Abstract
Vitreous hemorrhage is associated with a myriad of conditions such as proliferative diabetic retinopathy, proliferative retinopathy following vascular occlusion and vasculitis, trauma, retinal breaks, and posterior vitreous detachment without retinal break. Multiple pathological mechanisms are associated with development of vitreous hemorrhage such as disruption of abnormal vessels, normal vessels, and extension of blood from an adjacent source. The diagnosis of vitreous hemorrhage requires a thorough history taking and clinical examination including investigations such as ultra-sonography, which help decide the appropriate time for intervention. The prognosis of vitreous hemorrhage depends on the underlying cause. Treatment options include observation, laser photo-coagulation, cryotherapy, intravitreal injections of anti-vascular endothelial growth factor, and surgery. Pars plana vitrectomy remains the cornerstone of management. Complications of vitreous hemorrhage include glaucoma (ghost cell glaucoma, hemosiderotic glaucoma), proliferative vitreoretinopathy, and hemosiderosis bulbi.
Keywords: Diabetic retinopathy, laser photo-coagulation, proliferative retinopathy, Terson’s syndrome, valsalva retinopathy, vascular occlusion, vitrectomy, vitreous hemorrhage
The diagnosis of vitreous hemorrhage (VH) remained elusive because of the inability to visualize behind the pupil. Despite the development of the ophthalmoscope by Hermann von Helmholtz in 1851 and the monocular indirect ophthalmoscope by Felix Giraud-Teulon in 1861, speculation remained rife of what lay in the posterior segment.[1] The earliest experimental studies on VH were performed by Pröbsting[2] in 1892 when he injected blood into the vitreous cavity of rabbits and subsequently enucleated the animal eye to microscopically study the “mass of blood”. Eventually came the era of identification of retinal pathology with the use of the binocular indirect ophthalmoscope by Charles Schepens’ in 1947, but the management of VH remained elusive.
Non-resolving VH was considered irremediable until the introduction of pars plana vitrectomy in 1970 by Machemer. Machemer[3] was inspired by David Kasner’s concept of vitreous removal for treatment of a potentially blinding condition. Kasner performed a radical anterior vitrectomy which was an open-sky technique wherein a 300 degree limbal incision was used to fold back the cornea, followed by use of cellulose sponge and scissors for sub-total excision of vitreous.
Machemer intrigued by David Kasner attempted the open-sky vitrectomy on a patient with diabetic VH using a hollowed-out drill with a side opening allowing the battery-powered drill bit to cut small pieces of vitreous which were manually aspirated through the drill along with an infusion tube to maintain the globe as vitreous was removed. Improving on this concept of vitrectomy, Machemer introduced his “vitreous nibbler” through the pars plana route and termed it the vitreous infusion suction cutter (VISC). Improvements in VISC brought about the modern three-port pars plana vitrectomy as the absolute remedy to non-resolving VH.
Method of literature review
Literature selection for this article was undertaken using PubMed, Google Scholar, ePub, and the Cochrane library database. Comprehensive systematic literature search was performed using keywords such as vitreous hemorrhage, vitrectomy, diabetic retinopathy, proliferative retinopathy, Terson’s syndrome, vascular occlusion, and valsalva retinopathy. All relevant review articles, original articles, case series, and reports were reviewed. References contained in those articles were also reviewed and included if they provided relevant information on the subject. The search was not limited by the year of publication.
Anatomy of Vitreous Humor
Vitreous humor is the largest intra-ocular structure constituting 80% of ocular volume. It is a highly hydrated tissue with ~ 99% water and 0.9% salts. The remaining 0.1% is divided between proteins and polysaccharide components which are arranged in the form of a meshwork of collagen fibrils interspersed with glycosaminoglycan molecules.[4] Nearly all of the collagen is in the form of thin, uniform, and heterotypic (of mixed composition) fibrils containing collagen types II, IX, and V/XI [Fig. 1].
Figure 1.
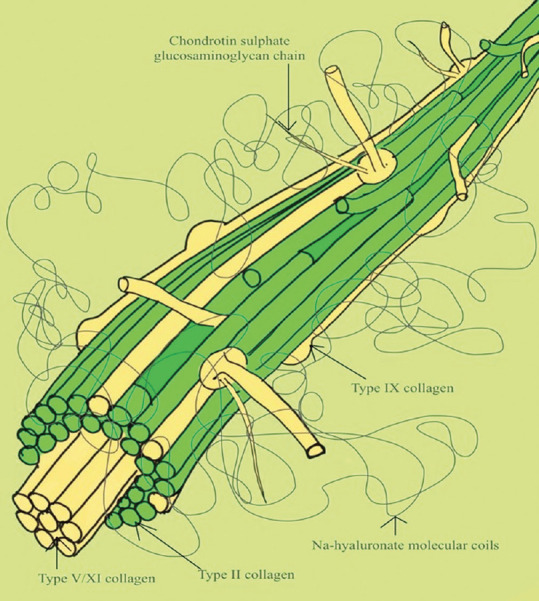
Diagramatic representation of the structure of vitreous humor
Other structural components demonstrated in the vitreous are fibrillin and opticin with hyaluronan being the predominant glycosaminoglycan (GAG). The distribution of collagen varies throughout the vitreous with the highest density of collagen filaments in the vitreous cortex at the vitreous base and ciliary epithelium, followed by the posterior vitreous cortex anterior to the retina, and then the next highest in the anterior vitreous cortex behind the posterior chamber. The lowest density is found in the central vitreous and adjacent to the anterior cortical gel.[5,6] Hyaluronic acid (HA) provides a stabilizing effect on the collagen network as it fills into the spaces between the collagen filaments.[7]
The total volume of vitreous is 4 ml. Vitreous attaches to the posterior aspect of the lens capsule in an annular fashion (8–9 mm in diameter), forming a hyaloideocapsular ligament of Weigert. Between the posterior lens capsule and anterior hyaloid face, surrounded by the ligament of Weigert, is a potential space known as Errgelet’s or Berger’s space. The canal of Cloquet arises from this space and courses posteriorly through the central vitreous. Posteriorly, the Cloquet canal connects over the area of the disc known as the area of Martegiani [Fig. 2].
Figure 2.
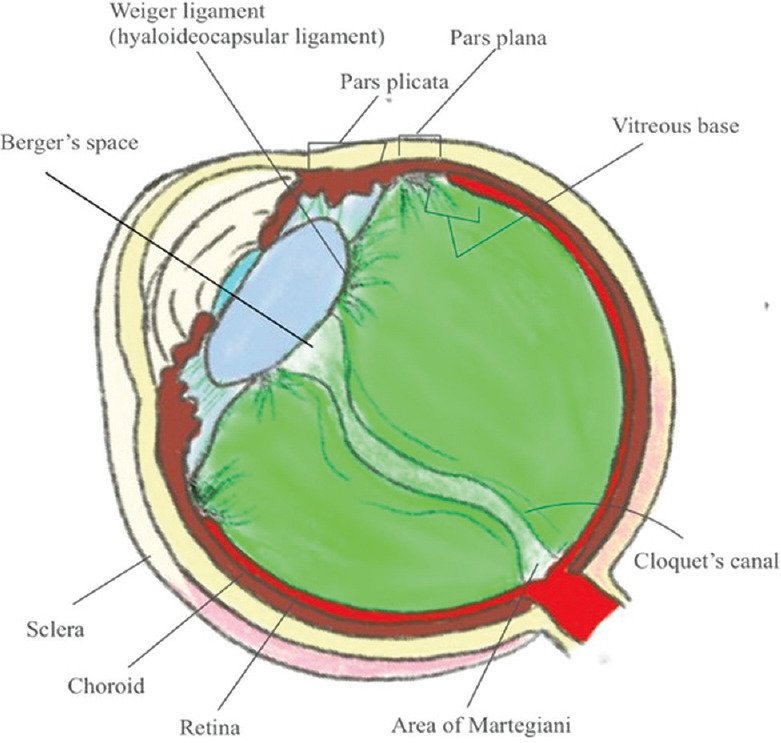
Schematic representation of anatomy of vitreous humor
Clinical implications
Aging gives way to posterior vitreous detachment where cortical vitreous gel splits away from the inner surface of the retina. Posterior vitreous detachment can be associated with pre-retinal, splinter, or flame-shaped hemorrhages because of delayed separation, resulting in persistent traction causing mechanical injury to the superficial retinal vessels, and sometimes may induce retinal tears associated with VH.[8]
Bleeding into the premacular bursa may result in a boat-shaped sub-hyaloid hemorrhage which shifts with changes in the patient head position unlike the sub-internal limiting membrane (ILM) hemorrhage which is under tension. Based on the degree of vitreous separation with or without vitreoschisis, it can form a scaffold for new vessel growth at different levels, resulting in hemorrhages at multiple levels.
Definition of Vitreous Hemorrhage
Vitreous hemorrhage is defined as the presence of extravasated blood in the vitreous cavity between the posterior lens capsule and zonules of the lens anteriorly, the non-pigmented epithelium of ciliary body and ILM laterally, and the ILM posteriorly. The term encompasses hemorrhage into the Cloquet canal, the canal of petit, and Bergers space and pre-retinal (sub-hyaloid and sub-ILM) and intra-vitreal or intra-gel hemorrhage.[9]
Pathomechanism of Vitreous Hemorrhage
Mechanisms resulting in VH are threefold.
Bleeding from abnormal vessels
This is the most commonly implicated mechanism of VH. It occurs secondary to neo-vascularization occurring in proliferative retinopathies such as proliferative diabetic retinopathy (PDR), retinal vascular occlusions, retinopathy of pre-maturity, retinal vasculitis, proliferative sickle cell retinopathy,[10] and so on. Retinal ischemia in the aforementioned etiologies results in a pro-angiogenic environment with increased levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor, and insulin-like growth factor, which lead to the formation of predominantly peripheral neo-vascularization.[11,12] These new vessels are not only fragile but also lack the endothelium and prone to bleeding microstructure, leading with vitreous traction and ultimately lead to VH [Figs. 3-5]. These new vessels may be present at the optic disc/within 1 disc diameter (NVD) or elsewhere (NVE) in the peripheral retina.
Figure 3.
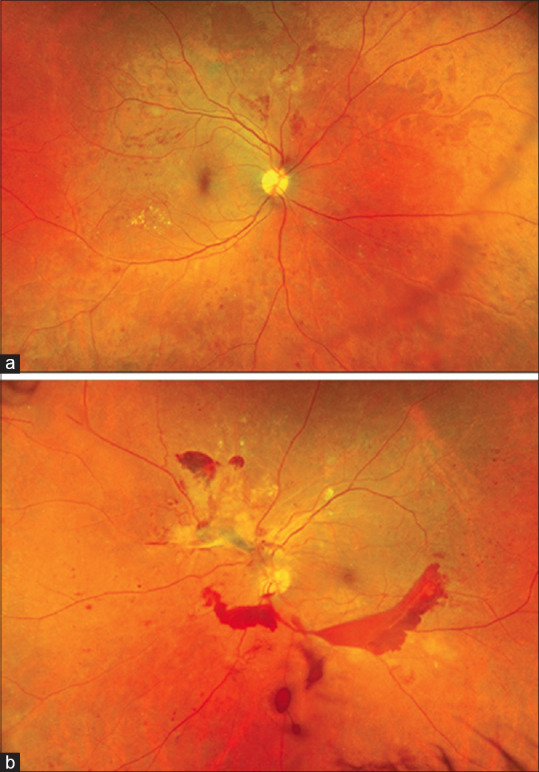
Ultra-widefield fundus photograph (Optos California) of a patient with diabetic vitreous hemorrhage (Panel b). Fundus examination of the fellow eye shows proliferative diabetic retinopathy giving a clue toward etiology of vitreous hemorrhage (Panel a)
Figure 5.
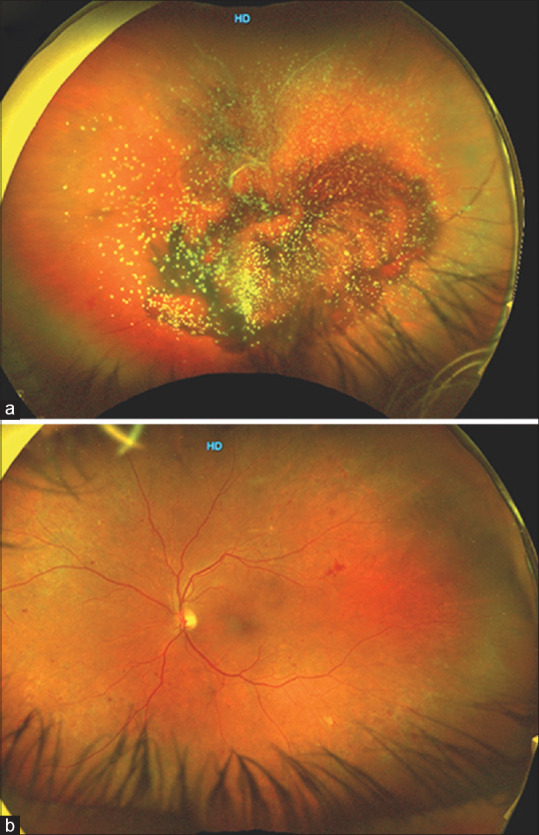
Ultra-widefield fundus photograph (Optos California) of a patient with diabetic vitreous hemorrhage along with asteroid hyalosis (Panel a). Fundus examination of the fellow eye shows non-proliferative diabetic retinopathy giving a clue toward etiology of vitreous hemorrhage likely because of vascular occlusion (Panel b)
Besides retinal ischemia secondary to retinal vascular disorders, long-standing retinal detachment may also develop neo-vascularization. Inflammatory disorders with possible retinal ischemia and subsequent neo-vascularization may be seen in sarcoidosis, pars planitis, and other retinal vasculitis with arteriolitis [Fig. 4]. Other conditions which may present with neo-vascularization and possible VH include hereditary incontinentia pigmenti, familial exudative vitreoretinopathy, and multiple sclerosis. Abnormal vessels in the absence of retinal ischemia may be seen in retinal artery macroaneurysm associated with systemic hypertension, retinal capillary hemangioma, retinal cavernous hemangioma, arteriovenous malformations, and congenital peripapillary arterial loops, which can also lead to VH.[13,14,15,16,17,18]
Figure 4.
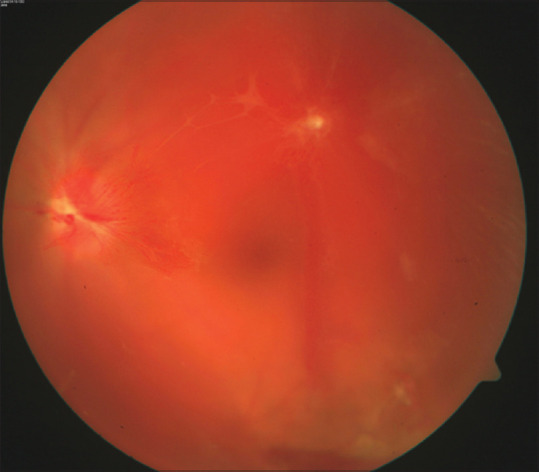
Vitreous hemorrhage with neo-vacularization of disc (NVD) in a patient with occlusive vasculitis
Disruption of normal retinal vessels
Blunt or penetrating trauma to the eye may directly lead to rupture of vessels and cause VH and is one of the leading causes of VH in patients younger than 40 years of age with a male preponderance.[19] Trauma may indirectly lead to VH because of acute posterior vitreous detachment. Posterior vitreous detachment (PVD) itself may lead to spontaneous VH because of avulsion of superficial and peripapillary retinal vessels. Development of PVD may lead to development of retinal tears which may additionally lead to VH because of avulsed retinal blood vessels traversing the tear. The risk of development of retinal tears may be as high as 70% in patients with acute PVD with VH.[20,21]
Another important cause of VH is Terson’s syndrome, characterized by intra-ocular hemorrhages (retinal, sub-hyaloid, and vitreous) in patients with intra-cranial hemorrhage secondary to head trauma or rupture of aneurysm.[22,23,24] The pathogenesis of VH in these patients appears to be because of increased intra-cranial pressure forcing blood into the sub-arachnoid space and along the optic nerve sheath into the pre-retinal space or an acute rise in intra-ocular pressure (IOP) causing venous stasis and VH. Alternatively, a raised optic nerve sheath pressure secondary to the increased intra-cranial pressure results in compression and raised hydrostatic pressure in the central retinal vein, leading to VH.[25] Valsalva retinopathy characterized by pre-retinal hemorrhages, especially sub-ILM hemorrhages, is seen because of raised intra-thoracic pressure, leading to rupture of superficial capillaries. The pre-retinal hemorrhage may break through into the vitreous gel and rarely present as VH.[26,27]
Hematological disorders such as idiopathic thrombocytopenic purpura, hypo-fibrinogenemia, disseminated intra-vascular coagulation, von Willebrands syndrome, and hemophilia may present as VH, which may be bilateral and massive in some cases.[28,29,30,31,32] Characteristically, the hematological disorders cause intra-retinal hemorrhages and vascular occlusion but may rarely present as VH. Vitreous veils in the setting of juvenile X-linked retinoschisis is also an important cause of VH.
Extension of hemorrhage through the retina from adjacent sources (‘break through’ hemorrhage)
Breakthrough VH is a less common occurrence. Sub-retinal bleed secondary to the choroidal neo-vascular membrane, idiopathic polypoidal choroidal vasculopathy, and tumors such as choroidal melanoma can extend through the ILM and retina via micro-breaks into the vitreous cavity usually several weeks after the sub-retinal hemorrhage.[33] Neo-vascular age-related macular degeneration (nAMD) is characterized by pathological choroidal vessels with disruption of Bruch’s membrane along with angiogenesis of the underlying choroid, resulting in sub-retinal bleed and/or vitreous hemorrhage.[34] Complicated intra-ocular procedures may also lead to VH secondary to extension of hemorrhage from any adjacent intra-ocular structure.
Natural History and Prognosis of Vitreous Hemorrhage
Resolution of VH has been found to be a gradual yet constant process occurring at the rate of 1% per day.[9] The prognosis depends on the etiology of VH. Spontaneous clearance of VH is more common in diseases which are unlikely to recur, such as following a PVD. Resolution occurs more rapidly because of vitreous syneresis in elderly, aphakes, and vitrectomized eyes. VH secondary to PDR frequently warrants intervention with recurrence and persistence being common. Factors which contribute to poor prognosis include associated tractional retinal detachment, neo-vascular glaucoma, and pre-retinal fibrosis.[35] The prognosis of VH associated with venous occlusions is the best with branch retinal vein occlusion, poorer in the case of hemi-central vein occlusion, and the worst in the case of associated central retinal vein occlusion.
VH secondary to nAMD has a poor prognosis because of associated sub-retinal hemorrhage and scarring involving the macula.
Pathophysiology of Resolution of Vitreous Hemorrhage
Certain distinct features of resolution of hemorrhage in the vitreous cavity include the following.
Rapid clot formation
Hemorrhage into the vitreous gel results in rapid formation of clot. This is facilitated by the molecular configuration of collagen and hyaluronic acid in the vitreous gel, which enhances platelet aggregation and membrane formation and inhibits passive diffusion of red blood cells (RBCs) and fibrin.
Slow lysis of fibrin
Factors which contribute to slow degradation of fibrin in vitreous are the low level of the tissue plasminogen activator and the lack of an early neutrophil response.
Extra-cellular lysis of RBCs
Extra-cellular lysis occurs to a greater extent in the vitreous cavity in comparison to intra-cellular lysis following ingestion by macrophages which predominates in other tissues.
Persistence of intact RBCs
This is proposed to occur because of failure of RBCs to elicit an adequate macrophage response.
Vitreous liquefaction is a common occurrence following VH. This is postulated to occur because of the presence of iron. Iron leads to generation of hydroxyl radicals, which in turn leads to de-polymerization of hyaluronic acid, thereby de-stabilizing the integrity of the vitreous microstructure, leading [Fig. 6] to its collapse. Breakdown of RBCs following VH may result in the formation of cholesterol crystals. Cholesterolosis involving the vitreous cavity has been termed synchysis scintillans. Hemoglobin spherulosis is a term coined to describe refractile brown spherules that occur in the vitreous following resolution of vitreous or more commonly sub-retinal hemorrhage. Vitreous cylinders are tubular structures that have been observed to develop from separated lamellae of vitreous following hemorrhage that condense to form cylindroid structures. The RBCs and debris suspended in vitreous collagen can clinically present as an ochre membrane. Fibrin deposition on the posterior hyaloid following resolution of sub-hyaloid hemorrhage has been described as silk-like veils.
Figure 6.
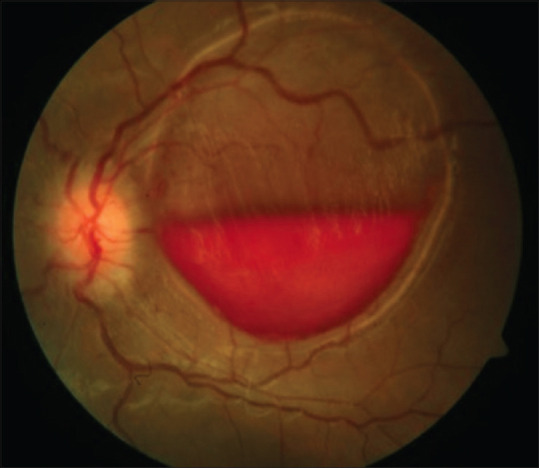
Vitreous hemorrhage in a patient with intra-cranial bleed (Terson’s syndrome)
Etiology
The three most common causes of VH are PDR, PVD with or without retinal tear, and ocular trauma. Together, these causes account for 59–88.5% of all cases presenting with vitreous hemorrhage.[9] Common causes of vitreous hemorrhage are enlisted in Table 1. Miscellaneous causes of VH are enlisted in Table 2.
Table 1.
Common causes of vitreous hemorrhage
| Common causes of vitreous hemorrhage | |
|---|---|
| Vitreouretinal PVD with/without retinal tear |
Choroidal – Choroidal neo-vascularization |
| Age-related macular degeneration | |
| Pathologic myopia | |
| Peripheral exudative hemorrhagic chorioretinopathy (PEHCR) Inflammatory/Infectious choroidopathies | |
| Retinovascular – With Neo-vascularization |
Ocular Trauma |
| Proliferative diabetic retinopathy | Closed globe injury |
| Retinal vein occlusion | Open globe injury |
| Sickle cell retinopathy | Valsalva Retinopathy |
| Radiation Retinopathy | Shaken baby syndrome |
| Retinopathy of Pre-maturity | |
| Occlusive vasculitis (e.g., Behcets diease) [Fig. 4] | |
| Retinovascular – Without Neo-vascularization |
Systemic causes |
| Macro-aneurysm | Blood disorders |
| Retinal Angioma | Terson’s syndrome |
| Arterio-venous malformation of the retina | |
| Persistent hyaloid artery | |
| Familial retinal arteriolar tortousity | |
Table 2.
Infrequent causes of vitreous hemorrhage
| Infrequent causes of vitreous hemorrhage | |
|---|---|
| Complicated surgical procedures | Inflammation |
| Complicated cataract surgery with or without IOL | Pars planitis |
| Trabeculectomy | Behcet’s disease |
| Globe perforation secondary to peri-bulbar/retro-bulbar anesthesia | Syphilitic retinitis |
| Penetrating keratoplasty | Multiple sclerosis with retinal vasculitis |
| Traumatic secondary IOL insertion | Systemic lupus erythematosus |
| Retinal and Choroidal tumors | Blood disorders |
| Retinoblastoma | Thrombocytopenia |
| Vasoproliferative tumors | Thrombotic thrombocytopenic purpura |
| Melanoma and melanocytoma | Protein C deficiency |
| Cavernous hemangioma | Anti-coagulant therapy |
| Vascular | Miscellaneous |
| Retinopathy of pre-maturity | Shaken baby syndrome |
| Coats disease | Newborn after vaginal delivery |
| Ocular ischemic syndrome | Retinoschisis (Senile and juvenile) |
Clinical Features and Evaluation
The diagnosis of VH is made upon clinical examination. Relevant and intelligent history from the patient can guide the physician to possible etiology of VH.
Clinical presentation, onset, and duration
Patients with VH present with an acute onset painless decrease in visual acuity. New onset floaters, shadows, “cobwebs”, or a seeing red hue to vision are common symptoms.
History taking
Pertinent questions regarding possible etiological causes of VH have to be asked and at the same time should be kept in mind while working up a patient with VH as enlisted in Table 3.
Table 3.
History pointing toward possible etiological causes
| History pointing toward possible etiological causes | |
|---|---|
| Ocular trauma: Closed/Open globe injury | Systemic illness: Diabetes Mellitus/Hypertension/Sickle retinopathy or blood dyscrasias |
| Floaters of Flashes of light: Acute posterior vitreous detachment | Straining/forceful cough or vomiting: Vaslalva Retinopathy |
| Redness/pain/recurrent floaters/decreased vision: Inflammatory pathology/Uveitis | Head injury/intra-cranial bleed: Terson’s syndrome |
Patient demographics
Systemic vascular conditions such as diabetes, hypertension, vascular occlusions, posterior vitreous detachment, and retinal tears are common causes of VH in middle-aged and older patients.[36,37,38] Exudative hemorrhagic chorioretinopathy may result in VH in patients older than 75 years.[39,40] Conversely, ocular trauma, inflammatory conditions/vasculitides, or hematologic conditions such as hemoglobinopathies, sickle cell disease, and so on are important causes of VH in young.[41,42] VH in childhood may be seen in patients with retinopathy of pre-maturity, persistent hyperplastic primary vitreous, Coat’s disease, and familial exudative vitreoretinopathy.
Clinical examination
Visual acuity varies depending upon the location, size, and degree of VH. Patients may present with dramatically reduced visual acuity in severe cases or may only present with sudden onset floaters with mild deterioration in vision.
Intra-ocular pressure and anterior segment examination
IOP should be noted in all cases. It may be raised in eyes with ocular trauma or neo-vascular glaucoma or because of RBCs clogging trabecular meshwork (Ghost cell glaucoma).
Relative afferent papillary defect (RAPD) should be ruled out. Disorders such as retinal detachment, macular disorder, or optic nerve disease may show the presence of RAPD, whereas VH alone does not have RAPD.
A careful and patient slit lamp examination can give clue toward possible etiology. The presence of neo-vascularization of iris (NVI) should be noted before dilating the pupil for fundus examination. Gonioscopy may be useful to detect neo-vascularization of the angle (NVA). NVI and NVA indicate severe ischemia and could be seen in patients with central retinal vein occlusion (CRVO), PDR, and severe occlusive vasculitis. In cases of ocular trauma, iris sphincter tear, iridodialysis, phacodonesis, and/or cyclodialysis may be present upon anterior segment examination. Signs of anterior segment inflammation such as cells, flare, posterior synechiae, iris nodules, and so on point toward possible inflammatory etiology (uveitis) of VH.
Dilated fundus examination
Dilated fundus exam may reveal diffuse VH or the blood confined to sub-hyaloid space (scaphoid/boat-shaped). In cases with dense VH, media may be hazy, making it difficult to visualize the retina. Alternatively, fundus details can be made out in cases with mild VH.
When retinal details are visible, careful observation should be made to pick up clinical signs of vascular occlusion, background diabetic retinopathy (DR) changes, retinal neo-vascularization or signs pointing toward inflammatory pathology (posterior uveitis) such as vitreous cells/snowballs, vascular sheathing, choroiditis, and so on. When VH is associated with acute PVD, it becomes important to rule out retinal tear or detachment by indirect ophthalmoscopy using scleral depression. Similarly, in eyes with ocular trauma, peripheral fundus examination by indirect ophthalmoscopy using scleral depression should be performed to rule out retinal tear/dialysis 2–3 weeks after the injury. Macular double ring sign may be seen in Terson’s syndrome, formed by sub-hyaloid membranes on the outside and sub-ILM bleed inside [Fig. 6].[43]
Examination of the fellow eye
Thorough examination of the fellow eye is important in eyes with VH as it may reveal important clinical clues pointing toward the possible etiology. This is especially important in the case of VH because of systemic conditions such as diabetes mellitus, hypertension, hemoglobinopathies, and so on [Fig. 5]. Signs of ocular inflammation such as vitreous cells, snowballs, vascular sheathing/obliteration, peri-vascular exuation, and choroiditis lesions/scars can be observed in fellow eyes of patients with vasculitic VH.
Diagnostic Procedures
Ultra-sonography
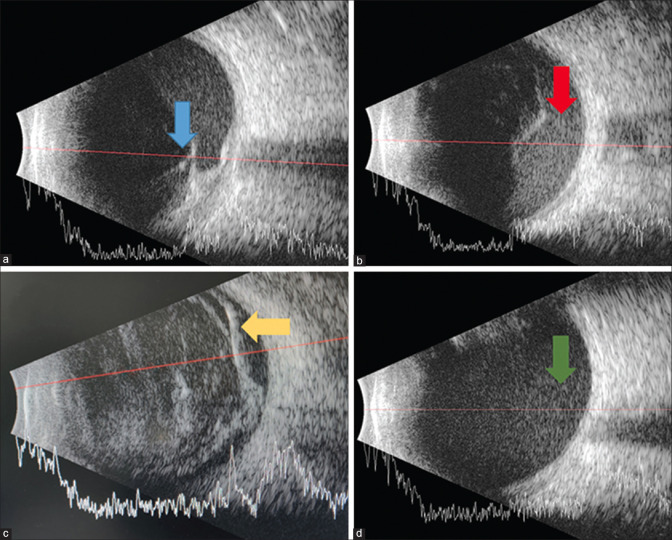
Ultra-sonography (USG) plays a very important role in detecting PVD, retinal detachment, tractional membranes, the presence of foreign bodies, or intra-ocular tumors in cases of dense VH where the posterior segment is not visible upon clinical examination. A-scan along with B-scan is useful in making the etiological diagnosis as well as study the characteristics of VH [Fig. 7]. Various characteristics of VH seen on USG are as follows.
Figure 7.
Cross-sectional B-scan image with overlying A-scan of the eye showing tractional retinal detachment with associated sub-hyaloid hemorrhage (Panel a, Blue arrow), sub-hyaloid hemorrhage (Panel b, Red arrow), vitreous hemorrhage associated with retinal detachment seen as a double membrane (Panel c, Yellow arrow), and dispersed rebleed in the vitrectomized eye (Panel d, Green arrow)
Fresh VH/VH in the vitrectomized eye: Multiple dot-like echoes of low reflectivity are better visualized on high gain. The echoes appear more mobile with the movement of the eyeball in a vitrectomized eye.[44]
Long-standing/old VH: Dot-like echoes coalesce to form a highly reflective membrane, which appear denser inferiorly. It is important to differentiate this finding from retinal detachment [Fig. 7].
Sub-hyaloid hemorrhage: Multiple dot-like echoes are seen behind thin membrane (PVD).
Clues to the underlying etiology: Ultra-sound helps in detecting underlying rhegmatogenous or tractional retinal detachment, retinal tear, intra-ocular foreign bodies, globe rupture/vitreous incarceration, and choroidal mound in the macular region, indicating polypoidal choroidal vasculopathy and mass lesion such as malignant melanoma.
Fundus fluorescein angiography
In patients with mild to moderate VH, fundus fluorescein angiography (FFA) is useful in localizing areas with capillary non-perfusion and neo-vascularization to plan laser therapy. This is especially useful in cases of PDR, vein occlusions, or occlusive vasculitis.
Optical coherence tomography
Optical coherence tomography (OCT) is useful in differentiating sub-hyaloid bleed from sub-ILM bleed in patients with pre-macular hemorrhage.[45]
Radio-imaging
X-ray/CT scans of orbit are useful in cases of ocular trauma to assess the integrity of the posterior eyewall and also when an intra-ocular foreign body is suspected.
Laboratory/blood investigations
Laboratory investigations are tailored according to the clinical suspicion for the diagnosis of diabetes mellitus, thrombocytopenia, leukemia, sickle cell disease, and other hematologic abnormalities.
Differential Diagnosis
Vitritis: Inflammation in patients with posterior uveitis may mimic old VH. Infection may also mimic VH. Careful anterior and posterior segment evaluation with meticulous history taking allows us to differentiate vitritis from VH. Signs of vitritis include anterior chamber cells/flare, retrolental cells, retinitis patches, choroiditis patches, thickening of the tenons space, optic disc edema, and exudative retinal detachment.
Primary intra-ocular lymphoma: Primary intra-ocular lymphoma (PIOL) may masquerade as old VH. The older age group with the presence of vitreous cells and the presence of sub-retinal pigment epithelium (RPE) deposits in PIOL.[46]
Asteroid hyalosis: Asteroid hyalosis may mimic VH on USG, although clinically, it can be easily differentiated. Asteroid hyalosis on USG appears as multiple discrete highly reflective echoes with a hypoechoic space near the retina and optic nerve.[47]
Management
The management of VH depends on various factors such as etiology, duration, laterality, and associated ocular conditions. Hence, management has to be customized depending on the presentation of the patient.
The basic principles of management include the following.
Observation
Prior to the introduction of modern vitrectomy, the only management option available for VH was observation with head end elevation. Patients with VH usually complain of worsening of visual symptoms after waking up, which improves as the day progresses. Head-end elevation with patching of the eye to diminish eye movement allows VH to settle inferiorly. Hence, in patients with unilateral VH with no concurrent ocular morbidity (no retinal detachment, no iris/angle neo-vascularization), observation with head-end elevation and re-evaluation to assess the resolution at weekly intervals is a viable option. In patients with non-proliferative causes such as an acute PVD, the presence of retinal breaks and subsequent retinal detachment has to be kept in mind when advising observation as they are indications of early intervention. Resolution of VH requires liquefaction of the vitreous, which may be accelerated in the presence of exogenous ascorbic acid, although its utility is yet to be proven in clinical trials.[48]
Use of laser therapy
Management strategies of pre-macular hemorrhage (sub-hyaloid and sub-ILM) include observation, laser hyaloidotomy, and vitrectomy. Spontaneous recovery of pre-macular hemorrhage has been observed to occur, and observation may be advised up to 3 months.[49,50] Sequestrated blood over the macula can predispose to epi-retinal membrane formation, especially sub-ILM hemorrhage, which persists longer than a sub-hyaloid hemorrhage and hence mandates an intervention. Laser hyaloidotomy (Nd: YAG laser) is a safe and effective alternative to hasten the visual recovery but may rarely be associated with macular hole formation, parafoveal macular holes, and persistent pre-macular cavity.[51] The basic principle is the drainage of the sequestrated blood into the vitreous cavity allowing for clearance of the macula and its subsequent resorption.
Laser photo-coagulation remains the bedrock of management in proliferative retinopathies and should be initiated as soon as the retina is visible [Fig. 8]. Besides the slit-lamp delivery system of laser, the indirect ophthalmoscope delivery system may also be used to reach up to the retinal periphery. In patients with visible retinal breaks or avulsed vessels, use of the indirect ophthalmoscope delivery system is valuable. In PDR, the role of pan-retinal photo-coagulation (PRP) remains the basis of treatment in patients having PDR with high-risk characteristics (Moderate to severe NVD, NVE, with VH).[52,53]
Figure 8.
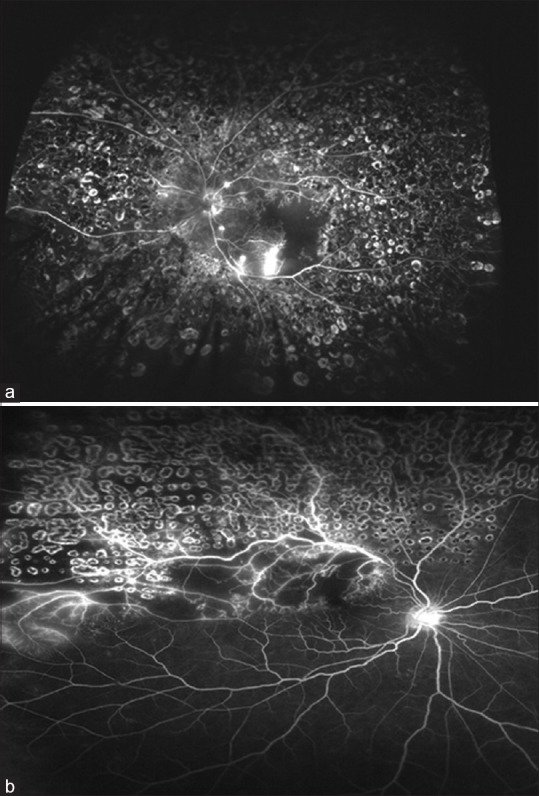
Fluorescein angiography showing laser marks in a patient with proliferative diabetic retinopathy (Panel a) and vascular occlusion (Panel b)
Laser photo-coagulation is also used in patients with retinal vascular occlusion (RVO) and retinal vasculitis. Sectoral scatter photo-coagulation is used in patients with branch retinal vein occlusion (BRVO) who have developed neo-vascularization (disc, retinal, iris) and reduces the risk of development of VH by 50%.[54] Similarly, pan-retinal photo-coagulation is performed in patients with development of neo-vascularization of iris (2-clock hours or more) or any angle neo-vascularization which reduces the risk of neo-vascular complications of CRVO such as neovascular glaucoma.[55] In the setting of VH with RVO, laser photo-coagulation essentially ablates the ischemic retina and reduces the VEGF load. The laser photo-coagulation allows the regression of neo-vascularization as the VH absorbs spontaneously.
Use of cryotherapy
Laser photo-coagulation requires a clear ocular media which may not always be present. Concurrent cataract formation and VH preclude completion of PRP in PDR patients, allowing the underlying pathological process to continue unchecked. An alternative management in such cases may be the injection of anti-angiogenic factors but is not possible in all cases (patients with a recent history of thrombo-embolic diseases, coronary artery disease, and tractional retinal detachment).[56] In some selected cases, anterior retinal cryopexy (ARC) may be employed to curb the angiogenic factors and minimize the proliferation of neo-vascular tissues. ARC acts by causing breakdown of the blood retinal barrier and macrophage activation which helps clear the VH, and at the same time, the peripheral ischemic retina is ablated to reduce the angiogenic load (VEGF). Cryotherapy may also be used in post-vitrectomy eyes with recurrent rebleed. Vitreous rebleed is seen in 13–40% of patients after vitrectomy with a common cause being port site proliferation of neo-vascular tissues. Port-site cryotherapy may reduce the risk of delayed vitreous rebleed.[57] With the advent of pars plana vitrectomy, use of cryotherapy is limited to post-vitrectomy rebleed with port-site neo-vascularization or in patients with anterior hyaloidal proliferation.
Use of anti-VEGF and pharmacologic agents
Observation and surgical intervention have remained the principle management of VH. Surgical intervention requires the patient to be systemically stable as well as associated with a longer post-operative recovery time as compared to an intravitreal injection, whereas an indefinite observation period would be required in patients who are not systemically fit for surgery. An alternative in such cases was required to hasten the resolution of VH.
Initial studies were performed to evaluate the role of intra-vitreal saline injection as well as use of enzymatic vitreolysis to hasten visual recovery by facilitating RBC lysis and phagocytosis.[58] Vitreolytic substances that were commonly employed were hyaluronidase, plasmin dispase, and the tissue plasminogen activator.[59] Enzymatic vitreolysis offers the advantage of early resolution of VH such that the underlying cause of VH may be identified and adequately treated unlike the period of observation where the underlying pathology remains untreated. Another advantage would be the lower treatment cost as vitrectomy requires sophisticated machines and the experience of a surgeon trained in vitreo-retinal surgery.
The rationale of using anti-VEGF agents in proliferative retinopathy (PDR, NVE in vasculitis sequelae) is to reduce neo-vascularization and vascular leak while the VH absorbs. Clearing of the VH would also allow the initiation and/or completion of laser photo-coagulation. Multiple studies have shown the efficacy of intra-vitreal anti-VEGF agents in resolution of VH in proliferative retinopathy including Eales vasculitis[60] and PDR[56,61,62,63] with clearance rates as high as 92% of cases in less than 3 months.[64] Anti-VEGF agents are also commonly used prior to vitrectomy in patients with PDR to regress fibro-vascular proliferation and neo-vascularization, hence facilitating dissection of membranes and reducing intra-operative bleeding and post-operative rebleed. However, their use in advanced PDR may be associated with “crunch syndrome”, which is defined as the worsening of tractional retinal detachment in patients associated with sudden loss of vision within 1 and 6 weeks of injection.[65] Anti-VEGF crunch syndrome is commonly associated with intra-vitreal bevacizumab injection with associated risk factors being a ring-shaped fibro-vascular membrane, the absence of laser photo-coagulation, use of a higher dose of intra-vitreal bevacizumab (2.5 mg), and a prolonged interval between injection and vitrectomy (>13 days).[65]
Vitrectomy
The definitive management of VH is pars plana vitrectomy. It is commonly indicated in patients with dense non-clearing VH/VH associated with retinal tears and retinal detachment. Proliferative causes of VH such as PDR may warrant an early vitrectomy, especially in the setting of a tractional retinal detachment involving the macula, whereas watchful observation may be employed in patients with VH with lasered PDR. Similarly, non-proliferative causes such as an acute posterior vitreous detachment with VH may be observed, unless complicated with a retinal tear or a retinal detachment which would mandate an early vitrectomy.
Over the past 2 decades, there has been a tremendous change toward early surgery in patients with vitreous hemorrhage. Advances in the instrumentation and techniques have reduced the risks of complications such as cataract, endophthalmitis, retinal tears, retinal detachment, and neo-vascular glaucoma dramatically in patients undergoing vitrectomy for vitreous hemorrhage.[66,67] Vitrectomy allows the removal of media opacities to not only improve visual acuity but also remove the vitreous scaffold, hence reducing the complications such as development of tractional retinal detachment, macular pucker, and the epi-retinal membrane in patients with proliferative retinopathy.
The role of vitrectomy in PDR has been well established with similar results in other causes of VH.[33,68,69,70,71,72] The combination of phaco-emulsification with intra-ocular lens implantation with pars plana vitrectomy is not only safe but also an effective procedure which shortens the recovery time, reduces the number of trips to the hospital and operating room, and provides good post-operative visibility for augmentation of laser if required with drawbacks such as increased inflammation and a transient increase in IOP.[73,74,75]
The micro-incision vitrectomy system (MIVS) in the form of 23G, 25G, and 27G vitrectomy systems has become increasingly popular with its advantages of better patient comfort, reduced astigmatism, and a faster recovery time. However, these systems have their own complications in the form of higher incidence of post-operative hypotony and endophthalmitis. In the 20G system, sclerotomy closure with sutures was a rule, whereas the MIVS system boasts of transconjunctival sutureless surgery with the rates of transient post-operative hypotony being as high as 10% with the 23G system[76,77,78,79] but < 1% with the 25G system with the scleral tunnel.[80] Another complication associated with the MIVS system is endophthalmitis. The rate of endophthalmitis associated with the 20G system ranges from 0.018% to 0.03%.[81,82] In contrast, the endophthalmitis rate associated with 23G vitrectomy ranged from 0.0% to 0.03%, whereas the rate associated with 25G vitrectomy ranged from 0.13% to 0.23%.[81,82,83]
Complications
Hemosiderosis bulbi: This occurs secondary to iron (Fe3+) released following the catabolism of hemoglobin. Clinical features are similar to siderosis following intra-ocular iron foreign bodies; however, it is a rare complication because of the slow catabolism of RBCs and the iron-binding capacity of vitreous proteins.
Proliferative vitreoretinopathy: This is a rare complication that may be seen following VH in Terson’s syndrome. Damage to the internal limiting membrane results in an exaggerated wound healing response with glial and fibro-vascular proliferation. VH in patients with proliferative retinopathy precludes intervention allowing the pathological processes to continue unchecked, causing severe fibro-vascular proliferation and tractional retinal detachment.
Glaucoma: Ghost cell glaucoma is a secondary open-angle glaucoma that occurs at 1–3 months following VH. Ghost cells are degenerated, smaller, khakhi-colored, less pliable RBCs which pass into the anterior chamber, occlude the trabecular meshwork, and reduce the outflow facility. These ghost cells remain in the vitreous even months after resolution of the VH and do not form in the anterior chamber because of rapid circulation and high levels of oxygen.[84,85] Hemolytic and hemosiderotic glaucoma are rare, secondary to obstruction of the trabecular meshwork by free hemoglobin or hemosiderin-laden macrophages.
Conclusion
Vitreous hemorrhage can be a result of a variety of ocular diseases and can lead to severe visual loss. With thorough history, systemic, and ocular evaluation, one can ascertain the cause of VH. Pars plana vitrectomy remains the definitive treatment, although the treatment should be individualized to each patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Keeler CR. The ophthalmoscope in the lifetime of Hermann von Helmholtz. Arch Ophthalmol. 2002;120:194–201. doi: 10.1001/archopht.120.2.194. [DOI] [PubMed] [Google Scholar]
- 2.Pröbsting A. Ueber blutinjectionen in den glaskörper. Albrecht von Graefes Archiv für Ophthalmologie. 1892;38:114–44. [Google Scholar]
- 3.Blodi CF. David Kasner, MD, and the road to pars plana vitrectomy:Supplementary issue:Ophthalmic history. Ophthalmol Eye Dis. 2016;8 doi: 10.4137/OED.S40424. OED-S40424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res. 2000;19:323–44. doi: 10.1016/s1350-9462(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 5.Balazs EA, Laurent TC, Laurent UBG, Deroche MH, Bunney OM. Studies on the structure of the vitreous body. Viii. Comparative biochemistry. Arch Biochem Biophys. 1959;81:464–79. doi: 10.1016/0003-9861(59)90227-9. [DOI] [PubMed] [Google Scholar]
- 6.Balazs EA. Importance of the vitreous body in retina surgery with special emphasis on reoperations. In:Physiology of the vitreous body. 1960:29–48. [Google Scholar]
- 7.Balazs EA. Molecular morphology of the vitreous body. In: Smelser GK, editor. The Structure of the Eye. New York: Academic Press; 1961. pp. 293–310. [Google Scholar]
- 8.Cibis GW, Watzke RC, Chua J. Retinal haemorrhages in posterior vitreous detachment. Am J Ophthalmol. 1975;80:1043–56. doi: 10.1016/0002-9394(75)90334-7. [DOI] [PubMed] [Google Scholar]
- 9.Spraul CW, Grossniklaus HE. Vitreous haemorrhage. Surv Ophthalmol. 1997;42:3–9. doi: 10.1016/s0039-6257(97)84041-6. [DOI] [PubMed] [Google Scholar]
- 10.Parekh PK, Miller MA, Russell SR. Sickle cell retinopathy. EyeRounds.org. Mar 29, 2016. Available from:http://EyeRounds.org/cases/233-Sickle-Cell-Retinopathy.htm .
- 11.Jampol LM, Ebroon DA, Goldbaum MH. Peripheral proliferative retinopathies:An update on angiogenesis, etiologies and management. Surv Ophthalmol. 1994;38:519–40. doi: 10.1016/0039-6257(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 12.Cabral T, Mello LG, Lima LH, Polido J, Regatieri CV, Belfort R, et al. Retinal and choroidal angiogenesis:A review of new targets. Int J Retina Vitreous. 2017;3:1–3. doi: 10.1186/s40942-017-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster AR, Maher ER, Moore AT. Clinical characteristics of ocular angiomatosis in von Hippel-Lindau disease and correlation with germline mutation. Arch Ophthalmol. 1999;117:371–8. doi: 10.1001/archopht.117.3.371. [DOI] [PubMed] [Google Scholar]
- 14.Singh AD, Nouri M, Shields CL, Shields JA, Perez N. Treatment of retinal capillary hemangioma. Ophthalmology. 2002;109:1799–806. doi: 10.1016/s0161-6420(02)01177-6. [DOI] [PubMed] [Google Scholar]
- 15.Messmer E, Laqua H, Wessing A, Spitznas M, Weidle E, Ruprecht K, et al. Nine cases of cavernous hemangioma of the retina. Am J Ophthalmol. 1983;95:383–90. doi: 10.1016/s0002-9394(14)78309-6. [DOI] [PubMed] [Google Scholar]
- 16.Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous haemorrhage and venous retinal macrovessel. Retina. 1996;16:74. doi: 10.1097/00006982-199616010-00014. [DOI] [PubMed] [Google Scholar]
- 17.Accou GP, Nerinckx F, Leroy BP, De Zaeytijd J. Vitreous haemorrhage as presenting sign of retinal arteriovenous malformation. Case Rep Ophthalmol Med. 2020;2020:8858242. doi: 10.1155/2020/8858242. doi:10.1155/2020/8858242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavin MJ, Marsh RJ, Peart ST, Rehman A. Retinal arterial macroaneurysms:A retrospective study of 40 patients. Br J Ophthalmol. 1987;71:817–25. doi: 10.1136/bjo.71.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dana MR, Werner MS, Viana MA, Shapiro MJ. Spontaneous and traumatic vitreous haemorrhage. Ophthalmology. 1993;100:1377–83. doi: 10.1016/s0161-6420(93)31472-7. [DOI] [PubMed] [Google Scholar]
- 20.Benson WE. 2 ed. Philadelphia: Harper &Row Publishers, Inc; 1988. Retinal Detachment:Diagnosis and Management; p. 4. [Google Scholar]
- 21.Byer NE. Natural history of posterior vitreous detachment with early management as the premier line of defense against retinal detachment. Ophthalmology. 1994;101:1503–13. 1513–4. doi: 10.1016/s0161-6420(94)31141-9. [DOI] [PubMed] [Google Scholar]
- 22.McCarron MO, Alberts MJ, McCarron P. A systematic review of Terson's syndrome:Frequency and prognosis after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2004;75:491–3. doi: 10.1136/jnnp.2003.016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tripathy K. Dissociated optic nerve fiber layer in a case of Terson syndrome. Eur J Ophthalmol. 2020;30:NP11–4. doi: 10.1177/1120672119853465. [DOI] [PubMed] [Google Scholar]
- 24.Czorlich P, Skevas C, Knospe V, Vettorazzi E, Richard G, Wagenfeld L, Westphal M, Regelsberger J. Terson syndrome in subarachnoid hemorrhage, intracerebral hemorrhage, and traumatic brain injury. Neurosurg Rev. 2015;38:129–36. doi: 10.1007/s10143-014-0564-4. discussion 136. [DOI] [PubMed] [Google Scholar]
- 25.Gress DR, Wintermark M, Gean AD. A case of Terson syndrome and its mechanism of bleeding. J Neuroradiol. 2013;40:312–4. doi: 10.1016/j.neurad.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Jones WL. Valsalva maneuver induced vitreous haemorrhage. J Am Optom Assoc. 1995;66:301–4. [PubMed] [Google Scholar]
- 27.Tripathy K, Chawla R, Vekaria L, Sharma YR. Sub-internal limiting membrane cavity following valsalva retinopathy resembling central serous chorioretinopathy. J Ophthalmic Vis Res. 2018;13:83–4. doi: 10.4103/jovr.jovr_192_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goel N, Arora S, Jain P, Ghosh B. Massive subretinal and vitreous haemorrhages at presentation in idiopathic thrombocytopenic purpura:Report of a case and review of literature. Clin Exp Optom. 2014;97:270–3. doi: 10.1111/cxo.12072. [DOI] [PubMed] [Google Scholar]
- 29.Mansour AM, Jaroudi MO. Recurrent vitreous haemorrhage and epidural haematoma in a child with hypofibrinogenaemia. Case Rep. 2012;2012:bcr2012006478. doi: 10.1136/bcr-2012-006478. doi:10.1136/bcr-2012-006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azar P, Smith RS, Greenberg MH. Ocular findings in disseminated intravascular coagulation. Am J Ophthalmol. 1974;78:493–6. doi: 10.1016/0002-9394(74)90237-2. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann WA, Lohmann CP, Demmler-Hackenberg M, Gabel VP. Von Willebrand's disease type I as a cause for subvitreal, retinal and subretinal haemorrhages. Graefe's Arch Clin Exp Ophthalmol. 2005;243:383–5. doi: 10.1007/s00417-004-0999-3. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi H, Honda Y. Intraocular haemorrhage in a patient with hemophilia. Metab Ophthalmol Pediatr Systemic. 1984;8:27–30. [PubMed] [Google Scholar]
- 33.Ziemianski MC, McMeel JW, Franks EP. Natural history of vitreous hemorrhage in diabetic retinopathy. Ophthalmology. 1980;87:306–12. doi: 10.1016/s0161-6420(80)35232-9. [DOI] [PubMed] [Google Scholar]
- 34.American Academy of Ophthalmology. Age-Related Macular Degeneration, Preferred Practice Pattern 2018. Available from:https://www.aao.org/ppp .
- 35.Tolls DB. Peripheral retinal hemorrhages:A literature review and report on thirty-three patients. J Am Optom Assn. 1998;69:563–74. [PubMed] [Google Scholar]
- 36.Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994;117:429–41. doi: 10.1016/s0002-9394(14)70001-7. [DOI] [PubMed] [Google Scholar]
- 37.Retina, Bagheri N, Wajda B, Calvo C, Durrani A. The Wills Eye Manual:Office and Emergency Room Diagnosis and Treatment of Eye Disease. 7th ed. Philadelphia: Wolters Kluwer; 2017. pp. 279–81. [Google Scholar]
- 38.Tranos P, Vacalis A, Asteriadis S, Koukoula S, Vachtsevanos A, Perganta G, et al. Resistance to antivascular endothelial growth factor treatment in age-related macular degeneration. Drug Design Dev Ther. 2013;7:485–90. doi: 10.2147/DDDT.S43470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khurshid G. Peripheral exudative hemorrhagic chorioretinopathy. JAMA Ophthalmol. 2017;135:e165491. doi: 10.1001/jamaophthalmol.2016.5491. [DOI] [PubMed] [Google Scholar]
- 40.Reddy SC, Jackson N. Retinopathy in acute leukaemia at initial diagnosis:Correlation of fundus lesions and haematological parameters. Acta Ophthalmol Scand. 2004;82:81–5. doi: 10.1046/j.1600-0420.2003.00197.x. [DOI] [PubMed] [Google Scholar]
- 41.Miller NR, Walsh FB, Hoyt WF, Newman NJ. Leukemias/Lymphomas. Walsh and Hoyt's Clinical Neuro-Ophthalmology. Philadelphia: Lippincott Williams and Wilkins; 2005.. pp. 1613–30. [Google Scholar]
- 42.Berges O, Torrent M. Écographie de l'oeil et de l'orbite. Paris, France: Vogot; 1986. [Google Scholar]
- 43.Shukla D, Naresh KB, Kim R. Optical coherence tomography findings in valsalva retinopathy. Am J Ophthalmol. 2005;140:134–6. doi: 10.1016/j.ajo.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 44.Jang HS, Sepah YJ, Sophie R, Bittencourt MG, Ferraz D, Hanout M, et al. Longitudinal spectral domain optical coherence tomography changes in eyes with intraocular lymphoma. J Ophthal Inflamm Infect. 2013;3:1–8. doi: 10.1186/1869-5760-3-59. doi:10.1186/1869-5760-3-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khoshnevis M, Rosen S, Sebag J. Asteroid hyalosis—a comprehensive review. Surv Ophthalmol. 2019;64:452–62. doi: 10.1016/j.survophthal.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Chattopadhyay D, Akiba J, Ueno N, Chakrabartia B. Metal ion catalyzed liquefaction of vitreous by ascorbic acid:Role of radicals and radical ions. Ophthalmic Res. 1992;24:1–7. doi: 10.1159/000267137. doi:10.1159/000267137. [DOI] [PubMed] [Google Scholar]
- 47.Heydenreich A. Treatment of preretinal haemorrhages by means of photocoagulation. Klin Monatsbl Augenheilkd. 1973;16:3671–6. [PubMed] [Google Scholar]
- 48.Meier P, Schmitz F, Wiedemann P. Vitrectomy for pre-macular hemorrhagic cyst in children and young adults. Graefe's Arch Clin Exp Ophthalmol. 2005;24:3824–8. doi: 10.1007/s00417-005-1213-y. [DOI] [PubMed] [Google Scholar]
- 49.Bypareddy R, Chawla R, Azad SV, Takkar B. Iatrogenic parafoveal macular hole following Nd-YAG posterior hyaloidotomy for premacular haemorrhage. BMJ Case Rep. 2016;2016:e217234. doi: 10.1136/bcr-2016-217234. doi:10.1136/bcr-2016-217234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy:Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- 51.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy:ETDRS report number 9. Ophthalmology. 1991;98:766–85. [PubMed] [Google Scholar]
- 52.Branch Vein Occlusion Study Group. Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. Branch Vein Occlusion Study Group. Arch Ophthalmol. 1986;104:34–41. doi: 10.1001/archopht.1986.01050130044017. [DOI] [PubMed] [Google Scholar]
- 53.Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischermic central vein occlusion:The Central Vein Occlusion Study Group N Report. Ophthalmology. 1995;102:1434–44. [PubMed] [Google Scholar]
- 54.Wu L, Martínez-Castellanos MA, Quiroz-Mercado H, Arevalo JF, Berrocal MH, Farah ME, et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin):Results of the Pan-American Collaborative Retina Study Group (PACORES) Graefes Arch Clin Exp Ophthalmol. 2008;246:81–7. doi: 10.1007/s00417-007-0660-z. [DOI] [PubMed] [Google Scholar]
- 55.Mahalingam P, Topiwalla TT, Ganesan G. Vitreous rebleed following sutureless vitrectomy:Incidence and risk factors. Indian J Ophthalmol. 2018;66:558–61. doi: 10.4103/ijo.IJO_770_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuppermann BD, Thomas EL, de Smet MD, Grillone LR Vitrase for Vitreous Hemorrhage Study Groups. Pooled efficacy results from two multinational randomized controlled clinical trials of a single intravitreous injection of highly purified ovine hyaluronidase (Vitrase®) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005;140:573–84. doi: 10.1016/j.ajo.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 57.Bhisitkul RB. Anticipation for enzymatic vitreolysis. Br J Ophthalmol. 2001;85:1–2. doi: 10.1136/bjo.85.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chanana B, Azad RV, Patwardhan S. Role of intravitreal bevacizumab in the management of Eales'disease. Int Ophthalmol. 2010;30:57–61. doi: 10.1007/s10792-009-9292-0. [DOI] [PubMed] [Google Scholar]
- 59.Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–8. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Diabetic Retinopathy Clinical Research Network. Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmol. 2013;131:283–93. doi: 10.1001/jamaophthalmol.2013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Antoszyk AN, Glassman AR, Beaulieu WT, Jampol LM, Jhaveri CD, Punjabi OS, et al. Effect of intravitreous aflibercept vs vitrectomy with panretinal photocoagulation on visual acuity in patients with vitreous hemorrhage from proliferative diabetic Retinopathy:A randomized clinical trial. JAMA. 2020;324:2383–95. doi: 10.1001/jama.2020.23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wirkkala J, Bloigu R, Hautala NM. Intravitreal bevacizumab improves the clearance of vitreous haemorrhage and visual outcomes in patients with proliferative diabetic retinopathy. BMJ Open Ophthalmol. 2019;4:e000390. doi: 10.1136/bmjophth-2019-000390. doi:10.1136/bmjophth-2019-000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan Y, Fukutomi A, Sun M, Durkin S, Gilhotra J, Chan W. Anti-VEGF crunch syndrome in proliferative diabetic retinopathy:A review. Surv Ophthalmol. 2021;66:926–32. doi: 10.1016/j.survophthal.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Jackson TL, Donachie PH, Sparrow JM, Johnston RL. United Kingdom national ophthalmology database study of vitreoretinal surgery:Report 1;case mix, complications, and cataract. Eye. 2013;27:644–51. doi: 10.1038/eye.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen GH, Tzekov R, Jiang FZ, Mao SH, Tong YH, Li WS. Iatrogenic retinal breaks and postoperative retinal detachments in microincision vitrectomy surgery compared with conventional 20-gauge vitrectomy:A meta-analysis. Eye. 2019;33:785–95. doi: 10.1038/s41433-018-0319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Four-year results of a randomized trial. Diabetic Retinopathy Study report 5. Arch Ophthalmol. 1990;108:958–64. doi: 10.1001/archopht.1990.01070090060040. [DOI] [PubMed] [Google Scholar]
- 67.Zhang T, Zhang J, Sun X, Tian J, Shi W, Yuan G. Early vitrectomy for dense vitreous hemorrhage in adults with non-traumatic and non-diabetic retinopathy. J Int Med Res. 2017;45:2065–71. doi: 10.1177/0300060517708942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar A, Tiwari HK, Singh RP, Verma L, Prasad N. Comparative evaluation of early vs. deferred vitrectomy in Eales'disease. Acta Ophthalmologica Scandinavica. 2000;78:77–8. doi: 10.1034/j.1600-0420.2000.078001077.x. [DOI] [PubMed] [Google Scholar]
- 69.Jung JH, Lee JK, Lee JE, Oum BS. Results of vitrectomy for breakthrough vitreous hemorrhage associated with age-related macular degeneration and polypoidal choroidal vasculopathy. Retina. 2010;30:865–73. doi: 10.1097/IAE.0b013e3181c969e9. [DOI] [PubMed] [Google Scholar]
- 70.Garweg JG, Koerner F. Outcome indicators for vitrectomy in Terson syndrome. Acta Ophthalmol. 2009;87:222–6. doi: 10.1111/j.1755-3768.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- 71.Mason JO, Colagross CT, Vail R. Diabetic vitrectomy:Risks, prognosis, future trends. Current opinion in ophthalmology. 2006;17:281–5. doi: 10.1097/01.icu.0000193098.28798.18. [DOI] [PubMed] [Google Scholar]
- 72.Treumer F, Bunse A, Rudolf M, Roider J. Pars plana vitrectomy, phacoemulsification and intraocular lens implantation. Comparison of clinical complications in a combined versus two-step surgical approach. Graefe's Arch Clin Exp Ophthalmol. 2006;244:808–15. doi: 10.1007/s00417-005-0146-9. [DOI] [PubMed] [Google Scholar]
- 73.Park DH, Shin JP, Kim SY. Comparison of clinical outcomes between 23-gauge and 20-gauge vitrectomy in patients with proliferative diabetic retinopathy. Retina. 2010;30:1662–70. doi: 10.1097/IAE.0b013e3181d95261. [DOI] [PubMed] [Google Scholar]
- 74.Hikichi T, Matsumoto N, Ohtsuka H, Higuchi M, Matsushita T, Ariga H, et al. Comparison of one-year outcomes between 23-and 20-gauge vitrectomy for preretinal membrane. Am J Ophthalmol. 2009;147:639–43. doi: 10.1016/j.ajo.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 75.Tewari A, Shah GK, Fang A. Visual outcomes with 23-gauge transconjunctival sutureless vitrectomy. Retina. 2008;28:258–62. doi: 10.1097/IAE.0b013e318159ec5a. [DOI] [PubMed] [Google Scholar]
- 76.Shimada H, Nakashizuka H, Mori R, Mizutani Y, Hattori T. 25-gauge scleral tunnel transconjunctival vitrectomy. Am J Ophthalmol. 2006;142:871–3. doi: 10.1016/j.ajo.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 77.Scott IU, Flynn HW JR, Dev S, Shaikh S, Mittra RA, Arevalo JF, et al. Endophthalmitis after 25-gauge and 20-gauge pars plana vitrectomy:Incidence and outcomes. Retina. 2008;28:138–42. doi: 10.1097/IAE.0b013e31815e9313. [DOI] [PubMed] [Google Scholar]
- 78.Kunimoto DY, Kaiser RS, Service WE. Incidence of endophthalmitis after 20-and 25-gauge vitrectomy. Ophthalmology. 2007;114:2133–7. doi: 10.1016/j.ophtha.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 79.Parolini B, Romanelli F, Prigione G, Pertile G. Incidence of endophthalmitis in a large series of 23-gauge and 20-gauge transconjunctival pars plana vitrectomy. Graefe's Arch Clin Exp Ophthalmol. 2009;247:895–8. doi: 10.1007/s00417-009-1063-0. [DOI] [PubMed] [Google Scholar]
- 80.Scott IU, Flynn HW, Acar N, Dev S, Shaikh S, Mittra RA, et al. Incidence of endophthalmitis after 20-gauge vs 23-gauge vs 25-gauge pars plana vitrectomy. Graefe's Arch Clin Exp Ophthalmol. 2011;249:377–80. doi: 10.1007/s00417-010-1505-8. [DOI] [PubMed] [Google Scholar]
- 81.Campbell DG. Ghost cell glaucoma following trauma. Ophthalmology. 1981;88:1151–8. doi: 10.1016/s0161-6420(81)34892-1. [DOI] [PubMed] [Google Scholar]
- 82.Campbell DG, Essigmann EM. Hemolytic ghost cell glaucoma:Further studies. Arch Ophthalmol. 1979;97:2141–6. doi: 10.1001/archopht.1979.01020020459011. [DOI] [PubMed] [Google Scholar]
- 83.Koinzer S, Heckmann J, Tode J, Roider J. Long-term, therapy-related visual outcome of 49 cases with retinal arterial macroaneurysm:A case series and literature review. Br J Ophthalmol. 2015;99:1345–53. doi: 10.1136/bjophthalmol-2014-305884. [DOI] [PubMed] [Google Scholar]
- 84.Srinivasan S, Kyle G. Subinternal limiting membrane and subhyaloid haemorrhage in Terson syndrome:The macular 'double ring'sign. Eye (Lond) 2006;20:1099–101. doi: 10.1038/sj.eye.6702134. [DOI] [PubMed] [Google Scholar]
- 85.Rivas-Aguino P, García-Amaris RA, Berrocal MH, Sánchez JG, Rivas A, Arévalo JF. Pars plana vitrectomy, phacoemulsification and intraocular lens implantation for the management of cataract and proliferative diabetic retinopathy:Comparison of a combined versus two-step surgical approach. Arch Soc Esp Oftalmol. 2009;84:31–8. doi: 10.4321/s0365-66912009000100005. [DOI] [PubMed] [Google Scholar]