Abstract
Background and Objectives
Neuropathy with antibodies to myelin-associated glycoprotein (MAG) is the most common paraproteinemic IgM neuropathy. Recently, the mutational profile of the MYD88 and CXCR4 genes has been included in the diagnostic workup of IgM monoclonal gammopathies. The objective of our study was to assess the prevalence of MYD88L265P and CXCR4S338X gene variants in patients with anti-MAG antibody neuropathy. Secondary aims were to evaluate possible correlations between the mutational profile and neuropathy severity, antibody titers, and treatment response.
Methods
Seventy-five patients (47 men, mean age at molecular analysis 70.8 ± 10.2 years; mean disease duration 5.1 ± 4.9 years) with anti-MAG antibody neuropathy were recruited. Among them, 38 (50.7%) had IgM monoclonal gammopathy of undetermined significance, 29 (38.7%) Waldenstrom macroglobulinemia (WM), and 8 (10.6%) chronic lymphocytic leukemia/marginal zone lymphoma/hairy cell leukemia variant. Molecular analysis was performed on DNA from the bone marrow mononuclear cells in 55 of 75 patients and from peripheral mononuclear cells in 18 of 75 patients. Forty-five patients were treated with rituximab, 6 with ibrutinib, 2 with obinutuzumab-chlorambucil, and 3 with venetoclax-based therapy. All the patients were assessed with the Inflammatory Neuropathy Cause and Treatment (INCAT) Disability Scale, INCAT Sensory Sum Score, and MRC Sum Score at baseline and follow-up. We considered as responders, patients who improved by at least 1 point in 2 clinical scales.
Results
Fifty patients (66.7%) carried the MYD88L265P variant, with a higher frequency in WM and naive patients (77.2% vs 33.3%, p = 0.0012). No patients harbored the CXCR4S338X variant. There were no significant differences in hematologic data (IgM levels, M protein, and anti-MAG antibody titers), neuropathy severity, or response to rituximab in MYD88-altered and MYD88 wild-type patients. Nine of 11 (81.8%) patients treated with novel targeted drug, according to the MYD88 status, responded to treatments.
Discussion
MYD88L265P variant has a high prevalence (66.7%) in anti-MAG antibody neuropathy representing a potential effective mutational target for Bruton tyrosine kinase inhibitors. MYD88L265P variant, however, does not seem to be a prognostic factor of neuropathy severity or response to rituximab. In patients not responding or becoming refractory to rituximab, a tailored therapy with new effective target therapies should be considered.
Anti–myelin-associated glycoprotein (MAG) antibody neuropathy is a chronic sensorimotor demyelinating polyneuropathy, associated with either an IgM monoclonal gammopathy of undetermined significance (MGUS) or lymphoproliferative disorder (Waldenstrom macroglobulinemia [WM], marginal zone lymphoma [MZL], and chronic lymphocytic leukemia [CLL]).1,2 Despite being slowly progressive, the neuropathy may severely influence patients' functionality and quality of life.3 Among possible therapies, rituximab, an anti-CD20 chimeric monoclonal antibody, remains the most used treatment efficacious in almost half of the patients and capable of improving disability scales and the response to questionnaires in the global impression of the disease.4-8 Recently, the discovery of the mutational profile of the MYD88 and CXCR4 genes has radically changed the diagnostic and prognostic evaluation of IgM monoclonal gammopathies.
Specifically, MYD88L265P has been found to be the most common variant reported in WM and IgM-MGUS.9 Since MYD88L265P interacts with nuclear factor kB signaling, it plays a crucial role in the response to ibrutinib, the first in-class inhibitor of Bruton tyrosine kinase (BTK), which acts by inhibiting the downstream signaling after the interaction between altered MYD88 protein and BTK.10 In addition, somatic variants in the C-terminal domain of CXCR4 have been reported in WM and shown to be associated with a more aggressive disease. More important, MYD88/CXCR4 status has been shown to be predictive of the response to ibrutinib in WM.9
In a prospective study, WM patients with MYD88-altered and CXCR4 wild-type have been shown to have better and longer response to ibrutinib.9 Among the 63 studied patients, 9—3 of whom with anti-MAG antibodies—had received ibrutinib for progressive IgM paraproteinemic neuropathy. All 9 patients had a response, with subjective improvement of peripheral neuropathy in 5 patients and stability in 4 patients during the treatment course.
In a subsequent study, 4 of 31 patients with WM had been treated with ibrutinib for the neuropathy: 2 remained stable and 2 had subjective improvement starting from week 9 of treatment, with subsequent complete recovery in 1 patient.11
Preliminary data on 20 patients with anti-MAG antibody neuropathy have shown that 60% of the patients carry the MYD88L265P suggesting the use of BTK inhibitors in anti-MAG polyneuropathy.12 Accordingly, we first reported on 3 patients with WM and anti-MAG antibody neuropathy, who had a subjective, objective, and hematologic response to ibrutinib, 2 after the loss of response to rituximab.13
Since the response to ibrutinib strictly depends on the IgM paraprotein alteration profile, the aim of our prospective study was to assess the mutational profile of the MYD88 and CXCR4 genes in patients with anti-MAG antibody neuropathy, irrespective of the underlying hematologic conditions.
The results might help identify the presence of a potential mutational target for new therapies (ibrutinib, second generation BTK inhibitors or other target treatments). Moreover, we aimed at assessing possible correlations between the mutational profile of MYD88 and CXCR4 genes and neuropathy severity, antibody titers, and treatment response.
Methods
Clinical Evaluation
This is an observational prospective study, involving the Departments of Neurosciences of the University of Padova and University of Pisa. Inclusion criteria were clinical and neurophysiologic diagnosis of anti-MAG antibody neuropathy associated with histologically confirmed IgM monoclonal gammopathy (IgM MGUS or lymphoproliferative diseases); anti-MAG antibody titer >7,000 Bühlmann titre units (BTU).14,15 All the patients had neurophysiologic evidence of distal acquired demyelinating symmetric neuropathy with low conduction velocities and markedly prolonged distal latencies and no conduction blocks. Patients with atypical forms of anti-MAG antibody neuropathy16 were not included.
The primary endpoint was to identify the rate of MYD88L265P variant in patients with anti-MAG antibody neuropathy. Secondary aims were to evaluate the presence of CXCR4S338X variant, and the correlation between the mutational profile with neuropathy severity, antibody titers, and treatment response.
All the patients were assessed with the Inflammatory Neuropathy Cause and Treatment (INCAT) Disability Scale,17 INCAT Sensory Sum Score (ISS),18 and Medical Research Council (MRC) Sum Score (in 6 muscles, deltoid, biceps, wrist extensor, iliopsoas, quadriceps femoris, tibialis anterior, bilaterally) at baseline and after treatment. We considered as responders, patients who improved by at least 1 point in 2 clinical scales.
Standard Protocol Approvals, Registrations, and Patient Consents
The study did not need ethical committee approval being the genetic assessment and treatments performed part of standard of care in the Hematological unit. Signed informed consent was obtained from all the patients.
Anti-MAG Antibody Testing
Anti-MAG antibodies were tested per standard care using a commercially available enzyme linked immunosorbent assay (Bühlmann Laboratories, Schönenbuch, Switzerland) and expressed as BTU/L.
Molecular Analysis
Molecular analysis was performed in all the 75 enrolled patients. In 57 patients on DNA extracted by an automated system (Maxwell 16, Promega Italia, Milano, Italy) from bone marrow mononuclear cells after density gradient separation by Ficoll Hypaque (Sigma-Aldrich). MYD88L265P variants were searched by allele-specific PCR (AS-PCR), as previously described19 with a reported sensitivity of 0.1%. For detecting the most common CXCR4 variant, i.e., S338X, a highly sensitive AS-PCR assay was developed.20,e3 Sanger sequencing of CXCR4 gene (less sensitive) was still be required for both nonsense and frameshift mutations of the C-terminal domain in S338X-negative samples. In 18 patients, molecular analysis was performed from circulating mononuclear cells after density gradient separation by centrifugation. DNA was extracted from isolated cells by an EZ1Qiagen automated system. MYD88L265P variants were searched by real time PCR “qBiomarker Somatic Mutation PCR Assay” (Qiagen, 7,900 Applied Biosistem) in combination with amplification refractory mutation system PCR.21
Treatments
Forty-five patients were treated with rituximab (375 mg/m2 IV weekly for 4 consecutive weeks), 6 with ibrutinib (420 mg daily, orally), 2 with obinutuzumab-chlorambucil (obinutuzumab was given IV at 100 mg on day +1, 900 mg on day +2, then at 1,000 mg on day 8 and 15 of cycle 1 and day 1 of cycles 2–6; chlorambucil orally at 0.5 mg/kg at day 1 and 15 of cycles 1–6), and 3 patients were treated with venetoclax-based therapy (venetoclax 400 mg/d orally after a lead-in weekly ramp-up phase) including 1 with rituximab after the ramp-up phase, at 375 mg/m2 for the second month and then monthly at 500 mg/m2 for months 3–7, 1 with obinutuzumab (at the above reported doses) and 1 single agent.
Data Availability
Anonymized data not published within this article will be made available on reasonable request from any qualified investigator.
Statistical Analysis
Continuous variables were compared with the Mann-Whitney test, while categorial variables with the Fisher exact test or χ2 test. Comparison of neurologic scales was compared with Kruskal-Wallis tests.
Results
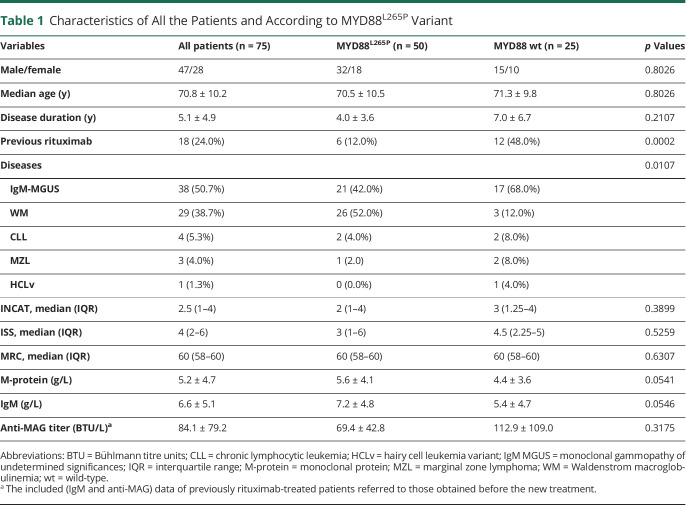
We enrolled 75 consecutive patients, 47 men and 28 women, with a mean age at the time of molecular analysis of 70.8 ± 10.2 years, and mean disease duration of anti-MAG antibody neuropathy at the time of molecular analysis of 5.1 ± 4.9 years (Table 1). Of them, 38 (50.7%) had IgM-MGUS, 29 (38.7%) WM, 4 (5.3%) CLL, 3 (4.0%) MZL, and 1 (1.3%) hairy cell leukemia-variant. All the 75 patients were assessed for MYD88L265P variant, while 47 of 75 patients were assessed for CXCR4S338X variant, by AS-PCR as mentioned above. Although none of the tested patients harbored the CXCR4 variant, 50 of 75 patients (66.7%, 32 men, mean age 70.5 ± 10.5 years, mean disease duration 4.0 ± 3.6 years) carried the MYD88L265P variant. Conversely, 25 of 67 patients (33.3%, 15 men, mean age 71.3 ± 9.8 years, mean disease duration 7.0 ± 6.7 years) were MYD88 wild-type. The 2 groups were homogeneous regarding sex, age, and duration of follow-up (Table 1). Considering the underlying hematologic disease, the alteration was present in 26 of 29 (89.7%) patients with WM, 21 of 38 (55.3%) IgM MGUS, and 3 of 8 (37.5%) CLL/MZL. According to the literature,9 MYD88L265P variant was significantly more common in patients with WM (p = 0.0107) (Table 1). In addition, there was no significant difference between MYD88-altered and MYD88-wild-type patients regarding anti-MAG antibody titer and neuropathy severity (Table 1). In particular, the median INCAT Disability Scale was 2 in MYD88-altered patients and 3 in MYD88 wild-type patients; the median ISS was 3 in MYD88-altered and 4.5 in MYD88 wild-type; the median MRC was 60 in both groups (Table 1). We found a trend for slightly higher IgM and M-protein levels in patients MYD88L265P compared with wild-type patients (both p = 0.054, Table 1).
Table 1.
Characteristics of All the Patients and According to MYD88L265P Variant
At the time of molecular analysis, 18 patients (6 MYD88L265P-altered patients and 12 wild-type) had already been treated with rituximab, while 57 (44 MYD88L265P-altered and 13 wild-type) were therapy-naive. MYD88L265P variant was significantly more common in therapy-naive patients (p = 0.0002). Of the 75 enrolled patients, 44 of the 57 (77%) therapy-naive patient carried the MYD88L265P variant, vs 6 of the 18 (33.3%) previously treated patients (p = 0.0012). In the latter group, the mean delay between treatments and molecular analyses was 5.6 ± 4.1 years (5.7 ± 4.9 years in MYD88 wild-type, 5.2 ± 1.9 years in MYD88L265P-altered) (eTable 1 in eAppendix 1, links.lww.com/NXI/A855).
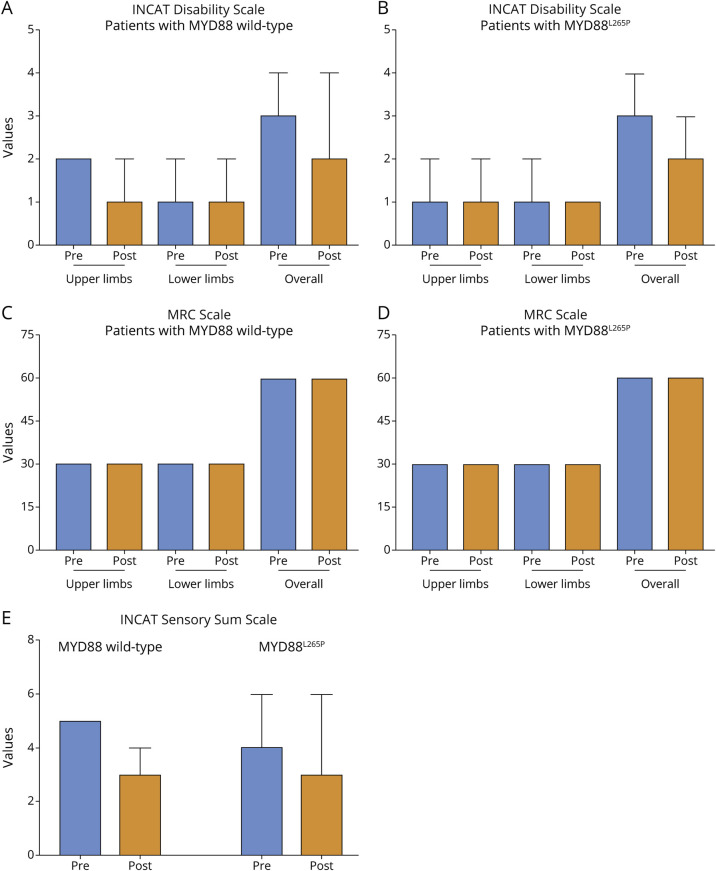
Of the 57 therapy-naive patients, 21 MYD88L265P-altered and 6 wild-type were treated with rituximab after molecular analysis. Overall, among the 45 patients treated with rituximab, 40 (23 MYD88-altered and 17 wild-type) had a follow-up of at least 12 months for evaluating the treatment response. Twenty-six of 40 (65%) were responders to treatment, including 14 of 23 (61%) MYD88-altered and 12 of 17 (70.6%) wild-type (p = 0.7385). Detailed analysis of the adopted clinical scales is reported in Figure 1. Among the 18 patients genetically tested after treatment with rituximab, 14 (18.7% of all patients) needed additional cycles because of relapse: 5 of 50 (10.0%) MYDD88-altered and 9 of 25 (36.0%) wild-type (p = 0.0109).
Figure 1. Assessment of INCAT, ISS, and MRC Scales Before and After Treatment With Rituximab.
In the upper panels, the INCAT Disability Scale is applied in patients with MYD88 wild-type (A) and MYD88-altered (B). The Dunn multiple comparison test was applied to upper limbs, lower limbs, or both limbs (each comparison had a p > 0.05). In the middle panel, the MRC muscle scale is applied in patients with MYD88 wild-type (C) and MYD88-altered (D). The Dunn multiple comparison test was applied to upper limbs, lower limbs, or both limbs (each comparison had p > 0.05). In the lower panels (E), the INCAT Sensory Scale (ISS) is applied in patients with MYD88 wild-type and MYD88-altered. The Dunn multiple comparison test was applied to upper limbs, lower limbs, or both limbs (each comparison had p > 0.05). All data are reported as median and interquartile range. INCAT = Inflammatory Neuropathy Cause and Treatment.
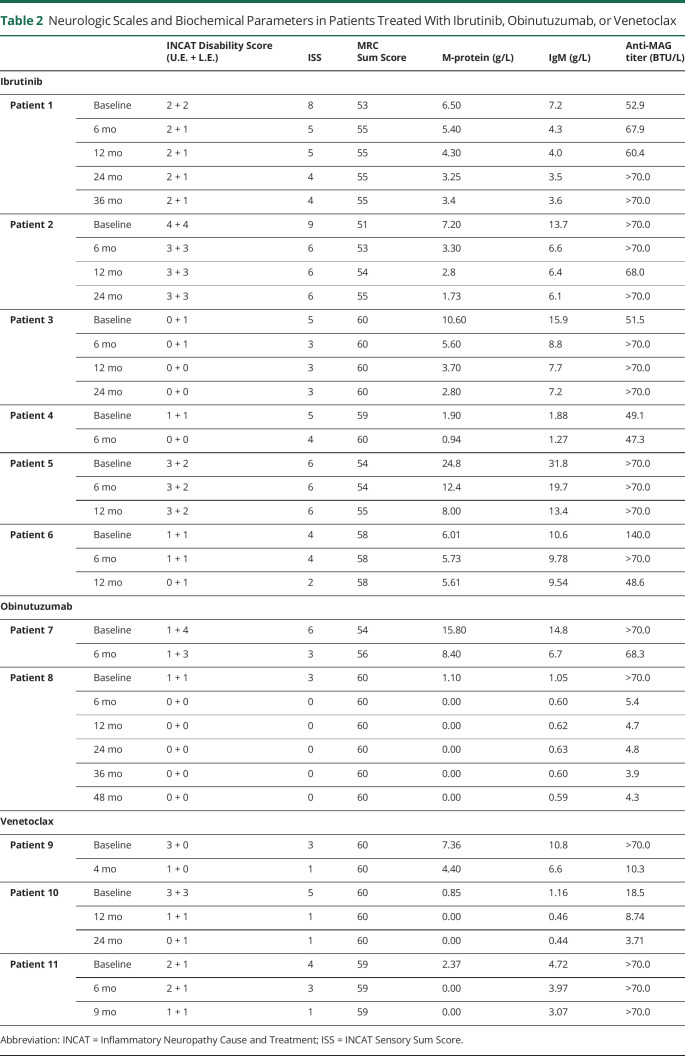
Six patients (all with WM, MYD88L265P-altered and CXCR4 wild-type, previously treated with rituximab with lack or loss of benefit) were treated with ibrutinib 420 mg/d orally, with early and persistent clinical benefit as shown by improvement in clinical scales (Table 2). We now have a longer follow-up of the first 3 treated and described patients13 showing a maintenance of the response up to 36 months.
Table 2.
Neurologic Scales and Biochemical Parameters in Patients Treated With Ibrutinib, Obinutuzumab, or Venetoclax
Three additional patients have been treated with ibrutinib. One patient (4, Table 2) was a 79-year-old man with anti-MAG antibody neuropathy and WM, who was treated with rituximab in September 2020 with only transient (3 months) benefit. For worsening of the gait stability and several falls, the patient was started on ibrutinib in June 2021. At neurologic evaluation 6 months later, the patient reported reduced hypoesthesia and improved motility at feet, and absence of falls. Unfortunately, the patient, who had been suffering for several years from severe depression, died 2 months later of unrelated causes.
The fifth patient (5, Table 2) was a 76-year-old man previously (2014–2015) treated with benefit with rituximab and bendamustine for anti-MAG antibody neuropathy in WM. From 2019, the gait instability worsened forcing the patient to use unilateral support for walking; he also developed inability to botton and perform precision tasks (INCAT lower limbs 2, upper limbs 3). An additional rituximab cycle (September 2020) had no benefit. The patient was started (June 2021) on ibrutinib with no amelioration, but clinical stability was achieved.
The sixth patient (6, Table 2) was a 46-year-old man who complained of distal sensory loss (toes and fingers) with neurophysiologic evidence of diffuse symmetric demyelinating polyneuropathy. Three years earlier, he was accidently found to carry an IgM/k (0.59 g/L) monoclonal gammopathy. Bone marrow biopsy showed the presence of MYD88-altered and CXCR4 wild-type WM. Anti-MAG antibody was positive (140.000 BTU). For worsening of symptoms at lower limbs (with the onset of ataxic gait) and occurrence of symptoms at the hands (INCAT 1 + 1), the patient was treated with rituximab with subjective worsening, despite neurologically he was unchanged. Ten months later, however, a further worsening of sensory symptoms and onset of stepping gait occurred, so the patient was started on ibrutinib with arrest of deterioration. Neurologic evaluation 12 months after the beginning of ibrutinib showed a mild improvement (INCAT 0 + 1).
In all the 6 patients, treatment with ibrutinib was well tolerated. Interesting enough, M-protein and IgM levels showed a constant decrease in all the 6 patients, while anti-MAG antibody titers showed a fluctuating pattern, despite clinical improvement (Table 2).
Two therapy-naive patients with CLL received obinutuzumab-chlorambucil. Patient 7 was MYD88 wild-type, whereas patient 8 was MYD88L265P-altered; both were CXCR4 wild-type. Both patients had improvement in clinical scales and hematologic data (Table 2). Unfortunately, patient 7 developed grade 4 neutropenia, and patient 8 died of pneumonia 2 months after the end of treatment. At the last follow-up after 4 years from therapy, patient 7 persisted improved (INCAT 0); also neurophysiologic evaluation had ameliorated compared with baseline.22 Three patients were treated with venetoclax alone or in combination with anti-CD20 monoclonal antibody (Table 2).
Patients 9 was an elderly man with untreated CLL and concurrent anti-MAG antibody neuropathy with severe impairment at upper limbs (distal tremor, sensory loss, and incapability of writing and buttoning without help; INCAT upper limbs 3), while gait was quite preserved. The patient had MYD88-altered and CXCR4 wild-type. He was treated with venetoclax-obinutuzumab. Despite a fast improvement, being able of buttoning up independently already after the first month, he developed a severe SARS-CoV-2 pneumonia during the fourth month of treatment and died (month +7).
Patient 10 was a 62-year-old woman affected by anti-MAG neuropathy and CLL, both MYD88 and CXCR4 wild-type, previously treated with rituximab-cyclophosphamide with only partial benefit and subsequent relapse was treated with venetoclax-rituximab, with dramatic clinical and hematologic response (Table 2). Treatment was well tolerated. Early assessment has been already reported.23 According to the treatment schedule, venetoclax was stopped after 2 years. After 24 months, the patient continues to show a clinical response with further improvement at upper limbs (no longer tremor, the patient is now capable of buttoning up, she regained the capability of knitting and using the computer keyboard).
Patient 11, a 74-year-old man, with a long history of anti-MAG antibody neuropathy and MGUS IgM, had been treated with 4 cycles of rituximab (2 in 2007, 1 in 2008, and the latest in 2010), followed by plasma-exchange, that was discontinued for side effects. When we first evaluated the patient, he had an ataxic unstable gait, left steppage. Bone marrow biopsy revealed a WM without MYD88 and CXCR4 alterations, so he was started treatment with venetoclax. After 6 months of follow-up, neuropathy stabilized, and at month 9, he improved in gait stability, Sensory Sum Score, and upper limbs functionality (Table 2).
In conclusion, 65% of patients were responders to rituximab treatment, regardless of MYD88 alteration. Nine of 11 (81.8%) patients treated with novel targeted drug responded to treatments, being able to improve at least 1 point in 2 different neurologic scales. Caution is however warranted for potential adverse effects in aged patients, especially risks of infection that may be lethal. In the first report by Rakocevic et al.,24 obinutuzumab was ineffective but safe in 2 patients with anti-MAG antibody neuropathy, whereas in our experience, obinutuzumab combined with either chlorambucil or venetoclax was effective in all the 3 patients, but burdened with serious side effects. For unknown reasons, obinutuzumab-based treatment seems to be highly toxic in patients with anti-MAG neuropathy as compared with other real-world evidence studies in patients with CLL.25,26
Discussion
Anti-MAG antibody neuropathy is a chronic, potentially disabling demyelinating polyneuropathy for which adequate immunotherapy is eagerly needed.27 According to clinical trials and case reports, rituximab is effective in nearly 50% of patients, but no clear predicting factors of therapy response have so far been identified.8 A recent systematic review on rituximab in chronic immune-mediated neuropathies showed that rituximab was effective in 48% of anti-MAG antibody neuropathy.28 The review included 23 studies, of which 2 were randomized controlled trials, and 6 prospective and 15 retrospective studies. Neurophysiologic improvement was evident in 40% of patients with anti-MAG neuropathy.
MYD88L265P variant has been shown to be the most frequent alteration in patients with WM and IgM MGUS.19 In this study, we confirm, in the largest population so far assessed, the high prevalence of the MYD88L265P variant in patients with anti-MAG antibody neuropathy, especially in therapy-naive patients (77%). These data are interesting because the MYD88L265P variant represents a potential effective mutational target for ibrutinib that has been demonstrated to be effective in patients with anti-MAG neuropathy also after failure or loss of efficacy of previous therapies.13 MYD88L265P variant, however, does not seem to be a prognostic factor of neuropathy severity or response to rituximab. In our study, as expected, we did not find a significant difference in clinical scales or in hematologic parameters, or in neurophysiologic findings (data not shown) between MYD88-altered and wild-type patients indeed. However, assessment of MYD88 alteration might be useful for hematologic diagnosis and for selecting the optimal target therapy besides rituximab.
In MYD88 wild-type patients, likely nonresponders to ibrutinib, venetoclax, an oral inhibitor of BCL2, alone or in combination with rituximab has been shown to be highly active in ibrutinib-resistant hematologic malignancies and has so far been used with benefit in a single patient with anti-MAG antibody neuropathy.23
Limitations of our study are the different genetic assessment in a subgroup (24%) of patients, in which MYD88 alteration was assessed in the peripheral blood rather than from bone marrow cells. A comparative study showed a high, but not complete concordance between the peripheral blood and bone marrow tests.29 Possible future strategies to avoid bone marrow assessment might be the use of droplet digital PCR rather than AS-PCR on peripheral blood or plasma cell-free DNA.21,29
Moreover, the rarest CXCR4 variants have not been searched for in all the patients. However, data coming from the literature report that only a small percentage of patients with anti-MAG antibody neuropathy carry the CXCR4 variation.30 Although neurophysiologic evaluation was performed in all the patients at the diagnosis and in most also after therapy (almost 50% of patients),13,23,31 still neurophysiologic data were not included among the criteria of response, considering that neurophysiologic assessment was often performed in different centers with different laboratory reference values. Moreover, often axonal loss at lower limbs is present before starting therapy, so neurophysiologic improvement may be difficult to be demonstrated if not in the more spared regions (e.g., upper limbs).4
In conclusion, the mutational analysis of IgM-paraprotein associated with anti-MAG antibody neuropathy has revealed that MYD88L265P is the most frequent variant, opening new avenues to potential efficacious therapy, especially in patients who are not responsive or become refractory or might be unfit to rituximab, due to comorbidities. Currently, rituximab should be the first-line therapy in patients with anti-MAG antibody neuropathy, with the awareness that efficacy may occur up to 6 months from the administration.7
Genetic assessment should therefore be performed in patients with anti-MAG antibody neuropathy to help identify possible mutational targets for potential new therapies (ibrutinib, second-generation BTK inhibitors or other target treatments) for the most common and often disabling paraproteinemic neuropathy. In our center, genetic assessment is routinely performed on bone marrow cells because this compartment showed a higher sensibility than peripheral blood assessment. However, ongoing studies are evaluating the new role of non–invasive serum cell-free DNA in IgM monoclonal gammopathies.29
The lower rate of MYD88L265P alteration in rituximab-relapsed patients poses some questions because we do not know if MYD88 wild-type patients might have a higher relapsed rate than MYD88-altered, despite a similar response rate. Furthermore, it is not known whether rituximab might eradicate MYD88-altered cells favoring the occurrence of a MYD88 wild-type B-cell clone. Therefore, prospective sequential studies are warranted.
In conclusion, rituximab is still the standard of care for the treatment of anti-MAG antibody neuropathy because of the presence of prospective clinical trials and the longer follow-up.
In patients who relapse after rituximab, if not done at diagnosis, we suggest to evaluate MYD88 alteration and to tailor the next treatment based on the genetic results, considering ibrutinib for MYD88L265P-altered patients and venetoclax for MYD88 wild-type cases. In addition, rituximab-responsive patients may benefit also from retreatment in case of a late relapse.32
Caution should be used in elderly patients with obinutuzumab-based therapy especially for potentially life-threatening infections. The availability of new more selective BTK inhibitors, such zanubrutinib, with a safer profile and activity also in MYD88 wild-type patients, further support the use of target therapies in patients with anti-MAG neuropathy.33,34
Acknowledgment
The authors thank Associazione Italia Ricerca sul Cancro (AIRC) and Ricerca per credere nell Vita (RCV) odv to L. Trentin. Chiara Briani is a member of the European Reference Network for Neuromuscular Diseases.
Glossary
- AS-PCR
allele-specific PCR
- BTK
Bruton tyrosine kinase
- CLL
chronic lymphocytic leukemia
- INCAT
Inflammatory Neuropathy Cause and Treatment
- ISS
INCAT Sensory Sum Score
- MAG
myelin-associated glycoprotein
- MGUS
monoclonal gammopathy of undetermined significance
- MZL
marginal zone lymphoma
- WM
Waldenstrom macroglobulinemia
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
F. Castellani reports no disclosures; A. Visentin is in the advisory board of Janssen and BeiGene; E. Schirinzi, A. Salvalaggio, M. Cacciavillani, C. Candiotto, C. Baratè, A. Cellini, R. Bertorelle, and G. Siciliano report no disclosures; L. Trentin is in the advisory board of Janssen and BeiGene; C. Briani reports no disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Latov N, Sherman WH, Nemni R, et al. Plasma-cell dyscrasia and peripheral neuropathy with a monoclonal antibody to peripheral-nerve myelin. N Engl J Med. 1980;303(11):618-621. doi. 10.1056/nejm198009113031105 [DOI] [PubMed] [Google Scholar]
- 2.Briani C, Visentin A, Campagnolo M, et al. Peripheral nervous system involvement in lymphomas. J Peripher Nerv Syst. 2019;24(1):5-18. doi. 10.1111/jns.12295 [DOI] [PubMed] [Google Scholar]
- 3.Campagnolo M, Ruiz M, Falzone YM, et al. Limitations in daily activities and general perception of quality of life: long term follow-up in patients with anti-myelin-glycoprotein antibody polyneuropathy. J Peripher Nerv Syst. 2019;24(3):276-282. doi. 10.1111/jns.12342 [DOI] [PubMed] [Google Scholar]
- 4.Parisi M, Dogliotti I, Clerico M, et al. Efficacy of rituximab in anti-myelin-associated glycoprotein demyelinating polyneuropathy: clinical, hematological and neurophysiological correlations during 2 years of follow-up. Eur J Neurol. 2022;29(12):3611-3622. doi. 10.1111/ene.15553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svahn J, Petiot P, Antoine JC, et al. , Francophone anti-MAG cohort Group. Anti-MAG antibodies in 202 patients: clinicopathological and therapeutic features. J Neurol Neurosurg Psychiatry. 2018;174:S165-S505. doi. 10.1016/j.neurol.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 6.Leger JM, Viala K, Nicolas G, et al. , RIMAG Study Group France and Switzerland. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein neuropathy. Neurology. 2013;80(24):2217-2225. doi. 10.1212/wnl.0b013e318296e92b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalakas MC, Rakocevic G, Salajegheh M, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein antibody demyelinating neuropathy. Ann Neurol. 2009;65(3):286-293. doi. 10.1002/ana.21577 [DOI] [PubMed] [Google Scholar]
- 8.Dalakas MC. Advances in the diagnosis, immunopathogenesis and therapies of IgM-anti-MAG antibody-mediated neuropathies. Ther Adv Neurol Disord. 2018;11:175628561774664. doi. 10.1177/1756285617746640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenstrom's macroglobulinemia. N Engl J Med. 2015;372(15):1430-1440. doi. 10.1056/nejmoa1501548 [DOI] [PubMed] [Google Scholar]
- 10.Visentin A, Pravato S, Castellani F, et al. From biology to treatment of monoclonal gammopathies of neurological significance. Cancers (Basel). 2022;14(6):1562. doi. 10.3390/cancers14061562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimopoulos MA, Trotman J, Tedeschi A, et al. , iNNOVATE Study Group and the European Consortium for Waldenström's Macroglobulinemia. Ibrutinib for patients with rituximab-refractory Waldenstrom's macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017;18(2):241-250. doi. 10.1016/s1470-2045(16)30632-5 [DOI] [PubMed] [Google Scholar]
- 12.Vos JM, Notermans NC, D'Sa S, et al. High prevalence of the MYD88 L265P mutation in IgM anti-MAG paraprotein-associated peripheral neuropathy. J Neurol Neurosurg Psychiatry. 2018;89(9):1007-1009. doi. 10.1136/jnnp-2017-316689 [DOI] [PubMed] [Google Scholar]
- 13.Castellani F, Visentin A, Campagnolo M, et al. The Bruton tyrosine kinase inhibitor ibrutinib improves anti-MAG antibody polyneuropathy. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e720. doi. 10.1212/nxi.0000000000000720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latov N. Antibody testing in neuropathy associated with anti-Myelin-associated Glycoprotein antibodies: where we are after 40 years. Curr Opin Neurol. 2021;34(5):625-630. doi. 10.1097/wco.0000000000000975 [DOI] [PubMed] [Google Scholar]
- 15.Liberatore G, Giannotta C, Sajeev BP, et al. Sensitivity and specificity of a commercial ELISA test for anti-MAG antibodies in patients with neuropathy. J Neuroimmunol. 2020;345:577288. doi. 10.1016/j.jneuroim.2020.577288 [DOI] [PubMed] [Google Scholar]
- 16.Doneddu PE, Ruiz M, Bianchi E, et al. A diagnostic score for anti-myelin-associated-glycoprotein neuropathy or chronic inflammatory demyelinating polyradiculoneuropathy in patients with anti-myelin-associated-glycoprotein antibody. Eur J Neurol. 2023;30(2):501-510. doi. 10.1111/ene.15296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes R, Bensa S, Willison H, et al. , Inflammatory Neuropathy Cause and Treatment INCAT Group. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 2001;50(2):195-201. doi. 10.1002/ana.1088 [DOI] [PubMed] [Google Scholar]
- 18.Merkies IS, Schmitz PI, van der Meche FG, van Doorn PA. Psychometric evaluation of a new sensory scale in immune-mediated polyneuropathies. Neurology. 2000;54(4):943-949. doi. 10.1212/wnl.54.4.943 [DOI] [PubMed] [Google Scholar]
- 19.Varettoni M, Arcaini L, Zibellini S, et al. Prevalence and clinical significance of the MYD88 (L265P) somatic mutation in Waldenstrom's macroglobulinemia and related lymphoid neoplasms. Blood. 2013;121(13):2522-2528. doi. 10.1182/blood-2012-09-457101 [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Hunter ZR, Tsakmaklis N, et al. Clonal architecture of CXCR4 WHIM-like mutations in Waldenstrom macroglobulinaemia. Br J Haematol. 2016;172(5):735-744. doi. 10.1111/bjh.13897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drandi D, Genuardi E, Dogliotti I, et al. Highly sensitive MYD88(L265P) mutation detection by droplet digital polymerase chain reaction in Waldenstrom macroglobulinemia. Haematologica. 2018;103(6):1029-1037. doi. 10.3324/haematol.2017.186528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briani C, Visentin A, Salvalaggio A, Cacciavillani M, Trentin L. Obinutuzumab, a new anti-CD20 antibody, and chlorambucil are active and effective in anti-myelin-associated glycoprotein antibody polyneuropathy. Eur J Neurol. 2019;26(2):371-375. doi. 10.1111/ene.13838 [DOI] [PubMed] [Google Scholar]
- 23.Briani C, Visentin A, Castellani F, Cacciavillani M, Trentin L. The BCL2 inhibitor venetoclax plus rituximab is active in MYD88 wild-type polyneuropathy with anti-MAG antibodies. Neurol Neuroimmunol Neuroinflamm. 2022;9(4):e1181. doi. 10.1212/nxi.0000000000001181 [DOI] [PubMed] [Google Scholar]
- 24.Rakocevic G, Martinez-Outschoorn U, Dalakas MC. Obinutuzumab, a potent anti-B-cell agent, for rituximab-unresponsive IgM anti-MAG neuropathy. Neurol Neuroimmunol Neuroinflamm. 2018;5(4):e460. doi. 10.1212/nxi.0000000000000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visentin A, Mauro FR, Catania G, et al. Obinutuzumab plus chlorambucil versus ibrutinib in previously untreated chronic lymphocytic leukemia patients without TP53 disruptions: a real-life CLL campus study. Front Oncol. 2022;12:1033413. doi. 10.3389/fonc.2022.1033413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fresa A, Autore F, Piciocchi A, et al. Relative dose intensity of obinutuzumab-chlorambucil in chronic lymphocytic leukemia: a multicenter Italian study. Blood Adv. 2022;6(13):3875-3878. doi. 10.1182/bloodadvances.2022006964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lunn MP, Nobile-Orazio E. Immunotherapy for IgM anti-myelin-associated glycoprotein paraprotein-associated peripheral neuropathies. Cochrane Database Syst Rev. 2016;10(10):CD002827. doi. 10.1002/14651858.cd002827.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaganti S, Hannaford A, Vucic S. Rituximab in chronic immune mediated neuropathies: a systematic review. Neuromuscul Disord. 2022;32(8):621-627. doi. 10.1016/j.nmd.2022.05.013 [DOI] [PubMed] [Google Scholar]
- 29.Ferrante M, Furlan D, Zibellini S, et al. MYD88(L265P) detection in IgM monoclonal gammopathies: methodological considerations for routine implementation. Diagnostics (Basel). 2021;11(5):779. doi. 10.3390/diagnostics11050779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allain JS, Thonier F, Pihan M, et al. IGHV segment utilization in immunoglobulin gene rearrangement differentiates patients with anti-myelin-associated glycoprotein neuropathy from others immunoglobulin M-gammopathies. Haematologica. 2018;103(5):e207-e210. doi. 10.3324/haematol.2017.177444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campagnolo M, Zambello R, Nobile-Orazio E, et al. IgM MGUS and Waldenstrom-associated anti-MAG neuropathies display similar response to rituximab therapy. J Neurol Neurosurg Psychiatry. 2017;88(12):1094-1097. doi. 10.1136/jnnp-2017-315736 [DOI] [PubMed] [Google Scholar]
- 32.Benedetti L, Garnero M, Demichelis C, et al. Outcomes after single-cycle rituximab monotherapy in patients with anti-MAG polyneuropathy: a bi-center experience with an average follow-up of 11 years. J Neuroimmunol. 2019;337:577081. doi. 10.1016/j.jneuroim.2019.577081 [DOI] [PubMed] [Google Scholar]
- 33.Brown JR, Eichhorst B, Hillmen P, et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2023;388(4):319-332. doi. 10.1056/nejmoa2211582 [DOI] [PubMed] [Google Scholar]
- 34.Tam CS, Opat S, D'Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood. 2020;136(18):2038-2050. doi. 10.1182/blood.2020006844 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available on reasonable request from any qualified investigator.