Purpose of review
The purpose of this article is to provide an overview of currently recommended treatment approaches for anemia during pregnancy, with a special focus on iron deficiency and iron deficiency anemia (IDA).
Recent findings
As consistent patient blood management (PBM) guidelines in obstetrics are still lacking, recommendations regarding the timing of anemia screening and the treatment recommendations for iron deficiency and IDA during pregnancy are still controversial. Based on increasing evidence, early screening for anemia and iron deficiency should be recommended at the beginning of each pregnancy. To reduce maternal and fetal burden, any iron deficiency, even without anemia, should be treated as early as possible during pregnancy. While oral iron supplements administered every other day are the standard treatment in the first trimester, the use of intravenous iron supplements is increasingly suggested from the second trimester onwards.
Summary
The treatment of anemia, and more specifically iron deficiency anemia during pregnancy, holds many possibilities for improvement. The fact that the period of risk is known well in advance and thus there is a long optimization phase is per se an ideal prerequisite for the best possible therapy of treatable causes of anemia. Standardization of recommendations and guidelines for screening and treatment of IDA in obstetrics is required for the future. In any case, a multidisciplinary consent is the precondition for a successfully implementation of anemia management in obstetrics to establish an approved algorithm easily enabling detection and treatment of IDA during pregnancy.
Keywords: iron deficiency anemia, obstetrics, patient blood management, peripartum hemorrhage, pregnancy
INTRODUCTION
Nearly all modern concepts in preoperative anemia management in obstetrics based on patient blood management (PBM) strategies, aim to maintain hemoglobin concentration, optimize hemostasis, minimize blood loss, and limit blood transfusions during delivery to improve maternal and fetal outcomes. PBM is primarily described as a patient-focused, evidence-based, and multidisciplinary approach to optimize safety and care of patients who are at risk to require blood transfusion during surgical interventions and has recently been implemented in several medical areas according to the World Health Organization (WHO) recommendation in 2010 [1]. Recent studies demonstrated that adhering to a PBM algorithm significantly minimizes perioperative bleeding, reduces the requirement of blood transfusion, decreases perioperative morbidity, mortality, as well as length of stay, and costs [2]. However, implementation of PBM strategies in obstetrics is challenging and thus is still underrepresented in many hospitals, even though the high prevalence of iron deficiency and iron deficiency anemia (IDA) during pregnancy as well as the risk of peripartum hemorrhage (PPH) for every woman indicate that implementation of PBM in obstetrics is promising. Especially the long period of time between a possible detection of iron deficiency or IDA during pregnancy care and a potential blood loss at delivery represents an almost perfect prerequisite for the implementation of the first pillar of PBM in obstetrics, the detection and correction of antenatal anemia [3].
According to PBM strategies in surgical procedures with an estimated blood loss of >500 ml, PBM should be realized for any intervention where there is a probability of excessive bleeding, including vaginal delivery or nonelective caesarean section [4▪]. A multidisciplinary consensus is the precondition for a successfully implementation of PBM in obstetrics, involving midwives, resident gynecologists, obstetricians, anesthetists, and hematologists to establish an approved algorithm to easily enable detection and treatment of iron deficiency and IDA during pregnancy.
During the past years, several national and international guidelines for PPH management and PBM programs in obstetrics have been published [5,6]. However, comparing these guidelines, substantive inconsistencies could be observed [6]. Therefore, many obstetrical departments are still in need of reliable PBM recommendations for their daily practice that fit to their individual patient flow and current process of care [3].
Box 1.
no caption available
PERIPARTUM HEMORRHAGE
Although mortality rates from PPH have significantly declined in the developed countries over the last years [7], PPH remains one of the leading causes of maternal mortality worldwide, with an incidence of more than 15% [8] and contributes to maternal mortality ranging from 16% in developed regions to more than 30% in some African areas [9]. Usually, PPH is defined as a blood loss of >500 ml within 24 h during vaginal delivery or of >1000 ml during caesarean section [8,10,11]. However, the definition of PPH has recently been updated by the Committee on Practice Bulletins-Obstetrics defining PPH as a blood loss leading to signs of hypovolemia regardless of the method of delivery [12]. Apart from the measured or estimated blood loss, other signs (indicating shock) should also be considered in making the diagnosis [11].
Irrespective of which definition is used, severe PPH increases the risk of the need for red blood cell transfusions associated with potential complications for the mother [5,8,13▪]. This risk is increased in patients suffering from preexisting anemia.
Considering merely risk factors for PPH with an odds ratio >2, the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis (NATA) recently published the following list to be considered when assessing patients [5]:
multiple pregnancies (odds ratio [OR] 2.3-4.7);
a history of PPH (OR 3.3);
pregnancy-induced hypertension (OR 1.9-2.5);
chorioamnionitis (OR 2.5);
episiotomy (OR 1.4–2.2);
prelabor caesarean section (OR 1.3-2.3);
caesarean section during labor (OR 1.7–3.6);
macrosomia (OR 1.7–3.5);
operative vaginal delivery (OR 2.3).
In addition to the above, uterine atony, placental anomalies, preeclampsia, coagulopathies, and a preexisting anemia have been described as the most common obstetrical risk factors for PPH [3,8,10,13▪,14]. It is of utmost importance, however, to take into consideration that PPH can occur during every pregnancy and the majority of women developing a PPH have none of the described risk factors [3,8]. Even using a scoring system to identify women at risk often fails in daily practice due to their lack of reliability, thus there is need for further improvement [10,13▪]. In recent years, artificial intelligences and machine learning has been applied to perform an unbiased approach to hemorrhage risk prediction, which eventually may be superior to human risk assessment and represents an area for future research [15▪]. These facts emphasize the importance of a PBM algorithm in obstetrics, since a preexisting anemia, one of the commonest risk factors for PPH associated with maternal and fetal morbidities, can easily be assessed and corrected during pregnancy care.
IRON DEFICIENCY AND IRON DEFICIENCY ANEMIA DURING PREGNANCY
According to the WHO, the prevalence of anemia in the general population is about 25% worldwide, whereas significantly less people are affected in industrialized countries. In pregnant women, the prevalence of anemia is estimated to be about 42% worldwide and still around 25% in Europe [16]. In contrast to an Hb-threshold of 12 g/dl in nonpregnant women, anemia in pregnant women has been defined by the WHO as Hb-values <11 g/dl irrespective of the gestational age. In contrast to this definition, the Centers for Disease Control and Prevention (CDC) define anemia of pregnancy as Hb <11 g/dl during the first and third trimesters, and Hb <10.5 g/dl in the second trimester [17–19]. This definition relates to the recognition that the Hb-value is reduced by 0.5 g/dl between the third and sixth months of pregnancy [3]. However, these limits are based only on population-based distributions of Hb values and do not indicate whether it would not be more appropriate to treat anemia before it occurs in the presence of iron deficiency. After all, anemia is only a consequence of iron deficiency that usually develops late.
Globally, iron deficiency is the most common micronutrient deficiency and accordingly anemia during pregnancy is most commonly caused by iron deficiency [20▪▪]. Although the prevalence of isolated iron deficiency is unknown and still being researched, the results of recently published studies indicate that 42% of women in the first trimester had isolated iron deficiency [21] and even 75% of women had iron deficiency or IDA by the third trimester [22]. In general, iron deficiency, not only during pregnancy, remains underdiagnosed, understudied, and undertreated, in spite of its high prevalence and the associated negative effects on both mother and fetus [23]. Iron deficiency and IDA during pregnancy are known to increase maternal morbidity and mortality (Table 1). Maternal IDA is associated with preterm labor, placental abruption, preeclampsia, increased risk of infection, additional cardiovascular stress, fatigue, reduced physical and mental performance, headache, dizziness, prolonged hospitalization, reduced milk production, increased transfusion rates in PPH, increased risk of postpartum depression and even maternal death [20▪▪,24]. Fetal complications include a higher rate of premature birth, low birth weight, fetal distress, intrauterine growth retardation, unfavorable impact on placental development, memory disorders, intellectual disability, autism and reduced fetal iron stores [24,25]. According to Georgieff, newborns with iron deficiency have compromised recognition memory, slower speed of processing and poorer bonding that persists despite postnatal iron repletion [25]. Reduced concentration, cognition, and motor function are still detectable 25 years after delivery in children born with iron deficiency, compared to those born with sufficient iron stores [26]. Although there is growing evidence that fetal iron deficiency can affect the growth and function of multiple major organ systems, particularly the heart, muscles, gastrointestinal tract and the brain [20▪▪], only the SGGG (Swiss Society of Gynaecologist and Obstetrics) guideline recommends routine screening for iron deficiency for nonanemic first trimester women during antenatal care [27▪▪]. To reduce maternal complications caused by iron deficiency and IDA and to ensure the safety of fetal development, especially neurodevelopment during late gestation, early screening for anemia and iron deficiency at the beginning of each pregnancy should be strongly recommended.
Table 1.
| Maternal risks | Neonatal risks |
| • Preterm labor • Placental abruption • Preeclampsia • Increased risk of infection • Additional cardiovascular stress • Fatigue, headache, dizziness • Reduced mental performance • Prolonged hospitalization • Reduced milk production, • Increased transfusion rates in PPH • Increased risk of postpartum depression • Maternal death |
• Higher rate of premature birth • Low birth weight • Fetal distress • Intrauterine growth retardation • Unfavorable impact on placental development • Reduced fetal iron stores • Memory disorders • Autism • Intellectual disability |
IRON METABOLISM DURING PREGNANCY
The so-called physiological anemia of pregnancy is hypothesized to be an adaptive process serving the purpose to increase placental blood flow by a reduced maternal blood viscosity and to facilitate the oxygen and nutrient supplies to the fetus by increasing the erythrocyte mass. From the sixth week of pregnancy, the plasma volume increases disproportionately compared to the erythrocyte mass reaching a maximum around the 24th week of pregnancy. At this time, the plasma volume of the pregnant woman is about 40–50% higher than at the beginning of pregnancy, whereas the erythrocyte mass only increases by 15–25%, which explains the drop in Hb-values due to the dilutional effect [28].
Increased erythropoiesis is the main reason for increased iron requirements and is estimated at 500 mg during pregnancy. Approximately 350 mg of iron is required for fetal and placental development and 250 mg are associated with blood loss at delivery, which results in an extra maternal iron requirement of approximately 1 g over the entire course of pregnancy [20▪▪,28]. The iron requirement of pregnant women depends on the gestational age and increases from 0.8 mg/day in early pregnancy to 7.5 mg/day in late pregnancy, so that an average iron requirement of 4.4 mg/day is assumed during pregnancy [28]. In industrialized countries, a balanced diet contains about 12–18 mg of iron per day. However, to meet the increased iron requirement, the recommended daily intake of iron during pregnancy is about 27 mg, considering, that the proportion of iron absorbed is only 10–15% of elemental iron [20▪▪]. Preexisting iron deficiency often leads to IDA during pregnancy, even in developed countries, suggesting that physiological adaptations are often inadequate to meet increased demands and iron intake is often below nutritional requirements. IDA in pregnancy, if undiagnosed and untreated, can have significant effects on maternal and fetal health.
SCREENING RECOMMENDATIONS
To reduce the possible negative effects of iron deficiency and IDA, several international guidelines suggest adjusted screening in pregnant women. Considering that the incidence of anemia and iron deficiency is associated with geographic and ethnic factors, different screening methods are recommended in different guidelines worldwide. This applies not only to the selection of the laboratory parameters to be determined, but also to their thresholds and the timing of screening [29▪]. The recommendations of some associations on screening and therapy for iron deficiency and IDA during pregnancy are summarized in Table 2. Noticeably, not all countries routinely recommend anemia diagnosis at the beginning of pregnancy, and only professional societies in Switzerland and Australia suggest to screen for iron deficiency.
Table 2.
Screening and therapy for iron deficiency and IDA during pregnancy according to different international guidelines (according to Helmer [29▪])
| Recommendations | WHO [30] | NICE [31] UK | SGGG [27▪▪] Switzerland | DGEM [32] Germany | NBA [33] Australia | Health Canada [34] |
| 1. Trimester | – | CBC | CBC + ferritin | – | CBC + ferritin (women at risk) | – |
| 2. Trimester | CBC (28. WOP) | Hb | ||||
| 3. Trimester | – | Hb | ||||
| Iron replacement | ||||||
| prophylactic | 30–60 mg/day | No | No | No | No | 16–20 mg/day |
| Therapeutic | 30–60 mg/day | Yes | Yes | Yes | Yes | Yes |
| Folate replacement | ||||||
| Before pregnancy | 400 μg/day | 400 μg/day | 400 μg/day | 400 μg/day up to the 12th WOP | – | 400 μg/day |
| During pregnancy | 400 μg/day | 400 μg/day up to the 12th WOP | 400 μg/day up to the 12th WOP | 800 μg/day up to the 12th WOP | 400 μg/day | |
CBC, complete blood count; Hb, hemoglobin; WOP, week of pregnancy.
Serum ferritin, as a marker of reticuloendothelial iron stores, and transferrin saturation (TSAT) being below the normal range in the first trimester was able to detect women who subsequently had anemia predelivery, with ferritin being most discriminatory. Both were superior to hemoglobin concentration [35]. A recent systematic review of serum ferritin thresholds for iron deficiency in pregnancy reported that most studies used ferritin values of 12–15 μg/l but that thresholds ranged as high as 30 μg/l, thus further evaluation and investigations are needed to hopefully be able to standardize recommendations in the future [36]. As lower ferritin thresholds are associated with significantly decreased sensitivity for iron deficiency with a minimal increase in sensitivity, and serum ferritin <30 μg/l indicates that there is a 90% probability that iron stores are depleted [24], a threshold of < 30 μg/l seems to represent a reasonable threshold to detect iron deficiency during pregnancy. Although low serum ferritin always indicates iron deficiency, serum ferritin is an acute-phase reactant that may be elevated out of proportion to iron stores due to infection or inflammation. Although inflammatory markers such as C-reactive protein can be helpful in this case, if iron deficiency is assumed despite normal or elevated ferritin levels, the diagnosis can be made using additional parameters of iron metabolism.
Both a decreased transferrin saturation (TSAT < 15–20%) and an elevated serum soluble transferrin receptor (sTfR) concentration correlate with other indicators of iron deficiency in pregnancy and may indicate iron deficiency in patients with normal ferritin concentrations who are clinically suspected of having iron deficiency [28]. Although TSAT may be reduced in inflammatory disease, sTfR is not affected by an infection [37].
Additional parameters of iron metabolism are promising but not yet widely available and not in use for routine diagnosis of iron-deficiency anemia. For example, elevated values of zinc protoporphyrin can reliably indicate iron deficiency in more complex patients. Further, reticulocyte parameters including reticulocyte hemoglobin equivalent (Ret-He) and immature reticulocyte fraction (IRF) are newly recognized hematological parameters that are being used for diagnosis and follow-up of anemic patients. Although these parameters can be valuable and helpful in difficult cases, the determination of the Hb value, the percentage of transferrin saturation and the serum ferritin are sufficient to assess the iron status in the majority of otherwise healthy young pregnant women [17].
Another reason for anemia during pregnancy is folate deficiency, however, due to the widespread supplementation for the prophylaxis of neural tube defects, the manifestation of anemia caused by folate deficiency during pregnancy is rare [29▪]. If there is evidence of hyperchromic anemia, a Vit B12 deficiency should be ruled out.
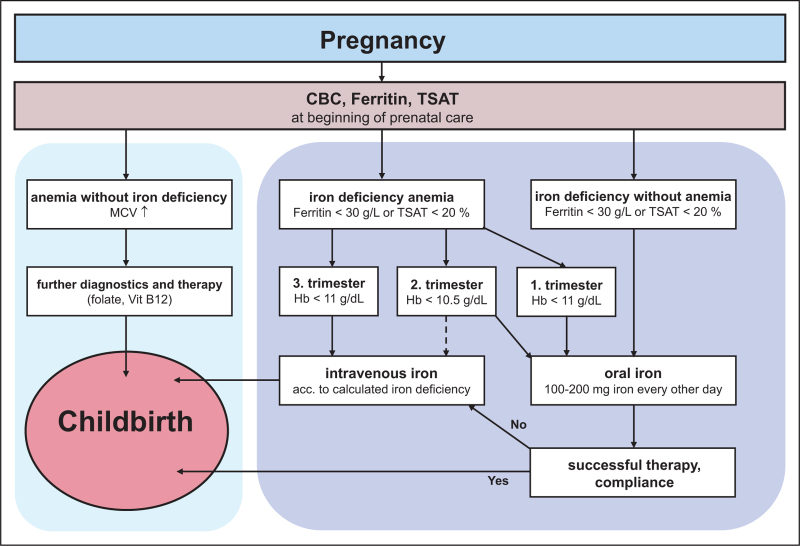
If the proven PBM concept for major elective surgery is applied to anemia screening in pregnant women, anemia diagnostics with iron status should be recommended at the beginning of every pregnancy. As consistent guidelines in obstetrics are still lacking, a simple algorithm that is easy to implement should be developed in consent with the obstetricians to ensure the safe care of mother and fetus in daily practice (Fig. 1). The determination of Hb, serum ferritin and TSAT is inexpensive and should be sufficient to diagnose iron deficiency or IDA in most pregnant women. In addition, it should be recommended to check these values at the beginning of each trimester and immediately before delivery. For patients at risk, e.g. vegans, patients with a history of abnormal bleeding, women with multiple pregnancies, very young mothers and if the last pregnancy was less than a year ago, Jehovah's Witnesses, or for patients with an anemia of unclear cause, further laboratory parameters should be determined during the screening examination [29▪]. Nevertheless, the limit values and intervention thresholds given can only provide guidance and should be supplemented by individual considerations. If in the third trimester a considerable drop in hemoglobin level is detected even hemoglobin levels >11 g/dl may justify i.v. iron supplementation to avoid a further drop in the subsequent weeks until the period at risk during birth.
FIGURE 1.
Proposed algorithm for diagnosing and treating iron deficiency anemia in pregnancy (modified according to Helmer [29▪]). CBC, complete blood count; TSAT, transferrin saturation; Hb, hemoglobin; MCV, mean corpuscular volume.
TREATMENT OF IRON DEFICIENCY AND IRON DEFICIENCY ANEMIA DURING PREGNANCY
While the guidelines of some national professional societies such as the American College of Obstetricians and Gynecologists (ACOG) and the CDC, but also the WHO, recommend the prophylactic administration of oral iron during every pregnancy [20▪▪], most guidelines recommend against it. The reasons against prophylactic administration during each pregnancy are the relatively high prevalence of hemochromatosis in some industrialized countries [29▪] and the increased risk of infection with iron-dependent microorganisms and parasites, including malaria, in several developing countries. However, iron supplementation is considered to be at low risk and a daily iron supplement of 65 mg of elemental iron is usually sufficient to prevent iron deficiency during pregnancy [28].
Any iron deficiency, even without anemia, should be treated during pregnancy, with oral iron supplementation in the first trimesters as the standard treatment. The recommendations for oral iron supplementation vary from 60 to 200 mg/day of elemental iron, given once to three times daily. Unfortunately, up to 70% of women experience significant gastrointestinal adverse effects such as nausea, constipation, diarrhea, indigestion or metallic taste, which prevent compliance with treatment [38]. Although these complaints do not necessarily seem to be dose-dependent, the results of recently published studies demonstrate that oral iron doses ≥60 mg/day trigger a rise in hepcidin levels that subsides after 48 h, and are associated with lowered fractional iron absorption on the following day. Therefore, to maximize fractional iron absorption, and to reduce side effects to enhance compliance, oral doses ≥60 mg should be given every other day [39,40]. As the SGGG considers iron doses < 100 mg/day ineffective for isolated iron deficiency [24], the recommended two-day iron dose for iron deficiency and IDA should not be <100 mg. This dosing seems to be very low, especially if a severe IDA is present. In this case, twice the daily target iron dose may be given every other day, but it must be taken into account that fractional iron absorption decreases considerably with increasing iron doses and unabsorbed luminal iron likely has adverse effects on the gut. [39]. Currently however, oral iron represents the only approved option for iron supplementation during the first trimester of pregnancy, and the administration of intravenous iron remains restricted to the second and third trimesters due to lack of safety data in early pregnancy.
Recent studies have shown that parenteral iron administration is superior to oral therapy in terms of efficacy and duration of therapy for IDA in pregnancy [41]. There are a number of intravenous iron preparations with different dosing regimens, the latest of which allow the use of high doses in a single administration. Due to the ease of use and associated patient satisfaction, single-dose infusions should be used nowadays, which require only one patient visit and are therefore more cost-effective than older formulations [20▪▪]. Iron dextran preparations are no longer recommended because of the increased risk of anaphylactic reactions compared to the newer intravenous iron preparations [29▪], even though more recent data has shown a similar safety profile of low molecular weight iron dextran to other common products [20▪▪], but still requires a test dose. Both, ferric carboxymaltose and iron isomaltoside are based on carbohydrates with reduced immunogenic properties [17] and should be currently the treatment options of first choice. The higher rate of hypophosphatemia associated with ferric carboxymaltose administration appears to be short-lived in pregnant individuals (usually receiving only a single intravenous infusion) if it occurs [20▪▪,42]. On the other hand, a potential advantage of ferric carboxymaltose can be seen with a lower risk for hypersensitivity reactions compared with iron isomaltoside [43].
So far, intravenous iron has been recommended if oral iron preparations are not tolerated due to gastrointestinal side effects, Hb-values do not rise sufficiently due to intestinal absorption disorders or poor compliance, severe anemia (Hb < 9 g/dL) is present, or if rapid anemia treatment is required due to advanced gestational age [3]. With the advent of new intravenous iron formulations that allow complete replacement dosing in just 15–60 min associated with more favorable safety profiles, recent recommendations place a higher priority for the use of intravenous iron as a treatment for iron deficiency during pregnancy [17,20▪▪,26]. These recommendations include treating iron deficiency and IDA with intravenous iron as early as possible in pregnancy. Accordingly, all pregnant women with an iron deficiency in the second trimester who have Hb-values <10.5 g/dl, and all women in the third trimester could be treated with intravenous iron. Administration of 1000 mg ferric carboxymaltose in the second or third trimester of pregnancy maintains iron stores more consistently and reduces the need for repeat infusion or subsequent monitoring [44▪].
CONCLUSION
PBM strategies in obstetrics are still underrepresented, although the high prevalence of iron deficiency and IDA during pregnancy as well as the risk of PPH for every woman indicate that implementation of PBM in obstetrics might be promising. As most of the available PBM-guidelines in obstetrics are inconsistent, the timing of anemia screening and the thresholds for anemia and iron diagnostics during pregnancy should be standardized and performed on a regular basis. To reduce maternal complications caused by iron deficiency and IDA and to ensure the safety of fetal development, early screening for iron deficiency at the beginning of each pregnancy should be strongly recommended. Any iron deficiency, even without anemia, should be treated during pregnancy, with oral iron supplements given every other day being the standard treatment in the first trimester. With the advent of new intravenous iron formulations associated with more favorable safety profiles, it has recently been recommended that iron deficiency and IDA should be treated with intravenous iron as early as possible in pregnancy. A multidisciplinary consent – at least on a departmental level – is the precondition for a successfully implementation of PBM in obstetrics to establish an approved algorithm to easily enable detection and treatment of iron deficiency and IDA during pregnancy.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
C.W. received speaking fees from CSL Vifor. P.K. received speaker fees from CSL Vifor, CSL Behring and Pharmacosmos. PM's Department received research grants from the German Research Foundation (ME 3559/1-1, ME 3559/3–1, ME 6094/3-2), BMBF (01KG1815), BMG (ZMVI1-2520DAT10E); PM received honoraria for scientific lectures from Biotest AG, CSL Behring, Haemonetics, Pharmacosmos GmbH, Vifor Pharma, Werfen GmbH. The authors declare that there are no further competing interests.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1. WHO, WHA63.12 availability, safety and quality of blood products. WHO resolution the sixty-third world health assembly. Geneva: WHO; 2010. [Google Scholar]
- 2.Goodnough LT, Maggio P, Hadhazy E, et al. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion 2014; 54:2753–2759. [DOI] [PubMed] [Google Scholar]
- 3.Surbek D, Vial Y, Girard T, et al. Patient blood management (PBM) in pregnancy and childbirth: literature review and expert opinion. Arch Gynecol Obstet 2020; 301:627–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪.Halvorsen S, Mehilli J, Cassese S, et al. 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery. Eur Heart J 2022; 43:3826–3924. [DOI] [PubMed] [Google Scholar]; The current ESC recommendations include a separate chapter on patient blood management.
- 5.Muñoz M, Peña-Rosas JP, Robinson S, et al. Patient blood management in obstetrics: management of anaemia and haematinic deficiencies in pregnancy and in the postpartum period: NATA consensus statement. Transfus Med 2018; 28:22–39. [DOI] [PubMed] [Google Scholar]
- 6.Shaylor R, Weiniger CF, Austin N, et al. National and international guidelines for patient blood management in obstetrics: a qualitative review. Anesth Analg 2017; 124:216–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franchini M, Liumbruno GM. Implementation of a patient blood management programme in obstetrics: let's do it!. Blood Transfus 2019; 17:87–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zdanowicz JA, Schneider S, Mueller M, et al. Red blood cell transfusion in obstetrics and its implication for patient blood management: a retrospective analysis in Switzerland from 1998 to 2016. Arch Gynecol Obstet 2021; 303:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014; 2:e323–e333. [DOI] [PubMed] [Google Scholar]
- 10.Faysal H, Araji T, Ahmadzia HK. Recognizing who is at risk for postpartum hemorrhage: targeting anemic women and scoring systems for clinical use. Am J Obstet Gynecol MFM 2022; 6:100745. [DOI] [PubMed] [Google Scholar]
- 11. Peripartal haemorrhage, diagnosis and therapy. Guideline of the DGGG, OEGGG and SGGG, S2k-Level, AWMF Registry No. 015/063, August 2022. Available at: http://www.awmf.org/leitlinien/detail/ll/015–063.html. [Google Scholar]
- 12.Committee on Practice Bulletins-Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol 2017; 130:e168–e186. [DOI] [PubMed] [Google Scholar]
- 13▪.Delgado C, Komatsu R. Patient blood management programs for postpartum hemorrhage. Best Pract Res Clin Anaesthesiol 2022; 36:359–369. [DOI] [PubMed] [Google Scholar]; This review presents strategies for early preoperative identification of risk factors for PPH and detection of anemia before delivery.
- 14.Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med 2021; 384:1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Westcott JM, Hughes F, Liu W, et al. Prediction of maternal hemorrhage using machine learning: retrospective cohort study. J Med Internet Res 2022; 24:e34108. [DOI] [PMC free article] [PubMed] [Google Scholar]; Description of how to use machine learning techniques to identify patients at risk of postpartum hemorrhage at delivery.
- 16.McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 2009; 12:444–454. [DOI] [PubMed] [Google Scholar]
- 17.Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin, and folate. Blood 2017; 129:940–949. [DOI] [PubMed] [Google Scholar]
- 18.Garzon S, Cacciato PM, Certelli C, et al. Iron deficiency anemia in pregnancy: novel approaches for an old problem. Oman Med J 2020; 35:e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanu FA, Hamner HC, Scanlon KS, Sharma AJ. Anemia among pregnant women participating in the special supplemental nutrition program for women, infants, and children – United States, 2008–2018. MMWR Morb Mortal Wkly Rep 2022; 71:813–819. [DOI] [PubMed] [Google Scholar]
- 20▪▪.Benson AE, Shatzel JJ, Ryan KS, et al. The incidence, complications, and treatment of iron deficiency in pregnancy. Eur J Haematol 2022; 109:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent overview on the topic of iron deficiency during pregnancy.
- 21.Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med 2021; 34:1002–1005. [DOI] [PubMed] [Google Scholar]
- 22.Tang G, Lausman A, Abdulrehman J, et al. Prevalence of iron deficiency and iron deficiency anemia during pregnancy: a single Centre Canadian study. Blood 2019; 134:3389. [Google Scholar]
- 23.Teichman J, Nisenbaum R, Lausman A, Sholzberg M. Suboptimal iron deficiency screening in pregnancy and the impact of socioeconomic status in a high-resource setting. Blood Adv 2021; 5:4666–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breymann C, Honegger C, Hösli I, Surbek D. Diagnosis and treatment of iron-deficiency anaemia in pregnancy and postpartum. Arch Gynecol Obstet 2017; 296:1229–1234. [DOI] [PubMed] [Google Scholar]
- 25.Georgieff MK. Iron deficiency in pregnancy. Am J Obstet Gynecol 2020; 223:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auerbach M, Gafter-Gvili A, Macdougall IC. Intravenous iron: a framework for changing the management of iron deficiency. Lancet Haematol 2020; 7:e342–e350. [DOI] [PubMed] [Google Scholar]
- 27▪▪.Breymann C, Honegger C, Hösli I, Surbek D. Diagnostik und Therapie der Eisenmangelanämie in der Schwangerschaft und postpartal. Schweizerische Gesellschaft für Gynäkologie und Geburtshilfe 2022. [Google Scholar]; First recommendation by a national professional society for routine screening for anemia and iron deficiency at the beginning of each pregnancy.
- 28.Means RT. Iron deficiency and iron deficiency anemia: implications and impact in pregnancy, fetal development, and early childhood parameters. Nutrients 2020; 12:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪.Helmer P, Schlesinger T, Hottenrott S, et al. Patient blood management in the preparation for birth, obstetrics and postpartum period. Anaesthesist 2022; 71:171–180. [DOI] [PubMed] [Google Scholar]; Contains a simple and practical algorithm for the detection and treatment of iron deficiency and IDA during pregnancy.
- 30.WHO. Daily iron and folic acid supplementation in pregnant women. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 31.NICE. Antenatal care for uncomplicated pregnancies. 2019. [PubMed] [Google Scholar]
- 32. DEGEM. Available at: https://www.dge.de/ernaehrungspraxis/bevoelkerungsgruppen/schwangere-stillende/handlungsempfehlungen-zur-ernaehrung-in-der-schwangerschaft/#:∼:text=Neben%20Fols%C3%A4ure%20besteht%20auch%20die,%C2%B5g%20Fols%C3%A4ure%2FTag%20supplementiert%20werden. [Google Scholar]
- 33. National_Blood_Authority. Patient blood management guidelines: module 5-obstetrics and maternity. National Blood Authority; 2015. [Google Scholar]
- 34. Guidelines EaGONP. Prenatal nutrition guidelines for health professional-iron contributes to a healthy pregnancy. HealthCanada; 2009. [Google Scholar]
- 35.Crispin P, Stephens B, McArthur E, Sethna F. First trimester ferritin screening for predelivery anaemia as a patient blood management strategy. Transfus Apher Sci 2019; 58:50–57. [DOI] [PubMed] [Google Scholar]
- 36.Daru J, Allotey J, Peña-Rosas JP, Khan KS. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: a systematic review. Transfus Med 2017; 27:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urrechaga E, Borque L, Escanero JF. Biomarkers of hypochromia: the contemporary assessment of iron status and erythropoiesis. Biomed Res Int 2013; 2013:603786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One 2015; 10:e0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015; 126:1981–1989. [DOI] [PubMed] [Google Scholar]
- 40.Stoffel NU, von Siebenthal HK, Moretti D, Zimmermann MB. Oral iron supplementation in iron-deficient women: how much and how often? Mol Aspects Med 2020; 75:100865. [DOI] [PubMed] [Google Scholar]
- 41.Breymann C, Milman N, Mezzacasa A, et al. Ferric carboxymaltose vs. oral iron in the treatment of pregnant women with iron deficiency anemia: an international, open-label, randomized controlled trial (FER-ASAP). J Perinat Med 2017; 45:443–453. [DOI] [PubMed] [Google Scholar]
- 42.Wolf M, Rubin J, Achebe M, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA 2020; 323:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulder MB, van den Hoek HL, Birnie E, et al. Comparison of hypersensitivity reactions of intravenous iron: iron isomaltoside-1000 (Monofer®) versus ferric carboxy-maltose (Ferinject®). A single center, cohort study. Br J Clin Pharmacol 2019; 85:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44▪.Froessler B, Schubert KO, Palm P, et al. Testing equivalence of two doses of intravenous iron to treat iron deficiency in pregnancy: a randomised controlled trial. BJOG 2023; 130:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]; A single 1000 mg dose of intravenous iron represents an efficient and effective method to manage iron deficiency and IDA clinically in pregnancy.