Abstract
Evolutionary medicine has been a fast-growing field of biological research in the past decade. One of the strengths of evolutionary medicine is to use non-traditional model organisms which often exhibit unusual characteristics shaped by natural selection. Studying these unusual traits could provide valuable insight to understand biomedical questions, since natural selection likely discovers solutions to those complex biological problems. Because of many unusual traits, the naked mole-rat (NMR) has attracted attention from different research areas such as aging, cancer, and hypoxia- and hypercapnia-related disorders. However, such uniqueness of NMR physiology may sometimes make the translational study to human research difficult. Damaraland mole-rat (DMR) shares multiple characteristics in common with NMR, but shows higher degree of similarity with human in some aspects of their physiology. Research on DMR could therefore offer alternative insights and might bridge the gap between experimental findings from NMR to human biomedical research. In this review, we discuss studies of DMR as an extension of the current set of model organisms to help better understand different aspects of human biology and disease. We hope to encourage researchers to consider studying DMR together with NMR. By studying these two similar but evolutionarily distinct species, we can harvest the power of convergent evolution and avoid the potential biased conclusions based on life-history of a single species.
Keywords: Damaraland mole-rat, Fukomys damarensis, Evolutionary medicine, Biomedical research, Hypoxia, Aging, Metabolism, Neurology, Endocrinology
1. Introduction
Evolutionary medicine is a set of concepts and approaches first considered by Williams & Nesse (Williams and Nesse, 1991) about three decades ago. The essence of evolutionary medicine is to attempt to understand health and disease in the context of natural selection and to apply the principles and tools of evolutionary biology to help our understanding of human health and disease (Stearns, 2012). Using these concepts and approaches, evolutionary medicine has contributed to a better understanding of topics in human health including reproductive health (Ellison, 2003), mental illness (Keller and Nesse, 2006; Nesse, 1999, 2004), immune function and inflammation (Cooper and Herrin, 2010; Litman and Cooper, 2007; Straub, 2012), cancer (Greaves, 2010; Merlo et al., 2006), aging (Austad, 2009; Miller et al., 2011; Stearns et al., 2000), nutrition, and exercise (Chakravarthy and Booth, 2004; Eaton et al., 1999; Leonard, 2008).
One of the promises of evolutionary medicine is to use non-traditional model organisms, which could be more suitable for a particular health or disease question (Maher, 2009). Traditional animal models, which are simple, fast-growing, and tractable model systems (such as worms, flies, zebra fish, and laboratory mice and rats), allow the study of normal and pathological processes in a controlled and systematic way to investigate specific hypothesis or test the effects of drugs and genes that, can be turned on and off with genetic approaches (Magalhães, 2015; O’Connor et al., 2002). Research using model organisms has generated useful knowledge and significant breakthroughs regarding the mechanisms that contribute to different human diseases. However, these model organisms have been primarily chosen for convenience, rather than for specific features pertinent to human health and diseases (Buffenstein, 2005). This resulted in caveats which limit the information obtained from model organisms that could be translated to humans. For example, model organisms are typically short-lived and lack genetic diversity (Gasch et al., 2016). Using these model organisms in aging and/or oncology research could be insufficient because of the confounding biological difference between short-lived model organisms and long-lived human. As a result, more and more non-traditional model organisms have been employed to study specific human health and disease questions (Bolker, 2012). One such group of animals are the African mole-rats.
African mole-rats, are burrowing rodents of the family Bathyergidae and Heterocephalidae (Bennett and Faulkes, 2000). These mole-rat species represent a distinct evolution of subterranean lifestyles and have attracted the attention of biogerontologists due to their generally high longevity (Buffenstein, 2005; Buffenstein et al., 2021). Even though Bathyergidae and Heterocephalidae contain more than 20 species, our understanding of these mole-rats is heavily biased towards the naked mole-rat (NMR), Heterocephalus glaber (Faulkes et al., 2004). NMR have been intensively studied in a wide range of biomedical research because of their extreme characteristics such as long lifespan, cancer resistance, hypoxia- and hypercapnia-resistance, oxidative stress tolerance and high reproductive outputs for breeders (Buffenstein et al., 2021). Unfortunately, the unique physiology of naked mole-rats might make it difficult for translation into human biomedical research. For example, unlike other mammals including human, NMR is defined as heterothermic endotherms where their body temperature was the lowest in mammals (Buffenstein and Yahav, 1991). NMRs also exhibit extreme changes in the opioid system compared to other mammals (Park et al., 2003; Smith et al., 2015; St John Smith et al., 2012). Additionally, NMR have fewer C-fibers than laboratory rodents and other mole-rat species and do not produce the pain-relaying neuropeptides substance P and calcitonin gene-related peptide (Park et al., 2003; St John Smith et al., 2012). Moreover, mutations in Nav1.7 protein in NMR allow the organism to tolerate against acid or capsaicin in injested food and surrounding environments, which may offer advantages in food selection and survival in subterranean habitat (Smith et al., 2020). These unique features of NMR make it challenging to reconcile the discrepancy between the physiological and pathological responses in human and NMR. In this regard, other African mole-rat species, such as the Damaraland mole-rat (DMR; Fukomys damarensis; Fig. 1), who share many similar characteristics with NMR, could provide alternative explanations and might bridge NMR research to human biomedical research (Smith et al., 2015).
Fig. 1.
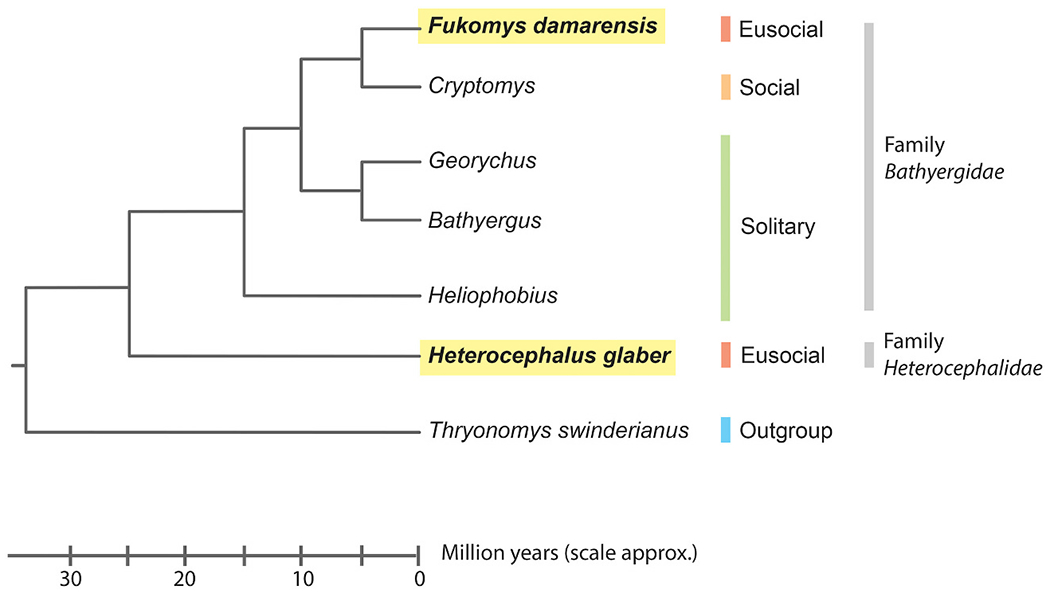
Phylogeny for the Bathyergidae and the Heterocephalidae, together with a close outgroup (the cane rat Thryonomys swinderianus. Adapted from (Fang et al., 2014; Faulkes and Bennett, 2016; Ivy et al., 2020).
Just like NMR, DMR is a fully fossorial rodent living under eusocial structure (Faulkes and Bennett, 2016). It is important to note here that eusociality was evolved independently in NMR and DMR (Fig. 1). DMR reside in an arid habitat with unpredictable rainfall in southern Africa. Much of their distribution is characterized by red Kalahari arenosols, but they can also be found in a wide range of coarse sandy soils (Bennett and Jarvis, 2004; Faulkes and Bennett, 2016). DMR and NMR diverged ~26 million years ago (Fang et al., 2014) and exhibit similar unusual adaptations due to their comparable habitats and social structure (Fig. 1). The divergences between DMR and NMR could make DMR an alternative system to better understand the biology of some human diseases or conditions (Fang et al., 2014; Faulkes and Bennett, 2016; Smith et al., 2015). For instance, similar to humans, DMR is a homeothermic mammal that tightly controls its body temperature at a slightly lower temperature of 35 °C (Buffenstein and Yahav, 1991; Hislop and Buffenstein, 1994). Under hypoxia, DMR’s responses resemble that of a non-fossorial species (including human), in that a ventilatory response was mounted and no metabolic depression was observed (Zhang and Pamenter, 2019a). Therefore, as a distant cousin of NMR, DMR exists as a system to investigate the adaptations to extreme environmental conditions while maintaining physiology similar to other mammalian species and humans. On the other hand, studying these two similar but also very different species together would allow us to harvest the power of convergent evolution to better understand the effects of natural selection and avoid the potential bias of conclusions based on life-history of a single species (Sackton and Clark, 2019). Studying DMR could also help us connect the knowledge between NMR and human physiology, and therefore enhancing the translatability of the scientific findings from NMR physiology and human biomedical research. The use of NMR in biomedical research has been intensively reviewed (Buffenstein et al., 2021), however, to the best of our knowledge, such a review is still lacking for DMR. In this review, we discussed the use of DMR as an extension of our current set of model organisms to help us better understand human biology, particularly, in aging, hypoxic/hypercapnic response as well as the neuroendocrine control of mammalian reproduction (Fig. 2).
Fig. 2.
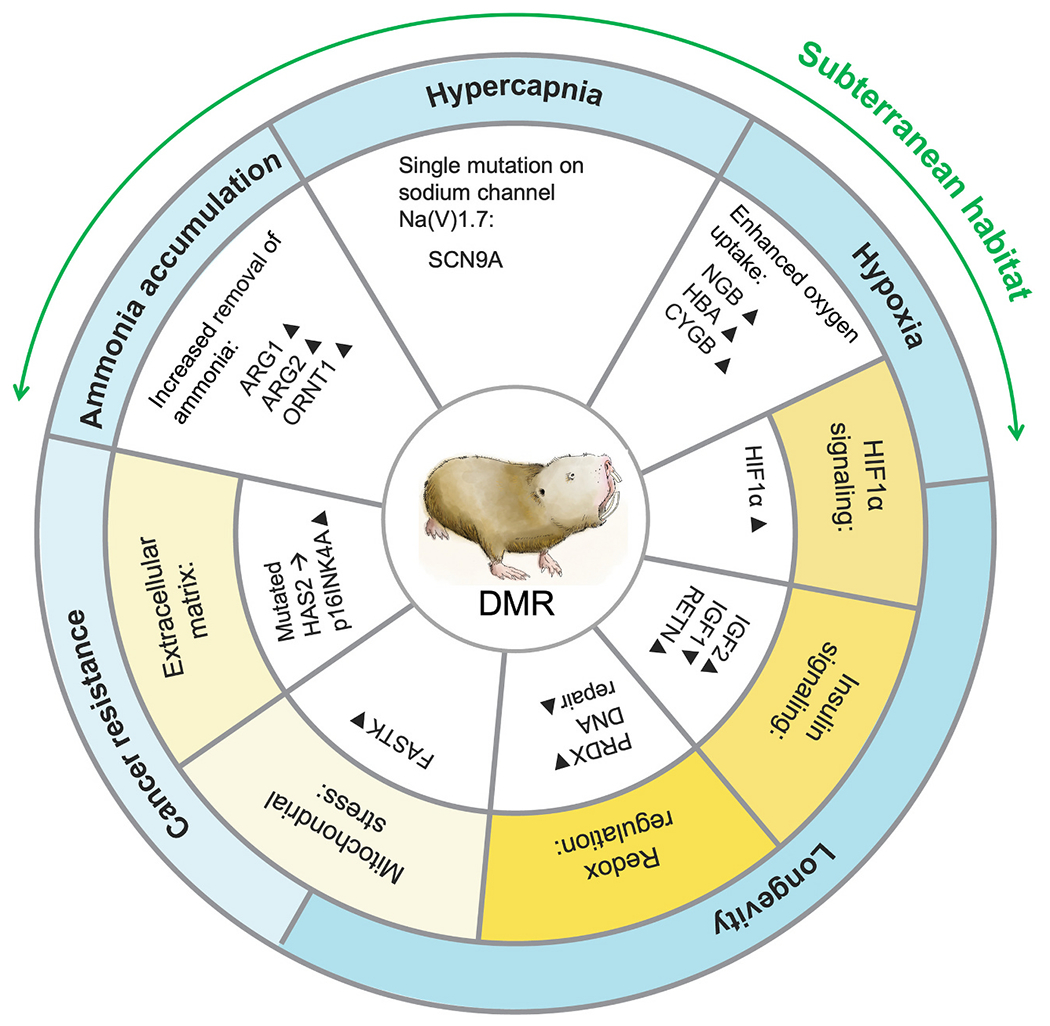
Areas of interests in biomedical research in Damaraland mole-rat.
1.1. Aging research – Insights on the rate of living theory, free radical theory of aging, DNA damage theory of aging and epigenetic oxidative redox shift theory of aging
Subterranean rodents have very long lifespans for their body weight, among which, fully fossorial DMR is one of the representatives (Edrey et al., 2011). The longest-lived DMR survived for more than 20 years in a laboratory setting (Dammann and Burda, 2007). DMR demonstrate a similar longevity quotient to that of humans and therefore present excellent models to study the biology of aging and age-related diseases (Dammann and Burda, 2007; Edrey et al., 2011).
Physiological research of DMR was pioneered on investigating their metabolic rates by Dr. Barry Lovegrove more than 30 years ago (Lovegrove, 1989; Lovegrove, 1986). These studies have documented that the resting metabolic rate was 43% lower than size-matched rodents, and 29% lower than other size-matched subterranean rodents. Together with other African mole-rat species, the fossorial rodents have low basal metabolic rates (BMR) compared to those expected for their size (McNab, 1966). These results support the Rate-of-Living theory which postulates that the slower an organism’s metabolism, the longer its lifespan (Rubner, 1908). However, challenges to the Rate-of-Living theory indicated that BMR might not be a valid marker for total daily energy expenditure (DEE), where the ratio of BMR to total daily energy expenditure can vary between 1.6 and 8.0 among mammals (Speakman et al., 2002). Using doubly labelled water, DEE and sustained metabolic scope (the ratio of daily energy expenditure to basal metabolic rate) was measured, and metabolic scopes were lower for DMR (around 2) compared with other rodents (about 4.1) (Karasov, 1992; Scantlebury et al., 2006). More interesting, BMR does not vary among young, middle age, and old DMRs where BMR typically decreases with age for other laboratory rodents and humans (O’Connor et al., 2002; Yap et al., 2022). Besides lower but constant BMR at the organismal level, a recent study from our labs also demonstrated that DMR primary lung fibroblasts depended on glycolysis rather than oxidative phorsphorlation (OXPHOS) for ATP production in cells, and low basal proton conductance through mitochondria inner membrane in isolated lung mitochondria (Yap et al., 2022). These unique bioenergetic properties might result from adaptation to hypoxic environments since hypoxia-inducible factor 1 alpha subunit (HIF-1α) was upregulated (Yap et al., 2022). These metabolic adaptations and upregulated HIF-1α pathways, on the other hand, might contribute to their extreme longevity.
As inspired by the Free Radical Theory of Aging (Harman, 1972), the oxidative status of DMR have been studied to investigate the relationship between redox homeostasis and longevity (Labinskyy et al., 2006; Lambert et al., 2007). In a study conducted using isolated mitochondria, there was a lower capacity of superoxide and/or hydrogen peroxide production by mitochondrial Complex I during reverse electron transport in DMR compared to short-lived rodents (Lambert et al., 2007). The authors also reported a highly significant correlation between maximum lifespan and the capacity of mitochondrial Complex I H2O2 production during reverse electron transport in species of different lifespan (Lambert et al., 2007). These findings seem to echo the notion that aging is caused by accumulation of damage inflicted by reactive oxygen species (ROS). However, it is important to note that the capacity of ROS production measured from isolated mitochondria might not represent physiologically relevant mitochondrial ROS production in cells, tissues or in vivo (Zhang and Wong, 2021). In fact, low levels of ROS production in isolated mitochondria did not transfer to lower oxidative damage in tissues even when the antioxidant capacities were similar when compared to short-lived rodents (Lewis et al., 2013). Moreover, levels of antioxidant markers were similar between breeders and non-breeders, but both reduced (Schmidt et al., 2014) and constant (Jacobs et al., 2021) levels of oxidative damage makers had been shown for breeders in DMR. Interestingly, when experimentally increased cooperative contributions in breeding DMRs, oxidative stress was increased, without changing body condition (Mendonça et al., 2020). Consequently, it has been proposed that these long-lived mole-rats can tolerate high oxidative damage, maintaining somatic tissue function, without affecting their healthspan and lifespan (Lewis et al., 2013).
One of the mechanisms for tolerating high oxidative damage focused on resistance to DNA fragmentation and DNA repair pathways. In an ex vivo model of oxidative stress-induced vascular aging, epithelial and smooth muscle cells of DMR carotid artery were found to be resistant to H2O2-induced DNA fragmentation and caspase activation (Labinskyy et al., 2006). The study also demonstrated using NMRs, DMRs, guinea pigs and mice a negative correlation between the extent of oxidant-induced DNA fragmentation and maximal lifespan potential (Labinskyy et al., 2006). These findings suggest a more efficient DNA repair mechanism in DMR in response to oxidative stress compared to short-lived rodents. The notion of an improved DNA repair mechanism was supported by transcriptomic analysis of DMR (Fang et al., 2014). The transcriptome of DMR liver revealed differential expression and enrichment of several genes associated with oxidoreduction. Despite downregulated expression (peroxiredoxins) and activity (glutathione peroxidases) of antioxidant enzymes, several genes associated with DNA damage repair and responses to stress also exhibited higher expression in DMR even during normoxia, suggesting improved DNA repair is an intrinsic mechanism of adaptation to an underground environment (Fang et al., 2014).
Besides DNA repair pathways, genomic and transcriptomic analyses also revealed an inactivation of Fas-activated serine/threonine kinase (FASTK) in the livers of DMR compared with short-lived rodents (Fang et al., 2014). FASTK serves as a regulator of Fas-mediated apoptosis and is located at the inner mitochondrial membrane (Jourdain et al., 2017). While FASTK has an important role in governing cell survival, its overexpression has been demonstrated to be involved in age-related pathological conditions such as tumorigenesis and immune-mediated inflammatory diseases (Simarro et al., 2010; Zhi et al., 2013). The role of FASTK in aging was corroborated by its effect on neuron elongation and regeneration. The ability of neurons to regenerate and their rate of elongation decrease with age whereas a statistically significant improvement of such was observed in FASTK null subjects (Loh et al., 2008). Therefore, the beneficial effect of FASTK knockdown on neuronal development may in part explain the outstanding longevity of DMR, particularly the integrity of neurological functions of DMR.
The longevity of DMR may also attribute to their distinct endocrine regulation of glucose homeostasis. DMR, like other mole-rats, harbor a divergent insulin β-chain sequence (Chan et al., 1984; Kramer and Buffenstein, 2004). Mutations in insulin β-chain are known to cause misfolded insulin and reduced insulin signaling in humans. The mutation Arg22Gln was found in DMR insulin and was reported to be associated with insulin misfolding (Liu et al., 2010). The alteration in insulin structure was also found to be associated with a decreased insulin growth factors 1 (IGF1)/growth hormone signaling (Fang et al., 2014). As an alternative mechanism, DMR expresses insulin growth factor 2 (IGF2) and insulin growth factor 2 binding protein and the autocrine/paracrine actions of IGF2 in the liver substitutes for insulin for glucose handling in DMR (Edrey et al., 2011; Fang et al., 2014). In humans, a reduced insulin level, an improved insulin sensitivity and an inhibition of the growth hormone/IGF1 axis are often considered to provide beneficial effects on longevity, as was the case, for example, in caloric restriction (Bartke et al., 2003; Janssen and Lamberts, 2004; Tatar et al., 2003; Yamaza et al., 2002). It is therefore hypothesized that the outstanding longevity of DMR, may in part be accredited to the divergence in insulin signaling.
1.2. Cancer resistance – the significant role of very high molecular mass hyaluronan in tumorigenesis
Consistent with other African mole-rats, the longevity of DMR also comes along with a rare incidence of cancers. This may partly be explained by the aforementioned inactivation of FASTK since its overexpression is strongly associated with tumor formation (Zhi et al., 2013). Another explanation for cancer resistance is related to the enzyme hyaluronan synthase 2 (Fang et al., 2014; Tian et al., 2013). Hyaluronan is an extracellular matrix polysaccharide that serves as an extracellular signal for early contact inhibition (Tian et al., 2013). A single amino acid substitution in a highly conserved region of hyaluronan synthase 2 in DMR leads to the synthesis of very high molecular mass hyaluronan which may confer cancer resistance by sensitizing DMR cells tocontact inhibition and triggering the downstream induction of the tumor suppressor P16INK4A (Fang et al., 2014; Tian et al., 2013). The discovery of anti-tumorigenesis activity of very high molecular mass hyaluronan has been a breakthrough in oncology research and opens up new avenues for anti-cancer therapies (Fisher, 2015; Gorbunova et al., 2020).
1.3. Hypoxia, hypercapnia, and ammonia metabolism – enhanced efficiencies of oxygen uptake, bicarbonate buffering and ammonia metabolism
Similar to NMR, DMR are also fully fossorial and live in densely populated, poorly ventilated underground burrows, within which they likely experience intermittent periods of hypoxia and hypercapnia (Faulkes and Bennett, 2016; Zhang and Pamenter, 2019a). Even though both species face similar hypoxic environments, the physiological responses to hypoxia are divergent. For NMR, hypoxia tolerance is achieved by depressions in metabolic rate, body temperature, and activity level with little change in ventilation. This hypoxia metabolic response is a hallmark of hypoxia adaptation and has been observed in most hypoxia-tolerance species. But DMR employed different strategy which resembles that of a non-fossorial species (including human). Upon exposure to acute and progressive hypoxia, DMR demonstrate a significant increase in ventilation and an absence of hypoxic metabolic response (Zhang and Pamenter, 2019a). The observation was consistent with most adult mammals of which the initial physiological response to low O2 environments is a reflex increase in minute ventilation (Zhang and Pamenter, 2019a). This lack of hypoxic metabolic response but instead reliance on a robust hypoxic ventilation in DMR are similar to some other fossorial species, such as Middle East blind mole-rat (Spalax ehrenbergi). With chronic hypoxia, DMR demonstrated a further increase in ventilation upon ventilatory acclimatization to hypoxia compared to normoxia-acclimated animals (Zhang and Pamenter, 2019a). This observation indicates the occurrence of neuroplasticity within the ventilatory control circuits in DMR during prolonged exposure to hypoxia (Zhang and Pamenter, 2019a).
To better survive in a hypoxic environment, another important adaptation is an improved oxygen uptake to high oxygen-demanding tissues. The transcriptome and proteome of DMR revealed enhanced expression of proteins of the globin family (Fang et al., 2014). These proteins are responsible for the delivery and storage of oxygen in cells and tissues (Vinogradov and Moens, 2008). Under both normoxic and hypoxic conditions, there were higher protein expression of hemoglobin α and neuroglobin in the brains of DMR when compared to above-ground rodents (Fang et al., 2014). There was also a trend of elevated mRNA expression of cytoglobin, a member of the globin family that facilitates diffusion of oxygen through tissues, scavenges nitric oxide or reactive oxygen species to protect against oxidative stress in the brains of DMR (Fang et al., 2011, 2014; Hodges et al., 2008; Li et al., 2007; Reuss et al., 2016; Singh et al., 2014; Xu et al., 2006). These diverse physiological responses highlight the wide variety of adaptations that can mitigate systemic hypoxia. Importantly, the primary criterion of hypoxia tolerance is not the manifestation of physiological responses to a hypoxic stimuli, but rather the ability to successfully match metabolic demand to metabolic supply when oxygen is limited (Buck and Pamenter, 2006). Therefore, the true measure of hypoxia tolerance is the ability to tolerate prolonged and/or severe hypoxic exposure without detriment. Such concept provides a potential novel route for human studies in hypoxia condition, where increase hypoxia tolerance in organisms should target matching metabolic supply to metabolic demand regardless of physiological responses.
The underground burrows of DMRs present a gaseous environment range from normocapnic (0.1% CO2) to hypercapnic (6% CO2) conditions (Faulkes and Bennett, 2016; Zhang and Pamenter, 2019b). Surprisingly, unlike most burrow dwelling animals which exhibit delayed or blunted hypercapnic responses as an adaptation to reduce the energetic costs associated with hyperpnoea in hypercapnia (Barros et al., 2004; Boggs et al., 1984), the response of DMR to hypercapnia mirrors that of above-ground rodents. It was found that DMRs underwent hyperventilation along with the lack of change in metabolic rate during hypercapnia. The respiratory exchange ratio was also unchanged in hypercapnia suggesting DMRs do not adjust their metabolic fuel source in acute hypercapnia (Zhang and Pamenter, 2019b). Elevated CO2 in the body may result in respiratory and/or metabolic acidosis. When dissolved in blood, CO2 dissociates into equilibrium between carbonic acid and bicarbonate, resulting in increased acidity (Branigan et al., 2018; Heinemann and Goldring, 1974). Dissolution of inhaled CO2 in fluids of the upper respiratory tract and nasal mucosa also triggers painful, burning sensations that lead to behavioral avoidance (Brand et al., 2010). Despite the lack of metabolic adaptation, DMR were found to exhibit no sign of behavioral avoidance as manifested by no differences in movement velocity, distance travelled, spatial exploration, or body temperature at any level of environmental hypercapnia (Branigan et al., 2018). This ability of DMR is mediated by bicarbonate buffering at the level of the kidney or within the blood as acetazolamide, an inhibitor of carbonic anhydrase that potentiates whole animal acidosis, was able to sensitize DMR to hypercapnia-mediated behavioral avoidance (Branigan et al., 2018). Applications of such inhibitor on whole animal acidosis should be studied in other organisms including human.
The build up of CO2 also evokes acid-induced pain. A negatively charged motif in the sodium channel Nav1.7 protein (SCN9A), which is highly expressed in nociceptor neurons, prevents acid-induced pain signaling to the NMR brain (Smith et al., 2011). Such negatively charged motif is also present in the DMR, as well as other family Bathyergidae species (Fang et al., 2014; Smith et al., 2011). Interestingly, even though the negatively charge constellations [(−)(+)(−)] of the motif are the same between NMR and DMR, the exact mutation in Nav1.7 protein are different, with Glu-Lys-Asp (EDK) in DMR compared to Glu-Lys-Glu (EDE) in NMR (Eigenbrod et al., 2019; Fang et al., 2014). Moreover, a closer look at the Nav1.7 sequences revealed that domain III also carries two negative charges in NMR, but not in DMR (Eigenbrod et al., 2019). As a matter of fact, when screening through eight African rodent species, DMR showed robust pain behaviors to painful substances capsaicin, acid (HCl, pH 3.5), and allyl isothiocyanate, similar to laboratory rodents and humans; Whereas NMR showed no behavioral response to capsaicin and acid (Eigenbrod et al., 2019). Other than differences in Nav1.7 protein, NMR also has fewer C-fibers (small unmyelinated axons associated with slow pain signaling) than other rodents, including the DMR. Both of these mechanisms have been exploited as targets for new pain therapies in human (Kingwell, 2019; Lynn, 1990). Consequently, these evidence serve as good examples that studies on DMR and other mole-rat species could bridge NMR research to human biomedical studies.
Living in densely populated underground burrows, the accumulation of ammonia which gives rise to the potent irritants, nitrogen and methane (Burda et al., 2007; LaVinka et al., 2009), presents a significant challenge for DMR survival. To cope with this, DMRs have evolved a divergent arginase 1, an enzyme that catalyzes the final step of the hepatic urea cycle, to enhance the efficiency of ammonia removal (Jenkinson et al., 1996). The single replacement of leucine/tyrosine residue by histidine at position 254 improves ammonia removal efficiency of arginase 1 by strengthening the assembly of arginase 1 homotrimer (Dowling et al., 2008; Fang et al., 2014; Lavulo et al., 2001; Sabio et al., 2001). Moreover, mitochondrial ornithine transporter (SLC25A15), another critical enzyme of the urea cycle, was also found to be expressed at high level in the livers of DMR (Fang et al., 2014). All together, these findings suggest enhanced ammonia detoxification of DMR as an adaptation to their natural habitat.
1.4. Neuroendocrine regulation – the control of reproduction in response to environmental changes
Being one of the two widely accepted eusocial mammals, the physiological and behavioral regulations of reproduction in DMR have been extensively studied. DMR exhibit a strong skew in lifetime reproductive success, with breeding restricted to a single female and one or two males (Clarke et al., 2001; Cooney and Bennett, 2000; Faulkes and Bennett, 2016). Non-breeding NMRs of both sexes are physiologically inhibited from reproducing, while in DMRs only the non-breeding females are physiologically suppressed (Bennett et al., 2018). Even under reproductive suppression, non-breeding members of the colony possess intact reproductive machineries and were shown to be able to switch from reproductively quiescent to reproductively active once the breeding female has died and a new, unrelated, individual becomes available or the fragmentation of the colony occurs (Faulkes et al., 1994; Molteno and Bennett, 2000; Snyman et al., 2006). For NMR, eusociality might be achieved by elevated circulating prolactin levels in non-breeders, but this is not the case for DMR (Bennett et al., 2018). In fact, the ovaries of female non-breeding DMRs have varying levels of follicular development, although they do not ovulate. Consequently, the reproductive suppression of non-breeding DMR is largely influenced by a self-restraint mechanism in order to minimize inbreeding and prevent incest until an unrelated male is present (Bennett, 1996; Clarke et al., 2001).
The underlying mechanisms for reproductive suppression of DMR might happen locally at tissue level. Inhibition of hypothalamopituitary-gonadal axis has been highlighted to be responsible for the neuroendocrine changes in DMR females (Bennett, 1996; Molteno and Bennett, 2000; Voigt et al., 2014; Voigt and Bennett, 2019; Voigt and Bennett, 2017). In DMR, the suppression of reproduction is achieved by the inhibition of ovulation, which is a consequence of a reduced level of gonadotropin-releasing hormone (GnRH)-induced luteinizing hormone from the pituitary (Bennett, 1996; Molteno and Bennett, 2000; Voigt et al., 2014). While the GnRH perikarya were found to be structurally and functionally intact in non-breeding DMR females, differential expression of GnRH was observed in different brain regions of non-breeding or breeding DMR females (Molteno and Bennett, 2000). Intriguingly, in situ hybridization revealed the elevated expression of GnRH mRNA along the rostral preoptic region of DMR breeding females compared to non-breeding females whereas the latter had increased GnRH mRNA level at the caudal level of the anterior hypothalamus (Voigt and Bennett, 2017). The delicate regulation of GnRH expression in specific neuron subpopulations is mediated by the region-specific expressions of neuropeptides such as dynorphin, neurokinin B, kiss-peptin and RFamide-related peptide-3, and is considered as the key mechanism governing the switch between reproductive statuses (Voigt and Bennett, 2019).
1.5. Hearing – the importance of the precise arrangement/orientation of hair bundles in hearing
Subterranean rodent families show elevated auditory thresholds and restricted frequency ranges of hearing compared with other surface-dwelling rodents (Gerhardt et al., 2017; Heffner and Heffner, 1993; Heffner and Heffner, 1992; Okanoya et al., 2018; Pyott et al., 2020). DMRs hear within a narrow frequency range, between 125 cHz and 4 kHz, with the best hearing at around 1 kHz. Even at their best frequencies, audiograms indicate thresholds are elevated by 25 to 35 dB in DMR compared with above-ground rodents (Barone et al., 2019). Previous studies attempted to relate the elevated auditory threshold of DMR to the lack of pinnae which is known to be a feature that contributes to the poor sound localization (Heffner and Heffner, 1993). However, pinnae in rodents were shown to be responsible for acoustical gain at frequencies above 8 kHz, therefore the missing pinnae could not account for the elevated auditory thresholds at low frequency (Koka et al., 2011; Lauer et al., 2011). Furthermore, in NMR findings from microcomputed tomography also suggest that the gross dimensions of the middle and inner ear may not be sufficient to explain altered hearing (Mason et al., 2016). Based on well-curated hearing genotype-phenotype database, amino acid substitutions that matches pathogenic mutations in humans in the hair bundle link proteins were identified in DMR. These substitutions are consistent with abnormal hair bundle morphology observed by scanning electron microscopy and reduced cochlear amplification measured in vivo in DMR (Pyott et al., 2020). Missing and disorganized hair bundles from outer hair cells in the apical and apical/middle turns were observed in DMR (Pyott et al., 2020). Precise arrangement and orientation of hair bundles across the sensory epithelium are critical for hearing as they allows efficient deflection of the OHC hair bundles, depolarization of the OHCs and prestin activation (McPherson, 2018). The impaired hearing ability of DMR is largely attributed to the disrupted hair bundles in the outer hair cells. The impaired hearing ability of DMR is largely attributed to the disrupted hair bundles in the outer hair cells, of which is consistent with many hearing pathologies in human. Currently, chinchillas, rabbits, paca, rats and mice of various genetic variations are the main animal models used to understand the biology of human auditory system (Chatterjee and Lufkin, 2011; Reis et al., 2017; Salvi et al., 2021). Identify gene mutations in DMR would help to understand physiological mechanisms associated with hearing, then further understand human hearing pathologies.
Recently, it has been shown that NMR has a colony-specific greeting (dialects) that is modulated by the “queen” of the colony and learned by other members of the colony in early life (Barker et al., 2021). Such vocal dialect facilitates recognition of colony members and thereby helps maintain colony cohesiveness (Buffenstein, 2021). DMR is another highly social, yet xenophobic, animals which can also make the birdlike chirps similar to NMR (Jacobs et al., 1998). It is still an open question on whether other eusocial mammals use similar strategies to maintain their highly organized social structure (Leedale et al., 2021).
1.6. Conclusion and future directions
Here we discussed some interesting aspects of the physiology of DMR as adaptations to their unique habitat and specific social structure, all of which provide valuable information and insights to the current biomedical research (Fig. 2). In the field of aging research, the use of NMR and to a lesser extent DMR has become popular due to the longevity of these mole-rat species. With extended human life expectancy, an unprecedented number of resources have been dedicated to aging research to promote health span by understanding the biology of age-related diseases. Based on the current use of DMR, several signaling pathways, as listed above, have been highlighted to be heavily involved in and may serve as therapeutic targets to slow down our aging process. This is also observed inoncology research in which the anti-tumorigenic molecule, very high molecular weight hyaluronan, was first identified to be naturally expressed in DMR. Furthermore, the effort to understand the reproductive skew of DMR females has broadened our knowledge of the delicate neuroendocrine regulation of reproduction in other mammals and human. This is useful information as female reproductive health has become an increasingly popular area of research.
However, challenges often result when scientists tried to validate these findings. Compared to laboratory rodents and othercommonly used model systems, the molecular biology and physiology of DMR are less defined and therefore the interpretation of results might not be as straightforward as it is for the more well-defined model systems. Fewer scientific tools and support are available for less defined systems as well. Reagents, such as antibodies for protein analysis or methodologies for functional assessments, may need to be re-validated and re-developed before conducting actual experiments. This makes the studies with DMR and NMR highly time-and resource-intensive. In this regard, this current review also intends to encourage researchers in evolutionary biology and biomedicine to allocate effort to the comprehensive characterizations of DMR and other non-traditional model organisms. Luckily, annotated genome for DMR is available for use (Fang et al., 2014), which provides bases for other approaches such as qPCR, transcriptomic study and designing transfection plasmid and virus (Fang et al., 2014; Johnston et al., 2021). Protocols for establishing certain primary cells from DMR is also available to use (Johnston et al., 2021; Yap et al., 2022). Even though published transfection protocol specifically for DMR cells is still unavailable, however, methods used for NMR cells should apply for DMR cells as well (Seluanov et al., 2009). Expressing exogenous genes in primary DMR cells might be difficult because of mole-rats genome are relatively stable compared to other model organisms (Petruseva et al., 2017; Sahm et al., 2018). In short, establishing protocols for DMR might be difficult at first, but these lines of study will enable the subsequent development of a wealth of tools and resources to support further research with these organisms. Moreover, it should be of note that the eusocial structure of DMR has been reported to elicit considerable influence on multiple aspects of biology, these include but are not limited to the aging process (Healy, 2015; Lucas and Keller, 2020) and neurodevelopment (Anyan et al., 2011; Oosthuizen, 2020; Oosthuizen and Amrein, 2016). Therefore, there are potential caveats when trying to translate the findings from DMR to human and one should interpret these data with care.
Compared to NMR, DMR has attracted less attention from researchers even though these two species share many similar unusual traits together. As discussed throughout this review, the underlying physiological mechanisms for these unusual traits often differ between DMR and NMR. Comparative genomic study between NMR and DMR indicated both differences and similarities of their genomes (Fang et al., 2014; Lewis et al., 2016). The major differences between these two genomes lied in response to hypoxia, body temperature regulation, pain sensitivity, processing of rRNA (Fang et al., 2014). For example, neuroglobin is often elevated in the brains of subterranean rodents even during normoxia (Avivi et al., 2010). Higher neuroglobin expression was observed NMR brain, but not in the DMR brain. But DMR exhibited higher cytoglobin expression. This indicated species-specific expression of globins in response to hypoxia (Avivi et al., 2010; Fang et al., 2014). However, when comparing to above ground rodents, DMR and NMR genome showed lots of similarities. For example, because of the ammonia environment, arginase 1, which catalyzes the last step of the hepatic urea cycle, has changed in both the NMR and DMR: His254 replaces Leu/Tyr. This amino acid change was also detected in the subterranean coruro (Spalacopus cyanus) and the semi-subterranean degu (Octodon degus)(Fang et al., 2014). As a result, this mutation could be the adaptation for subterranean habitat both DMR and NMR occupied. Many areas of the “unique” underlying physiological mechanisms for different adaptations in NMR still remain untested for DMR. For some biomedical research topics, such as hypoxia adaptation, DMR could help bridge translational research to human health from NMR research. Consequently, results from studies on DMR are not only supplementary evidence to strengthen findings from NMR, but would also offer a unique perspective that will shed light on alternative physiological adaptations for similar evolutionary force. By studying these two similar but evolutionarily distinct species, we can avoid the potential bias of conclusions based on life-history of a single species. In addition to a holistic view of DMR, we also encourage researchers to consider the similarities and differences within the whole family Bathyergidae, or perhaps all subterranean rodents, particularly including the family Spalacidae (Fang et al., 2014). Understanding the broader phylogenetic context will be essential for understanding the evolution of these adaptations (Eigenbrod et al., 2019; Ivy et al., 2020; Smith et al., 2011; St John Smith et al., 2012).
Here, we reviewed a collection of studies with DMR and their potential influence on the development of current biomedical research. For complex biological questions, evolutionary approach is a powerful tool. The fundamental promise of an evolutionary approach is that natural selection is likely to discover solutions to complex biological problems, and by studying these solutions, we might discover mechanisms that differ from, and are superior to, those elucidated by the study of traditional model species. Though this is a powerful approach, we should always bear in mind that the findings of these studies may, to a certain extent, be confounded due to the lack of a comprehensive characterization of DMR and the discrepancies between DMR and human. More effort is warranted to broaden our understanding of DMR and to establish a good collection of experimental resources to support future research.
Acknowledgement
We would like to thank the vivarium staff from University of Memphis for their excellent animal care. We appreciate the invitation from guest editor Matthew Pamenter to submit this review. We would also like to dedicate this review to Dr. Barry Lovegrove (Dec 5, 1956 – Mar 6, 2022), a pioneer in studying Damaraland mole-rat, who recently passed away. Y.Z. was supported by US National Science Foundation grant IOS – 2037735.
Footnotes
Declaration of Competing Interest
The authors declare no competing financial interests.
References
- Anyan JJ, Seney ML, Holley A, Bengston L, Goldman BD, Forger NG, Holmes MM, 2011. Social status and sex effects on neural morphology in Damaraland mole-rats, Fukomys damarensis. Brain Behav. Evol 77, 291–298. 10.1159/000328640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN, 2009. Comparative biology of aging. J. Gerontol. A Biol. Sci. Med. Sci 64, 199–201. 10.1093/gerona/gln060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avivi A, Gerlach F, Joel A, Reuss S, Burmester T, Nevo E, Hankeln T, 2010. Neuroglobin, cytoglobin, and myoglobin contribute to hypoxia adaptation of the subterranean mole rat Spalax. Proc. Natl. Acad. Sci. U. S. A 107, 21570–21575. 10.1073/pnas.1015379107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AJ, Veviurko G, Bennett NC, Hart DW, Mograby L, Lewin GR, 2021. Cultural transmission of vocal dialect in the naked mole-rat. Science 371, 503–507. 10.1126/science.abc6588. [DOI] [PubMed] [Google Scholar]
- Barone CM, Douma S, Reijntjes DOJ, Browe BM, Köppl C, Klump G, Park TJ, Pyott SJ, 2019. Altered cochlear innervation in developing and mature naked and Damaraland mole rats. J. Comp. Neurol 527, 2302–2316. 10.1002/cne.24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros RCH, Abe AS, Cárnio EC, Branco LGS, 2004. Regulation of breathing and body temperature of a burrowing rodent during hypoxic-hypercapnia. Comp. Biochem. Physiol. A. Mol. Integr. Physiol 138, 97–104. 10.1016/j.cbpb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Bartke A, Chandrashekar V, Dominici F, Turyn D, Kinney B, Steger R, Kopchick JJ, 2003. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology 4, 1–8. 10.1023/a:1022448532248. [DOI] [PubMed] [Google Scholar]
- Bennett NC, 1996. Reproductive suppression in subordinate, non-breeding female Damaraland mole-rats: two components to a lifetime of socially induced infertility. Proc. R. Soc. Lond. B Biol. Sci 263, 1599–1603. 10.1098/rspb.1996.0234. [DOI] [PubMed] [Google Scholar]
- Bennett NC, Faulkes CG, 2000. African Mole-Rats: Ecology and Eusociality. Cambridge University Press. [Google Scholar]
- Bennett NC, Jarvis JUM, 2004. Cryptomys damarensis. Mamm. Species 1–5. 10.1644/756. [DOI] [Google Scholar]
- Bennett NC, Ganswindt A, Ganswindt SB, Jarvis JUM, Zöttl M, Faulkes CG, 2018. Evidence for contrasting roles for prolactin in eusocial naked mole-rats, Heterocephalus glaber and Damaraland mole-rats, Fukomys damarensis. Biol. Lett 14, 20180150. 10.1098/rsbl.2018.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DF, Kilgore DL, Birchard GF, 1984. Respiratory physiology of burrowing mammals and birds. Comp. Biochem. Physiol. A Physiol 77, 1–7. 10.1016/0300-9629(84)90003-3. [DOI] [PubMed] [Google Scholar]
- Bolker J, 2012. Model organisms: there’s more to life than rats and flies. Nature 491, 31–33. 10.1038/491031a. [DOI] [PubMed] [Google Scholar]
- Brand A, Smith ESJ, Lewin GR, Park TJ, 2010. Functional neurokinin and NMDA receptor activity in an animal naturally lacking substance P: the naked mole-rat. PLoS One 5, e15162. 10.1371/journal.pone.0015162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branigan T, Elkhalifa S, Pamenter ME, 2018. Behavioural responses to environmental hypercapnia in two eusocial species of African mole rats. J. Comp. Physiol. A 204, 811–819. 10.1007/s00359-018-1283-z. [DOI] [PubMed] [Google Scholar]
- Buck LT, Pamenter ME, 2006. Adaptive responses of vertebrate neurons to anoxia–matching supply to demand. Respir. Physiol. Neurobiol 154, 226–240. 10.1016/j.resp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, 2005. The naked mole-rat: a new long-living model for human aging research. J. Gerontol. A Biol. Sci. Med. Sci 60, 1369–1377. 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, 2021. Colony-specific dialects of naked mole-rats. Science 371, 461–462. 10.1126/science.abf7962. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Yahav S, 1991. Is the naked mole-rat Hererocephalus glaber an endothermic yet poikilothermic mammal? J. Therm. Biol 16, 227–232. 10.1016/0306-4565(91)90030-6. [DOI] [Google Scholar]
- Buffenstein R, Park TJ, Holmes MM, 2021. The Extraordinary Biology of the Naked Mole-Rat. Springer. [Google Scholar]
- Burda H, šumbera R, Begall S, 2007. Microclimate in burrows of subterranean rodents — Revisited. In: Begall S, Burda H, Schleich CE (Eds.), Subterranean Rodents: News from Underground. Springer, Berlin, Heidelberg, pp. 21–33. 10.1007/978-3-540-69276-8_3. [DOI] [Google Scholar]
- Chakravarthy MV, Booth FW, 2004. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J. Appl. Physiol. Bethesda Md 1985 (96), 3–10. 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- Chan SJ, Episkopou V, Zeitlin S, Karathanasis SK, MacKrell A, Steiner DF, Efstratiadis A, 1984. Guinea pig preproinsulin gene: an evolutionary compromise? Proc. Natl. Acad. Sci. U. S. A 81, 5046–5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Lufkin T, 2011. The sound of silence: mouse models for hearing loss. Genet. Res. Int 2011, e416450 10.4061/2011/416450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke FM, Miethe GH, Bennett NC, 2001. Reproductive suppression in female Damaraland mole-rats Cryptomys damarensis : dominant control or self-restraint? Proc. R. Soc. Lond. B Biol. Sci 268, 899–909. 10.1098/rspb.2000.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney R, Bennett NC, 2000. Inbreeding avoidance and reproductive skew in a cooperative mammal. Proc. R. Soc. B Biol. Sci 267, 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MD, Herrin BR, 2010. How did our complex immune system evolve? Nat. Rev. Immunol 10, 2–3. 10.1038/nri2686. [DOI] [PubMed] [Google Scholar]
- dos Reis A, Dalmolin SP, Dallegrave E, 2017. Animal models for hearing evaluations: a literature review. Rev. CEFAC 19, 417–428. 10.1590/1982-021620171932117. [DOI] [Google Scholar]
- Dammann P, Burda H, 2007. Senescence patterns in African mole-rats (Bathyergidae, Rodentia). In: Begall S, Burda H, Schleich CE (Eds.), Subterranean Rodents: News from Underground. Springer, Berlin, Heidelberg, pp. 251–263. 10.1007/978-3-540-69276-8_18. [DOI] [Google Scholar]
- Dowling DP, Costanzo LD, Gennadios HA, Christianson DW, 2008. Evolution of the arginase fold and functional diversity. Cell. Mol. Life Sci. CMLS 65, 2039–2055. 10.1007/s00018-008-7554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton SB, Eaton SBI, Konner M, 1999. Paleolithic nutrition revisited. In: Evolutionary Medicine. Oxford University Press, pp. 313–332. [Google Scholar]
- Edrey YH, Hanes M, Pinto M, Mele J, Buffenstein R, 2011. Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical research. ILAR J. 52, 41–53. 10.1093/ilar.52.1.41. [DOI] [PubMed] [Google Scholar]
- Eigenbrod O, Debus KY, Reznick J, Bennett NC, Sánchez-Carranza O, Omerbaţić D, Hart DW, Barker AJ, Zhong W, Lutermann H, Katandukila JV, Mgode G, Park TJ, Lewin GR, 2019. Rapid molecular evolution of pain insensitivity in multiple African rodents. Science 364, 852–859. 10.1126/science.aau0236. [DOI] [PubMed] [Google Scholar]
- Ellison P, 2003. On Fertile Ground: A Natural History of Human Reproduction. Harvard University Press, Cambridge, MA. [Google Scholar]
- Fang J, Ma I, Allalunis-Turner J, 2011. Knockdown of cytoglobin expression sensitizes human glioma cells to radiation and oxidative stress. Radiat. Res 176, 198–207. 10.1667/rr2517.1. [DOI] [PubMed] [Google Scholar]
- Fang X, Seim I, Huang Z, Gerashchenko MV, Xiong Z, Turanov AA, Zhu Y, Lobanov AV, Fan D, Yim SH, Yao X, Ma S, Yang L, Lee S-G, Kim EB, Bronson RT, Sumbera R, Buffenstein R, Zhou X, Krogh A, Park TJ, Zhang G, Wang J, Gladyshev VN, 2014. Adaptations to a subterranean environment and longevity revealed by the analysis of mole rat genomes. Cell Rep. 8, 1354–1364. 10.1016/j.celrep.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkes CG, Bennett NC, 2016. Damaraland and naked mole-rats: Convergence of social evolution. In: Koenig WD, Dickinson JL (Eds.), Cooperative Breeding in Vertebrates. Cambridge University Press, Cambridge, pp. 338–352. 10.1017/CB09781107338357.020. [DOI] [Google Scholar]
- Faulkes CG, Trowell SN, Jarvis JUM, Bennett NC, 1994. Investigation of numbers and motility of spermatozoa in reproductively active and socially suppressed males of two eusocial African mole-rats, the naked mole-rat (Heterocephalus glaber) and the Damaraland mole-rat (Cryptomys damarensis). Reproduction 100, 411–416. 10.1530/jrf.0.1000411. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Verheyen E, Verheyen W, Jarvis JUM, Bennett NC, 2004. Phylogeographical patterns of genetic divergence and speciation in African mole-rats (family: Bathyergidae). Mol. Ecol 13, 613–629. 10.1046/j.1365-294x.2004.02099.x. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, 2015. Cancer resistance, high molecular weight hyaluronic acid, and longevity. J. Cell Commun. Signal 9, 91–92. 10.1007/sl2079-015-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Payseur BA, Pool JE, 2016. The power of natural variation for model organism biology. Trends Genet. TIG 32, 147–154. 10.1016/j.tig.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt P, Henning Y, Begall S, Malkemper EP, 2017. Audiograms of three subterranean rodent species (genus Fukomys) determined by auditory brainstem responses reveal extremely poor high-frequency hearing. J. Exp. Biol 220, 4377–4382. 10.1242/jeb.164426. [DOI] [PubMed] [Google Scholar]
- Gorbunova V, Takasugi M, Seluanov A, 2020. Hyaluronan goes to great length. Cell Stress 4, 227–229. 10.15698/cst2020.09.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M, 2010. Cancer stem cells: back to Darwin? Semin. Cancer Biol 20, 65–70. 10.1016/j.semcancer.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Harman D, 1972. Free radical theory of aging: dietary implications. Am. J. Clin. Nutr 25, 839–843. 10.1093/ajcn/25.8.839. [DOI] [PubMed] [Google Scholar]
- Healy K, 2015. Eusociality but not fossoriality drives longevity in small mammals. Proc. R. Soc. B Biol. Sci 282, 20142917. 10.1098/rspb.2014.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE, 1992. Hearing and sound localization in blind mole rats (Spalax ehrenbergi). Hear. Res 62, 206–216. 10.1016/0378-5955(92)90188-s. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE, 1993. Degenerate hearing and sound localization in naked mole rats (Heterocephalus glaber), with an overview of central auditory structures. J. Comp. Neurol 331, 418–433. 10.1002/cne.903310311. [DOI] [PubMed] [Google Scholar]
- Heinemann HO, Goldring RM, 1974. Bicarbonate and the regulation of ventilation. Am. J. Med 57, 361–370. 10.1016/0002-9343(74)90131-4. [DOI] [PubMed] [Google Scholar]
- Hislop MS, Buffenstein R, 1994. Noradrenaline induces nonshivering thermogenesis in both the naked mole-rat (Heterocephalus glaber) and the Damara mole-rat (Cryptomys damarensis) despite very different modes of thermoregulation. J. Therm. Biol 19, 25–32. 10.1016/0306-4565(94)90006-X. [DOI] [Google Scholar]
- Hodges NJ, Innocent N, Dhanda S, Graham M, 2008. Cellular protection from oxidative DNA damage by over-expression of the novel globin cytoglobin in vitro. Mutagenesis 23, 293–298. 10.1093/mutage/gen013. [DOI] [PubMed] [Google Scholar]
- Ivy CM, Sprenger RJ, Bennett NC, van Jaarsveld B, Hart DW, Kirby AM, Yaghoubi D, Storey KB, Milsom WK, Pamenter ME, 2020. The hypoxia tolerance of eight related African mole-rat species rivals that of naked mole-rats, despite divergent ventilatory and metabolic strategies in severe hypoxia. Acta Physiol. Oxf. Engl 228, el3436 10.1111/apha.13436. [DOI] [PubMed] [Google Scholar]
- Jacobs DS, Reid S, Kuiper S, 1998. Out-breeding behaviour and xenophobia in the damaraland mole-rat, Cryptomys damarensis. South Afr. J. Zool 33, 189–194. 10.1080/02541858.1998.11448470. [DOI] [Google Scholar]
- Jacobs PJ, Hart DW, Bennett NC, 2021. Plasma oxidative stress in reproduction of two eusocial African mole-rat species, the naked mole-rat and the Damaraland mole-rat. Front. Zool 18, 45. 10.1186/sl2983-021-00430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen JA, Lamberts SWJ, 2004. Igf-I and longevity. Horm. Res 62 (Suppl. 3), 104–109. 10.1159/000080508. [DOI] [PubMed] [Google Scholar]
- Jenkinson CP, Grody WW, Cederbaum SD, 1996. Comparative properties of arginases. Comp. Biochem. Physiol. B Biochem. Mol. Biol 114, 107–132. 10.1016/0305-0491(95)02138-8. [DOI] [PubMed] [Google Scholar]
- Johnston RA, Vullioud P, Thorley J, Kirveslahti H, Shen L, Mukherjee S, Karner CM, Clutton-Brock T, Tung J, 2021. Morphological and genomic shifts in mole-rat “queens” increase fecundity but reduce skeletal integrity. eLife 10, e65760. 10.7554/eLife.65760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain AA, Popow J, de la Fuente MA, Martinou J-C, Anderson P, Simarro M, 2017. The FASTK family of proteins: emerging regulators of mitochondrial RNA biology. Nucleic Acids Res. 45, 10941–10947. 10.1093/nar/gkx772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov WH, 1992. Daily energy expenditure and the cost of activity in mammals. Am. Zool 32, 238–248. 10.1093/icb/32.2.238. [DOI] [PubMed] [Google Scholar]
- Keller MC, Nesse RM, 2006. The evolutionary significance of depressive symptoms: different adverse situations lead to different depressive symptom patterns. J. Pers. Soc. Psychol 91, 316–330. 10.1037/0022-3514.91.2.316. [DOI] [PubMed] [Google Scholar]
- Kingwell K, 2019. Nav1.7 withholds its pain potential. Nat. Rev. Drug Discov 10.1038/d41573-019-00065-0. [DOI] [PubMed] [Google Scholar]
- Koka K, Jones HG, Thornton JL, Lupo JE, Tollin DJ, 2011. Sound pressure transformations by the head and pinnae of the adult Chinchilla (Chinchilla lanigera). Hear. Res 272, 135–147. 10.1016/j.heares.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer B, Buffenstein R, 2004. The pancreas of the naked mole-rat (Heterocephalus glaber): an ultrastructural and immunocytochemical study of the endocrine component of thermoneutral and cold acclimated animals. Gen. Comp. Endocrinol 139, 206–214. 10.1016/j.ygcen.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z, 2006. Comparison of endothelial function, O2.− and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am. J. Physiol. Heart Circ. Physiol 291, 7. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD, 2007. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell 6, 607–618. 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Lauer AM, Slee SJ, May BJ, 2011. Acoustic basis of directional acuity in laboratory mice. J. Assoc. Res. Otolaryngol 12, 633–645. 10.1007/sl0162-011-0279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVinka PC, Brand A, Landau VJ, Wirtshafter D, Park TJ, 2009. Extreme tolerance to ammonia fumes in African naked mole-rats: animals that naturally lack neuropeptides from trigeminal chemosensory nerve fibers. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol 195, 419–427. 10.1007/S00359-009-0420-0. [DOI] [PubMed] [Google Scholar]
- Lavulo LT, Sossong TM, Brigham-Burke MR, Doyle ML, Cox JD, Christianson DW, Ash DE, 2001. Subunit-subunit interactions in trimeric arginase. Generation of active monomers by mutation of a single amino acid. J. Biol. Chem 276, 14242–14248. 10.1074/jbc.M010575200. [DOI] [PubMed] [Google Scholar]
- Leedale AE, Thorley J, Clutton-Brock T, 2021. Odour-based social recognition in Damaraland mole-rats, Fukomys damarensis. Anim. Behav 179, 83–96. 10.1016/j.anbehav.2021.06.019. [DOI] [Google Scholar]
- Leonard WR, 2008. Lifestyle, diet, and disease: comparative perspectives on the determinants of chronic health risks. In: Evolution in Health and Disease, 2. Oxford University Press, pp. 265–276. [Google Scholar]
- Lewis KN, Andziak B, Yang T, Buffenstein R, 2013. The naked mole-rat response to oxidative stress: just deal with it. Antioxid. Redox Signal 19, 1388–1399. 10.1089/ars.2012.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Soifer I, Melamud E, Roy M, Mclsaac RS, Hibbs M, Buffenstein R, 2016. Unraveling the message: insights into comparative genomics of the naked mole-rat. Mamm. Genome off. J. Int. Mamm. Genome Soc 27, 259–278. 10.1007/S00335-016-9648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Chen XQ, Li W-J, Yang Y-H, Wang J-Z, Yu ACH, 2007. Cytoglobin upregulated by hydrogen peroxide plays a protective role in oxidative stress. Neurochem. Res 32, 1375–1380. 10.1007/s11064-007-9317-x. [DOI] [PubMed] [Google Scholar]
- Litman GW, Cooper MD, 2007. Why study the evolution of immunity? Nat. Immunol 8, 547–548. 10.1038/ni0607-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Hodish I, Haataja L, Lara-Lemus R, Rajpal G, Wright J, Arvan P, 2010. Proinsulin misfolding and diabetes: mutant INS gene-induced diabetes of youth. Trends Endocrinol. Metab. TEM 21, 652–659. 10.1016/j.tem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh SHY, Francescut L, Lingor P, Bähr M, Nicotera P, 2008. Identification of new kinase clusters required for neurite outgrowth and retraction by a loss-of-function RNA interference screen. Cell Death Differ. 15, 283–298. 10.1038/sj.cdd.4402258. [DOI] [PubMed] [Google Scholar]
- Lovegrove BG, 1986. The metabolism of social subterranean rodents: adaptation to aridity. Oecologia 69, 551–555. 10.1007/BF00410361. [DOI] [PubMed] [Google Scholar]
- Lovegrove BG, 1989. The cost of burrowing by the social mole rats (Bathyergidae) Cryptomys damarensis and Heterocephalus glaber: the role of soil moisture. Physiol. Zool 62, 449–469. 10.1086/physzool.62.2.30156179. [DOI] [Google Scholar]
- Lucas ER, Keller L, 2020. The co-evolution of longevity and social life. Funct. Ecol 34, 76–87. 10.1111/1365-2435.13445. [DOI] [Google Scholar]
- Lynn B, 1990. Capsaicin: actions on nociceptive C-fibres and therapeutic potential. Pain 41, 61–69. 10.1016/0304-3959(90)91110-5. [DOI] [PubMed] [Google Scholar]
- Magalhães JP, 2015. The big, the bad and the ugly: extreme animals as inspiration for biomedical research. EMBO Rep. 16, 771–776. 10.15252/embr.201540606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher B, 2009. Evolution: biology’s next top model? Nature 458, 695–698. 10.1038/458695a. [DOI] [PubMed] [Google Scholar]
- Mason MJ, Cornwall HL, Smith ESJ, 2016. Ear structures of the naked mole-rat, Heterocephalus glaber, and its relatives (Rodentia: Bathyergidae). PLoS One 11, e0167079. 10.1371/journal.pone.0167079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab BK, 1966. The metabolism of fossorial rodents: a study of convergence. Ecology 47, 712–733. 10.2307/1934259. [DOI] [Google Scholar]
- McPherson DR, 2018. Sensory hair cells: an introduction to structure and physiology. Integr. Comp. Biol 58, 282–300. 10.1093/icb/icy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça R, Vullioud P, Katlein N, Vallat A, Glauser G, Bennett NC, Helfenstein F, 2020. Oxidative costs of cooperation in cooperatively breeding Damaraland mole-rats. Proc. R. Soc. B Biol. Sci 287, 20201023. 10.1098/rspb.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo LMF, Pepper JW, Reid BJ, Maley CC, 2006. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 6, 924–935. 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- Miller RA, Williams JB, Kiklevich JV, Austad S, Harper JM, 2011. Comparative cellular biogerontology: primer and prospectus. Ageing Res. Rev 10, 181–190. 10.1016/j.arr.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteno AJ, Bennett NC, 2000. Anovulation in non-reproductive female Damaraland mole-rats (Cryptomys damarensis). J. Reprod. Fertil 119, 35–41. 10.1530/jrf.0.1190035. [DOI] [PubMed] [Google Scholar]
- Nesse R, 1999. What Darwinian medicine offers psychiatry. In: Evolutionary Medicine. Oxford University Press, pp. 351–374. [Google Scholar]
- Nesse RM, 2004. Natural selection and the elusiveness of happiness. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 359, 1333–1347. 10.1098/rstb.2004.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TP, Lee A, Jarvis JUM, Buffenstein R, 2002. Prolonged longevity in naked mole-rats: age-related changes in metabolism, body composition and gastrointestinal function. Comp. Biochem. Physiol. A. Mol. Integr. Physiol 133, 835–842. 10.1016/s1095-6433(02)00198-8. [DOI] [PubMed] [Google Scholar]
- Okanoya K, Yosida S, Barone CM, Applegate DT, Brittan-Powell EF, Dooling RJ, Park TJ, 2018. Auditory-vocal coupling in the naked mole-rat, a mammal with poor auditory thresholds. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol 204, 905–914. 10.1007/s00359-018-1287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuizen MK, 2020. Exploratory behaviour, memory and neurogenesis in the social Damaraland mole-rat (Fukomys damarensis). J. Exp. Biol jeb.221093 10.1242/jeb.221093. [DOI] [PubMed] [Google Scholar]
- Oosthuizen MK, Amrein I, 2016. Trading new neurons for status: adult hippocampal neurogenesis in eusocial Damaraland mole-rats. Neuroscience 324, 227–237. 10.1016/j.neuroscience.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Park TJ, Comer C, Carol A, Lu Y, Hong H-S, Rice FL, 2003. Somatosensory organization and behavior in naked mole-rats: II. Peripheral structures, innervation, and selective lack of neuropeptides associated with thermoregulation and pain. J. Comp. Neurol 465, 104–120. 10.1002/cne.10824. [DOI] [PubMed] [Google Scholar]
- Petruseva IO, Evdokimov AN, Lavrik OI, 2017. Genome stability maintenance in naked mole-rat. Acta Nat. 9, 31–41. [PMC free article] [PubMed] [Google Scholar]
- Pyott SJ, van Tuinen M, Screven LA, Schrode KM, Bai J-P, Barone CM, Price SD, Lysakowski A, Sanderford M, Kumar S, Santos-Sacchi J, Lauer AM, Park TJ, 2020. Functional, morphological, and evolutionary characterization of hearing in subterranean, eusocial African mole-rats. Curr. Biol 30, 4329–4341.e4. 10.1016/j.cub.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss S, Wystub S, Disque-Kaiser U, Hankeln T, Burmester T, 2016. Distribution of cytoglobin in the mouse brain. Front. Neuroanat 10, 47. 10.3389/fnana.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubner M, 1908. Das problem det lebensdaur und seiner beziehunger zum wachstum und ernarnhung. Oldenberg, Munich, Germany. [Google Scholar]
- Sabio G, Mora A, Rangel MA, Quesada A, Marcos CF, Alonso JC, Soler G, Centeno F, 2001. Glu-256 is a main structural determinant for oligomerisation of human arginase I. FEBS Lett. 501, 161–165. 10.1016/S0014-5793(01)02650-3. [DOI] [PubMed] [Google Scholar]
- Sackton TB, Clark N, 2019. Convergent evolution in the genomics era: new insights and directions. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 374, 20190102. 10.1098/rstb.2019.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm A, Bens M, Henning Y, Vole C, Groth M, Schwab M, Hoffmann S, Platzer M, Szafranski K, Dammann P, 2018. Higher gene expression stability during aging in long-lived giant mole-rats than in short-lived rats. Aging 10, 3938–3956. 10.18632/aging.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R, Lobarinas E, Chen G-D, Stolzberg D, Ding D, Radziwon K, Manohar S, 2021. Animal models of hearing loss, tinnitus, and Hyperacusis. In: Handbook of Laboratory Animal Science. CRC Press. [Google Scholar]
- Scantlebury M, Speakman JR, Oosthuizen MK, Roper TJ, Bennett NC, 2006. Energetics reveals physiologically distinct castes in a eusocial mammal. Nature 440, 795–797. 10.1038/nature04578. [DOI] [PubMed] [Google Scholar]
- Schmidt CM, Blount JD, Bennett NC, 2014. Reproduction is associated with a tissue-dependent reduction of oxidative stress in eusocial female Damaraland mole-rats (Fukomys damarensis). PLoS One 9, el03286. 10.1371/journal.pone.0103286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seluanov A, Hine C, Azpurua J, Feigenson M, Bozzella M, Mao Z, Catania KC, Gorbunova V, 2009. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc. Natl. Acad. Sci. U. S. A 106, 19352–19357. 10.1073/pnas.0905252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simarro M, Giannattasio G, De la Fuente MA, Benarafa C, Subramanian KK, Ishizawar R, Balestrieri B, Andersson EM, Luo HR, Orduna A, Boyce J, Anderson P, 2010. Fas-activated serine/threonine phosphoprotein promotes immune-mediated pulmonary inflammation. J. Immunol. Baltim. Md 1950 (184), 5325–5332. 10.4049/jimmunol.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Canseco DC, Manda SM, Shelton JM, Chirumamilla RR, Goetsch SC, Ye Q, Gerard RD, Schneider JW, Richardson JA, Rothermel BA, Mammen PPA, 2014. Cytoglobin modulates myogenic progenitor cell viability and muscle regeneration. Proc. Natl. Acad. Sci. U. S. A 111, E129–E138. 10.1073/pnas.l314962111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ESJ, Omerbaţić D, Lechner SG, Anirudhan G, Lapatsina L, Lewin GR, 2011. The molecular basis of acid insensitivity in the African naked mole-rat. Science 334, 1557–1560. 10.1126/science.1213760. [DOI] [PubMed] [Google Scholar]
- Smith ESJ, Schuhmacher L-N, Husson Z, 2015. The naked mole-rat as an animal model in biomedical research: current perspectives. Open Access Anim. Physiol 137 10.2147/OAAP.S50376. [DOI] [Google Scholar]
- Smith E, Park TJ, Lewin GR, 2020. Independent evolution of pain insensitivity in African mole-rats: origins and mechanisms. J. Comp. Physiol. A 206, 313–325. 10.1007/s00359-020-01414-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyman P, Jackson C, Bennett N, 2006. Do dispersing non-reproductive female Damaraland mole-rats, Cryptomys damarensis (Rodentia: Bathyergidae) exhibit spontaneous or induced ovulation? Physiol. Behav 87, 88–94. 10.1016/j.physbeh.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Selman C, McLaren JS, Harper EJ, 2002. Living fast, dying when? The link between aging and energetics. J. Nutr 132 10.1093/jn/132.6.1583S, 1583S–97S. [DOI] [PubMed] [Google Scholar]
- St John Smith E, Purfiirst B, Grigoryan T, Park TJ, Bennett NC, Lewin GR, 2012. Specific paucity of unmyelinated C-fibers in cutaneous peripheral nerves of the African naked-mole rat: comparative analysis using six species of Bathyergidae. J. Comp. Neurol 520, 2785–2803. 10.1002/cne.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC, 2012. Evolutionary medicine: its scope, interest and potential. Proc. Biol. Sci 279, 4305–4321. 10.1098/rspb.2012.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC, Ackermann M, Doebeli M, Kaiser M, 2000. Experimental evolution of aging, growth, and reproduction in fruitflies. Proc. Natl. Acad. Sci. U. S. A 97, 3309–3313. 10.1073/pnas.97.7.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, 2012. Evolutionary medicine and chronic inflammatory state-known and new concepts in pathophysiology. J. Mol. Med. Berk Ger 90, 523–534. 10.1007/s00109-012-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A, 2003. The endocrine regulation of aging by insulin-like signals. Science 299, 1346–1351. 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A, 2013. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499, 346–349. 10.1038/naturel2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov SN, Moens L, 2008. Diversity of globin function: enzymatic, transport, storage, and sensing. J. Biol. Chem 283, 8773–8777. 10.1074/jbc.R700029200. [DOI] [PubMed] [Google Scholar]
- Voigt C, Bennett NC, 2017. Gnrh mRNA expression in the brain of cooperatively breeding female Damaraland mole-rats. Reproduction 153, 453–460. 10.1530/REP-16-0471. [DOI] [PubMed] [Google Scholar]
- Voigt C, Bennett N, 2019. Reproductive status-dependent dynorphin and neurokinin B gene expression in female Damaraland mole-rats. J. Chem. Neuroanat 102, 101705 10.1016/j.jchemneu.2019.101705. [DOI] [PubMed] [Google Scholar]
- Voigt C, Gahr M, Leitner S, Lutermann H, Bennett N, 2014. Breeding status and social environment differentially affect the expression of sex steroid receptor and aromatase mRNA in the brain of female Damaraland mole-rats. Front. Zool 11, 38. 10.1186/1742-9994-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC, Nesse RM, 1991. The dawn of Darwinian medicine. Q. Rev. Biol 66, 1–22. 10.1086/417048. [DOI] [PubMed] [Google Scholar]
- Xu R, Harrison PM, Chen M, Li L, Tsui T, Fung PCW, Cheung P, Wang G, Li H, Diao Y, Krissansen GW, Xu S, Farzaneh F, 2006. Cytoglobin overexpression protects against damage-induced fibrosis. Mol. Ther. J. Am. Soc. Gene Ther 13, 1093–1100. 10.1016/j.ymthe.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Yamaza H, Chiba T, Higami Y, Shimokawa I, 2002. Lifespan extension by caloric restriction: an aspect of energy metabolism. Microsc. Res. Tech 59, 325–330. 10.1002/jemt.10212. [DOI] [PubMed] [Google Scholar]
- Yap KN, Wong HS, Ramanathan C, Rodriguez-Wagner CA, Roberts MD, Freeman DA, Buffenstein R, Zhang Y, 2022. Naked mole-rat and Damaraland mole-rat exhibit lower respiration in mitochondria, cellular and organismal levels. Biochimica et Biophysica Acta (BBA)-Bioenergetics 148582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Pamenter ME, 2019a. Ventilatory, metabolic, and thermoregulatory responses of Damaraland mole rats to acute and chronic hypoxia. J. Comp. Physiol. B 189, 319–334. 10.1007/s00360-019-01206-y. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Pamenter ME, 2019b. Fossorial Damaraland mole rats do not exhibit a blunted hypercapnic ventilatory response. Biol. Lett 15, 20190006. 10.1098/rsbl.2019.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wong HS, 2021. Are mitochondria the main contributor of reactive oxygen species in cells? J. Exp. Biol 224, jeb221606. 10.1242/jeb.221606. [DOI] [PubMed] [Google Scholar]
- Zhi F, Zhou G, Shao N, Xia X, Shi Y, Wang Q, Zhang Y, Wang R, Xue L, Wang S, Wu S, Peng Y, Yang Y, 2013. miR-106a-5p inhibits the proliferation and migration of astrocytoma cells and promotes apoptosis by targeting FASTK. PLoS One 8, e72390. 10.1371/journal.pone.0072390. [DOI] [PMC free article] [PubMed] [Google Scholar]


