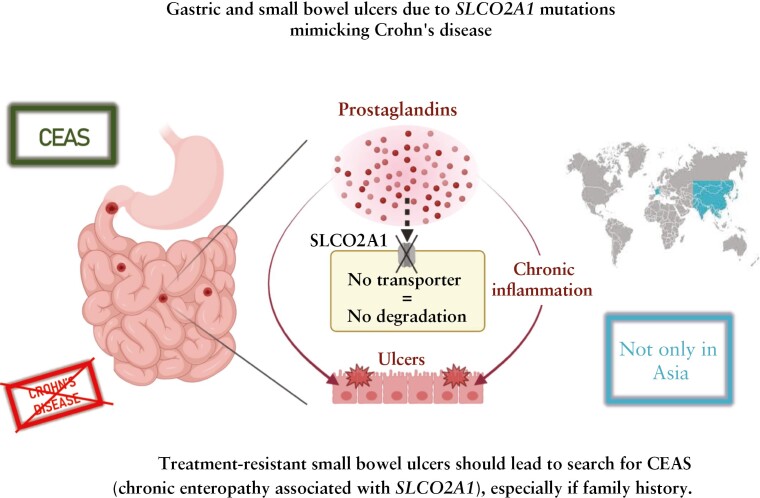
Abstract
Introduction
Multiple chronic ulcers of small intestine are mainly ascribed to Crohn’s disease. Among possible differential diagnoses are chronic ulcers of small bowel caused by abnormal activation of the prostaglandin pathway either in the archetypal but uncommon non-steroidal anti-inflammatory drug [NSAID]-induced enteropathy, or in rare monogenic disorders due to PLA2G4A and SLCO2A1 mutations. SLCO2A1 variants are responsible for CEAS [chronic enteropathy associated with SLCO2A1], a syndrome which was exclusively reported in patients of Asian origin. Herein, we report the case of two French female siblings, P1 and P2, with CEAS.
Case report
P1 underwent iterative bowel resections [removing 1 m of small bowel in total] for recurrent strictures and perforations. Her sister P2 had a tight duodenal stricture which required partial duodenectomy. Next-generation sequencing was performed on P1’s DNA and identified two compound heterozygous variants in exon 12 in SLCO2A1, which were also present in P2.
Conclusion
CEAS can be detected within the European population and raises the question of its incidence and recognition outside Asia. Presence of intractable recurrent ulcerations of the small intestine, mimicking Crohn’s disease with concentric strictures, should motivate a genetic search for SLCO2A1 mutations, particularly in the context of family history or consanguinity.
Keywords: Small bowel ulcerations, small bowel stricture, crohn-like ulcers, monogenic enteropathy, prostaglandines
Graphical Abstract
Graphical Abstract.
1. Introduction
Inflammatory bowel disease [IBD], encompassing Crohn’s disease [CD] and ulcerative colitis, are generally complex multifactorial diseases in which genetically predisposed individuals develop disease upon exposure to a spectrum of environmental factors. Yet an increasing number of human monogenic diseases that can present with IBD-like intestinal inflammation are being reported. Distinguishing monogenic IBD from classical IBD can be challenging but is essential, as some mutations can be targeted with tailored treatment.1
Along this line, small intestinal ulcers associated with chronic iron-deficient anaemia and protein-losing enteropathy mimicking CD may occur in the archetypal but uncommon non-steroidal anti-inflammatory drug [NSAID]-induced enteropathy, and in recently described monogenic disorders due to autosomal recessive loss-of-function [LOF] variants in PLA2G4A or SLCO2A1, which cause cryptogenic multifocal ulcerous stricturing enteritis [CMUSE]2,3 or chronic enteropathy associated with SLCO2A1 [CEAS],4 respectively. The three entities result from the abnormal metabolism of prostaglandins and share some clinical features. Intestinal ulcers due to NSAID-induced enteropathy5 or due to mutations in PLA2G4A and SLCO2A1 are usually thinner than CD ulcers, with a linear or circumferential diaphragm-like configuration, although these features are not specific. To date, all reported CEAS patients have been of Asian origin.6SLCO2A1 mutations can also give rise to primary hypertrophic osteoarthropathy [PHO], characterised by pachydermia, periostitis and digital clubbing.7 In contrast to CEAS that predominantly affects females, complete PHO phenotype is almost exclusively seen in males.8
To the best of our knowledge, no case of CEAS has yet been described outside Asia. Herein, we report the case of two French female siblings with CEAS.
2. Case Report
A 43-year old Caucasian female [P1], born from non-related parents of French origin [Alsace] without Asian ancestors, was admitted to our gastroenterology department for expertise in 2021.
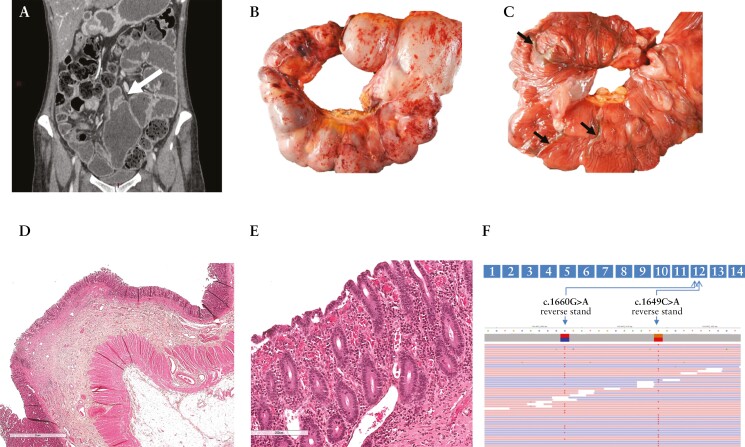
She displayed Raynaud’s disease, chronic abdominal pain, and, since adolescence, had chronic iron deficiency anaemia. Between 2012 to 2020, the patient underwent five iterative segmental small bowel resections [removing 1 m of small bowel in total] due to ulcerative strictures complicated three times by perforations on anastomosis. Severe iron deficiency anaemia preceded each surgery. As diagnosis of CD was suggested, she was treated with a combination of infliximab and azathioprine 6 months before her admission without any clinical and biological response. At admission, she presented with vomiting, alternating diarrhoea and constipation, severe malnutrition (body mass index [BMI] of 19 with a loss of 12 kg in 7 months), iron deficiency anaemia [haemoglobin 85 g/L, ferritin 5 ng/mL], and peripheral oedema due to hypoalbuminaemia [albumin 14 g/L]. Alpha-1 anti-trypsin clearance showed moderate protein exudation [58 ml/24 h, N < 20 ml/24 h]. Upper and lower endoscopies with biopsies were normal. Positron emission tomography [PET] did not show any abnormal metabolic activity. Magnetic resonance [MR] enterography was normal with no mucosal thickening. Yet, a patency capsule remained blocked in the small bowel and computed tomography [CT] enterography showed segmental proximal ileal dilation [up to diameter of 77 mm] upstream a stricturing anastomosis containing the patency capsule, with enhancement of intestinal wall [Figure 1A]. A 60-cm small bowel resection was performed to remove strictures [45 cm up the ileo-caecal valve] with a temporary small bowel stoma. Macroscopically, the resected loops showed alternation of short strictures and dilations [Figure 1B] with thin mucosal diaphragms mimicking the ‘diaphragm disease’ [Figure 1C] resulting from NSAIDs use.5 Microscopically [Figure 1D and E], only the mucosa was congestive and ulcerated at the top of diaphragms but lacked inflammation, discarding a diagnosis of CD. Fibrosis was limited to the submucosa and the nearby intestinal wall was strictly normal. Diarrhoea, anaemia, and hypoalbuminaemia were transiently improved after the surgery. However, abdominal pain and iron deficiency resumed following stoma closure. In 2022, capsule endoscopy identified a relapse with circumferential ulcerations of the ileo-ileal anastomosis and small circular ulcerations in the terminal ileum [Supplementary Figure 2A and B].
Figure 1.
Imaging, histology, and genetics of Patient 1 [P1] with chronic enteropathy associated with SLCO2A1 gene mutation [CEAS]. A: Coronal presurgical computed tomographic enterography showing a tight anastomotic stricture [arrow] with upstream dilatation of the ileal loops. B: Small bowel resection with petechial subserosa without sclerolipomatosis and multifocal short strictures alternating with saccular dilatation of the loop. C: Circular shallow ulcers delimiting each stricture [arrows] as shown after opening the ileal loops [scale mark of 2 cm]. D, E: Section through a stricture displaying superficial erosion with villous blunting and congestion limited to this area with no further lesion [haematein eosin and saffron stains] .F: Schematic representation of SLCO2A1 gene, highlighting both variants in exon 12. Each variant is localised on a different allele as shown on the IGV screenshot [Integrative Genomics Viewer, https://software.broadinstitute.org].
P2, her sister, was admitted in 2016 [at 24 years of age] for obstructive symptoms with complaints of vomiting and abdominal pain for 1 month, without any prior medical history. At admission, she had iron deficiency [haemoglobin 124 g/L and ferritin 10 ng/ml] with no other biological abnormalities. Upper endoscopy with biopsies revealed a tight duodenal [D2] stricture without specificity or histological cause. There was no evidence of Helicobacter pylori gastritis or NSAID use. Proton pump inhibitors and corticoids were ineffective. Partial duodenectomy was performed and revealed a non-specific narrow ulcer in the centre of a short circular stricture, without histological signs of inflammation [Supplementary Figure 1]. In 2022, P2 has no digestive symptoms but iron deficiency anaemia remains. Capsule endoscopy identified a relapse with circumferential ulcerations of the duodeno-jejunal anastomosis [Supplementary Figure 2C]. One of the two sisters has been healthy but one had a history of duodenal stricture [lost for follow-up].
Given disease severity and lack of exposure to NSAIDs or aspirin, a genetic screen was carried out in P1 by next-generation sequencing of a targeted panel of genes involved in monogenic enteropathies.9 Two composite variants of SLCO2A1 were found in exon 12: a premature stop codon [c.1649C > A p.Ser550*] and a missense variant [c.1660G > A p.Gly554Arg, Figure 1F], the second already described as disease-causing.10,11 P2 carried both variants as well. Neither P1 nor P2 carried variants on NOD2.
Skin examination and bone Xrays [head, hands, and lower limbs] did not show any evidence of PHO in either patient.
3. Discussion
Here we report the first two related cases of CEAS secondary to SLCO2A1 mutations in a European population.
Multiple chronic ulcers of the small intestine are a hallmark of CD. Possible differential diagnoses are infectious, eosinophilic, drug-induced, autoimmune, or ischaemic enteritis and, more rarely, refractory coeliac disease type 2, Behçet disease, or Zollinger-Ellison syndrome. Singular entities due to monogenic disorders were recently described: CMUSE due to PLA2G4A mutations and CEAS due to SLCO2A1 mutations. Currently, 98 different variants of SLCO2A1 have been described in patients with either CEAS or PHO or both.12 Review of 73 reported patients with CEAS indicates higher frequency in females [70%] and median age at onset of 18 years [range 1–69 years] [Table 1; and Supplementary Table 1]. Interestingly, SLCO2A1-associated CEAS can involve any part of the small bowel including ileum [97.4%], duodenum [46.7%], jejunum [38.7%], and stomach [26.0%]. More than half of the patients [54.8%] underwent surgery. Importantly, all patients previously reported with CEAS were exclusively of Asian origin.
Table 1.
Clinical features of chronic enteropathy associated with SLCO2A1 [CEAS] in the two reported cases and in the 73 published cases.
| Patient 1 | Patient 2 | Reported cases n = 73 |
||
|---|---|---|---|---|
| Gender | Female Male |
1 0 |
1 0 |
51 [70%] 22 [30%] |
| Ethnicity | Japan China Korea France |
0 0 0 1 |
0 0 0 1 |
51 [70%] 7 [10%] 15 [20%] 0 |
| Age of symptom onset, years [median, range minimum–maximum] |
15 | 24 | 18 [1–69] | |
| Age of diagnosis, years [median, range minimum–maximum] |
43 | 29 | 39 [4–69] | |
| Association with primary hypertrophic osteoarthritis [PHO] | 0 | 0 | 23 [31%] | |
| Disease site | Gastric Duodenal Jejunal Ileal |
0 0 1 1 |
0 1 0 0 |
19 [26%] 34 [47%] 28 [39%] 70 [97%] |
| Surgery | 1 | 1 | 40 [55%] |
Distinguishing between CD and CEAS is challenging. CEAS should be considered in case of family history, consanguinity, refractory pain with iron deficiency anaemia, and absence of lesions in colon. Whereas terminal ileum is affected in more than 90% of small bowel CD, ulcers localised in the terminal ileum are rare in CEAS (only five patients reported in the literature [6.8%], see Table 1). MR enterography can fail to detect abnormality in CEAS, as in P1. At endoscopy, CEAS ulcers appear superficial, circular, and circumferential with healthy margins, whereas ulcers in CD are usually deep, longitudinal, and more rarely circumferential.8 Moreover histologically, CEAS ulcers are narrow, sharp, and abrupt without inflammation on the edges in contrast to ulcers in CD.
In keeping with the strong overlap between the clinical, endoscopic, and histological presentation of CEAS and CMUSE with NSAID-induced enteropathy, the three entities share a common pathophysiology ascribed to impaired prostaglandin [PGE2] metabolism.13SLCO2A1 encodes a prostaglandin transporter [PGT] located at the cell membrane of endothelial cells in many tissues.14 After synthesis, PGE2 is secreted in the extracellular medium or blood and is rapidly inactivated by the 15-hydroxyprostangladin dehydrogenase [15-PGDH] that is highly active in endothelium. Accordingly, PGE2 must be internalised within endothelial cells to be inactivated by 15-PGDH. SLCO2A1 mutations, by reducing expression or function of PGT, lessen intracellular uptake of PGE2. Thereby SLCO2A1 mutations prevent PGE2 inactivation and result in an excess of active PGE2 in the extracellular compartment, which nurtures inflammation.12 Therefore CEAS patients develop a chronic inflammatory disease which does not respond to immunosuppressive treatments classically used in CD, including prednisolone, azathioprine, and anti-tumour necrosis factor [TNF].8 Moreover COX2 inhibitors, which have a good efficacy with PHO symptoms, do not resolve CEAS.15 Better characterisation of CEAS may allow development of rationale-based therapy.
In conclusion, our results show that CEAS can be detected in the European population, raising questions on its incidence and recognition outside Asia. Presence of intractable or recurrent sharp ulcerations of the small intestine and concentric strictures, in combination with iron deficiency anaemia, should motivate genetic search for SLCO2A1 mutations, particularly in the context of family history or consanguinity. PHO features involvement should be systematically searched for, even if their absence in female patients is expected. Additional studies are needed to better delineate an effective treatment for CEAS.
The original data generated in the course of the study are available upon request.
Supplementary Material
Contributor Information
Annick Hamon, Department of Gastroenterology, Beaujon Hospital, University of Paris, Paris, France.
Dominique Cazals-Hatem, Department of Pathology, Beaujon Hospital, University of Paris, Paris, France.
Carmen Stefanescu, Department of Gastroenterology, Beaujon Hospital, University of Paris, Paris, France; Groupe Hospitalier Ambroise Paré – Hartmann, Institut des MICI, Neuilly sur Seine, France.
Mathieu Uzzan, Department of Gastroenterology, Mondor Hospital, University of Paris, Paris, France.
Xavier Treton, Department of Gastroenterology, Beaujon Hospital, University of Paris, Paris, France; Groupe Hospitalier Ambroise Paré – Hartmann, Institut des MICI, Neuilly sur Seine, France.
Alain Sauvanet, Department of Hepato-biliary Surgery, Beaujon Hospital, University of Paris, Paris, France.
Yves Panis, Department of Hepato-biliary Surgery, Beaujon Hospital, University of Paris, Paris, France.
Marie Monsinjon, Department of Colorectal Surgery, Beaujon Hospital, Paris, France.
Fanny Bonvalet, Department of Radiology, Beaujon Hospital, University of Paris, Paris, France.
Olivier Corcos, Department of Gastroenterology, Beaujon Hospital, University of Paris, Paris, France.
Emilie Azouguene, Department of Genomic Medecine for Rare Diseases, Necker-Enfants Malades Hospital, University of Paris-Cité, Paris, France.
Nadine Cerf-Bensussan, INSERM UMR1163, Intestinal Immunity, Institut Imagine, Paris, France.
Yoram Bouhnik, Department of Gastroenterology, Beaujon Hospital, University of Paris, Paris, France; Groupe Hospitalier Ambroise Paré – Hartmann, Institut des MICI, Neuilly sur Seine, France.
Fabienne Charbit-Henrion, Department of Genomic Medecine for Rare Diseases, Necker-Enfants Malades Hospital, University of Paris-Cité, Paris, France; INSERM UMR1163, Intestinal Immunity, Institut Imagine, Paris, France.
Funding
No specific funding has been received.
Conflict of Interest
MU declares counselling, board, and transport fees from Abbvie, Celltrion, Galapagos, Janssen, and Takeda
Author Contributions
Concept and design of the study: DCH, YB, FCH. Acquisition of data, or analysis and interpretation of data: AH, CS, MU, XT, AS, YP, MM, OC, DCH. Drafting the article or revising it critically for important intellectual content: DCH, YB, NCB, FCH, AH. Final approval of the version to be submitted: all authors revised and approved final version of the manuscript.
References
- 1. Uhlig HH, Charbit-Henrion F, Kotlarz D, et al. Clinical genomics for the diagnosis of monogenic forms of inflammatory bowel disease: a position paper from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2021;72:456–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perlemuter G. Cryptogenetic multifocal ulcerous stenosing enteritis: an atypical type of vasculitis or a disease mimicking vasculitis. Gut 2001;48:333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adler DH, Cogan JD, Phillips JA, et al. Inherited human cPLA2α deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest 2008;118:2121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Umeno J, Hisamatsu T, Esaki M, et al. A hereditary enteropathy caused by mutations in the SLCO2A1 gene, encoding a prostaglandin transporter. PLoS Genet 2015;11:e1005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lang J, Price AB, Levi AJ, Burke M, Gumpel JM, Bjarnason I.. Diaphragm disease: pathology of disease of the small intestine induced by non-steroidal anti-inflammatory drugs. J Clin Pathol 1988;41:516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang H, Wang X, Ou D, et al. Four variants of SLCO2A1 identified in three Chinese patients with chronic enteropathy associated with the SLCO2A1 gene. Dig Dis Sci 2021;66:2992–3001. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Z, Xia W, He J, et al. Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am J Hum Genet 2012;90:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Umeno J, Esaki M, Hirano A, et al. Clinical features of chronic enteropathy associated with SLCO2A1 gene: a new entity clinically distinct from Crohn’s disease. J Gastroenterol 2018;53:907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charbit-Henrion F, Parlato M, Hanein S, et al. Corrigendum to: Diagnostic yield of next-generation sequencing in very early-onset inflammatory bowel diseases: a multicentre study . J Crohns Colitis 2021;15:517–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li SS, He JW, Fu WZ, Liu YJ, Hu YQ, Zhang ZL.. Clinical, biochemical, and genetic features of 41 Han Chinese families with primary hypertrophic osteoarthropathy, and their therapeutic response to Etoricoxib: results from a six-month prospective clinical intervention: Han Chinese families with PHO and therapeutic response to etoricoxib. J Bone Miner Res 2017;32:1659–66. [DOI] [PubMed] [Google Scholar]
- 11. Hou Y, Lin Y, Qi X, et al. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and phenotypic comparison between two subtypes of primary hypertrophic osteoarthropathy [PHO]: a single-center study. Bone 2018;106:96–102. [DOI] [PubMed] [Google Scholar]
- 12. Nakanishi T, Nakamura Y, Umeno J.. Recent advances in studies of SLCO2A1 as a key regulator of the delivery of prostaglandins to their sites of action. Pharmacol Ther 2021;223:107803. [DOI] [PubMed] [Google Scholar]
- 13. Hosoe N, Ohmiya N, Hirai F, et al. Chronic enteropathy associated with SLCO2A1 gene [CEAS]—characterisation of an enteric disorder to be considered in the differential diagnosis of Crohn’s disease. J Crohns Colitis 2017;11:1277– 81. [DOI] [PubMed] [Google Scholar]
- 14. Nakata R, Nakamura Y, Hosomi S, et al. SLCO2A1 deficiency exacerbates experimental colitis via inflammasome activation in macrophages: a possible mechanism of chronic enteropathy associated with SLCO2A1 gene. Sci Rep 2020;10:4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Q, Li Y, Lin G, et al. Primary hypertrophic osteoarthropathy related gastrointestinal complication has distinctive clinical and pathological characteristics: two cases report and review of the literature. Orphanet J Rare Dis 2019;14:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun X, Hosoe N, Miyanaga R, et al. A male Korean who was diagnosed with chronic enteropathy associated with SLCO2A1 [CEAS]: case report with literature review. BMJ Open Gastroenterol 2018;5:e000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu P, He H, Dai N, Zhang S, Deng L.. Chronic enteropathy associated with SLCO2A1 gene: a case report and literature review. Clin Res Hepatol Gastroenterol 2019;43:e68–72. [DOI] [PubMed] [Google Scholar]
- 18. Sonoda A, Wada Y, Togo K, et al. Characteristic facial appearance was the key to diagnosing chronic enteropathy associated with SLCO2A1-associated primary hypertrophic osteoarthropathy. Intern Med 2020;59:491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jimbo K, Okuno T, Ohgaki R, et al. A novel mutation in the SLCO2A1 gene, encoding a prostaglandin transporter, induces chronic enteropathy. PLoS One 2020;15:e0241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fang Y, Gu W, Luo Y, Chen J.. Obscure gastrointestinal bleeding caused by congenital enteropathy in a Chinese young child: a case report. BMC Pediatr 2020;20:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsuzuki Y, Aoyagi R, Miyaguchi K, et al. Chronic enteropathy associated with SLCO2A1 with pachydermoperiostosis. Intern Med 2020;59:3147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hong HS, Baek J, Park JC, et al. Clinical and genetic characteristics of Korean patients diagnosed with chronic enteropathy associated with SLCO2A1 gene: a KASID multicenter study. Gut Liver 2022;16:942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ariake C, Hosoe N, Sakurai H, et al. Chronic enteropathy associated with Solute Carrier Organic Anion Transporter Family, Member 2A1 [SLCO2A1] with positive immunohistochemistry for SLCO2A1 protein. Intern Med 2022;61:2607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.