Abstract
Therapeutic induction of collateral flow as a means to salvage tissue and improve outcome from acute ischemic stroke is a promising approach in the era in which endovascular therapy is no longer time-dependent but collateral-dependent. The importance of collateral flow enhancement as a therapeutic for acute ischemic stroke extends beyond those patients with large amounts of salvageable tissue. It also has the potential to extend the time window for reperfusion therapies in patients who are ineligible for endovascular thrombectomy. In addition, collateral enhancement may be an important adjuvant to neuroprotective agents by providing a more robust vascular route for which treatments can gain access to at risk tissue. However, our understanding of collateral hemodynamics, including under co-morbid conditions that are highly prevalent in the stroke population, has hindered the efficacy of collateral flow augmentation for improving stroke outcome in the clinical setting. This review will discuss our current understanding of pial collateral function and hemodynamics, including vasoactivity that is critical for enhancing penumbral perfusion. In addition, mechanisms by which collateral flow can be increased during acute ischemic stroke to limit ischemic injury, that may be different depending on the state of the brain and vasculature prior to stroke, will also be reviewed.
Introduction
Stroke is the second leading cause of death worldwide and the leading cause of disability in the US.[1,2] Approximately one-third of all ischemic strokes are large vessel occlusions, which accounts for the majority of stroke-related morbidity and mortality.[3,4] Treatment of large vessel occlusion focuses on restoration of blood flow to the penumbra, a region of reduced blood supply surrounding the core infarction, because tissue within the penumbra is potentially salvageable if reperfusion occurs.[5–7] Traditionally, stroke treatment with either thrombolysis or endovascular thrombectomy has been limited by time windows for safety and efficacy. However, recent clinical trials (DAWN and DEFUSE 3) demonstrated major benefit of reperfusion up to 24 hours in patients with salvageable tissue.[8,9] These trials showed that more robust collateral grade was linked to lower baseline National Institutes of Health Stroke Scale (NIHSS), smaller core infarct volumes, better clinical outcomes, and importantly was unrelated to time to recanalization, i.e., good collaterals offset any time dependency of revascularization treatment effects.
The importance of collateral flow during acute ischemic stroke (AIS) cannot be overstated. Flow through the leptomeningeal anastomoses (LMA), also known as pial collaterals, as an alternate pathway of perfusion during large vessel occlusion has been shown in numerous clinical and animal studies to offset the potential ischemic brain injury. Patients with good collaterals respond better to reperfusion therapies, have better outcomes, and less hemorrhagic complications. Indeed, the association between good collaterals on baseline imaging (determined by a graded scale), better reperfusion and more favorable outcome from AIS suggests better overall cerebrovascular and brain health. However, while the lack of time-dependency for endovascular therapy has revolutionized stroke treatment, its use is essentially limited to patients with small ischemic cores and large penumbral tissue that can be salvaged by reperfusion, i.e., patients with good collateral flow.[9–11] In addition, delays or inaccessibility to endovascular thrombectomy further highlight the need for interventions that sustain penumbral tissue until reperfusion can occur. [12]
Enhancement of collateral flow as a therapeutic target has been around for decades without showing success in the long-term. Revisiting this approach seems appropriate in an age in which endovascular thrombectomy has no time window and brain injury from AIS is no longer time-dependent but collateral-dependent. However, patients with good collaterals are few compared to those with insufficient amounts of salvageable tissue that are ineligible for endovascular thrombectomy. These patients not only demonstrate the importance of good collaterals for more favorable outcome in AIS, but also highlight the need for additional or adjunct treatments. This review will focus on collateral enhancing strategies and therapies for augmenting collateral perfusion during AIS. We will also consider recent studies demonstrating functional (vasoactive) LMAs and the influence of co-morbid conditions that may affect infarct expansion and the efficacy of collateral enhancing therapies.
Cerebral hemodynamics and the pial collateral circulation
The central concept behind collateral therapies for improvement of AIS outcome is to increase cerebral blood flow (CBF) in regions that are hypoperfused with the hope that these regions can be salvaged. This concept is supported by numerous clinical studies showing a strong association between good collateral status on imaging and better outcome from AIS.[9–11] Patients with robust collaterals have smaller baseline core infarct volumes, suggesting that LMAs support more salvageable tissue and a larger penumbra volume.[9–11] In addition, patients with good collaterals have more favorable outcome from endovascular thrombectomy further highlighting the important influence of collateral perfusion in sustaining viable tissue until reperfusion can occur. However, whether collateral perfusion can be induced after the onset of AIS in a way that is beneficial in the long-term is the challenge and has, to date, not been successfully accomplished. A better understanding of LMA function and collateral hemodynamics, including its heterogeneity, may help improve the benefit of collateral enhancing therapies.
LMAs are anastomotic connections between distal branches of major arterial territories, connecting middle (MCA), anterior (ACA), and posterior cerebral arteries (PCA) of the pial circulation supplying the cerebral cortex (Figure 1). Pial collaterals are unique vessels in that they are highly pressurized but have low flow and low shear stress under physiological conditions because of a lack of a pressure differential in adjacent arterial trees, e.g., ACA and MCA.[13,14] However, a considerable pressure differential is created during large vessel occlusion between the patent and obstructed arteries. This sharply increases collateral flow by ~15-fold and luminal shear stress in LMAs by ~20-fold.[15] The increase in LMA flow drives collateral perfusion through penetrating arterioles that can salvage the penumbra. When recanalization occurs, the pressure differential in LMAs is eliminated and flow and shear stress return to their low physiological levels. In the acute setting when the occlusion is distal to the circle of Willis, the increase in penumbral flow would necessarily be retrograde through pial collaterals, another unique feature of LMAs, making these small arterioles central to the efficacy of collateral enhancing therapies. In fact, the number and caliber of LMAs largely determines penumbral flow that is highly variable in humans and contributes to the high variability in stroke outcome. [16–19]
Figure 1:
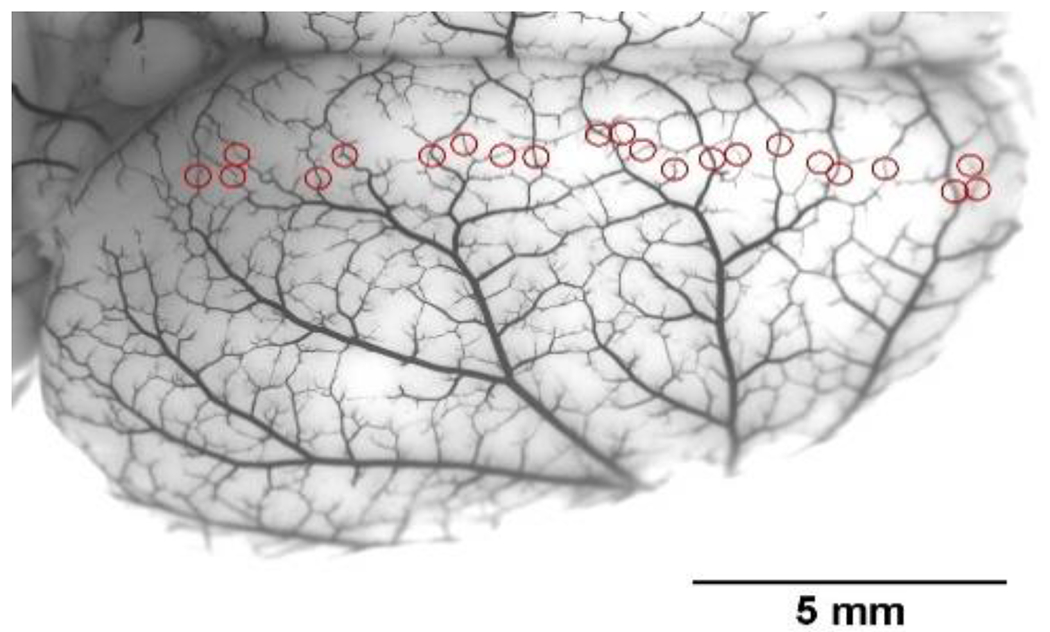
Photomicrograph of the dorsal surface of a rat brain perfused with India Ink to show leptomeningeal anastomoses (LMAs) or pial collaterals (red circles). Reproduced from Li Z et al., Am J Physiol 2018;315:H1703-H1712.
Animal studies have shown that several factors can influence LMA diameter and hence collateral perfusion. Mechanical forces including intravascular pressure, luminal flow and shear stress are potent vasoactive stimuli in the cerebral circulation that contribute to cerebrovascular resistance and control of CBF. Under hypoxic/ischemic conditions, metabolic factors including oxygen tension, carbon dioxide and pH fall outside the physiological range and promote vasodilation of cerebral arteries and arterioles. This serves to increase CBF but also may impair autoregulation of CBF. Endothelial mediators including nitric oxide (NO), intermediate-conductance calcium-activated potassium channels (IKCa) and transient receptor potential vanilloid type 4 (TRPV4) channels are factors involved in flow-induced vasodilation; their activity also opposes myogenic tone and, in that regard, contribute to changes in cerebrovascular resistance and flow. [20,21]. Endothelial dysfunction, present in a number of disease states prominent in stroke patients, impairs endothelium-dependent vasodilation, and promotes vasoconstriction, that could limit collateral flow and contribute to larger infarcts.[22–24] Interestingly, LMAs from rats do not have perivascular innervation and do not respond to norepinephrine, suggesting little neurogenic influence on collateral diameters.[21] However, perivascular innervation of arteries and arterioles proximal to LMAs may have an influence on collateral flow through changing resistance upstream (see Sphenopalatine Ganglion Stimulation). In healthy rats, LMAs have been studied isolated and pressurized and shown to have less basal myogenic tone than pial arterioles that do not anastomose (non-LMA; Figure 2). Methodology was also developed to study isolated LMAs under flow conditions and were shown to dilate substantially to increases in flow (Figure 3). Both the lower levels of tone and dilation to increased flow would be conducive to retrograde increased perfusion during large vessel occlusion.[19,20]
Figure 2:
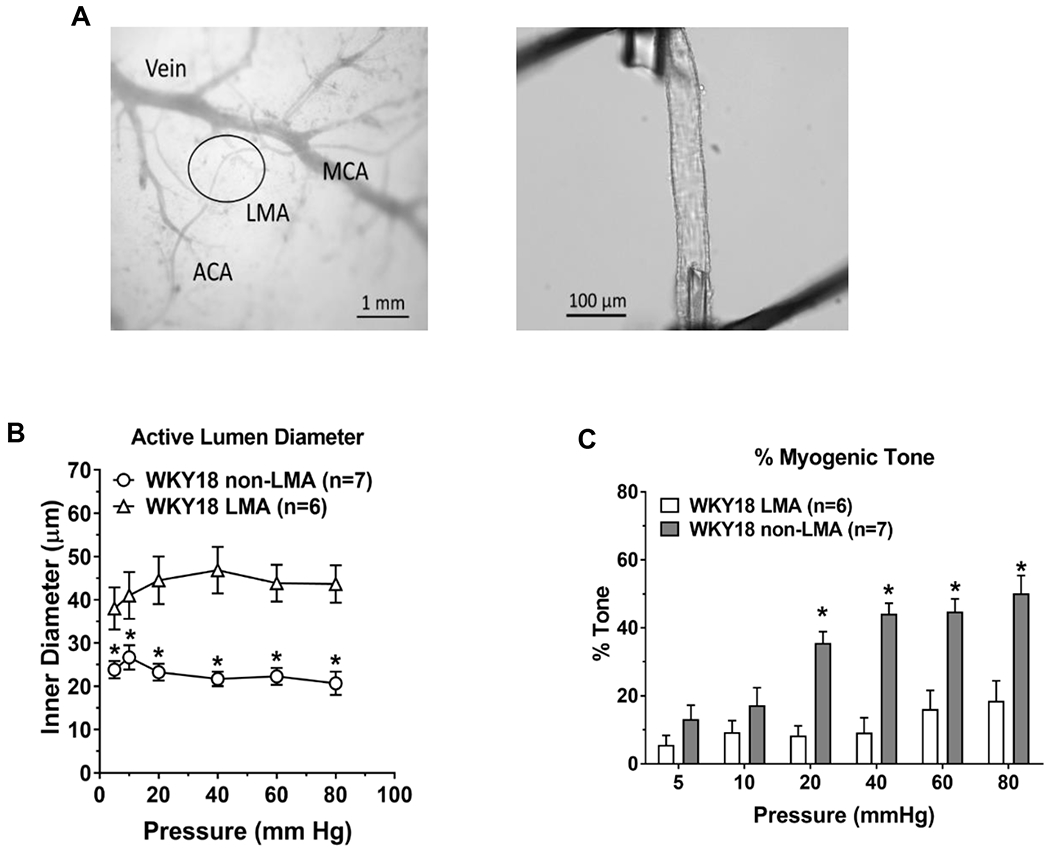
The study of isolated and pressurized leptomeningeal anastomoses (LMAs) in an arteriograph system has allowed a greater understanding of the functional properties of these unique vessels. (A) Left, photomicrograph showing leptomeningeal arterioles (black circle) and how are identified as connecting distal branches of middle cerebral artery (MCA) and anterior cerebral artery (ACA) for study isolated and pressurized. Right, LMA isolated, mounted on glass cannulas within an arteriograph chamber and pressurized. Reproduced from Chan et al. Stroke 2016;47:1618-1625; Open access CC BY-NC-ND 4.0 B) Active myogenic vasoconstriction of LMAs and non-LMAs from 18 week old normotensive WKY rats. *P<0.05 vs. LMA. C) Myogenic tone of LMA and non-LMAs from 18 week old WKY rats. *P<0.05 vs. LMA. Reproduced from Cipolla MJ. Stroke 2021;52:2465-2477
Figure 3:
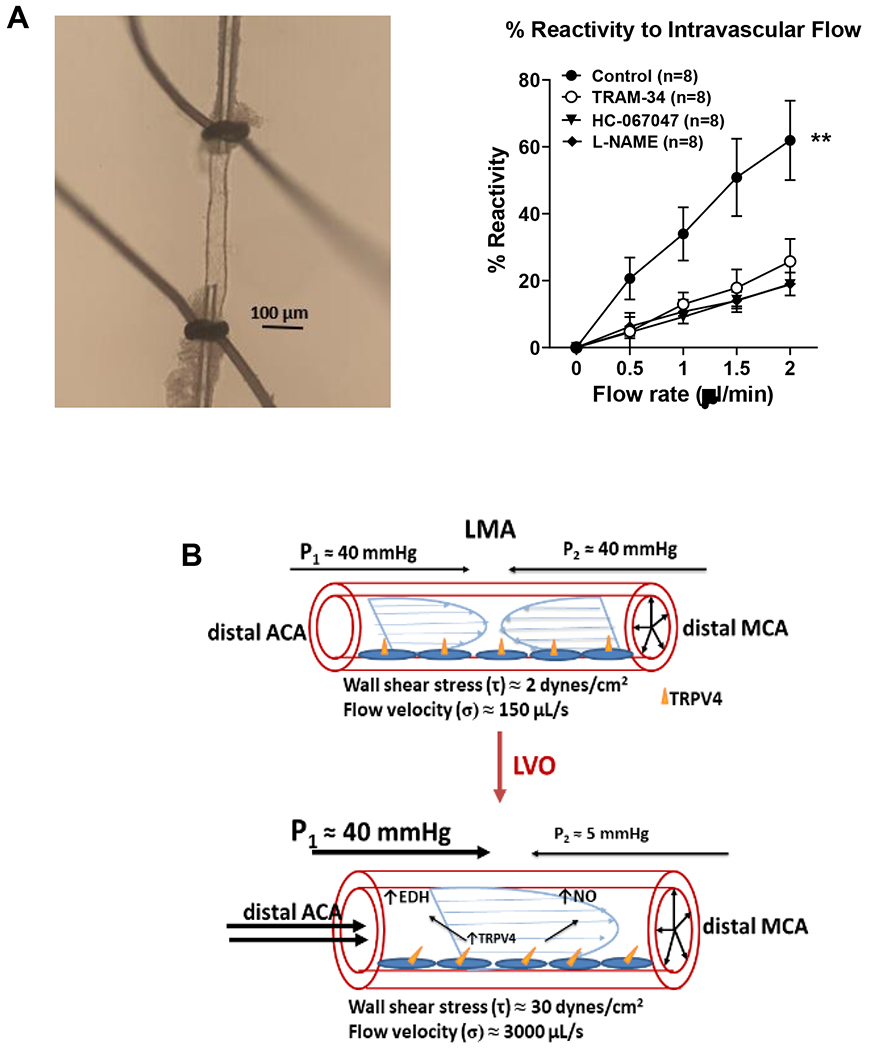
Leptomeningeal anastomoses have little to no flow under normal physiological conditions. However, shear stress and flow are increased substantially during MCAO making this vasoactive force an important mediator of collateral flow. Flow-mediated responses were studied in isolated and pressurized LMAs using an arteriograph system. (A) Left, schematic showing set up for flow-mediated responses of isolated LMAs. Cannula resistance was measured based on Ohm’s law: R=(P2-P1)/Q; where R is cannula resistance, P is inflow/outflow pressure and Q is flow. Experimentally, P1 was controlled by an inline pressure transducer and pressure servo controller such that pressure could be set between 0-200 mmHg; P2 was manipulated by changing the level of a water column connected to the distal cannula (adjustable pressure ranges −74 – 37 mmHg). During an experiment, the syringe pump was set at a certain flow rate (0.5-5 μl/min) and P1 and P2 were adjusted to obtain 40 mmHg of luminal pressure (Pvessel). Shear stress (τ) was calculated for each vessel depending on the flow rate and LMA diameter by the equation: τ = 4ηQ/πr3. Where η=viscosity of the physiological saline solution (PSS) in the perfusate, measured using a viscometer and set as 1.086 centipoise (1 cP = 1 mPa*s); Q=flowrate, and r=vessel lumen diameter. Right, photomicrograph of isolated and pressurized LMA used to measure % reactivity to flow. (B) Percent reactivity to increased flow of LMAs measured using the system described under (A). Nontreated LMAs dilated to increased flow that was inhibited by inhibitors of TRPV4 (HC067047), NO (L-NAME), and IKCa channels (TRAM-34). **p<0.01 vs. all. (C) Diagram demonstrating change in flow and shear stress in LMAs during large vessel occlusion. Upper, under normal physiological conditions, there is no pressure differential from ACA and MCA providing little flow and shear stress. During large vessel occlusion, a pressure differential is created from the proximal occlusion of the MCA, driving a substantial increase in flow and shear stress that activates mechanosensitive TRPV4 and its downstream vasodilatory pathways (EDH, endothelium-dependent hyperpolarization, NO). Data will be made available upon reasonable request.
Luminal flow and wall shear stress are also major forces that regulate vessel diameter. Shear stress is the tangential frictional force on the endothelium produced by blood flow. Shear stress is a potent vasodilator of LMAs governed by NO and activation of TRPV4 and IKCa channels.[20] Shear stress and luminal flow increase substantially in LMAs during large vessel occlusion and therefore mediators of flow- and shear stress-induced vasodilation may be important targets for enhancing collateral flow. We recently studied mechanisms of flow-mediated dilation in isolated and pressurized LMAs (Figure 3A). In untreated control LMAs from normotensive rats, increased flow caused significant vasodilation, demonstrating that flow is a potent vasodilator in LMAs (Figure 3A). When the NO synthase inhibitor L-NAME (10−3 M) was given to LMAs prior to measuring flow-induced dilation, it largely prevented the dilation Similarly, when flow-induced dilation was measured in the presence of 1.0 µmol/L TRAM-34, an IKCa channel inhibitor or GSK067047, a selective inhibitor of TRPV4 channels, they prevented the dilation of LMAs (Figure 3A). These results show that under healthy physiological conditions, increased flow and shear stress can promote significant dilation of LMAs that is blocked by inhibition of endothelial NO, TRPV4 and IKCa channels.
The importance of understanding determinants of LMA vasoactivity is that luminal diameter is the most powerful determinant of vascular resistance and hence flow.[25] Vasodilation of LMAs from their preconstricted state with basal myogenic tone is a potential means by which collateral flow could increase during large vessel occlusion. Importantly, the hemodynamic response during large vessel occlusion seems to favor LMA vasodilation through several means. First, the penumbra, by definition, is hypoperfused that would decrease pO2 and increase pCO2 in the tissue, both vasodilators of cerebral arteries and arterioles. Second, intravascular pressure likely decreases due to the occlusion that decreases not only flow, but pressure downstream. Decreased intravascular pressure promotes myogenic vasodilation to preserve flow in the face of decreased pressure. However, in healthy mice, diameters of LMAs do not change much during large vessel occlusion, suggesting vasodilation of downstream arterioles that undergo myogenic vasodilation in response to reduced pressure.[26] Third, retrograde flow through LMAs increases substantially in response to occlusion and promotes vasodilation through flow- and shear shear-mediated mechanisms. Under normal physiological conditions, LMAs are larger than pial arterioles, both actively with tone and structurally under passive conditions, that would provide less resistance when proximal and distal segments vasodilate.[21] Thus, LMAs are lower resistance vessels spanning higher resistance arterioles proximally and distally, i.e., LMAs are importantly not a bottleneck to flow. This is not necessarily the case for disease states and co-morbid conditions in which LMAs are highly vasoconstricted and have impaired vasodilation to flow and other mediators. [21,27–29]
Influence of co-morbid conditions on pial collaterals and collateral flow
Most stroke patients are not healthy adults but have multiple comorbid conditions such as hypertension, diabetes and hyperlipidemia. These conditions are associated with considerable cerebrovascular dysfunction that could potentially influence LMA structure and function and hence collateral perfusion. The effect of disease states on stroke and stroke outcome relates to how stroke hemodynamics are affected including perfusion deficit, collateral flow and extent of reperfusion. In addition, many co-morbid conditions are states of high oxidative stress, inflammation and endothelial dysfunction, making the brain more susceptible to hypoxic/ischemic injury.[22–24] In other words, ischemic tolerance is potentially lower in patients with certain co-morbidities.
There is clinical evidence that co-morbidities are associated with poor collateral flow and worse outcomes. Treatment of stroke risk factors, including hyperlipidemia and hypertension, not only reduces the risk of stroke, but is associated better collateral flow and outcome from AIS. For example, patients on lipid lowering statins have more favorable outcome from AIS, including smaller final infarct volume, that has been associated with better collateral flow. [30–36] Statins have pleiotropic beneficial effects on the vasculature that extend beyond that of lipid lowering. These include upregulation of endothelial NO synthase and NO production, reduced inflammation and less oxidative stress. [37–39] Therefore, the association between statin use and better collateral flow during AIS is likely multifactorial and may relate to better overall cerebrovascular health. In addition, statin-induced upregulation of NO may provide for a more robust collateral flow response to shear stress that is NO-dependent. The greater collateral flow response to AIS in patients on statins also suggests that hyperlipidemia impairs collateral flow to worsen outcome.
Antihypertensive use has also been associated with better collateral flow during AIS, suggesting hypertension has a negative impact on collateral perfusion. In fact, numerous animal studies have shown that compared to normotensive animals, chronic hypertensive animals are more susceptible to ischemic injury [40–45]. One of the primary mechanisms by which infarction is larger in chronic hypertension is the relative lack of salvageable tissue, poor collateral status, and rapid evolution of infarct to encompass the penumbra.[41–43] Induction of collateral flow during AIS is also impaired in animal models of chronic hypertension (Figure 4). This has been shown to be associated with LMAs that are highly vasoconstricted and respond to pressure myogenically (Figure 5).
Figure 4:
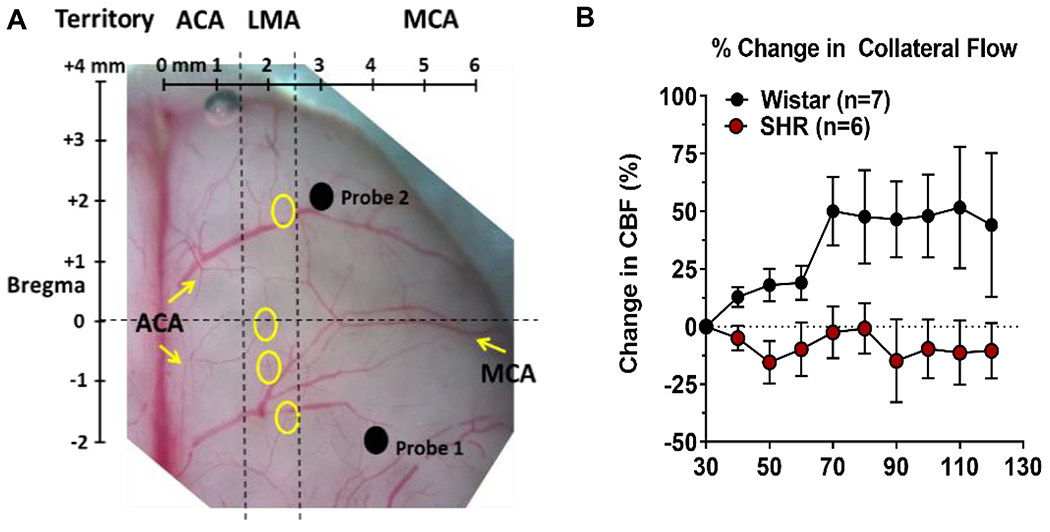
Measurement of collateral flow using multi-site laser Doppler in rats and the effect of chronic hypertension. (A) Photomicrograph of the dorsal surface of a rat brain with overlay of skull coordinates. Probe 1 was placed over the core MCA territory and Probe 2 lateral to LMAs (yellow circles) to measure collateral flow. Reproduced from Cipolla et al., J Cerebr Blood Flow Metab. 2018;38:755-766. (B) Graph showing % change in collateral flow calculated from filament insertion in normotensive Wistar and hypertensive SHR rats. Collateral flow increased in response to MCAO in Wistar but not SHR. Data will be made available upon reasonable request.
Figure 5:
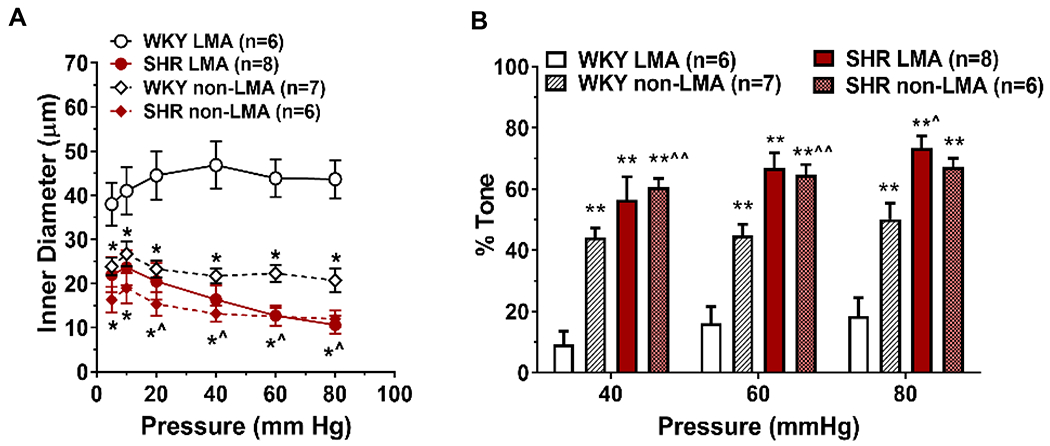
Vasoconstriction of LMAs and non-LMAs from spontaneously hypertensive rats (SHR) compared to normotensive Wistar Kyoto (WKY) rats. LMAs were isolated and studied pressurized in an arteriograph chamber. Intravascular pressure was increased stepwise and lumen diameter measured to study the myogenic response. (A) Inner diameter was greater and myogenic reactivity was considerably less in LMAs from WKY rats compared to LMAs from SHR that were more similar to non-LMA pial arterioles. *p<0.05 vs. WKY non-LMA; ^p<0.05 vs. WKY LMA. Data will be made available upon reasonable request.
Clinical studies have also shown a positive association between poor collateral status on imaging and untreated chronic hypertension. [46] One clinical study compared collateral status in patients with AIS who had untreated chronic hypertension vs. those who were treated with antihypertensive medications (calcium channel blockers, β blockers, ACE inhibitors or AT1R blockers) or did not have hypertension. The results showed a graded, stepwise association between collateral status and treatment of hypertension. Patients without antihypertensive medications had the worst collateral status whereas patients without hypertension had the best; hypertensive patients treated with antihypertensive medications were in between.[46] Thus, similar to statin use, antihypertension treatments have a beneficial effect on collaterals [27].
Other co-morbid conditions and aging are also associated with less favorable outcome from AIS and poor collateral flow, including diabetes, metabolic syndrome, and small vessel disease.[47–53] These conditions are all associated with cerebrovascular disease and may have common underlying mechanisms of poor collateral flow, including endothelial dysfunction and impaired NO that reduces shear stress and flow-mediated vasodilation of LMAs. In addition, structural inward remodeling and rarefaction of LMAs may reduce vasodilatory reserve and impair recruitment of pial collaterals during large vessel occlusion.
Considerations for collateral enhancing therapies
Understanding the structure and vasoactive state of LMAs under conditions such as aging and co-morbid conditions (hypertension, hyperlipidemia, diabetes) is likely important for successfully increasing collateral flow during occlusion. Although pretreatment with statins or antihypertensive medications may provide a healthier cerebrovasculature that is more conducive to collateral flow during large vessel occlusion, these medications are not indicated for all those who have stroke. Co-morbidities that are highly prevalent in the stroke population may influence the efficacy of collateral enhancing therapies. For example, therapies that attempt to increase collateral flow hemodynamically through increasing the pressure differential between systemic and cerebral circulations or other hemodynamic means (e.g., aortic occlusion, induced hypertension) may not work in patients with vasoactive LMAs that respond myogenically to increases in intravascular pressure such as chronic hypertension. Similarly, treatments that rely on vasodilation (e.g., sphenopalatine ganglion stimulation (SPG), inhaled NO) may not be effective in patients with pial collaterals that are already maximally vasodilated through either autoregulation (myogenic vasodilation), metabolic factors (low pO2 and high pCO2) or have reduced vasodilatory reserve from rarefaction or inward remodeling. In addition, for vasodilators to increase collateral perfusion, the pial circulation needs to be able to respond with vasodilation, i.e., it needs to be partially constricted and have enough vasodilatory reserve to increase flow. Pial arteries and arterioles, including LMAs, also need to be able to activate vasodilatory pathways in response to the vasodilating agent. This requires expression and function of appropriate receptors, ion channels, intracellular signaling pathways, etc. Thus, the “one size fits all” approach to stroke treatment may not work for collateral enhancing therapies.
Collateral enhancing therapies
A number of interventions have been used experimentally and clinically to enhance collateral perfusion and improve outcome from AIS. Described below are some of these treatments, their purported mechanism of action, and efficacy in AIS, if known. This is not meant to be exhuastive but to provide a review of the various means by which collateral enhancement has been tried.
PP-007 (Sanguinate™)
PP-007 (previously Sanguinate™, SG) is a PEGylated carboxyhemoglobin (PEG-COHb) gas transfer agent with pleiotropic effects on the brain and cerebral circulation. Protection during ischemia is thought to be due to several beneficial effects of this compound, including release of small amounts of carbon monoxide (CO).[54] Release of CO has been shown to have vasodilatory, anti-inflammatory, and anti-apoptotic effects.[55] In addition, the unloading of CO from PEG-COHb in ischemic tissue may also increase oxyhemoglobin and aid in oxygenating the ischemic tissue. Several preclinical studies have shown PEG-COHb provides robust protection of AIS, including decreased neurological deficit and reduced infarct volume.[54,56,57] Protection by PEG-COHb was shown to be related to the sustained vasodilation of pial arterioles at the ischemic boarder region.[56] CO is a known vasodilator and it is likely that CO released from PEG-COHb in the ischemic region provided sustained dilation that was not seen in non-ischemic animals.[55] While collaterals were not specifically targeted in this study, a subsequent study in spontaneously hypertensive rats (SHR) showed that SG, also a PEG-COHb gas transfer agent similar to the one used previously, increased collateral flow and reduced early infarct.[57] A recent study in dogs confirmed the collateral enhancing properties of SG.[58]. The success of these preclinical studies has led to an ongoing Phase 1 multi-center clinical trial for treatment of large vessel occlusion in patients undergoing endovascular thrombectomy (HEMERA-1).
Inhaled NO (iNO)
NO is a potent vasodilator gas that when mixed with 30% O2 at low concentrations can be inhaled and was originally used as a selective treatment for persistent pulmonary hypertension. iNO was shown to increase cerebral blood volume in healthy pigs without increasing CBF [59]. Subsequent studies in mice showed indeed iNO did not increase CBF in non-ischemic mice, but selectively dilated arterioles within the penumbra and increased collateral flow.[60] The increase in collateral flow was associated with reduced infarction and better neurologic outcome.[60] The mechanism behind selective vasodilation in ischemic tissue is not clear, but may involve NO carriers such as nitrite or S-nitroso hemoglobin.[61] Regardless of the carrier, how iNO causes vasodilation seems important to understand since vascular dysfunction may impair classic NO vasodilatory pathways, including cyclic GMP and guanylyl cyclase activation in smooth muscle. For example, iNO does not appear to work in models of acute hyperglycemia and chronic hypertension.[62] In addition, dilation to the NO donor sodium nitroprusside was significantly impaired in LMAs from SHR. To date, no clinical trial has been performed on iNO for treatment of ischemic stroke.
Sphenopalatine ganglion stimulation (SPG)
Electrical stimulation of the SPG produces modest increases in CBF through activation of parasympathetic innervation in the anterior circulation. [63,64] When used in ischemic stroke models, SPG stimulation increases ipsilateral CBF and reduces infarct volume compared to untreated animals. [65–71] The mechanism by which SPG increases flow is likely multi-factorial. Parasympathetic fibers arise directly from the SPG and innervate the carotid artery and other cerebral vessels. Activation of the SPG promotes vasodilation through release of neurotransmitters including NO, acetylcholine and vasoactive intestinal peptide.[72] Mechanisms of action of SPG stimulation in acute stroke has been nicely reviewed. [73]
A clinical trial of SPG stimulation (ImpACT-24B Trial) in stroke patients (who were not treated with endovascular thrombectomy or thrombolysis) enrolled 303 patients of the planned 660 when refinement of the SPG stimulation treatment technique (inclusion of an optical navigation system) stopped the trial. However, analysis of the results of the initial 303 patients was done. It was found that SPG stimulation significantly improved outcomes in patients with cortical stroke, with improved modified Rankin scale (mRS) at 90 days.[74] Interestingly, the beneficial effect of SPG stimulation was location-specific and only occurred in patients with cortical but not in patients with stroke in other locations. Since collateral enhancement would increase CBF to a greater extent in cortical regions, this suggests SPG stimulation acted through increased collateral flow. In addition, SPG stimulation was performed around 18 hours after stroke symptom onset, suggesting collateral enhancement even at later time points can provide improvement.[74]
Remote Ischemic Conditioning (RIC)
RIC involves brief, intermittent events of mild ischemia and reperfusion applied to a peripheral tissue. Its mechanism of action is based on ischemic preconditioning in which brief periods of ischemia before or after more severe ischemia provides protection from tissue injury. [75] Ischemic preconditioning was subsequently found to be efficacious when applied to a remote organ or vessel from severe ischemia, i.e., RIC. [76] In humans, RIC is usually applied by inflating or deflating a cuff around the upper arm or leg. RIC for stroke has the advantage that it is safe, easy to perform, and non-invasive. For treatment of AIS, RIC can be applied during the ischemic event but prior to reperfusion (remote preconditioning) or after the ischemic event (remote post-conditioning). Several studies have shown benefits of remote perconditioning in animal models of stroke [77–82] In a rat model of distal middle cerebral artery occlusion (MCAO), remote perconditioning was shown to promote a sustained increase collateral perfusion and prevent “collateral failure” over time. [77] The increase in collateral flow with remote perconditioning reduced early infarction. However, the beneficial effect of this perconditioning on collateral flow and stroke outcome may not be as robust in co-morbid models or aging that have greater ischemic injury and collaterals that have impaired vasoreactivity. The mechanism by which RIC increases collateral flow in the acute phase is not clear but has been attributed to a combination of circulating humoral factors, activation of neural pathways, and peripheral immune system activity. This has been reviewed recently by Abbasi-Habashi, et al.[83]
Randomized clinical trials of RIC as an adjunct to tPA or endovascular thrombectomy have been done or are currently ongoing in AIS patients.[84–89] Results have shown that RIC is well-tolerated and showed improvement in small cohorts of select AIS patients [88–90]. However, the effect of RIC on collateral perfusion does not seem to be an endpoint in any previous or ongoing clinical trial. Therefore, the efficacy of RIC as a collateral enhancing therapy has yet to be determined.
Rapamycin
Several studies in experimental stroke models in rats have looked at giving a vasodilator to enhance collateral flow either during MCAO or post-treatment. Rapamycin is an inhibitor of mammalian target of rapamycin (mTOR) that has been shown to have beneficial effects on the cerebral vasculature.[91] Treatment with rapamycin can restore CBF in models of Alzheimer’s Disease and promotes vasodilaton through NO release.[92] In a rat model of stroke, rapamycin increased collateral perfusion during MCAO and during reperfusion[93]. This study also showed that rapamycin dilated LMAs that was inhibited by L-NAME, suggesting NO release as a mechanism of collateral enhancement by rapamycin.[93] Rapamycin treatment also improved infarct and sensorimotor deficit at 24 hours in normal healthy animals. [93,94] However, while rapamycin modestly increased collateral perfusion in a model of chronic hypertension (SHR) as well, it did not improve early infarction [93]. Post-treatment with rapamycin also improved collateral perfusion in mice that was associated with smaller infarct and improved neurobehavioral deficits after 14 days.[94] Rapamycin is a Food and Drug Administration approved drug for immune anti-rejection and therefore is known to be tolerated in humans, but to date no clinical trial of rapamycin for AIS is ongoing.
TM5441
Plasminogen activator inhibitor-1 (PAI-1) is an endogenous serine protease inhibitor that regulates fibrinolysis through reducing plasmin production by tissue-type and urokinase-type plasminogen activator (tPA, uPA) [95,96]. PAI-1 is increased in aging and hypertension and has been shown to have a role in stroke outcome through increased coagulation. [97] In addition, PAI-1 inhibition reduced infarct and increased reperfusion in a mouse model of AIS.[98] TM5441, a selective inhibitor of PAI-1 vasodilates LMAs through NO release [99], making it a promising collateral enhancing therapy. In young and aged SHR, i.v. infusion of TM5441 30 minutes into MCAO increased collateral perfusion and reduced early infarct. Improvement of stroke outcomes in the long-term have not been done with TM5441. There are also currently no clinical trials of TM5441 in AIS ongoing.
Induced hypertension
Pressor therapy has been used for decades in the neurocritical care setting to prevent delayed cerebral ischemia subsequent to subarachnoid hemorrhage. In healthy adults, increasing systemic blood pressure up to ~180 mmHg does not appreciably increase CBF because of autoregulatory mechanisms that keep brain blood flow relatively constant in the face of increased mean arterial pressure. Therefore, the mechanism by which induced hypertension increases collateral flow relies on the penumbra having impaired CBF autoregulation during large vessel occlusion so that the pial arterioles, including LMAs, are pressure-passive. Experimental studies in several different species, including dogs, baboons, mice and rats have shown that induced hypertension reduces infarction.[100–104]. Some studies also showed that more favorable stroke outcome was related to enhanced collateral flow [103,104] and increased cerebral metabolic rate of oxygen in the core and penumbra.[103] While promising, pial collaterals may not be pressure-passive under all conditions. We previously showed that LMA vasoconstriction in SHR persists in vivo during MCAO, suggesting autoregulation may be partially intact.[21] In the clinical setting, not all patients benefit from therapeutic induced hypertension. For example, in a clinical trial of therapeutic induced hypertension in AIS patients, using phenylephrine to raise blood pressure, found early neurologic improvement (better NIHSS at 7 days) in 58% of treated and 31% of untreated patients.[106] While promising, there were still 42% of patients that did not benefit from induced hypertension.[106] Collateral flow was not measured in this study and therefore the role of collaterals in improving outcome with induced hypertension is not clear.
Partial aortic occlusion
Augmentation of CBF by temporary occlusion of the abdominal aorta as a means to reduce ischemic injury has been investigated. The mechanism by which aortic occlusion increasing CBF is multi-factorial. Aortic occlusion augments mean arterial pressure in the carotid artery, promotes redistribution of blood volume, activates the sympathetic nervous system and is used clinically to prevent hemodynamic collapse during traumatic hemorrhage [107–109] Aortic occlusion was shown to reduce infarction in a model of embolic stroke.[110] In a previous clinical trial (SENTIS) a catheter (NeuroFlo) was used to restrict aortic flow in AIS patients and was shown to be safe, but did not show clinical benefit compared to standard care.[111–113] However, subgroup analysis found improved outcome in patients with moderate stroke severity when treated within 6 hours, suggesting these patients had salvageable tissue. [113] Thus, hemodynamic augmentation of collateral perfusion may be beneficial in a subset of patients but more studies are needed to determine factors that confer efficacy.
Summary and Future directions
Tissue within the core infarction is not readily salvageable, making the focus of neuroprotection during AIS penumbral tissue. Brain tissue within the penumbra may be salvageable if reperfusion occurs or neuroprotective agents are present to provide protection from cell death. In either case, collateral perfusion is important for preventing or offsetting ischemic injury during large vessel occlusion. Figure While some progress has been made experimentally at understanding LMA function under physiological and pathological conditions, more work is needed from a vascular biology standpoint to understand collateral failure and how to induce collateral flow. This includes understanding not only LMA function but also the function of penetrating arterioles that branch off pial collaterals that are the bottlenecks to flow to the penumbra. Even then, it is likely that combined therapies are needed to salvage penumbral tissue and improve outcome in a majority of AIS patients. A tertiary analysis of the URICO-ICTUS clinical trial revealed early ischemic worsening was prevented in patients who received uric acid, a known antioxidant, but only in patients that had good collaterals.[114] This is an important lesson – collateral flow is likely needed for neuroprotective agents to reach their target. Other clinical trials that treat with neuroprotective agents in combination with endovascular thrombectomy (e.g., HEMERA-1, ESCAPE NA1) may also provide insight into pleotropic effects of increasing collateral perfusion and providing neuroprotection to the penumbra. However, the utility of collateral enhancement for improving outcome from AIS needs to extend beyond those patients with large amounts of salvageable tissue. Increasing collateral perfusion in patients who are ineligible for reperfusion therapies is also critically important, either to extend the time window for reperfusion and make them eligible for endovascular thrombectomy or tPA or provide for neuroprotection.
Figure 6:
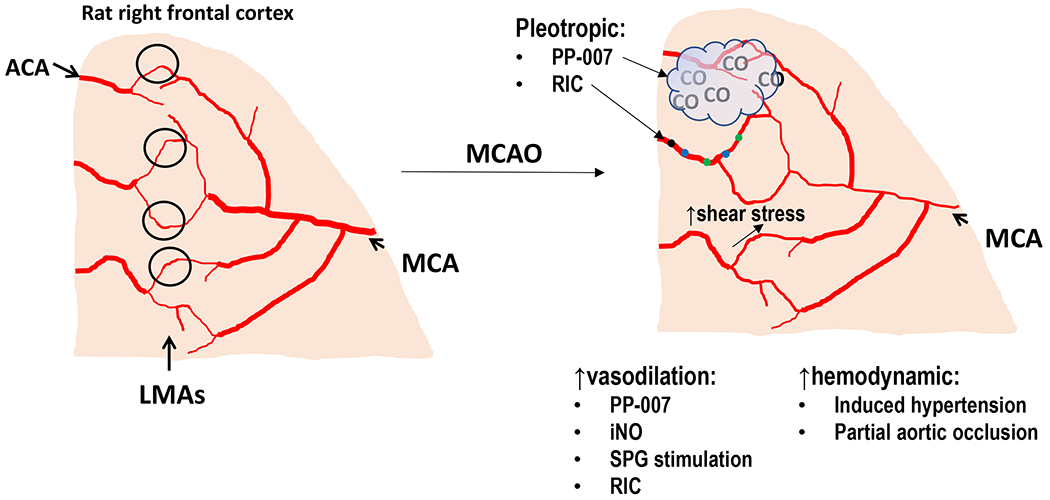
Summary of known and potential mechanisms of collateral enhancing therapies. (A) Diagram of the pial surface of the cortex showing branches of the MCA and ACA. LMAs are distal connections between these arterial territories (black circles). Under normal physiological conditions, there is little flow and shear stress within LMA due to a lack of pressure differential between MCA and ACA. As shown, LMAs are larger in diameter than pial arterioles that do not anastomose. (B) During MCAO, distal branches of the MCA are smaller in diameter due to proximal occlusion that decreases pressure and flow. Also shown are collateral enhancing therapies and potential mechanism by which collateral flow increases. They can be categorized into vasodilators that increase collateral flow through LMA vasodilation or hemodynamically increasing flow through increased cerebral perfusion pressure. Some therapies have multiple beneficial effects (pleotropic). PP-007 causes vasodilation of LMAs and also releases small amounts of CO gas that has anti-inflammatory effects. RIC also increases collateral flow through increased circulating humoral factors and enhanced peripheral immune system activity that likely has other beneficial effects on mitigating ischemic injury. Flow and shear stress are also potent vasodilators that increases collateral flow during MCAO. Flow-induced dilation appears mediated by shear stress-induced activation of TRPV4, NO and IKCa channels.
Funding:
We are grateful for the continued support of the National Institute of Neurologic Disorders and Stroke grants 2R01NS93289 and R21NS120419.
Footnotes
Disclosures: None.
Ethics approval: All studies were approved by the Institutional Animal Care and Use committee at the University of Vermont and complied with the National Institutes of Health guidelines for the care and use of laboratory animals.
References
- 1.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013; 369:448–457. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, et al. Prognosis of untreated strokes due to anterior circulation proximal intracranial arterial occlusions detected by use of computed tomography angiography. JAMA Neurol. 2014; 71:151–157. [DOI] [PubMed] [Google Scholar]
- 4.Chen CJ, Ding D, Starke RM, Mehndiratta P, Crowley RW, Liu KC, et al. Endovascular vs medical management of acute ischemic stroke. Neurology. 2015; 85:1980–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia – the ischemic penumbra. Stroke. 1981;12:723–725. [DOI] [PubMed] [Google Scholar]
- 6.Jung S, Gilgen M, Slotboom J, et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain. 2013;136:3554–3560. [DOI] [PubMed] [Google Scholar]
- 7.Winship IR, Armitage GA, Ramakrishnan G, et al. Augmenting collateral blood flow during ischemic stroke via transient aortic occlusion. J Cereb Blood Flow Metab. 2014;34:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albers GW et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378, 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nogueira RG et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018. 378; 11–21. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer PW, Barak ER, Kamalian S, Gharai LR, Schwamm L, Gonzalez RG, et al. Quantitative assessment of core/penumbra mismatch in acute stroke: CT and MR perfusion imaging are strongly correlated when sufficient brain volume is imaged. Stroke. 2008;39:2986–2992. [DOI] [PubMed] [Google Scholar]
- 11.Yoo AJ, Chaudhry ZA, Nogueira RG, Lev MH, Schaefer PW, Schwamm LH, et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke. 2012;43:1323–1330. [DOI] [PubMed] [Google Scholar]
- 12.Hossini J neuroimage. 2018.
- 13.Faber JE, Storz JF, Cheviron ZA, Zhang H. High-altitude rodents have abundant collaterals that protect against tissue injury after cerebral, coronary and peripheral artery occlusion. J Cereb Blood Flow Metab. 2020;41:731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toriumi H, Tatarishvili J, Tomita M, et al. Dually supplied T-junctions in arteriolo-arteriolar anastomosis in mice: key to local hemodynamic homeostasis in normal and ischemic states? Stroke. 2009;40:3378–3383. [DOI] [PubMed] [Google Scholar]
- 15.Chalothorn D and Faber JE. Formation and maturation or the murine native cerebral collateral circulation. J Molec Cell Cardiol. 2010;49:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke. 2003;34:2750–2762. [DOI] [PubMed] [Google Scholar]
- 17.Liebeskind DS. Collaterals in acute stroke: beyond the clot. Neuroimaging Clin N Am. 2005;15:553–573. [DOI] [PubMed] [Google Scholar]
- 18.Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol. 2006;26:1057–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30:923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Cipolla MJ. Mechanisms of Flow-Mediated Dilation of Pial Collaterals and the Effect of Hypertension. Hypertension. 2022;79:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan SL, Sweet JG, Bishop N, Cipolla MJ. Pial Collateral Reactivity During Hypertension and Aging: Understanding the Function of Collaterals for Stroke Therapy. Stroke. 2016;47:1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cipolla MJ, Liebeskind DS, Chan SL. The importance of comorbidities in ischemic stroke: Impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab. 2018;38:2129–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol. 2018;34:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Meyer GR, Herman AG. Vascular endothelial dysfunction. Prog Cardiovasc Dis. 1997;39:325–42. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler EC, Brenner ZR. Peripheral vascular anatomy, physiology, and pathophysiology. AACN Clin Issues. 1995;6:505–514. [DOI] [PubMed] [Google Scholar]
- 26.Murtha LA, McLeod DD, Pepperall D, McCann SK, Beard DJ, Tomkins AJ, Holmes WM, McCabe C, Macrae IM, Spratt NJ. Intracranial pressure elevation after ischemic stroke in rats: cerebral edema is not the only cause, and short-duration mild hypothermia is a highly effective preventive therapy. J Cereb Blood Flow Metab. 2015;35:592–600. Erratum in: J Cereb Blood Flow Metab. 2015;35:2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Lindner DP, Bishop NM, Cipolla MJ. ACE (Angiotensin-Converting Enzyme) Inhibition Reverses Vasoconstriction and Impaired Dilation of Pial Collaterals in Chronic Hypertension. Hypertension. 2020;76:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipolla MJ, Chan SL. Impact of Acute and Chronic Hypertension on Changes in Pial Collateral Tone In Vivo During Transient Ischemia. Hypertension. 2020;76:1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCabe C, Gallagher L, Gsell W, Graham D, Dominiczak AF, Macrae IM. Differences in the evolution of the ischemic penumbra in stroke-prone spontaneously hypertensive and Wistar-Kyoto rats. Stroke. 2009;40:3864–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsivgoulis G, Katsanos AH, Sharma VK, Krogias C, Mikulik R, Vadikolias K, Mijajlovic M, Safouris A, Zompola C, Faissner S, Weiss V, Giannopoulos S, Vasdekis S, Boviatsis E, Alexandrov AW, Voumvourakis K, Alexandrov AV. Statin pretreatment is associated with better outcomes in large artery atherosclerotic stroke. Neurology. 2016;86:1103–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sargento-Freitas J, Pagola J, Rubiera M, Flores A, Silva F, Rodriguez-Luna D, Pineiro S, Alvarez-Sabín J, Molina CA, Ribo M. Preferential effect of premorbid statins on atherothrombotic strokes through collateral circulation enhancement. Eur Neurol. 2012;68:171–176. [DOI] [PubMed] [Google Scholar]
- 32.Safouris A, Katsanos AH, Kerasnoudis A, Krogias C, Kinsella JA, Sztajzel R, Lambadiari V, Deftereos S, Kargiotis O, Sharma VK, Demchuk AM, Saqqur M, McCabe DJH, Tsivgoulis G. Statin Pretreatment and Microembolic Signals in Large Artery Atherosclerosis. Stroke. 2018;49:1992–1995. [DOI] [PubMed] [Google Scholar]
- 33.Ovbiagele B, Saver JL, Starkman S, Kim D, Ali LK, Jahan R, Duckwiler GR, Viñuela F, Pineda S, Liebeskind DS. Statin enhancement of collateralization in acute stroke. Neurology. 2007;68:2129–2131. [DOI] [PubMed] [Google Scholar]
- 34.Lee MJ, Bang OY, Kim SJ, Kim GM, Chung CS, Lee KH, Ovbiagele B, DS, Saver JL. Role of statin in atrial fibrillation-related stroke: an angiographic study for collateral flow. Cerebrovasc Dis. 2014;37:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liebeskind DS, Tomsick TA, Foster LD, Yeatts SD, Carrozzella J, Demchuk AM, Jovin TG, Khatri P, von Kummer R, Sugg RM, Zaidat OO, Hussain SI, Goyal M, Menon BK, Al Ali F, Yan B, Palesch YY, Broderick JP; IMS III Investigators. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke. 2014;45:759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malhotra K, Safouris A, Goyal N, Arthur A, Liebeskind DS, Katsanos AH, Sargento-Freitas J, Ribo M, Molina C, Chung JW, Bang OY, Magoufis G, Cheema A, Shook SJ, Uchino K, Alexandrov AV, Tsivgoulis G. Association of statin pretreatment with collateral circulation and final infarct volume in acute ischemic stroke patients: A meta-analysis. Atherosclerosis. 2019;282:75–79. [DOI] [PubMed] [Google Scholar]
- 37.Giannopoulos S, Katsanos AH, Tsivgoulis G, Marshall RS. Statins and cerebral hemodynamics. J Cereb Blood Flow Metab. 2012;32:1973–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asahi M, Huang Z, Thomas S, Yoshimura S, Sumii T, Mori T, Qiu J, Amin-Hanjani S, Huang PL, Liao JK, Lo EH, Moskowitz MA. Protective effects of statins involving both eNOS and tPA in focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oesterle A, Liao JK. The Pleiotropic Effects of Statins - From Coronary Artery Disease and Stroke to Atrial Fibrillation and Ventricular Tachyarrhythmia. Curr Vasc Pharmacol. 2019;7:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Letourneur A, Roussel S, Toutain J, et al. Impact of genetic and renovascular chronic arterial hypertension on the acute spatiotemporal evolution of the ischemic penumbra: A sequential study with MRI in the rat. J Cereb Blood Flow Metab. 2011; 31:504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCabe C, Gallagher L, Gsell W, et al. Differences in the evolution of the ischemic penumbra in stroke-prone spontaneously hypertensive and Wistar-Kyoto rats. Stroke. 2009;40:3864–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cipolla MJ, Sweet JG, Chan S-L. Effect of hypertension and peroxynitrite decomposition with FeTMPyP on CBF and stroke outcome. J Cerebr Blood Flow Metab. 2017;37:1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barone FC, Clark RK, Feuerstein G, et al. Quantitative comparison of magnetic resonance imaging (MRI) and histologic analyses of focal ischemic damage in the rat. Brain Res Bull.1991;26: 285–291. [DOI] [PubMed] [Google Scholar]
- 44.Kang BT, Leoni RF and Silva AC. Impaired CBF regulation and high CBF threshold contribute to the increased sensitivity of spontaneously hypertensive rats to cerebral ischemia. Neuroscience. 2014; 69:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dogan A, Baskaya MK, Rao VL, et al. Intraluminal suture occlusion of the middle cerebral artery in spontaneously hypertensive rats. Neurol Res. 1998;20:265–270. [DOI] [PubMed] [Google Scholar]
- 46.Fujita K, Tanaka K, Yamagami H, Ide T, Ishiyama H, Sonoda K, Satow T, Takahashi JC, Ihara M, Koga M, Yokota T, Toyoda K. Detrimental Effect of Chronic Hypertension on Leptomeningeal Collateral Flow in Acute Ischemic Stroke. Stroke. 2019;50:1751–1757. [DOI] [PubMed] [Google Scholar]
- 47.Nishijima Y, Akamatsu Y, Yang SY, Lee CC, Baran U, Song S, Wang RK, Tominaga T, Liu J. Impaired Collateral Flow Compensation During Chronic Cerebral Hypoperfusion in the Type 2 Diabetic Mice. Stroke. 2016;47:3014–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, Hill MD, Demchuk AM, Damani Z, Cho KH, Chang HW, Hong JH, Sohn SI. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. 2013;74:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akamatsu Y, Nishijima Y, Lee CC, Yang SY, Shi L, An L, Wang RK, Tominaga T, Liu J. Impaired leptomeningeal collateral flow contributes to the poor outcome following experimental stroke in the Type 2 diabetic mice. J Neurosci. 2015;35:3851–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishijima Y, Akamatsu Y, Weinstein PR, Liu J. Collaterals: Implications in cerebral ischemic diseases and therapeutic interventions. Brain Res. 2015;1623:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiegers EJA, Mulder MJHL, Jansen IGH, Venema E, Compagne KCJ, Berkhemer OA, Emmer BJ, Marquering HA, van Es ACGM, Sprengers ME, van Zwam WH, van Oostenbrugge RJ, Roos YBWEM, Majoie CBLM, Roozenbeek B, Lingsma HF, Dippel DWJ, van der Lugt A; MR CLEAN Trial and MR CLEAN Registry Investigators. Clinical and Imaging Determinants of Collateral Status in Patients With Acute Ischemic Stroke in MR CLEAN Trial and Registry. Stroke. 2020;51:1493–1502. [DOI] [PubMed] [Google Scholar]
- 52.Ma J, Ma Y, Shuaib A, Winship IR. Impaired collateral flow in pial arterioles of aged rats during ischemic Stroke. Transl Stroke Res. 2020;11:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin MP, Brott TG, Liebeskind DS, Meschia JF, Sam K, Gottesman RF. Collateral recruitment is impaired by cerebral small vessel disease. Stroke. 2020;51:1404–141. [DOI] [PubMed] [Google Scholar]
- 54.Klaus JA, Kibler KK, Abuchowski A, Koehler RC. Early treatment of transient focal cerebral ischemia with bovine PEGylated carboxy hemoglobin transfusion. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Motterlini R Carbon monoxide-releasing molecules (CO-RMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem Soc Trans. 2007;35:1142–1146. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Cao S, Kwansa H, Crafa D, Kibler KK, Koehler RC. Transfusion of hemoglobin-based oxygen carriers in the carboxy state is beneficial during transient focal cerebral ischemia. J Appl Physiol (1985). 2012;113:1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cipolla MJ, Linfante I, Abuchowski A, Jubin R, Chan SL. Pharmacologically increasing collateral perfusion during acute stroke using a carboxyhemoglobin gas transfer agent (Sanguinate™) in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2018;38:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christoforidis GA, Saadat N, Liu M, Jeong YI, Roth S, Niekrasz M, Carroll T. Effect of early Sanguinate (PEGylated carboxyhemoglobin bovine) infusion on cerebral blood flow to the ischemic core in experimental middle cerebral artery occlusion. J Neurointerv Surg. 2021:neurintsurg-2021–018239. [DOI] [PubMed] [Google Scholar]
- 59.Kuebler WM, Kisch-Wedel H, Kemming GI, Meisner F, Bruhn S, Koehler C, Flondor M, Messmer K, Zwissler B. Inhaled nitric oxide induces cerebrovascular effects in anesthetized pigs. Neurosci Lett. 2003;348:85–88. [DOI] [PubMed] [Google Scholar]
- 60.Terpolilli NA, Kim SW, Thal SC, Kataoka H, Zeisig V, Nitzsche B, Klaesner B, Zhu C, Schwarzmaier S, Meissner L, Mamrak U, Engel DC, Drzezga A, Patel RP, Blomgren K, Barthel H, Boltze J, Kuebler WM, Plesnila N. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ Res. 2012;110:727–738. [DOI] [PubMed] [Google Scholar]
- 61.Zhou J, Wu PF, Wang F, Chen JG. Targeting gaseous molecules to protect against cerebral ischaemic injury: mechanisms and prospects. Clin Exp Pharmacol Physiol. 2012;39:566–76. [DOI] [PubMed] [Google Scholar]
- 62.Biose IJ, Dewar D, Macrae IM, McCabe C. Impact of stroke co-morbidities on cortical collateral flow following ischaemic stroke. J Cereb Blood Flow Metab. 2020;40:978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seylaz J, Hara H, Pinard E, Mraovitch S, MacKenzie ET, Edvinsson L. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab. 1988;8:875–878. [DOI] [PubMed] [Google Scholar]
- 64.Goadsby PJ. Sphenopalatine ganglion stimulation increases regional cerebral blood flow independent of glucose utilization in the cat. Brain Res. 1990;506:145–8. [DOI] [PubMed] [Google Scholar]
- 65.Levi H, Schoknecht K, Prager O, Chassidim Y, Weissberg I, Serlin Y, Friedman A. Stimulation of the sphenopalatine ganglion induces reperfusion and blood-brain barrier protection in the photothrombotic stroke model. PLoS One. 2012;7:e39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bar-Shir A, Shemesh N, Nossin-Manor RYC. Late stimulation of the sphenopalatine-ganglion in ischemic rats: improvement in N-acetylaspartate levels and diff usion weighted imaging characteristics as seen by MR. J Magn Reson Imaging. 2010; 6: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 67.Henninger N, Fisher M. Stimulating circle of Willis nerve fibers preserves the diff usion-perfusion mismatch in experimental stroke. Stroke. 2007; 38: 2779–2786. [DOI] [PubMed] [Google Scholar]
- 68.Bang OY, Saver JL, Kim SJ, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42:2235–2239. [DOI] [PubMed] [Google Scholar]
- 69.Shuaib A, Hussain M. The past and future of neuroprotection in cerebral ischemic stroke. Eur Neurol. 2008;59:4–14. [DOI] [PubMed] [Google Scholar]
- 70.Siesjo BK. Mechanisms of ischemic brain damage. Crit Care Med. 1988;16:954–963. [DOI] [PubMed] [Google Scholar]
- 71.Astrup J, Siesjo B, Symon L. Thresholds in cerebral ischemia: the ischemic penumbra. Stroke. 1981;12:723–725. [DOI] [PubMed] [Google Scholar]
- 72.Borsody MK, Sacristan E. Facial nerve stimulation as a future treatment for ischemic stroke. Brain Circ. 2016;2:164–177. Erratum in: Brain Circ. 2017;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bahr-Hosseini M, Saver JL. Mechanisms of action of acute and subacute sphenopalatine ganglion stimulation for ischemic stroke. Int J Stroke. 2020;15:839–848. [DOI] [PubMed] [Google Scholar]
- 74.Bornstein NM, Saver JL, Diener HC, Gorelick PB, Shuaib A, Solberg Y, Devlin T, Leung T, Molina CA; ImpACT-24A Investigators. Sphenopalatine Ganglion Stimulation to Augment Cerebral Blood Flow: A Randomized, Sham-Controlled Trial. Stroke. 2019;50:2108–2117. [DOI] [PubMed] [Google Scholar]
- 75.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–48. [DOI] [PubMed] [Google Scholar]
- 76.Zhao W, Li S, Ren C, Meng R, Jin K, Ji X. Remote ischemic conditioning for stroke: clinical data, challenges, and future directions. Ann Clin Transl Neurol. 2018. 15;6:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma J, Ma Y, Dong B, Bandet MV, Shuaib A, Winship IR. Prevention of the collapse of pial collaterals by remote ischemic perconditioning during acute ischemic stroke. J Cereb Blood Flow Metab. 2017;37:3001–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma J, Ma Y, Shuaib A, Winship IR. Improved collateral flow and reduced damage after remote ischemic perconditioning during distal middle cerebral artery occlusion in aged rats. Sci Rep. 2020;10:12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hess DC, Blauenfeldt RA, Andersen G, Hougaard KD, Hoda MN, Ding Y, Ji X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol. 2015;11:698–710. [DOI] [PubMed] [Google Scholar]
- 80.Hess DC, Hoda MN, Khan MB. Humoral Mediators of Remote Ischemic Conditioning: Important Role of eNOS/NO/Nitrite. Acta Neurochir Suppl. 2016;121:45–8. [DOI] [PubMed] [Google Scholar]
- 81.Hoda MN, Siddiqui S, Herberg S, Periyasamy-Thandavan S, Bhatia K, Hafez SS, Johnson MH, Hill WD, Ergul A, Fagan SC, Hess DC. Remote ischemic perconditioning is effective alone and in combination with intravenous tissue-type plasminogen activator in murine model of embolic stroke. Stroke. 2012;43:2794–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoda MN, Bhatia K, Hafez SS, Johnson MH, Siddiqui S, Ergul A, Zaidi SK, Fagan SC, Hess DC. Remote ischemic perconditioning is effective after embolic stroke in ovariectomized female mice. Transl Stroke Res. 2014;5:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abbasi-Habashi S, Jickling GC, Winship IR. Immune Modulation as a Key Mechanism for the Protective Effects of Remote Ischemic Conditioning After Stroke. Front Neurol. 2021;12:746486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalakech H, Hibert P, Prunier-Mirebeau D, Tamareille S, Letournel F, Macchi L, et al. RISK and SAFE signaling pathway involvement in apolipoprotein a-i-induced cardioprotection. PLoS ONE. 2014;9:e107950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lecour S Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40. [DOI] [PubMed] [Google Scholar]
- 86.Tamareille S, Mateus V, Ghaboura N, Jeanneteau J, Croué A, Henrion D, et al. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol. 2011;106:1329–1339. [DOI] [PubMed] [Google Scholar]
- 87.Davidson SM, Selvaraj P, He D, Boi-Doku C, Yellon RL, Vicencio JM, Yellon DM. Remote ischaemic preconditioning involves signalling through the SDF-1α/CXCR4 signalling axis. Basic Res Cardiol. 2013;108:377. [DOI] [PubMed] [Google Scholar]
- 88.England TJ, Hedstrom A, O’Sullivan S, et al. Recast (remote ischemic conditioning after stroke trial): a pilot randomized placebo controlled phase II trial in acute ischemic stroke. Stroke. 2017;48:1412–1415. [DOI] [PubMed] [Google Scholar]
- 89.England TJ, Hedstrom A, O’Sullivan SE, Woodhouse L, Jackson B, Sprigg N, Bath PM. Remote Ischemic Conditioning After Stroke Trial 2: A Phase IIb Randomized Controlled Trial in Hyperacute Stroke. J Am Heart Assoc. 2019;8:e013572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao W, Che R, Li S, et al. Remote ischemic conditioning for acute stroke patients treated with thrombectomy. Ann Clin Transl Neurol 2018;5:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Towner RA, Gulej R, Zalles M, Saunders D, Smith N, Lerner M, Morton KA, Richardson A. Rapamycin restores brain vasculature, metabolism, and blood-brain barrier in an inflammaging model. Geroscience. 2021;43:563–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih YY, Muir E, Solano Fonseca R, Strong R, Richardson AG, Lechleiter JD, Fox PT, Galvan V. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33:1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beard DJ, Li Z, Schneider AM, Couch Y, Cipolla MJ, Buchan AM. Rapamycin induces an eNOS (endothelial nitric oxide synthase) dependent increase in brain collateral perfusion in Wistar and Spontaneously Hypertensive Rats. Stroke. 2020;51:2834–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Lin X, Mu Z, Shen F, Zhang L, Xie Q, Tang Y, Wang Y, Zhang Z, Yang GY. Rapamycin Increases Collateral Circulation in Rodent Brain after Focal Ischemia as detected by Multiple Modality Dynamic Imaging. Theranostics. 2019;9:4923–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamamoto K, Takeshita K, Saito H. Plasminogen activator inhibitor-1 in aging. Sem Thromb Hemostas. 2014;40:652–659. [DOI] [PubMed] [Google Scholar]
- 96.Vaughan DE, Rai R, Khan SS, Eren M, Ghosh AK. Plasminogen activator inhibitor-1 is a marker and a mediator of senescence. Arterioscl Thromb Vasc Biol. 2017;37:1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nagai N, Suzuki Y, Van Hoef B, Lijnen HR, Collen D. Effects of plasminogen activator inhibitor-1 on ischemic brain injury in permanent and thrombotic middle cerebral artery occlusion models in mice. J Thromb Haemost. 2005;3:1379–1384. [DOI] [PubMed] [Google Scholar]
- 98.Denorme F, Wyseure T, Peeters M, Vandeputte N, Gils A, Deckmyn H, et al. Inhibition of thrombin-activatable fibrinolysis inhibitor and plasminogen activator inhibitor-1 reduces ischemic brain damage in mice. Stroke. 2016;47:2419–2422. [DOI] [PubMed] [Google Scholar]
- 99.Chan SL, Bishop N, Li Z, Cipolla MJ. Inhibition of PAI (Plasminogen Activator Inhibitor)-1 Improves Brain Collateral Perfusion and Injury After Acute Ischemic Stroke in Aged Hypertensive Rats. Stroke. 2018;49:1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Drummond JC, Oh YS, Cole DJ, Shapiro HM. Phenylephrine-induced hypertension reduces ischemia following middle cerebral artery occlusion in rats. Stroke. 1989;20:1538–1544. [DOI] [PubMed] [Google Scholar]
- 101.Smrcka M, Ogilvy CS, Crow RJ, Maynard KI, Kawamata T, Ames A III. Induced hypertension improves regional blood flow and protects against infarction during focal ischemia: time course of changes in blood flow measured by laser Doppler imaging. Neurosurgery. 1998;42:617–624. [DOI] [PubMed] [Google Scholar]
- 102.Chileuitt L, Leber K, McCalden T, Weinstein PR. Induced hypertension during ischemia reduces infarct area after temporary middle cerebral artery occlusion in rats. Surg Neurol. 1996;46:229–234. [DOI] [PubMed] [Google Scholar]
- 103.Shin HK, Nishimura M, Jones PB, Ay H, Boas DA, Moskowitz MA, Ayata C. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke. 2008;39:1548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cole DJ, Drummond JC, Osborne TN, Matsumura J. Hypertension and hemodilution during cerebral ischemia reduce brain injury and edema. Am J Physiol. 1990;259:H211–217. [DOI] [PubMed] [Google Scholar]
- 105.Cole DJ, Drummond JC, Shapiro HM, Hertzog RE, Brauer FS. The effect of hypervolemic hemodilution with and without hypertension on cerebral blood flow following middle cerebral artery occlusion in rats anesthetized with isoflurane. Anesthesiology. 1989;71:580–585. [DOI] [PubMed] [Google Scholar]
- 106.Bang OY, Chung JW, Kim SK, Kim SJ, Lee MJ, Hwang J, Seo WK, Ha YS, Sung SM, Kim EG, Sohn SI, Han MK. Therapeutic-induced hypertension in patients with noncardioembolic acute stroke. Neurology. 2019;93:e1955–e1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bailey ZS, Cardiff K, Yang X, Gilsdorf J, Shear D, Rasmussen TE, Leung LY. The Effects of Balloon Occlusion of the Aorta on Cerebral Blood Flow, Intracranial Pressure, and Brain Tissue Oxygen Tension in a Rodent Model of Penetrating Ballistic-Like Brain Injury. Front Neurol. 2019;10:1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Long B, Hafen L, Koyfman A, Gottlieb M. Resuscitative Endovascular Balloon Occlusion of the Aorta: A Review for Emergency Clinicians. J Emerg Med. 2019;56:687–697. [DOI] [PubMed] [Google Scholar]
- 109.Liebeskind DS. Aortic occlusion for cerebral ischemia: from theory to practice. Curr Cardiol Rep. 2008;10:31–36. [DOI] [PubMed] [Google Scholar]
- 110.Noor R, Wang CX, Todd K, et al. Partial intra-aortic occlusion improves perfusion deficits and infarct size following focal cerebral ischemia. J Neuroimaging. 2010;20:272–276. [DOI] [PubMed] [Google Scholar]
- 111.Hammer MD, Schwamm L, Starkman S, Schellinger PD, Jovin T, Nogueira R, Burgin WS, Sen S, Diener HC, Watson T, Michel P, Shuaib A, Dillon W, Liebeskind DS. Safety and feasibility of NeuroFlo use in eight- to 24-hour ischemic stroke patients. Int J Stroke. 2012;7:655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Emery DJ, Schellinger PD, Selchen D, et al. Safety and feasibility of collateral blood flow augmentation after intravenous thrombolysis. Stroke 2011; 42: 1135–37. [DOI] [PubMed] [Google Scholar]
- 113.Shuaib A, Schwab S, Rutledge JN, Starkman S, Liebeskind DS, Bernardini GL, Boulos A, Abou-Chebl A, Huang DY, Vanhooren G, Cruz-Flores S, Klucznik RP, Saver JL, 2013. Importance of proper patient selection and endpoint selection in evaluation of new therapies in acute stroke: further analysis of the SENTIS trial. J. Neurointerv. Surg. 5 (Suppl. 1), i21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Amaro S, Laredo C, Renú A, Llull L, Rudilosso S, Obach V, Urra X, Planas AM, Chamorro Á; URICO-ICTUS Investigators. Uric acid therapy prevents early ischemic stroke progression: A tertiary analysis of the URICO-ICTUS Trial (Efficacy Study of Combined Treatment With Uric Acid and r-tPA in Acute Ischemic Stroke). Stroke. 2016;47:2874–2876. [DOI] [PubMed] [Google Scholar]


