Abstract
Although survival in pediatric acute myeloid leukemia (AML) has increased considerably over the past decades, refractory disease and relapse rates remain high. Refractory and relapsed disease are difficult to treat, with overall survival rates less than 40–50%. Preventing relapse should, therefore, be one of the highest priorities. Current conventional chemotherapy regimens are hard to intensify due to associated toxic complications, hence more effective therapies that do not increase toxicity are needed. A promising targeted agent is the CD33-directed antibody–drug conjugate gemtuzumab ozogamicin (GO). Because CD33 is highly expressed on leukemic cells in the majority of AML patients, GO can be useful for a broad range of patients. Better relapse-free survival (RFS) after therapy including GO has been reported in several pediatric clinical trials; however, ambiguity about the clinical value of GO in newly diagnosed children remains. Treatment with GO in de novo AML patients aged ≥1 month, in combination with standard chemotherapy is approved in the United States, whereas in Europe, GO is only approved for newly diagnosed patients aged ≥15 years. In this review, we aimed to clarify the clinical value of GO for treatment of newly diagnosed pediatric AML patients. Based on current literature, GO seems to have additional value, in terms of RFS, and acceptable toxicity when used in addition to chemotherapy during initial treatment. Moreover, in KMT2A-rearranged patients, the clinical value of GO was even more evident. Also, we addressed predictors of response, being CD33 expression and SNPs, PgP-1 and Annexin A5. The near finalized intent-to-file clinical trial in the MyeChild consortium investigates whether fractionated dosing has additional value for pediatric AML, which may pave the way for a broader application of GO in pediatric AML.
Keywords: antibody–drug conjugates, calicheamicin, immunotherapy, single-nucleotide polymorphism, tailored therapy
Introduction
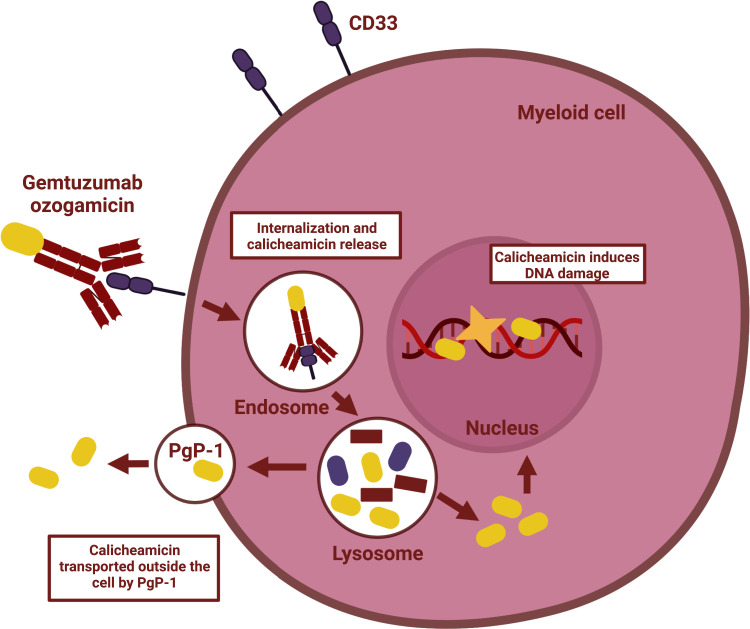
Over the past decades, the prognosis of patients with de novo pediatric acute myeloid leukemia (AML) has improved considerably, resulting in current survival rates around 75%.1,2 However, 5–10% of pediatric AML patients suffers from refractory disease, and 20–35% experiences disease relapse.2,3 In relapsed patients, overall survival (OS) rates are merely 40%, despite intensive therapies including hematopoietic stem cell transplantation (HSCT).2 To further improve OS, preventing the occurrence of relapse should be one of the highest priorities. However, intensifying the conventional chemotherapy regimens that are currently used for the treatment of newly diagnosed pediatric AML patients will likely increase treatment-related mortality (TRM). Hence, there is a need for more targeted, and thereby less toxic therapies that can be integrated safely into the current chemotherapy backbone. A therapy based on targeted delivery to leukemic cells that has been widely used for the treatment of AML is gemtuzumab ozogamicin (GO; Mylotarg®). GO is a CD33-directed monoclonal antibody linked to the cytotoxic agent calicheamicin.4,5 Once the monoclonal antibody is connected to the IgV domain of CD33, the antibody–drug conjugate (ADC) is rapidly internalized and transits to the lysosomes. In the lysosomes, calicheamicin is detached from the antibody and localizes to the nucleus, where it causes double-strand DNA breaks.4,5 These breaks are extremely toxic for proliferating cells and subsequently trigger apoptosis (Figure 1).4,5 Because CD33 is expressed on AML blasts in 85–90% of the patients,4 this therapy holds the potential to be used widely among pediatric AML patients despite the heterogeneous nature of the disease.
Figure 1.
Mechanism of action of gemtuzumab ozogamicin.
The clinical use of GO is dependent on its approval status, which has had a remarkable journey over the past years.6–14 GO was first approved in 2000 by the United States Food and Drug Administration (US FDA) for use in older patients with relapsed AML. However, in 2010 GO was voluntarily withdrawn from the market by the company, due to preliminary results from a Phase III study by the Southwest Oncology Group (SWOG). In hindsight, this withdrawal appeared to be premature.4,15 Specifically, the French ALFA-0701 trial led to re-approval of GO by the FDA in 2017 for adults, and to European Medicines Agency (EMA) approval in 2018 for patients ≥15 years. In this trial, the addition of GO in three fractionated doses of 3 mg/m2 led to a significant improvement in 3-year EFS of 31% (vs 19%) and 3-year RFS of 38% (vs 25%), both in favor of GO, without significant OS benefit. This was mainly caused by improvements in the favorable and intermediate cytogenetic risk group.16 Subsequently, results of the Children’s Oncology Group (COG) AAML0531 trial led to FDA approval for de novo AML patients aged ≥1 month.11,16,17 However, despite several studies suggesting the additional value of GO in pediatric AML, it is currently not registered for the treatment of children with de novo AML <15 years in Europe.6,7,11–13,18
The aim of this review is to address the clinical value of GO in combination with current chemotherapy regimens in the treatment of de novo pediatric AML. We summarize the findings of pediatric clinical trials, with a special focus on the optimal dose and time point during treatment to administer GO. Furthermore, we describe potential predictors of response of GO: CD33 expression, CD33 single-nucleotide polymorphisms (SNPs), the PgP-1 drug efflux transporter, and Annexin A5.19–26
Methods
Search Strategy
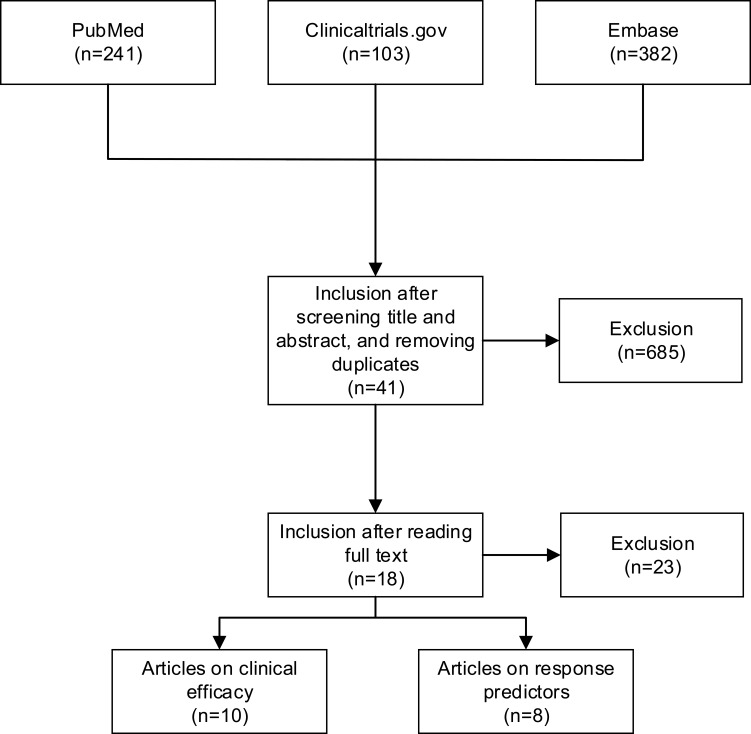
This literature review was conducted in May 2022, using a triplex approach. First, PubMed was searched using the following search terms: “gemtuzumab ozogamicin, mylotarg, GO, gemtuzumab”. In addition, ClinicalTrials.gov and Embase were searched using equal search terms, to cross-check previously identified clinical trials, and to identify any potentially missed or ongoing trials (Figure 2). Reference lists of the included articles were screened (which did not yield additional articles). Last, duplicates were removed.
Figure 2.
Flow chart of the literature selection process.
Eligibility
Articles were eligible for inclusion if they described clinical trials (randomized controlled trials [RCTs], prospective or retrospective cohort studies) that investigated either clinical efficacy (OS, event-free survival [EFS], relapse rate [RR], and relapse-free or disease-free survival [RFS/DFS]) as well as toxicity, or response predictors of GO in newly diagnosed pediatric AML patients. Studies were also eligible if the study population was a combination of pediatric and adult patients. Only English, peer-reviewed articles were included.
Screening
Endnote version X9 (Clarivate analytics) was used to process and to identify potential articles. Titles and abstracts were independently screened for eligibility (JK, NW). Discrepancies were discussed (JK, NW). Figure 2 shows a flow chart of the literature selection process.
Results
Clinical Trials Investigating the Clinical Efficacy of GO
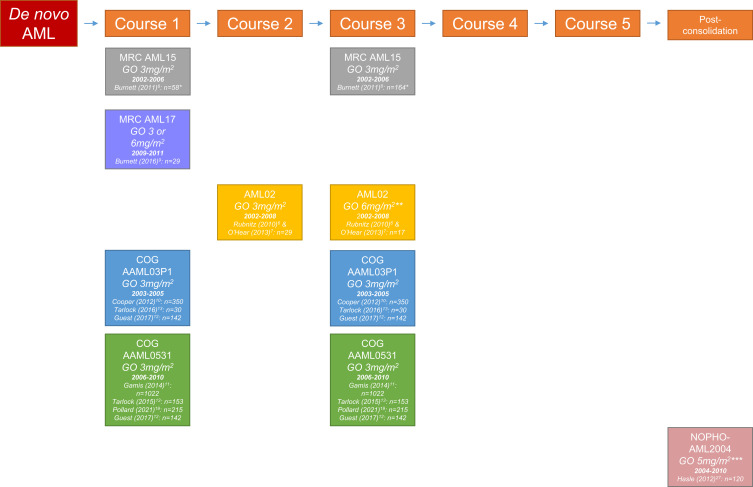
Over the past years, the clinical efficacy of GO for the treatment of newly diagnosed pediatric AML has been assessed in various clinical trials. Ten articles encompassing six trials met our inclusion criteria.6–13,19,27 These trials investigated the clinical efficacy of GO using different dosing regimens that were administered at different time points during treatment. Table 1 provides a summary of all the published trials that assessed the efficacy of GO in children with newly diagnosed AML. An overview of all trials and associated articles including the study group, dose, and treatment time point is presented in Figure 3.
Table 1.
Clinical Trials Investigating the Clinical Efficacy of Gemtuzumab Ozogamicin in Newly Diagnosed Pediatric Acute Myeloid Leukemia
| Study Name | Recruitment Period | Patients (n) | Age Range | GO Dose Regimen | Clinical Efficacy | Toxicity |
|---|---|---|---|---|---|---|
| NRCI MRC AML158 | July 2002 – June 2006 | 1113, 58 patients aged 0–14 years | 0–71 years | Patients were randomized to receive: Induction 1 (n=1113):
Consolidation 1 (n=948):
|
|
|
| NRCI MRC AML179 | June 2009 – October 2011 | 788, 29 patients aged 0–16 years | 0–60 years | Patients were randomized to receive: Induction 1 (n=788):
|
|
|
| St. Jude AML 026,7 | 2002–2008 | 216 | 2 days – 21.4 years | Patients were non-randomly assigned to receive: Induction 2:
Induction 3:
Amendment: Induction 2:
|
|
|
| COG AAML03P110,12,13 |
December 2003 – November 2005 | 350 | 25 days - 21.6 years | All patients non-randomly received (n=350): Induction 1 (day 6):
Consolidation 2 (day 7):
|
|
|
| COG AAML053111−13,18 |
August 2006 – June 2010 | 1022 | 30 days – 29 years | Patients were randomized to receive: Induction 1 (day 6):
Consolidation 2 (day 7):
Or standard five course chemotherapy alone (n=511) |
|
|
| NOPHO-AML 200427 | January 2004–2010 | 120 | 0–18 years | Patients were randomized to receive: Post-consolidation:
|
|
|
Abbreviations: ADE, Ara-C + daunorubicin + etoposide; AML, acute myeloid leukemia; CIR, cumulative incidence of relapse; COG, Children’s Oncology Group; CR, complete remission; DA, daunorubicin + cytarabine; EFS, event-free survival; GO, gemtuzumab ozogamicin; HSCT, hematological stem cell transplantation; FLAG-Ida, fludarabine, cytarabine, and idarubicin; MA, mitoxantrone + cytarabine; MACE, amsacrine, cytarabine and etoposide; MRC, Medical Research Council; NOPHO, Nordic Society of Paediatric Haematology and Oncology; NRCI, National Cancer Research Institute; OS, overall survival; RFS, relapse-free survival; RR, relapse rate; VOD, veno-occlusive disease.
Figure 3.
Overview of study groups assessing the effect of gemtuzumab ozogamicin during different time periods in treatment of pediatric acute myeloid leukemia.
Notes: The number of patients concerns pediatric patients who were randomized to either receive GO or not. *Patients 0–14 years. **Initially, the combination of ADE + GO was used only in patients with >25% bone marrow blasts after induction 1. Patients with an MRD level >0.1% after induction 2 were given GO at a dose of 6 mg/m2 as induction 3. The protocol was amended in 2005 to give ADE + GO as induction 2 to patients with an MRD level >1% after induction 1, and thereby eliminate the use of single-agent GO as induction 3. ***As post-consolidation therapy, repeated after 3 weeks.
Abbreviations: ADE, Ara-C + daunorubicin + etoposide; AML, acute myeloid leukemia; COG, Children’s Oncology Group; GO, gemtuzumab ozogamicin; MRC, Medical Research Council; NOPHO, Nordic Society of Paediatric Haematology and Oncology.
Gemtuzumab Ozogamicin During Induction 1 and Consolidation 1
The UK-based Medical Research Council (MRC) initiated two clinical trials investigating the efficacy of GO, in combined adult-pediatric studies. First, the randomized AML15 trial8 examined the addition of GO to chemotherapy in de novo AML patients <60 years. The trial enrolled 1113 patients, of whom 8 patients aged 0–14 years received GO during induction 1 (3 mg/m2; vs 6 no-GO), and 29 patients aged 0–14 years received GO during consolidation 1 (3 mg/m2; vs 29 no-GO in the same age group).8 In the total cohort, there were no statistically significant differences in OS, RFS and cumulative incidence of relapse (CIR) with the addition of GO. Although this trial demonstrated a significant benefit in terms of OS for the favorable cytogenetic subgroup28 (79% vs 51%, p<0.001), there was no observed OS benefit for the patients aged ≥30 years in the other subgroups, neither for all patients aged 0–29 years. Overall, the addition of GO was well tolerated with no significant increase in toxicity.8
The AML17 trial9 investigated the effect of adding GO to the first induction course in a randomized setting at a dose of either 6 mg/m2 or 3 mg/m2. In this trial, 788 patients were enrolled, of whom only 29 were <16 years. In the total cohort, a single dose of 6 mg/m2 GO as compared to 3 mg/m2 GO did not improve 4-year OS (47% vs 50%, p=0.3), or 4-year RFS (38% vs 44%, p=0.3). Concerning toxicity, the 30-day and 60-day mortality were both significantly increased in the 6 mg/m2 group in the total cohort (7% vs 3%, p=0.02, and 9% vs 5%, p=0.01, respectively). Causes of death mostly concerned infection (11 vs 10), hemorrhage (4 vs 3), resistant disease (6 vs 2) and veno-occlusive disease (VOD) (5 vs 0), in the the 6 mg/m2 vs 3 mg/m2 group, respectively. Among the 29 patients aged <16 years, there was no benefit for OS (OR: 0.56 [95% CI 0.14–2.23]). Other outcomes were not reported for this subgroup. Hence, the use of GO at 6 mg/m2 increased toxicity without improving clinical efficacy in comparison with 3 mg/m2 during induction therapy, indicating that 3 mg/m2 was the more favorable dose.9
Gemtuzumab Ozogamicin During Induction 2
The St. Jude Study group assessed the clinical efficacy of GO, either alone or in combination with chemotherapy, in a multi-intervention non-randomized clinical trial in AML patients ≤21 years. Initially, the combination of chemotherapy (ADE: Ara-C, daunorubicin and etoposide) and GO at a dose of 3 mg/m2 as induction 2 was used in patients with refractory disease (>25% bone marrow blasts after induction 1; n=9). Also, patients with a measurable residual disease (MRD) level >0.1% after induction 2 were given GO at a dose of 6 mg/m2 as induction 3. Three years after study initiation, the protocol was amended to give ADE plus GO at a dose of 3 mg/m2 as induction 2 to patients with an MRD level >1% after induction 1, and eliminated the use of GO monotherapy as induction 3. In total, 29 patients received ADE and GO (3 mg/m2) as induction 2, and 17 received GO monotherapy (6 mg/m2) as induction 3. Twenty-seven of the 29 patients who received GO during induction 2 had a decrease in MRD.6,7 Before the amendment, MRD became negative in 4 of the 9 refractory patients receiving GO. After the amendment, MRD became negative in 9 of 20 patients receiving GO with MRD levels >1%.7 The 5-year OS and 5-year EFS from start of induction 2 were not significantly improved using GO (OS: 55.0% vs 36.4%, p=0.28, EFS: 50.0% vs 31.8%, p=0.28, respectively). Of the 17 patients who received GO monotherapy at 6 mg/m2 as induction 3 (patients with MRD >0.1% after induction 2), 13 became MRD negative.7 Even though the efficacy of GO was not the main focus of this multi-intervention study, the administration of GO resulted in a decrease in MRD in most patients, without significantly improving OS. Notably, GO was exclusively administered to patients with poor early responses, which limits the generalizability of the findings to all newly diagnosed AML patients.6,7
Gemtuzumab Ozogamicin as Remission Induction and Post-Remission Consolidation
The COG AAML03P1 pilot trial primarily investigated the safety of the addition of two doses of GO (3 mg/m2) to chemotherapy in a non-randomized setting, one during induction and one during consolidation, in patients ≤21 years (n=340) (Table 1).10 The second dose of GO during consolidation was administered to children not undergoing HSCT. In this study, 3-year OS was 66%, and 3-year EFS was 53%. Toxicity was similar compared with conventional AML chemotherapy regimens. The most frequently reported adverse events were infections (grade ≥3).10 This pilot study demonstrated that the addition of GO was safe and feasible, and that the outcomes were more favorable compared to other clinical trials in that era reporting 5-year OS of around 67% and 5-year EFS of around 55%.10
Based on these results, the aim of the subsequent randomized AAML0531 phase III trial was to determine whether GO added to standard chemotherapy improved OS and EFS in children with newly diagnosed AML. The study design and backbone chemotherapy were similar to the AAML03P1 trial, although patients in the AAML0531 trial were randomized to receive GO or not (Table 1).11 In total, 1022 patients aged 0–29 years were included (n=1009 aged 0–20 years) and half of these patients received two doses of GO (3 mg/m2). The 3-year EFS in this group was significantly higher compared with controls (53.1 vs 46.9%, p=0.04), mainly because of a reduction in RR (32.8% vs 41.3%, p=0.01), while 3-year OS was similar (69.4% vs 65.4%, p=0.39). This may in part be explained by the somewhat increased 5-year TRM in the GO group compared to the no-GO group (8.6% vs 5.9%, p=0.09). Specifically, there was significantly more TRM in the low-risk group (t(8;21) or inv(16)): 7.5% vs 1.8%, p=0.04, respectively.11 Furthermore, Pollard et al18 reported a sub-analysis of 215 patients with KMT2A-rearranged AML who showed similar TRM with or without GO, but with a trend towards improved OS (63% vs 53%, p=0.05, respectively), together with improved EFS (48% vs 29%, p<0.01, respectively) and reduced RR (40% vs 66%, p<0.001, respectively).18 Likewise, a sub-analysis on the efficacy of GO in 142 infants ≤1 year showed similar TRM in both groups, and a non-significant 5-year OS and EFS (OS: 66% vs 57%, p=0.22; EFS: 47% vs 37%, p=0.19).12 In FLT3-ITD positive patients within the AAML03P1 and AAML0531 trials (n=30 and n=153, of whom 112 received GO), findings were similar to what was reported for the total cohort: RR was significantly decreased (37% vs 59%, p=0.02), but OS did not differ (50% vs 49%, p=0.74, respectively), while TRM was significantly increased in the GO group (16% vs 0%, p<0.001).13
Gemtuzumab Ozogamicin as Post-Consolidation Therapy
The Nordic Society of Paediatric Haematology and Oncology (NOPHO) AML 2004 study investigated the effect of GO (5 mg/m2) as post-consolidation therapy in de novo pediatric AML patients, in a randomized setting, at least 4 weeks after the last consolidation course, and repeated once after 3 weeks.27 In patients receiving GO (n=59) compared to no further therapy (n=61), the median time to relapse increased non-significantly (15 months vs 11 months), while the number of relapses was similar in both groups (24 [41%] vs 25 [41%]). Outcome was similar for both the GO and no-GO groups (5-year OS: 74% vs 80% and 5-year EFS: 55% vs 51%, respectively). Regarding toxicity, GO appeared to be well tolerated with only modest elevations of transaminase and bilirubin levels in the intervention group, and no signs of VOD.27
Clinical Trials Addressing Response Predictors
In order to aid the selection of pediatric AML patients that will most likely benefit from GO, it is crucial to investigate possible response predictors to GO. In this review, we included 8 articles19–26 that identified several response predictors: CD33 expression, CD33 SNPs, the drug transporter PgP-1, and Annexin A5.
CD33 Expression
The relation between CD33 expression and clinical benefit of GO in both adults and children has been addressed in multiple studies, often showing that a higher CD33 expression correlates with better GO response.19,25,29–31 In children with AML, Pollard et al25 prospectively quantified CD33 expression levels in samples of 825 patients in the AAML0531 trial, and found that patients in the lowest quartile of CD33 expression had no clinical benefit from GO, whereas patients in quartiles 2–4 had a significant improvement in 5-year EFS (53% vs 41%, p<0.01), a reduction in RR (32% vs 49%, p<0.001) and an improvement in 5-year RFS (47% vs 61%, p<0.01). There was no difference in OS in both groups.19 Although this study did not establish an absolute CD33 expression threshold, it does provide evidence that the expression level of CD33 is a relevant predictive factor for response to GO. In the MRC15 trial (n=418), the researchers differentiated CD33 expression levels between either positive (>20% of the blasts expressed CD33) or negative, and demonstrated a lower RR (OR: 0.80 95% CI 0.64–0.99) and increased 5-year RFS (OR: 0.81 95% CI 0.67–0.98) in CD33-positive patients that received GO. However, the group classified as negative was very small (n=43), possibly distorting adequate analysis.8
CD33 Single-Nucleotide Polymorphisms
Another response predictor of GO concerns the contribution of host genetic factors, such as single-nucleotide polymorphisms (SNPs), which have been identified by several studies.20–22,26 SNP rs12459419 C>T was found to lead to the loss of the V-set antibody binding domain.4,20 Because most diagnostic antibodies are directed at this V-set domain, these patients would appear to be CD33 negative, while actually being CD33 positive. Most importantly, the lack of this V-set domain impacts the GO binding capacity and hence, the efficacy of GO. Thus, this SNP C>T influences the ability to adequately identify the level of CD33 expression, but also the binding capacity of GO, making the CD33 expression non-functional. This was demonstrated in the AAML0531 trial. In this trial, patients with the CC genotype in rs12459419, hence without the T allele, had nearly a 50% reduction in RR (26% vs 49%, p<0.001) and an improved DFS (65% vs 46%, p<0.01) when treated with GO compared to those who were not, respectively. OS did not differ significantly (66% vs 58%, p=0.16).20 In contrast, in patients with at least one T allele, the addition of GO did not result in any clinical benefit.20,22 These results suggest that this loss of V-set domain compromises the efficacy of GO.4 This finding was supported by Lamba et al,26 who found that the presence of the T allele was significantly associated with poor response to GO in pediatric AML patients.26
Drug Transporter PgP-1
The efficacy of GO also relies on the intracellular levels of calicheamicin, which can be influenced by PgP-1, a drug efflux transporter encoded by the ABCB1 gene.32 Due to its mechanism of action, the expression of the ABCB1 gene inversely correlates with GO response. Rafiee et al24 evaluated the impact of ABCB1-SNPs on GO response in DNA samples of patients in the AAML0531 study (n=942).24 The rs1045642 SNP CT/TT allele was associated with lower ABCB1 expression and consequently, lower PgP-1 expression. Patients with this genotype had better outcome in terms of EFS (p=0.02) and RR (p=0.01), compared to patients with the CC genotype.24 Hence, the CT/TT genotype may increase intracellular levels of calicheamicin due to reduced activity of the PgP-1 drug transporter.4,24
Annexin A5
Lastly, another proposed response predictor is the cellular protein annexin A5, encoded by ANAX5. Annexin A5 plays a role in the regulation of cellular growth, differentiation, inflammation and signaling.23 It also functions as an anti-inflammatory and anti-apoptotic agent under stress, aiming to protect cells from dying.23 In line with this, high expression levels of ANXA5 have been associated with adverse outcomes in pediatric AML patients upon conventional chemotherapy.23 Zhang et al23 re-analyzed data from the AAML03P1 and AAML0531 trials, and identified that high ANXA5 expression was an independent favorable factor for OS and EFS upon GO treatment, compared with low ANXA5 expression (p<0.01 and p<0.001, respectively; exact numbers not reported). Thus, the addition of GO to standard chemotherapy may be a way to overcome the adverse prognostic effect of high expression of ANXA5 in pediatric AML patients.23
Discussion
In this review, we aimed to clarify the clinical value of GO in newly diagnosed pediatric AML. Currently, there is no EMA market approval for GO for children with de novo AML aged <15 years, in contrast to FDA approval in the USA.4,5,16,33 In the largest, randomized trial (AAML0531) that investigated the use of GO in addition to chemotherapy in pediatric AML, an improved EFS and reduced RR were reported, although this did not result in superior OS. The lack of OS benefit may be explained at least in part by the increased TRM in the low-risk group that received GO. This is supported by a sub-analysis in KMT2A-rearranged patients, where TRM was similar in both groups, and OS showed a trend towards improvement (63% vs 53%, p=0.05).11 Despite the lack of benefit in terms of OS in the total cohort, the results of this trial ultimately led to FDA approval for de novo AML patients aged ≥1 month. Likewise, all other, smaller trials in de novo pediatric AML, and several sub-analyses in the AAML0531 trial, showed similar results in terms of EFS and RR, without OS benefit with the addition of GO.10,11,13,19,27 Overall, the results in pediatric AML were in line with those in adult AML. For instance, in the French ALFA-0701 trial, the addition of GO resulted in a 3-year EFS of 31% (vs 19%, p<0.01) and a RFS of 38% (vs 25%, p=0.01), both in favor of GO, without significant OS benefit.16,33 Moreover, the NCRI AML 16 trial demonstrated an improved 2-year OS (35% vs 29%, p=0.04) and an improved 2-year RFS (28% vs 23%, p=0.03).34 These findings may suggest that GO is most effective early in treatment, in combination with chemotherapy.35–37
One of the major concerns with the use of GO is liver toxicity, VOD in particular, which has been reported in a previous adult trial.38 Overall, GO given at a dose of 3 mg/m2 was well tolerated in the studies included in this review, with no increased VOD rate and no increased overall toxicity.6–8,10,11,13,18 Albeit TRM was increased in both low-risk and FLT3-ITD patients that received GO in the AML0531 trial, TRM in the no-GO groups was remarkably low (1.8% and 0%, respectively) as compared to what is common (4–5%).11,13 Altogether, a dose of 3 mg/m2 of GO appears to be safe and feasible when used in combination with most conventional chemotherapy regimens. In the MRC17 trial, the use of a single dose of 6 mg/m2 GO as compared to a single dose of 3 mg/m2, led to an increased VOD rate (5.6% vs 0.5%, p<0.0001), with no clinical benefit. Hence, GO at a dose of 3 mg/m2 was considered the more favorable dose in terms of toxicity.9 Whether fractionated dosing has additional value for pediatric AML is unknown, but this is currently being investigated in the intent-to-file MyeChild study (EudraCT Number: 2014–005066-30), in the context of a Pediatric Investigational Plan. Whether GO is useful as monotherapy, often given on compassionate use basis in the relapsed and refractory disease (R/R) setting, has been extensively studied.39–44 For instance, treatment with single-agent high-dose GO was effective for bridging children with advanced AML to HSCT in different Phase I and II trials.40,44 Moreover, Parigger et al45 reviewed the dose-related efficacy and toxicity of GO, mainly in R/R patients, and reported favorable outcomes and acceptable toxicity using a dose of 3 mg/m2, while doses of >6 mg/m2 led to more adverse events.45
Additionally, the different toxicity profile of GO, due to its targeted delivery to leukemic cells, raises the question whether parts of the backbone therapy can be replaced by GO in patients that experience severe toxicity with conventional chemotherapy, for example in patients with cardiotoxicity due to anthracyclines. Currently, the use of the cardioprotectant dexrazoxane was and will be studied in (future) clinical trials, although another approach could be to at least partly replace anthracyclines by GO, without deteriorating OS or EFS.46,47 This is already being applied in individual patients.
To improve treatment benefit, one could consider to determine CD33 expression levels on AML blasts and associated SNPs, since data from the COG AAML0531 and MRC15 trial showed that a higher CD33 expression correlates with better GO response. This is in line with findings in adult trials.8,25 The French ALFA-0701 trial showed that adults with higher CD33 expression (>70% blasts were CD33 positive) had an improved outcome in terms of EFS and RFS, compared to patients with <70% blasts being CD33 positive.30 In addition, the MRC16 and MRC17 trials showed that adults with the lowest quartile of CD33 expression had no benefit from GO, unlike patients in higher quartiles.31 Additionally, SNP rs12459419 C allele has been found to be associated with significant clinical benefit of GO, whereas patients with the T genotype did not derive clinical benefit from GO, due to the loss of the V-set domain.20,26 The latter impacts the GO binding capacity and thereby the efficacy of GO. The other biomarkers PgP-1 (encoded by the ABCB1) and ANXA5 expression are also associated with poorer or better response to GO, respectively.48 Taken together, by identifying predictors of response, GO therapy has the potential to be personalized.
Conclusion
The addition of GO to conventional chemotherapy in the treatment of de novo pediatric AML may improve EFS and reduce RR, but does not seem to improve OS. Response predictors like CD33 expression and associated SNPs, PgP-1 and Annexin A5 may be used to select pediatric AML patients who are mostly likely to benefit from GO. Future studies on the addition of GO could focus on decreasing toxicity, thereby possibly leading to an OS benefit. We feel that the documented clinical efficacy of GO in the frontline treatment of pediatric AML warrants its use. Hopefully, the ongoing MyeChild study will further support its clinical utility and ultimately lead to EMA approval.
Acknowledgments
Figure 1 in this review was created with Biorender.com (accessed October 2022).
Abbreviations
ADC, Antibody–drug conjugate; ADE, Ara-C, daunorubicin, etoposide; AML, Acute myeloid leukemia; CIR, Cumulative incidence of relapse; COG, Children’s Oncology Group; CR, Complete remission; DA, Daunorubicin, cytarabine; DFS, Disease-free survival; EFS, Event-free survival; EMA, European Medicines Agency; FLAG-Ida, Fludarabine, cytarabine, and idarubicin; GO, Gemtuzumab ozogamicin; HSCT, Hematological stem cell transplantation; MA, Mitoxantrone, cytarabine; MACE, Amsacrine, cytarabine and etoposide; MRC, Medical Research Council; MRD, Measurable residual disease; NOPHO, Nordic Society of Paediatric Haematology and Oncology; NRCI, National Cancer Research Institute; OS, Overall survival; RCT, Randomized controlled trial; RFS, Relapse-free survival; RR, Relapse rate; R/R, Relapse and refractory disease; SNP, Single-nucleotide polymorphism; SWOG, Southwest Oncology Group; TRM, Treatment-related mortality; US FDA, United States Food and Drug Administration; VOD, Veno-occlusive disease.
Disclosure
Prof. Dr. C Michel Zwaan reports institutional funding to perform clinical trials from Pfizer, Jazz, Takeda, and AbbVie, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Rasche M, Zimmermann M, Borschel L, et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia. 2018;32(10):2167–2177. doi: 10.1038/s41375-018-0071-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein K, de Haas V, Kaspers GJ. Clinical challenges in de novo pediatric acute myeloid leukemia. Expert Rev Anticancer Ther. 2018;18(3):277–293. doi: 10.1080/14737140.2018.1428091 [DOI] [PubMed] [Google Scholar]
- 3.Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin oncol. 2015;33(27):2949. doi: 10.1200/JCO.2015.62.8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gbadamosi M, Meshinchi S, Lamba JK. Gemtuzumab ozogamicin for treatment of newly diagnosed CD33-positive acute myeloid leukemia. Future Oncol. 2018;14(30):3199–3213. doi: 10.2217/fon-2018-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottardi M, Simonetti G, Sperotto A, et al. Therapeutic Targeting of Acute Myeloid Leukemia by Gemtuzumab Ozogamicin. Cancers. 2021;13:18. doi: 10.3390/cancers13184566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543–552. doi: 10.1016/S1470-2045(10)70090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Hear C, Inaba H, Pounds S, et al. Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia. Cancer. 2013;119(22):4036–4043. doi: 10.1002/cncr.28334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369–377. doi: 10.1200/JCO.2010.31.4310 [DOI] [PubMed] [Google Scholar]
- 9.Burnett A, Cavenagh J, Russell N, et al. Defining the dose of gemtuzumab ozogamicin in combination with induction chemotherapy in acute myeloid leukemia: a comparison of 3 mg/m2 with 6 mg/m2 in the NCRI AML17 Trial. haematologica. 2016;101(6):724. doi: 10.3324/haematol.2016.141937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Cancer. 2012;118(3):761–769. doi: 10.1002/cncr.26190 [DOI] [PubMed] [Google Scholar]
- 11.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32(27):3021. doi: 10.1200/JCO.2014.55.3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guest EM, Aplenc R, Sung L, et al. Gemtuzumab ozogamicin in infants with AML: results from the Children’s Oncology Group trials AAML03P1 and AAML0531. Blood J Am Soc Hematol. 2017;130(7):943–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarlock K, Alonzo TA, Gerbing RB, et al. Gemtuzumab ozogamicin reduces relapse risk in FLT3/ITD acute myeloid leukemia: a report from the Children’s Oncology Group. Clin Cancer Res. 2016;22(8):1951–1957. doi: 10.1158/1078-0432.CCR-15-1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersdorf S, Kopecky K, Stuart RK, et al. Preliminary results of Southwest Oncology Group Study S0106: an international intergroup Phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood. 2009;114(22):790. [Google Scholar]
- 15.Ali S, Dunmore HM, Karres D, et al. The EMA review of mylotarg (gemtuzumab ozogamicin) for the treatment of acute myeloid leukemia. Oncologist. 2019;24(5):e171–e179. doi: 10.1634/theoncologist.2019-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert J, Pautas C, Terré C, et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica. 2019;104(1):113. doi: 10.3324/haematol.2018.188888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norsworthy KJ, Ko CW, Lee JE, et al. FDA approval summary: mylotarg for treatment of patients with relapsed or refractory CD33‐positive acute myeloid leukemia. oncologist. 2018;23(9):1103–1108. doi: 10.1634/theoncologist.2017-0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard JA, Guest E, Alonzo TA, et al. Gemtuzumab ozogamicin improves event-free survival and reduces relapse in pediatric KMT2A-rearranged AML: results from the phase III children’s oncology group trial AAML0531. J Clin oncol. 2021;39(28):3149–3160. doi: 10.1200/JCO.20.03048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollard JA, Loken M, Gerbing RB, et al. CD33 expression and its association with gemtuzumab ozogamicin response: results from the randomized phase III Children’s Oncology Group Trial AAML0531. J Clin oncol. 2016;34(7):747. doi: 10.1200/JCO.2015.62.6846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamba JK, Chauhan L, Shin M, et al. CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo acute myeloid leukemia: report from randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2017;35(23):2674. doi: 10.1200/JCO.2016.71.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauhan L, Shin M, Wang Y-C, et al. CD33_PGx6_score predicts gemtuzumab ozogamicin response in childhood acute myeloid leukemia: a report from the children’s oncology group. JCO Precision Oncol. 2019;3:1–15. doi: 10.1200/PO.18.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortland L, Alonzo TA, Walter RB, et al. Clinical significance of CD33 nonsynonymous single-nucleotide polymorphisms in pediatric patients with acute myeloid leukemia treated with gemtuzumab-ozogamicin–containing chemotherapy. Clin Cancer Res. 2013;19(6):1620–1627. doi: 10.1158/1078-0432.CCR-12-3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang N, Zhang Y, Zhang P, et al. Overexpression of annexin A5 might guide the gemtuzumab ozogamicin treatment choice in patients with pediatric acute myeloid leukemia. Ther Adv Med Oncol. 2020;12:1758835920927635. doi: 10.1177/1758835920927635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafiee R, Chauhan L, Alonzo TA, et al. ABCB1 SNP predicts outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin: a report from Children’s Oncology Group AAML0531 Trial. Blood Cancer J. 2019;9(6):1–8. doi: 10.1038/s41408-019-0211-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollard JA, Alonzo TA, Loken M, et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood J Am Soc Hematol. 2012;119(16):3705–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamba JK, Pounds S, Cao X, et al. Coding polymorphisms in CD33 and response to gemtuzumab ozogamicin in pediatric patients with AML: a pilot study. Leukemia. 2009;23(2):402–404. doi: 10.1038/leu.2008.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasle H, Abrahamsson J, Forestier E, et al. Gemtuzumab ozogamicin as postconsolidation therapy does not prevent relapse in children with AML: results from NOPHO-AML 2004. Blood J Am Soc Hematol. 2012;120(5):978–984. [DOI] [PubMed] [Google Scholar]
- 28.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1612 patients entered into the MRC AML 10 trial. Blood J Am Soc Hematol. 1998;92(7):2322–2333. [PubMed] [Google Scholar]
- 29.Renneville A, Abdelali RB, Chevret S, et al. Clinical impact of gene mutations and lesions detected by SNP-array karyotyping in acute myeloid leukemia patients in the context of gemtuzumab ozogamicin treatment: results of the ALFA-0701 trial. Oncotarget. 2014;5(4):916. doi: 10.18632/oncotarget.1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olombel G, Guerin E, Guy J, et al. The level of blast CD33 expression positively impacts the effect of gemtuzumab ozogamicin in patients with acute myeloid leukemia. Blood J Am Soc Hematol. 2016;127(17):2157–2160. [DOI] [PubMed] [Google Scholar]
- 31.Khan N, Hills RK, Virgo P, et al. Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia. 2017;31(5):1059–1068. doi: 10.1038/leu.2016.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goemans B, Zwaan C, Vijverberg S, et al. Large interindividual differences in cellular sensitivity to calicheamicin may influence gemtuzumab ozogamicin response in acute myeloid leukemia. Leukemia. 2008;22(12):2284–2285. doi: 10.1038/leu.2008.147 [DOI] [PubMed] [Google Scholar]
- 33.Castaigne S, Pautas C, Terré C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–1516. doi: 10.1016/S0140-6736(12)60485-1 [DOI] [PubMed] [Google Scholar]
- 34.Burnett AK, Hills RK, Hunter A, et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013;27(1):75–81. doi: 10.1038/leu.2012.229 [DOI] [PubMed] [Google Scholar]
- 35.Molica M, Breccia M, Foa R, Jabbour E, Kadia TM. Maintenance therapy in AML: the past, the present and the future. Am J Hematol. 2019;94(11):1254–1265. doi: 10.1002/ajh.25620 [DOI] [PubMed] [Google Scholar]
- 36.Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood J Am Soc Hematol. 2013;121(24):4854–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Löwenberg B, Beck J, Graux C, et al. Gemtuzumab ozogamicin as postremission treatment in AML at 60 years of age or more: results of a multicenter phase 3 study. Blood J Am Soc Hematol. 2010;115(13):2586–2591. [DOI] [PubMed] [Google Scholar]
- 38.Wadleigh M, Richardson PG, Zahrieh D, et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood. 2003;102(5):1578–1582. doi: 10.1182/blood-2003-01-0255 [DOI] [PubMed] [Google Scholar]
- 39.Niktoreh N, Lerius B, Zimmermann M, et al. Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: a report by Berlin-Frankfurt-Münster study group. haematologica. 2019;104(1):120. doi: 10.3324/haematol.2018.191841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zwaan CM, Reinhardt D, Corbacioglu S, et al. Gemtuzumab ozogamicin: first clinical experiences in children with relapsed/refractory acute myeloid leukemia treated on compassionate-use basis. Blood J Am Soc Hematol. 2003;101(10):3868–3871. [DOI] [PubMed] [Google Scholar]
- 41.Liu AP-Y, Leung AW-K, Cheuk DK-L, Lee V, Ha S-Y. Gemtuzumab ozogamicin containing chemotherapy for relapsed or refractory acute myeloid leukemia (AML) in children. J Pediatr Hematol Oncol. 2018;40(2):163–168. doi: 10.1097/MPH.0000000000001010 [DOI] [PubMed] [Google Scholar]
- 42.Zwaan CM, Reinhardt D, Zimmerman M, et al. Salvage treatment for children with refractory first or second relapse of acute myeloid leukaemia with gemtuzumab ozogamicin: results of a Phase II study. Br J Haematol. 2010;148(5):768–776. doi: 10.1111/j.1365-2141.2009.08011.x [DOI] [PubMed] [Google Scholar]
- 43.Reinhardt D, Diekamp S, Fleischhack G, et al. Gemtuzumab ozogamicin (Mylotarg®) in children with refractory or relapsed acute myeloid leukemia. Oncol Res Treatment. 2004;27(3):269–272. doi: 10.1159/000075606 [DOI] [PubMed] [Google Scholar]
- 44.Arceci RJ, Sande J, Lange B, et al. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood. 2005;106(4):1183–1188. doi: 10.1182/blood-2004-10-3821 [DOI] [PubMed] [Google Scholar]
- 45.Parigger J, Zwaan C, Reinhardt D, Kaspers G. Dose-related efficacy and toxicity of gemtuzumab ozogamicin in pediatric acute myeloid leukemia. Expert Rev Anticancer Ther. 2016;16(2):137–146. doi: 10.1586/14737140.2016.1129903 [DOI] [PubMed] [Google Scholar]
- 46.Getz KD, Sung L, Alonzo TA, et al. Effect of dexrazoxane on left ventricular systolic function and treatment outcomes in patients with acute myeloid leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2020;38(21):2398. doi: 10.1200/JCO.19.02856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubnitz JE, Kaspers GJ. How I treat pediatric acute myeloid leukemia. blood. 2021;138(12):1009–1018. doi: 10.1182/blood.2021011694 [DOI] [PubMed] [Google Scholar]
- 48.Boyer T, Gonzales F, Barthélémy A, et al. Clinical significance of ABCB1 in acute myeloid leukemia: a comprehensive study. Cancers. 2019;11(9):1323. doi: 10.3390/cancers11091323 [DOI] [PMC free article] [PubMed] [Google Scholar]