Abstract
Opioid withdrawal’s physiological effects are a major impediment to recovery from opioid use disorder (OUD). Prior work has demonstrated that transcutaneous cervical vagus nerve stimulation (tcVNS) can counteract some of opioid withdrawal’s physiological effects by reducing heart rate and perceived symptoms. The purpose of this study was to assess the effects of tcVNS on respiratory manifestations of opioid withdrawal – specifically, respiratory timings and their variability. Patients with OUD (N = 21) underwent acute opioid withdrawal over the course of a two-hour protocol. The protocol involved opioid cues to induce opioid craving and neutral conditions for control purposes. Patients were randomly assigned to receive double-blind active tcVNS (n = 10) or sham stimulation (n = 11) throughout the protocol. Respiratory effort and electrocardiogram-derived respiration signals were used to estimate inspiration time (Ti), expiration time (Te), and respiration rate (RR), along with each measure’s variability quantified via interquartile range (IQR). Comparing the active and sham groups, active tcVNS significantly reduced IQR(Ti) – a variability measure – compared to sham stimulation (p = .02). Relative to baseline, the active group’s median change in IQR(Ti) was 500 ms less than the sham group’s median change in IQR(Ti). Notably, IQR(Ti) was found to be positively associated with post-traumatic stress disorder symptoms in prior work. Therefore, a reduction in IQR(Ti) suggests that tcVNS downregulates the respiratory stress response associated with opioid withdrawal. Although further investigations are necessary, these results promisingly suggest that tcVNS – a non-pharmacologic, non-invasive, readily implemented neuromodulation approach – can serve as a novel therapy to mitigate opioid withdrawal symptoms.
Keywords: opioid use disorder, non-invasive, vagus nerve stimulation, respiration, variability
I. Introduction
The opioid epidemic has taken a considerable toll on the United States. In 2021 alone, over 100,000 people lost their lives due to drug overdoses, the majority secondary to opioids [1]. A major impediment to recovery from opioid use disorder (OUD) is opioid withdrawal, where severe behavioral and physiological symptoms are experienced due to abstinence from opioids. In fact, the avoidance of withdrawal symptoms remains a primary factor for long-term opioid use [2]. Although medication for OUD can help, the barriers to care remain high [3]–[5]. New paradigms are therefore necessary for managing withdrawal in the treatment of OUD.
In recent work, our group demonstrated that transcutaneous cervical vagus nerve stimulation (tcVNS) reduces perceived withdrawal symptoms, pain, and distress in comparison to sham stimulation [6]. Notably, this double-blind study was performed in patients with OUD undergoing acute opioid withdrawal. We also observed a significant reduction in heart rate but a reduction in craving that was nonsignificant, suggesting that the dominant mechanism for withdrawal symptom reduction was via the autonomic nervous system rather than via executive control. In particular, tcVNS may counteract withdrawal’s effects by reducing sympathetic arousal (“fight or flight”) and/or increasing parasympathetic activity (“rest and digest”) (Fig. 1). However, the necessity exists to investigate other physiological endpoints to study this hypothesis further.
Fig. 1.
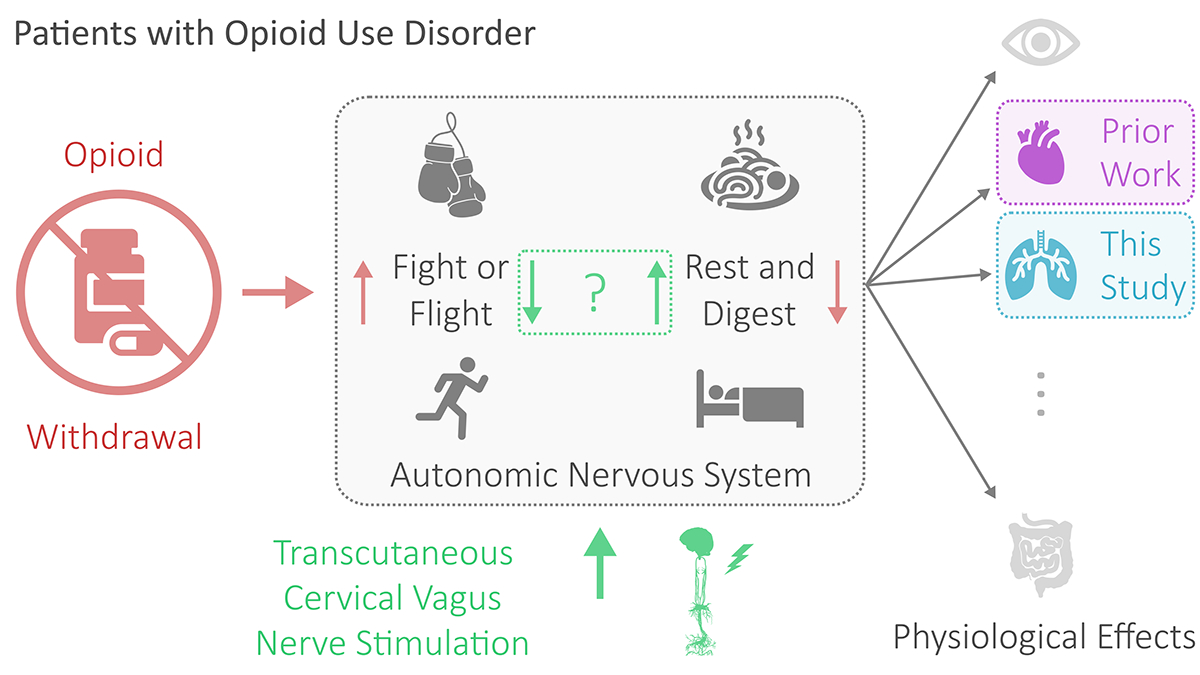
Overview of the work. For patients with opioid use disorder, opioid withdrawal manifests physiologically via the autonomic nervous system. Manifestations include changes in the pupillary system, cardiovascular system, respiratory system, and/or gastrointestinal system. Prior work investigated the cardiac effects of transcutaneous cervical vagus nerve stimulation–believed to reduce fight or flight and increase rest and digest–in the context of opioid withdrawal. This study investigates the respiratory effects.
In this study, we build upon this prior work by analyzing the respiratory effects of tcVNS in the context of opioid withdrawal. The premise of this investigation is illustrated in Fig. 1. To thoroughly assess the effects of tcVNS on respiratory physiology, we compare tcVNS and sham stimulation effects not only on respiration rate, but also on inspiration time, expiration time, and their variability. We have found in recent work that inspiration time, expiration time, and respiration pattern variability (RPV) are potent markers of varying autonomic nervous system activity (e.g., induced by tcVNS, traumatic stressors, etc.) [7]–[9]. This paper contributes to the literature by detailing the first analysis of tcVNS-induced respiratory effects in patients with OUD experiencing opioid withdrawal.
II. Methods
A. Study Cohort
As part of a study approved by the Institutional Review Boards of Emory University (IRB00117320) and the Georgia Institute of Technology (H20203), N = 21 patients with OUD (6 females) with a mean age of 35 (SD 11) years and body mass index of 28.1 (SD 7.3) were recruited and written informed consent was obtained. All patients underwent acute opioid withdrawal during the study by abstaining from opioid use for a minimum of eight hours prior to study onset. Participants wore masks to protect against Coronavirus Disease 2019 (COVID-19) and remained seated in a reclining chair throughout. For further details, please see Gazi et al. [6].
B. Experimental Protocol
The experimental protocol is illustrated on the right in Fig. 2. Neutral videos lasted approximately 90–120 s and consisted of a mailwoman describing her job to elicit neutral affect for control purposes (baseline). Opioid cue audios lasted approximately 240–300 s and consisted of a guided breathing exercise followed by instructions to recollect prior opioid use in as vivid a manner as possible. Opioid cue videos lasted approximately 90–120 s and contained snippets of opioid imagery and use. Opioid cue audios and videos were designed according to prior research to elicit opioid craving [10].
Fig. 2.
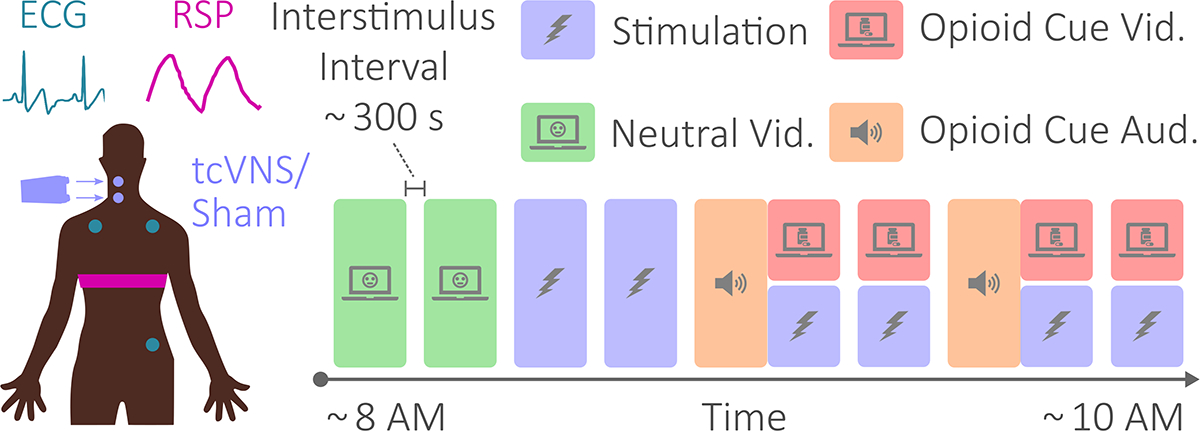
Physiological sensing, stimulation, and protocol. The electrocardiogram and respiratory effort signals were measured at the locations shown on the left. Transcutaneous cervical vagus nerve stimulation (tcVNS) or sham stimulation was administered targeting the right carotid artery. The diagram on the right illustrates the protocol’s chronology. Protocol conditions included neutral videos, stimulation, and opioid cue audio and videos. The protocol lasted approximately two hours. Interstimulus intervals, illustrated using empty space between conditions, lasted approximately five minutes.
Conforming to a double-blind protocol (ClinicalTrials.gov NCT04556552), either active tcVNS (n = 10) or sham stimulation (n = 11) was delivered to the right cervical vagus for 120 s simultaneously with each of the opioid cue videos, as well as twice separately to assess stimulation-specific effects. The active and sham devices (gammaCore, electroCore, Basking Ridge, NJ, USA) were indistinguishable and operated identically, differing only in output voltage waveform.
C. Physiological Sensing
Respiratory effort (RSP) and electrocardiogram (ECG) signals were measured at the locations shown on the left in Fig. 2. RSP was transduced using a belt worn around the chest that measured thoracic expansion and contraction, while ECG was measured using adhesive Ag/AgCl electrodes in a three-lead configuration. Data were transmitted using the Biopac RSPECR system (Biopac Systems, Goleta, CA, USA) and acquired at 2 kHz using the Biopac MP150 data acquisition system.
D. Respiration Signal Processing
The respiration signal processing and feature extraction pipeline is illustrated in Fig. 3, along with example data from a patient in the active group. ECG signals were processed as in prior work to extract two ECG-derived respiration signals: the respiration-induced intensity variation (RIIV) and respiration-induced amplitude variation (RIAV) [7]. The RSP, RIIV, and RIAV signals were then linearly resampled to 50 Hz with a finite impulse response (FIR) antialiasing low-pass filter. This was followed by FIR band-pass filtering (0.1 – 0.72 Hz) [7]. The three filtered signals produced were then segmented into 60 s windows with 2 s overlap for peak detection via adaptive thresholding; peak detection thresholds were kept equivalent as in prior work [7]. The minimum value between each pair of peaks was treated as the end of the first breath’s expiration and the beginning of the second breath’s inspiration. Expiration (Te) and inspiration times Ti were then computed as the time difference between peak and subsequent minimum and the time difference between peak and previous minimum, respectively. Respiration rate (RR) was computed by taking the reciprocal of the time difference between pairs of peaks.
Fig. 3.
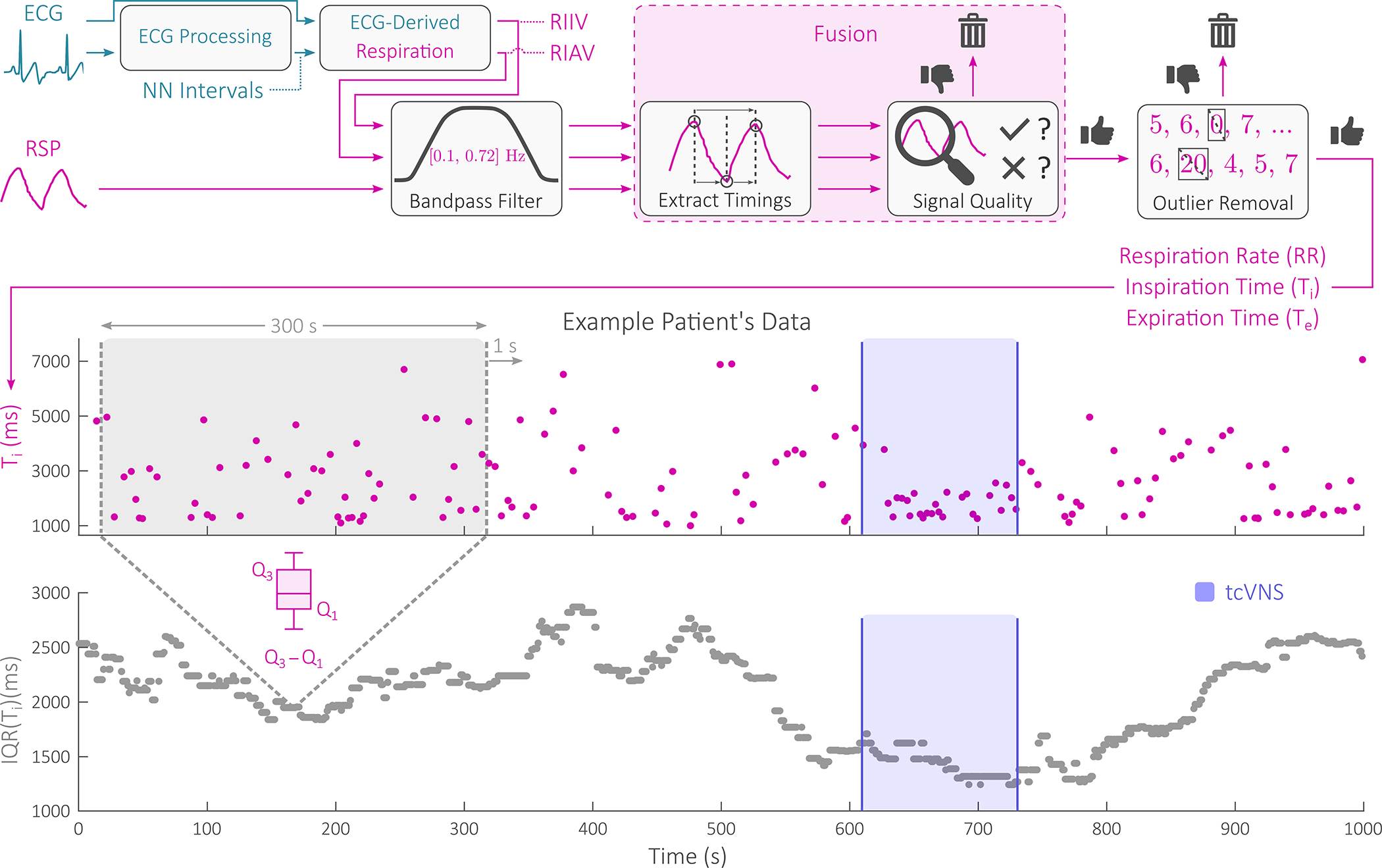
Signal processing and feature extraction block diagram, with example data shown. Two electrocardiogram (ECG)-derived respiration signals were fused with the respiratory effort (RSP) signal for respiration rate, inspiration time, and expiration time estimation. From these three measures, an additional three variability measures were extracted by computing an interquartile range (IQR) time series for each on a 300 s moving window basis with a 299 s overlap. Example patient data are shown before, during, and after an example administration of transcutaneous cervical vagus nerve stimulation (tcVNS).
Feature-level fusion of RR, Ti, and Te and quality assessment were then performed by returning back to the RSP, RIIV, and RIAV signals themselves. These signals were first resampled to 4 Hz and equivalently bandpass filtered (0.1 – 0.72 Hz). Two respiration quality indices (RQIs) were then computed for each 16 s non-overlapping window of data using a power spectral density-based RQI and an autocorrelation-based RQI. These two RQIs were then averaged to compute window’s final RQI [7]. For each 16 s window, if all three signals’ final RQIs fell below an empirically tuned threshold of 0.4, the window’s features were rejected. Otherwise, the features extracted from the signal with maximum RQI were stored [7]. For windows with ECG data deemed unusable via signal quality assessment, only the RSP signal was considered. The aforementioned feature extraction along with the described respiration signal quality assessment and fusion process produced a single set of RR, Ti, and Te for each patient. Outliers were then removed using the default thresholds of prior work to produce a final set of RR, Ti, and Te used for all subsequent analysis [7].
E. Respiration Pattern Variability Extraction
With the RR, Ti, and Te time series, RPV was extracted by computing the interquartile range (IQR) with a moving window. This produced three RPV time series used in subsequent analysis: IQR(Ti), IQR(Te), and IQR(RR). As in prior work [7] and as shown in Fig. 3, a 300 s window was used with 299 s overlap, centered with respect to the RPV datapoint produced.
F. Active vs. Sham Analysis
Stimulation effects on Ti, Te, RR, IQR(Ti), IQR(Te), and IQR(RR) were compared between active and sham groups across all administrations. For each participant, for each feature, the average value during the second neutral video served as baseline, as in prior work [6]. This baseline was subtracted from the average value during each stimulation administration to produce a Δ feature value for each stimulation. The final stimulation’s average Δ values were statistically compared between the active and sham groups. This was to capture the overall effects of repeated tcVNS vs. sham stimulation in the context of the 2-hour opioid withdrawal protocol.
Statistical tests were performed treating device group (active vs. sham) as the independent variable and each of the six respiration features as dependent variables. The final stimulation’s Δ feature values were compared using unpaired t-tests or Mann-Whitney U tests for normally and non-normally distributed variables, respectively. Normality was assessed using the Shapiro-Wilk test. All statistical tests were two tailed and performed with a significance level of α = .05.
III. Results
Fig. 4 depicts this study’s primary finding. Specifically, ΔIQR(Ti) – an RPV measure – was significantly lower during the final stimulation for the active group (mean ± SD: −368.5 ± 514.1 ms) than the sham group (74.2 ± 253.1 ms). The effect size for this comparison was d = 1.11; t = 2.54; p = .02.
Fig. 4.
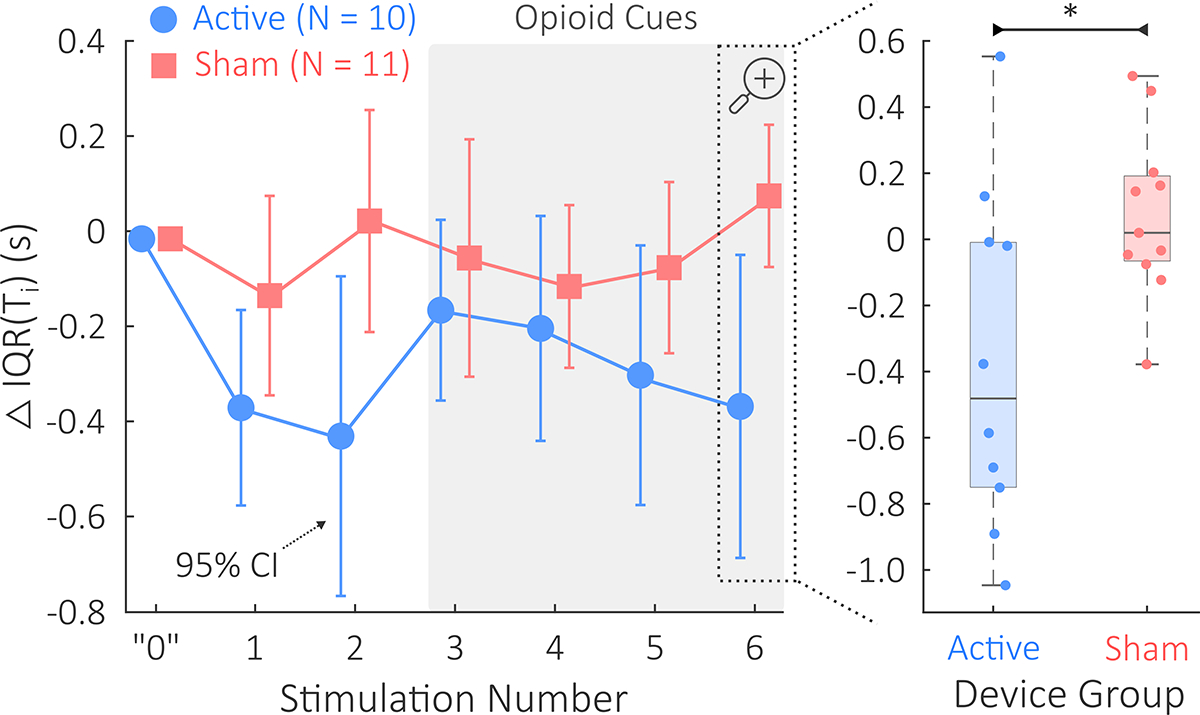
This study’s primary finding: IQR(Ti) comparison between the active tcVNS and sham stimulation groups. Δ denotes changes relative to baseline. On the left, mean ΔIQR(Ti) and 95% confidence intervals are shown during each of the six administrations of active or sham stimulation. The horizontal shift between active and sham data is used for the purpose of visualization; measurements were taken equivalently. A gray background is used to highlight opioid cue presentation during stimulations 3–6. The “0” shown represents baseline. On the right, we see that the final ΔIQR(Ti) was significantly lower in the active group compared to the sham group (p < .05, denoted by *).Horizontal jitter is used to overlay the patient-specific datapoints.
None of the remaining five measures significantly differed between groups during the final stimulation. ΔIQR(Te) did not significantly differ (d = 0.21, t = 0.47, p = .64) between the active group (−39.5 ± 458.0 ms) and the sham group (38.4 ± 289.0 ms); and ΔIQR(RR) did not significantly differ (d = 0.79, t = 1.81, p = .09) between the active group (1.56 ± 1.67 bpm) and the sham group (0.21 ± 1.75 bpm). ΔTi did not significantly differ (f = .67, U = 36, p = .20) between the active group (−408.7 ± 539.5 ms) and the sham group (−83.3 ± 294.0 ms); ΔTe did not significantly differ (d = 0.25, t = 0.58, p = .57) between the active group (−101.2 ± 399.5 ms) and the sham group (10.0 ± 475.2 ms); and ΔRR did not significantly differ (d = 0.12, t = 0.27, p = .79) between the active group (1.09 ± 2.29 bpm) and the sham group (0.76 ± 3.28 bpm).
IV. Discussion
This double-blind study of tcVNS found reductions in respiratory variability compared to sham stimulation in patients with OUD undergoing acute opioid withdrawal. Specifically, a significant reduction in IQR(Ti) was observed following a 2-hour opioid cue protocol for patients receiving active tcVNS in comparison to those receiving sham stimulation. Interestingly, the effects of tcVNS seemed to gradually accumulate over the course of the protocol, with the exception of the initial opioid cue presentation (i.e., stimulation 3). During each stimulation, the active group’s mean ΔIQR(Ti) remained lower than the sham group’s mean ΔIQR(Ti), and the difference between the two groups steadily increased over time. These findings add to the evidence of tcVNS’s autonomic effects during opioid withdrawal (i.e., reduced heart rate) [6]. It also agrees with our prior observation of reduced respiratory variability following tcVNS in the context of traumatic stress [7].
tcVNS-induced reductions in respiratory variability suggest that tcVNS counteracts some of the respiratory manifestations of increased sympathetic arousal and decreased parasympathetic activity associated with opioid withdrawal. In prior work, increases in respiratory variability have corresponded with neural and psychological outcomes characteristic of traumatic stress [7], [8]. Physiological modeling studies have also demonstrated that decreased respiratory variability would imply decreased sympathetic arousal and increased parasympathetic activity [11]. Since opioid withdrawal induces the opposite effect on the autonomic nervous system, this study’s primary finding implicates respiratory variability as a respiratory biomarker of tcVNS’s autonomic effects when used to mitigate opioid withdrawal.
This study is not without limitations. The small sample size limited the statistical power of the analysis and increased margin of error. Future efforts should look to replicate this analysis in a larger sample of patients with OUD undergoing opioid withdrawal, bearing in mind the difficulty in recruitment for this clinical population. This study also focused only on the initial phase of opioid withdrawal; symptoms are known to progressively worsen. Longitudinal studies will be necessary to further our understanding of the respiratory effects of tcVNS and withdrawal over longer periods of abstinence. Future work should also explore alternate physiological measures pertinent to VNS and withdrawal (e.g., gastrointestinal measures).
V. Conclusion
In this double-blind study, we compared the effects of tcVNS and sham stimulation on RR, Ti, Te, and their variability in patients with OUD experiencing opioid withdrawal. At the end of a two-hour protocol involving opioid cues, the 10 patients who received tcVNS exhibited reduced respiratory variability compared to the 11 in the sham group. This suggests that tcVNS downregulates some of the respiratory stress response associated with opioid withdrawal. Although further investigation is necessary to address the study’s small sample size and protocol’s short time course, these results promisingly suggest that tcVNS can act as a non-pharmacologic, non-invasive neuromodulation approach to mitigate opioid withdrawal symptoms. Given that withdrawal symptoms are a primary factor of long-term opioid use [2], this could ultimately disrupt the therapeutic landscape for OUD.
Acknowledgments
A. H. Gazi was supported by a National Science Foundation (NSF) Graduate Research Fellowship (DGE-2039655), and T. P. Lambert was supported by the National Institutes of Health (NIH) (UG3 DA048502-01A1S2). This research was supported by the NIH (UG3 DA048502 and R01HL155711), and in part, by the Julian T. Hightower Chair (C. J. Rozell) and the Linda J. and Mark C. Smith Chair (O. T. Inan) at the Georgia Institute of Technology.
Contributor Information
Asim H. Gazi, School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA, USA..
Anna B. Harrison, School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA, USA..
Tamara P. Lambert, Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA, USA.
Malik Obideen, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine..
Justine W. Welsh, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine.
Viola Vaccarino, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA, USA, and the Department of Medicine, Division of Cardiology, Emory University School of Medicine, Atlanta, GA, USA..
Amit J. Shah, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA, USA, and the Department of Medicine, Division of Cardiology, Emory University School of Medicine, Atlanta, GA, USA. Atlanta Veterans Affairs Health Care System, 1670 Clairmont Road, Decatur, GA, USA.
Sudie E. Back, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA.
Christopher J. Rozell, School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA, USA..
J. Douglas Bremner, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine.; Atlanta Veterans Affairs Health Care System, 1670 Clairmont Road, Decatur, GA, USA. Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, USA.
Omer T. Inan, School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA, USA.; Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA, USA.
References
- [1].“Drug Overdose Deaths in the U.S. Top 100,000 Annually.” [Online]. Available: https://bit.ly/cdcPressReleases2021
- [2].Cicero TJ and Ellis MS, “The prescription opioid epidemic: a review of qualitative studies on the progression from initial use to abuse,” Dialogues in Clinical Neuroscience, vol. 19, no. 3, p. 259, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Volkow ND et al. , “Medication-Assisted Therapies — Tackling the Opioid-Overdose Epidemic,” New England Journal of Medicine, vol. 370, no. 22, pp. 2063–2066, 2014. [DOI] [PubMed] [Google Scholar]
- [4].Beetham T et al. , “Access to Office-Based Buprenorphine Treatment in Areas With High Rates of Opioid-Related Mortality: An Audit Study,” Annals of internal medicine, vol. 171, no. 1, pp. 1–9, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sigmon SC et al. , “Opioid Detoxification and Naltrexone Induction Strategies: Recommendations for Clinical Practice,” The American journal of drug and alcohol abuse, vol. 38, no. 3, p. 187, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gazi AH et al. , “Transcutaneous Cervical Vagus Nerve Stimulation Reduces Behavioral and Physiological Manifestations of Withdrawal in Patients with Opioid Use Disorder: A Double-Blind, Randomized, Sham-Controlled Pilot Study,” under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].——, “Robust Estimation of Respiratory Variability Uncovers Correlates of Limbic Brain Activity and Transcutaneous Cervical Vagus Nerve Stimulation in the Context of Traumatic Stress,” IEEE Transactions on Biomedical Engineering, vol. 69, no. 2, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].——, “Association Between Posttraumatic Stress Disorder Symptoms and Respiratory Variability in the Context of Traumatic Stress: A Co-Twin Control Study,” under review. [Google Scholar]
- [9].——, “Transcutaneous Cervical Vagus Nerve Stimulation Lengthens Exhalation in the Context of Traumatic Stress,” in IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Back SE et al. , “Laboratory-induced cue reactivity among individuals with prescription opioid dependence,” Addictive behaviors, vol. 39, no. 8, pp. 1217–1223, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jaworski J and Bates JH, “Sources of breathing pattern variability in the respiratory feedback control loop,” Journal of Theoretical Biology, vol. 469, pp. 148–162, 2019. [DOI] [PubMed] [Google Scholar]


