Abstract
Background:
Persons with HIV (PWH) on antiretroviral therapy (ART) have persistent immune activation associated with increased risk for non-AIDS related diseases. Latent tuberculosis infection (LTBI), endemic in Africa, may contribute to this immune dysregulation. We evaluated the impact of HIV and TB co-infection on plasma pro- and anti-inflammatory cytokines among Kenyan adults.
Methods:
We compared data from 221 PWH on long-term ART and 177 HIV-negative adults examining biomarkers of pro-[sCD14, interleukin (IL)-2, IL-6, interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), IL-12p70, IL-17A] and anti(IL-4, IL-5, IL-13) inflammatory cytokines, by HIV/LTBI status (HIV+LTBI+, HIV+LTBI−, HIV−LTBI+, HIV−LTBI−). LTBI was diagnosed based on a positive QuantiFERON TB Gold-Plus test in the absence of active TB symptoms. Linear regression was used to evaluate the associations of HIV, LTBI, and HIV/LTBI status with biomarkers adjusting for clinical factors including HIV-specific factors.
Results:
Half of the participants were women and 52% had LTBI. HIV was independently associated with higher sCD14, IL-15, IL-6, IL-4, IL-5. LTBI was independently associated with higher TNF-α, IL-12p70, IL-17A, IL-4, IL-13 in adjusted models (P < 0.05). LTBI status was associated with higher IL-4 and IL-12p70 only among PWH, but not HIV-negative participants (P < 0.05 for interactions). In multivariate analysis, only HIV+LTBI+ demonstrated elevated levels of TNF-α, IL-6, IL-12p70, IL-15, IL-17A, IL4, IL-5, IL-13 in comparison to the HIV−LTBI− (P < 0.05 for all). The effect of LTBI on cytokines among PWH was independent of CD4+ T-cell count and ART duration.
Conclusions:
Despite viral suppression, persons with HIV and LTBI exhibit abnormal cytokine production accompanied by high concentrations of pro- and anti-inflammatory cytokines.
Keywords: Africa, cytokines, HIV, immune activation, latent, sex, T-helper cells, tuberculosis
Introduction
The introduction of antiretroviral therapy (ART) has increased the lifespan of people with HIV (PWH) in sub-Saharan Africa (SSA). However, in the same period, the burden of non-AIDS related comorbidities such as cardiovascular diseases (CVDs) have increased possibly due to residual inflammation. We have recently demonstrated that virally suppressed PWH continue to have persistent inflammation [1,2]. Therefore, understanding the drivers of residual inflammation and defining targets to reverse inflammation remains a major goal toward restoring health and lifespan in PWH.
The etiology of HIV-associated immune activation is not clear and may include HIV itself, microbial translocation, ART toxicity, comorbidities, and co-infections such as tuberculosis (TB) [3–6]. In TB endemic regions such as SSA, PWH are more likely to acquire TB, resulting in a spectrum of outcomes from asymptomatic to active or fulminant TB disease. Latent tuberculosis infection (LTBI) is characterized by the presence of immune responses to Mycobacterium tuberculosis without clinical evidence of active TB. Although the prevalence of LTBI is variable throughout SSA, it is estimated that the prevalence rates are up to 50% in regions with high TB transmission, including Kenya [7,8]. Like HIV, LTBI is also characterized by immunologic dysregulation. Thus, it may not be benign, as indicated by the increased risk of CVD in persons with LTBI [9–11]. One possible common mechanistic pathway for the increased risk for CVD in PWH and LTBI is the immune dysregulation resulting from periodic activity of some component (i.e. mRNA, protein) or low-level replication of mycobacterium and HIV [12–14].
Cytokines secreted by monocytes/macrophages and CD4+ T-lymphocytes of T helper (Th) type are important initiators and regulators of the immune response to both HIV and TB [15,16]. Monocytes produce cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-12p70. Of the CD4+ T-cell types, Th1 cells producing IL-2, TNF-α, and interferon (IFN)-γ, and IL-17A-producing Th17 cells may be the most critical in the control of TB [17]. On the other hand, Th2 responses are accompanied by an increase in anti-inflammatory cytokines IL-4, IL-5, and IL-13. These type 2 cytokines and other anti-inflammatory cytokines may enhance susceptibility to infections [15,18]. HIV infection of Th cells causes further imbalance in cytokine production; however, ART may reverse this immune dysregulation by restoring the ability of T cells to respond to antigens [19].
Although the cytokine profiles of those with LTBI or HIV mono-infection have been well explored, to our knowledge, there are no studies that have assessed thoroughly the combined effects of HIV and LTBI co-infections on the systemic inflammatory milieu. In this study, we compared cytokine profiles by HIV/LTBI status in plasma samples of adult men and women from Kenya. We hypothesized that LTBI/HIV co-infection would be associated with greater cytokine levels compared to either condition alone or the absence of both conditions, and that these association would be stronger in women compared to men.
Methods
Study population and procedures
We utilized cross-sectionally collected samples from a study which assessed risk factors for CVD and pulmonary diseases in PWH on long-term ART in Kenya [8]. Data were collected between December 2018 and December 2019 and included demographic and anthropometric measurements. Study procedures have been described elsewhere [8]. Briefly, a convenience sample of men and women 30 years or older with and without HIV were recruited from the Kisumu District Hospital HIV clinic and voluntary HIV testing centers respectively. Pregnant women or individuals with history of CVD, neoplasia, active infections, and those on immunosuppressive agents were excluded from the study. For this analysis, we further excluded individuals with previous diagnosis of TB or those presently on treatment for TB.
Data on covariates such as age, education, alcohol use, smoking status, ART duration and nadir CD4+ T-cell count (CD4) were obtained from the participants and confirmed using medical records. Anthropometric measurements such as weight, height, and blood pressure (BP) were measured. Body mass index (BMI) was calculated from weight and height measurements. Fasting blood samples were used to assess for blood glucose (FBG), lipids, current CD4+ T-cell count, and HIV RNA viral load (VL). Elevated BP was defined by systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg and/or if the participant was on antihypertensives medications. Viral suppression was defined as VL <1000 copies/ml per Kenyan guidelines [20]. Data included in this manuscript was obtained and stored in compliance with regulations of the Ethics and Research Committee of Kenyatta National Hospital and the Institutional Review Board at University of Washington. All participants provided written informed consent.
Latent tuberculosis Infection assessment
A QuantiFERON TB Gold-Plus (QFT-Plus) test was performed at CDC/KEMRI in Kenya in accordance with manufacturer's instructions (Qiagen). We defined LTBI by a positive QFT-Plus and the absence of symptoms or signs suggestive of active TB. Prior TB infection was self-reported. Study participants were asked whether they had previously been told they have TB. All participants were screened for symptoms of active TB (ongoing productive cough, hemoptysis, weight loss, chest pain, fevers, chills, or night sweats) using World Health Organization symptom screening form. Those with symptoms of active TB were not enrolled. Eighty percentage of PWH had received prior Isoniazid preventive therapy (IPT) for treatment of latent TB at the time of recruitment and all were included in these analyses. Individuals with indeterminate QuantiFERON values on were also excluded in the analysis.
Immunological biomarker analysis
Plasma levels of IL-2, IL-4, IL-5, IL-6, IL-2p70, IL-13, IL-15, IL-17A, IFN-γ, and TNF-α were measured using the multiplex ELISA-based assay (Meso-Scale Discovery). Samples with a coefficient of variation >30% were re-ran. Plasma biomarkers of intestinal barrier dysfunction [intestinal-fatty acid binding protein (I-FABP)] and monocyte/macrophage activation markers (soluble CD14) were quantified using ELISA (Quantikine ELISA kit, R&D Systems). Assays were performed in duplicate and in accordance with manufacturers’ protocols.
Statistical analysis
We first compared demographic and clinical characteristics within and across groups [HIV+LTBI+(HIV+LTBI+), HIV+LTBI−(HIV), HIV−LTBI+(LTBI), and HIV−LTBI− (healthy controls, HC)] using t-tests, Wilcoxon rank sum tests or Kruskal–Wallis test for continuous variables and chi-squared or Fisher test for categorical variables. Univariable and multivariable linear regression models were used to investigate the association of biomarkers with LTBI and HIV in two ways. First, we assessed independent association of HIV or LTBI with biomarkers. We regressed biomarkers on HIV and LTBI status before and after adjustment of demographics (age, sex, education level), and clinical factors (body mass index, HDL, hypertension, alcohol intake, and smoking status). An interaction term between LTBI and HIV status was introduced in the model to test the difference of these associations by LTBI. Second, HIV and LTBI were combined into a single variable with four groups: HIV+LTBI, HIV, LTBI, HC and we evaluated the synergistic effects of LTBI/HIV status on biomarkers adjusting for demographics and clinical risk factors listed above. In a similar manner, we conducted a multivariable linear regression analysis to examine the association between HIV/LTBI status with biomarkers among male and females separately. These covariates were selected based on prior work suggesting an association with the biomarkers of interest [1,2,21].
Among PWH only, linear regression analyses were performed to evaluate the relationships between biomarkers and LTBI with further adjustment for nadir and current CD4, ART regimen, and ART duration.
All biomarkers were log-transformed before model fitting due to their non-normal distribution. We report the exponentiated β-coefficients and their calculated 95% confidence intervals (CIs) representing the fold increase/decrease in mean level of the biomarkers. Significance was set at a P value <0.05. Analyses were performed using STATA version 13 (San Antonio, Texas, USA).
Results
Study participants characteristics
Of the 398 participants enrolled, 101 were HIV+LTBI+, 120 HIV, 108 LTBI, and 69 HC (Table 1). Overall, the median [interquartile range (IQR)] age was 46 (38,57) years and 51% were women. The prevalence of LTBI was 46% in PWH and 61% in the HIV-negative participants (P = 0.002). There were differences in age, BMI, rates of hypertension, and diabetes by HIV/LTBI status. Participants with LTBI were younger regardless of HIV status. Women were more likely to be overweight/obese (51% vs. 29%; P < 0.001), and less likely to use alcohol (13% vs. 18%; P < 0.001) or smoke tobacco (1% vs. 6%; P < 0.001) than men.
Table 1.
Characteristics of participants stratified by HIV and latent TB status.
| HIV-negative | HIV-positive | P, across groups | P, LTBI+ vs. LTBI− | P, HIV+ vs. HIV− | |||
| LTBI (n = 108) | HC (n = 69) | HIV+LTBI (n = 101) | HIV (n = 120) | ||||
| Demographics | |||||||
| Age, years | 43 (33,5) | 48 (35,60) | 45 (39,54) | 49 (42,58) | 0.01 | 0.003 | 0.05 |
| Female | 58 (54) | 32 (47) | 57 (56) | 55 (49) | 0.36 | 0.08 | 0.92 |
| Cardiovascular risk factors | |||||||
| Diabetes | 5 (5) | 8 (12) | 2 (2) | 5 (4) | 0.04 | 0.10 | 0.06 |
| Hypertension | 33 (31) | 26 (38) | 16 (16) | 19 (16) | <0.001 | 0.93 | <0.001 |
| Current smoker | 4 (4) | 2 (3) | 2 (2) | 4 (4) | 0.92 | 0.78 | 0.87 |
| Current alcohol | 13 (13) | 8 (13) | 14 (15) | 22 (20) | 0.32 | 0.57 | 0.07 |
| Other characteristics | |||||||
| BMI, kg/m2 | 25 (22,29) | 24 (21,29) | 23 (21, 26) | 23 (20, 26) | 0.003 | 0.43 | 0.0004 |
| Waist circumference, cm | 83 ± 22 | 84 ± 21 | 84 ± 15 | 84 ± 15 | 0.56 | 0.69 | 0.22 |
| Laboratory values | |||||||
| HDL-cholesterol, mg/dl | 50 ± 11 | 52 ± 14 | 53 ± 14 | 53 ± 13 | 0.47 | 0.58 | 0.10 |
| LDL- cholesterol, mg/dl | 103 ± 31 | 99 ± 29 | 94 ± 32 | 98 ± 34 | 0.29 | 0.76 | 0.07 |
| Total cholesterol, mg/dl | 171 ± 38 | 166 ± 35 | 166 ± 37 | 171 ± 41 | 0.78 | 0.76 | 1.00 |
| HIV related characteristics | |||||||
| Nadir CD4+ T-cell count, cells/μl | 365 (200,552) | 335 (200,571) | 0.90 | ||||
| CD4+ T-cell count, cells/μl | 543 (406,695) | 475 (351,614) | 0.05 | ||||
| Viral unsuppressed RNA >1000 copies/ml | 4 (4) | 3 (3) | 0.56 | ||||
| Total ART duration, years | 10 (6,11) | 10 (6,12) | 0.65 | ||||
| Current integrase inhibitor | 44 (44) | 70 (58) | 0.10 | ||||
| Nevirapine-based treatment | 24 (24) | 22 (18) | 0.11 | ||||
| Efavirenz-based treatment | 22 (22) | 14 (12) | 0.04 | ||||
| PI-based treatment | 6 (6) | 11 (9) | 0.09 | ||||
Data reported as mean ± standard deviation, percentage, or median [interquartile range (IQR)]. HIV+LTBI+ [HIV+LTBI+], HIV+LTBI− [HIV], HIV−LTBI+ [LTBI], and HIV-LTBI− [healthy controls, HC].
ART, antiretroviral therapy; LTBI, latent tuberculosis infection; BP, blood pressure; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein. The Kruskal–Wallis or Pearson exact and χ2 tests for comparisons of more than two groups and the Wilcoxon rank sum or χ2 test for comparison between two groups.
Of the participants with HIV, the median (IQR) ART duration was 9 (6,12) years. The median current CD4+ T-cell count was 508 cells/μl and was borderline higher for the HIV/LTBI co-infected individuals (P = 0.05) compared to the HIV mono-infected individuals. Women with HIV had significantly higher median CD4+ T-cell count (565 vs. 443 cells/μl; P < 0.001) and nadir CD4+ T-cell count (356 vs. 333 cells/μl; P = 0.02) than the men. Almost all PWH (97%) were virally suppressed.
Impact of latent tuberculosis infection on plasma gut permeability and monocyte activation biomarkers in HIV
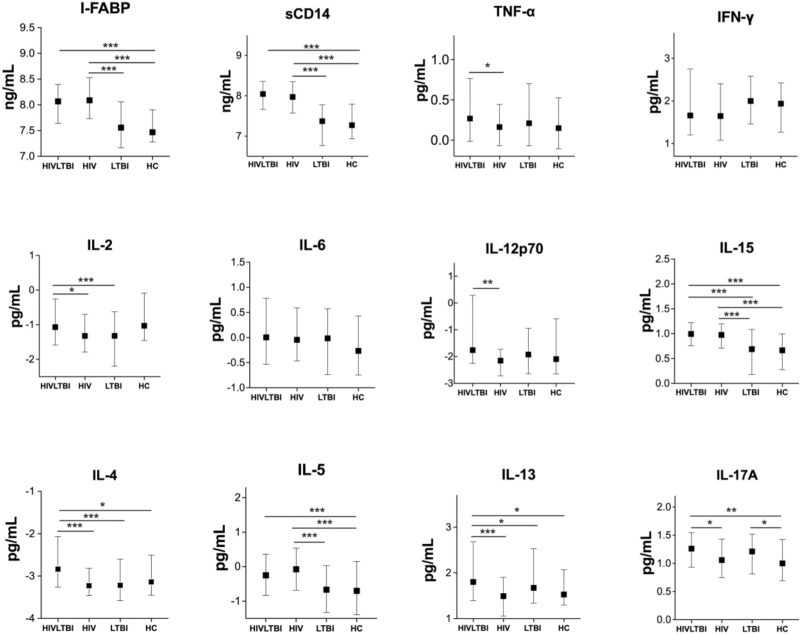
To determine the influence of LTBI on intestinal barrier function and monocyte activation, we compared plasma levels of I-FABP and sCD14 in persons with and without LTBI by HIV status. As shown in Fig. 1, plasma levels of I-FABP and sCD14 were significantly higher in the HIV+LTBI+ and HIV groups compared to the LTBI and the HC participants (P < 0.001 for all).
Fig. 1.
Log10 plasma levels of biomarkers by HIV serostatus and LTBI status.
LTBI/HIV co-infection is associated with elevated levels of monocyte activation and pro-inflammatory cytokines. Symbols depict median log10 levels and interquartile ranges for each biomarker by LTBI/HIV status. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. HIV+LTBI+ [HIV+LTBI+], HIV+LTBI− [HIV], HIV−LTBI+ [LTBI], and HIV−LTBI− [healthy controls, HC]. IFN-γ, interferon gamma; IL, interleukin. I-FABP, intestinal fatty acid-binding protein; sCD14, cluster of differentiation 14; TNF-α, tumor necrosis factor alpha; LTBI, latent tuberculosis infection
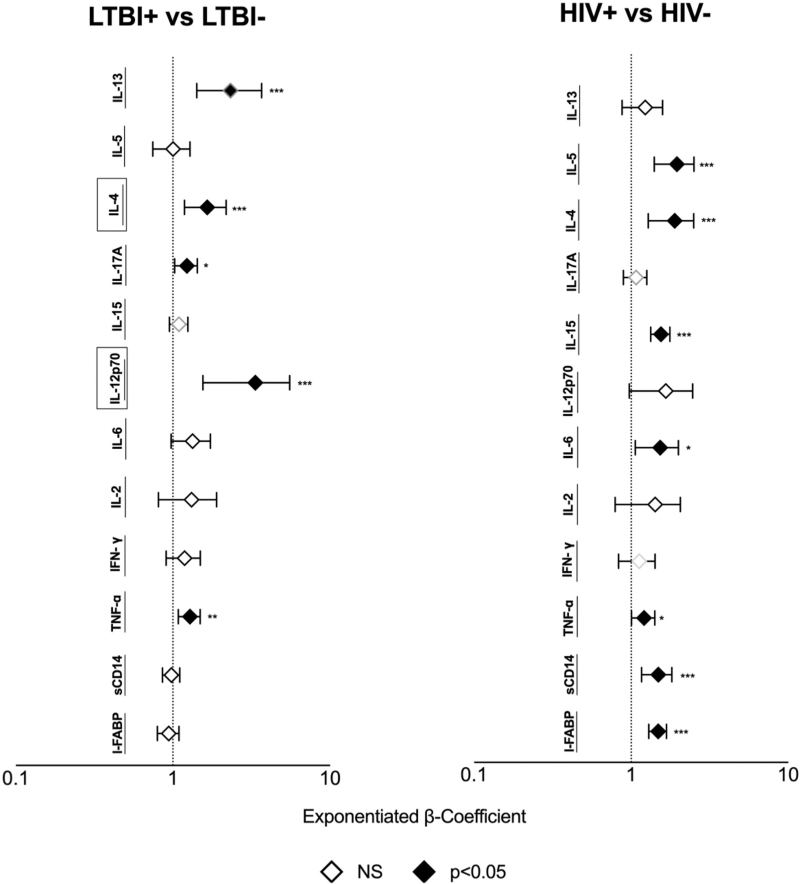
We evaluated the independent effect of HIV and LTBI separately by regressing biomarkers on HIV status, LTBI status, and their interaction controlling for demographic and clinical factors (Fig. 2). HIV but not LTBI was independently associated with higher I-FABP (P < 0.0001) and sCD14 (P < 0.001) in a fully adjusted model. HIV status did not modify the effect of LTBI on I-FABP or sCD14 (P = 0.36 and P = 0.92, respectively for LTBI by HIV status interaction).
Fig. 2.
Association of LTBI and HIV with plasma biomarkers.
Markers were regressed on LTBI status, HIV status, and their interaction, age, sex, education HDL, hypertension, BMI, smoking, and alcohol use status. Black boxes surrounding a y-axis variable indicate that there is effect modification by HIV status for this marker, that is, P < 0.05 for LTBI by an HIV status interaction term. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. LTBI, latent tuberculosis infection; NS, not significant.
We next evaluated the effect of LTBI on plasma biomarkers by combining HIV and LTBI into a single variable with four subgroups. In fully adjusted models, both HIV+LTBI+ and HIV+ remained associated with higher I-FABP and sCD14 (P < 0.001 for all) compared to the HC (Table 2). When stratified the analyses by sex, men, and women with HIV, regardless of their LTBI status, had significantly higher sCD14 and I-FABP compared to the HC in fully adjusted models (P > 0.05 for all; Table 3).
Table 2.
Association of latent tuberculosis status with biomarkers by HIV serostatus and LTBI status.
| I-FABP exponentiated β (95% CI) | sCD14 exponentiated β (95% CI) | TNF-α exponentiated β (95% CI) | IFN-γ exponentiated β (95% CI) | IL-2 exponentiated β (95% CI) | IL-6 exponentiated β (95% CI) | |
| Stratified by HIV and LTBI status | ||||||
| Unadjusted | ||||||
| HC | Reference | Reference | Reference | Reference | Reference | Reference |
| LTBI | 0.99 (0.88, 1.19) | 0.96 (077, 1.18) | 1.16 (0.89, 1.52) | 1.39 (0.78, 2.49) | 1.09 (1.00, 1.89) | 1.33 (0.84, 2.12) |
| HIV | 1.61 (1.34, 1.92) | 1.97 (1.58, 2.44) | 1.01 (0.78, 1.30) | 1.06 (0.59, 1.91) | 2.14 (1.07, 2.02) | 1.35 (0.86, 2.12) |
| HIV+LTBI+ | 1.47 (1.22, 1.22) | 1.93 (1.55, 2.41) | 1.34 (1.03, 1.75) | 1.57 (0.88, 2.80) | 1.90 (1.25, 2.33) | 1.65 (1.03, 2.61) |
| a Fully adjusted | ||||||
| HC | Reference | Reference | Reference | Reference | Reference | Reference |
| LTBI | 1.03 (0.85, 1.24) | 1.01 (0.81, 1.26) | 1.19 (0.90, 1.59) | 1.16 (0.75, 1.80) | 1.24 (0.87, 1.77) | 1.35 (0.80, 2.26) |
| HIV | 1.55 (1.29, 1.83) | 1.81 (1.45, 2.28) | 1.12 (0.84, 1.48) | 1.08 (0.70, 1.66) | 1.32 (0.93, 1.88) | 1.52 (0.91, 2.53) |
| HIV+LTBI+ | 1.37 (1.19, 1.73) | 1.82 (1.45, 2.29) | 1.48 (1.11, 1.97) | 1.27 (0.81, 1.99) | 1.40 (0.98, 2.02) | 1.97 (1.17, 3.33) |
| IL-12p70 exponentiated β (95% CI) | IL-15 exponentiated β (95% CI) | IL-17A exponentiated β (95% CI) | IL-4 exponentiated β (95%CI) | IL-5 exponentiated β (95% CI) | IL-13 exponentiated β (95% CI) | |
| Stratified by HIV and LTBI status | ||||||
| Unadjusted | ||||||
| HC | Reference | Reference | Reference | Reference | Reference | Reference |
| LTBI | 1.27 (0.61, 2.64) | 1.08 (0.88, 1.35) | 1.35 (1.04, 1.76) | 1.04 (0.63, 1.72) | 1.09 (0.70, 1.68) | 1.36 (0.88, 2.12) |
| HIV | 0.68 (0.33, 1.40) | 1.49 (1.22, 1.82) | 1.19 (0.92, 1.55) | 1.03 (0.63, 1.68) | 2.14 (1.39, 3.29) | 0.77 (0.49, 1.19) |
| HIV+LTBI+ | 2.18 (1.05, 4.48) | 1.59 (1.27, 1.92) | 1.42 (1.09, 1.84) | 2.10 (1.29, 3.50) | 1.90 (1.23, 2.94) | 1.60 (1.04, 2.45) |
| a Fully adjusted | ||||||
| HC | Reference | Reference | Reference | Reference | Reference | Reference |
| LTBI | 1.23 (0.55, 2.71) | 1.03 (0.82, 1.32) | 1.51 (1.12, 2.03) | 0.93 (0.54, 1.24) | 1.02 (0.62, 1.67) | 1.49 (0.90, 1.59) |
| HIV | 0.82 (0.37, 1.80) | 1.48 (1.16, 1.87) | 1.31 (0.97, 1.75) | 1.08 (0.63, 1.83) | 1.95 (1.20, 3.18) | 0.93 (0.84, 1.48) |
| HIV+LTBI+ | 2.57 (1.15, 5.70) | 1.63 (1.28, 2.08) | 1.48 (1.08, 1.95) | 2.23 (1.29, 1.73) | 2.22 (1.16, 3.12) | 1.48 (1.11, 1.97) |
HIV+LTBI+; HIV+LTBI+, HIV; HIV+LTBI−, LTBI; HIV−LTBI+, and HC; HIV−LTBI−.
I-FABP, intestinal fatty acid-binding protein; IFN-γ; interferon gamma; IL, interleukin; sCD14, cluster of differentiation 14; TNF-α, tumor necrosis factor alpha.
Fully adjusted for age, sex, HDL, body mass index, hypertension, body mass index, smoking, and alcohol use, and education status.
Results are presented as exponentiated β coefficients and 95% CI. Interpret the coefficient as fold increase in mean level of the biomarkers of interest comparing the groups with HC for example 1.63 will be interpreted as HIV+LTBI+ have 63% higher mean IL-15 level in comparison to HC.
Sensitivity analysis restricted to integrase inhibitor users showed similar trend in the association between biomarkers and HIV/LTBI status.
Table 3.
Association of LTBI infection with biomarkers by HIV serostatus and LTBI status, stratified by gender.
| A | I-FABP exponentiated β (95% CI) | sCD14 exponentiated β (95% CI) | IFN-γ exponentiated β (95% CI) | TNF-α exponentiated β (95% CI) | ||||
| Female | Male | Female | Male | Female | Male | Female | Male | |
| Unadjusted | ||||||||
| HIV−, LTBI− | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| HIV−, LTBI+ | 0.97 (0.74, 1.27) | 1.04 (0.82, 1.32) | 0.87 (0.64, 1.19) | 1.04 (0.77, 1.41) | 1.39 (0.78, 2.49) | 1.10 (0.64, 1.90) | 1.20 (079, 1.83) | 1.11 (0.81, 1.54) |
| HIV+, LTBI− | 1.58 (1.19, 2.07) | 1.64 (1.31, 2.06) | 2.06 (1.51, 2.81) | 1.88 (1.41, 2.51) | 1.06 (0.59, 1.91) | 0.98 (0.58, 1.65) | 1.00 (0.65, 1.53) | 1.03 (0.76, 1.33) |
| HIV+, LTBI+ | 1.54 (1.17, 2.03) | 1.41 (1.11, 1.64) | 1.78 (1.31, 2.42) | 2.14 (1.57, 2.92) | 1.57 (0.88, 2.80) | 0.84 (0.50, 1.44) | 1.44 (0.94, 2.19) | 1.24 (0.90, 1.69) |
| a Fully adjusted | ||||||||
| HIV−, LTBI− | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| HIV−, LTBI+ | 0.99 (0.73, 1.33) | 1.03 (0.81, 1.32) | 0.98 (0.72, 1.36) | 1.03 (0.75, 1.40) | 1.11 (0.58, 2.14) | 1.19 (0.65, 2.17) | 1.20 (076, 1.82) | 1.26 (0.88, 1.82) |
| HIV+, LTBI− | 1.50 (1.11, 2.03) | 1.64 (1.32, 2.06) | 1.81 (1.31, 2.50) | 1.73 (1.27, 2.34) | 0.92 (0.48, 1.78) | 1.22 (0.68, 2.18) | 1.09 (0.69, 1.68) | 1.17 (0.82, 1.68) |
| HIV+, LTBI+ | 1.58 (1.17, 2.14) | 1.28 (1.01, 1.64) | 1.63 (1.18, 2.26) | 2.06 (1.49, 2.87) | 1.38 (0.71, 2.66) | 1.08 (0.59, 1.98) | 1.60 (1.01, 2.02) | 1.39 (0.96, 2.02) |
| B | IL-2 exponentiated β (95% CI) | IL-6 exponentiated β (95% CI) | IL-12p70 exponentiated β (95% CI) | IL-15 exponentiated β (95% CI) | ||||
| Female | Male | Female | Male | Female | Male | Female | Male | |
| Unadjusted | ||||||||
| HC | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| LTBI+ | 1.59 (1.05, 2.43) | 1.17 (0.72, 1.89) | 1.38 (0.69, 2.75) | 1.25 (0.67, 2.31) | 1.54 (0.55, 4.35) | 1.02 (0.38, 2.76) | 1.21 (0.90, 1.64) | 0.97 (0.73, 1.30) |
| HIV+ | 1.66 (1.08, 2.53) | 1.31 (0.83, 2.08) | 1.28 (0.64, 2.55) | 1.42 (0.79, 2.56) | 0.88 (0.32, 2.48) | 0.53 (0.32, 1.41) | 1.82 (1.35, 2.45) | 1.23 (0.94, 1.67) |
| HIV+, LTBI+ | 2.25 (0.98, 3.41) | 1.26 (0.78, 2.03) | 2.05 (1.04, 4.08) | 1.23 (0.66, 2.27) | 4.20 (1.51, 11.67) | 0.91 (0.33, 2.51) | 1.89 (1.41, 2.53) | 1.28 (0.96, 1.70) |
| a Fully adjusted | ||||||||
| HC | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| LTBI+ | 1.35 (0.85, 2.15) | 1.15 (0.66, 2.08) | 1.18 (0.56, 2.52) | 1.56 (0.75, 3.22) | 0.43 (0.35, 3.33) | 1.19 (0.39, 3.64) | 1.18 (0.84, 1.67) | 0.90 (0.65, 1.26) |
| HIV+ | 1.46 (0.92, 2.33) | 1.18 (0.69, 2.02) | 1.23 (0.58, 2.63) | 1.90 (0.94, 3.85) | 0.97 (0.32, 3.01) | 0.72 (0.24, 2.18) | 1.80 (1.26, 2.54) | 1.21 (0.88, 1.67) |
| HIV+, LTBI+ | 1.81 (0.99, 2.87) | 1.08 (0.62, 1.91) | 2.25 (1.06, 4.81) | 1.78 (0.84, 3.68) | 4.39 (1.41, 13.64) | 1.46 (0.46, 4.64) | 1.88 (1.33, 2.66) | 1.39 (0.99, 1.95) |
| IL-4 exponentiated β (95%CI) | IL-5 exponentiated β (95%CI) | IL-13 exponentiated β (95%CI) | IL-17A exponentiated β (95%CI) | |||||
| Female | Male | Female | Male | Female | Male | Female | Male | |
| Unadjusted | ||||||||
| HC | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| LTBI+ | 1.19 (0.52, 2.48) | 0.92 (0.51, 1.63) | 1.76 (1.01, 3.11) | 0.67 (0.34, 1.33) | 1.88 (0.95, 3.71) | 0.75 (0.54, 1.62) | 0.50 (0.12, 0.89) | 1.08 (0.75, 1.55) |
| HIV+ | 1.15 (0.49, 2.56) | 0.91 (0.52, 1.60) | 3.09 (1.77, 5.41) | 1.44 (0.75, 2.76) | 0.94 (0.47, 1.88) | 0.58 (0.34, 1.00) | 0.34 (-0.04, 0.73) | 1.02 (0.73, 1.49) |
| HIV+, LTBI+ | 3.03 (1.37, 6.75) | 1.36 (0.77, 2.23) | 3.09 (1.80, 5.43) | 1.16 (0.75, 2.29) | 2.44 (1.25, 4.76) | 0.98 (0.57, 1.68) | 0.58 (0.19, 0.96) | 1.11 (0.77, 158) |
| a Fully adjusted | ||||||||
| HC | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| LTBI+ | 0.92 (0.39, 2.18) | 0.83 (0.41, 5.31) | 1.99 (1.07, 3.78) | 0.51 (0.23, 1.12) | 1.98 (0.91, 4.25) | 0.93 (0.48, 1.80) | 1.73 (1.11, 2.79) | 1.32 (0.89, 1.94) |
| HIV+ | 1.44 (0.63, 3.32) | 0.85 (0.41, 1.59) | 3.38 (1.81, 6.40) | 1.13 (0.53, 2.42) | 1.31 (0.61, 2.82) | 0.62 (0.32, 1.19) | 1.38 (0.89, 2.17) | 1.23 (0.85, 1.80) |
| HIV+, LTBI+ | 3.46 (1.49, 7.92) | 1.43 (0.70, 2.91) | 3.90 (2.07, 7.34) | 0.86 (0.39, 1.88) | 3.29 (1.53, 6.96) | 1.01 (0.52, 1.98) | 1.68 (1.08, 2.64) | 1.22 (0.82, 1.82) |
HIV+LTBI+; HIV+LTBI+, HIV; HIV+LTBI−, LTBI; HIV−LTBI+, and HC; HIV-LTBI−.
IL, interleukin. I-FABP, intestinal fatty acid-binding protein; sCD14, cluster of differentiation 14; TNF-α, tumor necrosis factor alpha; IFN-γ; interferon gamma.
Fully adjusted for age, HDL, body mass index, hypertension, body mass index, smoking, and alcohol use, and education status.
Sensitivity analysis restricted to integrase inhibitor users showed similar trend in the association between biomarkers and HIV/LTBI status except for IL-6. We found no association between IL-6 and HIV+, LTBI+ status in women; β (95% CI) 1.61 (0.94–4.45), P = 0.65.
Effect of latent tuberculosis infection on pro-inflammatory cytokines in chronic HIV
To determine the influence of LTBI on pro-inflammatory cytokines in HIV, we compared the plasma levels of TNF-α, IFN-γ, IL-2, IL-6, IL-12p70, IL-15, IL-17A in PWH and negative participants with and without LTBI. As shown in Fig. 1, median levels of TNF-α and IL-12p70 were significantly higher in HIV+LTBI+ than the HIV group. Levels of IL-2 were higher in HIV+LTBI+ than those with HIV or LTBI mono-infection. Median levels IL-17A were elevated in both the HIV+LTBI+ and LTBI groups compared with HC (P < 0.01 for all) while levels of IL-15 were higher in both the HIV+LTBI+ and HIV participants than the LTBI or HC participants. We did not observe any differences in the levels of IFN-γ and IL-6 between the HIV+LTBI and HC participants.
We determined the independent influence of LTBI and HIV on these pro-inflammatory cytokines using multivariable linear regression models. Both HIV and LTBI were independently associated with higher TNF-α when adjusting for demographics and clinical factors (Fig. 2). LTBI remained independently associated with higher IL-12p70, and IL-17A. HIV but not LTBI was independently associated with higher IL-6 and IL-15 in adjusted models controlling for LTBI (P < 0.01). The effect of LTBI on IL-12p70 was modified by HIV (P = 0.049 for LTBI by HIV status interaction). Plasma IL-12p70 levels were higher in LTBI+ participants than LTBI- among those with HIV (P < 0.0001 in adjusted model), while IL-12p70 levels were comparable among those without HIV (LTBI vs. HC; P = 0.70). No significant interaction was observed between HIV and LTBI in relationship to TNF-α or IL-17A.
We next assessed the influence of LTBI on proinflammatory cytokines by LTBI/HIV status (Table 2). In a fully adjusted model, the HIV+LTBI+ status was associated with higher IL-6, TNF-α IL-12p70, and IL-17A (P < 0.05 for all) in comparison to the HC. Both HIV+LTBI+ and HIV exhibited higher IL-15 compared to the HC participants. In analyses stratified by sex, HIV+LTBI+ co-infected women demonstrated higher plasma IL-6, IL-17a, TNF-α, IL-12p70, IL-15 than the HC women (P < 0.05; Table 3). There were no significant associations between HIV/LTBI groups and the pro-inflammatory cytokines in male participants (P > 0.05).
Effect of latent tuberculosis infection on anti-inflammatory cytokines in chronic HIV infection
To determine the influence of LTBI on anti-inflammatory cytokines in PWH, we compared plasma Type 2 cytokines (IL-4, IL-5, and IL-13) in HIV positive and negative persons with and without LTBI. HIV+LTBI+ participants had significantly higher systemic median levels of IL-4 and IL-13 cytokines compared to the healthy controls, HIV, and LTBI mono-infected groups (P < 0.05 for all; Fig. 1). Levels of IL-5 were also increased in HIV+LTBI+ in comparison to the HIV-negative participants but were comparable to those of HIV mono-infected participants.
In multivariable analyses, HIV status was independently associated with higher IL-4 and IL-5. LTBI status but not HIV status was independently associated with IL-13 (P < 0.05 for all; Fig. 2). The effect of LTBI on IL-4 was modified by HIV (P = 0.02 for LTBI by HIV status interaction). Plasma IL-4 levels were higher in LTBI+ than LTBI- among those with HIV (HIV+LTBI+ vs. HIV; P = 0.0003), whereas levels were similar between LTBI+ and LTBI– among those without HIV (LTBI vs. HC; P = 0.45). The interaction between HIV and LTBI on IL-13 was not statistically significant (P = 0.52).
Next, we examined the relationship between LTBI/HIV status and IL-4, IL-5, and IL-13 (Table 2). In the fully adjusted models, only HIV+LTBI+ co-infection status remained significantly associated with higher IL-4 and IL-13 cytokines relative to the HC. Both HIV+LTBI+ and HIV had significantly higher IL-15 in comparison to the HC participants. We did not observe any significant differences in the levels of these biomarkers between the HC and any other groups (LTBI or HIV). In analyses stratified by sex, we show that only women HIV+LTBI+ participants exhibited higher IL-4, IL-5, and IL-13 cytokines than HC women (P < 0.001; Table 3). We did not observe any differences in the levels of these cytokines between the HIV/LTBI groups in men.
We conducted sensitivity analysis limiting our analysis to PWH and further adjusted for ART duration, nadir, and current CD4+ T-cell count. Even after adjustment for these HIV specific risk factors, LTBI remained independently associated with higher pro-(TNF-α, IL-12p70, and IL-17A) and anti (IL-4 and IL-13) inflammatory cytokines (Figure 1, Supplemental Digital Content).
Discussion
In this cohort of African adults, we show that LTBI is independently associated with higher pro- (TNF-α, IL-12p70, IL-17A) and anti (IL-4, IL-13) inflammatory markers. HIV is independently associated with higher monocyte activation and gut permeability biomarkers (sCD14 and I-FABP), pro-(IL-6) and anti(IL-4, IL-5) inflammatory markers. Importantly, we demonstrate for the first time that despite long-term ART, African persons with HIV and LTBI co-infection exhibit immune dysregulation characterized by heightened systemic levels of pro-(TNF-α, IL-6, IL-12p70, IL-15, IL-17A) and anti-inflammatory cytokines (IL-4, IL-5, 1L-13), women more so than men. While both HIV and LTBI have previously been reported to dysregulate cytokine production, the synergistic effect of LTBI/HIV co-infection on circulating cytokines after long-term ART using multivariate approaches has not been explored prior to our study. We provide the most direct evidence that coexisting LTBI may profoundly alter the immune response in PWH at the systemic level.
Immune dysregulation is a hallmark of HIV [1,2]. Our data demonstrate elevated levels of both pro- and anti-inflammatory cytokines in our participants with HIV on ART, consistent with prior reports from Italy [19], France [22], South Africa [12] and Nigeria [23,24]. Our study, however, contrasts with some other studies that showed normalization of plasma cytokines after ART. Though the root causes of persistent immune activation during ART are unclear, the fact that most of our participants had undetectable viral load implicates potential factors beyond HIV viremia such as co-infections, ART toxicity, and microbial translocation. Of interest, HIV is independently associated with higher monocyte activation and gut permeability indicating that gut microbial translocation may partially contribute to this heightened immune activation. The fact that only HIV and LTBI+ co-infection was associated with an exaggerated production of certain cytokines as compared to HIV alone in our cohort suggests that LTBI may also play an important role. Apart from I-FAPB, sCD14, IL-5, and IL-15, which were also increased in the HIV group, we did not find increased measures of other biomarkers among the HIV and LTBI mono-infected participants in comparison to the healthy controls. We also found that LTBI modulation of cytokine response was largely dependent on HIV, as HIV−LTBI+ individuals failed to exhibit significant differences in levels of most biomarkers in comparison to the healthy controls. This indicates that co-infection with LTBI may enhance abnormal cytokine production in PWH possibly through greater expression of pro- and anti-inflammatory cytokines by the macrophages and Th cells. Our study further expands previous reports in the US that found high sCD14, IL-6 and MCP-1 but not TNF-α in HIV+LTBI+ individuals on long-term ART [25,26]. In another study from South Africa, plasma biomarkers of inflammation were not elevated, but HIV+LTBI+ had higher T-cell activation [12]. A study in Peru reported distinct monocyte alteration suggestive of an exacerbated inflammatory response among individuals with LTBI compared with non-LTBI individuals [27]. Some differences between our data and these prior studies may be explained by the reference group used and differences in study size and population.
Early onset of protective type 1 and 17 cytokines (IFN-γ, TNF-α, IL-2, IL-17A) in the latent stage appears to be key in long-term protection and host resistance to TB reactivation and development of active disease [16]. IFN-γ and TNF-α are critically important for controlling Mycobacterium tuberculosis bacterial growth due to their phagocyte activating functions and their important role in granuloma formation [28,29]. Anti-inflammatory cytokines (IL-4, IL-5, IL-10, and IL-13) appear to regulate type 1 cytokine production and high circulating levels may be counterproductive when infected with TB [30,31]. Thus, alteration in type 1/2 cytokine balance could lead to increased risk of reactivation of TB. Potential drivers for overexpression of Th2 cytokines may be parasitic co-infection endemic in Kenya. It has been previously reported that asymptomatic helminth infection in active TB was associated with marked decreases in dual-functional Th1 and Th17 cell frequencies [32] and a marked increase in Treg and Th2 responses [33]. The heightened anti-inflammatory milieu in HIV+LTBI+ individuals may explain why PWH in TB-endemic countries tend to have increased risk of LTBI reactivation [34,35].
Globally, TB rates are significantly higher in men than women [36]. Although the mechanism underlying this disparity is unclear, sex dimorphism in immune response likely play a role [37]. Sex differences in the immune response have long been observed with vaccines and infections,[38]. This heightened immune response in women may be protective against co-infections but may be maladaptive in the long run since immune activation may increase the risk of non-communicable diseases (NCD). A study in Uganda among adults on long-term ART demonstrated that HIV-positive women had significantly higher levels of sCD14, sCD163, IL-6, IFAPB, and hsCRP than men. In our study, we show for the first time that HIV positive African women co-infected with LTBI in our cohort exhibited heightened plasma anti and pro-inflammatory cytokine response compared to the HIV-/LTBI- women [39]. This cytokine responses were not observed in our male participants. Because we lack past studies for comparison, future validation of these findings in other cohorts and populations is warranted.
Our finding of heightened immune activation among otherwise healthy PWH with LTBI is of clinical importance since immune activation is associated non-AIDS related comorbidities, and mortality in this population [9,25]. LTBI-induced inflammation may lead to increased risk of atherosclerotic CVD in HIV [9,25]. Biomarkers such as TNF-α IL-4, IL-6, and IL-17A, elevated in HIV+LTBI+ individuals in our cohort have all been linked in CVD pathogenesis. Systemic TNF-α and IL-17A are thought to contribute to increased CVD risk by mediating endothelial damage [40,41]. We also recently demonstrated an association between IL-4 with carotid plaque burden in PWH, suggesting a direct role of type 2 type response in atherosclerosis plaque development [42]. Our observation that the cytokine response was significantly increased in women is concerning and could explain the higher risk of CVD observed among the HIV-positive women with HIV in the SSA compared to the men [43,44]. Our results are particularly relevant in the context of Kenya and other TB-endemic settings and provides support for LTBI treatment to reduce inflammation in persons with HIV infection. It is important to point out that the potential effects of LTBI treatment on the measured cytokine is currently unknown and is an area of ongoing investigation.
Our study has several strengths and limitations. It is the first study to assess the combined effects of HIV and LTBI on type 1, 2, and 17 cytokines in a cohort of adult men and women on suppressive ART. An equal number of men and women were included in the analysis allowing us to robustly assess for sex differences. The large sample size enables us to adjust for multiple confounders. The inclusion of HIV-negative individuals for comparison is also a key strength. As with any cross-sectional studies, we cannot assume causality. We used QFT Plus, an IGRA based assay, for the diagnosis of LTBI. There is no gold standard test for LTBI however IGRA based assays are preferred over tuberculin skin test (TST) as these assays are more sensitive than TST [45]. The definition of LTBI using IGRA may include a heterogenous group of individuals; possibly including those with subclinical and incipient TB [46]. Given this heterogeneity, inflammatory marker profiles may vary in those defined as having LTBI.
Conclusion
In summary, we report here that asymptomatic latent tuberculosis infection is common in African adults, may be a potential driver of the persistent inflammation and immune activation observed in virally suppressed African PWH. Our results provide further support for screening and treatment for TB infection targeting this high-risk group to reduce inflammatory associated disease risk and mortality in this population. As LTBI and HIV are both prevalent in SSA, more prospective studies are needed to define health risks associated with LTBI among Africans with HIV.
Acknowledgements
We thank Celestine Adogo, Geoffrey Omondi, and Juliet Aldo, for their contributions. T.M.T., C.F., S.T.P., J.S.Z., and C.N.W. contributed to the conception and design of the study, the supervision, data acquisition, analysis and interpretation, and the critical revision of the manuscript. J.S.Z., C.N.W., H.J., S.J.P., S.D., B.C., B.G., C.L., N.A.M., S.S., J.N.M., S.M.L., K.C., J.O., and S.M. contributed to the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript. All authors contributed to editing of the manuscript and approved submission of the final draft for publication.
Source of funding: This project was supported by National Institutes of Health (NIH) grant R21TW010459, P30AI027757, and R21TW010459-02S1; The GlaxoSmithKline R&D grant number 3001388945; and EDCTP2 program grant TMA-2016-1598-Kenya CVHIV. T.M.T. is supported by NIH grant 1K01HL147723. J.S.Z. was supported by the NIH D43TW009345 and K12 HD043451 and the Firland Foundation 20180018 and 20180017C. The funders did not participate in data collection or any activity that is directly related to the execution of the research.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Carey Farquhar and Jerry S. Zifodya contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.Masyuko SJ, Page ST, Polyak SJ, Kinuthia J, Osoti AO, Otieno FC, et al. Human immunodeficiency virus is associated with higher levels of systemic inflammation among Kenyan adults despite viral suppression. Clin Infect Dis 2020; 73:e2034–e2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Temu TM, Zifodya JS, Polyak SJ, Wagoner J, Wanjalla CN, Masyuko S, et al. Antiretroviral therapy reduces but does not normalize immune and vascular inflammatory markers in adults with chronic HIV infection in Kenya. AIDS 2020; 35:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009; 51:268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr 2015; 68:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-inflammatory markers in relation to cardiovascular disease in HIV infection. A systematic review. PLoS One 2016; 11:e0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friis-Møller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349:1993–2003. [DOI] [PubMed] [Google Scholar]
- 7.Health KMo. Kenya latent tuberculosis infection policy; 2020. [Google Scholar]
- 8.Zifodya JS, Temu TM, Masyuko SJ, Nyale G, Kinuthia J, Page ST, et al. HIV, pulmonary infections, and risk of chronic lung disease among Kenyan adults. Ann Am Thorac Soc 2021; 18:2090–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huaman MA, De Cecco CN, Bittencourt MS, Ticona E, Kityo C, Ballena I, et al. Latent tuberculosis infection and subclinical coronary atherosclerosis in Peru and Uganda. Clin Infect Dis 2021; 73:e3384–e3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huaman MA, Ticona E, Miranda G, Kryscio RJ, Mugruza R, Aranda E, et al. The relationship between latent tuberculosis infection and acute myocardial infarction. Clin Infect Dis 2018; 66:886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheu JJ, Chiou HY, Kang JH, Chen YH, Lin HC. Tuberculosis and the risk of ischemic stroke: a 3-year follow-up study. Stroke 2010; 41:244–249. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan ZA, Wong EB, Ndung’u T, Kasprowicz VO, Bishai WR. Latent and active tuberculosis infection increase immune activation in individuals co-infected with HIV. EBioMedicine 2015; 2:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulougoura A, Sereti I. HIV infection and immune activation: the role of coinfections. Curr Opin HIV AIDS 2016; 11:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan J, Pandey S, Filion LG, Angel JB, Kumar A, Cameron DW. Comparison of interferon-γ-, interleukin (IL)-17- and IL-22-expressing CD4 T cells, IL-22-expressing granulocytes and proinflammatory cytokines during latent and active tuberculosis infection. Clin Exp Immunol 2012; 167:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etna MP, Giacomini E, Severa M, Coccia EM. Pro- and anti-inflammatory cytokines in tuberculosis: a two-edged sword in TB pathogenesis. Semin Immunol 2014; 26:543–551. [DOI] [PubMed] [Google Scholar]
- 16.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol 2013; 31:475–527. [DOI] [PubMed] [Google Scholar]
- 17.Mayer-Barber KD, Barber DL. Innate and adaptive cellular immune responses to Mycobacterium tuberculosis infection. Cold Spring Harb Perspect Med 2015; 5:a108424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rook GA. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med 2007; 7:327–337. [DOI] [PubMed] [Google Scholar]
- 19.Bordoni V, Sacchi A, Casetti R, Cimini E, Tartaglia E, Pinnetti C, et al. Impact of ART on dynamics of growth factors and cytokines in primary HIV infection. Cytokine 2020; 125:154839. [DOI] [PubMed] [Google Scholar]
- 20.Ministry of Health NASCP. Guidelines on use of antiretroviral drugs for treating and preventing HIV infection in Kenya 2018. NASCOP; 2018. [Google Scholar]
- 21.Borges AH, O’Connor JL, Phillips AN, Ronsholt FF, Pett S, Vjecha MJ, et al. Factors associated with plasma IL-6 levels during HIV infection. J Infect Dis 2015; 212:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinon F, Michelet C, Peguillet I, Taoufik Y, Lefebvre P, Goujard C, et al. Persistent alterations in T-cell repertoire, cytokine and chemokine receptor gene expression after 1 year of highly active antiretroviral therapy. AIDS 1999; 13:185–194. [DOI] [PubMed] [Google Scholar]
- 23.Clerici M, Shearer GM. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today 1993; 14:107–111. [DOI] [PubMed] [Google Scholar]
- 24.Osuji FN, Onyenekwe CC, Ahaneku JE, Ukibe NR. The effects of highly active antiretroviral therapy on the serum levels of pro-inflammatory and anti-inflammatory cytokines in HIV infected subjects. J Biomed Sci 2018; 25:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaVergne S, Umlauf A, McCutchan A, Heaton R, Benson C, Kumarasamy N, et al. Impact of latent tuberculosis infection on neurocognitive functioning and inflammation in HIV-infected and uninfected South Indians. J Acquir Immune Defic Syndr 2020; 84:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroeze S, Wit FW, Rossouw TM, Steel HC, Kityo CM, Siwale M, et al. Plasma biomarkers of human immunodeficiency virus-related systemic inflammation and immune activation in sub-Saharan Africa before and during suppressive antiretroviral therapy. J Infect Dis 2019; 220:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feria MG, Chang C, Ticona E, Moussa A, Zhang B, Ballena I, et al. Pro-inflammatory alterations of circulating monocytes in latent tuberculosis infection. Open Forum Infect Dis 2022; 9:ofac629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahey T, Sheth S, Matee M, Arbeit R, Horsburgh CR, Mtei L, et al. Interferon γ responses to mycobacterial antigens protect against subsequent HIV-associated tuberculosis. J Infect Dis 2010; 202:1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavalcanti YV, Brelaz MC, Neves JK, Ferraz JC, Pereira VR. Role of TNF-alpha, IFN-gamma, and IL-10 in the development of pulmonary tuberculosis. Pulm Med 2012; 2012:745483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rook GA, Hernandez-Pando R, Dheda K, Teng Seah G. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol 2004; 25:483–488. [DOI] [PubMed] [Google Scholar]
- 31.Ordway DJ, Costa L, Martins M, Silveira H, Amaral L, Arroz MJ, et al. Increased interleukin-4 production by CD8 and gammadelta T cells in health-care workers is associated with the subsequent development of active tuberculosis. J Infect Dis 2004; 190:756–766. [DOI] [PubMed] [Google Scholar]
- 32.George PJ, Anuradha R, Kumar NP, Sridhar R, Banurekha VV, Nutman TB, et al. Helminth infections coincident with active pulmonary tuberculosis inhibit mono- and multifunctional CD4+ and CD8+ T cell responses in a process dependent on IL-10. PLoS Pathog 2014; 10:e1004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abate E, Belayneh M, Idh J, Diro E, Elias D, Britton S, et al. Asymptomatic helminth infection in active tuberculosis is associated with increased regulatory and Th-2 responses and a lower sputum smear positivity. PLoS Negl Trop Dis 2015; 9:e0003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med 1989; 320:545–550. [DOI] [PubMed] [Google Scholar]
- 35.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 2010; 50: (Suppl 3): S201–S207. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Global tuberculosis report 2019. 2019. Available at: https://www.who.int/publications/i/item/9789241565714. [Google Scholar]
- 37.McClelland EE, Smith JM. Gender specific differences in the immune response to infection. Arch Immunol Ther Exp (Warsz) 2011; 59:203–213. [DOI] [PubMed] [Google Scholar]
- 38.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–638. [DOI] [PubMed] [Google Scholar]
- 39.Siedner MJ, Zanni M, Tracy RP, Kwon DS, Tsai AC, Kakuhire B, et al. Increased systemic inflammation and gut permeability among women with treated HIV infection in rural Uganda. J Infect Dis 2018; 218:922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karbach S, Croxford AL, Oelze M, Schüler R, Minwegen D, Wegner J, et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol 2014; 34:2658–2668. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009; 116:219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wanjalla CN, Temu TM, Mashayekhi M, Warren CM, Shepherd BE, Gangula R, et al. IL-17A is associated with flow-mediated dilation and IL-4 with carotid plaque in persons with HIV. AIDS 2022; 36:963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kipke J, Margevicius S, Kityo C, Mirembe G, Buggey J, Yun CH, et al. Sex, HIV status, and measures of cardiac stress and fibrosis in Uganda. J Am Heart Assoc 2021; 10:e018767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longenecker CT, Bogorodskaya M, Margevicius S, Nazzinda R, Bittencourt MS, Erem G, et al. Sex modifies the association between HIV and coronary artery disease among older adults in Uganda. J Int AIDS Soc 2022; 25:e25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu P, Chen X, Zhu LM, Yang HT. Interferon-gamma release assays for the diagnosis of tuberculosis: a systematic review and meta-analysis. Lung 2016; 194:447–458. [DOI] [PubMed] [Google Scholar]
- 46.Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev 2018; 31:e00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.