Abstract
Background
Dermatosis is a general term for diseases of the skin and skin appendages including scleroderma, psoriasis, bullous disease, atopic dermatitis, basal cell carcinoma, squamous cell carcinoma, and melanoma. These diseases affect millions of individuals globally and are a serious public health concern. However, the pathogenesis of skin diseases is not fully understood, and treatments are not optimal. Yes‐associated protein (YAP) is a transcriptional coactivator that plays a role in the regulation of gene transcription and signal transduction.
Aims
To study the role of Yes‐associated protein in skin diseases.
Materials and Methods
The present review summarizes recent advances in our understanding of the role of YAP in skin diseases, current treatments that target YAP, and potential avenues for novel therapies.
Results
Abnormal YAP expression has been implicated in occurrence and development of dermatosis. YAP regulates the cell homeostasis, proliferation, differentiation, apoptosis, angiopoiesis, and epithelial‐to‐mesenchymal transition, among other processes. As well as, it serves as a potential target in many biological processes for treating dermatosis.
Conclusions
The effects of YAP on the skin are complex and require multidimensional investigational approaches. YAP functions as an oncoprotein that can promote the occurrence and development of cancer, but there is currently limited information on the therapeutic potential of YAP inhibition for cancer treatment. Additional studies are also needed to clarify the role of YAP in the development and maturation of dermal fibroblasts; skin barrier function, homeostasis, aging, and melanin production; and dermatosis.
Keywords: dermatosis, hippo signaling pathway, signal transduction, skin physiology, Yes‐associated protein
Abbreviations
- BCC
basal cell carcinoma
- cSCC
cutaneous squamous cell carcinoma
- ECM
extracellular matrix
- EMT
epithelial‐to‐mesenchymal transition
- JEB
junctional epidermolysis bullosa
- ROS
reactive oxygen species
- SSc
systemic sclerosis
- VP
verteporfin
1. INTRODUCTION
The skin is the largest organ and first line of defense of the human body. Its functions include acting as a barrier, providing immune protection, maintaining electrolyte balance, and melanin metabolism, among others. However, the skin is also the most susceptible organ to external stimuli, which can lead to the development of skin diseases such as scleroderma, psoriasis, basal cell carcinoma (BCC), melanoma, cutaneous squamous cell carcinoma (cSCC), and bullous skin disease. With the aging of the global population, changes in living conditions, environmental pollution, and other factors in the last few decades, the incidence of skin diseases has increased significantly; dermatosis—a general term for diseases of the skin and skin appendages—constitutes a considerable threat to public health. 1
Yes‐associated protein (YAP) is a mammalian transcriptional accessory protein first identified in Drosophila with a relative molecular mass of ∼65 kDa. The human YAP gene is located on chromosome 11q22. 2 YAP is the main effector of the Hippo signaling pathway but has functions that are independent of Hippo. In human and mouse embryo, YAP is mainly localized in the nucleus and cytoplasm of the basal layer of the epidermis. YAP expression in the nucleus declines postnatally, but it continues to be expressed in epidermal stem cells and in the nucleus of keratinocytes. 3 , 4 , 5 YAP functions as a transcriptional coactivator that binds to transcription factors to induce the transcription of target genes. The intracellular localization of YAP determines its transcriptional activity. In the nucleus, YAP contributes to intracellular signal transduction in collaboration with connexins to regulate proliferation, cell growth, and epithelial‐to‐mesenchymal transition (EMT), whereas phosphorylated YAP binds to 14‐3‐3 protein and accumulates in the cytoplasm, and does not participate in transcriptional coactivation. 6
2. OVERVIEW OF YAP FUNCTION
YAP regulates cell proliferation, apoptosis, and differentiation via multiple pathways and plays an important regulatory role in the development and maintenance of skin tissue structure. YAP overexpression in mice resulted in thickening of the acanthocyte and granulosa cell layers and hyperkeratosis of the interfollicular epidermis, thereby increasing epidermal thickness. 5 Meanwhile, YAP knockout mice showed thinner and more fragile skin, with loss of epidermal tissue in distal limbs, thinning of the epidermis and stratum corneum, disordered epidermal structure, basal lamina dysplasia, and decreased proliferation of epidermal stem cells. 7 In HaCaT human keratinocytes, the downregulation of YAP was associated with decreased cell proliferation and cell cycle arrest in G0/G1 phase, 8 whereas YAP activation enhanced keratinocyte proliferation via activation of WNT16/β‐catenin signaling or increased expression of plasminogen activator urokinase (Plau) and transforming growth factor beta (TGF‐β) type III receptor. 9 , 10 , 11 However, simultaneous overexpression of YAP resulted in downregulation of 14‐3‐3 and upregulation of DNp63a, which restored immortality in human keratinocytes. 12 Integrin/Src and epidermal growth factor receptor (EGFR)/phosphatidylinositol 3‐kinase (PI3K) signaling induced YAP nuclear localization and promoted basal cell proliferation. 5 YAP was shown to interact with proteins such as baculoviral inhibitor of apoptosis (IAP) repeat‐containing 5 (BIRC5), deoxyribo nucleic acid (DNA) damage‐inducible transcript 4 (DDIT4), tumor necrosis factor‐related apoptosis‐inducing ligand, nucleosome remodeling and deacetylase (NuRD), B cell lymphoma 2 (Bcl‐2), p53, caspase‐3, cyclooxygenase‐2 (COX2), and p21 to inhibit the apoptosis of fibroblasts. However, nuclear YAP can also combine with p73 to promote the transcription of tumor protein 53 (TP53)‐regulated apoptosis inducing protein 1 (p53AIP1), Bcl2‐associated X protein (Bax), death receptor 5 (DR5), promyelocytic leukemia, p21, and P53 upregulated modulator of apoptosis (PUMA), leading to fibroblast apoptosis. 13 , 14 This apparent paradox of YAP function can be explained by the binding of YAP to different transcription factors, although the precise mechanisms of transcriptional activation or inhibition are unclear.
YAP is known to play a role in intracellular mechanotransduction. Excessive division of epidermal cells leads to cell crowding and uneven stress distribution; high cell density or low mechanical tension affects actin contraction through the E‐cadherin/β‐catenin complex and acts on α‐catenin, vinculin, Merlin, and LIM (Lin‐11, Isl1, MEC‐3) domain‐containing 1 (LIMD1) to inhibit YAP activity, which in turn suppresses cell proliferation. 15 However, YAP knockout in mice did not cause pathologic changes, suggesting that the role of YAP in the regulation of epidermal regeneration is nonessential 16 (Figure 1). YAP expression is regulated by the polarity of epidermal cells, which in turn impacts skin cell differentiation. When basal cells lose contact with and delaminate from the basement membrane, they downregulate integrin/Src and EGFR/PI3K signaling, thereby inhibiting nuclear translocation of YAP and promoting cell differentiation. 5 YAP induces the proliferation of basal epidermal progenitor cells and inhibits their terminal differentiation along with the differentiation of mouse embryonic stem cells and premature differentiation of basal epidermal keratinocytes; it also regulates T cell differentiation. 17 However, the regulatory effect of YAP on the production of skin extracellular matrix (ECM) and dermis remains to be elucidated.
FIGURE 1.
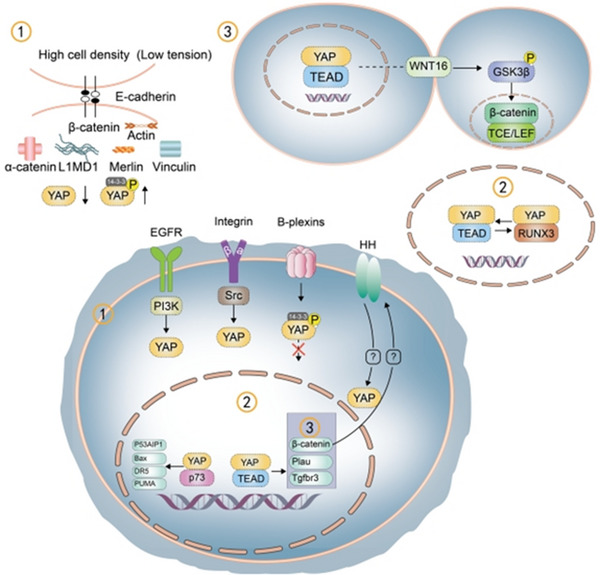
Regulatory pathways in cell proliferation and differentiation involving Yes‐associated protein (YAP). (1) YAP is phosphorylated at high cell density and low mechanical tension. (2) The switch between the YAP/TEAD (transcriptional enhanced associate domain) and YAP/RUNX3 complexes—which promotes and inhibits cell proliferation, respectively—regulates the cell cycle. After entering the nucleus, YAP competes with VGLL4 for binding to the transcription factor TEAD, thereby maintaining cell proliferation. 18 (3) The YAP/TEAD complex activates WNT16/GSK3β/β‐catenin to bind to TCF/LEF (transcription factor/ lymphoid enhancer binding factor), promoting cell proliferation. The integrin/Src and EGFR/PI3K pathways promote YAP nuclear localization; B‐plexin phosphorylates YAP; Hedgehog (HH) signaling activates YAP; and nuclear YAP binds to β‐catenin to activate HH signaling. YAP also binds to p73, increasing p53AIP1 and Bax expression, while the upregulation of DR5 and PUMA promotes cell apoptosis. YAP increases the expression of Plau and Tgfbr3 to enhance keratinocyte proliferation.
In light of the increasing evidence regarding the role of YAP in skin homeostasis, in this review, we summarize the relationship between YAP dysregulation and skin diseases and discuss the development of pharmacologic agents targeting YAP and their therapeutic potential for the treatment of dermatosis.
3. RELATIONSHIP BETWEEN YAP AND SKIN DISEASES
Dysregulation of YAP expression is a feature of multiple skin diseases. YAP overexpression results in the overproliferation of cells, which is associated with malignancies. 3 YAP induces EMT via the PI3K/protein kinase B (Akt), angiomotin (AMOT), TGF‐β, and WNT/β‐catenin pathways, accelerating tumor invasion and metastasis and increasing mortality in patients with malignant tumors. 19 , 20 , 21 However, it was also shown that YAP overexpression alone is not sufficient to promote complete EMT. 12 Excessive activation of YAP inhibits epidermal differentiation while promoting cell and vascular proliferation, expression of inflammatory factors, and T cell differentiation, which are pathophysiologic processes that are closely related to the occurrence and development of skin diseases.
3.1. Relationship between YAP and wound healing
YAP plays an important role in skin wound repair. YAP was shown to be upregulated in the basal cell layer of injured skin 5 and localized in the nucleus of the dermis. 22 Nuclear YAP regulates cell proliferation, fibroblast activation, vascular proliferation, 23 and ECM remodeling to promote wound healing. It has been demonstrated that YAP enhanced the activation of fibroblasts and angiogenesis by increasing the expression of the target gene connective tissue growth factor (CTGF/CCN [cellular communication network]2), suppressing that of Mothers against decapentaplegic 7 (Smad‐7), and enhancing TGF‐β/Smad2/3 signaling. 22 YAP interacts with Smad2/3 through TGF‐β, leading to the accumulation of phosphorylated Smad2/3 and promoting tissue repair 24 (Figure 2). In a cellular model of wound healing, HaCaT cell migration was decreased by YAP inhibition 17 ; and in mouse models of type I and II diabetes, overexpression of growth differentiation factor 11 (GDF11) stimulated dermal fibrosis and accelerated skin wound closure via YAP/Smad2/3/CTGF signaling. 25 Nuclear localization of YAP was shown to be increased by treatment with calcipotriol, which induced EMT through the YAP/TGF‐β/Smad pathway to accelerate tissue repair. 26 YAP/interleukin 33 (IL‐33)‐mediated autophagy was identified as a potential pharmacologic target for accelerating wound healing. 27 The mechanism of YAP regulation observed in spiny mice (Acomys cahirinus) applied to human fibroblasts cultured in vitro was shown to prevent and rescue TGF‐β1–mediated myofibroblast differentiation, providing a basis for preventing scar formation during wound healing. 28 However, treatment with the YAP inhibitor verteporfin (VP) promoted wound repair without scarring. Most of the research to date has focused on the downstream effects of YAP in wound healing, and it is not known how skin damage induces the activation of YAP. Clarifying this mechanism can reveal potential strategies for stimulating tissue repair following skin injury.
FIGURE 2.
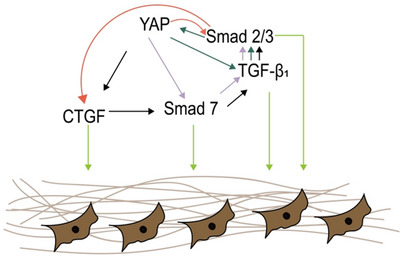
Mechanism of Yes‐associated protein (YAP)/transforming growth factor beta 1 (TGF‐β1)/Smad regulation in wound healing. YAP induces connective tissue growth factor (CTGF) and inhibits Smad7, thereby increasing the expression of TGF‐β1 and Smad 2/3; acts directly on Smad7; and modulates TGF‐β1 and Smad 2/3. YAP and TGFβ1/Smad2/3 engage in a positive feedback loop. These signaling cascades act on fibroblasts to stimulate wound healing.
3.2. Role of YAP in scleroderma
High YAP expression promotes skin thickening and fibrosis in systemic sclerosis (SSc) as a result of EMT of skin epithelial cells to fibroblasts (mesenchymal‐like cells). Single‐cell sequencing identified YAP as a potential therapeutic target for SSc. 29 Transcriptome analysis in a scleroderma model showed that YAP knockdown resulted in the downregulation of the profibrotic genes TGF‐β1, endothelin 1 (ET‐1), IL‐6, TEAD1, plasminogen activator inhibitor 1 (PAI‐1), cysteine‐rich 61 (Cyr61/CCN1), and CCN2. 30 As a critical effector of mechanotransduction signaling, YAP nuclear accumulation and activity increased with matrix stiffness and in fibroblasts; a pathologic increase in ECM stiffness activated YAP, inducing the expression of profibrotic mediators such as PAI‐1 and ECM proteins, leading to fibroblast activation and tissue fibrosis. 31 VP reduced TGF‐β1–induced expression of alpha‐smooth muscle actin, CCN2, and human collagen I (COLI), thereby preventing the occurrence of scleroderma fibrosis. 32 Dimethyl fumarate (DMF) exerts an antifibrotic effect by targeting YAP via the PI3K/Akt1 and TGF‐β1/Akt1/glycogen synthase kinase 3‐beta (GSK3β) pathways. 30 Pharmacotherapies for scleroderma mainly target inflammation, autoimmunity, vascular disease, and fibrosis. However, the precise role of YAP in immune dysregulation and vascular lesions in scleroderma is poorly understood, and additional studies are needed to determine whether pharmacologic targeting of YAP is a potential treatment strategy for SSc.
3.3. Role of YAP in psoriasis
YAP is highly expressed in the skin of psoriasis patients, mainly in the basal and lower spinous layers. 8 The pathogenesis of psoriasis is related to keratinocyte activation by YAP, abnormal proliferation of blood vessels, and inflammatory cell infiltration. YAP promotes keratinocyte proliferation through positive regulation of amphiregulin (AREG) 8 ; and YAP is closely related to angiogenesis, and the increase of nuclear YAP can affect the expression of other angiogenesis factors, which can promote endothelial cell proliferation, angiogenesis. 33 IL‐17α was shown to promote the development of psoriasis via activation of the YAP/AREG axis. 34 Meanwhile, IL‐38 inhibited the transcriptional activity of YAP to suppress keratinocyte proliferation. 35 Inhibiting YAP expression improved skin lesions by inducing cell cycle arrest in G0/G1 phase and reducing the levels of inflammatory factors as well as activation of extracellular signal‐regulated kinase (ERK), signal transducer and activator of transcription 3 (STAT3), and nuclear factor kappa B signaling. 36 The mechanism by which YAP inhibits the inflammatory response is not well understood.
Mechanical stress contributes to the pathogenesis of psoriasis; continuously increasing mechanical stimulation resulted in YAP‐mediated inhibition of Notch signaling and stimulated proliferation while inhibiting the differentiation of epidermal keratinocytes, leading to the formation of psoriatic skin. 37 Danshensu, a naturally derived polyphenol compound that is used in traditional Chinese medicine for its antitumor and antiangiogenic properties, was shown to prevent psoriasis by downregulating YAP expression, 38 highlighting its therapeutic potential in the treatment of psoriasis.
3.4. Role of YAP in bullous skin disease
YAP is overexpressed around pemphigus lesions. 39 Desmoglein 3 (Dsg3) and immunoglobulin G (IgG) are essential for the induction and progression of bullous skin disease; Dsg3 is destroyed by IgG to generate reactive oxygen species (ROS), which acts directly on various signaling pathways including α‐catenin, Dsg3, p38 mitogen‐activated protein kinase (MAPK), c‐Jun N‐terminal kinase, and protein kinase C to upregulate YAP. Increased YAP expression contributes to intraepidermal blister formation by affecting calcium channels, Dsg3, and adherens junctions. 39 , 40 Dsg3 was shown to form a complex with phosphorylated YAP under high mechanical tension, contradicting a previous report that phosphorylated YAP is inactive. 41 However, the function of phosphorylated YAP in keratinocytes remains to be determined. Autoantibodies against epidermal cadherin that are known to be pathogenic include anti‐Dsg1 and ‐Dsg3 antibodies, but there have been no studies examining the relationship between Dsg1 and YAP. Unlike in pemphigus lesions, YAP expression is significantly reduced in keratinocytes of the junctional epidermolysis bullosa (JEB). 42 Laminin 332 and α6β4 integrin‐regulated YAP and the downstream target gene Forkhead box M1 (FOXM1) are involved in the repair of human epidermal stem cells, which has important implications for the treatment of JEB. 42 , 43 Most studies to date have focused on the effect of oxidative stress on YAP and intercellular linkages; future research directions include the impact of YAP on the apoptosis, proliferation, and T cell regulation of keratinocytes in bullous skin disease.
3.5. Role of YAP in rosacea and atopic dermatitis
Decreased YAP expression has been reported in skin lesions of patients with atopic dermatitis. YAP promotes the differentiation of progenitor CD4+ T cells into T helper 17 cells or regulatory T cells, and its downregulation alters the ratio of these two cell types, leading to the transition of atopic dermatitis from the acute to the chronic phase. 17 At the same time, YAP expression in the epidermis is decreased, and the rate of keratinocyte proliferation is reduced, while apoptosis is accelerated, resulting in skin erosion and the progression of atopic dermatitis. 17 The microRNA miR‐375‐3p was shown to attenuate inflammation by targeting the YAP/lympho‐epithelial Kazal‐type‐related inhibitor pathway in HaCaT cells, 44 although the detailed mechanism requires clarification. The upregulation of YAP is closely related to angiogenesis, cell proliferation and maturation, and tissue remodeling, 23 , 45 , 46 which are dysregulated in rosacea. Telangiectasia and erythema in rosacea were shown to be improved upon VP‐induced inhibition of vascular endothelial growth factor expression. 47 As mentioned above, angiogenesis is closely related to chronic inflammatory skin diseases. Elucidating the site of action of YAP in blood vessels in diseases such as urticaria, hidradenitis suppurativa, angioedema, and atopic dermatitis can provide a basis for developing novel treatment strategies.
3.6. Role of YAP in epidermal BCC
Genome‐wide analysis of BCC samples has revealed an upregulation of YAP target genes. 48 In YAP‐overexpressing mouse BCC cell lines, the rate of proliferation rate was found to be increased 2.1 fold relative to control cells. 49 Aberrant WNT and Hedgehog signaling is thought to underlie BCC. 50 The interaction between Hedgehog and YAP also controls epidermis development and homeostasis, 51 and a positive feedback interaction between these factors can promote the development of BCC. 52 Disruption of the Gαs/PKA interaction induced cell‐autonomous Hedgehog signaling and activation of YAP, leading to the development of mouse and human BCC. 53 Hyperactivation of Hippo/YAP and WNT pathways was observed in intrinsically resistant BCC. 49 At the same time, YAP promotes the development of BCC through the c‐JUN/activator protein 1 (AP1) axis independent of the WNT and Hedgehog pathways. 16 YAP and its downstream effectors CCN1 and CCN2 were found to be upregulated in the skin of patients with BCC, and elevated levels of CCN family proteins were significantly correlated with tumor malignancy. CCN1 regulates the proliferation of abnormal keratinocytes whereas CCN2 regulates tumor stromal cell activation and remodeling. 54 Epidermal stem cells lacking B‐plexins are unable to sense mechanical compression, leading to disinhibition of YAP, cell hyperproliferation, and tissue overgrowth. 37 , 55 B‐plexins and protein tyrosine phosphatase nonreceptor type 14 (PTPN14) inhibit YAP to inhibit the development of BCC. 37 , 48 An analysis of skin samples of patients with BCC found no difference in YAP expression levels between recurrent and nonrecurrent disease, 56 and activating mutations of YAP did not result in the development of BCC‐like lesions. Although the precise role of YAP in BCC requires clarification, targeting YAP or its up‐ or downstream factors may be a promising treatment approach.
3.7. Role of YAP in cSCC
YAP is highly expressed in poorly differentiated (precancerous) cSCC, 57 , 58 , 59 and the expression level is positively correlated with the progression of cSCC and actinic keratosis as well as Bowen disease, a precancerous lesion of squamous cell carcinoma. 60 Another study found that at the site of epithelial scratches, YAP induced the transformation of cSCC to spindle cell carcinoma through zinc finger E‐box binding homeobox 1 (ZEB1)‐mediated EMT. 61 Additionally, YAP activation was observed in most cSCC patients who experienced treatment failure and relapse. 62 Following α‐catenin/YAP overexpression in the dermis, tumors showed signs of EMT, leading to the development of tumors that were morphologically similar to human cSCC. 7 Loss of YAP resulted in the arrest of cSCC cells in G1/S phase and the cell cycle regulators cyclin A, B1, D1, and E and cyclin‐dependent kinase 2 (CDK2) and CD25A were all downregulated, resulting in decreased cell proliferation. 57 YAP was also found to promote EGFR/RAS signaling by regulating TP53, NOTCH1, NOTCH2, histone‐lysine N‐methyltransferase 2D (KMT2D), fat atypical cadherin 1−4 (FAT1−4), and other genes 63 or AREG transcription to enhance EGFR/RAS signaling; RAS further activated the RAF/MEK (MAP/ERK kinase)/ERK and PI3K/Akt pathways, 57 thereby enhancing the proliferation and invasion of cSCC. Oncogenic activation of YAP expression by integrin β4‐src signaling was shown to be inhibited by ε‐catenin. 64 YAP/NUAK2 is a potential target in the treatment of cSCC. 58 Knockdown of the long noncoding RNA p38‐inhibited cutaneous squamous cell carcinoma‐associated lincRNA (PICSAR) suppressed the proliferation and invasion of cSCC cells through regulation of the miR‐125b/YAP1 axis and promoted the apoptosis of cSCC cells, 65 suggesting another avenue for cSCC treatment.
3.8. Role of YAP in melanoma
YAP regulates the proliferation, apoptosis, and invasion of melanocytes through various pathways. The main driving force of melanoma development is the activation of BRAF and MAPK signaling. BRAF mutations are detected in about half of melanomas, and YAP causes T cell exhaustion by inducing the upregulation of programmed death ligand 1, resulting in immune evasion and BRAF inhibitor resistance. 66 Silencing ubiquitin‐specific peptidase 22 expression resulted in the upregulation of YAP, leading to BRAF inhibitor resistance. 67 As YAP activation has been linked to increased resistance to BRAF and/or MEK inhibitors, 68 , 69 simultaneous BRAF/MEK inhibition is a potential treatment for BRAF‐mutant melanoma.
The TGFβ/Smad and YAP signaling pathways interact to induce melanoma cell proliferation and their transformation to an aggressive phenotype; thus, these pathways are suitable therapeutic targets for blocking melanoma progression, metastasis, and drug resistance. 70 YAP activation may promote the proliferation and migration of melanoma cells through MAPK, Akt, and other signaling pathways. 71 As a classic mechanical sensor, YAP is modulated by static mechanical stimulation of the actin cytoskeleton. In melanoma models, semaphorin 6A (SEMA6A) was shown to activate the RhoA/YAP axis, which remolded the actin cytoskeleton and predicted shorter recurrence‐free interval in patients. 72 Upstream of RhoA/YAP, PPARG coactivator 1 alpha inhibition increased the expression of WNT5A and activated RhoA to increase the level of YAP, thereby increasing melanoma invasion 73 through the regulation of actin‐related protein 2/3 complex subunit 5, which is associated with melanocyte adhesion. 74
YAP is also affected by dynamic mechanical stress, which plays a role in the pathology of melanoma. In plantar melanoma, continuous mechanical stress caused by weight‐bearing activities activated the expression of YAP, stimulating the proliferation of melanoma cells. 75 YAP activity is also positively regulated by the ECM 76 ; ECM‐mediated signaling induced by changes in cytoskeletal structure promoted tumor development through increased collagen production and remodeling, while the HU177 epitope was shown to promote melanoma cell metastasis through CDK5/YAP signaling. 77 High fibromodulin expression in the tumor microenvironment stimualted ECM remodeling, activated the integrin/focal adhesion kinase pathway, and induced YAP nuclear translocation and metastatic growth and vasculogenic mimicry. 78 Fibroblast reticulocyte relaxation depends on constitutive inhibition of Janus kinase 1 (JAK1)/STAT3 and YAP signaling, which resulted in lymph node expansion and increased melanoma invasiveness. 79 DMF combined with vemurafenib improved therapeutic response in melanoma by inhibiting YAP 80 ; however, VP was found to be ineffective in inhibiting melanoma progression in a mouse model. 81 Additional studies are needed to resolve these contradictory findings.
4. YAP IN CLINICAL DIAGNOSIS AND TREATMENT
Immunohistochemical detection of the YAP1 C terminus has allowed the classification of hole‐like lesions in NUT carcinoma. 82 Elevated YAP expression may be a useful indicator for clinical staging, monitoring postoperative survival, and predicting prognosis of malignant melanoma. 83 YAP is a downstream effector of C‐Jun–mediated apoptosis following cisplatin treatment and may be a key predictor of chemotherapy response and treatment outcome. 84 Aberrant YAP expression is observed in many skin diseases and its clinical potential for disease diagnosis, staging, and prognostic assessment is warranted to guide clinical treatment. The YAP inhibitor VP is approved for the treatment of age‐related macular degeneration 85 , 86 and its antitumor potential has also been reported. YAP is involved in apoptosis signaling downstream of chemotherapeutic agents such as cisplatin, ionizing radiation, and Fas ligand‐targeted therapy. 87 , 88 , 89 In an in vitro study, dabrafafenib alone or in combination with trametinib induced the expression of SEMA6A and activated the RhoA/YAP axis. 68 The combination of Neratinib and pemetrexed increased the phosphorylation of YAP resulting in the loss of YAP transcriptional activity. 90 Topical administration of the YAP inhibitor danshensu had a therapeutic effect in psoriasis model mice 38 ; and topical application of GDF1 increased YAP expression to accelerate skin wound healing in a mouse model of diabetes 91 . DMF injection targeting YAP relieved symptoms of scleroderma. 20 Inhibiting YAP activity in T cells is an important mechanism in T cell therapy for cancer and other diseases 92 ; YAP agonists such as XMU‐MP‐1 93 have not been applied to skin diseases but are worth exploring in the future. The evidence to date indicates that targeting YAP or its up‐ and downstream genes is a promising treatment strategy for skin diseases. However, as circulating levels of YAP are low, treatment response monitoring is currently limited to epidermal sampling. Identifying biomarkers for this purpose is an important future research direction.
5. CONCLUSION AND OUTLOOK
The effects of YAP on the skin are complex and require multidimensional investigational approaches. YAP functions as an oncoprotein that can promote the occurrence and development of cancer (Table 1), but there is currently limited information on the therapeutic potential of YAP inhibition for cancer treatment. Additional studies are also needed to clarify the role of YAP in the development and maturation of dermal fibroblasts; skin barrier function, homeostasis, aging, and melanin production; and dermatosis. This will require systematic investigation of the molecular pathways and mechanisms of YAP in skin development and maintenance, which would provide a basis for the development of pharmacotherapies targeting YAP and related pathways for the prevention and treatment of skin diseases.
TABLE 1.
Summary of YAP pathways and functions.
| YAP pathway or related process | Effect | Reference |
|---|---|---|
| YAP/P73/p53AIP1, BAX, DR5, PUMA | Promote apoptosis | 13 , 14 |
| Integrin/Src/YAP, EGFR/PI3K/YAP | Promote proliferation | 5 |
| YAP/WNT16/β‐catenin | Promote proliferation | 9 |
| YAP/PLAU, YAP/TgFbr3 | Promote proliferation | 11 |
| Conversion between YAP‐TEAD4 and YAP‐RUNX3 | Cell cycle regulation | 18 |
| Hedgehog/YAP | Control tissue regeneration | 51 |
| High cell density or low mechanical tension | Inhibit excess cell proliferation | 15 |
| B‐plexins/YAP | Inhibit excess cell proliferation | 37 |
| YAP/PI3K/Akt, YAP/AMOT, YAP/TGFβ‐Smad, YAP/WNT/β‐catenin | Induce EMT occurrence, accelerate tumorigenesis and metastasis | 19 , 20 , 21 |
| YAP/CTGF/Smad7/TGF‐β/Smad | Promote wound healing | 22 |
| YAP/Smad2/3/CTGF | Promote wound healing | 24 |
| YAP/IL‐33autophagy pathway | Promote wound healing | 27 |
| PI3K/Akt1/YAP, TGF‐β1/Akt1/GSK3β/YAP | Target YAP to inhibit fibrosis | 30 |
| YAP/AREG | Promote keratinocyte proliferation | 8 |
| YAP/ERK, STAT3, NF‐KB | Proinflammatory effect | 36 |
| YAP/Th17, Treg | Associated with atopic dermatitis | 17 |
| YAP/VEGF | Associated with rosacea | 47 |
| YAP‐TEAD/c‐JUN/AP1 | Promote BCC tumorigenesis | 16 |
| YAP/CCN1, CCN2 | Promote BCC tumorigenesis | 54 |
| PTPN14/YAP, B‐plexin/YAP | Inhibition of YAP and BCC generation and development | 23 , 76 |
| YAP/AREG/EGFR/RAS/RAF/MEK/ERK | Promote CSCC occurrence and development | 57 |
| YAP/AREG/EGFR/RAS/PI3K/Akt | Promote CSCC occurrence and development | 57 |
| YAP/TP53, NOTCH1, NOTCH2, KMT2D, FAT1‐4 | Promote CSCC occurrence and development | 63 |
| YAP/NUAK2 | Promote CSCC occurrence and development | 58 |
| USP22/YAP/CCN1, CCN2 | Promote melanoma tumorigenesis | 67 |
| YAP/PD‐L1/T cell | T cell immune escape, BRAF inhibitor resistance | 66 |
| YAP/LRP1/MAPK, Akt | Promote melanoma proliferation and migration | 71 |
| PGC1α/WNT5A/RhoA GTPase/YAP | Inhibit melanoma cell invasion | 73 |
Abbreviations: BCC, basal cell carcinoma; cSCC, cutaneous squamous cell carcinoma; EMT, epithelial‐to‐mesenchymal transition; Treg, regulatory T cell; VEGF, vascular endothelial growth factor.
Jia X, He L, Yang Z. Recent advances in the role of Yes‐associated protein in dermatosis. Skin Res Technol. 2023;29:e13285. 10.1111/srt.13285
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept. 2017;7(2):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size‐control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang H, Pasolli HA, Fuchs E. Yes‐associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci U S A. 2011;108(6):2270‐2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beverdam A, Claxton C, Zhang X, James G, Harvey KF, Key B. Yap controls stem/progenitor cell proliferation in the mouse postnatal epidermis. J Invest Dermatol. 2013;133(6):1497‐1505. [DOI] [PubMed] [Google Scholar]
- 5. Elbediwy A, Vincent‐Mistiaen ZI, Spencer‐Dene B, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143(10):1674–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283(9):5496‐5509. [DOI] [PubMed] [Google Scholar]
- 7. Schlegelmilch K, Mohseni M, Kirak O, et al. Yap1 acts downstream of α‐catenin to control epidermal proliferation. Cell. 2011;144(5):782‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jia J, Li C, Yang J, et al. Yes‐associated protein promotes the abnormal proliferation of psoriatic keratinocytes via an amphiregulin dependent pathway. Sci Rep. 2018;8(1):14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendoza‐Reinoso V, Beverdam A. Epidermal YAP activity drives canonical WNT16/β‐catenin signaling to promote keratinocyte proliferation in vitro and in the murine skin. Stem Cell Res. 2018;29:15–23. [DOI] [PubMed] [Google Scholar]
- 10. Akladios B, Mendoza‐Reinoso V, Samuel MS, et al. Epidermal YAP2‐5SA‐ΔC drives β‐catenin activation to promote keratinocyte proliferation in mouse skin in vivo. J Invest Dermatol. 2017;137(3):716–726. [DOI] [PubMed] [Google Scholar]
- 11. Corley SM, Mendoza‐Reinoso V, Giles N, et al. Plau and Tgfbr3 are YAP‐regulated genes that promote keratinocyte proliferation. Cell Death Dis. 2018;9(11):1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Addario I, Abbruzzese C, Lo Iacono M, Teson M, Golisano O, Barone V. Overexpression of YAP1 induces immortalization of normal human keratinocytes by blocking clonal evolution. Histochem Cell Biol. 2010;134(3):265‐276. [DOI] [PubMed] [Google Scholar]
- 13. Zhang X, Abdelrahman A, Vollmar B, Zechner D. The ambivalent function of YAP in apoptosis and cancer. Int J Mol Sci. 2018;19(12):3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu WW, Wang F, Li C, et al. Silibinin relieves UVB‐induced apoptosis of human skin cells by inhibiting the YAP‐p73 pathway. Acta Pharmacol Sin. 2022;43(8):2156–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dasgupta I, McCollum D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. J Biol Chem. 2019;294(46):17693–17706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maglic D, Schlegelmilch K, Dost AF, et al. YAP‐TEAD signaling promotes basal cell carcinoma development via a c‐JUN/AP1 axis. EMBO J. 2018;37(17):e98642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jia J, Mo X, Yan F, et al. Role of YAP‐related T cell imbalance and epidermal keratinocyte dysfunction in the pathogenesis of atopic dermatitis. J Dermatol Sci. 2021;101(3):164–173. [DOI] [PubMed] [Google Scholar]
- 18. Jang JW, Kim MK, Lee YS, et al. RAC‐LATS1/2 signaling regulates YAP activity by switching between the YAP‐binding partners TEAD4 and RUNX3. Oncogene. 2017;36(7):999–1011. [DOI] [PubMed] [Google Scholar]
- 19. Liu Y, He K, Hu Y, et al. YAP modulates TGF‐β1‐induced simultaneous apoptosis and EMT through upregulation of the EGF receptor. Sci Rep. 2017;7:45523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karvonen H, Barker H, Kaleva L, Niininen W, Ungureanu D. Molecular mechanisms associated with ROR1‐mediated drug resistance: crosstalk with hippo‐YAP/TAZ and BMI‐1 Pathways. Cells. 2019;8(8):812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seton‐Rogers S. Epithelial‐mesenchymal transition: untangling EMT's functions. Nat Rev Cancer. 2016;16(1):1. [DOI] [PubMed] [Google Scholar]
- 22. Lee MJ, Byun MR, Furutani‐Seiki M, Hong JH, Jung HS. YAP and TAZ regulate skin wound healing. J Invest Dermatol. 2014;134(2):518–525. [DOI] [PubMed] [Google Scholar]
- 23. Kim J, Kim YH, Kim J, et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J Clin Invest. 2017;127(9):3441–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varelas X, Samavarchi‐Tehrani P, Narimatsu M, et al. The Crumbs complex couples cell density sensing to Hippo‐dependent control of the TGF‐β‐SMAD pathway. Dev Cell. 2010;19(6):831‐844. [DOI] [PubMed] [Google Scholar]
- 25. Li Q, Jiao L, Shao Y, et al. Topical GDF11 accelerates skin wound healing in both type 1 and 2 diabetic mouse models. Biochem Biophys Res Commun. 2020;529(1):7–14. [DOI] [PubMed] [Google Scholar]
- 26. Wang D, Lin L, Lei K, et al. Vitamin D3 analogue facilitates epithelial wound healing through promoting epithelial‐mesenchymal transition via the Hippo pathway. J Dermatol Sci. 2020;100(2):120–128. [DOI] [PubMed] [Google Scholar]
- 27. Gao Y, Luo C, Rui T, et al. Autophagy inhibition facilitates wound closure partially dependent on the YAP/IL‐33 signaling in a mouse model of skin wound healing. FASEB J. 2021;35(10):e21920. [DOI] [PubMed] [Google Scholar]
- 28. Brewer CM, Nelson BR, Wakenight P, et al. Adaptations in Hippo‐Yap signaling and myofibroblast fate underlie scar‐free ear appendage wound healing in spiny mice. Dev Cell. 2021;56(19):2722–2740.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu D, Wang W, Li X, Yin B, Ma Y. Single‐cell sequencing reveals the antifibrotic effects of YAP/TAZ in systemic sclerosis. Int J Biochem Cell Biol. 2022;149:106257. [DOI] [PubMed] [Google Scholar]
- 30. Toyama T, Looney AP, Baker BM, et al. Therapeutic targeting of TAZ and YAP by dimethyl fumarate in systemic sclerosis fibrosis. J Invest Dermatol. 2018;138(1):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu F, Lagares D, Choi KM, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308(4):L344‐L357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shi‐Wen X, Racanelli M, Ali A, et al. Verteporfin inhibits the persistent fibrotic phenotype of lesional scleroderma dermal fibroblasts. J Cell Commun Signal. 2021;15(1):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen A, Hoang V, Laquer V, Kelly KM. Angiogenesis in cutaneous disease: part I. J Am Acad Dermatol. 2009;61(6):921‐942. [DOI] [PubMed] [Google Scholar]
- 34. Yu Z, Yu Q, Xu H, et al. IL‐17A promotes psoriasis‐associated keratinocyte proliferation through ACT1‐dependent activation of YAP‐AREG axis. J Invest Dermatol. 2022;142(9):2343–2352. [DOI] [PubMed] [Google Scholar]
- 35. Mermoud L, Shutova M, Diaz‐Barreiro A, et al. IL‐38 orchestrates proliferation and differentiation in human keratinocytes. Exp Dermatol. 2022;31(11):1699–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jia J, Wang N, Zheng Y, et al. RAS‐association domain family 1A regulates the abnormal cell proliferation in psoriasis via inhibition of Yes‐associated protein. J Cell Mol Med. 2021;25(11):5070–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Totaro A, Castellan M, Battilana G, et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat Commun. 2017;8:15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jia J, Mo X, Liu J, et al. Mechanism of danshensu‐induced inhibition of abnormal epidermal proliferation in psoriasis. Eur J Pharmacol. 2020;868:172881. [DOI] [PubMed] [Google Scholar]
- 39. Rehman A, Huang Y, Wan H. Evolving mechanisms in the pathophysiology of pemphigus vulgaris: a review emphasizing the role of desmoglein 3 in regulating p53 and the Yes‐associated protein. Life (Basel). 2021;11(7):621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang Y, Jedličková H, Cai Y, et al. Oxidative stress‐mediated YAP dysregulation contributes to the pathogenesis of pemphigus vulgaris. Front Immunol. 2021;12:649502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uttagomol J, Ahmad US, Rehman A, et al. Evidence for the desmosomal cadherin desmoglein‐3 in regulating YAP and phospho‐YAP in keratinocyte responses to mechanical forces. Int J Mol Sci. 2019;20(24):6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Rosa L, Secone Seconetti A, De Santis G, et al. Laminin 332‐dependent YAP dysregulation depletes epidermal stem cells in junctional epidermolysis bullosa. Cell Rep. 2019;27(7):2036–2049.e6. [DOI] [PubMed] [Google Scholar]
- 43. Enzo E, Secone Seconetti A, Forcato M, et al. Single‐keratinocyte transcriptomic analyses identify different clonal types and proliferative potential mediated by FOXM1 in human epidermal stem cells. Nat Commun. 2021;12(1):2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng S, Di Z, Hirman AR, et al. MiR‐375‐3p alleviates the severity of inflammation through targeting YAP1/LEKTI pathway in HaCaT cells. Biosci Biotechnol Biochem. 2020;84(10):2005–2013. [DOI] [PubMed] [Google Scholar]
- 45. Boopathy G, Hong W. Role of hippo pathway‐YAP/TAZ signaling in angiogenesis. Front Cell Dev Biol. 2019;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Azad T, Ghahremani M, Yang X. The role of YAP and TAZ in angiogenesis and vascular mimicry. Cells. 2019;8(5):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee J, Jung Y, Jeong SW, Jeong GH, Moon GT, Kim M. Inhibition of hippo signaling improves skin lesions in a rosacea‐like mouse model. Int J Mol Sci. 2021;22(2):931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bonilla X, Parmentier L, King B, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48(4):398–406. [DOI] [PubMed] [Google Scholar]
- 49. Yurchenko AA, Pop OT, Ighilahriz M, et al. Frequency and genomic aspects of intrinsic resistance to vismodegib in locally advanced basal cell carcinoma. Clin Cancer Res. 2022;28(7):1422–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brinkhuizen T, van den Hurk K, Winnepenninckx VJ, et al. Epigenetic changes in basal cell carcinoma affect SHH and WNT signaling components. PLoS One. 2012;7(12):e51710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Swiderska‐Syn M, Xie G, Michelotti GA, et al. Hedgehog regulates yes‐associated protein 1 in regenerating mouse liver. Hepatology. 2016;64(1):232‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Akladios B, Mendoza Reinoso V, Cain JE, et al. Positive regulatory interactions between YAP and Hedgehog signalling in skin homeostasis and BCC development in mouse skin in vivo. PLoS One. 2017;12(8):e0183178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iglesias‐Bartolome R, Torres D, Marone R, et al. Inactivation of a Gα(s)‐PKA tumour suppressor pathway in skin stem cells initiates basal‐cell carcinogenesis. Nat Cell Biol. 2015;17(6):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Quan T, Xu Y, Qin Z, et al. Elevated YAP and its downstream targets CCN1 and CCN2 in basal cell carcinoma: impact on keratinocyte proliferation and stromal cell activation. Am J Pathol. 2014;184(4):937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jiang C, Javed A, Kaiser L, et al. Mechanochemical control of epidermal stem cell divisions by B‐plexins. Nat Commun. 2021;12(1):1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vornicescu C, Șenilă SC, Bejinariu NI, et al. Predictive factors for the recurrence of surgically excised basal cell carcinomas: a retrospective clinical and immunopathological pilot study. Exp Ther Med. 2021;22(5):1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jia J, Li C, Luo S, et al. Yes‐associated protein contributes to the development of human cutaneous squamous cell carcinoma via activation of RAS. J Invest Dermatol. 2016;136(6):1267–1277. [DOI] [PubMed] [Google Scholar]
- 58. Al‐Busani H, Al‐Sobaihi S, Nojima K, et al. NUAK2 localization in normal skin and its expression in a variety of skin tumors with YAP. J Dermatol Sci. 2020;97(2):143–151. [DOI] [PubMed] [Google Scholar]
- 59. Neinaa Y, El‐Aziz Mohamed DA, Ali S, Gaballah HH, El‐Tatawy RA. YAP1 expression in lichen planus and squamous cell carcinoma: role in disease pathogenesis and potential therapeutic target. Am J Dermatopathol. 2022;44(5):348–354. [DOI] [PubMed] [Google Scholar]
- 60. Fania L, Didona D, Di Pietro FR, et al. Cutaneous squamous cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2021;9(2):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Debaugnies M, Sánchez‐Danés A, Rorive S, et al. YAP and TAZ are essential for basal and squamous cell carcinoma initiation. EMBO Rep. 2018;19(7):e45809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nan Y, Luo Q, Wu X, et al. DLGAP1‐AS2‐mediated phosphatidic acid synthesis activates yap signaling and confers chemoresistance in squamous cell carcinoma. Cancer Res. 2022;82(16):2887–2903. [DOI] [PubMed] [Google Scholar]
- 63. Maehama T, Nishio M, Otani J, Mak TW, Suzuki A. The role of Hippo‐YAP signaling in squamous cell carcinomas. Cancer Sci. 2021;112(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li P, Silvis MR, Honaker Y, Lien WH, Arron ST, Vasioukhin V. αE‐catenin inhibits a Src‐YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes Dev. 2016;30(7):798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lu X, Gan Q, Gan C, et al. Long non‐coding RNA PICSAR knockdown inhibits the progression of cutaneous squamous cell carcinoma by regulating miR‐125b/YAP1 axis. Life Sci. 2021;274:118303. [DOI] [PubMed] [Google Scholar]
- 66. Kim MH, Kim CG, Kim SK, et al. YAP‐induced PD‐L1 expression drives immune evasion in BRAFi‐resistant melanoma. Cancer Immunol Res. 2018;6(3):255–266. [DOI] [PubMed] [Google Scholar]
- 67. Wei Y, Jiang Z, Lu J. USP22 promotes melanoma and BRAF inhibitor resistance via YAP stabilization. Oncol Lett. 2021;21(5):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pisanu ME, Maugeri‐Saccà M, Fattore L, et al. Inhibition of stearoyl‐CoA desaturase 1 reverts BRAF and MEK inhibition‐induced selection of cancer stem cells in BRAF‐mutated melanoma. J Exp Clin Cancer Res. 2018;37(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fattore L, Mancini R, Ciliberto G. Cancer stem cells and the slow cycling phenotype: how to cut the Gordian knot driving resistance to therapy in melanoma. Cancers (Basel). 2020;12(11):3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lüönd F, Pirkl M, Hisano M, et al. Hierarchy of TGFβ/SMAD, Hippo/YAP/TAZ, and Wnt/β‐catenin signaling in melanoma phenotype switching. Life Sci Alliance. 2022;5(2):202101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xiong H, Yu Q, Gong Y, et al. Yes‐associated protein (YAP) promotes tumorigenesis in melanoma cells through stimulation of low‐density lipoprotein receptor‐related protein 1 (LRP1). Sci Rep. 2017;7(1):15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Loria R, Laquintana V, Scalera S, et al. SEMA6A/RhoA/YAP axis mediates tumor‐stroma interactions and prevents response to dual BRAF/MEK inhibition in BRAF‐mutant melanoma. J Exp Clin Cancer Res. 2022;41(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Luo C, Balsa E, Perry EA, et al. H3K27me3‐mediated PGC1α gene silencing promotes melanoma invasion through WNT5A and YAP. J Clin Invest. 2020;130(2):853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lui JW, Moore S, Huang L, Ogomori K, Li Y, Lang D. YAP facilitates melanoma migration through regulation of actin‐related protein 2/3 complex subunit 5 (ARPC5). Pigment Cell Melanoma Res. 2022;35(1):52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Seo J, Kim H, Min KI, et al. Weight‐bearing activity impairs nuclear membrane and genome integrity via YAP activation in plantar melanoma. Nat Commun. 2022;13(1):2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yap AS, Duszyc K, Viasnoff V. Mechanosensing and mechanotransduction at cell‐cell junctions. Cold Spring Harb Perspect Biol. 2018;10(8):a028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Caron JM, Han X, Contois L, Vary C, Brooks PC. The HU177 collagen epitope controls melanoma cell migration and experimental metastasis by a CDK5/YAP‐dependent mechanism. Am J Pathol. 2018;188(10):2356–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Oria VO, Zhang H, Zito CR, et al. Coupled fibromodulin and SOX2 signaling as a critical regulator of metastatic outgrowth in melanoma. Cell Mol Life Sci. 2022;79(7):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lund AW. Be easy and chill: melanoma cells tell lymph node fibroblasts to relax. Cancer Res. 2022;82(9):1692–1694. [DOI] [PubMed] [Google Scholar]
- 80. Li H, Wang Y, Su R, et al. Dimethyl fumarate combined with vemurafenib enhances anti‐melanoma efficacy via inhibiting the hippo/YAP, NRF2‐ARE, and AKT/mTOR/ERK pathways in A375 melanoma cells. Front Oncol. 2022;12:794216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lui JW, Xiao S, Ogomori K, Hammarstedt JE, Little EC, Lang D. The efficiency of verteporfin as a therapeutic option in pre‐clinical models of melanoma. J Cancer. 2019;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Prieto‐Granada C, Morlote D, Pavlidakey P, et al. Poroid adnexal skin tumors with YAP1 fusions exhibit similar histopathologic features: a series of six YAP1‐rearranged adnexal skin tumors. J Cutan Pathol. 2021;48(9):1139–1149. [DOI] [PubMed] [Google Scholar]
- 83. Feng Q, Guo P, Kang S, Zhao F. High expression of TAZ/YAP promotes the progression of malignant melanoma and affects the postoperative survival of patients. Pharmazie. 2018;73(11):662–665. [DOI] [PubMed] [Google Scholar]
- 84. Danovi SA, Rossi M, Gudmundsdottir K, Yuan M, Melino G, Basu S. Yes‐associated protein (YAP) is a critical mediator of c‐Jun‐dependent apoptosis. Cell Death Differ. 2008;15(1):217‐219. [DOI] [PubMed] [Google Scholar]
- 85. Rizzi M, Tonello S, Estevão BM, Gianotti E, Marchese L, Renò F. Verteporfin based silica nanoparticle for in vitro selective inhibition of human highly invasive melanoma cell proliferation. J Photochem Photobiol B. 2017;167:1–6. [DOI] [PubMed] [Google Scholar]
- 86. Martins Estevão B, Miletto I, Marchese L, Gianotti E. Optimized Rhodamine B labeled mesoporous silica nanoparticles as fluorescent scaffolds for the immobilization of photosensitizers: a theranostic platform for optical imaging and photodynamic therapy. Phys Chem Chem Phys. 2016;18(13):9042‐9052. [DOI] [PubMed] [Google Scholar]
- 87. Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes‐associated protein, YAP, to induce interaction with 14‐3‐3 and attenuation of p73‐mediated apoptosis. Mol Cell. 2003;11(1):11–23. [DOI] [PubMed] [Google Scholar]
- 88. Strano S, Monti O, Pediconi N, et al. The transcriptional coactivator Yes‐associated protein drives p73 gene‐target specificity in response to DNA damage. Mol Cell. 2005;18(4):447‐459. [DOI] [PubMed] [Google Scholar]
- 89. Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c‐Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol Cell. 2008;29(3):350‐361. [DOI] [PubMed] [Google Scholar]
- 90. Dent P, Booth L, Poklepovic A, et al. Osimertinib‐resistant NSCLC cells activate ERBB2 and YAP/TAZ and are killed by neratinib. Biochem Pharmacol. 2021;190:114642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhou K, Muroyama A, Underwood J, et al. Actin‐related protein2/3 complex regulates tight junctions and terminal differentiation to promote epidermal barrier formation. Proc Natl Acad Sci U S A. 2013;110(40):E3820‐E3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stampouloglou E, Cheng N, Federico A, et al. Yap suppresses T‐cell function and infiltration in the tumor microenvironment. PLoS Biol. 2020;18(1):e3000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang M, Dong Y, Gao S, et al. Hippo/YAP signaling pathway protects against neomycin‐induced hair cell damage in the mouse cochlea. Cell Mol Life Sci. 2022;79(2):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


