Abstract
Chemotherapeutics continue to play a central role in the treatment of a wide variety of cancers. Conventional chemotherapy involving bolus intravenous doses results in severe side effects - in some cases life threatening - delayed toxicity and compromised quality-of-life. Attempts to deliver small drug molecules using liposomes, polymeric nanoparticles, micelles, lipid nanoparticles, etc. have produced limited nanoformulations for clinical use, presumably due to a lack of biocompatibility of the material, costs, toxicity, scalability, and/or lack of effective administration. Naturally occurring small extracellular vesicles, or exosomes, may offer a solution and a viable system for delivering cancer therapeutics. Combined with their inherent trafficking ability and versatility of cargo capacity, exosomes can be engineered to specifically target cancerous cells, thereby minimizing off-target effects, and increasing the efficacy of cancer therapeutics. Exosomal formulations have mitigated the toxic effects of several drugs in murine cancer models. In this article, we review studies related to exosomal delivery of both small molecules and biologics, including siRNA to inhibit specific gene expression, in the pursuit of effective cancer therapeutics. We focus primarily on bovine milk and colostrum exosomes as the cancer therapeutic delivery vehicles based on their high abundance, cost effectiveness, scalability, high drug loading, functionalization of exosomes for targeted delivery, and lack of toxicity. While bovine milk exosomes may provide a new platform for drug delivery, extensive comparison to other nanoformulations and evaluation of long-term toxicity will be required to fully realize its potential.
1. Current Standard of Care for Cancer
Cancer is the second leading cause of death in the western world. According to the National Cancer Institute (NCI), more than 1.9 million new cancer cases were diagnosed in the United States in 2022, and more than 600,000 people died from cancer in the same year [1]. Based on data from 2015 to 2017, the NCI estimates that nearly 40% of all people in the US will be diagnosed with cancer at some point during their lifetimes [2]. In addition to the personal and family burdens of caring for and losing cancer patients, the treatment of cancer applies an enormous burden on the healthcare system, costing more than $150 billion in 2018, a number likely only to grow as the average age of the population increases and more expensive treatments are adopted.
As we in the scientific community continue to understand better the genetic anomalies that lead to cancer development, we are constantly reminded of the heterogeneity of the disease in terms of genetic mutations and clinical manifestations, which directly affect susceptibility or resistance to standard chemotherapeutic practices. Despite these known differences, most cancer patients are left with limited options when it comes to choosing a treatment regimen [3]. Four standard primary treatments are surgery, radiation, chemotherapy, and immunotherapy [4]. Many patients receive multiple treatments during their fight with cancer, and still, in many cases, face the prospect of low 5-year survival rates and even lower probability of disease-free survival. In 1973, the five-year survival rate for lung cancer patients was just over 10%; now, nearly 50 years later, the five-year survival rate is just over 20% [5]. In 1975, the five-year survival rate for pancreatic cancer patients was under 1%, and today is still under 5% [6]. The prognosis of patients with some forms of pancreatic cancer is so grim that a drug with horrific side effects that increases survival by only 2 months is considered a breakthrough.
Prognosis of some cancer types has improved more dramatically, thanks to early screening and intervention, but the incremental progress toward effective cancer treatment emphasizes the need for a new approach. With a new focus toward personalized cancer treatment, many anticancer drugs are currently in various stages of preclinical and clinical trials. The most significant hurdle to clinical efficacy is always effective delivery of these therapeutics to the tumor or disease site without causing adverse side effects that can reduce patient compliance with treatment and overall prognosis. The use of nanoparticles, such as liposomes, polymeric nanoparticles, micelles, lipid nanoparticles (LNPs), exosomes, small extracellular vesicles, microvesicles, and others as delivery vesicles has garnered a lot of attention over the past decade. For cancer therapeutics, nanoparticles offer better delivery platforms and targetability with fewer side effects in healthy tissue [7]. However, not all nanoparticles are created equal, and each offers its own set of benefits and drawbacks with respect to cancer therapeutics.
2. Conventional Nanoformulations
A variety of nanoparticles are currently being investigated for their potential to be used as drug delivery vesicles. Based on information at clinicaltrials.gov, there are currently 71 clinical trials recruiting patients to study nanoparticles to deliver cancer therapeutics in various disease models (Retrieved 02/21/23). Despite the interest in nanomedicine applications for cancer, the number of Food and Drug Administration (FDA)-approved nanoparticle drug delivery systems has not matched the enthusiasm. To date, only 16 nanoformulations have been approved by the FDA and/or the European Medicines Agency (EMA) [8].
Liposomes were the first nanoparticles to receive FDA approval for drug delivery [9]. Liposomes are particles produced from natural or synthetic phospholipids forming a lipid bilayer that can be manipulated to encapsulate various small molecules. In 1995, Doxil®, the PEGylated liposomal doxorubicin, became the first FDA-approved nanoformulation for cancer treatment [10]. Like many chemotherapeutics, doxorubicin has many adverse side effects, potentially the worst being irreversible congestive heart failure [10]. In safety assessment done by independent clinical evaluations, the use of a liposomal formulation of doxorubicin significantly decreased the risk of heart failure associated with high doses [11], supporting the potential of nanoformulations to mitigate the toxicity of the primary drug.
The FDA approval of this liposomal formulation has been viewed as the pivotal threshold moment for the development of nanomedicine for cancer [12]. Although approved in 1995, the usage of Doxil® remains limited, as it has only been approved for a short list of cancers and is administered like conventional chemotherapy, and bolus intravenous dosing. Additional chemotherapeutic liposomal formulations have reached the clinic, but their usage is still limited with marginal efficacy [13–15].
Although these liposomal formulations seem to mitigate some of the most severe adverse side effects of these standard-of-care chemotherapeutics, the potential healthy tissue damage by these broad-spectrum pharmaceuticals is not addressed; however, it is potentially mitigated using gene-based therapy [16]. While liposomes have successfully delivered small molecules like doxorubicin, the advent of gene therapy based on the delivery of nucleic acids required a new generation of carriers, namely LNPs. Compared to the first-generation liposomes, LNPs have a more complex internal structure for binding to and protecting siRNA and other nucleic acids and enhanced stability at physiological conditions [17]. LNPs also have a greater loading capacity for both small molecules and nucleic acids and require less complex manufacturing [17].
Clinical trials have been completed or are underway for liposome-delivered cancer gene therapeutics, particularly siRNA. In a first-in-human trial, siRNA delivered by liposomes targeting protein kinase N3, called Atu027, in patients with solid tumors demonstrated promising initial results but has not moved substantially toward widespread use [18–20]. Another siRNA-liposomal drug, EPHARNA, targeting EphA2 tyrosine kinase, is in clinical trial [21], but no publications have emerged besides preclinical animal data.
Like earlier generations of chemotherapeutics, these new types of pharmaceuticals must also be delivered intravenously, usually in a clinical setting. While synthetic lipid particle drug delivery has decreased the intensity of side effects caused by chemotherapeutic drugs like doxorubicin, the use of liposomes has created a new set of side effects, such as skin toxicity and mucositis [22, 23]. The consistent production of liposomes is also a complex process [22], making the cost of liposomal drug formulations prohibitively high for many patients. The development of a successful nanocarrier for cancer therapeutics with minimal side effects is the next great barrier to overcome for personalized cancer treatment.
Extracellular vesicles (EVs) produced by many different cell types are emerging as novel nanocarriers and provide an alluring alternative to the synthetic nanoparticles like liposomes and LNPs. These particles include exosomes and other small EVs that function naturally in cell-to-cell communication. Their natural trafficking ability and endogenous cargo inherently render them excellent carriers, which can be further engineered for targeted drug delivery. The subpopulation of extracellular particles that have gained the most attention in drug delivery are exosomes, the focus of this review.
3. Classifications of Extracellular Vesicles (EVs)
EVs comprise a heterogenous population in which new classes of particles continue to be discovered. As nanoparticles continue to be characterized, their natural biological function is more precisely defined. Once thought to all be a means of eliminating cellular waste, EVs can be classified as microvesicles (MVs), apoptotic bodies, and exosomes. Some studies have further subdivided exosomes into small and large exosomes based on size and endogenous cargo [24]. A distinct class of nanoparticles, namely exomeres, has recently been isolated by prolonged ultracentrifugation, although its role in the natural system is not entirely understood [24]. Described as non-membranous vesicles, exomeres appear to comprise the smallest population of extracellular vesicles bearing detectible protein markers yet discovered. The newest characterized subpopulation of EVs, supermeres, has been found to carry the majority of extracellular RNA and potentially participate in cancer metastasis [25]; although the discovery is so recent, the field lacks clarity on the specific biological function.
EVs as group tend to share many characteristics, making the clear delineation of a preparation isolated from bodily fluids or other biomass as only one type of vesicle extremely challenging. The International Society for Extracellular Vesicles issued a position statement in 2014 [26], revised in 2018 [27], to guide researchers and readers regarding the classification of EVs. Exosomes are believed to have the most promise as far as therapeutic benefit, and a lot of the work in the field of EVs has been dedicated to separating the different types of particles present in bodily fluids. The three major types of EVs, MVs, apoptotic bodies, and exosomes, all endogenously carry nucleic acids and proteins, and all participate in intercellular communication, so the current paradigm is to distinguish the particle types based on their biogenesis [28]. The different development pathways produce particles with specific surface markers, endogenous cargo, and size, allowing for further characterization of each EV subcategory.
3.1. Microvesicles (MVs)
MVs range from 100 nm to a few microns in diameter, formed by outward budding from the cell surface [29], and include cytoplasmic material [30]. These large particles carry specific surface markers, like β1-integrin and MHC class I molecules, not found on the other two classes of EVs [31], as a result of their formation from the releasing cell’s plasma membrane [32]. They are also characterized by tetraspanins, which are found in exosomes. MVs are enriched in CD63 and CD9, often found closely associated with the cell membranes, but demonstrate a lower abundance of CD81 than exosomes [33]. Like exosomes, MVs also participate in cell-to-cell communication [34]. It is unclear how the formation of MVs is triggered by the releasing cells, making their biological function in both diseased and health cells elusive. Their large size in many ways makes them unsuitable for drug deliver vesicles as they are trapped by elimination systems.
3.2. Apoptotic Bodies
The second type of EVs, apoptotic bodies, have a more clearly defined biological function. These particles are released by dying cells during late-phase apoptosis as the cell undergoes random blebbing to release these particles [35]. They are generally the easiest EVs to distinguish based on the presence of DNA and histones [35]. They are the largest EVs, ranging from 500 nm to 2 mm [30]. After release into the extracellular space, apoptotic bodies are scavenged by macrophages and degraded in phagolysosomes [30]. Like microvesicles, the large size of apoptotic bodies, and their natural clearance from the body by phagocytosis, makes them unsuitable for drug delivery
3.3. Exosomes
The third type of EVs, exosomes, continue to garner the most attention in the field of drug delivery. Unlike MVs, exosomes are derived from endocytosis when the multivescular body (MVB) membrane buds inward and are secreted following the fusion of MVBs with the cell membrane [36]. Unlike synthetically produced nanocarriers, exosomes have naturally associated proteins that facilitate endocytosis of the particle and its cargo [37, 38]. Exosomes offer many advantages over synthetically assembled nanoparticles. Based on their natural conformation, exosomes are built for the trafficking and delivery of various payloads throughout the body. Thus, they are well-suited for further engineering to enhance drug delivery of therapeutics against systemic diseases like cancer. As a result of their biogenesis and intrinsic function for delivering cargo such as proteins and nucleic acids between different cells, exosomes are inherently designed for crossing the cell membrane and exhibit increased systemic circulation due to their lack of immunogenicity.
The word “exosomes” first appeared in the literature in 1981 as a proposed term to describe microvesicles, ranging in size from 40–1000 nm, shed or exfoliated, from various cell lines with 5’ nucleotidase activity and specific composition of their surrounding phospholipid bilayer [39]. By the early 2000s, exosomes had been isolated from culture media of many different cell types, including immune cells, like T lymphocytes [40] and mast cells [41], as well as intestinal epithelial cells [42] and many cancer cell lines [43]. Exosomes are now believed to be secreted by all cell types. They have been isolated from most bodily fluids, including blood [44], urine [45], saliva [46], cerebral spinal fluid [47], breast milk [48], amniotic fluid [49], lymph [50], and bile [51]. There is no universal consensus on the size range that encompasses all exosomes; however, the most frequently published size range is between 30 and 150 nm [52]. Exosomes isolated from different sources may vary more narrowly in their size range.
4. Characteristics of Exosomes
Exosomes contain various constitutive elements such as proteins, lipids and nucleic acids derived from their producing cells. They share many characteristics with the cells that produce them. Approximately 4400 proteins, 194 lipids, 1639 mRNAs, and 764 miRNAs have been reported in exosomes from various cell types, demonstrating their complexity and possible functional diversity [53, 54].
4.1. Proteins
The precise endogenous cargo depends on the cell type of origin, but all exosomes possess common proteins, including tetraspanins, endosomal sorting complexes required for transport (ESCRT)-I associated proteins like Tsg101, and MVB-associated proteins (Alix) [55]. Exosomes also carry surface proteins involved in intracellular signaling [56] and various enzymes that promote membrane fusion with their target cells [57]. An important consideration for drug delivery, exosomes are also dotted with CD47, a “don’t eat me” protein known to protect from phagocytosis [58].
4.2. Nucleic Acids
In addition to characteristic protein profiles, exosomes carrying several other endogenous payloads – nucleic acids - that are naturally delivered to target cells as a form of cell-to-cell communication. The discovery of miRNA and mRNA in exosomes derived from mast cells shed further light on the biological function of these EVs. Mast cell-derived exosomes were found to carry mRNA, not found in the cytoplasm of the progenitor cell, which could be delivered to another cell for the purpose of altered gene expression [59]. Following this breakthrough discovery, the endogenous miRNA and RNA content of other exosomes was characterized and found to vary greatly between different EVs populations, with the source of exosomes defining their biological function [60]. A third class of RNA, long-noncoding RNA or lncRNA, have also been packaged in exosomes and may play a role in coordinating gene expression in target cells [61]. tsRNA, a small regulating RNA derived from tRNA, has been found in exosomes derived from cancer patients [62]. Many types of exosomes, including those derived from tumors, also contain double stranded DNA [63] and there are some indications that exosomes may help to maintain homeostasis in the cell by removing potentially harmful DNA in other cases [64].
4.3. Lipids
Although the specific cargo varies greatly amongst exosomes with respect to RNA populations, like with their protein components, exosomes also have characteristic lipid profiles. As with the protein markers and the nucleic acid cargo, the nature of lipids found within exosomes is a product of their cells of origin. Additionally, several lipids have been found in exosomes from various sources, including ceramides, sphingolipids, and phosphoglycerides [56], which may or may not be found in the parental cell.
4.4. Intrinsic Activity of Exosomes
As a product of the diverse characteristics of the cells that produce them, exosomes comprise a heterogenous population of EVs with unique combinations of miRNA, RNA, DNA, and other nucleic acids, lipids, and protein compositions that enhance the fidelity of their natural function. Despite these physiological differences, all exosomes play pivotal roles in intracellular communication, functioning in such processes as the maintenance of homeostasis [65] and both activation [66] and suppression of immune responses [67].
The transfer of mRNA cargo within exosomes to recipient cells has been demonstrated to regulate gene expression in those targets. Transfer of miRNA from cell-to-cell as delivered by exosomes has been shown to result in down regulation of gene expression in the target cell [68]. The ability of exosomes to transfer biologics like RNA is so central to its biological function that some viruses, such as HIV and EBV, have evolved mechanisms of inserting their own miRNA into these nanocarriers derived from infected B cells to be delivered to susceptible cells [68].
In order to deliver their payload to target cells, exosomes have a variety of mechanisms, depending both on the progenitor cell of the nanocarrier and the nature of the recipient cell [69]. In the immune system, exosomes express various adhesion molecules, including integrins, which allow the particles to interact with the extracellular matrix and target cells, providing a form of cell-to-cell communication in the absence of cellular contact [70]. In other instances, the internalization of the exosome and its cargo into the target cell is mediated by clathrin-mediated endocytosis and micropinocytosis [71]. A third mechanism in the entry of exosomes into recipient cells is via interaction of a wide variety of ligands on the surface of the exosome with cellular receptors (reviewed in [72]), a relationship dictated by exosome origin and the destination of its delivery. The inherent targetability using surface ligands of exosomes as a nano delivery system is an attractive quality for drug delivery.
As well as targetability, exosomes possess natural trafficking capabilities throughout the body. Exosomes released into circulation from various cell types travel to other regions of the body for long-distance communication [73]. The miRNA profile of peripheral blood mononuclear cells has been found to match the same profile from serum-isolated exosomes, supporting the proposal that circulating exosomes carrying their cargo far away from the microenvironment in which they are released [74]. Once inside their target cell, exosomes are transported along with the cytoskeleton or diffused in local microenvironments of cytoplasm [75].
4.5. Cargo and Activity of Bovine Milk Derived Exosomes
The characteristics of exosomes are specific to their progenitor cells, as well as the species of origin. Exosomes in milk from all mammals are produced by mammary gland epithelial cells [76]. The endogenous cargo of colostrum, milk produced in the first few days following giving birth, and mature milk, vary. In bovine samples, while both mature milk and colostrum derived exosomes contained canonical exosomal markers, these markers were enriched in colostrum as compared to mature milk [77]. Moreover, bovine colostrum exosomes carry proteins related to innate immune responses, while bovine mature milk exosomes bear proteins functioning in transport and apoptosis [77]. The nucleic acids, specifically miRNA, carried by bovine milk-derived exosomes are species-specific, but the vast majority have nucleotide sequences identical to those found in humans [76].
Given the endogenous cargo of bovine-derived exosomes, there is always a question of if and how these nucleic acids, lipids, proteins, etc could potentially affect a patient received exosome-delivered therapeutics. The average American adult consumes 200 mL of cow’s milk daily [78]. An early study on the potential ability of endogenous miRNA in particular digested during dietary consumption of cow’s milk to modulate human gene expression found that plasma concentrations of some miRNA change and the targeted protein expression modulated as well [79]. This study was relatively small (n=5) and the specific miRNA monitored were homologous between humans and cows, with no attempt to distinguish between the two when assessing abundance [80]. A second study using the same samples derived from the same 5 individuals differentiated between human and bovine miRNA and found no significant difference in plasma levels of the original miRNA monitored or 200 additional nucleic acids assessed and offered the hypothesis that the observed difference was due to some other endogenous mechanism, not the consumption of bovine miRNA [80]. Even given the contradictory results of these two studies, it is not beyond the realm of possibility that miRNA from bovine exosomes could interact with human mRNA, given the highly conserved nature of these miRNA sequences across all mammals and potential toxic or gene modulation effects must be assessed before declaring a particular formulation safe for human administration. While specific alterations in global gene expression have not been assessed following administration of milk exosomes, several studies have assessed general toxicity. Milk exosomes have been reported to be well tolerated across species, showing little immunogenicity [81–83]. In multiple short-term and long-term toxicity studies, administration of milk exosomes to both mice and rats did not result in significant changes in liver or kidney function or other hematological toxicity parameters or evoke inflammatory responses [81].
5. Exosomes as Nanocarriers for Cancer Therapeutics
5.1. Production of Exosomes for Therapeutic Use
To be a viable drug nanodelivery platform for cancer therapeutics, exosomes must be isolated in bulk quantity at a high degree of purity. Exosomal production is not limited to the animal kingdom as these particles were first discovered in fungi [84] and plants [85]. Despite numerous natural sources of exosomes, identifying a source for mass isolation of these nanoparticles has proven to be one of the limitations of using exosomes in cancer treatment and drug delivery. The volume of cell culture medium or plant biomass that must be processed to obtain meaningful amounts of purified exosomes can equate to extensive production time and difficult translatability to large-scale manufacturing.
Typical bulk manufacturing of exosomes for therapeutic use relies on growing cells in large volumes of culture media and then isolated from the conditioned media. A variety of cell types have been used, including monocyte-derived dendritic cells (MDDC) [86], bone marrow-mesenchymal cells (bmMSC) [87], adipose-derived stem cells (ADSC) [88], and umbilical cord mesenchymal stem cells [89]. Although there likely is variation across isolation methods and cell types used for the production of conditioned media, the yield of exosomes from conditioned media has been reported to average approximately 1–2.5 μg of exosomal protein per 1 mL of conditioned media [90–93]. In vivo studies require 10–100 mg (extrapolated based on drug load reported) for efficacy [94] and substantially more for use in human clinical trials. In most cases, primary cell lines are used, which may have diminished exosome-producing capacity and are challenging to maintain for long periods. Immortalization of cell lines, such as the overexpression of the oncogene MYC, has been used to overcome the longevity obstacle [95]. Still, there is an associated risk, such that MYC mRNA will be packaged into the exosome and delivered to a potential target cell. Overall, the production of exosomes by all cell lines in synthetic growth conditions is low, making it difficult to achieve the production necessary for commercialization [96].
Regardless of type, the growth of sufficient cells for bulk quantities of exosomes to develop pharmaceuticals requires enormous volumes of media. Because the standard culture flasks used in most laboratories produce only small volumes of conditioned media, the focus of most scale-up efforts has been increased surface area for cell attachment. The use of stirred bioreactors [97] or hollow fiber bioreactors [98] has dramatically increased the volume of conditioned cell media that can be produced. It has eliminated the possibility of human error during flask transfer affecting reproducibility. Still, alterations in the conditions, such as agitation speed, can alter the cell phenotype and, therefore the characteristics of the exosomes produced [99].
As exosomes are the products of living cells, there is always a risk of changing to composition and/or content of these vesicles when they are produced in an artificial environment [99, 100]. Most cell culture media contain serum containing exosomes and extracellular particles that can potentially contaminate the target exosome population. Preparation of exosome-free serum is time-consuming and increases the already higher serum cost. The lack of serum does not provide optimal conditions for the cells and can induce formation of more apoptotic bodies and diminish the therapeutic benefit of isolated exosomes [101]. A comparison of exosomes produced in serum-containing or serum-free media found no alteration in the size of particles produced but an enrichment of stress-related molecules, like reactive oxygen species, in exosome isolations from serum-free media [102]. As the regulation of biogenesis of exosomes is not yet fully understood, replicating the conditions in vitro has proven to be complex.
Even if these limitations are overcome, the yield of exosomes from conditioned cell culture media is still low compared to the volume of media that must be gathered, stored, and processed (Table 1). Isolation of exosomes from bovine colostrum and mature milk overcomes the low yield and possible alterations in exosome characteristics because of being produced outside of a natural system, such as in a bioreactor, not within a living organism. Several techniques, including ultracentrifugation and size exclusion chromatography, can produce highly purified exosomes [81, 103]. The presence of exosomes in milk was first reported in human breast milk [104], but was later reported in the milk of all mammals tested. The readily available nature of bovine milk makes it ideal for commercial-scale production. The average dairy cow produces more than twice as much colostrum as her calf will require [110, 111] and tens of thousands of dairy farms in the United States, which makes colostrum and mature milk an easily obtainable raw material for exosome isolation.
Table 1:
Exosome abundance in various sources based on purification protocol.
| Source | Method of Isolation | Concentration** | Reference |
|---|---|---|---|
|
| |||
| Human Origin | |||
| Adipose | Sucrose-Cushion Ultracentrifugation | 1.86×1011 particles/L growth media | [105] |
| Stem Cells | Ultracentrifugation | 1.02×1011 particles/L growth media | [105] |
|
| |||
| Bone | Ultracentrifugation | 6.53–10.4 ×1012 particles/L growth media | [106] |
| Marrow | Sucrose-Cushion Ultracentrifugation | 2.33×1011 particles/L growth media | [105] |
| MSC* Media | Ultracentrifugation | 6.66×1010 particles/L growth media | [105] |
|
| |||
| Breast Milk | Ultracentrifugation | 8.06×1013 particles/L milk | [48] |
|
| |||
| Saliva | Commercial Exosome Isolation Reagent (ExoQuick) | 3.1×1013 particles/L saliva | [46] |
|
| |||
| Semen | Commercial Exosome Isolation Reagent (ExoQuick) | approx. 1015 particles/L semen | [107] |
|
| |||
| Urine | Ultracentrifugation/S ize Exclusion Chromatography | 8.1×1012 particles/L urine | [45] |
|
| |||
| Wharton′s Jelly-Derived MSC* Media | Sucrose-Cushion Ultracentrifugation | 8.66×109 particles/L growth media | [108] |
| Total Exosome Isolation Reagent (Thermofisher) | 2.93×109 particles/L growth media | [108] | |
| Ultracentrifugation | 6.93×109 particles/L growth media | [108] | |
|
| |||
| Whole Blood | Separation by Acoustofluidics | 8.42×1013 particles/L whole blood*** | 109] |
|
| |||
| Bovine Origin | |||
| Raw Milk | Ultracentrifugation | 1.4×1014 particles/L milk | [48] |
| Ultracentrifugation | 2.8×1014 particles/L milk**** | ***** | |
MSC = mesenchymal stem cell
Exosomes have been isolated from many different bodily fluids and conditioned media from various cell cultures. Different methods can affect the yield of isolation. To facilitate easier comparison, reported abundances were all converted to particles per liter.
Authors report in the text and in the figure legend quantification in particles per microliter. In the figure itself, the axis is labeled particles per milliliter. The figure label is likely a typo so particles per microliter was used for calculation.
The particle concentration per liter of raw milk is higher since only 70% of the material was subjected to the final ultracentrifugation step.
Personal communication with Jeyaprakash Jeyabalan, Margaret Wallen, Wendy Spencer, and Ramesh Gupta of 3P Biotechnologies, Inc.
The exosome isolation protocol developed in our laboratory has thus far relied on differential ultracentrifugation to remove other milk components, like casein and fat globules [112]. The average particle size for isolated exosomes from this preparation is between 65 and 80 nm [112]. Each isolation is also characterized by probing for exosomal markers, like CD81, TSG101, and Alix, and other measures, like assessing zeta potential of purified particles and confirming size by AFM or TEM. Finally, the assessment of preparations is completed by testing each new preparation in cell culture for its drug delivery and transfection efficiency. Other groups isolating exosomes from the same source also report using ultracentrifugation, which can be followed by additional purification steps like sucrose density gradient centrifugation or size exclusion chromatography [113–116]. Commercially available exosome precipitation reagents, such as ExoQuick™ (System Biosciences) have also been used to prepare smaller quantities of bovine milk exosomes [114].
5.2. Loading Payload into and onto Exosomes for Therapeutic Use
The rate-limiting step in delivering cargo for cancer therapeutics is loading the payload onto or into the exosomes. Several methods have been tried to load exosomes with small and macro-molecules. Strategies for incorporation of exogenous cargo include both active and passive mechanisms.
Active drug loading methods include electroporation, sonication, extrusion, saponin treatment, hypotonic dialysis, and drug conjugation [117]. Electroporation, sonication, and extrusion involve disrupting the lipid bilayer surrounding the nanoparticle, allowing the drug to diffuse into the lumen, theoretically without disturbing membrane-associated proteins [118]. Electroporation uses the application of a current to make the bilayer more unstable and has been widely used to load biologics such as siRNA [119] and dsDNA [120]; however, the use of this technique has some adverse side effects on the exosomes themselves, causing morphological changes and aggregation [121]. In the specific case of siRNA, electroporation has been found to decrease siRNA retention into exosomes as a result of the formation of insoluble precipitates and may be less efficient than has been reported [122].
Sonication uses vibration to disrupt interactions between phospholipids in the membrane, allowing drug molecules to move into the interior of the particle. This technique has been used to load such molecules as catalase [123] and paclitaxel [124], and nucleic acids like siRNA [125]. As with electroporation, sonication is not without unwanted effects and has been shown to alter the size distribution of particles, disrupt membrane integrity, and decrease cellular update of exosomes loaded with cargo by using this method [126].
Extrusion employs physical force to push exosomes mixed with drug solution via syringe through membranes with 100–400 nm pores, disrupting the exosomal membrane and allow the drug molecules to enter the vesicles [127]. As with other membrane disruption techniques, extrusion can change the physical characteristics of exosomes, altering their natural molecule signature and trafficking abilities. Chemically permeabilizing exosomes have also been used as a strategy for drug loading. Saponin is a detergent that can form complexes with cholesterol in the exosome membrane, generating pores and increasing permeability to various exogenous loads [128]. It has been used to load catalase [123] and small hydrophilic molecules like porphyrin [129]. Despite facilitating high loading efficiency, saponin and other surfactants present a challenge due to their known cytotoxicity and hemolytic activity [130]. Hypotonic dialysis applies osmotic pressure via incubation solution, increasing the permeability of increased loading of intended cargo. Doxorubicin has been successfully loaded using this technique [131].
Because of the diversity and abundance of proteins present on the surface of exosomes, many small molecules can be directly conjugated. These conjugations have most notably been used to attach tags to monitor targeting and distribution of exosomes in vivo. In our laboratory, we have extensively used covalent conjugation of AlexaFluor-750 to exosomes to compare the biodistribution of various exosomal formulations. The attachment of such moieties does not appear to alter the size or other morphological traits dramatically and would not be expected to change natural trafficking or cellular uptake.
Although not universally true, active methodologies for loading exosomes with exogenous cargo often risk alterations of the natural characteristics of the exosomes themselves, which potentially alters their cellular uptake and value as a therapeutic. Small molecules and nucleic acids can be loaded using passive techniques (Fig. 1). Passive drug loading methods are much more straightforward, typically involving the simple incubation of purified exosomes with the drug.
Figure 1. Loading of exosomes with exogenous cargo.
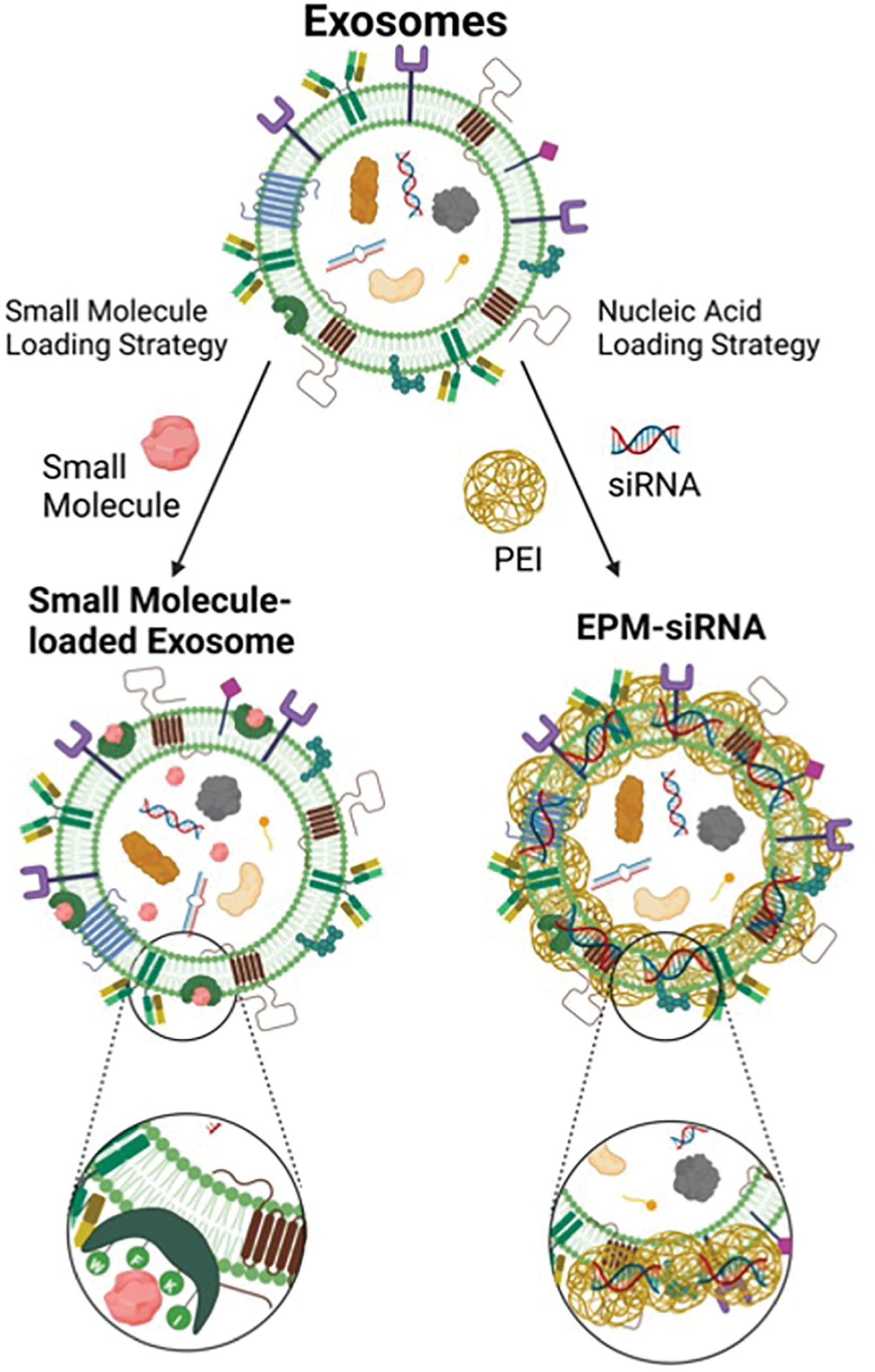
Bovine milk- and colostrum-derived exosomes carry a variety of endogenous cargos, including proteins, lipids, and nucleic acids. The figure shows that small molecules associate with exosomes by interacting with the hydrophobic domains of proteins on the vesicle surface. Conversely, loading of negatively charged biologics requires a polycationic mediator in the form of polyethyleneimine (PEI) to first condense and encapsulate the nucleic acids, and then associate with the negatively charged surface areas of the exosome particles, forming the EPM-siRNA.
5.3. Loading Small Molecules for Cancer Therapeutics
All of these loading strategies have been used for the incorporation of therapeutic molecules into and onto exosomes derived from a variety of origins for cancer treatment. Multiple critical areas of analysis are lacking in the literature, namely comparison of the efficacy, and toxicity of exosomal formulations to liposomal or other nanoparticle formulations and consistent reporting of drug loading and/or encapsulation efficiency of exosomal formulations of small molecules. Table 2 summarizes the variety of small molecules that have been loaded onto/into bovine milk exosomes to specifically target cancerous tissues in a variety of models, and where available, the drug load of each molecule. As this review focuses on milk-derived exosomes, we will focus on studies using this source of raw material for exosome isolation to compare study findings and methodologies better.
Table 2:
Exosome loaded small molecules for cancer treatment
| Anticancer Small Molecule | Source of Exosomes | Model System | Drug Load* | Reference |
|---|---|---|---|---|
|
| ||||
| Anthocyanidins | Raw bovine milk |
In vitro ovarian cancer cell lines In vivo ovarian cancer in mice |
~20% | [136] |
| Raw bovine milk |
In vitro lung, prostate, colon, breast, pancreatic, and ovarian cancer In vivo lung cancer xenografts in mice |
~20% | [137] | |
| Raw bovine milk | In vivo colon cancer in mice | ~20% | [138] | |
|
| ||||
| Celastrol | Raw bovine milk |
In vitro lung cancer cell lines In vivo lung cancer xenografts in mice |
18–20% | [139] |
|
| ||||
| Curcumin | Raw bovine milk |
In vitro breast, lung, and cervical cancer cell lines In vivo cervical tumor xenografts |
18–24% | [135] |
| Raw bovine milk | In vitro breast cancer cell lines | 2 μM | [116] | |
| Pasteurized skim milk | In vitro colorectal cancer cell lines | 9% | [140] | |
|
| ||||
| Raw Bovine Milk | In vitro breast cancer cell lines | EE ~70% | [141] | |
| Doxorubicin | Raw Bovine Milk | In vitro breast and lung cancer cell lines | None reported | [142] |
| Raw Bovine Milk | In vitro and in vivo oral squamous cell carcinoma | 13.4% | [143] | |
|
| ||||
| Paclitaxel | Raw bovine milk | In vitro lung cancer cells | ~8% | [81] |
| Raw bovine milk | In vitro breast cancer cell lines | ~30% | [144] | |
| Bovine colostrum |
In vitro lung cancer cell lines In vivo lung cancer in mice |
25–75% | [145] | |
|
| ||||
| Resveratrol | Raw bovine milk | In vitro breast cancer cell lines | 15 M | [116] |
|
| ||||
| Withaferin | Raw bovine milk |
In vitro lung cancer lung cancer tumors in mice |
[81] | |
Reported as w/w percent drug load unless otherwise indicated. EE = encapsulation efficiency.
5.3.1. Curcumin
Curcumin is a hydrophobic polyphenol isolated from Curcuma longa (turmeric) with known anti-inflammatory and anti-cancer properties. While inexpensive and nontoxic [132], low bioavailability and short half-life in circulation have limited its clinical use [133]. Although not initially delivered with milk exosomes, curcumin was the first small molecule associated stably with exosomes derived from cancer cells by brief coincubation [134]. Exosomal curcumin separation by sucrose gradient was comparable to unloaded exosomes in terms of surface markers and morphology [134]. Binding efficiency from this study was reported at 2.9 g curcumin per 1 g exosomal protein [134], although more recent studies using bovine milk exosomes have reported more modest drug loadings of 18–24% (w/w) [135]. In a head-to-head comparison of the loading efficiency of curcumin on bovine milk-derived exosomes versus cancer cell-derived exosomes, curcumin was loaded at a rate three times higher on milk-derived exosomes than those harvested from conditioned media (9% vs 3% loading) [140]. In this case, the milk source was skim, pasteurized milk purchased at a grocery store [140], which may account for the lower drug load than others have reported on raw milk. The loading of curcumin into bovine milk exosomes did not dramatically alter particle size [135].
Exosomal formulations of curcumin were demonstrated to show specific antiproliferative effects via induction of apoptosis against breast cancer cells in vitro. In contrast, this effect was absent when treated non-tumorigenic cells [116]. While free curcumin did not accumulate in the mammary glands of rats, exosomal formulations deliver curcumin to the mammary tissues at therapeutically meaningful levels [116]. Our own work has demonstrated that the tissue accumulation of curcumin in the lung, liver, and kidney is enhanced by milk-derived exosomal delivery compared to free curcumin [135]. This greater bioavailability correlated with greater tumor inhibition in a mouse cervical cancer model [135]. Antiproliferative effects of curcumin-loaded bovine milk exosomes has also been studied in colorectal cancer cell lines, resulting in significantly impaired metabolic activity of cancer cells compared to the free compound [140]. This same study demonstrated that milk exosome-encapsulated curcumin was also bioavailable after oral delivery, as it could permeate the intestinal wall [140].
Other delivery modalities have been used to encapsulate curcumin for the purpose of targeting tumors. A study using dimethyldioctadecylammonium bromide (DDAB) to produce curcumin-loaded liposomes found the formulation had antiproliferation effects on cervical cancer cell lines greater than free curcumin, but the presence of DDAB was highly cytotoxic [146]. The use of a different lipid, 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC), produced liposomal curcumin formulations with antiproliferation effects in a pancreatic cancer model, both in vitro and in vivo [147]. In vitro, liposomal curcumin performed equal to or better than free curcumin; however, in vivo, while the liposomal formulation showed anti-tumor effects, it was not compared to free curcumin [147]. Additional synthetic vesicles have been used to encapsulate curcumin, including micelles [148] and chitosan-based particles [149].
Based on the available literature, comparing the efficacy of exosomal formulations to synthetic particles is difficult. While studies comparing the two curcumin formulations in cancer models are not available, in a study comparing the anti-inflammatory properties, mice treated with liposomal-curcumin showed significantly decreased survival compared to mice treated with exosomal-curcumin following inflammatory challenge [134]. It is also of note that this study utilized conditioned media to isolate exosomes, not bovine milk.
5.3.2. Paclitaxel
The efficacy of standard chemotherapeutics like paclitaxel, another hydrophobic compound, has also been improved when delivered via bovine milk exosomal formulations [115, 145]. Paclitaxel, first isolated from the park of the Pacific yew, Taxus brevifiola, like curcumin, suffers low bioavailability and is not well tolerated in many cancer patients, leading to allergic responses, toxicity, and other adverse side effects [115, 150–152]. However, due to its interference with the spindle apparatus during cell division, it is a potent and widely used anti-cancer therapeutic.
Paclitaxel can be loaded onto/into bovine exosomes via simple incubation. Multiple investigators have reported that while particle size is marginally affected, the loading of paclitaxel somewhat alters the surface charge, or zeta potential, of the bovine milk exosomes [115, 144, 145]. Drug loading of bovine colostrum exosomes, specifically, was achieved over a broad range, from 24–75%, as determined with fluorescence quenching assays correlated with drug load, indicating that paclitaxel is surface-associated [145]. Paclitaxel loading of bovine mature milk exosomes has also varied across investigators, from approximately 8% [115] to about 30% [144].
Delivery of paclitaxel via bovine milk exosomes mitigated many of the toxic effects of the free drug or vehicle (cremophor EL) used for its solubility and administration. Using immunocompetent mice in a short-term toxicity study, delivery of paclitaxel via milk exosomes abolished toxicity of the free drug with respect to decreased leukocytes and bone marrow cells [115]. In a similar study, bovine milk paclitaxel formulations lacked hemopoietic and hematopoietic toxicity associated with the administration of free paclitaxel [145].
The efficacy of paclitaxel against cancer cells and tumors has also been improved via exosomal delivery as shown against breast cancer cell lines [144]. Similar paclitaxel formulations showed greater tumor reduction than free paclitaxel in multiple subcutaneous and orthotopic lung tumor model [115, 145] and demonstrated a high degree of penetration in the tumor sphere [153]. Taxol-resistant tumor cell growth was also inhibited in vitro when treated with exosomal paclitaxel formulations [145]. The bioavailability of paclitaxel following oral administration was also greatly improved with exosomal delivery, compared to the free drug [115, 145]. While the bioavailability and associated toxicity have been explored with exosomal formulations of paclitaxel, the efficacy against metastatic forms of cancer is an area that has not yet been thoroughly explored.
Several drug delivery systems have been developed to attempt to improve the bioavailability and decrease the toxicity of paclitaxel, including albumin-bound Abraxane®, liposomal (Lipusu®), and other conjugated forms (reviewed by Chou et al [154]). As described with curcumin, few comparisons have been made with these formulations and bovine milk exosomal formulations. Intravenously delivered Abraxane® has been compared to bovine colostrum exosomal formulations of paclitaxel. When administered orally the exosome-delivered dose was as effective as Abraxane™ in against lung xenografts, and when administered intravenously, inhibition of tumor growth was significantly improved by exosomal delivery [145]. Exosomes isolated from breast tumors have been compared to liposomes with respect to paclitaxel delivery. They have been found to result in a high accumulation of paclitaxel in breast cancer cells, accompanied by more significant changes in cancer-related gene expression, such as Bcl-2 and p21 [155].
5.3.3. Doxorubicin
Doxorubicin is an anthracycline antibiotic with known anti-cancer effects. However, its clinical use is also associated with adverse side effects, including cardiotoxicity [156]. Like paclitaxel, doxorubicin has been used extensively to treat multiple cancer types, and nanoformulations have been developed to mitigate the severe adverse reactions. The compound can be associated with milk exosomes either by simple incubation [142] or covalent attachment [143]. In both cases, the size of the loaded particles increased slightly with doxorubicin loading.
Bovine milk exosomal formulations of doxorubicin have shown preclinical success in targeting and inhibiting breast cancer cells in vitro, with greater cytotoxicity than free doxorubicin [142]. Bovine milk exosomes have also demonstrated better delivery of doxorubicin to target cells in vitro and tumors in vivo compared to the free compound and resulted in greater inhibition of oral squamous cell carcinoma xenografts in mice [143]. The improved efficacy of exosomal doxorubicin was also accompanied by decreased cardiotoxicity compared to free drug [143].
As the first FDA-approved liposomal formulation, Doxil™ has existed for some time. Still, as unfortunately is the case for other compounds, there is a very little comparison of exosomal formulations to this or any different liposomal formulation of doxorubicin. Exosomes of bovine and other origins have been shown to mitigate the associated toxicity and have shown equal if not better efficacy compared to free doxorubicin in breast, ovarian, and other cancer models [157–160], but a side-by-side comparison of liposomal to exosomal formulations is still needed.
5.3.4. Other Small Molecules
A handful of other small molecules have been delivered by bovine milk exosomes for the purpose of developing anticancer therapeutics. The general theme is the same: the exosomal formulations show greater efficacy compared to the free drug alone and a comparison with other formulations of the same compounds is missing. We have loaded other plant-derived compounds, such as withaferin A, potent cancer therapeutic from Withania somnifera [81], anthocyanidins, blueberry-derived therapeutics [136], and celastrol, a triterpenoid with known anti-inflammatory properties [139], onto/into bovine milk exosomes, with non-significant alteration in the size and zeta potential of the resultant formulation and delivered them successfully against tumors in mouse cancer models [135–139]. Exosome-loaded anthocyanidins showed increased tumor inhibition and modulation of inflammation compared to the free compound [135]. Although free celastrol shows some inhibition of tumor growth, the effect is intensified by exosome encapsulation [139], and so is the case with withaferin A [81].
Resveratrol, a stilbenoid found in the skin of grapes and some berries with antioxidant properties, has been loaded into/onto milk exosomes with passive incubation, albeit at a lower drug load than others polyphenols [116]. It has shown antiproliferative effects in vitro, similar to curcumin but at a higher concentration [116].
5.5. Tissue/tumor targeting with the Milk Exosomes
Bovine exosomes from milk and colostrum provide a multipurpose platform for loading a variety of molecules, including plant-derived polyphenolics and nucleic acids. The natural trafficking or higher uptake of the exosomal particles carries these cargos to tissues of interest rather than being degraded in circulation or excreted as waste. The composition of the membrane of exosomes lends itself to being functionalized with various targeting ligands for more tissue-specific and even cell-specific delivery. The idea of organ specific or tissue specific “addressing” of nanoparticles has been found naturally in exosomes [161] and is further enhanced by the addition of targeting moieties. For example, folate receptors are highly expressed in several cancer cell lines and in clinical tumor isolates. Bovine exosomes, functionalized with activated folic acid (FA), have been shown to increase the delivery of the payload to the tumor and the efficacy of anticancer therapy [81, 112, 145]. Using a similar approach in both subcutaneous and orthotopic murine cancer models (Fig. 2A), delivery of siRNA against oncogenic mutant KRAS by exosome-PEI matrix (EPM) resulted in modest inhibition of tumor growth. In contrast, delivery via FA-functionalized EPM significantly inhibited >60% of tumor growth compared to untreated animals.
Figure 2: Inhibition of tumors using bovine exosomes as delivery particles.
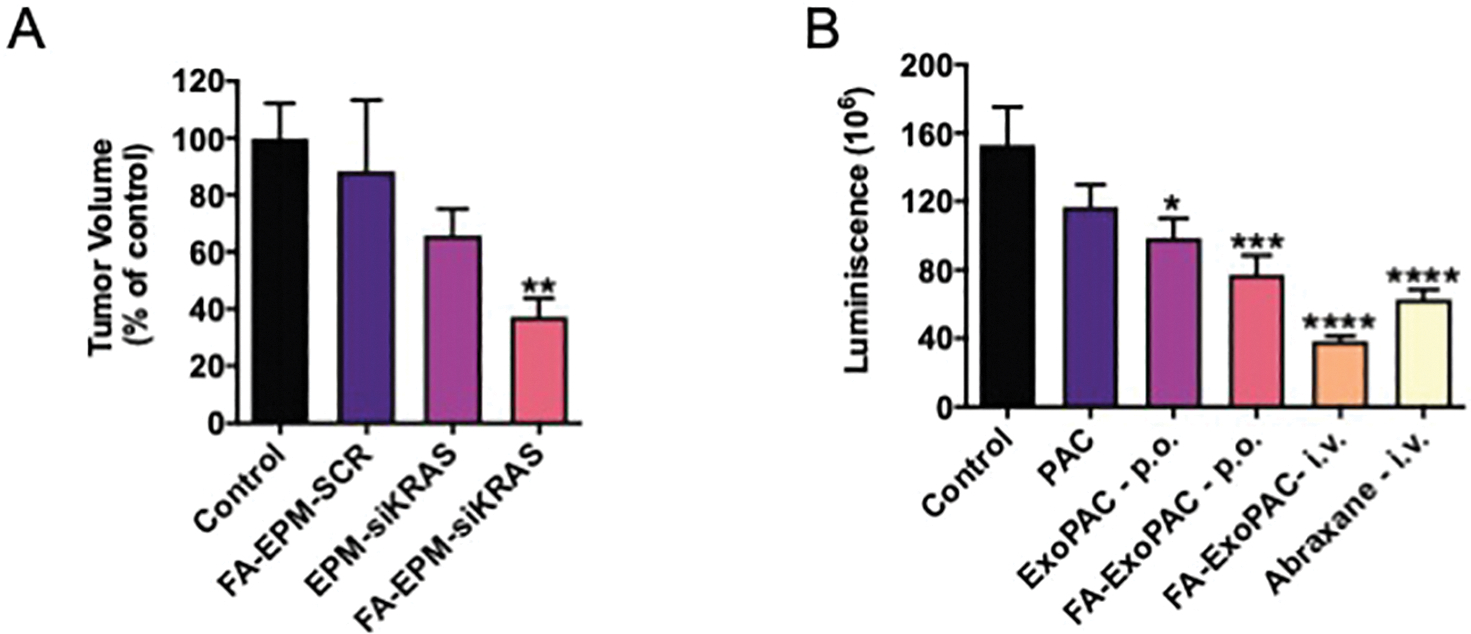
A) Folic acid (FA) functionalized EPM-delivered siRNA against oncogenic mutant KRAS significantly inhibited tumor growth in an orthotopic lung cancer model, while non-functionalized EPM-delivered siKRAS only resulted in modest inhibition, indicating the tumor targeting by FA. The delivery of a scrambled siRNA (siSCR) by the same vehicle had no effect on tumor volume. Tumor volume of treated animals calculated as a percentage of tumor volume in untreated animals. (B) NOD SCID mice were inoculated with luciferase-expressing A549 lung cancer cells intrathoracially to establish orthotopic lung tumors. Tumor growth was monitored via chemiluminescent signal. Ten days after injection, intervention began. Intravenous administration of free paclitaxel (PAC) and Abraxane™ were included as comparators. Exosomal formulation of paclitaxel (ExoPAC) administered orally (ExoPAC-p.o), FA-functionalized ExoPAC administered orally (FA-ExoPAC-p.o.), and FA-ExoPAC administered intravenously (FA-ExoPAC-i.v.) all showed significant inhibition of tumor growth, matching or outperforming inhibition by Abraxane™. Data was analyzed by one-way ANOVA followed by Dunnet’s multiple comparison of means (*p<.05, **p<.01, ***p<.001, ****p<.0001). Figure in Panel A is reproduced from Munagala, et. al. 2021 [111] with permission from Elsevier and in Panel B adopted from Kandimalla, et. al. [139] with permission from the authors.
Lactoferrin interacts with many receptors in the body, and these receptors are upregulated in specific cell types and during certain physiological conditions. Lactoferrin receptors are expressed to a high degree on bronchial epithelial cells of the lung but not on alveolar cells [162], particularly during inflammation. Using passive loading techniques, bovine exosomes can also be functionalized with another ligand, lactoferrin. When therapeutics are delivered intranasally to target the lung specifically, this functionalization allows for specific delivery to cells within a tissue expressing a particular phenotype for increased treatment efficacy. The loading of such targeting moieties does not interfere with the ability of the exosome to form the EPM complex with PEI and nucleic acids and successfully deliver both siRNA and plasmid DNA [163]. The functionalized EPM does not show subchronic systemic or immunotoxicity [112]. In our most recent studies, we use lactoferrin-functionalized exosomes to deliver a plasmid to facilitate CRISPR/Cas9-mediated genome editing to ameliorate symptoms related to chronic inflammation in the lung. In our preliminary in vitro work, lactoferrin does not interfere with the gene knockdown associated with CRISRP/Cas9-introduced mutations.
5.6. Diversity of Administration Routes for the Exosomes
The standard practice for administering chemotherapeutic agents continues to be an intravenous infusion. Moreover, systemic administration can lead to off-target effects on otherwise healthy tissue. For anticancer drugs like paclitaxel, low solubility in water required injectable doses to contain solvents such as Cremophor EL, causing adverse side effects like hypersensitivity and neutropenia [186]. Abraxane™, the albumin-bound paclitaxel, lacks these solvents but is still administered intravenously and is extremely expensive for cancer patients.
The administration of most exosome and other nanoparticle-based drugs continues to be intravenous, although alternative routes such as intraperitoneal, oral, and intranasal have been explored. Each administrative route offers its advantages and disadvantages and can be exploited to further direct the formulation to a tissue or body region of interest. For example, intranasal delivery may offer the best delivery to the brain and lung [164] and may help overcome obstacles of poor bioavailability of systemically administered drugs [165]; however, the function of the nasal passages is to keep the delicate lungs free of contaminants, so this route is not designed to deliver cargo to the body but to move it out. Intravenous delivery makes the drug immediately available systemically, leaving delivery vesicles prone to clearance and accumulation in the liver.
Oral delivery is the elusive “gold medal” for all cancer therapeutics. This administration route provides many advantages, chief among them being patient compliance and the flexibility of dosage and administrate regimen. The gastrointestinal (GI) tract offers an extensive surface area over which drugs can be absorbed [166]. There are drawbacks, including the conditions an orally delivered drug must survive, such as the acidic conditions of the stomach or the proteolytic enzymes and molecules found in the small intestines. Although the GI tract is designed for absorption, it is also geared toward the exclusion of potentially harmful pathogens that may enter the body, so penetrating the epithelial barrier is an obstacle for many oral drugs. However, colostrum and milk are both naturally designed to deliver payload to babies of all mammalian species that must be absorbed through the GI tract (REF)
Our laboratory has demonstrated that various potential cancer therapeutics can be developed for oral delivery using an exosomal delivery platform. Bovine milk exosomes loaded with paclitaxel (PAC) inhibited tumor growth in a lung cancer model when delivered orally, outperforming free PAC through its conventional intravenous administration route [115]. Further targeting of the PAC-loaded exosomes to the tumor by FA-functionalization could also be accomplished with oral delivery [145] (Fig. 2B).
The successful oral delivery of nucleic acids is an accomplishment that has not yet been reported. The EPM technology developed in our laboratory using bovine exosomes and PEI has been found to inhibit tumor growth in orthotopic lung cancer models by delivering anti-oncogenic siRNA when administered orally. Presumably, the entrapment of the siRNA in PEI that then associates with the exosome coupled with the natural absorption of milk/colostrum exosomes is sufficiently exploited to protect the siRNA to the intended target. These exosomes can also be functionalized with FA, targeting the tumor for even more significant inhibition. This FA- functionalization also helps with the crossing over of the epithelial barrier in the GI tract, as both the duodenum and jejunum show elevated folate receptor expression [167], allowing for the absorption of the exosomes and their cargo.
6. Future Perspectives
Exosomes function in cell-to-cell communication, presumably delivering their endogenous payload. Based on this notion, we and others have used exosomes for delivery of exogenous payloads including small molecules and biologics. These emerging natural particles hold a great promise as nanodelivery vehicles. Bovine milk and colostrum are sources of abundant particles compared to more conventional isolation from conditioned cell culture media. The exosomes from these sources can be isolated in bulk at comparatively low cost and are created in their natural production environment without the creation of synthetic growth conditions. Because of natural role in intracellular communication, exosomes are dotted with proteins that can be exploited as nanocarriers to further functionalize and target cargo delivered by these particles. Our research group has also demonstrated the ability of the EPM to deliver plasmids for the purpose of exogenous gene expression, making it a possible nanoparticle for cancer vaccinations.
Drug delivery aims to administer therapeutics precisely at their target at the lowest dose possible without interfering with normal tissues in the body. The versatility of the bovine exosome platform for small molecule loading and the EPM for the entrapment of biologics like RNA and DNA lends itself to modifications facilitating a “zip code” delivery system. The “addressing” of exosomes is based on selective targeted with specific tumor or organ specific ligand. This concept was demonstrated by functionalization of exosome vector for the purpose of targeting tumors and lactoferrin receptors for lung targeting. Simple modifications of the surface exosomal proteins can be used to target diseased tissues, much as FA can be used to target drug delivery to tumors. Other ligands can be used for several other tissue/cell types based on different expression patterns of cell receptors.
As promising as the use of bovine exosomes in the EPM nanoplatform as a delivery vehicle for hydrophobic small molecules and nucleic acids is, additional safety assessment is required before widespread use in the clinic can be realized. As exosomes naturally carrying miRNA and other forms of nucleic acids, is it possible that these can modulate human gene expression as a product of cross-species reactivity? It has been documented that biological active miRNA from food products such as chicken eggs and milk are present in circulation in the human body [168], so it is certainly not beyond the realm of possibility. The systemic toxicity and immunotoxicity studies conducted with bovine milk exosomes and the EPM formulations thus far have been short term (28 days treatment), indicating that these exosomes are well tolerated. This regimen would not exactly parallel a patient receiving cancer therapy, so longer term assessments as well as toxicity studies in larger mammals and non-human primates are needed. Although the successful oral delivery of small molecules and siRNA would revolutionize cancer therapeutics, there is still some work to fully develop this exosome-based delivery system.
Highlights:
Exosomes, produced by most cells, play role in intracellular communication.
Bovine milk and colostrum provide an abundant source of exosomes.
Exosomes have been used to load small molecules for the treatment of cancer.
siRNA can be loaded into or onto exosomes and delivered as a cancer therapeutics.
Exosomes functionalized with targeting ligands can be used for tissue/organ targeting.
ACKNOWLEDGMENTS
This work was supported by multiple funding sources: USPHS grants CA-118114, CA-125152 and R41-CA-189517, Kentucky Lung Cancer Research Program, and Helmsley Funds, 3P Biotechnologies, Inc. and in part, from the NCI SBIR grant R44-CA-221487 and Agnes Brown Duggan Endowment (to R.C.G.). We would like to acknowledge key contributors in the development of the bovine milk/colostrum exosome delivery platform for small molecules and biologics discussed in this review, including Drs. Radha Munagala, Ashish Agrawal, and Raghuram Kandimalla, and Mr. Jeyaprakash Jeyabalan.
Footnotes
CONFLICT OF INTERESTS
Dr. Ramesh C. Gupta holds positions both at the University of Louisville and 3P Biotechnologies.
The authors have filed an international patent application (PCT) based on part of the results reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].A.C. Society, 2022 Cancer Facts and Figures, 2022.
- [2].Harding C, Stahl P, Transferrin recyling in reticulocytes: pH and iron are important determinants of ligand binding and processing, Biochemical and Biophysical Research Communications, Volume 112 (1983) 650–658. [DOI] [PubMed] [Google Scholar]
- [3].Krzyszczyk P, Acevedo A, Davidoff EJ, Timmins LM, Marrero-Berrios I, Patel M, . . . Yarmush ML, The growing role of precision and personalized medicine for cancer treatment, TECHNOLOGY, 06 (2018) 79–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].N.C. Institute, Types of cancer treatment, 2017. [Google Scholar]
- [5].Lu T, Yang X, Huang Y, Zhao M, Li M, Ma K, . . . Wang Q, Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades, Cancer Manag Res, 11 (2019) 943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bengtsson A, Andersson R, Ansari D, The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data, Scientific Reports, 10 (2020) 16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mundekkad D, Cho WC, Nanoparticles in Clinical Translation for Cancer Therapy, International Journal of Molecular Sciences, 23 (2022) 1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rodríguez F, Caruana P, De la Fuente N, Español P, Gámez M, Balart J, . . . Céspedes MV, Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges, Biomolecules, 12 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anselmo AC, Mitragotri S, Nanoparticles in the clinic: An update, Bioeng Transl Med, 4 (2019) e10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barenholz Y, Doxil® — The first FDA-approved nano-drug: Lessons learned, Journal of Controlled Release, 160 (2012) 117–134. [DOI] [PubMed] [Google Scholar]
- [11].Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, . . . Gabizon A, Pegylated liposomal doxorubicin (doxil): Reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2, Annals of Oncology, 11 (2000) 1029–1034. [DOI] [PubMed] [Google Scholar]
- [12].Fan Y, Marioli M, Zhang K, Analytical characterization of liposomes and other lipid nanoparticles for drug delivery, Journal of Pharmaceutical and Biomedical Analysis, 192 (2021) 113642. [DOI] [PubMed] [Google Scholar]
- [13].Waterhouse DN, Tardi PG, Mayer LD, Bally MB, A Comparison of Liposomal Formulations of Doxorubicin with Drug Administered in Free Form, Drug Safety, 24 (2001) 903–920. [DOI] [PubMed] [Google Scholar]
- [14].Frampton JE, Liposomal Irinotecan: A Review in Metastatic Pancreatic Adenocarcinoma, Drugs, 80 (2020) 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tzogani K, Penttilä K, Lapveteläinen T, Hemmings R, Koenig J, Freire J, . . . Pignatti F, EMA Review of Daunorubicin and Cytarabine Encapsulated in Liposomes (Vyxeos, CPX-351) for the Treatment of Adults with Newly Diagnosed, Therapy-Related Acute Myeloid Leukemia or Acute Myeloid Leukemia with Myelodysplasia-Related Changes, The Oncologist, 25 (2020) e1414–e1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Das SK, Menezes ME, Bhatia S, Wang XY, Emdad L, Sarkar D, Fisher PB, Gene Therapies for Cancer: Strategies, Challenges and Successes, J Cell Physiol, 230 (2015) 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tenchov R, Bird R, Curtze AE, Zhou Q, Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement, ACS Nano, 15 (2021) 16982–17015. [DOI] [PubMed] [Google Scholar]
- [18].Schultheis B, Strumberg D, Santel A, Vank C, Gebhardt F, Keil O, . . . Drevs J, First-in-Human Phase I Study of the Liposomal RNA Interference Therapeutic Atu027 in Patients With Advanced Solid Tumors, Journal of Clinical Oncology, 32 (2014) 4141–4148. [DOI] [PubMed] [Google Scholar]
- [19].Schultheis B, Strumberg D, Kuhlmann J, Wolf M, Link K, Seufferlein T, . . . Pelzer U, A phase Ib/IIa study of combination therapy with gemcitabine and Atu027 in patients with locally advanced or metastatic pancreatic adenocarcinoma, Journal of Clinical Oncology, 34 (2016) 385–385. [Google Scholar]
- [20].Schultheis B, Strumberg D, Kuhlmann J, Wolf M, Link K, Seufferlein T, . . . Pelzer U, Safety, Efficacy and Pharcacokinetics of Targeted Therapy with The Liposomal RNA Interference Therapeutic Atu027 Combined with Gemcitabine in Patients with Pancreatic Adenocarcinoma. A Randomized Phase Ib/IIa Study, Cancers, 12 (2020) 3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Naing A, Lopez-Berestein G, Fu S, Tsimberidou AM, Pant S, Piha-Paul SA, . . . Coleman RL, EphA2 gene targeting using neutral liposomal small interfering RNA (EPHARNA) delivery: A phase I clinical trial, Journal of Clinical Oncology, 35 (2017) TPS2604–TPS2604. [Google Scholar]
- [22].Ibrahim M, Abuwatfa WH, Awad NS, Sabouni R, Husseini GA, Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review, Pharmaceutics, 14 (2022) 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ansari L, Shiehzadeh F, Taherzadeh Z, Nikoofal-Sahlabadi S, Momtazi-borojeni AA, Sahebkar A, Eslami S, The most prevalent side effects of pegylated liposomal doxorubicin monotherapy in women with metastatic breast cancer: a systematic review of clinical trials, Cancer Gene Therapy, 24 (2017) 189–193. [DOI] [PubMed] [Google Scholar]
- [24].Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, . . . Lyden D, Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation, Nat Cell Biol, 20 (2018) 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang Q, Jeppesen DK, Higginbotham JN, Graves-Deal R, Trinh VQ, Ramirez MA, . . . Coffey RJ, Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets, Nature Cell Biology, 23 (2021) 1240–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, . . . Théry C, Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles, J Extracell Vesicles, 3 (2014) 26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, . . . Zuba-Surma EK, Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines, J Extracell Vesicles, 7 (2018) 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zaborowski MP, Balaj L, Breakefield XO, Lai CP, Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study, Bioscience, 65 (2015) 783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].D’Souza-Schorey C, Clancy JW, Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers, Genes Dev, 26 (2012) 1287–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Battistelli M, Falcieri E, Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication, Biology, 9 (2020) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schorey C, ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles, Curr Biol, 19 (2009) 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Østergaard O, Nielsen CT, Iversen LV, Jacobsen S, Tanassi JT, Heegaard NHH, Quantitative Proteome Profiling of Normal Human Circulating Microparticles, Journal of Proteome Research, 11 (2012) 2154–2163. [DOI] [PubMed] [Google Scholar]
- [33].Barranco I, Padilla L, Parrilla I, Álvarez-Barrientos A, Pérez-Patiño C, Peña FJ, . . . Roca J, Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles, Scientific Reports, 9 (2019) 11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Doyle LM, Wang MZ, Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J, The Methods of Choice for Extracellular Vesicles (EVs) Characterization, International Journal of Molecular Sciences, 18 (2017) 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].El Andaloussi S, Mäger I, Breakefield XO, Wood MJA, Extracellular vesicles: biology and emerging therapeutic opportunities, Nature Reviews Drug Discovery, 12 (2013) 347–357. [DOI] [PubMed] [Google Scholar]
- [37].Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M, A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy, Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1846 (2014) 75–87. [DOI] [PubMed] [Google Scholar]
- [38].Vader P, Mol EA, Pasterkamp G, Schiffelers RM, Extracellular vesicles for drug delivery, Advanced drug delivery reviews, 106 (2016) 148–156. [DOI] [PubMed] [Google Scholar]
- [39].Trams EG, Lauter CJ, Salem Norman Jr., Heine U, Exfoliation of membrane ecto-enzymes in the form of micro-vesicles, Biochimica et Biophysica Acta (BBA) - Biomembranes, 645 (1981) 63–70. [DOI] [PubMed] [Google Scholar]
- [40].Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C, TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/ζ complex, The Journal of Immunology, 168 (2002) 3235–3241. [DOI] [PubMed] [Google Scholar]
- [41].Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C, Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation, Molecular biology of the cell, 8 (1997) 2631–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf–Bensussan N, Heyman M, Intestinal epithelial cells secrete exosome–like vesicles, Gastroenterology, 121 (2001) 337–349. [DOI] [PubMed] [Google Scholar]
- [43].Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, . . . T. Tursz, Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming, Nature medicine, 7 (2001) 297–303. [DOI] [PubMed] [Google Scholar]
- [44].Hornick NI, Huan J, Doron B, Goloviznina NA, Lapidus J, Chang BH, Kurre P, Serum exosome microRNA as a minimally-invasive early biomarker of AML, Scientific reports, 5 (2015) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cho S, Yang HC, Rhee WJ, Development and comparative analysis of human urine exosome isolation strategies, Process Biochemistry, 88 (2020) 197–203. [Google Scholar]
- [46].Comfort N, Bloomquist TR, Shephard AP, Petty CR, Cunningham A, Hauptman M, . . . Baccarelli A, Isolation and characterization of extracellular vesicles in saliva of children with asthma, Extracell Vesicles Circ Nucl Acids, 2 (2021) 29–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Otake K, Kamiguchi H, Hirozane Y, Identification of biomarkers for amyotrophic lateral sclerosis by comprehensive analysis of exosomal mRNAs in human cerebrospinal fluid, BMC Medical Genomics, 12 (2019) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vaswani K, Mitchell MD, Holland OJ, Qin Koh Y, Hill RJ, Harb T, . . . Peiris H, A Method for the Isolation of Exosomes from Human and Bovine Milk, J Nutr Metab, 2019 (2019) 5764740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dixon CL, Sheller-Miller S, Saade GR, Fortunato SJ, Lai A, Palma C, . . . Menon R, Amniotic fluid exosome proteomic profile exhibits unique pathways of term and preterm labor, Endocrinology, 159 (2018) 2229–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Milasan A, Tessandier N, Tan S, Brisson A, Boilard E, Martel C, Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis, Journal of extracellular vesicles, 5 (2016) 31427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yoon SB, Chang JH, Extracellular vesicles in bile: a game changer in the diagnosis of indeterminate biliary stenoses?, Hepatobiliary Surgery and Nutrition, 6 (2017) 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Martins TS, Vaz M, Henriques AG, A review on comparative studies addressing exosome isolation methods from body fluids, Analytical and Bioanalytical Chemistry, (2022). [DOI] [PubMed] [Google Scholar]
- [53].Mathivanan S, Fahner CJ, Reid GE, Simpson RJ, ExoCarta 2012: database of exosomal proteins, RNA and lipids, Nucleic Acids Res, 40 (2012) D1241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kim DK, Kang B, Kim OY, Choi DS, Lee J, Kim SR, . . . Gho YS, EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles, J Extracell Vesicles, 2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wei H, Chen Q, Lin L, Sha C, Li T, Liu Y, . . . Zhu X, Regulation of exosome production and cargo sorting, Int J Biol Sci, 17 (2021) 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Boriachek K, Islam MN, Möller A, Salomon C, Nguyen NT, Hossain MSA, . . . Shiddiky MJA, Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles, Small, 14 (2018). [DOI] [PubMed] [Google Scholar]
- [57].Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, . . . Freitas RP, Rab27a and Rab27b control different steps of the exosome secretion pathway, Nature cell biology, 12 (2010) 19–30. [DOI] [PubMed] [Google Scholar]
- [58].Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, . . . Weissman IL, CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis, Cell, 138 (2009) 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO, Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells, Nature Cell Biology, 9 (2007) 654–659. [DOI] [PubMed] [Google Scholar]
- [60].Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, . . . Zöller M, Cell Surface Tetraspanin Tspan8 Contributes to Molecular Pathways of Exosome-Induced Endothelial Cell Activation, Cancer Research, 70 (2010) 1668–1678. [DOI] [PubMed] [Google Scholar]
- [61].Dragomir M, Chen B, Calin GA, Exosomal lncRNAs as new players in cell-to-cell communication, Transl Cancer Res, 7 (2018) S243–s252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D, . . . Peng Y, Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis, Molecular Cancer, 18 (2019) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, . . . Lyden D, Double-stranded DNA in exosomes: a novel biomarker in cancer detection, Cell Research, 24 (2014) 766–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, . . . Hara E, Exosomes maintain cellular homeostasis by excreting harmful DNA from cells, Nature Communications, 8 (2017) 15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lee Y, EL Andaloussi S, Wood MJA, Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy, Human Molecular Genetics, 21 (2012) R125–R134. [DOI] [PubMed] [Google Scholar]
- [66].Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z, Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2, Cancer research, 67 (2007) 7458–7466. [DOI] [PubMed] [Google Scholar]
- [67].Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J, . . . Ratajczak MZ, Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells, Experimental hematology, 30 (2002) 450–459. [DOI] [PubMed] [Google Scholar]
- [68].Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MAJ, Hopmans ES, Lindenberg JL, . . . Middeldorp JM, Functional delivery of viral miRNAs via exosomes, Proceedings of the National Academy of Sciences, 107 (2010) 6328–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Aryani A, Denecke B, Exosomes as a Nanodelivery System: a Key to the Future of Neuromedicine?, Molecular Neurobiology, 53 (2016) 818–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB, Adhesion and signaling by B cell-derived exosomes: the role of integrins, The FASEB Journal, 18 (2004) 977–979. [DOI] [PubMed] [Google Scholar]
- [71].Tian T, Zhu Y-L, Zhou Y-Y, Liang G-F, Wang Y-Y, Hu F-H, Xiao Z-D, Exosome Uptake through Clathrin-mediated Endocytosis and Macropinocytosis and Mediating miR-21 Delivery*, Journal of Biological Chemistry, 289 (2014) 22258–22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gonda A, Kabagwira J, Senthil GN, Wall NR, Internalization of Exosomes through Receptor-Mediated Endocytosis, Molecular Cancer Research, 17 (2019) 337–347. [DOI] [PubMed] [Google Scholar]
- [73].Fatima F, Nawaz M, Long Distance Metabolic Regulation through Adipose-Derived Circulating Exosomal miRNAs: A Trail for RNA-Based Therapies?, Frontiers in Physiology, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, . . . Marsh CB, Detection of microRNA Expression in Human Peripheral Blood Microvesicles, PLOS ONE, 3 (2008) e3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tian T, Zhu Y-L, Hu F-H, Wang Y-Y, Huang N-P, Xiao Z-D, Dynamics of exosome internalization and trafficking, Journal of Cellular Physiology, 228 (2013) 1487–1495. [DOI] [PubMed] [Google Scholar]
- [76].Zempleni J, Aguilar-Lozano A, Sadri M, Sukreet S, Manca S, Wu D, . . . Mutai E, Biological Activities of Extracellular Vesicles and Their Cargos from Bovine and Human Milk in Humans and Implications for Infants, J Nutr, 147 (2017) 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Samuel M, Chisanga D, Liem M, Keerthikumar S, Anand S, Ang C-S, . . . Mathivanan S, Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth, Scientific Reports, 7 (2017) 5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mullie P, Pizot C, Autier P, Daily milk consumption and all-cause mortality, coronary heart disease and stroke: a systematic review and meta-analysis of observational cohort studies, BMC Public Health, 16 (2016) 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Baier SR, Nguyen C, Xie F, Wood JR, Zempleni J, MicroRNAs Are Absorbed in Biologically Meaningful Amounts from Nutritionally Relevant Doses of Cow Milk and Affect Gene Expression in Peripheral Blood Mononuclear Cells, HEK-293 Kidney Cell Cultures, and Mouse Livers, The Journal of Nutrition, 144 (2014) 1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Auerbach A, Vyas G, Li A, Halushka M, Witwer K, Uptake of dietary milk miRNAs by adult humans: a validation study, F1000Res, 5 (2016) 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Munagala R, Aqil F, Jeyabalan J, Gupta RC, Bovine milk-derived exosomes for drug delivery, Cancer Letters, 371 (2016) 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].del Pozo-Acebo L, López de las Hazas M-C, Tomé-Carneiro J, Gil-Cabrerizo P, San-Cristobal R, Busto R, . . . Dávalos A, Bovine Milk-Derived Exosomes as a Drug Delivery Vehicle for miRNA-Based Therapy, International Journal of Molecular Sciences, 22 (2021) 1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sanwlani R, Fonseka P, Chitti SV, Mathivanan S, Milk-derived extracellular vesicles in inter-organism, cross-species communication and drug delivery, Proteomes, 8 (2020) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].MARCHANT R, PEAT A, BANBURY GH, THE ULTRASTRUCTURAL BASIS OF HYPHAL GROWTH, New Phytologist, 66 (1967) 623–629. [Google Scholar]
- [85].Halperin W, Jensen WA, Ultrastructural changes during growth and embryogenesis in carrot cell cultures, Journal of Ultrastructure Research, 18 (1967) 428–443. [DOI] [PubMed] [Google Scholar]
- [86].Lamparski HG, Metha-Damani A, Yao J-Y, Patel S, Hsu D-H, Ruegg C, Le Pecq J-B, Production and characterization of clinical grade exosomes derived from dendritic cells, Journal of immunological methods, 270 (2002) 211–226. [DOI] [PubMed] [Google Scholar]
- [87].Pachler K, Lener T, Streif D, Dunai ZA, Desgeorges A, Feichtner M, . . . Gimona M, A Good Manufacturing Practice–grade standard protocol for exclusively human mesenchymal stromal cell–derived extracellular vesicles, Cytotherapy, 19 (2017) 458–472. [DOI] [PubMed] [Google Scholar]
- [88].Andriolo G, Provasi E, Lo Cicero V, Brambilla A, Soncin S, Torre T, . . . Turchetto L, Exosomes from human cardiac progenitor cells for therapeutic applications: development of a GMP-grade manufacturing method, Frontiers in physiology, 9 (2018) 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kuang MJ, Huang Y, Zhao XG, Zhang R, Ma JX, Wang DC, Ma XL, Exosomes derived from Wharton’s jelly of human umbilical cord mesenchymal stem cells reduce osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head in rats via the miR-21-PTEN-AKT signalling pathway, Int J Biol Sci, 15 (2019) 1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yamashita T, Takahashi Y, Nishikawa M, Takakura Y, Effect of exosome isolation methods on physicochemical properties of exosomes and clearance of exosomes from the blood circulation, European Journal of Pharmaceutics and Biopharmaceutics, 98 (2016) 1–8. [DOI] [PubMed] [Google Scholar]
- [91].Charoenviriyakul C, Takahashi Y, Morishita M, Matsumoto A, Nishikawa M, Takakura Y, Cell type-specific and common characteristics of exosomes derived from mouse cell lines: Yield, physicochemical properties, and pharmacokinetics, European Journal of Pharmaceutical Sciences, 96 (2017) 316–322. [DOI] [PubMed] [Google Scholar]
- [92].Wang F, Cerione RA, Antonyak MA, Isolation and characterization of extracellular vesicles produced by cell lines, STAR Protocols, 2 (2021) 100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shi X, Cheng Q, Hou T, Han M, Smbatyan G, Lang JE, . . . Zhang Y, Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy, Molecular Therapy, 28 (2020) 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH, Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo ABH, . . . Lim SK, Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs, Journal of translational medicine, 9 (2011) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, . . . Ma Y, Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation, Nature biomedical engineering, 4 (2020) 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Fernandes A, Fernandes T, Diogo M, da Silva CL, Henrique D, Cabral J, Mouse embryonic stem cell expansion in a microcarrier-based stirred culture system, Journal of biotechnology, 132 (2007) 227–236. [DOI] [PubMed] [Google Scholar]
- [98].Gerlach JC, Lin Y-C, Brayfield CA, Minteer DM, Li H, Rubin JP, Marra KG, Adipogenesis of human adipose-derived stem cells within three-dimensional hollow fiber-based bioreactors, Tissue Engineering Part C: Methods, 18 (2012) 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Colao IL, Corteling R, Bracewell D, Wall I, Manufacturing Exosomes: A Promising Therapeutic Platform, Trends in Molecular Medicine, 24 (2018) 242–256. [DOI] [PubMed] [Google Scholar]
- [100].Whitford W, Guterstam P, Exosome manufacturing status, Future Medicinal Chemistry, 11 (2019) 1225–1236. [DOI] [PubMed] [Google Scholar]
- [101].Chen Y-S, Lin E-Y, Chiou T-W, Harn H-J, Exosomes in clinical trial and their production in compliance with good manufacturing practice, Tzu-Chi Medical Journal, 32 (2020) 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Li J, Lee Y, Johansson HJ, Mäger I, Vader P, Nordin JZ, . . . Andaloussi SE, Serum-free culture alters the quantity and protein composition of neuroblastoma-derived extracellular vesicles, Journal of extracellular vesicles, 4 (2015) 26883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Vaswani K, Koh YQ, Almughlliq FB, Peiris HN, Mitchell MD, A method for the isolation and enrichment of purified bovine milk exosomes, Reprod Biol, 17 (2017) 341–348. [DOI] [PubMed] [Google Scholar]
- [104].Admyre C, Johansson SM, Qazi KR, Filén J-J, Lahesmaa R, Norman M, . . . Gabrielsson S, Exosomes with immune modulatory features are present in human breast milk, The Journal of immunology, 179 (2007) 1969–1978. [DOI] [PubMed] [Google Scholar]
- [105].Gupta S, Rawat S, Arora V, Kottarath SK, Dinda AK, Vaishnav PK, . . . Mohanty S, An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells, Stem Cell Res Ther, 9 (2018) 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, . . . Kalluri R, Generation and testing of clinical-grade exosomes for pancreatic cancer, JCI Insight, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Madison MN, Welch JL, Okeoma CM, Isolation of Exosomes from Semen for in vitro Uptake and HIV-1 Infection Assays, Bio Protoc, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chopra N, Dutt Arya B, Jain N, Yadav P, Wajid S, Singh SP, Choudhury S, Biophysical Characterization and Drug Delivery Potential of Exosomes from Human Wharton’s Jelly-Derived Mesenchymal Stem Cells, ACS Omega, 4 (2019) 13143–13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, . . . Huang TJ, Isolation of exosomes from whole blood by integrating acoustics and microfluidics, Proc Natl Acad Sci U S A, 114 (2017) 10584–10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Pritchett LC, Gay CC, Besser TE, Hancock DD, Management and Production Factors Influencing Immunoglobulin G1 Concentration in Colostrum from Holstein Cows1, Journal of Dairy Science, 74 (1991) 2336–2341. [DOI] [PubMed] [Google Scholar]
- [111].Geiger AJ, Colostrum: back to basics with immunoglobulins, Journal of Animal Science, 98 (2020) S126–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Munagala R, Aqil F, Jeyabalan J, Kandimalla R, Wallen M, Tyagi N, . . . Gupta RC, Exosome-mediated delivery of RNA and DNA for gene therapy, Cancer Lett, 505 (2021) 58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Vaswani K, Koh YQ, Almughlliq FB, Peiris HN, Mitchell MD, A method for the isolation and enrichment of purified bovine milk exosomes, Reproductive Biology, 17 (2017) 341–348. [DOI] [PubMed] [Google Scholar]
- [114].Wijenayake S, Eisha S, Tawhidi Z, Pitino MA, Steele MA, Fleming AS, McGowan PO, Comparison of methods for pre-processing, exosome isolation, and RNA extraction in unpasteurized bovine and human milk, PLOS ONE, 16 (2021) e0257633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, . . . Gupta RC, Milk-derived exosomes for oral delivery of paclitaxel, Nanomedicine: Nanotechnology, Biology and Medicine, 13 (2017) 1627–1636. [DOI] [PubMed] [Google Scholar]
- [116].González-Sarrías A, Iglesias-Aguirre CE, Cortés-Martín A, Vallejo F, Cattivelli A, del Pozo-Acebo L, . . . Espín JC, Milk-Derived Exosomes as Nanocarriers to Deliver Curcumin and Resveratrol in Breast Tissue and Enhance Their Anticancer Activity, International Journal of Molecular Sciences, 23 (2022) 2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Balachandran B, Yuana Y, Extracellular vesicles-based drug delivery system for cancer treatment, Cogent Medicine, 6 (2019) 1635806. [Google Scholar]
- [118].Patil SM, Sawant SS, Kunda NK, Exosomes as drug delivery systems: A brief overview and progress update, European Journal of Pharmaceutics and Biopharmaceutics, 154 (2020) 259–269. [DOI] [PubMed] [Google Scholar]
- [119].Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA, Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes, Nature Biotechnology, 29 (2011) 341–345. [DOI] [PubMed] [Google Scholar]
- [120].Lamichhane TN, Raiker RS, Jay SM, Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery, Molecular Pharmaceutics, 12 (2015) 3650–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Johnsen KB, Gudbergsson JM, Skov MN, Christiansen G, Gurevich L, Moos T, Duroux M, Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes, Cytotechnology, 68 (2016) 2125–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, . . . Vader P, Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles, Journal of Controlled Release, 172 (2013) 229–238. [DOI] [PubMed] [Google Scholar]
- [123].Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, . . . Batrakova EV, Exosomes as drug delivery vehicles for Parkinson’s disease therapy, Journal of Controlled Release, 207 (2015) 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, . . . Batrakova EV, Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells, Nanomedicine: Nanotechnology, Biology and Medicine, 12 (2016) 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, . . . Jay SM, Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication, Cellular and Molecular Bioengineering, 9 (2016) 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Nizamudeen ZA, Xerri R, Parmenter C, Suain K, Markus R, Chakrabarti L, Sottile V, Low-Power Sonication Can Alter Extracellular Vesicle Size and Properties, Cells, 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Kumar DN, Chaudhuri A, Aqil F, Dehari D, Munagala R, Singh S, . . . Agrawal AK, Exosomes as Emerging Drug Delivery and Diagnostic Modality for Breast Cancer: Recent Advances in Isolation and Application, Cancers, 14 (2022) 1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D, Engineering exosomes as refined biological nanoplatforms for drug delivery, Acta Pharmacol Sin, 38 (2017) 754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM, Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins, Journal of Controlled Release, 205 (2015) 35–44. [DOI] [PubMed] [Google Scholar]
- [130].Podolak I, Galanty A, Sobolewska D, Saponins as cytotoxic agents: a review, Phytochemistry Reviews, 9 (2010) 425–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wei H, Chen J, Wang S, Fu F, Zhu X, Wu C, . . . Lin J, A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro, Int J Nanomedicine, 14 (2019) 8603–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Chen Y, Wu Q, Zhang Z, Yuan L, Liu X, Zhou L, Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics, Molecules, 17 (2012) 5972–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Salehiabar M, Nosrati H, Javani E, Aliakbarzadeh F, Manjili HK, Davaran S, Danafar H, Production of biological nanoparticles from bovine serum albumin as controlled release carrier for curcumin delivery, International journal of biological macromolecules, 115 (2018) 83–89. [DOI] [PubMed] [Google Scholar]
- [134].Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, . . . H.-G. Zhang, A Novel Nanoparticle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes, Molecular Therapy, 18 (2010) 1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Gupta R, Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin, Aaps j, 19 (2017) 1691–1702. [DOI] [PubMed] [Google Scholar]
- [136].Aqil F, Jeyabalan J, Agrawal AK, Kyakulaga AH, Munagala R, Parker L, Gupta RC, Exosomal delivery of berry anthocyanidins for the management of ovarian cancer, Food Funct, 8 (2017) 4100–4107. [DOI] [PubMed] [Google Scholar]
- [137].Munagala R, Aqil F, Jeyabalan J, Agrawal AK, Mudd AM, Kyakulaga AH, . . . Gupta RC, Exosomal formulation of anthocyanidins against multiple cancer types, Cancer Letters, 393 (2017) 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Mudd AM, Gu T, Munagala R, Jeyabalan J, Egilmez NK, Gupta RC, Chemoprevention of Colorectal Cancer by Anthocyanidins and Mitigation of Metabolic Shifts Induced by Dysbiosis of the Gut Microbiome, Cancer Prev Res (Phila), 13 (2020) 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Aqil F, Kausar H, Agrawal AK, Jeyabalan J, Kyakulaga A-H, Munagala R, Gupta R, Exosomal formulation enhances therapeutic response of celastrol against lung cancer, Experimental and Molecular Pathology, 101 (2016) 12–21. [DOI] [PubMed] [Google Scholar]
- [140].Carobolante G, Mantaj J, Ferrari E, Vllasaliu D, Cow Milk and Intestinal Epithelial Cell-Derived Extracellular Vesicles as Systems for Enhancing Oral Drug Delivery, Pharmaceutics, 12 (2020) 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Pullan J, Dailey K, Bhallamudi S, Feng L, Alhalhooly L, Froberg J, . . . Mallik S, Modified Bovine Milk Exosomes for Doxorubicin Delivery to Triple-Negative Breast Cancer Cells, ACS Applied Bio Materials, 5 (2022) 2163–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Li D, Yao S, Zhou Z, Shi J, Huang Z, Wu Z, Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin, Carbohydrate Research, 493 (2020) 108032. [DOI] [PubMed] [Google Scholar]
- [143].Zhang Q, Xiao Q, Yin H, Xia C, Pu Y, He Z, . . . Wang Y, Milk-exosome based pH/light sensitive drug system to enhance anticancer activity against oral squamous cell carcinoma, RSC Advances, 10 (2020) 28314–28323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Kumar DN, Chaudhuri A, Dehari D, Shekher A, Gupta SC, Majumdar S, . . . Agrawal AK, Combination Therapy Comprising Paclitaxel and 5-Fluorouracil by Using Folic Acid Functionalized Bovine Milk Exosomes Improves the Therapeutic Efficacy against Breast Cancer, Life, 12 (2022) 1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Kandimalla R, Aqil F, Alhakeem SS, Jeyabalan J, Tyagi N, Agrawal A, . . . Gupta RC, Targeted Oral Delivery of Paclitaxel Using Colostrum-Derived Exosomes, Cancers (Basel), 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Saengkrit N, Saesoo S, Srinuanchai W, Phunpee S, Ruktanonchai UR, Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy, Colloids and Surfaces B: Biointerfaces, 114 (2014) 349–356. [DOI] [PubMed] [Google Scholar]
- [147].Li L, Braiteh FS, Kurzrock R, Liposome-encapsulated curcumin, Cancer, 104 (2005) 1322–1331. [DOI] [PubMed] [Google Scholar]
- [148].Mohanty C, Acharya S, Mohanty AK, Dilnawaz F, Sahoo SK, Curcumin-encapsulated MePEG/PCL diblock copolymeric micelles: a novel controlled delivery vehicle for cancer therapy, Nanomedicine, 5 (2010) 433–449. [DOI] [PubMed] [Google Scholar]
- [149].O’Toole MG, Henderson RM, Soucy PA, Fasciotto BH, Hoblitzell PJ, Keynton RS, . . . Gobin AS, Curcumin Encapsulation in Submicrometer Spray-Dried Chitosan/Tween 20 Particles, Biomacromolecules, 13 (2012) 2309–2314. [DOI] [PubMed] [Google Scholar]
- [150].Markman M, Managing taxane toxicities, Support Care Cancer, 11 (2003) 144–147. [DOI] [PubMed] [Google Scholar]
- [151].Mu Q, Jeon M, Hsiao MH, Patton VK, Wang K, Press OW, Zhang M, Stable and efficient Paclitaxel nanoparticles for targeted glioblastoma therapy, Advanced healthcare materials, 4 (2015) 1236–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X, Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles, Biomaterials, 33 (2012) 8167–8176. [DOI] [PubMed] [Google Scholar]
- [153].Chen J, Cao F, Cao Y, Wei S, Zhu X, Xing W, Targeted Therapy of Lung Adenocarcinoma by the Nanoplatform Based on Milk Exosomes Loaded with Paclitaxel, Journal of Biomedical Nanotechnology, 18 (2022) 1075–1083. [DOI] [PubMed] [Google Scholar]
- [154].Chou P-L, Huang Y-P, Cheng M-H, Rau K-M, Fang Y-P, Improvement of paclitaxel-associated adverse reactions (ADRs) via the use of nano-based drug delivery systems: A systematic review and network meta-analysis, International Journal of Nanomedicine, (2020) 1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kanchanapally R, Brown K, Cancer cell-derived exosomes as the delivery vehicle of paclitaxel to inhibit cancer cell growth, Journal of Cancer Discovery, (2022) 49–58. [Google Scholar]
- [156].Shafei A, El-Bakly W, Sobhy A, Wagdy O, Reda A, Aboelenin O, . . . Ellithy M, A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer, Biomedicine & Pharmacotherapy, 95 (2017) 1209–1218. [DOI] [PubMed] [Google Scholar]
- [157].Toffoli G, Hadla M, Corona G, Caligiuri I, Palazzolo S, Semeraro S, . . . Rizzolio F, Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin, Nanomedicine, 10 (2015) 2963–2971. [DOI] [PubMed] [Google Scholar]
- [158].Hadla M, Palazzolo S, Corona G, Caligiuri I, Canzonieri V, Toffoli G, Rizzolio F, Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models, Nanomedicine, 11 (2016) 2431–2441. [DOI] [PubMed] [Google Scholar]
- [159].Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, . . . Nie G, A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy, Biomaterials, 35 (2014) 2383–2390. [DOI] [PubMed] [Google Scholar]
- [160].Yang Y, Chen Y, Zhang F, Zhao Q, Zhong H, Increased anti-tumour activity by exosomes derived from doxorubicin-treated tumour cells via heat stress, International Journal of Hyperthermia, 31 (2015) 498–506. [DOI] [PubMed] [Google Scholar]
- [161].Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, . . . Lyden D, Tumour exosome integrins determine organotropic metastasis, Nature, 527 (2015) 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Elfinger M, Maucksch C, Rudolph C, Characterization of lactoferrin as a targeting ligand for nonviral gene delivery to airway epithelial cells, Biomaterials, 28 (2007) 3448–3455. [DOI] [PubMed] [Google Scholar]
- [163].Wallen M, Aqil F, Kandimalla R, Jeyabalan J, Auwardt S, Tyagi N, . . . Gupta RC, A model system for antiviral siRNA therapeutics using exosome-based delivery, Mol Ther Nucleic Acids, 29 (2022) 691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Gutierrez-Millan C, Calvo Díaz C, Lanao JM, Colino CI, Advances in Exosomes-Based Drug Delivery Systems, Macromolecular Bioscience, 21 (2021) 2000269. [DOI] [PubMed] [Google Scholar]
- [165].Djupesland PG, Nasal drug delivery devices: characteristics and performance in a clinical perspective—a review, Drug Delivery and Translational Research, 3 (2013) 42–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Sastry SV, Nyshadham JR, Fix JA, Recent technological advances in oral drug delivery–a review, Pharmaceutical science & technology today, 3 (2000) 138–145. [DOI] [PubMed] [Google Scholar]
- [167].Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, . . . Goldman ID, Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption, Cell, 127 (2006) 917–928. [DOI] [PubMed] [Google Scholar]
- [168].Wolf T, Baier SR, Zempleni J, The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells, J Nutr, 145 (2015) 2201–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]


