Abstract
Gliomas make up virtually 80% of all lethal primary brain tumors and are categorized based on their cell of origin. Glioblastoma is an astrocytic tumor that has an inferior prognosis despite the ongoing advances in treatment modalities. One of the main reasons for this shortcoming is the presence of the blood-brain barrier and blood-brain tumor barrier. Novel invasive and non-invasive drug delivery strategies for glioblastoma have been developed to overcome both the intact blood-brain barrier and leverage the disrupted nature of the blood-brain tumor barrier to target cancer cells after resection—the first treatment stage of glioblastoma. Exosomes are among non-invasive drug delivery methods and have emerged as a natural drug delivery vehicle with high biological barrier penetrability. There are various exosome isolation methods from different origins, and the intended use of the exosomes and starting materials defines the choice of isolation technique. In the present review, we have given an overview of the structure of the blood-brain barrier and its disruption in glioblastoma. This review provided a comprehensive insight into novel passive and active drug delivery techniques to overcome the blood-brain barrier, emphasizing exosomes as an excellent emerging drug, gene, and effective molecule delivery vehicle used in glioblastoma therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12035-023-03365-0.
Keywords: Glioblastoma, Blood-brain barrier, Drug delivery, Exosomes
Introduction
Glioblastoma multiform (GBM) is the most malignant type of primary astrocytoma, accounting for more than 60% of all brain tumors, which despite ongoing treatment advances, has remained incurable. The average life expectancy of GBM patients is approximately 14 months after diagnosis. The global prevalence of GBM is 3.19 per 100,000 people in the USA, and due to its inferior prognosis, it is considered the cause of 2.5% of deaths due to cancers [1]. Conventional treatment modalities for Gliomas (general term for defining primary brain tumors) are surgery, radiotherapy, and pharmacotherapy (typically with temozolomide). These modalities have not resulted in major survival improvement in GBM patients. This is largely due to invasive tumor growth- limiting local therapy- and the presence of the blood-brain barrier (BBB) [2, 3]. The GBM treatment market has an estimated Compound Annual Growth Rate (CAGR) of 17.4% from 2014 to 2024 and will reach 3.3$ billion. It is considered one of the fastest-growing disease treatment markets [4]. However, drug development for GBM has proved to be challenging to date, predominately as a result of the presence of BBB. This barrier forms a highly selective barrier that rigorously controls the entry of molecules to cerebral tissues and vice versa. The physicochemical properties of BBB refer to the characteristics of this membrane that determine its permeability to different substances [5]. The BBB is composed of tightly packed endothelial cells that form a continuous layer of cells with highly specialized properties. Tight junctions connect these cells, creating a barrier that prevents the free diffusion of many substances between the blood and the brain. Furthermore, pericytes and astrocytes surround the BBB, regulating its permeability and maintaining structural integrity [6]. Lipophilicity and size are two major types of physicochemical properties of BBB. Small lipophilic molecules or lipophilic drugs can pass through the BBB and diffuse through the endothelial cell membrane’s lipid bilayer. Conversely, due to their low lipophilicity, hydrophilic substances, such as polar molecules or large proteins, cannot cross the BBB [7]. The molecule’s size also influences BBB permeability. Paracellular pathways, which involve diffusion between the tight junctions of the endothelial cells, allow small molecules such as water, oxygen, and carbon dioxide to diffuse freely across the BBB. On the other hand, larger molecules, such as proteins or peptides, require a transcellular pathway to cross the BBB. This involves the active transport of molecules across the endothelial cell membrane through specific transporters, such as carrier-mediated transporters or receptor-mediated endocytosis [5, 8]. In addition to lipophilicity and size, hydrogen bonding capacity, charge, and polarity are also physicochemical properties of BBBs that influence permeability. Virtually all of the large therapeutic compounds and more than 98% of small molecule drugs are prevented from crossing this barrier [9]. To overcome this barrier, invasive and non-invasive techniques have been developed. Among non-invasive methods, nanoparticle-mediated drug delivery such as lipid-based, polymeric, and inorganic NPs have attracted the attention of many scientists; however, designing these synthetic carriers still remains challenging due to several issues, such as biotoxicity and inefficient BBB penetration ability. Exosomes are naturally derived nanocarriers with a 30–100 nm diameter distribution and facilitate cell-cell communication. These lipid bilayers’ extracellular vesicles are shed by almost all types of mammalian cells circulating in all bodily fluids and have low biotoxicity. There are at least 19,000 scientific articles published about exosomes indexed in Scopus and over 200 clinical trials investigating exosomes as viable diagnostic and therapeutic candidates, but no FDA-approved exosome product is available [10]. Nonetheless, due to its undeniable potential, rapidly growing numbers of companies are developing exosome-based products, such as Aegle Therapeutics, Aethlon Medical Inc, and Anjarium Biosciences AG. Their excellent biological barrier penetrability ability has made them excellent nano-vehicles for drug delivery. Isolation of exosomes has seen a great improvement over the past decades, and the emergence of microfabrication techniques is fueling this advancement. However, each isolation technique has its advantages and disadvantages and should be selected based on the type of starting material and intended use of the isolated exosomes. Exosomes have already been used in the delivery of effective therapeutics, such as genes and effective molecules, which are comprehensively included in Tables 2 and 3. Although cells naturally use exosomes to communicate with each other, there are currently no clinical trials investigating exosomes as drug delivery vehicles (clinicaltrials.gov) and no FDA-approved exosome delivery agents. In the following sections, we have provided an update on state-of-the-art drug delivery methods used to overcome BBB and blood-brain tumor barrier (BBTB) stringent control over molecule transportation from blood circulation to cerebral tissues and vice versa. Exosomes as novel, natural, biocompatible nano-vehicles have been reviewed in more detail. The biogenesis, composition, isolation, and therapeutic cargo loading of exosomes have been extensively studied here. We also highlight current GBM therapies using exosomes loaded with effective factors, molecules, and genes, alongside future opportunities in the field of developing novel drug delivery technologies to overcome the blood-brain barrier in GBM.
Table 2.
Effective Factors and Molecules Loaded into Exosomes for GBM Therapy
| Source of exosomes | Loading method | Chemotherapeutics | Outcome | References |
|---|---|---|---|---|
|
Mouse lymphoma cell line; Glioblastoma cell line; MSCs |
Incubation (with exosomes) | Curcumin | Anti-inflammatory | [87–89] |
| Cell prostate | Incubation (with exosomes) | Paclitaxel prostate | Cancer therapy | [90] |
| Brain endothelial cells | Incubation (with exosomes) | Paclitaxel and doxorubicin | Brain cancer therapy | [83] |
| Macrophages | Incubation (with exosomes) | Paclitaxel | Cancer therapy | [91] |
| Pancreatic cancer cells | Incubation (with exosomes) | Gemcitabine | Pancreatic cancer therapy | [92] |
| Macrophages | Incubation (with exosomes) | Doxorubicin, paclitaxel |
Triple-negative breast cancer therapy |
[93] |
| Mouse mammary cancer cells | Incubation (with exosomes) | Sinoporphyrin sodium | Sonodynamic cancer therapy | [94] |
| Breast and colorectal cancer cells | Incubation (with exosomes) | Aspirin | Breast and colorectal cancer therapy | [95] |
| Hela cells | Incubation (with exosomes) | Porphyrin and indocyanine green | Cancer therapy | [96] |
| MSCs; Macrophages | Incubation (with exosomes) | Doxorubicin | Breast cancer therapy | [97, 98] |
| Bovine milk | Incubation (with exosomes) |
Celastrol; Withaferin A, anthocyanidins, curcumin, paclitaxel and docetaxel |
Lung cancer therapy | [99–101] |
| Murine hepatocarcinoma cells |
Incubation (with exosome-secreting cells) |
Methotrexate, hydroxyl camptothecin and cisplatin | Ovarian cancer therapy | [102] |
|
Pancreatic cancer cells, pancreatic stellate cells, macrophages |
Incubation (with exosome-secreting cells) |
Doxorubicin | Pancreatic cancer therapy | [103] |
|
MSCs; Breast cancer cells, ovarian cancer cells, breast-to-lung metastatic cells |
Incubation (with exosome-secreting cells) |
Paclitaxel | Cancer therapy | [104, 105] |
| Macrophages |
Incubation (with exosome-secreting cells) |
Curcumin | Alzheimer’s disease therapy | [106] |
|
Immature dendritic cells; Human breast cancer cells |
Electroporation | Doxorubicin | Breast and ovarian cancer therapy | [107, 108] |
| Human breast cancer cells | Electroporation | Doxorubicin and indocyanine green | Cervical cancer therapy | [109] |
| Milk | Incubation | Paclitaxel | Oral | [99] |
| Mesenchymal stromal cells | Incubation | Paclitaxel | Inhibited growth of human pancreatic adenocarcinoma cell | [90] |
| Immature dendritic cells transfected with the vector expressing iRGD-Lamp2b fusion proteins | Electroporation | Doxorubicin | Specific drug delivery to the tumor site and inhibited tumor growth | [108] |
| Reticulocytes | Incubation | Doxorubicin | n/a | [110] |
|
Mouse lymphoma cell (EL-4) and RAW 264.7 cells |
Mixing | Curcumin | Enhanced anti-inflammatory activity | [87] |
|
Tumor cells (GL26-Luc, BV2, 3T3L1, 4T1, CT26, A20, and EL-4) |
Incubation | Curcumin | Inhibited brain inflammation and delayed brain tumor growth | [89] |
| Kunming mice blood | Incubation | Dopamine | Enhanced therapeutic effect due to brain-specific drug delivery | [111] |
Table 3.
Different genes loaded into exosomes for GBM therapy
| Source of exosomes | Loading method | Gene cargo (miRNAs, siRNAs, suicide genes) |
Outcome | References |
|---|---|---|---|---|
| T cells | Incubation (with exosomes) | miR-150 | Allergic cutaneous contact sensitivity inhibition | [120] |
| Human monocyte-derived macrophages | Incubation (with exosomes) | miR-159 | Triple-negative breast cancer therapy | [98] |
| Dendritic cells | Electroporation | BACE1 siRNA |
Specific gene knockdown after specific siRNA delivery to the brain for Alzheimer’s disease |
[121] |
| Epithelial prostate cells | Transfection | miR-143 | Prostate cancer therapy | [122] |
| Marrow stromal cells | Transfection | miR-150 | Glioma therapy | [123] |
| MSCs | Transfection | anti-miR-9 |
Increase chemo resistance of GBM |
[124] |
| HEK293 cells | Transfection | let-7a | Breast cancer therapy | [125] |
| Endothelial cells | Transfection | siRNA | N/A | [126] |
| Hepatic stellate cells | Transfection | miR-214 | Epigenetic regulation of connective tissue growth factor | [127] |
| MSCs | Transfection | miR-122 | Increase chemo-sensitivity of hepatocellular carcinoma | [128] |
| HEK293 cells | Transfection | siRNA | Chronic myeloid leukemia therapy | [129] |
| HEK293 cells | Transfection | mRNA | Parkinson’s disease therapy | [130] |
| Endothelial cells | Transfection | anti-miR-33a-5p | Atherosclerosis therapy | [131] |
| MSCs | Transfection | miR-125b | Hepatocellular carcinoma therapy | [132] |
| MSCs | Transfection | miR-181a |
Myocardium ischemia/reperfusion injury therapy |
[133] |
| MSCs | Transfection | miR-125b |
Myocardium ischemia/reperfusion injury therapy |
[134] |
| MSCs | Transfection | miR-126 | Spinal cord injury therapy | [135] |
| HEK293 cells | Transfection | CRISPR/CRISPR-associated protein 9 | Gene editing | [136] |
| HEK293 cells | Transfection | miR-497 | Lung cancer therapy | [137] |
| Breast cancer cells | Transfection | miR-126 | Lung cancer therapy | [138] |
| HEK293 cells | Transfection | siRNA | Colorectal cancer xenograft inhibition | [139] |
| Blood plasma, lung cancer cells, Hela cells | Transfection | siRNA |
Gene silencing of mitogen-activated protein kinase 1 |
[140] |
| Hela cells and human fibrosarcoma cells | Transfection | siRNA | Cancer therapy | [141] |
| Dendritic cells | Electroporation | siRNA | Alzheimer’s disease therapy | [121] |
| Dendritic cells | Electroporation | shRNA | Parkinson’s disease therapy | [142] |
| Alveolar basal epithelial and 3 T3 cells | Electroporation | siRNA | Lung cancer therapy | [143] |
| Colon cancer cells | Electroporation | miR-21i | Colon cancer therapy | [144] |
| MSCs | Electroporation | anti-miR-142-3p | Breast cancer therapy | [145] |
| Human renal epithelial cells | Electroporation | anti-miR-21 | Glioblastoma therapy | [146] |
| Blood plasma, lung cancer cells, Hela cells Gene | Electroporation | siRNA | Silencing of mitogen-activated | [140] |
| Hela cells and human fibrosarcoma cells | Electroporation | siRNA | Cancer therapy | [141] |
| MSCs | Electroporation | mRNA | Glioma therapy | [147] |
| HEK293T | Transfection | BCR-ABL siRNA | overcome pharmacological resistance in CML cells | [129] |
| Mouse fibroblasts Suppression of tumor growth in pancreatic | Electroporation | KRASG12D siRNA | Suppression of tumor growth in pancreatic cancer | [148] |
| Plasma (human) | Electroporation | MAPK siRNA | The MAPK-1 was down-regulated in monocytes and lymphocytes | [140] |
| Primary immature Dendritic cells Specific gene knockdown after specific siRNA | Electroporation | GAPDH siRNA | Specific gene knockdown after specific siRNA delivery to the brain for Alzheimer’s disease | [121] |
| Dendritic cell | Electroporation | VEGF siRNA | Suppression of tumor growth in breast cancer | [149] |
| HEK293 | Transfection | Let-7a mimic |
therapeutically to target EGFR-expressing cancerous tissues with nucleic acid drugs for breast cancer. |
[125] |
| Glioblastoma cells | Transfection | miRNA | Providing diagnostic information | [150] |
Blood-Brain Barrier and Blood-Brain Tumor Barrier: Two Major Limitations in GBM Drug Delivery
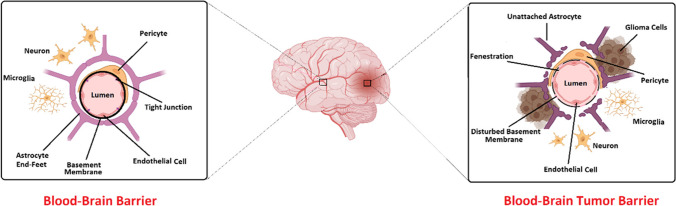
The treatment procedure for glioma, one of the most ordinary brain tumors, is limited because of two reasons. There are two important barriers, including the blood-brain barrier (BBB) and blood-brain tumor barrier (BBTB) (Fig. 1), which make the passage of many drugs difficult and limited [11]. Developing a drug delivery system will be possible to overcome these barriers. About 80% of brain tumors are glioma tumors, and common treatments include surgery, radiation therapy, temozolomide, and electric field therapy [12]. This tumor is invasive in nature and cannot be completely removed surgically, so treating Glioma is one of the major scientific challenges [12]. This type of brain tumor has an infiltrative growth pattern, so; its boundary becomes mixed with normal brain cells. This fusion of normal and cancerous cells has also made the healing process more difficult. Having a blood-brain barrier naturally prevents certain drugs and proteins from entering the brain [13]. This is the blood-brain barrier’s natural role in protecting the brain’s sensitive environment [14]. One of the important consequences of this barrier is the difficulty of the examination process with the help of, for example, fluorescent tracking and treatment of tumors [13]. The structure of BBB is built by a continuous, non-porous layer of capillary endothelial cells coated with a layer of the glycocalyx and tightly connected by a network of tight intercellular junctions (TJs), and adherens junctions, a basement membrane, pericytes, and perivascular astrocyte end-foot processes [15]. This complex BBB consists of three layers or barriers, including the glycocalyx, endothelium, and extravascular portion, which are organized in order from the blood to the brain. By understanding the structure and function of the BBB, it can be possible to overcome problems such as drug delivery in the treatment of brain tumors that are also hidden behind the BBTB. In the following, we will explain the three layers of the blood-brain barrier [15].
Fig. 1.
Schematic representation of the healthy blood-brain barrier (BBB) and the blood-brain tumor barrier (BBTB)
Glycocalyx
The thickness of this gel-like layer is approximately 300 nm. It has negatively charged proteoglycans, glycosaminoglycan, and glycoproteins and is located on the laminar membrane of the endothelium [16]. The components of this layer are anchored to the laminar membrane with the help of transmembrane proteins [17]. One of the most important roles of the glycocalyx layer is to prevent the adhesion of circulating cells and the passage of large molecules [18].
Endothelium
The endothelial layer of the blood-brain barrier has a different structure compared to the endothelium of other areas [19]. This layer has a specialized function with a thickness of approximately 200 nm. The cells are tightly connected in this layer and form a continuous, non-porous structure [19]. The number of pinocytic vesicles is reduced, so material transport is limited here [19]. Due to these limitations, the material transfer is done through paracellular diffusion and different intracellular mechanisms. Notably, the transfer of materials by the paracellular diffusion method is limited due to the presence of tight junctions (TJs) [20]. Tight junctions support this particular vascular structure with the help of the cytoskeleton. The two proteins occludin and claudin-5 are more expressed in the blood-brain barrier TJs than in other regions, leading to greater strength. Only lipophilic, neutral, and small molecules can pass through the intracellular pathway [20]. Studies have shown that the blood-brain barrier actually allows hydrophobic molecules smaller than 400 Da in size and form fewer than 8 to 10 hydrogen bonds with water to pass through [20]. Ideally, for the drug to be delivered effectively, it should have an octanol: water division ratio between 10:1 and 100:1. These specific endothelial transmission characteristics add to the limitations of drug delivery [21]. There are some transporters found in endothelial cells in this region, including solute carriers (SLC) of the family of passive transporters and other transporters which actively transport the adenosine triphosphate-binding cassette (ABC) [21]. However, various receptors are expressed in the endothelium of the BBB, such as the transferrin receptor, neonatal Fc receptor, and low-density lipoprotein receptor 1 (LRP1) [22].
Extravascular Compartment
Twenty-two to 32% of the surface of capillaries is covered by cells called pericytes [23]. Also, brain microvasculature, neuroglia, and neurons are coated by the processes of astrocytes [23]. In addition to secreting signals, these cells are essential for maintaining the integrity, physical, and metabolic support of the BBB [24]. As a result, this neurovascular unit is able to respond dynamically and continuously to physiological and environmental changes [25]. The glioma brain tumor is hidden behind another barrier called BBTB. Secreting vascular endothelial growth factor (VEGF) through high-grade gliomas is the main stimulator of BBB compromise. Production of VEGF increases under hypoxic conditions and up-regulation of hypoxia-inducible factor-1 [26]. Alteration of the BBB architecture and increased capillary growth occurs as a result of increased secretion of VEGF and leads to stimulation of tumor vascular endothelium and abnormal expression of various transporters and receptors. These changes occur to provide the metabolic needs of tumor cells [26]. New capillaries are even more permeable than peripheral vascular capillaries. Another point is that microvascular high-grade and non-solid brain tumors are different but more permeable than normal [27].
Increasing the number of pores as well as increasing the size of the gaps between the endothelium, are two important reasons for the high permeability of BBTB [26]. The degree of permeability of the BBB and the BBTB is measured with the help of fluorescently labeled dextrans. Endothelial pores of intracranial tumors are significantly smaller than extracranial tumors (210–550 nm compared to 380–2000 nm) [28]. Capillary permeability depends more on the number of pores than on their size. Medium-sized vessels in tumors have 30% fenestrations and 10% open junctions. Recent studies using nanoparticles have shown that molecules smaller than 330 kDa can pass through the BBTB pores [28]. Different types of endothelium are divided into three categories based on permeability. The endothelium in the skin, heart, and lungs is continuous and non-porous, and molecules larger than 4 to 6 nm do not pass through it [29]. However, this type of endothelium has 20-nm intercellular slits due to discontinuities in TJs [29]. The endothelium in the intestines, kidneys, and choroid plexus is a porous type through which 25 to 60 nm molecules pass [29]. The third type is a special endothelium found in the liver, spleen, and bone marrow that contains large pores that can pass 100–200 nm molecules without an underlying basement membrane [29]. Knowing these various types of endothelium could be helpful for the improvement of the drug delivery system. Anatomical location of the tumor, tumor type, stage, and volume cause changes in the BBB. The compromising range of BBB is different from serious disturbances that are present in the vasculature in solid, non-cerebral tumors compared to gentle compromise evident in neurodegenerative diseases, stroke, obesity, and diabetes, among other pathologies [30]. The structure and function of the BBTB in low-grade glioma are similar to what is seen in normal BBB [31]. In high-grade glioma, the blood-brain barrier changes due to edema and gadolinium-based contrast accumulation. In addition, in high-grade glioma, areas with high vascular density are seen in the white matter of the same area [32]. Penetration of glioma cancer cells causes astrocyte processes to migrate to the BBB and cause a fracture in it. On the other hand, infiltrating cancer cells are protected by a BBB [33]. Given the vessels that already exist in the BBTB and the new vessels that form in this area, it has been suggested that this barrier is involved in nourishing and oxygenating cancer cells. However, even in the presence of disruptions to the BBTB in the neoplasm core, BBB represents characteristics of intact BBB in certain areas. Therefore, while compromised, BBB still prevents the delivery of diagnostic agents to the tumor, which leads to reduced efficacy of intraoperative optical directive methods [34].
Main Strategies to Overcome the BBB and Their Limitations
Effective delivery of the drug to the central nervous system (CNS) has always been a challenge for scientists. This barrier rigorously controls the entry of molecules from circulation to cerebral tissues and vice versa so that virtually all of the large therapeutic compounds and more than 98% of small molecule drugs are prevented from crossing this barrier. Nowadays, several new technologies have been developed that can deliver suitable drugs to the CNS and treat glioma. These technologies can be classified into two major categories, invasive and non-invasive techniques [35, 36]. Moreover, in high-grade gliomas, the drug is more likely to reach the tumor core due to damage to the BBB. However, the more important challenge is the aggressive nature of this type of tumor and its unclear boundary [37, 38]. In the following, we will read about invasive and non-invasive techniques, including methods that have emerged due to compromised BBTBs, such as passive delivery and EPR, BBTB disruption, their applications, and clinical benefits.
Invasive Techniques
Intracerebroventricular and Intrathecal Infusion
Intracerebroventricular and intrathecal administration is invasive to deliver therapeutic drugs to the brain. In this method, therapeutic agents were directly injected into the ventricles via an outlet catheter and were administrated into the spinal cord lumbosacral subarachnoid space by intrathecal lumbar injection. This strategy has been used to deliver chemotherapy drugs for brain cancer treatment, opioids for pain control, and therapeutic agents to treat various neurological diseases [39].
Convention-Enhanced Delivery
In theory, convection-enhanced delivery (CED) is a minimally invasive technique for transferring a wide variety of agents into the brain. An infusion catheter is used in the CED system, which is positioned into the parenchyma, and then the desired therapeutics are injected into the target tissue through positive pressure micro-perfusion generated by a pump [39]. The CED technique may deliver a wide range of agents. These agents include low molecular weight compounds from imaging tracers and small molecule antineoplastic agents to macromolecules such as proteins, nanoparticles, and viruses. Unlike diffusion, the bulk flow generated by the pump homogenously distributes all the compounds in the target tissue regardless of molecular weight; at the same time, larger molecules are restricted because of the limitation of brain extracellular pore size. The major disadvantages of CED include (i) most macromolecules have not diffused from the injection site, which reduces efficacy and the volume of distribution; (ii) due to backflow, distribution of drugs is limited; and (iii) the safety and efficacy of this method have not yet been fully elucidated [40, 41].
Interstitial Polymer Wafers
The polymer wafers are placed into the resection cavity after surgery. They can be used for localized administration of therapeutic agents that cannot cross the BBB, for instance, in the glioblastoma treatment. This approach has increased attention to previously unused drugs due to their inability to cross the BBB or toxicity [41]. The Gliadel wafer, as a biodegradable polymer wafer, was approved by FDA about 18 years ago for glioma treatment. However, its widespread use has been limited because of infection risk, local toxicity, and expensive cost. Therefore, more investigations must be done to reduce its complications, and more methods must be developed for polymer delivery [41–43].
Disruption of BBB
Drug delivery in this method is done in different ways, which are explained below. One of them is hyperosmolar opening which is safe and effective for chemotherapeutics in cases of CNS lymphoma, anaplastic oligodendroglioma, and other brain malignancies [44]. In this method, due to the non-selective opening of the BBB, the entry of molecules through the blood leads to seizures, neurotoxicity, and other problems, so the clinical application of this method is limited [37]. In another way, drugs are injected into a specific area of the brain with the help of microbubbles based on ultrasound, with a lipid coating and a combination of intravenous injection methods [45]. The safety and effectiveness of this method need more investigation. Using the photodynamic method, the BBB can be opened with the help of ALA-5. After intravenous administration of ALA-5, laser irradiation with a wavelength of 635 nm increases the permeability of the BBB [46]. This technique uses toxic agents, focused ultrasounds, radiation, or hypertonic solutions (mannitol, urea, arabinose). These agents cause a shrinkage and tight-junction dysfunction in the brain’s endothelial cells and allow paracellular transport of therapeutic molecules into the brain parenchyma through the created window. However, this strategy has several limitations, including the following: (i) it is non-patient friendly, (ii) increasing the permeability of the BBB is done by a non-selective method which results in systemic toxicity in the CNS, and (iii) accumulation of unwanted blood components such as xenobiotic agents, neurotoxic, and exogenous materials that cause an injury to the CNS [47]. Nevertheless, recent investigations support the safety and efficacy of this method in humans. More studies are needed to understand better this technique’s safety and potential therapeutic value [41].
Non-invasive Techniques
Passive Delivery
In this method, by transferring drugs smaller than 40 kDa, it is possible to prevent blood-tumor diffusion gradient to some extent. The tumor-to-normal brain distribution ratio of drugs with low molecular weight is less than larger molecules [48]. Because of the quick removal of the small-molecule drugs from extracellular space and clearance from blood, the distribution of these drugs varies [48].
Enhanced Permeability and Retention
Drug delivery with this method is based on four components: hypervascularization of the tumor, increased permeability of tumor vasculature, hampered absorption of macromolecules back into the vasculature, and decreased drainage of molecules via the lymphatic system [48]. Experimental studies have shown that drug delivery by this mechanism relies on damage to the BBTB. Due to the high permeability of BBTB in high-grade gliomas, it is possible to deliver many drugs by this mechanism. The important point is the lack of a lymphatic system in tumors, which inhibits the secretion of large molecules and lipids from the extracellular space, and drugs will be available to the tissue for a longer period of time [49]. In this drug delivery mechanism, the increase in vascular density seen in this type of cancer plays an important role. For this mechanism to be effective, biocompatible molecules must not be eliminated through the reticuloendothelial system and must not be reactive with blood and endothelial cells [48]. In addition, to prevent glomerular filtration, the molecules must be larger than 40 kDa and preferably have a weakly negative or neutral charge [50]. Recent studies have shown that one-micrometer lactobacilli are transported to the tumor by inhibiting angiotensin-converting enzymes and opening the endothelial cell junctions [51]. Conjugated polymeric compounds such as immunoglobulin G (160 kDa) and liposome-encapsulated drugs bind to albumin to increase their molecular weight; therefore, they are delivered to the tumor by this mechanism [48]. High molecular weight drugs delivered by this mechanism accumulate within half an hour, and the bioavailability of these drugs is between a few hours to a few days [48]. Therefore, these large molecules are both safer than small molecules in terms of glomerular filtration and are available to the tissue and circulate for a long time [49].
Modification of Drugs
Lipidization
A common technique to promote BBB permeability against drugs is the chemical modifications of small molecules into their lipophilic analogs. For example, a fusion of a methyl group with morphine converts it to codeine, which increases its permeability from BBB up to 10-fold. However, lipidization causes molecules to be eliminated more quickly from the circulatory system via efflux transporters, thus having a negative effect on drug distribution. In addition, the lipidization of drugs can be used to modify drug structures and promote their affinity for endogenous endothelium transportation [39].
Substrate for CMT
Carrier-mediated transcytosis (CMT) represents a mechanism for the transport of molecules into the BBB. ATP-binding cassette (ABC) transporters have a high affinity for a wide range of solutes, particularly lipid-soluble molecules with oxygen and nitrogen atoms in their structure. These ABC transporters actively pump molecules across the membrane by ATP hydrolysis; therefore, they can transfer the solutes in contrast to the concentration gradient. For instance, levodopa and gabapentin, the phenylalanine analogs, are transported by large neutral amino acid transporters [39].
Substrate for RMT
Receptor-mediated transcytosis (RMT) is another class of transport technique that delivers defined substrates from the lumen of the endothelial cells into the cerebral tissue. Therapeutics such as anticancer drugs conjugated to relevant ligands or antibodies targeting RMT will allow delivery to the brain parenchyma [39, 52]. Some well-known receptors that have been utilized for this approach include insulin, insulin-like growth factor 1 receptor (IGF-1R), transferrin receptor (TfR), and low-density lipoprotein receptor-related protein (LRP1). In treating Alzheimer’s disease, TfR has been considered to transport therapeutic antibodies into the CNS [53, 54]. However, the side effects of this approach should be considered because the TfR is expressed in the intestine and liver [39].
Intranasal Delivery
The intranasal route is an effective non-invasive technique for the transport of therapeutics to the CNS. The drugs enter the brain by crossing the nasal mucosa or nasal olfactory epithelium in this approach. Intranasal administration has several advantages, including easy self-administration, rapid absorption, patient comfort and compliance, avoidance of hepatic first-pass effect, and bypassing gastrointestinal enzymatic degradation, thereby enhancing drug bioavailability and minimizing systemic adverse effects. Several therapeutic agents have been administrated using the intranasal routes, such as cytokines, chemotherapeutic drugs, proteins, plasmids, small molecules, and neuropeptides (insulin or interferon-β) [39, 40]. A neural pathway that directly connects the nasal mucosa to the brain allows the delivery of therapeutic agents to the CNS. Absorption occurs by different paths, including extracellular diffusion (via diffusion and/or convection), intraneuronal transport (via the olfactory sensory neuron), and intraneuronal transport (via the trigeminal nerve) [47]. Currently, a nicotine spray is in phase II clinical trial for treating the symptoms of Parkinson’s disease, and midazolam nasal spray received FDA approval for treating seizures [39].
Prodrug Bioconversion
In this method, inactive pro-drug compounds or inactive pro-agents can cross from the BBB and reach the brain parenchyma. After entering the target site, under enzymatic and/or chemical reactions, their structures become modified and are biologically active, exerting their pharmacological effects [47].
Use of Transport Carriers
Nanoparticle-Mediated BBB Delivery
One way to promote drug delivery through the BBB is to use a wide range of nanoparticles (NPs). NPs can pass through the BBB because they are administered intracerebrally and release the loaded drugs continuously. There are different types of NPs, including polymeric NPs, lipid-based NPs, and inorganic NPs. Small, less distributed therapeutic molecules can be included in nanoparticles by various chemical methods, such as adsorption and encapsulation. Moreover, large molecular agents can be bonded to the NP surface and improve their targeting [35]. The nano-delivery technique is a method that has attracted the attention of many scientists. Using this technique increases the bioavailability of drugs in various ways and increases the chances of the drug reaching the affected area. The use of liposomes as one of the modifiable nanomaterials that have two lipid membranes and have many applications has been widely adopted in this technique [55]. Pharmacokinetic optimization of liposomes is performed by changing the membrane’s size, composition, surface charge, and mechanical and biological properties. Consequently, Several parameters affect their performance, including size, shape, surface charges, stability, drug loading, and ligand density [56]. Some liposomal formulations have already been approved by FDA [55]. These liposomes escape phagocytosis with the help of cell-surface proteins derived from C6 glioma cells, which cause an effective liposomal interaction with tumor cells [57]. Also, using a family of peptides that possess structural homology to ligands that induce endothelial transcytosis is a vital and determining stage in receiving drugs across the entire BBB and BBTB endothelium [57]. Due to the diversity and complexity of gliomas, the clinical application of the techniques is limited. The difficulty of drug delivery in brain tumors, in addition to the existence of physical barriers, is due to the expression of a wide network of transporters and junctional proteins. Although the exact mechanisms of NPs crossing through the BBB are still not fully understood, different nanomaterial-based delivery systems are utilized to transport therapeutic agents or other molecules such as proteins, nucleic acids, or imaging agents across the BBB with no injury to the normal brain function [40]. Also, some challenges and crucial problems need to be further studied before using nanocarriers for further biomedical applications, which are discussed below:
-
i.
The biocompatibility and biodegradation of NPs are essential factors for clinical application. The interaction of NPs with the immune system is complicated, and the safety of these NPs is still unclear.
-
ii.
The surface charge of NPs has a critical role in crossing through the BBB and must be balanced. According to general knowledge, due to the negative charge of the endothelial cells, the cationic NPs pass more easily through the BBB. On the other hand, neutral NPs or NPs with negative charge display lower toxicity and higher stability in the circulation than cationic NPs. Besides, the presence of charge on the surface of NPs can cause non-specific adsorption with the circulation proteins, which disrupts the drug delivery process.
-
iii.
Despite advances in the use of nanoparticles for drug delivery, designing nanocarriers for better drug loading and effective drug release remains a significant challenge. For example, due to drug leakage during transportation, a limited number of drugs could be delivered to the tumor site in the brain. An ideal nanocarrier should have a high specific surface, powerful interactions with the loaded drugs, and release drugs in a controlled manner in the targeted area [56].
Virus-Mediated BBB Delivery
The use of viral vectors is another strategy for crossing from BBB and delivering the therapeutic drugs into the brain. In this strategy, tumor cells are targeted through cell surface receptors, and virus replication derivatives are used to overcome tumor growth. Previous in vivo studies showed that the use of the measles virus has cytotoxic effects on glioma stem cells and increases survival in a mouse model. Viral vectors, in combination with other routine treatments, such as radiation therapy or CED, may have synergistic effects [41]. Also, Huang et al. reported that Toca 511, a replicating retroviral vector, can successfully deliver a cytosine deaminase gene and significantly improve survival in orthotopic glioblastoma models. Combining this approach with radiation therapy or CED opens a new window for the future treatment of patients with diagnosed malignant glioma [58]. Although the use of viruses as vehicles has some advantages, such as small size and permeability across the BBB, it also has some limitations. The entry of viral capsid proteins into non-targeted tissues causes many complications, such as enhanced immune response and unwanted biochemical changes that increase the need for safer alternative methods. These viruses may also be lethal and carcinogenic [39].
Exosome-Mediated BBB Delivery
The use of exosomes as a novel non-cell-based strategy for drug delivery has recently received much attention. Exosomes can pass the BBB and can be used to transfer small pharmaceutical molecules to the brain tumor. A small but growing number of new studies are developing the use of exosomes as biological carriers for the treatment of brain tumors [59]. Therapeutic drugs can be loaded into exosomes by several techniques, including sonication, electroporation, freeze-thaw treatment, surfactant treatment, transfection, and the use of simple incubation [60]. Today the use of exosomes as delivery vehicles for brain tumor treatment has been considered by many researchers for some reasons: (1) exosomes affect many biological processes, comprising cell proliferation, apoptosis, differentiation, and immune response by released from donor cells and entering acceptor cells; (2) stability of exosomal RNA higher than cellular RNA; (3) during long-term storage, exosomes are highly resistant to degradation as well as to freeze-thaw cycles; (4) administered exosomes are non-immunogenic; (5) they do not have tumorigenic properties because they are non-viable; (6) unlike viral vectors, drug delivery via exosomes do not adversely increase the expression of genes; (7) exosome membranes or their cargoes can be used to target specific tumors or personalized therapy; and (8) exosomes can cross from BBB which makes them suitable drug delivery tools for the treatment of brain tumors such as glioblastoma. Despite the positive results of recent experiments and promising advances in recognizing the importance of exosomes, using these vehicles for treating brain malignancies is far from a clinical reality, and more studies are needed to develop exosome-based therapies [59] (Fig. 2).
Fig. 2.
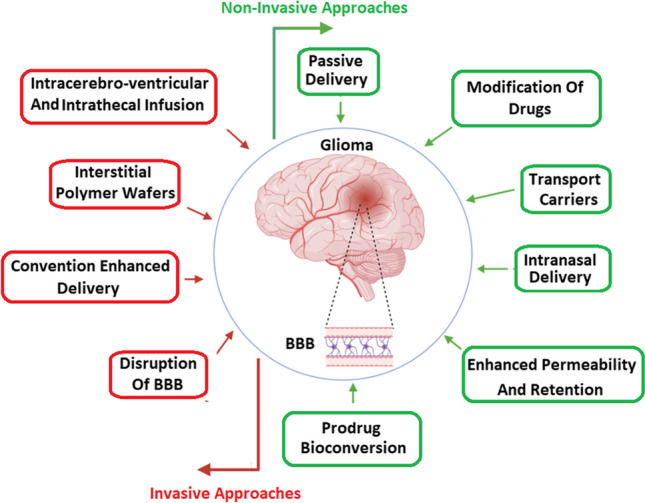
Novel invasive and non-invasive drug delivery strategies to overcome the intact BBB and leverage the disrupted nature of the BBTB to target cancer cells of GBM. BBB: blood-brain barrier, BBTB: blood-brain tumor barrier, GBM: glioblastoma multiform
Exosomes: Biogenesis, Composition, and Functions
Exosomes, as nanoscale vesicles with approximately 30–120 nm in size, circulate in virtually all body fluids, including plasma, saliva, urine, milk, amniotic fluid, and even cerebrospinal fluid [61]. Exosomes are generated within multivesicular bodies (MVB) with invagination of the late endosome membrane and biomolecule encapsulation. The fusion of MVBs with the plasma membrane leads to exosome secretion into the circulation, and then these vesicles migrate to the recipient cells. The bilayer membrane of the exosome is mainly composed of a high level of lipids—cholesterol, phosphatidylserine, sphingomyelin, gangliosides, unsaturated lipids—and proteins [62, 63]. The presence of high levels of sphingomyelins and unsaturated fatty acids in the exosome membrane is probably related to its rigidity, making it resistant to degradation when secreted out of the cell, and it has a stable structure as a carrier [64]. The exosomal lumen often contains active biomolecules, including various metabolites, proteins, lipids, mRNA, and different non-coding RNA (ncRNA) [65]. Exosomes directly connect to the receptors located on the recipient cell surface and fuse the contents of their membrane with its plasma membrane. This process plays a vital role in the regulation of recipient cell functions by carrying proteins, lipids, and nucleic acids [66]. In this way, exosomes play significant roles in maintaining normal conditions, such as intercellular communication, tissue repair, hematopoietic function, and organ development, and are involved in pathologic conditions, such as cancer progression and metastasis [62]. Exosomes can be utilized as a vehicle to transfer functional molecules such as proteins, lipids, nucleic acids, and various non-coding RNAs from one cell to another. In addition, their contents can rescue them from immune system attacks [67]. The effect of exosomes on recipient cells is different due to the expression of various receptors on the recipient cell surface that cause heterogeneity in the function of exosomes so that one set of exosomes induces apoptosis and others significantly promote cell proliferation. This heterogeneity also depends on the tissue and organ from which the exosome originated. The interaction between exosome formation and the regulation of secretory vesicles in neuronal cells suggested a new perspective on the relationship between these vesicles and neurological disease pathogenesis. Exosomes may increase or decrease the aggregation of unfolded proteins in the brain. Thereby, they can contribute to the progression of neurodegenerative diseases, and on the other hand, exert detoxifying and neuroprotective functions [68]. Recently, extracellular vesicles, particularly exosomes, have been used as a novel delivery system for gene or drug delivery. Exosomes have many advantages compared to other drug delivery systems due to the reasons that will be mentioned as follow. First, exosomes can introduce as versatile carriers. A wide range of biological cargoes, including mRNAs, small RNAs, and proteins, can be transferred by exosomes to the target cells. Second, exosomes can cross biological barriers such as BBB and move to the tissues without blood circulation, for instance, the dense cartilage. In addition, due to their low clearance rate, they can remain in the target tissue for a long time [69]. In spite of potential advantages, exosomes are at the primitive stages of clinical development despite being touted as a consolidated therapeutic approach for many diseases. There are several challenges associated with the use of exosomes in the clinical setting, including the lack of standard isolation and purification protocols, the risk of infectious agent transmission, the safety concerns, the pleiotropic effect, batch-to-batch inconsistencies, sterility, and the impact of storage conditions on exosome function and profile composition. In addition, the production of exosomes is currently a time-consuming and expensive process, which may limit their accessibility as a therapeutic option [70]. While exosomes hold great potential as therapeutic agents for brain diseases, their systemic application faces several challenges and limitations that must be addressed through rigorous research and development [71].
Due to their ability to transfer biological materials, including proteins, nucleic acids, and lipids, exosomes have gained significant attention in the last few years as a potential vehicle for targeted drug delivery. Furthermore, some of the limitations associated with conventional drug delivery systems can be overcome by using these systems, such as limited bioavailability and off-target effects [72]. Some additional sections and data related to exosomes and their role in drug delivery:
Exosome-mediated transfer of therapeutics: Exosomes have been shown to transfer therapeutic agents such as small interfering RNAs (siRNAs), microRNAs (miRNAs), and chemotherapeutic drugs between cells. This transfer can occur between cells of the same type or different types, including between cancer cells and normal cells. Using exosomes as vehicles for drug delivery can potentially improve the bioavailability and efficacy of therapeutics [73].
On-target delivery: Exosomes have been shown to selectively target specific cells and tissues, including cancer cells and the brain. This targeted delivery can be achieved by modifying exosome surface proteins, such as by engineering exosomes to express specific ligands that bind to receptors on the target cells. The ability of exosomes to target specific cells and tissues may also reduce off-target effects and toxicity associated with conventional drug delivery [74].
To methodically decipher the application of these extracellular vesicles and propel research in this field, exosomes should be isolated from various sources - cell debris and interfering components [75, 76]. The properties of exosomes largely depend on the isolation technique; therefore, until now, numerous studies have been developed for the isolation of exosomes from biological fluids and cells based on their shape, density, size, or surface components [77]. With each of these isolation techniques, we deal with a tradeoff between yield and specificity [75]. There is an urgent demand for developing techniques that yield a high quantity and purity of isolated exosomes. The current exosome isolation techniques developed will be discussed in detail in the following sections, along with their advantages and drawbacks [77].
Choosing the Proper Exosome Isolation Technique
The type of original sample and the intended use has a major influence on the choice of exosome isolation method. The type of starting material affects the success of exosome isolation, and it is evident in isolation from complex samples. The purity of isolated extracellular vesicles is low, especially in the case of exosomes obtained from plasma [78]. The high risk of protein contamination is a huge drawback of exosome isolation from these matrices. To overcome this issue, a combination of various methods, such as centrifugation, protein digestion, ultrafiltration, and SEC are used to improve exosome purity. However, additional steps complicate the isolation and slow down the process, leading to low recovery yields [75]. In tissue culture samples, ultracentrifugation is considered an adequate method for exosome isolation. Table 1 compiles the most frequently employed methods and their main characteristics.
Table 1.
Various methods of exosomes isolation
| Methods | Basis | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Ultracentrifugation | Density, size, and shape | Cost-effective, large sample capacity, does not require a high level of technical expertise, easy to carry out | High equipment cost, cumbersome, long run time, and labor-intensive, limited portability—therefore, is not available at point-of-care, high-speed centrifugation may damage exosomes thus impeding downstream analysis | [75–77] |
| Ultrafiltration | Based on the size difference between exosomes and other particulate constituents |
Fast, scalable, automatable, does not require special equipment, good portability, direct RNA extraction possible, minimal physical and chemical alteration |
Requires three steps, lack of specificity in separation, moderate purity, use of force possibly resulting in the deformation and breaking up of large vesicles, the possibility of clogging and vesicle trapping | [75–77] |
| Size-exclusion chromatography (SEC) | Based on size and Hydrophobicity |
High-purity exosomes, gravity flow preserves the integrity and biological activity; excellent reproducibility, and moderate sample capacity. Maintains vesicle integrity, good purification |
Requires dedicated equipment, not trivial to scale up, long run time, limited scalability, need to proceed with other methods, interfering problem between lipoprotein and protein aggregation | [75–77] |
| Precipitation with salts |
Altering the solubility or dispersibility or exosomes by the use of water-excluding polymers |
Easy to use, no specialized equipment required, low cost | High yields, potential for contamination with other sample components and precipitating reagents, long run time, require pre-and post-cleanup. | [75–77] |
| Immunoaffinity |
Based on specific interaction between membrane-bound antigens (receptors) of exosomes and immobilized antibodies (ligands) |
Excellent for the isolation of specific exosomes, highly purified exosomes much better than those isolated by other techniques, high possibility of subtyping. | High costs, lack of reliable markers, low capacity and low yields, only works with cell-free samples | [75–77] |
| Microfluidics |
Microscale isolation based on a variety of properties of exosomes like immunoaffinity, size, and density. |
Fast, low cost, portable, easy automation, and integration, high portability. good efficiency | Lack of standardization and largescale tests on clinical samples, lack of method validation, moderate to low sample capacity. | [75–77] |
Strategies for Loading Therapeutic Cargos into Exosomes
Exosomes are able to contain bioactive compounds that are either hydrophobic or hydrophilic due to their amphiphilic nature; these molecules can be incorporated on their surface as well [79]. Based on this agreeable feature of exosomes, many approaches have been developed to load cargoes in exosomes; among them, incubating cargoes with exosomes or exosome-secreting cells is a mild and easy-to-operate strategy. In this method, cargoes diffuse across the membrane without disrupting the integrity of exosomes based on concentration gradient. The ratio of cargoes and cells/exosomes, the concentration of cargoes, culture time, and environment influence the efficiency of this method. Even though these parameters have been strictly optimized for achieving optimum loading efficiency of incubation, the endpoint efficiency has remained considerably low. For instance, the loading efficiency of incubation of proteins (e.g., catalase) and chemotherapeutic drugs (e.g., paclitaxel) with exosomes at room temperature is only 4.9 ± 0.5% and 1.44 ± 0.38%. This can be due to several limiting factors, which are as follows: The first restricting factor is the limited concentration of cargoes in the incubation solution. The gradient-based diffusion is markedly curbed by the saturation concentration of certain cargoes and consequently restricts the upper limit of cargo loading; The second one is the exosomal membrane which acts as a barrier that restricts the diffusion of most hydrophilic molecules across the exosomes; and lastly, the large size of some cargoes such as proteins and nano-materials hinders their efficient diffusion across the exosomal membrane without external force [80]. There are various ways to overcome these limitations, such as physical treatments like sonication, electroporation, or surfactant treatment; mechanical shear force, electric field, and membrane molecule dissolution can also be utilized to create micropores in exosomal surfaces, too. Moreover, extrusion induces membrane recombination by employing stronger shear force, and freeze-thaw treatment enhances membrane fusion through rapid temperature fluctuations. Many studies have investigated the cargo loading efficiency of these approaches. Among all these treatments, physical approaches enhance exosome loading efficiencies more significantly than incubation. Several studies have pointed out that sonication is probably the most effective technique in this regard, whereas electroporation is not a suitable approach due to causing exosome damage. Besides the noticeable superiority of physical treatments in enhancing the loading efficiency of exosomes, they also have disadvantages. One of these drawbacks is the probable damage of physical treatments such as electroporation to the exosomal membrane that may lead to severe membrane aggregation. Second, surfactant treatment may lead to the increased presence of impurities and cause potential toxicity. Moreover, physical treatment may also damage or inactivate cargo and interfere with the biological functions of exosomes. These disadvantages bring up the importance of precise control of different parameters in physical treatments, such as shear force level, the voltage of the electric field, active agent concentration, and the number of freeze-thaw cycles to prevent or minimize adverse outcomes [79, 80]. Incubation or physical treatments are suitable for loading a plethora of different cargo. Several strategies have been developed to load specific categories of materials into exosomes. Transfection is a common approach for efficiently loading nucleic acids and proteins. In this method, cells/exosomes are efficiently transfected with protein-expressing plasmids or nucleic acids. This technique is laborious, time-consuming, and expensive for generating large batches of cargo-packaged exosomes; however, it is not suitable for loading drugs and has potential harm or contamination to cells and exosomes due to the presence of a transfection reagent. Another strategy only used for loading noble metals into exosomes is in situ assembly and synthesis, a synthetic chemical method to package desired nanoparticles into exosomes without physical damage to the exosomal surface. Its application for cargo loading is limited and restricts further application [80]. Overall, with the current exosomal loading techniques, simultaneous efficient loading of desired materials into exosomes and minimizing exosomal surface damage is a paradox. The important question that we are left with is how to utilize the advantages and strengths of the strategies mentioned above and avoid their disadvantages. To answer this question, we need to carry out comprehensive studies to expand our understanding of exosome biogenesis and content sorting/packaging mechanisms [76].
Role of Exosome in Delivery of Effective Therapeutics for GBM Therapy
Exosomes are extensively used as chemotherapeutics delivery for conventional drugs, genes, and other natural compounds in GBM. Exosomes have many privileges over other synthetic nanoparticles for gene or drug delivery [81, 82]. Furthermore, under both pathological and physiological conditions, exosomes can be used for chemotherapeutics because of their acceptable stability in circulation [81]. To evaluate the efficacy of exosomes for delivering therapeutics across the BBB, drugs were administered in the absence of exosomes as carriers and encapsulated in exosomes. In the absence of exosomes, therapeutics remained with the vasculature circulation and did not cross the BBB, whereas, in the presence of exosomes as carriers, a reduction of tumor progression was observed due to increased delivery of drugs across BBB. This highlights a prominent feature of exosomal-based therapy, allowing the transport of anti-tumor agents across the BBB, which is otherwise highly impermeable to many chemotherapeutic agents [83, 84]. Studying the pathophysiology of exosomes secreted from glioma cells can open up novel therapeutic avenues. For instance, it has been demonstrated that exosomes released by glioma cells can activate glycolysis in the human bone marrow mesenchymal stem cells (hBMSCs). This results in their tumor-like phenotype transformation and indicates that the mutual effect between exosomes and hBMSCs in the tumor microenvironment may be adopted as a therapy for glioma [85, 86]. Exosomes derived from brain endothelial and glioblastoma-astrocytoma cells (U-87) have been used as favorable drug delivery vehicles for model chemotherapeutics such as doxorubicin and paclitaxel [83]. In addition to the common chemotherapeutics (such as doxorubicin and paclitaxel) used in the treatment of GBM, effective factors and molecules in herbal medicines were loaded into exosomes (derived from different cells) for GBM and are listed in (Table 2).
Studies have demonstrated that exosomes have the ability to regulate a variety of complex signaling pathways by mediating small molecules such as miRNAs, which provides a powerful tool for GBM [112, 113]. The biological functions of exosomal microRNAs -gene regulatory factors -are different in glioma. Therefore, Gene therapy has the potential to be a novel and promising treatment strategy for GBM. Similar to chemical drugs, the low permeability of BBB impedes gene delivery to cerebral tissue [114]. As shown in Table 3, many studies have used different genes loaded into exosomes for GBM (Table 3). Exosomal microRNAs have been used successfully as biomarkers and therapeutic targets in other diseases. In recent studies, exosomal miRNA plays an important role in glioma occurrence, invasion, development, metastasis, and treatment resistance [112]. For precise glioma treatment, the use of exosomal miRNAs in combination with exosomes and transcriptomics is expected to be a new approach [112, 115]. Sakr et al. [116] have shown the transfection of miR-150-5p or miR-133a mimics into the exosomes generated from glioma cells and co-cultured these exosomes with glioma cells. Those exosomes inhibited the expression of membrane type 1 matrix metalloproteinases (MT1-MMP) and subsequently induced apoptosis of glioma cells [112, 116]. As a natural carrier of miRNAs, exosome is a nano-scale bilipid layered extracellular vesicle with good stability in circulation, excellent permeability to biological membranes, low immunogenicity, and toxicity. It can pass through the blood-brain and blood-cerebrospinal fluid barriers without inducing immune rejection. Recently, with the extensive and in-depth ongoing study of exosomal miRNAs and their molecular regulatory network, it has been confirmed that exosomes transporting specific miRNAs can regulate and alter the biological characteristics of glioma cells; consequently, they can effectively inhibit the malignant development of glioma [112, 117]. Different chemotherapeutics and miRNAs have been loaded into exosomes for GBM therapy. Lang et al. transfected bone marrow mesenchymal stem cells with miR-124a lentiviral vector and isolated exosomes generated by these cells from the medium supernatant. They co-cultured these exosomes with glioma stem cells and discovered a significant decrease in the proliferation and survival rate of glioma stem cells [118]. Furthermore, mice with intracranial glioma stem cell transplantation that were treated with isolated bone marrow mesenchymal cell exosomal miR-124a demonstrated prolonged survival time [112]. This finding showed that exosomes could selectively carry miR-124a as their cargo to inhibit glioma growth and invasion. The main mechanism associated with miR-124a is that its overexpression leads to a decrease in the level of target fork head box protein A2 (FOXA2) and causes lipid accumulation in cells, and therefore glioma stem cells lose their ability to metabolize lipids, resulting in cell poisoning effectively. Kim et al. also indicated that the overexpression of exosomal miR-584 in U87 cells was associated with an increased apoptosis rate and impaired proliferation and migration. Further animal studies have shown that U87 tumors transplanted into mice exposed to exosomes overexpressing miRNA-584 suppressed tumor growth [119] (Table 3). The studies mentioned above suggested that exosomes secreted by BMSCs can be used as delivery vehicles for chemotherapeutics in GBM. This particular treatment approach is quite effective due to its prominent effect on tumorigenesis and its long-term inhibitory effect. Generally, we can tap into the therapeutic potential of exosomes as natural delivery vehicles by adopting them for the selective knockout or inhibition of genes involved in tumor progression or overexpression of related key tumor suppressor genes. This can bring new hope to the field of targeted glioma therapy, whether through delivering chemotherapeutics, proteins or nucleic acids, and other effective therapeutic factors (Fig. 3).
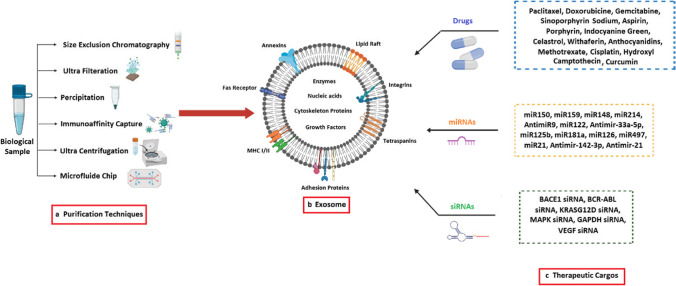
Fig. 3.
Exosomes are vesicles with a phospholipid bilayer membrane that contain many kinds of proteins, such as membrane transporters, and heat shock proteins. In addition, it also contains a lot of ncRNA, including miRNA and lncRNA with surface protein markers. a Current exosome isolation techniques; b Schematic representation of exosome structure, c: Effective factors, conventional drugs, genes, and other natural compounds loaded into exosome for GBM therapy. GBM: glioblastoma multiform, lncRNA: long non-coding RNA, ncRNA: non-coding RNA, miRNA: microRNA
Conclusion and Future Perspectives
The first step in malignant cerebral tumor treatment is resection. However, there still is a high risk of remaining single tumor cells in the outer layers of the brain tumor. Thus, subsequent chemotherapeutic intervention can be a successful strategy. Despite the advancement in drug development, the low penetrability of the intact blood-brain barrier is still a huge challenge, which the development of novel drug delivery techniques can overcome. A comprehensive list of invasive and non-invasive drug delivery techniques was covered here, mentioning each technique’s advantages, drawbacks, and limitations. Drug lipidization leads to a higher risk of elimination from the circulatory system, enhanced permeability, and retention technique, and carrier-mediated transcytosis has limitations in regard to the type of drugs they can deliver to cerebral tissues. Intranasal delivery can deliver drugs in a non-invasive, rapid, and self-administered manner to the brain. This technique can be adapted for delivering exosomes as already used in two clinical trials for the administration of mesenchymal stem cell-derived exosomes in healthy (NCT04313647) and severe COVID-19 patients (NCT04276987, NCT04602442) [151, 152]. In spite of the popularity of NPs among scientists, this technique has several significant drawbacks, such as immunogenicity, biocompatibility, biodegradability, and safety. Liposomes are the only FDA-approved NPs for drug discovery. This calls for extensive research to use other types of NPs in drug delivery safely. Viral delivery methods enable facile delivery of cargoes through the BBB but impose the risk of immunogenicity and carcinogenicity. Exosomes are amazing drug delivery candidates due to their non-immunogenic, non-tumorigenic, and natural propensity to penetrate biological barriers. Moreover, exosomal RNAs are more stable than cellular RNAs and can regulate signaling pathways in the brain. The downsides of exosomes are the lack of s standardization, high purity, low protein contamination, and high-throughput isolation methods. The current isolation methods listed in Table 1 all have huge trade-offs. Currently, ultrafiltration is widely used in producing exosomes through good manufacturing practices, sucrose gradient centrifugation, and commercial exosome isolation kits such as ExoQuick. Exosome production following GMP consists of three steps: cell expansion, exosome isolation, and exosome characterization, which leads to a cost of around 4500$ for experimental exosome treatment in the USA [153]. All of these steps need proper optimization to meet the FDA’s premarket review and approval requirements. In addition to this issue, current methods for loading effective molecules and genes into exosomes are not efficient and lead to low yield due to high exosome loss and damage to the surface of the exosomes. Therefore, despite the promising potential of exosomes as non-invasive methods for drug delivery to cerebral tissues, it is still far from reality, and further research is needed to bring exosomes to market.
Supplementary Information
(DOCX 648 kb)
Authors Contribution
Seyyed Hossein Khatami, Neda Karami, and Sina Taghvimi carried out the manuscript drafting and writing; Mortaza Taheri-Anganeh, Gholamhossein Tondro, and Marjan Khorsand carried out the manuscript critical editing; Elahe Soltani Fard, Khojaste Rahimi Jaberi, and Melika Moradi carried out the data collecting; Mohammad Hasan Darvishi, and Najmeh Sedighimehr, Parvaneh Nafisi Fard and Marzieh Kazemi Revised the article and Ahmad Movahedpour carried out the conceptualization and supervision.
Funding
This study is financially supported by the Behbahan Faculty of Medical Sciences (Grant Number: 401050).
Data availability
Not applicable.
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Seyyed Hossein Khatami and Neda Karami contributed equally as the first authors.
Contributor Information
Mohammad Hasan Darvishi, Email: darvishi@alumnus.tums.ac.ir.
Ahmad Movahedpour, Email: medical.biotechnology@yahoo.com.
References
- 1.Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. APJCP. 2017;18(1):3. doi: 10.22034/APJCP.2017.18.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humle N, Johnsen KB, Arendt GA, Nielsen RP, Moos T, Thomsen LB. Targeted vascular drug delivery in cerebral cancer. Curr Pharma Des. 2016;22(35):5487–5504. doi: 10.2174/1381612822666160726113907. [DOI] [PubMed] [Google Scholar]
- 3.Movahedpour A, Khatami SH, Khorsand M, Salehi M, Savardashtaki A, Mirmajidi SH, et al. Exosomal noncoding RNAs: key players in glioblastoma drug resistance. Mole Cell Biochem. 2021;476:4081–4092. doi: 10.1007/s11010-021-04221-2. [DOI] [PubMed] [Google Scholar]
- 4.Amarandi R-M, Ibanescu A, Carasevici E, Marin L, Dragoi B. Liposomal-based formulations: a path from basic research to temozolomide delivery inside glioblastoma tissue. Pharmaceutics. 2022;14(2):308. doi: 10.3390/pharmaceutics14020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mardi N, Salahpour-Anarjan F, Nemati M, Baher NS, Rahbarghazi R, Zarebkohan A. Exosomes; multifaceted nanoplatform for targeting brain cancers. Cancer Lett. 2023;557:216077. doi: 10.1016/j.canlet.2023.216077. [DOI] [PubMed] [Google Scholar]
- 6.de Lima LS, Mortari MR. Therapeutic nanoparticles in the brain: a review of types, physicochemical properties and challenges. International Journal of Pharmaceutics. 2022;612:121367. doi: 10.1016/j.ijpharm.2021.121367. [DOI] [PubMed] [Google Scholar]
- 7.Hersh AM, Alomari S, Tyler BM. Crossing the blood-brain barrier: advances in nanoparticle technology for drug delivery in neuro-oncology. Int J Mole Sci. 2022;23(8):4153. doi: 10.3390/ijms23084153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haqqani AS, Stanimirovic DB (2022) Brain delivery of therapeutics via transcytosis: types and mechanisms of vesicle-mediated transport across the BBB. Drug Deliv Brain: Physiol Concepts, Methodol Oaches 71–91
- 9.Taylor OG, Brzozowski JS, Skelding KA. Glioblastoma multiforme: an overview of emerging therapeutic targets. Front Oncol. 2019;9:963. doi: 10.3389/fonc.2019.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food U, Administration D (2019) Public safety notification on exosome products. Food Drug Admin
- 11.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019;21:v1. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor OG, Brzozowski JS, Skelding KA. Glioblastoma multiforme: an overview of emerging therapeutic targets. Front Oncol. 2019;9:963. doi: 10.3389/fonc.2019.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLong JC, Hoffman RM, Bouvet M. Current status and future perspectives of fluorescence-guided surgery for cancer. Exp Rev Antic Ther. 2016;16(1):71–81. doi: 10.1586/14737140.2016.1121109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi S, Tayebi Meybodi A, Belykh E, Cavallo C, Zhao X, Syed MP, Borba Moreira L, Lawton MT, Nakaji P, Preul MC. Survival outcomes among patients with high-grade glioma treated with 5-aminolevulinic acid–guided surgery: A systematic review and meta-analysis. Front Oncol. 2019;9:620. doi: 10.3389/fonc.2019.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutuzov N, Flyvbjerg H, Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood–brain barrier. Proc Nat Acad Sci. 2018;115(40):E9429–E9438. doi: 10.1073/pnas.1802155115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ando Y, Okada H, Takemura G, Suzuki K, Takada C, Tomita H, Zaikokuji R, Hotta Y, et al. Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Scientific reports. 2018;8(1):17523. doi: 10.1038/s41598-018-35976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reitsma S, Slaaf DW, Vink H. MAMJ v. Zandvoort and MG AO Egbrink. Pflügers Archiv. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haeren R, Van de Ven S, van Zandvoort M, Vink H, van Overbeeke JJ, Hoogland G, et al. Assessment and imaging of the cerebrovascular glycocalyx. Curr Neurovas Res. 2016;13(3):249–260. doi: 10.2174/1567202613666160504104434. [DOI] [PubMed] [Google Scholar]
- 19.Sweeney MD, Sagare AP, Zlokovic BVJNRN. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat RevNeurol. 2018;14(3):133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Biol. 2015;209(4):493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hediger MA, Clémençon B, Burrier RE, Bruford EA. The ABCs of membrane transporters in health and disease (SLC series): introduction. Mole Aspects Med. 2013;34(2-3):95–107. doi: 10.1016/j.mam.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163(5):1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharma Des. 2008;14(16):1581–1593. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- 24.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OPJG. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 25.Abbott NJ. Astrocyte–endothelial interactions and blood–brain barrier permeability. J Anatomy. 2002;200(5):523–534. doi: 10.1046/j.1469-7580.2002.00047_13.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am J Pathol. 2012;181(4):1126–1141. doi: 10.1016/j.ajpath.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plate KH, Scholz A, Dumont DJ. Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol. 2012;124:763–775. doi: 10.1007/s00401-012-1066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hobbs SK. Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aird WC (2007) Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100(2):158–73 [DOI] [PubMed]
- 30.Nir I, Levanon D, Iosilevsky GJN. Permeability of blood vessels in experimental gliomas: uptake of 99mTc-glucoheptonate and alteration in blood-brain barrier as determined by cytochemistry and electron microscopy. Neurosurgery. 1989;25(4):523–532. doi: 10.1227/00006123-198910000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Machein MR, Kullmer J, Fiebich BL, Plate KH, Warnke PCJN. Vascular endothelial growth factor expression, vascular volume, and, capillary permeability in human brain tumors. Neurosurgery. 1999;44(4):732–740. doi: 10.1097/00006123-199904000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Groothuis DR, Molnar P, Blasberg RG. Regional blood flow and blood-to-tissue transport in five brain tumor models. Brain. Tumor Biol. 1984;27:132–153. doi: 10.1159/000408227. [DOI] [PubMed] [Google Scholar]
- 33.Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H. Disruption of astrocyte–vascular coupling and the blood–brain barrier by invading glioma cells. Nat Commun. 2014;5(1):4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhan C, Lu W. The blood-brain/tumor barriers: challenges and chances for malignant gliomas targeted drug delivery. Curr Pharma Biotechnol. 2012;13(12):2380–2387. doi: 10.2174/138920112803341798. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F, Xu C-L, Liu C-M. Drug delivery strategies to enhance the permeability of the blood–brain barrier for treatment of glioma. Drug Des Devel Ther. 2015;9:2089. doi: 10.2147/DDDT.S79592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, Wang C, Wang L, Chen Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019;26(1):551–565. doi: 10.1080/10717544.2019.1616235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banks WA. From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15(4):275–292. doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 39.Pandit R, Chen L, Götz J. The blood-brain barrier: physiology and strategies for drug delivery. Adv Drug Del Rev. 2020;165:1–14. doi: 10.1016/j.addr.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Hersh S, Wadajkar SA, Roberts BN, Perez GJ, Connolly PN, Frenkel V, et al. Evolving drug delivery strategies to overcome the blood brain barrier. Curr Pharm Des. 2016;22(9):1177–1193. doi: 10.2174/1381612822666151221150733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azad TD, Pan J, Connolly ID, Remington A, Wilson CM, Grant GA. Therapeutic strategies to improve drug delivery across the blood-brain barrier. Neurosurg Focus. 2015;38(3):E9. doi: 10.3171/2014.12.FOCUS14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudnick JD, Sarmiento JM, Uy B, Nuno M, Wheeler CJ, Mazer MJ, et al. A phase I trial of surgical resection with Gliadel Wafer placement followed by vaccination with dendritic cells pulsed with tumor lysate for patients with malignant glioma. J Clin Neurosci. 2020;74:187–193. doi: 10.1016/j.jocn.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Champeaux C, Weller J. Implantation of carmustine wafers (Gliadel®) for high-grade glioma treatment. A 9-year nationwide retrospective study. J Neuro-Oncol. 2020;147(1):159–169. doi: 10.1007/s11060-020-03410-1. [DOI] [PubMed] [Google Scholar]
- 44.Priest R, Ambady P, Neweult E. ACTR-23 Safety of intra-arterial chemotherapy with osmotic opening of the blood-brain barrier. Neuro-Oncol. 2018;20:vi16. doi: 10.1093/neuonc/noy148.057. [DOI] [Google Scholar]
- 45.Song Y, Dzierzewski JM, Fung CH, Rodriguez JC, Jouldjian S, Mitchell MN, et al. Association between sleep and physical function in older veterans in an adult day healthcare program. J Am Geriatr Soc. 2015;63(8):1622–1627. doi: 10.1111/jgs.13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semyachkina-Glushkovskaya O, Kurths J, Borisova E, Sokolovski S, Mantareva V, Angelov I, et al. Photodynamic opening of blood-brain barrier. Biomed Opt Exp. 2017;8(11):5040–5048. doi: 10.1364/BOE.8.005040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellettato CM, Scarpa M. Possible strategies to cross the blood–brain barrier. Ital J Pediatr. 2018;44(2):127–133. doi: 10.1186/s13052-018-0563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maeda H, Tsukigawa K, Fang JJM. A retrospective 30 years after discovery of the enhanced permeability and retention effect of solid tumors: next‐generation chemotherapeutics and photodynamic therapy—problems, solutions, and prospects. Microcirculation. 2016;23(3):173–182. doi: 10.1111/micc.12228. [DOI] [PubMed] [Google Scholar]
- 49.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387. [PubMed] [Google Scholar]
- 50.Nakata H, Kikuchi Y, Tode T, Hirata J, Kita T, Ishii K, et al. Inhibitory effects of ginsenoside Rh2 on tumor growth in nude mice bearing human ovarian cancer cells. Jap J Cancer Res. 1998;89(7):733–740. doi: 10.1111/j.1349-7006.1998.tb03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang J, Liao L, Yin H, Nakamura H, Shin T, Maeda H. Enhanced bacterial tumor delivery by modulating the EPR effect and therapeutic potential of Lactobacillus casei. J Pharma Sci. 2014;103(10):3235–3243. doi: 10.1002/jps.24083. [DOI] [PubMed] [Google Scholar]
- 52.Parodi A, Rudzińska M, Deviatkin AA, Soond SM, Baldin AV, Zamyatnin AA. Established and emerging strategies for drug delivery across the blood-brain barrier in brain cancer. Pharmaceutics. 2019;11(5):245. doi: 10.3390/pharmaceutics11050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arvanitis CD, Ferraro GB, Jain RK. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parrish K, Sarkaria JN, Elmquist WF. Improving drug delivery to primary and metastatic brain tumors: strategies to overcome the blood–brain barrier. Clin Pharmacol Ther. 2015;97(4):336–346. doi: 10.1002/cpt.71. [DOI] [PubMed] [Google Scholar]
- 55.Hao Y, Wang L, Zhao Y, Meng D, Li D, Li H, et al. Targeted imaging and chemo‐phototherapy of brain cancer by a multifunctional drug delivery system. Macromol Biosci. 2015;15(11):1571–1585. doi: 10.1002/mabi.201500091. [DOI] [PubMed] [Google Scholar]
- 56.Ding S, Khan AI, Cai X, Song Y, Lyu Z, Du D, et al. Overcoming blood–brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater Today. 2020;37:112. doi: 10.1016/j.mattod.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demeule M, Regina A, Che C, Poirier J, Nguyen T, Gabathuler R, et al. Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther. 2008;324(3):1064–1072. doi: 10.1124/jpet.107.131318. [DOI] [PubMed] [Google Scholar]
- 58.Huang T, Hlavaty J, Ostertag D, Espinoza F, Martin B, Petznek H, et al. Toca 511 gene transfer and 5-fluorocytosine in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 2013;20(10):544–551. doi: 10.1038/cgt.2013.51. [DOI] [PubMed] [Google Scholar]
- 59.Katakowski M, Chopp M. Exosomes as tools to suppress primary brain tumor. Cell Mol Neurobiol. 2016;36(3):343–352. doi: 10.1007/s10571-015-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lundy DJ, Nguyễn H, Hsieh PC. Emerging nano-carrier strategies for brain tumor drug delivery and considerations for clinical translation. Pharmaceutics. 2021;13(8):1193. doi: 10.3390/pharmaceutics13081193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghaemmaghami AB, Mahjoubin-Tehran M, Movahedpour A, Morshedi K, Sheida A, Taghavi SP, et al. Role of exosomes in malignant glioma: microRNAs and proteins in pathogenesis and diagnosis. Cell Comm Sig. 2020;18:1–19. doi: 10.1186/s12964-020-00623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie X, Xiong Y, Panayi AC, Hu L, Zhou W, Xue H, et al. Exosomes as a novel approach to reverse osteoporosis: a review of the literature. Front Bioeng Biotechnol. 2020;8:594247. doi: 10.3389/fbioe.2020.594247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huda MN, Nafiujjaman M, Deaguero IG, Okonkwo J, Hill ML, Kim T, et al. Potential Use of exosomes as diagnostic biomarkers and in targeted drug delivery: progress in clinical and preclinical applications. ACS Biomat Sci Eng. 2021;7(6):2106. doi: 10.1021/acsbiomaterials.1c00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurian TK, Banik S, Gopal D, Chakrabarti S, Mazumder N. Elucidating methods for isolation and quantification of exosomes: a review. Mol Biotechnol. 2021;63:249. doi: 10.1007/s12033-021-00300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Najafi S, Zarch SMA, Majidpoor J, Pordel S, Aghamiri S, Rasul MF et al (2022) Recent insights into the roles of circular RNAs in human brain development and neurologic diseases. Int J Biol Macromol. 10.1016/j.ijbiomac.2022.11.166 [DOI] [PubMed]
- 66.Movahedpour A, Khatami SH, Karami N, Vakili O, Naeli P, Jamali Z, et al. Exosomal noncoding RNAs in prostate cancer. Clinica Chimica Acta. 2022;537:127. doi: 10.1016/j.cca.2022.10.018. [DOI] [PubMed] [Google Scholar]
- 67.Sadri Nahand J, Moghoofei M, Salmaninejad A, Bahmanpour Z, Karimzadeh M, Nasiri M, et al. Pathogenic role of exosomes and microRNAs in HPV‐mediated inflammation and cervical cancer: a review. Int J Cancer. 2020;146(2):305–320. doi: 10.1002/ijc.32688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science 367(6478):eaau6977 [DOI] [PMC free article] [PubMed]
- 69.Duan L, Xu L, Xu X, Qin Z, Zhou X, Xiao Y, et al. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13(3):1387–1397. doi: 10.1039/D0NR07622H. [DOI] [PubMed] [Google Scholar]
- 70.Rezabakhsh A, Sokullu E, Rahbarghazi R. Applications, challenges and prospects of mesenchymal stem cell exosomes in regenerative medicine. Stem Cell Res Ther. 2021;12:1–8. doi: 10.1186/s13287-021-02596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li X, Corbett AL, Taatizadeh E, Tasnim N, Little JP, Garnis C, et al. Challenges and opportunities in exosome research—perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3(1):011503. doi: 10.1063/1.5087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bashyal S, Thapa C, Lee S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J Controlled Rel. 2022;348:723–744. doi: 10.1016/j.jconrel.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 73.Allegra A, Petrarca C, Di Gioacchino M, Casciaro M, Musolino C, Gangemi S. Exosome-mediated therapeutic strategies for management of solid and hematological malignancies. Cells. 2022;11(7):1128. doi: 10.3390/cells11071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Souderjani AH, Rahimi F, Amoabediny G, Didandeh M, Montazeri M. Applications of exosomes in nanomedicine. Elsevier; 2023. pp. 407–414. [Google Scholar]
- 75.Gutierrez‐Millan C, Calvo Díaz C, Lanao JM, Colino CI. Advances in exosomes‐based drug delivery systems. Macromol Biosci. 2021;21(1):2000269. doi: 10.1002/mabi.202000269. [DOI] [PubMed] [Google Scholar]
- 76.Chen B-Y, Sung CW-H, Chen C, Cheng C-M, Lin DP-C, Huang C-T, et al. Advances in exosomes technology. Clinica Chimica Acta. 2019;493:14–19. doi: 10.1016/j.cca.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 77.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iwai K, Minamisawa T, Suga K, Yajima Y, Shiba K. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. J Extracell Ves. 2016;5(1):30829. doi: 10.3402/jev.v5.30829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu M, Yang Q, Sun X, Wang Y. Recent advancements in the loading and modification of therapeutic exosomes. Front Bioeng Biotechnol. 2020;8:586130. doi: 10.3389/fbioe.2020.586130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu S, Wang Y, Xia X, Zheng JC. Exosome engineering: Current progress in cargo loading and targeted delivery. Nano Impact. 2020;20:100261. [Google Scholar]
- 81.Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomed: Nanotechnol, Biol Med. 2018;14(1):195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 82.Mostafazadeh M, Samadi N, Kahroba H, Baradaran B, Haiaty S, Nouri M. Potential roles and prognostic significance of exosomes in cancer drug resistance. Cell Biosci. 2021;11(1):1–15. doi: 10.1186/s13578-020-00515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharma Res. 2015;32(6):2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gourlay J, Morokoff A, Luwor R, Zhu H-J, Kaye A, Stylli S. The emergent role of exosomes in glioma. J Clin Neurosci. 2017;35:13–23. doi: 10.1016/j.jocn.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y, Jin Y, Wu N. Role of tumor-derived extracellular vesicles in glioblastoma. Cells. 2021;10(3):512. doi: 10.3390/cells10030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Y, Zhang Y, Gong H, Luo S, Cui Y. The role of exosomes and their applications in cancer. Int J Mol Sci. 2021;22(22):12204. doi: 10.3390/ijms222212204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mole Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tian T, Zhang H-X, He C-P, Fan S, Zhu Y-L, Qi C, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 89.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mole Ther. 2011;19(10):1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle-and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Contr Rel. 2015;220:727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 91.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed : Nanotechnol , Biol Med. 2016;12(3):655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y-J, Wu J-Y, Wang J-M, Hu X-B, Cai J-X, Xiang D-X. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomat. 2020;101:519–530. doi: 10.1016/j.actbio.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 93.Haney MJ, Zhao Y, Jin YS, Li SM, Bago JR, Klyachko NL, et al. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J Neuroimm Pharmacol. 2020;15(3):487–500. doi: 10.1007/s11481-019-09884-9. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y, Bai L, Guo K, Jia Y, Zhang K, Liu Q, et al. Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics. 2019;9(18):5261. doi: 10.7150/thno.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tran PH, Wang T, Yin W, Tran TT, Nguyen TN, Lee B-J, et al. Aspirin-loaded nanoexosomes as cancer therapeutics. Int J Pharma. 2019;572:118786. doi: 10.1016/j.ijpharm.2019.118786. [DOI] [PubMed] [Google Scholar]
- 96.Luo C, Hu X, Peng R, Huang H, Liu Q, Tan W. Biomimetic carriers based on giant membrane vesicles for targeted drug delivery and photodynamic/photothermal synergistic therapy. ACS Appl Mat Inter. 2019;11(47):43811–43819. doi: 10.1021/acsami.9b11223. [DOI] [PubMed] [Google Scholar]
- 97.Gomari H, Moghadam MF, Soleimani M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. OncoTargets Ther. 2018;11:5753. doi: 10.2147/OTT.S173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gong C, Tian J, Wang Z, Gao Y, Wu X, Ding X, et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J Nanobiotechnol. 2019;17(1):1–18. doi: 10.1186/s12951-019-0526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed Nanotechnol, Biol Med. 2017;13(5):1627–1636. doi: 10.1016/j.nano.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Aqil F, Kausar H, Agrawal AK, Jeyabalan J, Kyakulaga A-H, Munagala R, et al. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp Mole Pathol. 2016;101(1):12–21. doi: 10.1016/j.yexmp.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 101.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Comm. 2012;3(1):1–11. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 103.Kanchanapally R, Deshmukh SK, Chavva SR, Tyagi N, Srivastava SK, Patel GK, et al. Drug-loaded exosomal preparations from different cell types exhibit distinctive loading capability, yield, and antitumor efficacies: a comparative analysis. Int J Nanomed. 2019;14:531. doi: 10.2147/IJN.S191313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Contr Rel. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 105.Wang J, Yeung BZ, Cui M, Peer CJ, Lu Z, Figg WD, et al. Exosome is a mechanism of intercellular drug transfer: application of quantitative pharmacology. J Contr Rel. 2017;268:147–158. doi: 10.1016/j.jconrel.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang H, Sui H, Zheng Y, Jiang Y, Shi Y, Liang J, et al. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale. 2019;11(15):7481–7496. doi: 10.1039/C9NR01255A. [DOI] [PubMed] [Google Scholar]
- 107.Hadla M, Palazzolo S, Corona G, Caligiuri I, Canzonieri V, Toffoli G, et al. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine. 2016;11(18):2431–2441. doi: 10.2217/nnm-2016-0154. [DOI] [PubMed] [Google Scholar]
- 108.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 109.Zhu L, Wang C, Pang D-W, Zhang Z-L. Controlled release of therapeutic agents with near-infrared laser for synergistic photochemotherapy toward cervical cancer. Anal Chem. 2019;91(10):6555–6560. doi: 10.1021/acs.analchem.8b05982. [DOI] [PubMed] [Google Scholar]
- 110.Lee H, Park H, Noh GJ, Lee ES. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohyd Polym. 2018;202:323–333. doi: 10.1016/j.carbpol.2018.08.141. [DOI] [PubMed] [Google Scholar]
- 111.Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Contr Rel. 2018;287:156–166. doi: 10.1016/j.jconrel.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 112.Aili Y, Maimaitiming N, Mahemuti Y, Qin H, Wang Y, Wang Z. The role of exosomal miRNAs in glioma: biological function and clinical application. Front Oncol. 2021;11:686369. doi: 10.3389/fonc.2021.686369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu Q, Ling X, Yang Y, Zhang J, Li Q, Niu X, et al. Embryonic stem cells‐derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv Sci. 2019;6(6):1801899. doi: 10.1002/advs.201801899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang X, Zhao L, Wu J, Dong H, Xu F, Gong G, et al. Current advances in vehicles for brain gene delivery. Curr Gene Ther. 2012;12(5):423–436. doi: 10.2174/156652312802762590. [DOI] [PubMed] [Google Scholar]
- 115.Peng Z, Liu C, Wu M. New insights into long noncoding RNAs and their roles in glioma. Mole Cancer. 2018;17(1):1–10. doi: 10.1186/s12943-018-0812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sakr M, Takino T, Sabit H, Nakada M, Li Z, Sato H. miR-150-5p and miR-133a suppress glioma cell proliferation and migration through targeting membrane-type-1 matrix metalloproteinase. Gene. 2016;587(2):155–162. doi: 10.1016/j.gene.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 117.Li Z, Ye L, Wang L, Quan R, Zhou Y, Li X. Identification of miRNA signatures in serum exosomes as a potential biomarker after radiotherapy treatment in glioma patients. Ann Diag Pathol. 2020;44:151436. doi: 10.1016/j.anndiagpath.2019.151436. [DOI] [PubMed] [Google Scholar]
- 118.Lang FM, Hossain A, Gumin J, Momin EN, Shimizu Y, Ledbetter D, et al. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro-ogy. 2018;20(3):380–390. doi: 10.1093/neuonc/nox152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim R, Lee S, Lee J, Kim M, Kim WJ, Lee HW, et al. Exosomes derived from microRNA-584 transfected mesenchymal stem cells: novel alternative therapeutic vehicles for cancer therapy. BMB Rep. 2018;51(8):406. doi: 10.5483/BMBRep.2018.51.8.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bryniarski K, Ptak W, Jayakumar A, Püllmann K, Caplan MJ, Chairoungdua A, et al. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J Allergy Clin Immunol. 2013;132(1):170–181. doi: 10.1016/j.jaci.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 122.Kosaka N, Iguchi H, Yoshioka Y, Hagiwara K, Takeshita F, Ochiya T. Competitive interactions of cancer cells and normal cells via secretory microRNAs. J Biol Chem. 2012;287(2):1397–1405. doi: 10.1074/jbc.M111.288662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell–derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther-Nuc Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohno S-i, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Banizs AB, Huang T, Dryden K, Berr SS, Stone JR, Nakamoto RK, et al. In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int J Nanomed. 2014;9:4223. doi: 10.2147/IJN.S64267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, et al. Epigenetic regulation of connective tissue growth factor by MicroRNA‐214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59(3):1118–1129. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8(1):1–11. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, et al. Interleukin 3-receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics. 2017;7(5):1333. doi: 10.7150/thno.17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kojima R, Bojar D, Rizzi G, Hamri GC-E, El-Baba MD, Saxena P, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Comm. 2018;9(1):1–10. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stamatikos A, Knight E, Vojtech L, Bi L, Wacker BK, Tang C, et al. Exosome-mediated transfer of anti-miR-33a-5p from transduced endothelial cells enhances macrophage and vascular smooth muscle cell cholesterol efflux. Human Gene Ther. 2020;31(3-4):219–232. doi: 10.1089/hum.2019.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baldari S, Di Rocco G, Magenta A, Picozza M, Toietta G. Extracellular vesicles–encapsulated MicroRNA-125b produced in genetically modified Mesenchymal stromal cells inhibits hepatocellular carcinoma cell proliferation. Cells. 2019;8(12):1560. doi: 10.3390/cells8121560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei Z, Qiao S, Zhao J, Liu Y, Li Q, Wei Z, et al. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci. 2019;232:116632. doi: 10.1016/j.lfs.2019.116632. [DOI] [PubMed] [Google Scholar]
- 134.Chen Q, Liu Y, Ding X, Li Q, Qiu F, Wang M, et al. Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Mol Cell Biochem. 2020;465(1):103–114. doi: 10.1007/s11010-019-03671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang J-H, Xu Y, Yin X-M, Lin F-Y. Exosomes derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience. 2020;424:133–145. doi: 10.1016/j.neuroscience.2019.10.043. [DOI] [PubMed] [Google Scholar]
- 136.Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, et al. Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci. 2018;5(4):1700611. doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jeong K, Yu YJ, You JY, Rhee WJ, Kim JA. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab on a Chip. 2020;20(3):548–557. doi: 10.1039/C9LC00958B. [DOI] [PubMed] [Google Scholar]
- 138.Nie H, Xie X, Zhang D, Zhou Y, Li B, Li F, et al. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale. 2020;12(2):877–887. doi: 10.1039/C9NR09011H. [DOI] [PubMed] [Google Scholar]
- 139.Pi F, Binzel DW, Lee TJ, Li Z, Sun M, Rychahou P, et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat Nanotechnol. 2018;13(1):82–89. doi: 10.1038/s41565-017-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wahlgren J, Karlson TDL, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nuc Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Comm Sig. 2013;11(1):1–10. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Izco M, Blesa J, Schleef M, Schmeer M, Porcari R, Al-Shawi R, et al. Systemic exosomal delivery of shRNA minicircles prevents parkinsonian pathology. Mol Ther. 2019;27(12):2111–2122. doi: 10.1016/j.ymthe.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jhan Y-Y, Prasca-Chamorro D, Zuniga GP, Moore DM, Kumar SA, Gaharwar AK, et al. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. Int J Pharma. 2020;573:118802. doi: 10.1016/j.ijpharm.2019.118802. [DOI] [PubMed] [Google Scholar]
- 144.Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnol. 2020;18(1):1–15. doi: 10.1186/s12951-019-0560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oskuee RK, Jaafari MR. Delivery of LNA-antimiR-142-3p by mesenchymal stem cells-derived exosomes to breast cancer stem cells reduces tumorigenicity. Stem Cell Rev Rep. 2020;16(3):541–556. doi: 10.1007/s12015-019-09944-w. [DOI] [PubMed] [Google Scholar]
- 146.Kim G, Kim M, Lee Y, Byun JW, Lee M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J Contr Rel. 2020;317:273–281. doi: 10.1016/j.jconrel.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 147.Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporatin. Nat Biomed Eng. 2020;4(1):69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y, Chen X, Tian B, Liu J, Yang L, Zeng L, et al. Nucleolin-targeted extracellular vesicles as a versatile platform for biologics delivery to breast cancer. Theranostics. 2017;7(5):1360. doi: 10.7150/thno.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Skog J, Würdinger T, Van Rijn S, Meijer DH, Gainche L, Curry WT, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pocsfalvi G, Mammadova R, Juarez APR, Bokka R, Trepiccione F, Capasso G. COVID-19 and extracellular vesicles: An intriguing interplay. Kidney Blood Pres Res. 2020;45(5):661–670. doi: 10.1159/000511402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tyumina O. Safety and Efficiency of Method of Exosome Inhalation in COVID-19 Associated Pneumonia. 2020. [Google Scholar]
- 153.Chen Y-S, Lin E-Y, Chiou T-W, Harn H-J. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu-Chi Med J. 2020;32(2):113. doi: 10.4236/cm.2020.113007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 648 kb)
Data Availability Statement
Not applicable.