Abstract
Purpose
Behavioral interventions have been used with breast cancer survivors (BCS) in cancer pain management and post-treatment quality of life (QOL) studies. We studied the effects of an anti-inflammatory dietary intervention on QOL in BCS.
Methods
One hundred fifty-three overweight and obese (body mass index [BMI] ≥ 25 kg/m2), early stage (0-III), English-speaking BCS who had completed all cancer treatment 2 or more months prior to enrollment were recruited into a two-arm randomized controlled trial with a 2 (group) by 3 (time) repeated measures design. Intervention components included six monthly food-preparation workshops and twelve motivational interviewing telephone calls. Endpoints included the Perceived Stress Scale (PSS), the Functional Assessment of Cancer Therapy–General (FACT-G) and Breast Cancer (FACT-B), and the Center for Epidemiologic Studies Depression Scale (CES-D). Repeated measures analysis using PROC MIXED in SAS version 9.4 was used.
Results
On repeated measures analysis (intent to treat), there were no differences between groups on any of the QOL outcomes except the PSS total scores. These were significantly different in the intervention group (IG; n = 76) compared to control group (CG; n = 77), showing a main effect of assignment but no effect of time and no interaction effects.
Conclusion
There was an impact on QOL as measured by the PSS between groups. The intervention reduced perceived stress at 6-month follow-up, but the effects dissipated by 12 months. Sources and stress and stress reduction should be a focus of future studies. Future research should also identify appropriate QOL measures that are sensitive to changes brought about by behavioral interventions.
Keywords: Health-related quality of life, Mediterranean diet, Perceived Stress Scale
Introduction
The breast is the leading site of new cancer cases among US women, and the second leading cause of cancer death, according to 2021 estimates [1]. However, survival has increased due to improvements in both treatment and detection. Over 3.8 million American women were breast cancer survivors as of January 1, 2019 [2]. These women experience a plethora of symptoms as part of subclinical and clinical syndromes including chronic fatigue, depression, anxiety, and stress related to fear of cancer recurrence [3]. Challenging physical and mental health symptoms can negatively impact quality of life (QOL) and cancer prognosis [4–8].
Multiple evidence-based approaches have been developed to address these issues. Some have focused on increasing physical activity using the cardiac rehabilitation model [9]. Others have employed cognitive behavioral therapy [3] and naturopathic oncology complementary and alternative medicine [10]. Motivational interviewing (MI) has also been used, both in person [11] and via telephone [12, 13].
Nutrition intervention studies have largely focused on personalized diets recommended by a trained dietitian [14, 15]. Little has been published on the association of diet and QOL, and results have been mixed. A systematic review of breast cancer survivor interventions could not provide an overall estimate of effects on QOL due to the mixed nature of the studies (diet, physical activity, and weight loss) [16]. The ketogenic diet had short-term (6-week) positive effects on global QOL in a randomized controlled trial of Iranian breast cancer patients undergoing chemotherapy. The study was done using the validated European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) [17]. A literature review of the Mediterranean diet concluded that it could reduce breast cancer recurrence and improve mood and depressive symptoms among postmenopausal women [18].
This study used an anti-inflammatory nutritional intervention focused on education in and adherence to elements of the Mediterranean diet to promote and help maintain behavioral change in dietary habits among a group of ethnically mixed breast cancer survivors. Our hypothesis was that adherence to the intervention would alter inflammatory cytokine levels and improve QOL. There is corroboration for this hypothesis and evidence for this effect of the Mediterranean diet specifically on depression in a recent narrative review [19]. Positive effects of the intervention on diet adherence and caloric intake have been previously published [20], and a manuscript on cytokine outcomes is in development. In this paper we explored the intervention’s effect on QOL outcomes.
Although multiple general QOL instruments are available (e.g., the Short Form SF-36 and its derivative SF-12) [21], we chose the Functional Assessment of Cancer Therapy (FACT) scales developed by Cella and colleagues[22]. These include the 27-item FACT-General (FACT-G), one of the most widely used validated instruments assessing overall response to cancer treatment and survivorship [23, 24]; and the FACT-B, which includes all domains of FACT-G and adds a breast-cancer specific domain [25, 26]. FACT-B has been used to measure QOL-associated factors in multiple ethnic groups [6, 27, 28]. We also used the Perceived Stress Scale (PSS) [29], the Cancer Worry Scale (CWS) [30], and the Depression Scale of the CES-D [31, 32] due to the impact of these factors on BCS QOL [6, 7].
Methods
The study design and outcomes associated with the dietary intervention have been described in detail elsewhere [20, 33]. Briefly, 153 overweight and obese (BMI ≥ 25 kg/m2), early-stage (0-III), English-speaking breast cancer survivors who had completed their treatment 2 or more months prior to enrollment were recruited to a two-arm randomized controlled trial (RCT) with a 2 (group) by 3 (time) repeated measures design. Participants were randomized to intervention (IG; n = 76) and control (CG; n = 77) groups. IG participants were invited to participate in six monthly workshops that included culinary demonstrations, recipes, and meal planning, and 12 monthly motivational interviewing (MI) telephone calls. They also received monthly tailored newsletters personalized to individual readiness for change. CG participants received monthly American Institute for Cancer Research informational brochures and telephone calls (non-MI) prior to assessment appointments only. All participants completed questionnaires including items on cancer diagnosis, stage, and treatment data; depression, self-efficacy, and cancer worry; and the FACT-G and FACT-B scales, as well as the Perceived Stress Scale and a reduced version of the Center of Epidemiological Studies depression assessment (CES-D). This battery of instruments was repeated at baseline, 6, and 12 months.
The FACT-G includes domains of physical, functional, social, and emotional well-being scored on a Likert-type scale ranging from 0 (not at all) to 4 (very much). The FACT-B contains all FACT-G domains plus a breast cancer-specific domain. Each domain is scored by adding individual item scores then multiplying by the number of items in the domain. The total score for the instrument is obtained by summing the domain scores. Higher scores equate to better QOL [34].
The PSS is rated on a 5-point Likert scale ranging from 0 (never) to 4 (very often). The scores ranging from 0 to 18 represent low stress, 19–37 represent moderate stress, and 38–56 represent high stress [29, 35].
The CES-D’s usual scoring is a simple sum of item weights. A higher score indicates greater frequency and number of depression symptoms [32, 36].
MI telephone calls were conducted by research staff trained in MI by a certified member of the Motivational Interviewing Network of Trainers [37]. Calls occurred after each workshop for the first 6 months of the study, then approximately monthly thereafter. Research staff recorded all questionnaire and telephone call responses in the Research Electronic Data Capture (REDCap) system, a web-based survey administration and data collection service developed at Vanderbilt University and housed in the Department of Population Health Sciences at UT Health San Antonio [38, 39].
Statistical analyses
Data were exported from REDCap into SAS and cleaned. Univariate and bivariate statistical tests were run using SAS version 9.4 (Cary, NC) to determine differences between the intervention and control groups on the various QOL instruments. T-tests were used to explore differences on continuous measures between the intervention and control groups. Differences between intervention and control groups and across timepoints were examined by repeated measures analyses using PROC MIXED in SAS [40]. Repeated measures analysis is used when there are many subjects but the number of measurement points in time on any individual subject is not large. PROC MIXED is robust for the analysis of longitudinal data as it uses all available data and does not enact case deletion if data for any one time point is missing. The covariance structure was modeled for correlations among measurements made on the same subject using a random effects statement.
Results
Participant baseline characteristics have been described elsewhere [33]. Briefly, women had a mean age of 56 years, were predominantly White (43%) and Latino (~ 41%), had a college degree (36%) and private insurance (~ 80%), and had an income of $2000 or more per month (76%) (Table 1). There were no significant differences between groups on demographic characteristics. Baseline scores on the quality of life (QOL) outcome measures comparing the intervention and control groups, including means and standard deviations, can be found in Table 2. There were no differences at baseline on any of the QOL measures, including the sub-scales of the FACT-B and FACT-G, PSS, or CES-D. There was some attrition (loss to follow-up) from baseline to 6-month and 12-month follow-up, with most loss to follow-up occurring between baseline and 6-month follow-up (Fig. 1).
Table 1.
Demographics of treatment and control groups
| Variables | All (n = 153) n (%) | Intervention (n = 76) n (%) | Control (n = 77) n (%) |
|---|---|---|---|
|
| |||
| Age (years) | |||
| Below 50 | 36 (23.53%) | 19 (25.00%) | 17 (22.08%) |
| 50–60 | 53 (34.64%) | 31 (40.79%) | 22 (28.57%) |
| Above 60 | 63 (41.18%) | 25 (32.89%) | 38 (49.35%) |
| Missing | 1 (0.65%) | 1 (1.32%) | - |
| Ethnicity | |||
| Black or African American | 3 (2.00%) | 1 (1.30%) | 2 (2.60%) |
| US Latino | 63 (41.20%) | 29 (38.20%) | 34 (44.20%) |
| Other Latino (Mexican, Cuban, etc.) | 16 (10.50%) | 10 (13.20%) | 6 (7.80%) |
| Anglo | 66 (43.10%) | 33 (43.40%) | 33 (42.90%) |
| Native American | 2 (1.30%) | 2 (2.60%) | 0 (0.00%) |
| Asian | 1 (0.70%) | 1 (1.30%) | 0 (0.00%) |
| Other | 2 (1.30%) | 0 (0.00%) | 2 (2.60%) |
| Monthly income | |||
| Under $1500 | 11 (7.30%) | 5 (6.50%) | 6 (7.80%) |
| $1501–$2000 | 21 (13.70%) | 9 (11.80%) | 12 (15.60%) |
| Over $2,000 | 116 (75.80%) | 60 (78.90%) | 56 (72.70%) |
| Refused | 5 (3.30%) | 2 (2.60%) | 3 (3.90%) |
| Insurance type | |||
| Private insurance | 123 (80.90%) | 64 (85.30%) | 59 (76.60%) |
| Medicaid/other “safety net” assistance | 24 (15.80%) | 9 (12.00%) | 15 (19.50%) |
| No insurance—everything out of pocket | 5 (3.30%) | 2 (2.7%) | 3 (3.90%) |
| Missing | 1 (0.65%) | 1 (1.32%) | 0 (0.00%) |
Table 2.
Baseline scores on quality-of-life outcomes across treatment and control groups
| Variables | Intervention (n = 76) Mean (SD) | Control (n = 77) Mean (SD) | P |
|---|---|---|---|
|
| |||
| Perceived Stress Scale | 21.77 (7.63) | 19.75 (7.60) | .10 |
| Social Well-Being (SWB) sub-scale | 21.02 (4.94) | 20.90 (5.62) | .88 |
| Emotional Well-Being (EWB) sub-scale | 20.05 (3.30) | 20.37 (3.54) | .56 |
| Functional Well-Being (FWB) sub-scale | 20.81 (5.26) | 21.06 (5.53) | .77 |
| Physical Well-Being (PWB) sub-scale | 23.38 (4.23) | 23.23 (4.31) | .82 |
| FACT-G | 85.27 (13.25) | 85.57 (15.06) | .89 |
| BCS* | 24.01 (5.32) | 23.98 (5.80) | .97 |
| TOI** | 68.21 (12.21) | 68.28 (12.71) | .97 |
| CES-D | 3.07 (2.92) | 2.33 (2.40) | .08 |
BCS, Breast Cancer Scale;
TOI, Trial Outcome Index = PWB + FWB + BCS; FACTG = PWB + SWB + EWB + FWB. FACT-B encompasses FACT-G + BCS
Fig. 1.
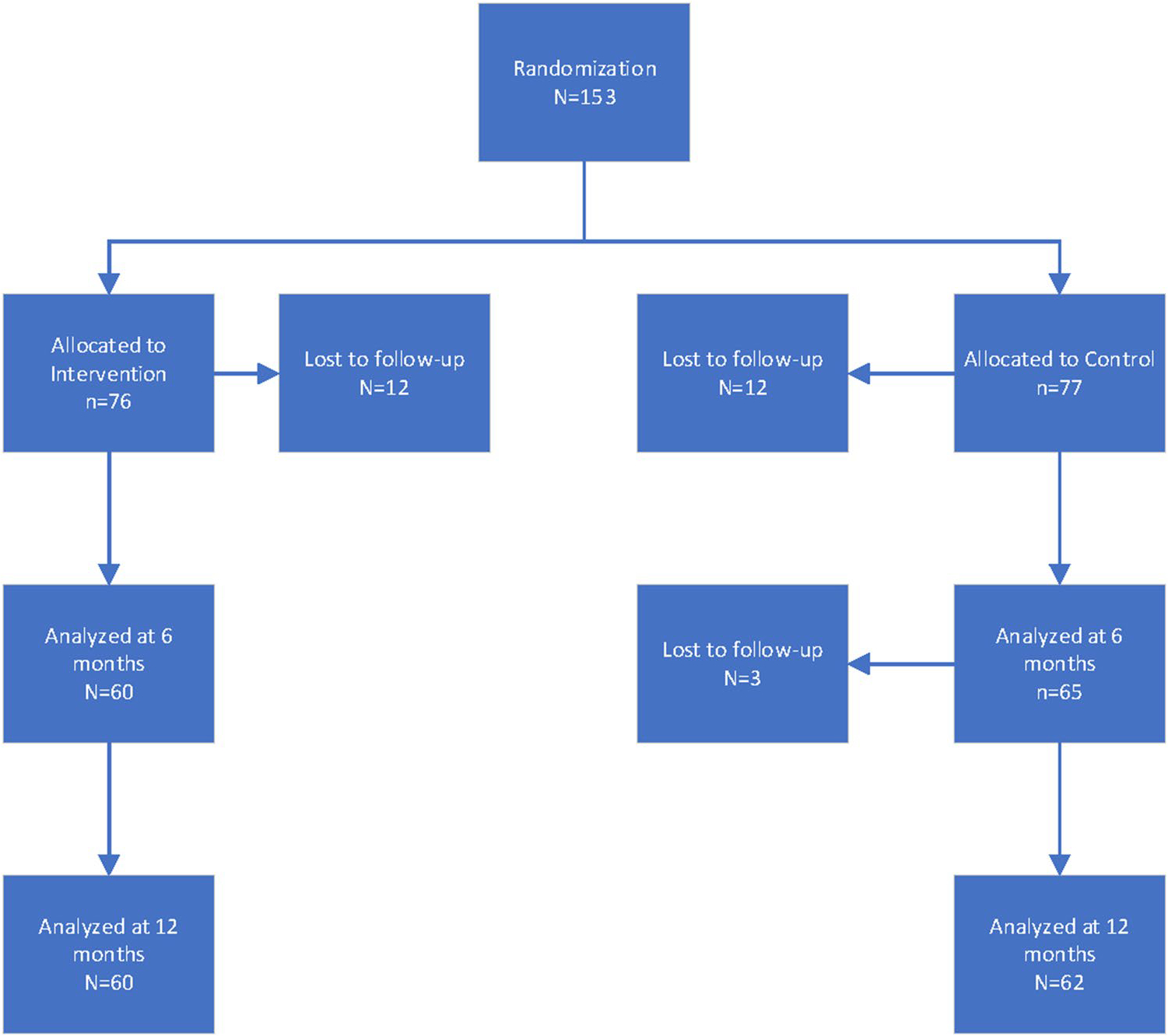
Study flow diagram
The results of the repeated measures analysis found that one outcome variable, the total score from the Perceived Stress Scale, showed a statistically significant main effect of group assignment F(1, 385) = 5.49, p = 0.019, but no main effect of time across the three data collection points (baseline, 6-month follow-up, 12-month follow-up), F (2, 385) = 0.03, p = 0.97, and no interaction between time and group assignment, F (2, 385) = 0.45, p = 0.63 (Fig. 2). No other significant findings were noted for the remaining QOL variables, total and sub-scale scores (Table 3).
Fig. 2.
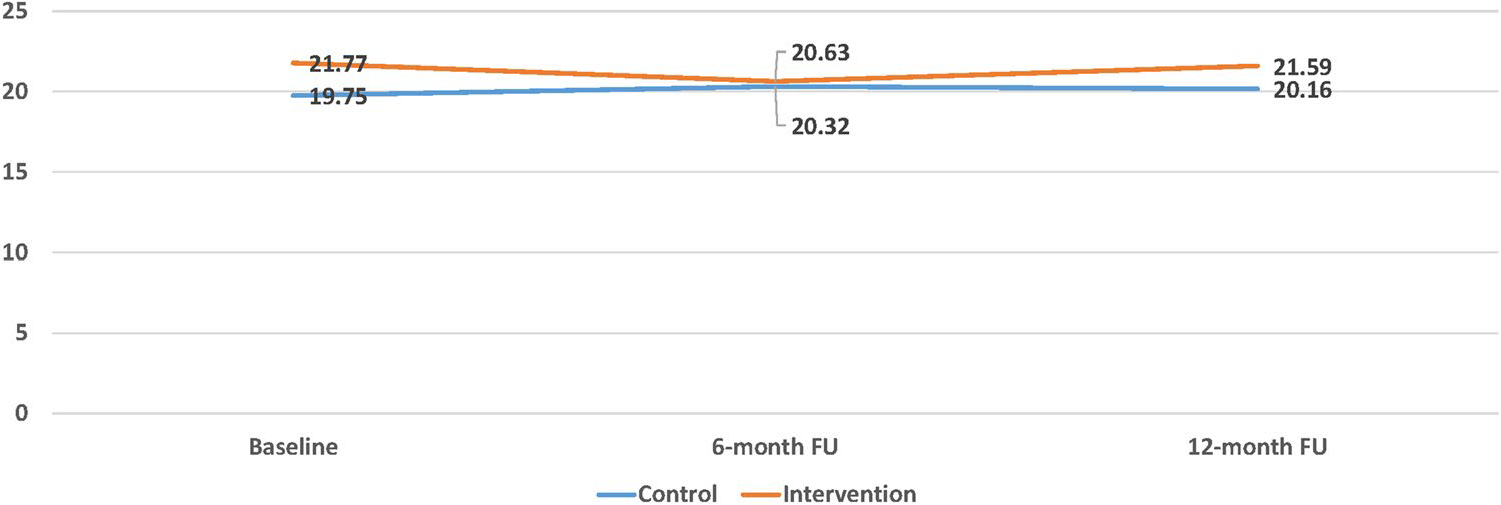
Perceived stress total score: main effect of group assignment F(1, 385) = 5.49, p = .019, but no main effect of time across baseline, 6-month follow-up, and 12-month follow-up, F (2, 385) = .03, p = .97, and no interaction between time and group assignment, F (2, 385) = .45, p = .63
Table 3.
Linear mixed model main effect results of quality-of-life outcomes across treatment and control groups, 6-month and 12-month follow-ups
| Variables | Intervention (n = 76) Mean (SD) | Control (n = 77) Mean (SD) | p |
|---|---|---|---|
|
| |||
| Perceived Stress Scale | |||
| 6-month follow-up | 20.64 (7.61) | 20.32 (8.31) | |
| 12-month follow-up | 21.59 (7.44) | 20.01 (8.23) | .01 |
| Social Well-Being (SWB) sub-scale | |||
| 6-month follow-up | 20.96 (5.37) | 20.54 (5.94) | |
| 12-month follow-up | 20.77 (5.15) | 20.46 (6.17) | .77 |
| Emotional Well-Being (EWB) sub-scale | |||
| 6-month follow-up | 20.91 (2.75) | 19.97 (3.67) | |
| 12-month follow-up | 20.22 (3.23) | 19.76 (3.98) | .76 |
| Functional Well-Being (FWB) sub-scale | |||
| 6-month follow-up | 21.76 (4.29) | 20.71 (5.24) | |
| 12-month follow-up | 20.60 (4.68) | 20.78 (5.42) | .98 |
| Physical Well-Being (PWB) sub-scale | |||
| 6-month follow-up | 24.13 (3.91) | 23.25 (4.42) | |
| 12-month follow-up | 23.60 (4.09) | 23.57 (4.13) | .62 |
| FACT-G | |||
| 6-month follow-up | 87.96 (12.48) | 84.47 (15.81) | |
| 12-month follow-up | 85.21 (13.38) | 84.57 (16.42) | .41 |
| BCS* | |||
| 6-month follow-up | 25.01 (5.38) | 24.15 (5.86) | |
| 12-month follow-up | 24.77 (5.34) | 24.31 (6.37) | .82 |
| TOI** | |||
| 6-month follow-up | 71.11 (11.32) | 68.12 (12.67) | |
| 12-month follow-up | 68.98 (11.51) | 68.67 (13.60) | .40 |
| CES-D | |||
| 6-month follow-up | 2.45 (2.18) | 2.65 (2.39) | |
| 12-month follow-up | 2.85 (2.74) | 2.88 (2.70) | .51 |
BCS, Breast Cancer Scale;
TOI, Trial Outcome Index = PWB + FWB + BCS; FACTG = PWB + SWB + EWB + FWB. FACT-B encompasses FACTG + BCS
Discussion
This study reported on the quality of life (QOL) outcomes assessed during a dietary intervention among breast cancer survivors. Baseline characteristics of participants and outcomes associated with the dietary intervention have been previously reported. The analysis revealed a reduction in perceived stress in the intervention group at 6-month follow-up; however, the effect had dissipated by the 12-month follow-up. None of the other quality of life indicators such as the FACT-G and FACT-B were significant.
Other randomized trials found differential effects when using the sub-scales and total scores of the FACT-G and FACT-B. A study of diet and exercise among breast cancer patients undergoing chemotherapy found no differences on either the FACT-G or the FACT-B [41]. Similarly, Thomas et al. found significant differences on one instrument but not the other [42]. The PSS has been successfully used with other samples of breast cancer patients and survivors, and the instrument has proven to be sensitive to assessment of perceived stress and reduction of perceived stress in a number of intervention studies [43–45].
One possible explanation for the findings related to QOL outcomes may be the construction of the intervention itself. The intervention group received an intense combination of food preparation and informational workshops during the first 6 months of the trial, motivational telephone interviewing, and monthly newsletters. Telephone contacts were structured such that they occurred after each of the monthly in-person workshops; after the first 6 months, no workshops were held and telephone contacts were either much shorter in duration or did not occur at all (e.g., participant did not pick up the telephone when the call was placed). Hence, any observable effects would probably have shown up at the 6-month follow-up, which is what occurred with the perceived stress scale, but not the other quality of life measures. It is also possible that some of the measures (FACT-G, FACT-B) are simply not sensitive enough to pick up changes from behavioral interventions such as the one employed in this trial. Other studies of populations with cancer and other long-term conditions have also found a decreased effect of interventions on some QOL measures at longer timepoints. A rapid systematic review demonstrated that psychological interventions aiming to improve QOL may not sustain physical QOL benefits over long-term follow-up of 12 months or more in patients with HIV and medically unexplained symptoms [46]. Likewise, a study of advanced prostate cancer survivors showed attenuated anxiety-reducing effects, as measured by the Memorial Anxiety Scale for Prostate Cancer, of an intervention at 12 months compared to 6-month follow-up [47]. A randomized controlled trial of men with advanced prostate cancer showed no effect on FACT-G scores at 12 months following two computer-based interventions [48].
Despite the fact our study was designed to achieve 90% power to detect a significant group-by-time interaction with α = 0.05 for all outcomes of interest, we ended with less than our initially estimated group sizes at 6- and 12-month follow-up. This may have affected our capacity to detect significant differences in QOL variables in our sample.
It is not possible to determine the exact nature of the reduction in stress occurring in the intervention. It could be that it was related to the participants’ feeling of empowerment from the intervention in that it provided guidance and hands on workshops to increase their intake of anti-inflammatory foods; this could have lowered feelings of stress related either to their previous diagnosis of breast cancer, stress related to body weight (as participants met criteria for overweight/obesity), some combination of both, or some other source of stress. However, exploration of the Cancer Worry Scale data shows that, for both groups, cancer worry decreased over time.
In conclusion, this study found a reduction in perceived stress as measured by the PSS among intervention participants. Future research should identify appropriate quality of life measures that are sensitive to changes brought about by behavioral interventions.
Acknowledgements
We thank Savitri Singh-Carlson, RN, PhD, FAAN, and Dennis G. Fisher, PhD, for their contributions to an earlier version of this manuscript.
Funding
This research was supported by Susan G. Komen for the Cure (SAB08-0005); the National Cancer Institute (P20 CA165589); and the Mays Cancer Center at UT Health San Antonio, through the NCI Cancer Center Support Grant (P30 CA054174).
Footnotes
Conflicts of interest The authors declare no competing interests.
Code availability Not applicable
Ethics approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate Informed consent was obtained from all individual participants included in the study.
Consent for publication Not applicable.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.American Cancer Society. Cancer facts and figures 2021. 2021. 04/01/2021]; Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
- 2.American Cancer Society. Breast cancer facts and figures 2019–2020. 2019. 04/01/2021]; Available from: https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html
- 3.Ye M, Du K, Zhou J, Zhou Q, Shou M, Hu B et al. (2018) A meta-analysis of the efficacy of cognitive behavior therapy on quality of life and psychological health of breast cancer survivors and patients. Psychooncology 27(7):1695–1703. 10.1002/pon.4687 [DOI] [PubMed] [Google Scholar]
- 4.Henneghan A (2016) Modifiable factors and cognitive dysfunction in breast cancer survivors: a mixed-method systematic review. Support Care Cancer 24(1):481–497. 10.1007/s00520-015-2927-y [DOI] [PubMed] [Google Scholar]
- 5.Jordan JH, Thwin SS, Lash TL, Buist DS, Field TS, Haque R et al. (2014) Incident comorbidities and all-cause mortality among 5-year survivors of stage I and II breast cancer diagnosed at age 65 or older: a prospective-matched cohort study. Breast Cancer Res Treat 146(2):401–409. 10.1007/s10549-014-3021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine EG, Yoo G, Aviv C (2017) Predictors of quality of life among ethnically diverse breast cancer survivors. Appl Res Qual Life 12(1):1–16. 10.1007/s11482-016-9447-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syrowatka A, Motulsky A, Kurteva S, Hanley JA, Dixon WG, Meguerditchian AN et al. (2017) Predictors of distress in female breast cancer survivors: a systematic review. Breast Cancer Res Treat 165(2):229–245. 10.1007/s10549-017-4290-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu AH, Kurian AW, Kwan ML, John EM, Lu Y, Keegan TH et al. (2015) Diabetes and other comorbidities in breast cancer survival by race/ethnicity: the California Breast Cancer Survivorship Consortium (CBCSC). Cancer Epidemiol Biomarkers Prev 24(2):361–368. 10.1158/1055-9965.Epi-14-1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolan LB, Barry D, Petrella T, Davey L, Minnes A, Yantzi A et al. (2018) The cardiac rehabilitation model improves fitness, quality of life, and depression in breast cancer survivors. J Cardiopulm Rehabil Prev 38(4):246–252. 10.1097/HCR.0000000000000256 [DOI] [PubMed] [Google Scholar]
- 10.Andersen MR, Sweet ES, Standish L (2018) Involvement in decision-making, and use of integrative oncology improve breast cancer survivor Hrqol in early survivorship. Ann Behav Med 52:S499–S499 [Google Scholar]
- 11.Kvale EA, Huang CS, Meneses KM, Demark-Wahnefried W, Bae S, Azuero CB et al. (2016) Patient-centered support in the survivorship care transition: outcomes from the Patient-Owned Survivorship Care Plan Intervention. Cancer 122(20):3232–3242. 10.1002/cncr.30136 [DOI] [PubMed] [Google Scholar]
- 12.Spector D, Deal AM, Amos KD, Yang H, Battaglini CL (2014) A pilot study of a home-based motivational exercise program for African American breast cancer survivors: clinical and quality-of-life outcomes. Integr Cancer Ther 13(2):121–132. 10.1177/1534735413503546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun A, Portner J, Grainger EM, Hill EB, Young GS, Clinton SK et al. (2018) Tele-motivational interviewing for cancer survivors: feasibility, preliminary efficacy, and lessons learned. J Nutr Educ Behav 50(1):19–32 e1. 10.1016/j.jneb.2017.05.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keaver L, Yiannakou I, Zhang FF (2020) Integrating nutrition into outpatient oncology care-a pilot trial of the NutriCare program. Nutrients 12(11):3590. 10.3390/nu12113590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Souza APS et al. (2021) Nutritional Intervention contributes to the improvement of symptoms related to quality of life in breast cancer patients undergoing neoadjuvant chemotherapy: a randomized clinical trial. Nutrients 13(2):589. 10.3390/nu1302058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barchitta M, Maugeri A, Magnano San Lio R, Quattrocchi A, Degrassi F, Catalano F et al. (2020) The effects of diet and dietary interventions on the quality of life among breast cancer survivors: a cross-sectional analysis and a systematic review of experimental studies. Cancers (Basel) 12(2):322. 10.3390/cancers12020322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khodabakhshi A, Seyfried TN, Kalamian M, Beheshti M, Davoodi SH (2020) Does a ketogenic diet have beneficial effects on quality of life, physical activity or biomarkers in patients with breast cancer: a randomized controlled clinical trial. Nutr J 19(1):87. 10.1186/s12937-020-00596-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cano A, Marshall S, Zolfaroli I, Bitzer J, Ceausu I, Chedraui P et al. (2020) The Mediterranean diet and menopausal health: an EMAS position statement. Maturitas 139:90–97. 10.1016/j.maturitas.2020.07.001 [DOI] [PubMed] [Google Scholar]
- 19.Pano O, Martínez-Lapiscina EH, Sayón-Orea C, Martinez-Gonzalez MA, Martinez JA, Sanchez-Villegas A (2021) Healthy diet, depression and quality of life: a narrative review of biological mechanisms and primary prevention opportunities. World J Psychiatry 11(11):997–1016. 10.5498/wjp.v11.i11.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuniga KE, Parma DL, Munoz E, Spaniol M, Wargovich M, Ramirez AG (2019) Dietary intervention among breast cancer survivors increased adherence to a Mediterranean-style, anti-inflammatory dietary pattern: the Rx for Better Breast Health Randomized Controlled Trial. Breast Cancer Res Treat 173(1):145–154. 10.1007/s10549-018-4982-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treanor C, Donnelly M (2015) A methodological review of the Short Form Health Survey 36 (SF-36) and its derivatives among breast cancer survivors. Qual Life Res 24(2):339–362. 10.1007/s11136-014-0785-6 [DOI] [PubMed] [Google Scholar]
- 22.Cella DF, Lent L, Bredle J, Arnold B, Pickard L and Romo S. Functional Assessment of Chronic Illness Therapy (FACIT) system overview. 2020. 04-08-2019 03/24/2020]; Available from: https://www.facit.org/FACITOrg/Overview
- 23.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A et al. (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11(3):570–579 [DOI] [PubMed] [Google Scholar]
- 24.Crew KD, Capodice JL, Greenlee H, Brafman L, Fuentes D, Awad D et al. (2010) Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol 28(7):1154–1160. 10.1200/jco.2009.23.4708 [DOI] [PubMed] [Google Scholar]
- 25.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR et al. (1997) Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 15(3):974–986. 10.1200/jco.1997.15.3.974 [DOI] [PubMed] [Google Scholar]
- 26.Hamer J, McDonald R, Zhang L, Verma S, Leahey A, Ecclestone C et al. (2017) Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support Care Cancer 25(2):409–419. 10.1007/s00520-016-3417-6 [DOI] [PubMed] [Google Scholar]
- 27.Graves KD, Jensen RE, Canar J, Perret-Gentil M, Leventhal KG, Gonzalez F et al. (2012) Through the lens of culture: quality of life among Latina breast cancer survivors. Breast Cancer Res Treat 136(2):603–613. 10.1007/s10549-012-2291-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janz NK, Friese CR, Li Y, Graff JJ, Hamilton AS, Hawley ST (2014) Emotional well-being years post-treatment for breast cancer: prospective, multi-ethnic, and population-based analysis. J Cancer Surviv 8(1):131–142. 10.1007/s11764-013-0309-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R (1983) A global measure of perceived stress. J Health Soc Behav 24(4):385–396 [PubMed] [Google Scholar]
- 30.Custers JA, van den Berg SW, van Laarhoven HW, Bleiker EM, Gielissen MF, Prins JB (2014) The Cancer Worry Scale: detecting fear of recurrence in breast cancer survivors. Cancer Nurs 37(1):E44–50. 10.1097/NCC.0b013e3182813a17 [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1(3):385–401 [Google Scholar]
- 32.Radloff LS (1991) The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. J Youth Adolesc 20(2):149–166. 10.1007/BF01537606 [DOI] [PubMed] [Google Scholar]
- 33.Ramirez AG, Parma DL, Munoz E, Mendoza KD, Harb C, Holden AEC et al. (2017) An anti-inflammatory dietary intervention to reduce breast cancer recurrence risk: Study design and baseline data. Contemp Clin Trials 57:1–7. 10.1016/j.cct.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FACIT.org. Funcional Assessment of Cancer Therapy-Breast. 2022. 01–07–22]; Available from: https://wizard.facit.org/index.php?option=com_facit&view=search&searchPerformed=1 [Google Scholar]
- 35.Ali S, Tauqir S, Farooqi FA, Al-Jandan B, Al-Janobi H, Alshehry S et al. (2021) Psychological Impact of the COVID-19 pandemic on students, assistants, and faculty of a Dental Institute of Saudi Arabia. Int J Environ Res Public Health 18(24):13366. 10.3390/ijerph182413366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turvey CL, Schultz SK, Beglinger L, Klein DM (2009) A longitudinal community-based study of chronic illness, cognitive and physical function, and depression. Am J Geriatr Psychiatry 17(8):632–641. 10.1097/jgp.0b013e31819c498c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motivational Interviewing Network of Trainers. Motivational Interviewing Network of Trainers. 2016. 03/23/2016]; Available from: http://www.motivationalinterviewing.org/
- 38.Obeid JS, McGraw CA, Minor BL, Conde JG, Pawluk R, Lin M et al. (2013) Procurement of shared data instruments for Research Electronic Data Capture (REDCap). J Biomed Inform 46(2):259–265. 10.1016/j.jbi.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Littell RC, Milliken GA, Stroup WW, Schabenberger O and Wolfinger RD. SAS for mixed models. 2nd ed. 2006, Cary, NC: SAS Institute, Inc. [Google Scholar]
- 41.Djuric Z, Ellsworth JS, Weldon AL, Ren J, Richardson CR, Resnicow K et al. (2011) A diet and exercise intervention during chemotherapy for breast cancer. Open Obes J 3:87–97. 10.2174/1876823701103010087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas ML, Elliott JE, Rao SM, Fahey KF, Paul SM, Miaskowski C (2012) A randomized, clinical trial of education or motivational-interviewing-based coaching compared to usual care to improve cancer pain management. Oncol Nurs Forum 39(1):39–49. 10.1188/12.ONF.39-49 [DOI] [PubMed] [Google Scholar]
- 43.Golden-Kreutz DM, Browne MW, Frierson GM, Andersen BL (2004) Assessing stress in cancer patients: a second-order factor analysis model for the Perceived Stress Scale. Assessment 11(3):216–223. 10.1177/1073191104267398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golden-Kreutz DM, Thornton LM, Wells-Di Gregorio S, Frierson GM, Jim HS, Carpenter KM et al. (2005) Traumatic stress, perceived global stress, and life events: prospectively predicting quality of life in breast cancer patients. Health Psychol 24(3):288–96. 10.1037/0278-6133.24.3.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loprinzi CE, Prasad K, Schroeder DR, Sood A (2011) Stress Management and Resilience Training (SMART) program to decrease stress and enhance resilience among breast cancer survivors: a pilot randomized clinical trial. Clin Breast Cancer 11(6):364–368. 10.1016/j.clbc.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 46.Anderson N, Ozakinci G (2018) Effectiveness of psychological interventions to improve quality of life in people with long-term conditions: rapid systematic review of randomised controlled trials. BMC Psychol 6(1):11. 10.1186/s40359-018-0225-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouchard LC, Yanez B, Dahn JR, Flury SC, Perry KT, Mohr DC et al. (2019) Brief report of a tablet-delivered psychosocial intervention for men with advanced prostate cancer: acceptability and efficacy by race. Transl Behav Med 9(4):629–637. 10.1093/tbm/iby089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penedo FJ, Fox RS, Oswald LB, Moreno PI, Boland CL, Estabrook R et al. (2020) Technology-based psychosocial intervention to improve quality of life and reduce symptom burden in men with advanced prostate cancer: results from a randomized controlled trial. Int J Behav Med 27(5):490–505. 10.1007/s12529-019-09839-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


