K, Norouzi G, et al. Prevalence of color vision deficiency and its correlation with amblyopia and refractive errors among primary school children. J Ophthalmic Vis Res. 2015;10:130–8. doi: 10.4103/2008-322X.163778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Haddad C, Bou Ghannam A, El Moussawi Z, Rachid E, Ismail K, Atallah M, et al. Multifocal electroretinography in amblyopia. Graefes Arch Clin Exp Ophthalmol. 2020;258:683–91. doi: 10.1007/s00417-019-04558-x. [DOI] [PubMed] [Google Scholar]
- 17.Feng LX, Zhao KX. Study on anisometropic amblyopia by simultaneously recording multifocal VEP and multifocal ERG. Zhonghua Yan Ke Za Zhi. 2005;41:41–6. [PubMed] [Google Scholar]
- 18.Ji CN, Liu Y, Fei F, Zheng HY, Sun J, Wang ZT, et al. Analysis of multifocal electroretinogram first-order kernel P(1) wave in anisometropic amblyopia. Zhonghua Yan Ke Za Zhi. 2010;46:969–73. [PubMed] [Google Scholar]
- 19.Baraas RC, Carroll J, Gunther KL, Chung M, Williams DR, Foster DH, et al. Adaptive optics retinal imaging reveals S-cone dystrophy in tritan color-vision deficiency. J Opt Soc Am A Opt Image Sci Vis. 2007;24:1438–47. doi: 10.1364/josaa.24.001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song H, Rossi EA, Stone E, Latchney L, Williams D, Dubra A, et al. Phenotypic diversity in autosomal-dominant cone-rod dystrophy elucidated by adaptive optics retinal imaging. Br J Ophthalmol. 2018;102:136–41. doi: 10.1136/bjophthalmol-2017-310498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legras R, Gaudric A, Woog K. Distribution of cone density, spacing and arrangement in adult healthy retinas with adaptive optics flood illumination. PLoS One. 2018;13:e0191141. doi: 10.1371/journal.pone.0191141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SP, Chung JK, Greenstein V, Tsang SH, Chang S. A study of factors affecting the human cone photoreceptor density measured by adaptive optics scanning laser ophthalmoscope. Exp Eye Res. 2013;108:1–9. doi: 10.1016/j.exer.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li KY, Tiruveedhula P, Roorda A. Intersubject variability of foveal cone photoreceptor density in relation to eye length. Invest Ophthalmol Vis Sci. 2010;51:6858–67. doi: 10.1167/iovs.10-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]